Abstract
Background:
Managing Parkinson’s non-motor symptoms (PD-NMS) is challenging. Traditional methods have limited efficacy for NMS. Acupoint therapy offers a safe and personalized option. There has been a growing number of studies on acupoint therapies for PD-NMS. However, a systematic review of their effectiveness and safety is currently not available. Therefore, this study aims to evaluate the effectiveness and safety of acupoint therapy for PD-NMS.
Methods:
PubMed, Web of Science, Cochrane Library, Embase, China National Knowledge Infrastructure, Wanfang Database, China Science and Technology Journal Database, and Chinese Biomedical Literature Database were searched. Data were analyzed using fixed or random effects models. Randomized controlled trials (RCT) in Chinese and English relating to acupoint therapy for NMS in PD (PD-NMS), individuals diagnosed with PD, primary and secondary outcome measures are HAMD, MMSE, MoCA, PDSS, PSQI were included. Cochrane risk-of-bias tool (ROB 2) for RCT was used. Meta-analyses were performed to calculate the RR and WMD. Subgroup analyses, sensitivity analyses, and funnel plot analyses were conducted.
Results:
Forty-two studies (3120 PD-NMS patients) from database establishment to May 2023 were included. According to the ROB 2 evaluation, risks of bias in random sequence generation, missing data and selective reporting were low, moderate in allocation concealment, and high in blinding. Acupoint therapy combined with CM or NDT was more effective than single-therapy in most outcome measures like effective rate, HAMD, MoCA, PDSS and PSQI, but the MMSE difference was non-significant.
Conclusion:
Due to the large number of research subjects, differences in the severity of the diseases, and possible variations of the intervention details, there is a certain degree of heterogeneity in the research results. However, acupoint therapy or acupoint therapy combined with CM could be an option for the treatment of PD-NMS in the future.
Systematic review registration:
This review protocol has been registered in PROSPERO (ID: CRD42023426305). https://www.crd.york.ac.uk/PROSPERO/.
Introduction
Parkinson’s disease (PD) is currently conceptualized as a complex neuropsychiatric disorder resulting from a reduction in the number of dopamine-producing cells in the substantia nigra (1). This disease is a common condition; in 2016, approximately 6.1 million people worldwide were affected (2, 3). Although the cause of PD remains unknown, various genetic factors have been identified (4). PD is currently described as a multisystem neurodegenerative disease because it simultaneously involves the central, enteric, and autonomic nervous systems; adaptive immune system; and gastrointestinal tract (5). Clinically, PD is characterized by the occurrence of both motor symptoms and non-motor symptoms (PD-NMS) (6–10). PD-related NMS (PD-NMS) represent a major source of disability, which may exceed the burden from PD-related motor impairments (11, 12). Furthermore, PD-NMS such as hyposmia/anosmia, rapid eye movement sleep behavior disorder, depression, anxiety, cardiac sympathetic denervation, and constipation may precede dopaminergic neuronal loss—and the consequent motor symptoms—by several years (13, 14).
Currently, the first-line therapy for patients with PD is either daily oral levodopa or a dopamine agonist. Levodopa offers good control of motor symptoms but does not modify disease evolution (11, 15). The effectiveness of pharmacological treatments tends to wear off over time, and patients need to take increasingly higher drug doses, thus imposing a heavy burden on families and society (16). Levodopa may induce or exacerbate the psychiatric symptoms in PD patients: such as hallucinations, delusions, depression, anxiety. Dopamine agonists have no significant effect on improving cognitive and sleep disorders and are associated with more side effects (e.g., nausea, orthostatic symptoms, and sleep disturbances) (17). In particular, neuropsychiatric PD-NMS may severely alter the quality of life of both patients and their caregivers (18, 19). Given the limitations of pharmacological therapy, either in combination with or as an alternative to dopamine replacement therapy, complementary and alternative medicine and integrative medicine approaches are widely used to improve brain and overall health in people with PD (20–22).
For example, lifestyle modifications can provide therapeutic benefits for patients with PD. Aerobic exercise continues to be a key treatment that is potentially neuroprotective in PD (23–25). Exercise promotes substances that have been termed “exerkines” which can influence homeostasis (26, 27). A clinical study confirmed long-term Tai Chi training can improve motor function, especially gait and balance in PD (28). Other complementary alternative therapies, such as thermal rehabilitative treatments statistically improved motor function, balance, QoL, and psychological well-being (29). Traditional Chinese medicine (TCM) has also been widely applied to improve PD symptoms and control disease evolution (18, 30, 31).
Acupoint therapy is an essential part of TCM and has many validated treatment effects. It includes many kinds of alternative treatments, such as acupuncture, moxibustion, massage, acupoint injection, acupressure, and cupping. These therapies can be considered complementary and alternative medicine (22, 32, 33). These treatments can have direct and positive effects on the body, both on motor symptoms and PD-NMS, when an acupoint is stimulated (34). Some studies have reported that acupuncture stimulation can inhibit an increase in α-synuclein and boost the survival rate of dopaminergic neurons in the substantia nigra (35, 36). Moreover, acupuncture can improve gut microbial dysregulation and inhibit neuroinflammation in the substantia nigra and striatum, all of which are associated with a PD phenotype (35). Acupuncture also inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of PD. Similarly, the stimulation of acupoints such as CV-12, RN-7, ST-36, and LR-3 via electroacupuncture reportedly regulates brain/gut levels of peptides such as neuropeptide Y, cholecystokinin, gastrin, and peptide YY, thereby reducing neuronal apoptosis, the nigral inflammatory response, and oxidative stress in the substantia nigra of PD model rats (37).
Notably, most of the published reviews on acupoint therapy for PD have focused on motor symptoms rather than PD-NMS. In addition, few reviews have systematically summarized the efficacy of different types of acupuncture in various functional disorders in PD. Researchers have also often overlooked the different categories of acupuncture and other types of acupoint therapy, thus neglecting the importance of acupuncture points in treating disease. We have therefore conducted a systematic review and meta-analysis of the available literature to determine the effects of acupoint therapy combined with conventional medication (CM) on the regulation of PD-NMS. Our study included different types of acupoint interventions for these patients. And CM therapies include Chinese medicine and western medicine.
Methods and analysis
Protocol and registration
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for systematic review protocols (38) and meta-analysis checklist (39). This review protocol has been registered in PROSPERO (ID: CRD42023426305).
Search methods
An electronic literature search was performed using the following databases for articles published in English and Chinese as of May 2023: PubMed, Web of Science, Cochrane Library, Embase, China National Knowledge Infrastructure, Wanfang Data, China Science and Technology Journal Database, and Chinese Biomedical Database. Medical Search Heading (MeSH) terms and their synonyms (free text) were combined as search terms using the Boolean operators “AND” and “OR.” The following MeSH terms were used: “Parkinson Disease,” “Acupuncture,” “Acupressure,” “Massage,” “Cupping Therapy,” “moxibustion,” “acupoint,” “acupoint injection,” “auricular acupuncture,” “scraping therapy,” and “randomized controlled trial.” The detailed search strategy is shown in the box. For more details in Supplementary material 3.
Search items in PubMed.
| No. | Search terms |
|---|---|
| #1 | Acupoint. Mesh |
| #2 | Acupuncture Points. Mesh |
| #3 | (Acupuncture Point) or (Point, Acupuncture) or (Acupoints) or (Acupoint). ti. ab |
| #4 | Acupuncture. Mesh |
| #5 | Acupuncture. ti. ab |
| #6 | Acupressure. Mesh |
| #7 | Acupressure. ti. ab |
| #8 | Acupuncture, auricular. Mesh |
| #9 | (Acupunctures, Ear) or (Ear Acupunctures) or (Auricular Acupuncture) or (Acupuncture, Auricular) or (Auricular Acupunctures). ti. ab |
| #10 | Massage. Mesh |
| #11 | Massage. ti. ab |
| #12 | (Manipulations, Musculoskeletal) or (Manipulation Therapy) or (Manipulative Therapies) or (Therapies, Manipulative) or (Therapy, Manipulation) or (Manipulation Therapies) or (Manual Therapies) or (Therapy, Manual). ti. ab |
| #13 | Cupping therapy. Mesh |
| #14 | (Cupping Therapies) or (Therapy, Cupping) or (Cupping Treatment) or (Treatment, Cupping). ti. ab |
| #15 | Moxibustion. Mesh |
| #16 | Moxibustion. ti. ab |
| #17 | Acupoint injection. Mesh |
| #18 | Acupoint injection.ti. ab |
| #19 | #1 or #2–18 |
| #20 | Randomized controlled trial. Mesh |
| #21 | Controlled clinical trial. ti. ab |
| #22 | Randomized. ti. ab |
| #23 | Randomly. ti. ab |
| #24 | Trial. ti. ab |
| #25 | #20 or #21–24 |
| #26 | Parkinson’s disease. Mesh |
| #27 | Parkinson’s disease. ti. ab |
| #28 | Parkinsonian disorders. ti. ab |
| #29 | #26 or #27–28 |
| #30 | #19 and #25 and #29 |
Criteria for consideration of studies in this review
Types of studies
RCTs on the combination treatment of acupoint therapy and CM for PD were included in the present review. Protocols, case reports, reviews, letters, cell experiments, animal experiments, and non-RCTs were excluded.
Types of participants
Individuals diagnosed with PD, with no restrictions on age, sex, race, or disease duration.
Types of interventions
Experimental groups with all types of acupoint therapy (including acupuncture, moxibustion, acupressure, massage, acupoint injection, cupping, skin scraping, transcutaneous acupoint electrical stimulation, and acupotomy) were included. The different types of acupoint therapy were then analyzed again as different interventions.
Types of comparisons
Control groups that received CM, which was defined as all types of anti-PD drugs used alone or in combination, were included. Control groups were also able to receive CM combined with a sham intervention.
To summarize, we included and classified the following comparisons:
-
Acupoint therapy combined with CM versus CM used alone or in combination.
-
Acupoint therapy combined with CM versus CM combined with a sham intervention.
Types of outcome measures
We extracted effect sizes at the first time point after the end of interventions. In the current review, we only included RCTs that reported the following main outcomes (i.e., that assessed PD-NMS using effective and validated scales): total effective rate, Hamilton Depression Scale (HAMD), Mini-Mental State Examination Scale (MMSE), Montreal Cognitive Assessment Scale (MoCA), PD Sleep Scale (PDSS), Pittsburgh Sleep Quality Index (PSQI), and Self-Assessment of Quality of Life for Constipation Patients (PAC-QoL). Only Chinese and English articles that met the aforementioned inclusion criteria were included.
Study selection process
Two independent authors (WH and HZ) revised the titles and abstracts of the searched papers to determine suitable studies. Then, the two authors revised the full texts of the retrieved reports independently. Any conflicts between authors were solved by the third author (ZW).
Data extraction
Two independent authors (WH and HZ) extracted data. The following data were extracted in a predefined data collection form: first author, year of publication, language, sample size, demographic data of participants, baseline characteristics of patients, diagnostic criteria, inclusion and exclusion criteria for participants, experimental and control interventions, course of treatment, frequency, location of the study, outcomes, and safety. Any conflicts between authors were solved by the third author (ZW).
Quality assessment
The Cochrane risk-of-bias tool (ROB 2) for RCTs was used to assess potential bias in the included studies (40). This risk-of-bias tool consists of the following seven domains: (I) random sequence generation, (II) allocation concealment, (III) blinding of participants and personnel, (IV) blinding of outcome assessment, (V) incomplete outcome data, (VI) selective reporting, and (VII) other bias (e.g., we assessed trials with no reported monitoring of self-acupressure procedures as having a high risk of compliance bias). Each RCT was categorized as having a low, high, or unclear risk of bias in each domain.
Data synthesis and statistical analysis
Review Manager (RevMan, version 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and STATA (version 17.0) software were used to quantitatively analyze the included studies, as follows. (I) Combination of effects: continuous outcomes (HAMD, MMSE, PDSS, MoCA, PSQI, and PAC-QoL) were evaluated using weighted mean differences (WMDs), whereas dichotomous outcomes (rates of improvement) were assessed using risk ratios (RRs). The 95% confidence interval (CI) was evaluated for all effect sizes, with a 95% CI excluding the point of no effect, indicating significance. When the median and first and third quartiles were provided, the mean and standard deviation were estimated using the formula developed by Wan et al. (41). (II) Heterogeneity test: the Q-test was performed to assess heterogeneity among studies. If p > 0.10, the results of multiple similar studies were considered homogeneous. If p > 0.10 and I2 ≥ 0 and ≤ 50%, a fixed effects model was used for the integrative analysis of studies. If p ≤ 0.10 or I2 > 50%, the results of multiple similar studies were considered heterogeneous, and a sensitivity analysis was performed (42). Articles were removed sequentially to observe changes in heterogeneity, WMDs, and RRs. If heterogeneity was altered after the removal of an article, it was considered a source of heterogeneity, and the underlying reason was analyzed. In contrast, if heterogeneity remained unaltered, a random effects model was used for a more conservative evaluation of the intervening effects. Subgroup analyses were performed based on acupoint methods (single acupuncture versus single moxibustion versus acupuncture combined with other therapies). When ≥10 studies were included in the meta-analysis, Egger’s test was used to assess publication bias, with p < 0.05 indicating significance (43).
Ethical considerations
Although the studied specimens were human, this is a secondary analysis of published articles and does not involve ethical issues.
Results
Study selection
From the eight databases, 1711 articles were selected. Of these, 731 articles were removed as duplicates. Of the remaining 980 articles, many articles were excluded because they failed to meet the inclusion criteria. Finally, 60 full-text articles that met the eligibility criteria were selected. According to the inclusion and exclusion criteria, 42 articles were eventually included in the review (44–85). The study selection process is illustrated in Figure 1.
Figure 1

Literature screening process.
Study characteristics and study quality
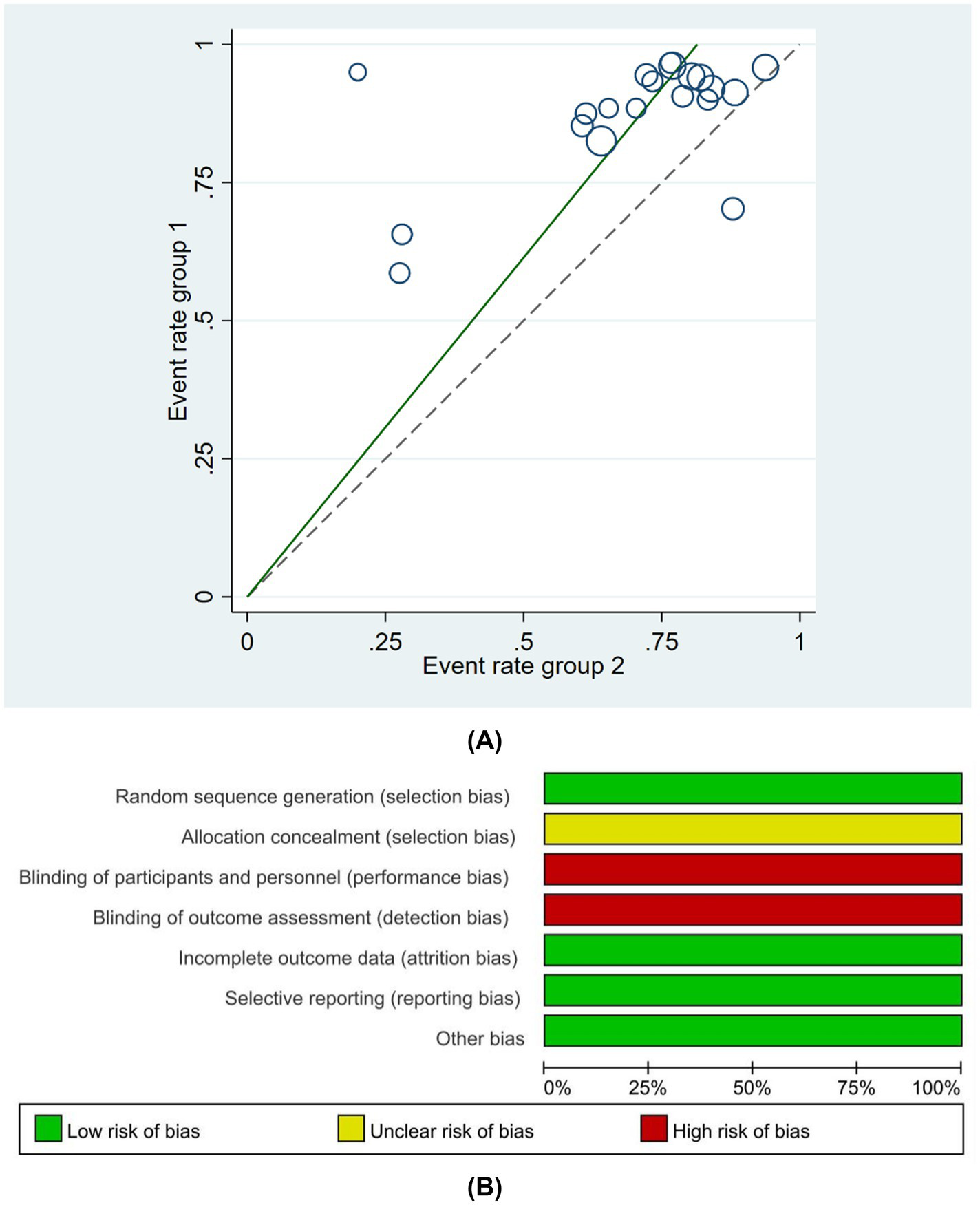
The baseline information of the 42 studies included in this systematic review and meta-analysis is summarized in Supplementary Table 1. Of the 3,120 patients with PD, 1556 were assigned to the experimental group and 1,564 were assigned to the control group, with 1743 male patients and 1,377 female patients. Of the 42 studies, 24 were randomized into groups using the random number table method (45–49, 52–78, 81–85), two were randomized according to the order of treatment (63, 73), two were grouped by computer random sampling (69, 72), one (50) was grouped by convenient sampling selection, one was grouped by numerical random sampling (64), and one was grouped by envelope (85). The remaining 11 studies (51, 55–59, 65, 68, 77, 78, 81) did not mention the specific randomization method. Of the 42 studies, none mentioned allocation concealment. Given the specificity of acupuncture procedures, the studies displayed a high risk of bias in terms of the blinding of participants and personnel; although one study (85) used a single-blinding method, no other studies mentioned the blinding method that was used. All 42 studies reported outcomes, and no studies demonstrated attrition bias. In summary, the risk of bias in random sequence generation was low. The risk of bias of allocation concealment was moderate. The risks of bias of blinding of participants and personnel and outcome assessment were high. The risks of bias of missing outcome data and selective reporting were low. The methodological quality of studies is shown in detail in Figure 2. A summary of the basic characteristics of the included studies is shown in Supplementary Table 2. All of the studies mentioned sample size. 41 studies mentioned the proportion of female in their research, and women were more than men in only five studies. 37 of 42 studies mentioned the disease duration, and 33 studies had patients with disease of less than 10 years.
Figure 2

(A) Risk of bias summary, (B) Risk of bias domain for each included study.
Meta-analysis findings
Effective rates
Twenty-eight RCTs reported the effective rates in PD-NMS (49–58, 60, 61, 63, 65, 68, 70, 71, 73–76, 78, 81–85). The effective rates were significantly better in patients treated with acupoint therapy combined with CM or non-drug treatment (NDT) than in those treated with CM or NDT alone (RR = 1.25; 95% CI, 1.16 to 1.35; Z = 5.67; p = 0). Heterogeneity was observed among studies (chi-squared = 93.24; df = 27 [p = 0]; I2 = 71%) (Figure 3).
Figure 3

Forest plot of comparison-acupoint therapy combined with CM or non-drug therapies vs. CM or non-drug therapies alone on response rate.
In the subgroup analysis, 20 studies (49, 52, 56, 60, 63–65, 65, 68, 70, 71, 73–76, 78, 81–85) reported a combination of acupoint therapy plus western drugs versus western drugs alone. The effective rates of a combination of acupoint therapy plus western drugs were significantly better than those of western drugs alone. A fixed effects model was used for the analysis (RR = 1.2; 95% CI, 1.11 to 1.30; Z = 4.38; p = 0; heterogeneity test: chi-squared = 56.05, df = 19, p = 0, I2 = 66%) (Figure 4). Further investigation using L’Abbe and Galbraith radial plots indicated that four articles had a strong possibility of heterogeneity (Figures 5A,B). A heterogeneity search was thus required. A sensitivity analysis of the 20 articles revealed that four studies (56, 63, 70, 84) had a relatively large influence on heterogeneity. After removing these studies, the random effects method was selected for the meta-analysis (RR = 1.15; 95% CI, 1.08 to 1.22; Z = 4.38; p < 0.00001; heterogeneity test: chi-squared = 26.64, df = 15, p = 0.032, I2 = 43.7%) (Supplementary Figure 1). A funnel plot was drawn to investigate publication bias; its symmetry indicated no publication bias (Supplementary Figure 2). However, the Egger’s bias test (with p < 0.05) indicated that there was publication bias in the 20 included studies (Supplementary Figure 3). The aforementioned asymmetrical funnel diagram was thus processed using the shear compensation method (Supplementary Figure 4); the seven points in the square represent the effect sizes of the literature that will need to be included in future studies. Combined with the aforementioned funnel diagram, the results of further inclusion are close to seven studies (52, 56, 63, 64, 70, 71, 84). The symmetry of the funnel plot can then be conducted, preventing publication bias.
Figure 4

Forest plot comparing the effects of acupoint therapies plus western drugs versus western drugs alone on effective rates.
Figure 5
(A) L’Abbe plot comparing the effects of acupoint therapies plus western drugs versus western drugs alone on effective rates. (B) Galbraith radial plot comparing the effects of acupoint therapies plus western drugs versus western drugs alone on effective rates.
Eight studies (50, 51, 53–55, 57, 58, 61) reported comparisons between a combination of acupoint therapy plus other therapies versus other therapies alone. The effective rates of acupoint therapy combined with other therapies were better than those of other therapies alone. A fixed effects model was used for the analysis (RR = 1.42; 95% CI, 1.30 to 1.55; Z = 7.72; p = 0.0002; heterogeneity test: chi-squared = 25.67, df = 7, p = 0.0006, I2 = 72.7%) (Figure 6). Further examination of L’Abbe and Galbraith radial plots indicated a strong possibility of heterogeneity in one article (48) (Supplementary Figures 5, 6). A heterogeneity search was therefore required. A sensitivity analysis of the eight articles revealed that one article had a large impact on heterogeneity. After removing this study, the combined effect variables of the meta-analysis were large. Furthermore, a subsequent heterogeneity test revealed no heterogeneity in the remaining seven articles. A meta-analysis with fixed effects was performed after exclusion (RR = 1.29; 95% CI, 1.18 to 1.40; Z = 5.88; p < 0.001; heterogeneity test: chi-squared = 6.37, df = 6, p = 0.38, I2 = 5.8% [<50%]) (Supplementary Figure 7). This finding suggests that the efficacy of acupoint therapy combined with other therapies is significantly better than that of other therapies alone. A funnel plot was drawn to investigate publication bias; its symmetry indicated no publication bias (Supplementary Figure 8). The Egger’s bias test (p > 0.05) also indicated no publication bias (Supplementary Figure 9).
Figure 6

Forest plot comparing the effects of acupoint therapies plus other therapies versus other therapies alone on effective rates.
HAMD scores
Thirteen RCTs reported the use of HAMD for assessing depression status (58, 59, 61, 65, 66, 73, 74, 76, 78–81, 84). HAMD scores were significantly better in patients treated with acupoint therapy combined with CM or NDT than in those treated with CM or NDT alone. A random effects model was used for the analysis (WMD = −2.14; 95% CI, −2.9 to −1.38; Z = 5.54; p < 0.00001). Heterogeneity was observed among the studies (chi-squared = 74.59; df = 12 [p < 0.00001]; I2 = 83.9%) (Figure 7). The results of a sensitivity analysis were not significantly different from the aforementioned results.
Figure 7

Forest plot comparing the effects of acupoint therapies plus CM or NDT versus CM or NDT alone on HAMD.
In the subgroup analysis, 10 RCTs (59, 65, 66, 73, 74, 76, 78, 79, 81, 84) reported comparisons between a combination of acupoint therapy with drugs versus drugs alone, and 3 RCTs (58, 61, 80) reported comparisons between acupoint therapy combined with other therapies versus other therapies alone. The fixed effects model results suggested strong heterogeneity in the 10 studies. After deleting two studies, heterogeneity still existed; a random effects model was therefore used for the analysis (WMD = −2.1; 95% CI, −3.15 to −1.05; Z = 3.91; p < 0.00001; heterogeneity test: chi-squared = 73.68, df = 9 [p = 0], I2 = 87.8%) (Supplementary Figure 10). The HAMD scores of patients treated with a combination of acupoint therapy and drugs were better than those of patients treated with drugs alone.
Acupoint therapy combined with other therapies was more effective than other therapies alone in improving HAMD scores (WMD = −2.26; 95% CI, −2.77 to −1.75; Z = 8.71; p < 0.00001; heterogeneity test: chi-squared = 0.9, df = 2 [p > 0.05], I2 = 0%) (Supplementary Figure 11). Heterogeneity was not assessed because only three studies reported comparisons between acupoint therapy combined with other therapies versus other therapies alone. Additionally, a meta-regression analysis of different acupoint therapies using “type of therapy” as an ordinal variable revealed no differences between acupuncture and moxibustion or acupoint sticking (Supplementary Figure 12).
A funnel plot was drawn to investigate publication bias; its symmetry indicated no publication bias (Supplementary Figure 13). Similarly, Egger’s test (p > 0.05) indicated no publication bias (Supplementary Figure 14).
MMSE scores
Two RCTs (45, 46) reported the use of MMSE, which assesses intellectual status and cognitive function. MMSE scores appeared to be better in patients treated with a combination of acupoint therapy and other therapies than in those treated with other therapies alone, but this effect was not significant. A random effects model was used for the analysis (WMD = 2.33; 95% CI, −0.99 to 5.65; Z = 1.37; p > 0.05). Heterogeneity was observed among studies (chi-squared = 14.45; df = 1 [p = 0.0001]; I2 = 93%) (Figure 8). The results of the sensitivity analysis were not significantly different from the aforementioned results.
Figure 8

Forest plot comparing the effects of acupoint therapies with other therapies versus other therapies alone on MMSE.
MoCA scores
Eight RCTs (45, 59, 68, 69, 71, 75, 77, 80) reported the use of MoCA, which assesses cognitive function. MoCA scores were significantly better in patients treated with a combination of acupoint therapy with other therapies than in those treated with other therapies alone. A random effects model was used for the analysis (WMD = 2.73; 95% CI, 0.63 to 4.84; Z = 2.54; p < 0.00001). Heterogeneity was observed among studies (chi-squared = 179.26; df = 7 [p < 0.0001]; I2 = 96%) (Figure 9). Furthermore, meta-regression analysis was conducted using “type of therapy” as an ordinal variable, there were no differences between acupuncture and acupoint sticking (Supplementary Figure 15).
Figure 9

Forest plot comparing the effects of acupoint therapies with other therapies versus other therapies alone on MoCA.
PDSS scores
Nine RCTs (44, 59, 60, 62, 64, 66, 82, 83, 85) reported the use of PDSS, which assesses cognitive function. PDSS scores were significantly better in patients treated with a combination of acupoint therapy with drugs than in those treated with drugs alone. A random effects model was used for the analysis (WMD = 15.87; 95% CI, 6.27 to 25.47; Z = 3.24; p = 0.001). Heterogeneity was observed among studies (chi-squared = 170.54; df = 8 [p < 0.0001]; I2 = 95.3%) (Figure 10). Meta-regression analysis using “type of therapy” as an ordinal variable was conducted, there were no differences between acupuncture and acupoint sticking (Supplementary Figure 16). The funnel plot test for publication bias is shown (Supplementary Figure 17). Egger’s bias test (p > 0.05) indicated no publication bias (Supplementary Figure 18).
Figure 10

Forest plot comparing the effects of acupoint therapies with drugs versus drugs alone on PDSS.
PSQI scores
Six RCTs reported the use of PSQI, which assesses sleep quality. A random effects model revealed that sleep quality was significantly better in patients treated with a combination of acupoint therapy with other therapies than in those treated with other therapies alone (WMD = −3.28; 95% CI, −4.55 to −2.00; Z = 5.03; p < 0.00001). Heterogeneity was observed among studies (chi-squared = 101.91; df = 5 [p < 0.0001]; I2 = 95%) (Figure 11). The results of the sensitivity analysis were not significantly different from the aforementioned results. When meta-regression analysis was conducted used “type of therapy” as an ordinal variable, the result showed no difference between acupuncture and acupoint sticking (Supplementary Figure 19). The funnel plot test for publication bias is shown (Supplementary Figure 20). Egger’s bias test indicated no publication bias (Supplementary Figure 21).
Figure 11

Forest plot comparing the effects of acupoint therapies with other therapies versus other therapies alone on PSQI.
PAC-QoL scores
Five RCTs reported the use of PAC-QoL, which assesses quality of life. A random effects model revealed that PAC-QoL was significantly better in patients treated with a combination of acupoint therapy with other therapies than in those treated with other therapies alone (WMD = −19.48; 95% CI, −30.47 to −8.49; Z = 3.47; p = 0.0005). Heterogeneity was observed among studies (chi-squared = 149.55; df = 4 [p < 0.00001]; I2 = 97.3%) (Figure 12). Meta-regression analysis using “type of therapy” as an ordinal variable,” showed there were no differences between treatments (Supplementary Figure 22). The results of the sensitivity analysis were not significantly different from the aforementioned results. Egger’s bias test indicated no publication bias (Supplementary Figure 23). The results of the meta-analysis for all outcome indicators were summarized in Supplementary Table 3.
Figure 12

Forest plot comparing the effects of acupoint therapies with other therapies versus other therapies alone on PAC-QoL scores.
Adverse events
Safety conditions were reported in 12 RCTs (44, 64, 65, 66, 69, 71, 74, 75, 78, 79, 82, 85) and adverse effects were not mentioned in 30 studies. Of the 12 studies that mentioned adverse effects, seven trials (21, 65, 69, 70, 78, 79, 82) reported a negative association with acupoint therapies; the other five trials (64, 66, 71, 75, 85) reported no such association. The observed adverse effects were mainly gastrointestinal symptoms (such as discomfort, vomiting, and nausea), subcutaneous hematoma, headache, or were related to the insertion of needles. All of these adverse effects were mild and showed signs of reversibility.
Discussion
Medication is the main treatment for PD, but its long-term use has many potential complications. Dopaminergic treatments also have limitations in the treatment of PD-NMS (86). In the present study, we reviewed the feasibility, efficacy, and safety of acupoint therapies for managing PD-NMS using 42 included studies. To the best of our knowledge, this is the first meta-analysis to address this specific question. Our preliminary data indicated the feasibility of acupoint therapies for PD-NMS without serious adverse events. The meta-analysis revealed that acupoint therapy combined with CM was superior to CM alone in terms of the total effective rate, as well as HAMD, MMSE, MoCA, PDSS, PSQI, and PAC-QoL scores. However, only two included studies measured MMSE.
One previous review (87) reported that acupoint therapy is a helpful intervention for relieving the motor symptoms of PD, and another indicated that acupuncture can relieve PD-NMS (88). To date, however, there have been no reviews of acupoint therapy other than acupuncture for PD-NMS treatment. Furthermore, the evidence for acupoint therapy in the management of PD-NMS remains inconclusive because of the limited availability of clinical trials, often with small sample sizes and suboptimal methodological quality (87–89). Compared with previous reviews, the present study used a more comprehensive search by including more recently published trials. Although evidence for the use of acupoint therapy to alleviate PD-NMS was mixed in the current study, likely because of high heterogeneity in participant characteristics and study designs, some interesting observations were noted. First, thirteenth studies used HAMD to evaluate anxiety and depression, nine studies used PDSS and six studies used PSQI to evaluate sleep quality, two studies used MMSE and eight studies used MoCA to evaluate intellectual status and cognitive function. This finding indicates that sleep disturbances, anxiety, depression, and reduced cognitive function are common in patients with PD. Second, only one study (44) evaluated acupoint sticking therapy in terms of PDSS scores, and one study evaluated this therapy in terms of PAC-QoL scores (50). These findings indicate that acupoint sticking for the treatment of PD-NMS is relatively uncommon, and conclusive conclusions cannot be made about its efficacy and safety. Further exploration is therefore required to explore whether PD-NMS patients can benefit from acupoint sticking therapy.
In terms of effective rates, our meta-analysis results indicated that acupoint therapy combined with CM or NDT showed more benefits than treatment with CM or NDT alone. Effective rates is the percentage of improvement in the patient’s condition with therapy. Studies using this indicator are based on TCM clinical symptom improvement criteria. In other previous studies, acupoint therapy combined with CM or NDT was also reportedly more effective, consistent with our findings (49–51, 53–58, 60, 61, 63–65, 68, 70, 71, 73–76, 78, 81–85). In addition, our subgroup analysis based on intervention type indicated that a combination of acupoint therapy plus western drugs was significantly better than western drugs alone, and that a combination of acupoint therapy plus other therapies was better than other therapies alone. Also, we performed subgroup analysis according to the treatment method in control group and the heterogeneity still remained high. We considered that this might be due to the large number of included studies and the relative diversity of acupoint selection or treatment method in control group. In conclusion, acupoint therapy may be applicable for the treatment of PD-NMS.
Depression is a common feature in PD, with an estimated prevalence of 30–50% (90). HAMD is most commonly applied to evaluate depression (96). Our findings revealed that acupoint therapy combined with CM or NDT resulted in a significant improvement in depression compared with CM or NDT alone. This finding is consistent with some previous studies (59, 65, 66, 73, 74, 76, 78, 79, 81, 84). Moreover, a previous qualitative review focusing on PD-NMS reported that acupuncture likely has a positive effect on PD-related depression (91). Our results showed the heterogeneity still remained high in these researches of acupoint therapy with drugs versus drugs alone after conducting subgroup analysis, though there was no heterogeneity in these researches of acupoint therapy combined with other therapies. We considered that this might be due to the large number of included studies and the relative diversity of acupoint selection or treatment method in control group. Although acupoint therapy was able to effectively ameliorate PD-related depressive symptoms, more evidence is needed because the sensitivity analysis suggested relative instability in the results.
In terms of cognition, we found evidence of an overall greater effect of acupoint therapy combined with CM than CM alone. Eight studies (45, 59, 68, 69, 71, 75, 77, 80) reported that acupoint therapy combined with CM improved MoCA scores more than CM alone. And we hypothesized that the high heterogeneity was due to the diversity of treatment method and duration differences. Two studies (45, 46) demonstrated that acupuncture combined with CM improved MMSE scores more than CM alone, but the effect was not significant. We speculate that the discrepancy between MMSE scores and MoCA scores may be attributed to the fact that only two studies using MMSE scores as an outcome measure were retrieved, which showed significant heterogeneity. Therefore, more related research is needed to support this finding. Other studies have also indicated that acupuncture exerts a positive effect on global cognitive function (92, 93).
In terms of PD-related sleep quality, nine RCTs (44, 59, 60, 62, 64, 66, 82, 83, 85) reported the use of PDSS for assessing sleep quality. PDSS scores were significantly better in patients treated with a combination of acupoint therapy with drugs than in those treated with drugs alone. However, the heterogeneity was high. We reviewed the nine studies and concluded that the reason may be the diversity of acupoint selection and the difference of treatment course. Six RCTs reported the use of PSQI for assessing sleep quality. Similar to the PDSS results, PSQI scores were significantly better in patients treated with a combination of acupoint therapy with CM or other therapies than in those treated with CM or other therapies alone. The high heterogeneity may be due to the diversity of acupoint therapy method or treatment method in control group. In addition, acupuncture is reportedly effective for various types of insomnia (94). However, the effects of types of acupoint therapy other than acupuncture on this PD-NMS need to be further studied and discussed.
Constipation is a predominant early PD-NMS, and some evidence suggests that the initial pathology of PD arises inside the central nervous system (95). Our results regarding PAC-QoL revealed that the effects of acupoint therapy combined with CM for PD-related constipation were better than with CM alone. The high heterogeneity may be due to the diversity of treatment method in control group or the difference of treatment course. Although acupuncture is considered an effective and safe treatment for constipation in patients without PD on the basis of previous randomized trials (95, 96), more high-quality evidence-based studies are needed to confirm the effectiveness of acupoint therapy on PD-related constipation.
Our study showed acupoint therapy combined with CM or NDT yielded superior results. According to meridian and acupoints theory in TCM, acupoint therapy regulates Qi (energy) and blood and maintains the Yin and Yang (two energy subdivisions) balance of internal organs by stimulating acupoints corresponding to symptomatic organs or body areas, thereby relieving symptoms. Empirical studies have found that acupoint therapy improves PD-NMS by modulating the levels of 5-hydroxytryptamine (5-HT), dopamine (DA) and brain-derived neurotrophic factor (BDNF) in serum (77, 84).
In the aforementioned results, almost all outcome indicators showed large heterogeneity. This may be attributable to the following reasons: (i) the relatively small sample sizes of the included articles; (ii) differences in the professional levels of acupuncturists or practitioners, the selection of acupuncture points, and/or the frequency of therapies; (iii) the different initial severity levels of patients included in the trials, which may have resulted in clinical heterogeneity between studies; and (iv) differences in the duration of each acupoint therapy and the intervention intensity across all studies. In addition, most studies had a short intervention time, meaning that their results only reflect the effects of short-term acupoint therapy for PD-NMS. Therefore, there is a need for large-sample, multicenter, long-term RCTs in the future. PD is a chronic progressive disease. At present, the challenge is not only to relieve symptoms but also to delay the progression of the disease. Therefore, the long-term effects of acupoint therapy in treating PD-NMS should also be taken into consideration. However, the present review highlights the possible therapeutic effectiveness of acupuncture therapies for PD-NMS.
Limitations and strengths
To our knowledge, our study is the first to comprehensively examine acupoint therapy for PD-NMS, although some symptoms (e.g., dizziness, bladder urgency, and apathy) were not reviewed because of a lack of original data. Our study combined data from 42 eligible studies that covered an array of common PD-NMS; our findings provide potential evidence for several non-pharmaceutical therapies for PD-NMS. Previous studies have mostly focused on motor symptoms (97, 98) or have reviewed the effects of complementary therapies (including acupuncture) for motor and non-motor symptoms but did not provide a quantitative analysis (99). Therefore, our study represents a comprehensive assessment of the currently available evidence regarding PD-NMS.
Given that acupoint therapy is a manipulated intervention, blinding for practitioners is hard to implement in most studies (even in studies outside of PD) (100). Consequently, concerns about the risk of bias in our study mostly existed in the domains of blinding—particularly in participant and personnel blinding—and weakened the strength of the evidence, and thus of our conclusions. Furthermore, in the present study, we included acupoint sticking therapy as an intervention method, and confirmed that acupoint sticking combined with conventional care was more effective for PD-NMS than conventional care alone. Some of the pooled results also continued to exhibit high levels of heterogeneity and were unstable in the sensitivity analyses, despite restricting the criteria for study enrollment and performing subgroup analyses. Another major limitation of the study was that all trials were conducted in China; this may affect the generalizability of our findings. In addition, there were no studies on Guasha, cupping, or acupressure interventions in PD-NMS, so we were unable to assess the efficacy of these treatments for PD-NMS. Our findings on the effectiveness of acupoint therapy in PD-NMS should therefore be interpreted with caution.
Implications for clinical practice
Some results from the present study may provide guidance for clinical applications. First, for patients with PD-related depression or decreased quality of life or cognition, acupoint therapy may be a good choice. Second, acupoint therapy combined with CM may be suitable for treating PD-related depression, cognitive function, insomnia, and constipation. Third, acupoint sticking therapy may be used to treat constipation.
Conclusion
The results of the present meta-analysis indicate that acupoint therapy may be associated with improvements in various PD-NMS including depression, intellectual status/cognitive function, sleep quality, and constipation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WH: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. WZ: Data curation, Formal analysis, Investigation, Software, Writing – original draft. SX: Data curation, Formal analysis, Software, Writing – original draft. YZ: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. ZW: Conceptualization, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Science and Technology Program (No. 2024NSFSC0560), Traditional Chinese Medicine Intelligent Diagnosis and Treatment Equipment Development: Meridian acupoint instrument for neurodegenerative diseases (No. 2022ZDZX0033), Clinical intervention of “sleep aid decoction” combined with Estazolam on subhealthy sleep disorder population (No. 2022-YF05-01827-SN), Sichuan Province Chinese Medicine sub-health industry product development and innovation team (No. 2022C006), Study on traditional Chinese medicine management strategy of “Internet +” coordinated management for insomnia in elderly hospitalized patients under normal epidemic prevention and control (No. 2023MS437), Study on the safe withdrawal and synergistic effect of acupuncture on drug-dependent insomnia from liver (No. 2023MS225) and Evaluation of clinical advantages of integrated intervention of Chinese and Western medicine on “reducing side effects” in elderly patients with refractory insomnia (No. 2022147), Study on the mechanism of multiple regulatory effects of acupuncture therapy on drug-dependent insomnia from the liver based on multimodal fMRI technology (No. 2022NSFSC0858).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1381500/full#supplementary-material
References
1.
Balestrino R Schapira A . Parkinson disease. Eur J Neurol. (2020) 27:27–42. doi: 10.1111/ene.14108
2.
Bloem BR Okun MS Klein C . Parkinson’s disease. Lancet. (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
3.
Tolosa E Garrido A Scholz SW Poewe W . Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. (2021) 20:385–97. doi: 10.1016/S1474-4422(21)00030-2
4.
Huq AJ Sexton A Lacaze P Masters CL Storey E Velakoulis D et al . Genetic testing in dementia-A medical genetics perspective. Int J Geriatr Psychiatry. (2021) 36:1158–70. doi: 10.1002/gps.5535
5.
Nandipati S Litvan I . Environmental Exposures and Parkinson's Disease. Int J Environ Res Public Health. (2016) 13:881. doi: 10.3390/ijerph13090881
6.
Costa HN Esteves AR Empadinhas N Cardoso SM . Parkinson's Disease: A Multisystem Disorder. Neurosci Bull. (2023) 39:113–24. doi: 10.1007/s12264-022-00934-6
7.
Johnson ME Stecher B Labrie V Brundin L Brundin P . Triggers, Facilitators, and Aggravators: Redefining Parkinson's Disease Pathogenesis. Trends Neurosci. (2019) 42:4–13. doi: 10.1016/j.tins.2018.09.007
8.
Kustrimovic N Comi C Magistrelli L Rasini E Legnaro M Bombelli R et al . Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation. (2018) 15:205. doi: 10.1186/s12974-018-1248-8
9.
Chen Z Chen S Liu J . The role of T cells in the pathogenesis of Parkinson's disease. Prog Neurobiol. (2018) 169:1–23. doi: 10.1016/j.pneurobio.2018.08.002
10.
Postuma RB Berg D Stern M Poewe W Olanow CW Oertel W et al . MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
11.
Gallagher DA Lees AJ Schrag A . What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them?Mov Disord. (2010) 25:2493–500. doi: 10.1002/mds.23394
12.
Committee TGPsDSGS . Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord. (2002) 17:60–7. doi: 10.1002/mds.10010
13.
Schapira A Chaudhuri KR Jenner P . Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:435–50. doi: 10.1038/nrn.2017.62
14.
Marinus J Zhu K Marras C Aarsland D van Hilten JJ . Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol. (2018) 17:559–68. doi: 10.1016/S1474-4422(18)30127-3
15.
Muller T . Pharmacokinetics and pharmacodynamics of levodopa/carbidopa cotherapies for Parkinson's disease. Expert Opin Drug Metab Toxicol. (2020) 16:403–14. doi: 10.1080/17425255.2020.1750596
16.
Cilia R Cereda E Akpalu A Sarfo FS Cham M Laryea R et al . Natural history of motor symptoms in Parkinson's disease and the long-duration response to levodopa. Brain. (2020) 143:2490–501. doi: 10.1093/brain/awaa181
17.
Armstrong MJ Okun MS . Diagnosis and treatment of Parkinson disease: a review. JAMA. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
18.
Angelopoulou E Anagnostouli M Chrousos GP Bougea A . Massage therapy as a complementary treatment for Parkinson's disease: a systematic literature review. Complement Ther Med. (2020) 49:102340. doi: 10.1016/j.ctim.2020.102340
19.
Weintraub D Aarsland D Chaudhuri KR Dobkin RD Leentjens AFG Rodriguez-Violante M et al . The neuropsychiatry of Parkinson's disease: advances and challenges. Lancet Neurol. (2022) 21:89–102. doi: 10.1016/S1474-4422(21)00330-6
20.
Bishop FL Yardley L Lewith GT . A systematic review of beliefs involved in the use of complementary and alternative medicine. J Health Psychol. (2007) 12:851–67. doi: 10.1177/1359105307082447
21.
Zesiewicz TA Bezchlibnyk Y Dohse N Ghanekar SD . Management of Early Parkinson Disease. Clin Geriatr Med. (2020) 36:35–41. doi: 10.1016/j.cger.2019.09.001
22.
Saeed U Lang AE Masellis M . Neuroimaging Advances in Parkinson's Disease and Atypical Parkinsonian Syndromes. Front Neurol. (2020) 11:572976. doi: 10.3389/fneur.2020.572976
23.
Rodriguez MA Albillos-Almaraz L Lopez-Aguado I Crespo I Del Valle M Olmedillas H . Vigorous Aerobic Exercise in the Management of Parkinson Disease: A Systematic Review. PM R. (2021) 13:890–900. doi: 10.1002/pmrj.12500
24.
Gronek P Haas AN Czarny W Podstawski R Delabary MS Clark CCT et al . The Mechanism of Physical Activity-induced Amelioration of Parkinson's Disease: A Narrative Review. Aging Dis. (2021) 12:192–202. doi: 10.14336/AD.2020.0407
25.
Lamotte G Rafferty MR Prodoehl J Kohrt WM Comella CL Simuni T et al . Effects of endurance exercise training on the motor and non-motor features of Parkinson's disease: a review. J Parkinsons Dis. (2015) 5:21–41. doi: 10.3233/JPD-140425
26.
Pedersen BK . Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. (2019) 15:383–92. doi: 10.1038/s41574-019-0174-x
27.
Tari AR Norevik CS Scrimgeour NR Kobro-Flatmoen A Storm-Mathisen J Bergersen LH et al . Are the neuroprotective effects of exercise training systemically mediated?Prog Cardiovasc Dis. (2019) 62:94–101. doi: 10.1016/j.pcad.2019.02.003
28.
Li G Huang P Cui SS Tan YY He YC Shen X et al . Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson's disease patients. Transl Neurodegener. (2022) 11:6. doi: 10.1186/s40035-022-00280-7
29.
Maccarone MC Masiero S . Can spa rehabilitative interventions play a role for patients suffering from neurodegenerative disorders at the early stages? A scoping review. Int J Biometeorol. (2022) 66:2369–77. doi: 10.1007/s00484-022-02369-0
30.
Deuel LM Seeberger LC . Complementary Therapies in Parkinson Disease: a Review of Acupuncture, Tai Chi, Qi Gong, Yoga, and Cannabis. Neurotherapeutics. (2020) 17:1434–55. doi: 10.1007/s13311-020-00900-y
31.
Pan XW Zhang XG Chen XC Lu Q Hu YS Han LY et al . A Survey of Application of Complementary and Alternative Medicine in Chinese Patients with Parkinson's Disease: A Pilot Study. Chin J Integr Med. (2020) 26:168–73. doi: 10.1007/s11655-018-2560-y
32.
Crowley EK Nolan YM Sullivan AM . Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson's disease: Evidence from rodent models. Prog Neurobiol. (2019) 172:2–22. doi: 10.1016/j.pneurobio.2018.11.003
33.
Vellata C Belli S Balsamo F Giordano A Colombo R Maggioni G . Effectiveness of Telerehabilitation on Motor Impairments, Non-motor Symptoms and Compliance in Patients With Parkinson's Disease: A Systematic Review. Front Neurol. (2021) 12:627999. doi: 10.3389/fneur.2021.627999
34.
Zhang J Li Z Li Z Li J Hu Q Xu J et al . Progress of Acupuncture Therapy in Diseases Based on Magnetic Resonance Image Studies: A Literature Review. Front Hum Neurosci. (2021) 15:694919. doi: 10.3389/fnhum.2021.694919
35.
Yeo S Song J Lim S . Acupuncture Inhibits the Increase in Alpha-Synuclein in Substantia Nigra in an MPTP- Induced Parkinsonism Mouse Model. Adv Exp Med Biol. (2020) 1232:401–8. doi: 10.1007/978-3-030-34461-0_51
36.
Ma J Yuan L Wang SJ Lei J Wang Y Li YN et al . Electroacupuncture improved locomotor function by regulating expression of tyrosine hydroxylase and alpha-synuclein proteins and transcription activating factor 6 and transcription factor X box binding protein 1 mRNAs in substantia nigra of rats with Parkinson's disease. Zhen Ci Yan Jiu. (2019) 44:805–9. doi: 10.13702/j.1000-0607.180600
37.
Yu J Min D Bai Y Qu L Zou T Wang S . Electroacupuncture alleviates Parkinson disease and regulates the expression of brain-gut peptides. Exp Anim. (2020) 69:448–60. doi: 10.1538/expanim.19-0153
38.
Cornell JEC . The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med. (2015) 162:797–8. doi: 10.7326/M15-0930
39.
Furukawa TA Salanti G Atkinson LZ Leucht S Ruhe HG Turner EH et al . Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. (2016) 6:e10919. doi: 10.1136/bmjopen-2015-010919
40.
Barcot O Ivanda M Buljan I Pieper D Puljak L . Enhanced access to recommendations from the Cochrane Handbook for improving authors' judgments about risk of bias: A randomized controlled trial. Res Synth Methods. (2021) 12:618–29. doi: 10.1002/jrsm.1499
41.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
42.
Higgins JPT Thomas J Chandler J Cumpston M Li T Page MJ et al . Cochrane handbook for systematic reviews of interventionsJohn Wiley and Sons Ltd (2019).
43.
Zhang J Zhang Z Huang S Qiu X Lao L Huang Y et al . Acupuncture for cancer-related insomnia: A systematic review and meta-analysis. Phytomedicine. (2022) 102:154160. doi: 10.1016/j.phymed.2022.154160
44.
Yangfan Cai LLTR . Effects of integrated Chinese and Western medicine on nocturia frequency and sleep quality in patients with Parkinson's disease accompanied by nocturia. World J Sleep Med. (2021) 11:1891–3.
45.
Cao L Li X Li G Wang Z Ke Z Wan J et al . Effects of acupuncture combined with cognitive function training on cognitive function and motor function in patients with Parkinson's disease. J Acupunc Moxibustion. (2018) 34:9–12.
46.
Chai Q . Effect of acupuncture combined with probiotics on intestinal flora disturbance in patients with Parkinson's disease. Med Theory Pract. (2022) 35:2030–2.
47.
Shen L-R Wang Q Shen L-P et al . Observation on the curative effect of acupuncture combined with Maren softgel in the treatment of constipation in advanced Parkinson's disease. Shanghai J Acupuncture Moxibustion. (2018) 37:1381–5.
48.
Jang JH Yeom MJ Ahn S . Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson's disease. Brain Behav Immun. (2020) 89:641–55. doi: 10.1016/j.bbi.2020.08.015
49.
Liu A Xiuling W Jinghong X Baoquan L . Clinical observation of Zusanli electroacupuncture for treating constipation in 48 patients with Parkinson's disease. J Chinese Med. (2018) 24:101–103+108.
50.
Liu F Gao L Chang H Zhao J Zhuang W Meng Q et al . Application effect of self-made Chinese medicine acupoint paste in patients with Parkinson's constipation. J Cardio Cerebrovasc Dis. (2018) 16:3358–61.
51.
Lv J . Observation on the curative effect of Wuzhuyu Reanbao applied to Shenque Point in treating constipation of Parkinson's disease. J Yangtze Univ. (2014) 11:15–16+4.
52.
Luo S Zhang Y . Clinical effect of Zusanli electroacupuncture combined with conventional western medicine in the treatment of Parkinson's disease with constipation. J Clin Med Res Pract. (2019) 4:136–8.
53.
Xue X Ni X Jingliang X Wang Y Wei W Wang K . Curative effect of warm acupuncture and moxibustion combined with Simo Decoction on Parkinson's constipation. Wisdom Health. (2018) 4:96–7.
54.
Xiu Zhan YW . Clinical effect of acupuncture on constipation of Parkinson's disease. Asian Pac Traditi Med. (2019) 15:128–30.
55.
Zhang H. Clinical study of abdominal acupuncture in treating functional constipation in Parkinson's disease. Guangzhou University of Chinese Medicine (2020).
56.
Li Cai YLYC . Clinical study of Defibrillation Decoction combined with acupuncture in the treatment of constipation in patients with Parkinson's disease. J Cardio Cerebrovasc Dis Integr Chinese Western Med. (2019) 17:2034–7.
57.
Chen H . Clinical observation of Guipi Decoction combined with acupuncture in treating insomnia with deficiency of heart and spleen in Parkinson's disease. World Latest Med Inform Abstracts. (2021) 21:469–70.
58.
Junfeng Dai JXSL . Therapeutic effect of acupuncture combined with repeated transcranial magnetic stimulation on depression in Parkinson’s disease. J Pract Chinese Med. (2019) 35:115–6.
59.
Deng M . Effect of Dingdiao Anshen prescription combined with acupuncture on cognitive function and neurotransmitter level of patients with Parkinson's disease. Chinese Foreign Med Res. (2021) 19:128–31.
60.
Qinjian Dong XLMT . A randomized controlled study on scalp electro-acupuncture for the treatment of sleep disorders in Parkinson's disease. J Pract Hosp Clin. (2018) 15:214–6.
61.
Hao D . Clinical observation of acupuncture combined with repetitive transcranial magnetic stimulation in treatment of Parkinson's disease complicated with depression. Guangxi Traditi Chinese Med. (2018) 41:34–6.
62.
Wanqin HZSGS . To explore the clinical observation of the synergistic effect of acupuncture at Shu and Jing points of blood Wuzang in the treatment of Parkinson's disease. Kang Yi. (2021) 21:249–50.
63.
Na Huang LHJA . Clinical efficacy of Fong's head acupuncture in treating insomnia of Parkinson's disease. Shaanxi Chinese Med. (2014) 35:348–9.
64.
Huang Y. Clinical effect of warm acupuncture and moxibustion combined with Western medicine in treating sleep disorder of Parkinson's disease Fujian University of Chinese Medicine (2020).
65.
Liu L . Clinical effect of Duloxetine combined with acupuncture on Parkinson's anxiety. Inner Mongolia Traditi Chinese Med. (2020) 39:137–8.
66.
Li L . Clinical Observation on Guipi Decoction Combined with Acupuncture and Moxibustion in the Treatment of Insomnia Patients with Parkinson. Guangming Chinese Med. (2018) 33:2043–4.
67.
Hao Qian ZWHZ . Effects of electrocephalic acupuncture on emotional and cognitive disorders in patients with Parkinson's disease. Liaoning J Traditi Chinese Med. (2021) 48:168–71.
68.
Zhicheng Lin YYSY . Clinical observation on treating mild cognitive dysfunction of Parkinson's disease with electroacupuncture at Baihui point. J Anhui Univ Chinese Med. (2018) 37:31–5.
69.
Lu W. Clinical study on the treatment of anxiety disorder in Parkinson's disease by regulating spirit acupuncture. Guangzhou University of Chinese Medicine (2022).
70.
Qiu L. The clinical study of invigorating kidney and nourishing marrow acupuncture in the treatment of mild cognitive impairment in Parkinson's disease with insufficient myelin sea. Shandong University of Chinese Medicine (2021).
71.
Yu Z . Clinical Observation on Treatment of Parkinson's Patients with Addition and Detoxification of Dihuang Yinzi Combined with Acupuncture. Chinese Med Guide. (2019) 17:7–8.
72.
Yi Xia HWYD . Effect of electroacupuncture combined with drugs on Parkinson's disease with depression and serum BDNF. Chinese Acupunc Moxibustion. (2012) 32:1071–4.
73.
Zhou Lu HYHL . Clinical study of Qin's "first eight needles" in the treatment of mild to moderate Parkinson's disease [J]. J Acupunc Moxibustion. (2022) 38:5–10.
74.
Su M . Clinical study on mild cognitive impairment of Parkinson's disease with phlegm-turbid-Meng-Qiaotype treated by administering Spleen and turbid-HuoacupunctureShandong University of Chinese Medicine (2021).
75.
Yu Tian QDHL . Effect of electroacupuncture combined with drugs on Parkinson's disease with depression and its influence on serum BDNF. J Integr Chinese Western Med. (2016) 8:289–91.
76.
Xiaoli Zhang WFYC . Observation of therapeutic effect of combination of acupuncture and drugs on Parkinson's disease complicated with mild cognitive impairment. Shanghai J Acupunc. (2013) 32:263–5.
77.
Jing Gu LSLS . Clinical curative effect of acupuncture on Parkinson's disease complicated with compulsive state. Primary Health Care China. (2023) 37:89–91.
78.
Xiaomei Wang CRFZ . Clinical efficacy and mechanism of heat-sensitive moxibustion in the adjuvant treatment of Parkinson's disease depression. Chinese J Acupuncture Massage Med. (2020) 18:33–9.
79.
Yumei Wu WHDS . Influence of traditional Chinese medicine characteristic nursing intervention on non-motor symptoms of patients with Parkinson's disease. Chinese Med Clin Res. (2021) 13:26–9.
80.
Ying Yuan XCFC . Observation on the clinical effect of "seven points of skull base" in improving Parkinson's disease with depression. Chinese J Geriatr Med. (2020) 18:27–30.
81.
Chen Zhou WPLW . Clinical effect and safety analysis of acupuncture combined with metoba in the treatment of Parkinson's disease. Chinese J Med Sci. (2016) 6:11–3.
82.
Lei Li ZTXZ . Clinical study of electroacupuncture combined with Metoba in the treatment of sleep disorders in Parkinson's disease. New Chinese Med. (2021) 53:113–6.
83.
Yanping Zhao YW . Clinical observation of Tongdu Tiaoshen acupuncture combined with paroxetine in treating 32 cases of Parkinson's disease depression (2019) 36:58–62.
84.
Xu Y Cai X Qu S Zhang J Zhang Z Yao Z et al . Madopar combined with acupuncture improves motor and non-motor symptoms in Parkinson's disease patients: A multicenter randomized controlled trial. Eur J Integr Med. (2020) 34:101049. doi: 10.1016/j.eujim.2019.101049
85.
Lei Jiang SKJJ . Clinical effect of acupuncture on functional constipation of Parkinson’s disease. Nerve Injury Funct Reconstr. (2020) 15:749–50.
86.
Cipriani A Furukawa TA Salanti G Chaimani A Atkinson LZ Ogawa Y et al . Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
87.
Sterne J Savovic J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
88.
Muhammed K Manohar S Ben YM Chong TT Tofaris G Lennox G et al . Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain. (2016) 139:2706–21. doi: 10.1093/brain/aww188
89.
Lei S Fan J Liu X Xv X Zhang J Zhou Z et al . Qualitative and quantitative meta-analysis of acupuncture effects on the motor function of Parkinson’s disease patients. Front Neurosci. (2023) 17:1125626. doi: 10.3389/fnins.2023.1125626
90.
Li Q Wu C Wang X Li Z Hao X Zhao L et al . Effect of acupuncture for non-motor symptoms in patients with Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. (2022) 14:995850. doi: 10.3389/fnagi.2022.995850
91.
Wen X Li K Wen H Wang Q Wu Z Yao X et al . Acupuncture-Related Therapies for Parkinson’s Disease: A Meta-Analysis and Qualitative Review. Front Aging Neurosci. (2021) 13:676827. doi: 10.3389/fnagi.2021.676827
92.
Goodarzi Z Mrklas KJ Roberts DJ Jette N Pringsheim T Holroyd-Leduc J . Detecting depression in Parkinson disease: A systematic review and meta-analysis. Neurology. (2016) 87:426–37. doi: 10.1212/WNL.0000000000002898
93.
Hussein A Guevara CA Del Valle P Gupta S Benson DL Huntley GW . Non-motor symptoms of Parkinson’s disease: the neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist. (2023) 29:97–116. doi: 10.1177/10738584211011979
94.
Subramanian I . Complementary and alternative medicine and exercise in nonmotor symptoms of Parkinson’s disease. Int Rev Neurobiol. (2017) 134:1163–88. doi: 10.1016/bs.irn.2017.05.037
95.
Lee SH Lim SM . Acupuncture for insomnia after stroke: a systematic review and meta-analysis. BMC Complement Altern Med. (2016) 16:228. doi: 10.1186/s12906-016-1220-z
96.
Horsager J Knudsen K Sommerauer M . Clinical and imaging evidence of brain-first and body-first Parkinson's disease. Neurobiol Dis. (2022) 164:105626. doi: 10.1016/j.nbd.2022.105626
97.
Liu Z Yan S Wu J He L Li N Dong G et al . Acupuncture for chronic severe functional constipation: a randomized trial. Ann Intern Med. (2016) 165:761–9. doi: 10.7326/M15-3118
98.
Lv JQ Wang CW Liu MY Zhao Y Wen Q Li N . Observation on the efficacy of electroacupuncture for functional constipation. Zhen Ci Yan Jiu. (2017) 42:254–8. PMID:
99.
Yang Y Wang Y Gao T Reyila A Liu J Liu J et al . Effect of physiotherapy interventions on motor symptoms in people with Parkinson’s disease: a systematic review and meta-analysis. Biol Res Nurs. (2023) 25:586–605. doi: 10.1177/10998004231171587
100.
He Y Guo X May BH Zhang AL Liu Y Lu C et al . Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. (2020) 6:271–8. doi: 10.1001/jamaoncol.2019.5233
Summary
Keywords
Parkinson’s disease, acupoint therapy, non-motor symptom, randomized controlled trials, meta-analysis
Citation
Hu W, Zhou H, Zhu W, Xie S, Zeng Y and Wang Z (2025) Combination treatment with acupoint therapy and conventional medication for non-motor symptoms in Parkinson’s disease: a systematic review and meta-analysis. Front. Neurol. 16:1381500. doi: 10.3389/fneur.2025.1381500
Received
07 February 2024
Accepted
21 April 2025
Published
22 May 2025
Volume
16 - 2025
Edited by
Mya C. Schiess, University of Texas Health Science Center at Houston, United States
Reviewed by
Maria Chiara Maccarone, University of Padua, Italy
Wei Huang, Hubei Provincial Hospital of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Hu, Zhou, Zhu, Xie, Zeng and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyan Wang, tonywzy1981@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.