Abstract
Background:
Phosphate disorders, including hypophosphatemia and hyperphosphatemia, influence the prognosis of patients. We designed this study to explore the relationship between serum phosphate levels and mortality in traumatic brain injury (TBI) patients.
Methods:
TBI patients from the Medical Information Mart for Intensive Care-III (MIMIC-III) database were included in this study. Univariate logistic regression analysis was conducted to identify potential risk factors for TBI mortality. Restricted cubic spline (RCS) analysis was conducted to explore the non-linear relationship between serum phosphate level and TBI mortality, both before and after adjusting for risk factors identified in the univariate logistic regression analysis. Length of ICU stay, length of hospital stay, and 30-day mortality were compared between groups with different serum phosphate levels.
Results:
A total of 400 TBI patients died, resulting in an overall mortality rate of 17.6%. Utilizing the RCS analysis, both unadjusted and adjusted associations between serum phosphate levels and TBI mortality were shown as a U-shaped curve. We divided the serum phosphate levels into three groups: < 3.0, 3.0–4.0, and >4.0 mg/dl, according to the U-shaped curve. The multivariate logistic regression analysis revealed that levels < 3.0 mg/dl (p = 0.012) and >4.0 mg/dl (p = 0.024) were associated with a higher risk of mortality than 3.0–4.0 mg/dl. Mortality rates in these three groups were 19.1%, 14.4%, and 23.4%, respectively (p < 0.001). Kaplan–Meier analysis showed a significant difference in survival among the three groups (p < 0.001).
Conclusion:
Both higher and lower serum phosphate levels are associated with increased mortality in TBI patients. Evaluating serum phosphate levels is beneficial to identify TBI patients at high risk for poor prognosis.
1 Introduction
Traumatic brain injury (TBI), the leading cause of long-term disability among adults under 35 years of age, affects an estimated 69 million individuals globally each year (1–4). The 14-day mortality rate among TBI patients undergoing emergency neurosurgery has been reported to be 18% (5). The poor prognosis of TBI is attributable to both initial brain injury severity and complications during hospitalization. As a commonly observed complication among hospitalized patients, electrolyte disorders are particularly prevalent due to pathophysiological changes and a series of iatrogenic interventions. The incidence of electrolyte disturbances in TBI patients ranges from 21% to 82% (6–8). Certain electrolyte disorders, such as hypernatremia, hyponatremia, hyperchloremia, and hypochloremia, have been shown to be associated with increased mortality in TBI patients (9–13).
As an essential trace element, phosphate plays an essential role in maintaining human physiological functions, including cellular signal transduction, energy metabolism, and bone metabolism (14, 15). It is also important to maintain the integrity of the cell membrane and regulate the oxygen release from hemoglobin (14). Although serum phosphate accounts for only 1% of the overall phosphate storage in the human body, changes in the serum phosphate level are commonly observed in various patients and are considered a marker of disease severity. The widely acknowledged normal range of serum phosphate is 2.5–4.5 mg/dl. Serum phosphate levels below or above the normal range are considered hypophosphatemia or hyperphosphatemia. Previous research studies have demonstrated that critically ill TBI patients exhibited lower serum phosphate levels than non-TBI patients, along with a higher prevalence of hypophosphatemia (8, 16). However, to date, no study has explored the incidence of hyperphosphatemia and the effect of phosphate disorders, including hypophosphatemia and hyperphosphatemia, on the prognosis of TBI. Therefore, we designed this study to investigate the incidence of phosphate disorders and verify the prognostic value of abnormal serum phosphate levels in TBI patients.
2 Materials and methods
2.1 Patients
TBI patients recorded in the Medical Information Mart for Intensive Care-III (MIMIC-III) database were eligible for this study. Developed by researchers at the Massachusetts Institute of Technology (MIT) Laboratory for Computational Physiology (LCP) in collaboration with Beth Israel Deaconess Medical Center (BIDMC), the MIMIC-III database integrates electronic health records, ICU monitoring data, and administrative data of patients hospitalized in the BIDMC between 2001 and 2012. All patients in the MIMIC-III were anonymized and de-identified to protect privacy. To access the MIMIC-III, researchers should complete the “Data or Specimens Only Research” training, sign the data use agreement, agree to ethical use restrictions, and finally download the database from PhysioNet (https://mimic.mit.edu/).
The TBI diagnosis of patients included in our study was identified according to ICD-9 codes (80,000–80,199, 80,300–80,499, and 85,000–85,419). A total of 400 TBI patients were excluded from this study based on the following criteria: (1) age < 18, n = 32; (2) lack of records of GCS on admission, n = 65; and (3) lack of records of vital signs and laboratory test, n = 129; (4) AIS < 3, n = 187 (Figure 1). A total of 2,267 patients were finally included in this study. This study was performed according to the ethical standards of the Helsinki Declaration.
Figure 1
Flowchart of patients' inclusion.
2.2 Data collection
Baseline data included age, gender, and comorbidities, including diabetes, hypertension, hyperlipidemia, coronary heart disease, history of myocardial infarction, chronic liver disease, chronic renal disease, and cancer. Systolic blood pressure, diastolic blood pressure, pulse oxygen saturation (SpO2), Glasgow Coma Scale (GCS), and Injury Severity Score (ISS) were also recorded on admission. Intracranial injury types were classified into epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraparenchymal hemorrhage. Laboratory examination results of the first blood sample collected on the first day of hospitalization were analyzed, including white blood cell, platelet, red blood cell (RBC), hemoglobin, blood glucose, serum creatinine, serum calcium, serum phosphate, prothrombin time, and international normalized ratio (INR). Neurosurgical interventions and therapies administered during the first 24 h, including RBC transfusion, platelet transfusion, and vasopressor use, were recorded. The primary outcome was 30-day mortality. The length of ICU stay and length of hospital stay were collected and compared between groups.
2.3 Statistical analysis
The Kolmogorov–Smirnov test was utilized to verify the normality of the included variables. Normally distributed or non-normally distributed variables were presented in the form of mean ± standard deviation and median (interquartile range), respectively. The differences in variables with normal distribution or non-normal distribution were assessed using Student's t-test and Mann–Whitney U-test. The difference in categorical variables was analyzed using the chi-square test or Fisher's exact test. A univariate logistic regression analysis was conducted to identify potential risk factors for TBI mortality. Then, the restricted cubic spline (RCS) was conducted to explore the non-linear relationship between serum phosphate level and TBI mortality, both before and after adjusting for risk factors discovered in the univariate logistic regression analysis. Finally, overall TBI patients were divided into groups according to borderline serum phosphate levels discovered in the RCS curve demonstrating the relationship between serum phosphate level and mortality. Length of ICU stay, length of hospital stay, and 30-day mortality were compared between groups of different serum phosphate levels using the Kruskal–Wallis H-test and Fisher's exact test. Additionally, the difference in survival between groups of different serum phosphate levels was compared using the Kaplan–Meier method.
A two-sided p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using R (version 3.6.1; R Foundation).
3 Results
3.1 Baseline characteristics of enrolled TBI patients
Among 2,267 TBI patients selected for the study, 400 died, indicating an overall mortality rate of 17.6% (Table 1). Compared to survivors, non-survivors were older (p < 0.001) and had a higher incidence of complications such as diabetes (p = 0.002), chronic renal disease (p < 0.001), and cancer (p = 0.025). Moreover, non-survivors had lower GCS (p < 0.001) and higher ISS scores (p < 0.001) than survivors. Laboratory examination results revealed that WBC (p < 0.001), glucose (p < 0.001), serum creatinine (p < 0.001), prothrombin time (p < 0.001), and INR (p < 0.001) were significantly higher in non-survivors, while platelet count (p < 0.001), RBC (p < 0.001), hemoglobin (p < 0.001), and calcium levels (p < 0.001) were lower in non-survivors. Importantly, serum phosphate levels did not show a statistical difference between survivors and non-survivors (p = 0.715). Finally, compared to survivors, non-survivors had a higher incidence of RBC transfusion (p < 0.001), platelet transfusion (p < 0.001), vasopressor use (p < 0.001), and neurosurgery (p = 0.002), as well as a longer ICU stay (p < 0.001) but a shorter overall hospital stay (p < 0.001).
Table 1
| Variables | Overall patients (N = 2,267) | Survivors (N = 1,867, 82.4%) | Non-survivors (N = 400 (17.6%) | p |
|---|---|---|---|---|
| Age (years) | 64.9 (43.8–811) | 61.3 (41.5–79.4) | 77.5 (60.2–85.6) | <0.001 |
| Male gender (n, %) | 1391 (61.4%) | 1155 (61.9%) | 236 (59.0%) | 0.312 |
| Comorbidities | ||||
| Diabetes (n, %) | 349 (15.4%) | 267 (14.3%) | 82 (20.5%) | 0.002 |
| Hypertension (n, %) | 844 (37.2%) | 685 (36.7%) | 159 (39.8%) | 0.275 |
| Hyperlipidemia (n, %) | 298 (13.1%) | 243 (13.0%) | 55 (13.8%) | 0.754 |
| Coronary heart disease (n, %) | 291 (12.8%) | 234 (12.5%) | 57 (14.2%) | 0.396 |
| History of myocardial infarction (n, %) | 83 (3.7%) | 70 (3.7%) | 13 (3.2%) | 0.737 |
| Chronic liver disease (n, %) | 94 (4.1%) | 78 (4.2%) | 16 (4.0%) | 0.981 |
| Chronic renal disease (n, %) | 153 (6.7%) | 103 (5.5%) | 50 (12.5%) | <0.001 |
| Cancer (n, %) | 238 (10.5%) | 183 (9.8%) | 55 (13.8%) | 0.025 |
| Systolic blood pressure (mmHg) | 132 (117–147) | 132 (118–147) | 132 (113–148) | 0.184 |
| Diastolic blood pressure (mmHg) | 67 (56–77) | 67 (57–78) | 66 (53–75) | 0.003 |
| SpO2 (%) | 99 (97–100) | 99 (97–100) | 100 (98–100) | 0.017 |
| GCS | 12 (6–15) | 14 (7–15) | 6 (3–11) | <0.001 |
| ISS | 16 (16–25) | 16 (16–22) | 20 (16–25) | <0.001 |
| Intracranial injury types | ||||
| EDH (n, %) | 539 (23.8%) | 440 (23.6%) | 99 (24.8%) | 0.660 |
| SDH (n, %) | 1312 (57.9%) | 1080 (57.8%) | 232 (58.0%) | 1.000 |
| SAH (n, %) | 949 (41.9%) | 769 (41.2%) | 180 (45.0%) | 0.178 |
| IPH (n, %) | 447 (19.7%) | 377 (20.2%) | 70 (17.5%) | 0.246 |
| Laboratory tests | ||||
| WBC (109/L) | 11.60 (8.40–15.70) | 11.40 (8.30–15.35) | 12.80 (9.50–17.10) | <0.001 |
| Platelet (109/L) | 230 (183–285) | 234 (188–288) | 213 (162–262) | <0.001 |
| RBC (109/L) | 4.13 (3.67–4.57) | 4.17 (3.73–4.61) | 3.88 (3.41–4.36) | <0.001 |
| Hemoglobin (g/dL) | 12.8 (11.4–14.1) | 12.9 (11.6–14.3) | 12.0 (10.5–13.4) | <0.001 |
| Glucose (mg/dl) | 132 (110–165) | 127 (107–156) | 159 (129–192) | <0.001 |
| Serum creatinine (mg/dl) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 1.0 (0.8–1.3) | <0.001 |
| Calcium (mg/dl) | 8.2 (1.2–8.9) | 8.3 (1.2–8.9) | 7.9 (1.1–8.8) | <0.001 |
| Phosphate (mg/dl) | 3.2 (2.8–3.7) | 3.2 (2.8–3.7) | 3.3 (2.7–3.9) | 0.715 |
| Prothrombin time (s) | 13.0 (12.3–14.2) | 12.9 (12.3–13.9) | 13.6 (12.7–16.0) | <0.001 |
| INR | 1.1 (1.0– 1.3) | 1.1 (1.0–1.2) | 1.2 (1.1–1.6) | <0.001 |
| RBC transfusion during the first 24 h (n, %) | 176 (7.8%) | 116 (6.2%) | 60 (15.0%) | <0.001 |
| Platelet transfusion during the first 24 h (n, %) | 223 (9.8%) | 155 (8.3%) | 68 (17.0%) | <0.001 |
| Vasopressor during the first 24 h (n, %) | 150 (6.6%) | 83 (4.4%) | 67 (16.8%) | <0.001 |
| Neurosurgery (n, %) | 571 (25.2%) | 445 (23.8%) | 126 (31.5%) | 0.002 |
| Length of ICU stay (days) | 2.3 (1.2–5.7) | 2.1 (1.2–5.1) | 3.5 (1.6–6.7) | <0.001 |
| Length of hospital stay (days) | 6.4 (3.7–12.4) | 6.7 (3.8–13.5) | 4.8 (2.1–9.1) | <0.001 |
Baseline characteristics of included TBI patients.
SpO2, pulse oxygen saturation; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IPH, intraparenchymal hemorrhage; WBC, white blood cell; RBC, red blood cell; INR, international normalized ratio. Bold values indicated p value < 0.05.
3.2 Risk factors of mortality among TBI patients analyzed by logistic regression
A univariate logistic regression analysis indicated that age (p < 0.001), diabetes (p = 0.002), chronic renal disease (p < 0.001), cancer (p = 0.020), diastolic blood pressure (p < 0.001), SpO2 (p < 0.001), GCS (p < 0.001), ISS (p < 0.001), WBC (p < 0.001), platelet (p < 0.001), RBC (p < 0.001), hemoglobin (p < 0.001), glucose (p = 0.008), serum creatinine (p < 0.001), calcium (p < 0.001), prothrombin time (p < 0.001), INR (p < 0.001), RBC transfusion (p < 0.001), platelet transfusion (p = 0.001), and vasopressor use (p = 0.002) were potential risk factors of mortality in TBI patients (Table 2).
Table 2
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.025 | 1.019–1.031 | <0.001 |
| Male gender | 1.127 | 0.905–1.405 | 0.286 |
| Diabetes | 1.545 | 1.174–2.035 | 0.002 |
| Hypertension | 1.138 | 0.912–1.420 | 0.251 |
| Hyperlipidemia | 1.065 | 0.778–1.460 | 0.693 |
| Coronary heart disease | 1.160 | 0.849–1.584 | 0.352 |
| History of myocardial infarction | 0.862 | 0.472–1.574 | 0.630 |
| Chronic liver disease | 0.956 | 0.552–1.655 | 0.871 |
| Chronic renal disease | 2.447 | 1.712–3.495 | <0.001 |
| Cancer | 1.467 | 1.062–2.026 | 0.020 |
| Systolic blood pressure | 0.997 | 0.993–1.001 | 0.642 |
| Diastolic blood pressure | 0.990 | 0.983–0.996 | <0.001 |
| SpO2 | 0.995 | 0.976–1.015 | <0.001 |
| GCS | 0.821 | 0.800–0.843 | <0.001 |
| ISS | 1.047 | 1.035–1.059 | <0.001 |
| EDH | 1.067 | 0.830–1.371 | 0.614 |
| SDH | 1.006 | 0.809–1.252 | 0.955 |
| SAH | 1.168 | 0.940–1.452 | 0.161 |
| IPH | 0.838 | 0.633–1.111 | 0.220 |
| WBC | 1.038 | 1.022–1.054 | <0.001 |
| Platelet | 0.997 | 0.996–0.999 | <0.001 |
| RBC | 0.581 | 0.498–0.679 | <0.001 |
| Hemoglobin | 0.820 | 0.779–0.862 | <0.001 |
| Glucose | 1.008 | 1.006–1.010 | 0.008 |
| Serum creatinine | 1.258 | 1.114–1.420 | <0.001 |
| Calcium | 0.961 | 0.933–0.989 | <0.001 |
| Prothrombin time | 1.031 | 1.015–1.048 | <0.001 |
| INR | 1.262 | 1.108–1.437 | <0.001 |
| RBC transfusion during the first 24 h | 2.664 | 1.910–3.715 | <0.001 |
| Platelet transfusion during the first 24 h | 2.262 | 1.662–3.079 | 0.001 |
| Vasopressor during the first 24 h | 4.325 | 3.070–6.091 | 0.002 |
| Neurosurgery | 1.469 | 1.160–1.861 | 0.251 |
Univariate logistic regression analysis of risk factors for mortality in TBI patients.
OR, odds ratio; CI, confidence interval; SpO2, pulse oxygen saturation; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; IPH, intraparenchymal hemorrhage; WBC, white blood cell; RBC, red blood cell; INR, international normalized ratio. Bold values indicated p value < 0.05.
3.3 Association between serum phosphate level and TBI mortality
Utilizing the RCS, the unadjusted association between the serum phosphate level and the TBI mortality was shown as a U-shaped curve (Figure 2A). After adjusting for potential risk factors in the univariate logistic regression analysis, the adjusted association between the serum phosphate level and TBI mortality remained U-shaped (Figure 2B). Based on the U-shaped curve, we divided the serum phosphate level into three groups: <3.0, 3.0–4.0, and >4.0 mg/dl. The univariate logistic regression analysis indicated that <3.0 mg/dl (p = 0.007) and >4.0 mg/dl (p < 0.001) were both associated with higher risk of mortality than 3.0–4.0 mg/dl (Table 3). After adjusting for potential risk factors, the multivariate logistic regression analysis indicated that < 3.0 mg/dl (p = 0.012) and >4.0 mg/dl (p = 0.024) were still associated with a higher risk of mortality than 3.0–4.0 mg/dl. The mortality rate of these three groups was 19.1%, 14.4%, and 23.4%, respectively (p < 0.001; Table 4). Length of ICU stay (p = 0.077) and length of hospital stay (p = 0.720) did not show a significant difference between the three groups. The Kaplan–Meier analysis showed a significant difference in survival between the three groups (p < 0.001; Figure 3).
Figure 2
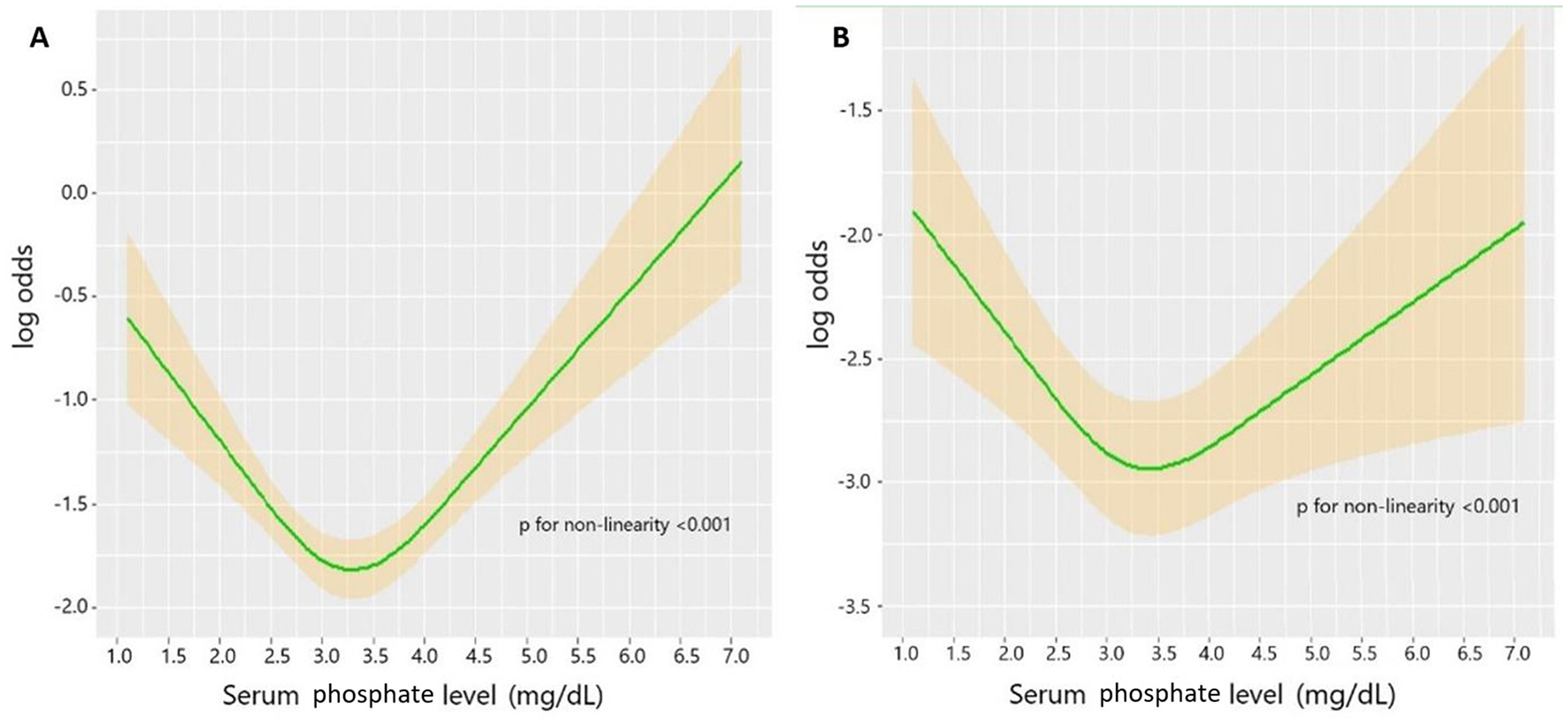
(A) Unadjusted association between serum phosphate level and mortality in TBI. (B) Adjusted association between phosphate level and mortality in TBI. Adjusted factors include age, diabetes, chronic renal disease, cancer, DBP, GCS, ISS, WBC, platelet, RBC, hemoglobin, glucose, serum creatinine, calcium, PT, INR, vasopressor, RBC transfusion, platelet transfusion, and neurosurgery.
Table 3
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Unadjusted | <0.001 | ||
| 3.0–4.0 mg/dl | 1.000 | Reference | |
| < 3.0 mg/dl | 1.408 | 1.101–1.802 | 0.007 |
| >4.0 mg/dl | 1.821 | 1.369–2.421 | <0.001 |
| Adjusted | 0.015 | ||
| 3.0–4.0 mg/dl | 1.000 | Reference | |
| <3.0 mg/dl | 1.454 | 1.086–1.946 | 0.012 |
| >4.0 mg/dl | 1.510 | 1.055–2.161 | 0.024 |
Multivariate logistic regression analysis of phosphate level and mortality in TBI patients.
OR, odds ratio; CI, confidence interval.
Adjusted for age, diabetes, chronic renal disease, cancer, diastolic blood pressure, SpO2, GCS, ISS, WBC, platelet, RBC, hemoglobin, glucose, serum creatinine, calcium, prothrombin time, INR, RBC transfusion (p < 0.001), platelet transfusion, vasopressor use.
Table 4
| Outcomes | Overall (n = 2267) | <3.0 mg/dl (n = 779, 34.4%) | 3.0-4.0 mg/dl (n = 1,078, 47.6%) | >4.0 mg/dl (n = 410, 18.1%) | p |
|---|---|---|---|---|---|
| Length of ICU stay (days) | 2.3 (1.2–5.7) | 2.3 (1.3–5.5) | 2.2 (1.1–5.5) | 2.6 (1.4–6.3) | 0.077 |
| Length of hospital stay (days) | 6.4 (3.7–12.4) | 6.2 (3.7–12.0) | 6.6 (3.7–12.0) | 6.5 (3.5–3.8) | 0.720 |
| 30-day mortality (n, %) | 400 (17.6%) | 149 (19.1%) | 155 (14.4%) | 96 (23.4%) | <0.001 |
Mortality comparison between different TBI patients grouped by serum phosphate level.
Figure 3
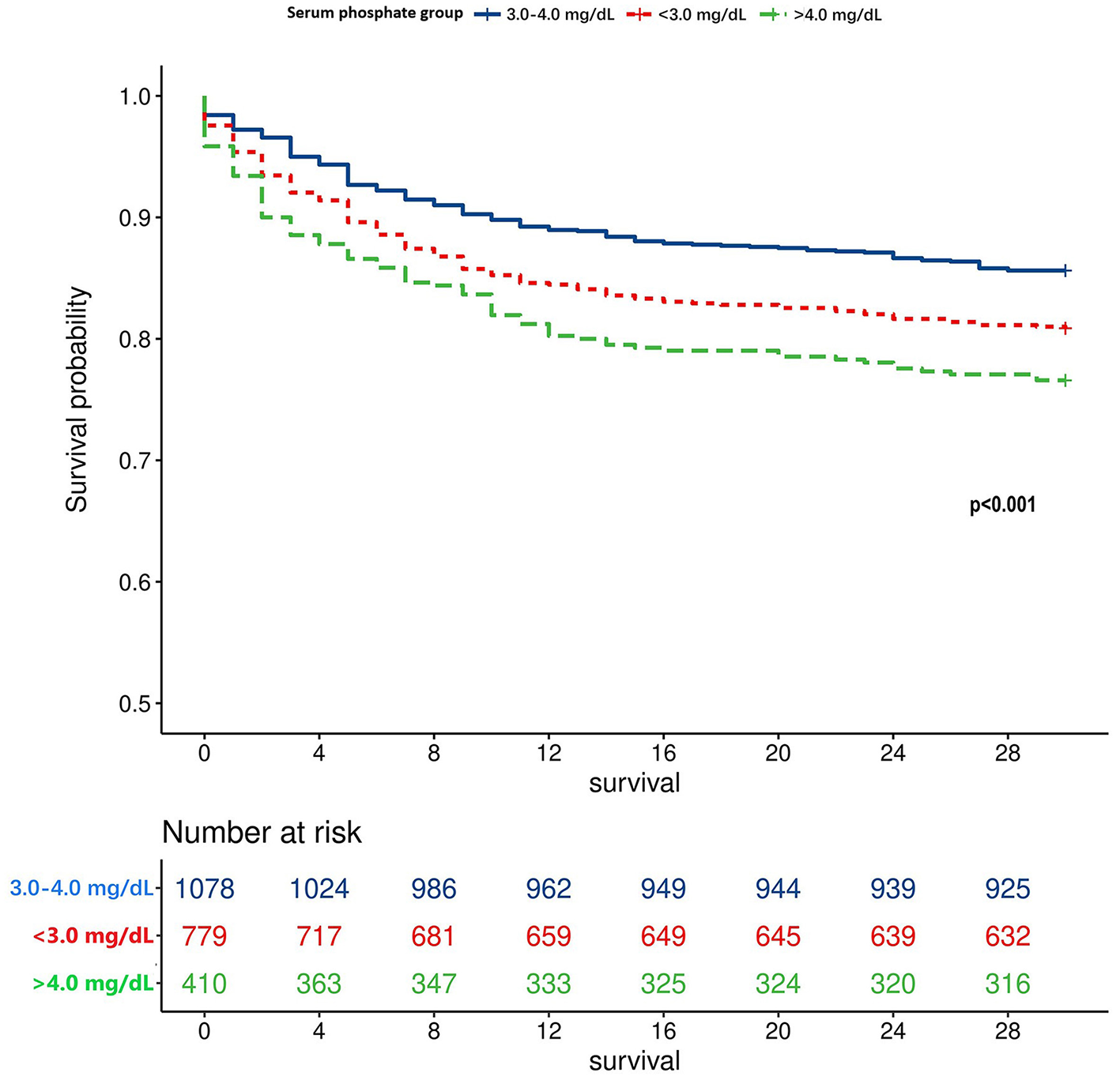
Survival curve of different TBI patients grouped by the serum phosphate level.
4 Discussion
Phosphate, which is absorbed from the small intestine and excreted by the kidney, plays a crucial role in multiple biological activities, including mineral metabolism, energy production, and cellular signal transduction (17). The reserve of phosphate is regulated by hormones such as 1,25-dihydroxy vitamin D, parathyroid hormone, and fibroblast growth factor 23. Some diseases can disrupt phosphate metabolism and lead to abnormal serum phosphate levels. Typically, serum phosphate levels <2.5 and >4.5 mg/dl are defined as hypophosphatemia and hyperphosphatemia, respectively. The abnormal serum phosphate level is frequently observed among critically ill patients, with the hypophosphatemia prevalence ranging from 10 to 80% and hyperphosphatemia incidence ranging from 9 to 45% (18–20). One previous study showed that the incidence of hypophosphatemia and hyperphosphatemia among TBI patients was 44 and 12%, respectively (8). Multiple trauma patients with TBI had lower serum phosphate levels than those without TBI (8, 16). The phosphate intake amounts were significantly greater in multiple trauma patients with TBI (21).
The abnormal serum phosphate level is attributable to multiple factors, including abnormal absorption, excretion, hormone levels, and disease pathophysiological changes (22). Hypophosphatemia is commonly caused by the transport of inorganic phosphate across cell membranes. The respiratory alkalosis with decreasing blood carbon dioxide level leads to increased intracellular pH, which in turn activates phosphofructokinase to promote glycolysis, incorporating inorganic phosphate transferred from the extracellular fluid (23). Respiratory alkalosis could develop in TBI patients as a result of ventilator-associated hypocapnia due to lower intracranial pressure or unintentional spontaneous hyperventilation (24). Other factors, including refeeding and insulin therapy, could also promote the shift of inorganic phosphate across cell membranes (25–27). Additionally, inadequate ingestion due to dysphagia and gastrointestinal dysfunction, massive loss due to mannitol therapy, excessive catecholamine surge, and increased energy requirements all decrease the serum phosphate level after suffering TBI. Conversely, impaired renal function and hypoparathyroidism led to the accumulation of phosphate in the body with subsequent hyperphosphatemia. Acute kidney injury is prevalent among TBI patients, which may promote the development of hyperphosphatemia.
The influence of hypophosphatemia and hyperphosphatemia on prognosis has been widely verified among various patients. Some studies have shown that hyperphosphatemia or increased serum phosphate level was correlated with higher mortality among patients with acute kidney injury undergoing continuous renal replacement therapy, ST-segment elevation myocardial infarction, blunt trauma, or sepsis (28–33). Hypophosphatemia has also been verified to be associated with poor prognosis of various kinds of diseases, including spontaneous intracerebral hemorrhage and acute kidney injury (34, 35). One previous study found that hypophosphatemia was effective in predicting brain death in severe TBI patients (36). Another confirmed higher serum glucose phosphate ratio reflected more severe injury and poorer prognosis in severe TBI patients (37). Moreover, some studies demonstrated that both hypophosphatemia and hyperphosphatemia were significantly related to the mortality of patients, such as patients with community-acquired pneumonia (38). Our study indicated that abnormally low (<3.0 mg/dl) and high (>4.0 mg/dl) levels of serum phosphate were both significantly correlated with the mortality of TBI patients. The association between the serum phosphate level and TBI mortality is U-shaped, which is similar to findings of previous studies, confirming that serum phosphate level shows a U-shaped relationship with mortality and functional outcome of ischemic stroke patients (39, 40). Although the cutoff values of 3.0 and 4.0 mg/dl do not meet the borderline value of the serum phosphate level defined for hypophosphatemia and hyperphosphatemia, several studies have indicated that even lower or higher phosphate levels within the normal range were associated with higher mortality risk (41, 42).
There are several potential mechanisms to reveal the relationship between phosphate disorder and TBI mortality. Phosphate is an essential component of 2,3-diphosphoglycerate in erythrocytes, which binds with hemoglobin and decreases its affinity between hemoglobin and oxygen, thereby promoting oxyhemoglobin to release oxygen. Therefore, hypophosphatemia would increase the affinity between hemoglobin and oxygen and decrease oxygen release with impaired brain energy metabolism (43). Additionally, the lack of phosphate impairs adenosine triphosphate (ATP) production with the shift from oxidative phosphorylation to glycolysis. The inadequate energy supply leads to the development of organ dysfunction and muscle weakness (44). Conversely, an abrupt increase in serum phosphate may increase reactive oxygen species and decrease nitric oxide by inhibiting endothelial nitric oxide synthase and endothelium-dependent vasodilation (45). Furthermore, hyperphosphatemia has been confirmed, leading to hypocalcemia, cardiac arrhythmia, and cardiac arrest (46).
5 Limitations
This study had several limitations. First, all TBI patients included in this study were sourced from a single medical center; thus, generalizability of the findings should be validated in other medical centers in future research. Second, we only analyzed the association between the initial serum phosphate level and the prognosis of TBI but did not record the sequential change in phosphate level and the mean level of serum phosphate during hospitalization. However, the effect of phosphate fluctuation on the prognosis of TBI patients should be explored in the future. Third, although several confounding factors were included, some factors influencing serum phosphate level were not included due to the lack of relevant data in the MIMIC-III, including parathyroid hormone, vitamin D, and phosphate-containing drugs. Finally, although the serum phosphate level was confirmed to be associated with mortality in TBI patients, whether correcting abnormal serum phosphate levels improves the prognosis warrants investigation in future studies.
6 Conclusion
Both higher and lower serum phosphate levels are associated with the mortality of TBI patients. Early identification of TBI patients with serum phosphate deviating from the normal value is beneficial to prevent unfavorable prognosis.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. XY: Data curation, Formal analysis, Writing – original draft. JX: Funding acquisition, Project administration, Supervision, Writing – review & editing. MH: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Sichuan Science and Technology Program (24QYCX0411, 2024YFHZ0070, 2023YFS0105), 1·3·5 projects for disciplines of excellence—Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH036), and the General Program of the National Natural Science Foundation of China (82173175).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Dewan MC Rattani A Gupta S Baticulon RE Hung YC Punchak M et al . Estimating the global incidence of traumatic brain injury. J Neurosurg. (2018) 130:1080–97. 10.3171/2017.10.JNS17352
2.
Hyder AA Wunderlich CA Puvanachandra P Gururaj G Kobusingye OC . The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. (2007) 22:341–53. 10.3233/NRE-2007-22502
3.
Langlois JA Rutland-Brown W Wald MM . The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. (2006) 21:375–8. 10.1097/00001199-200609000-00001
4.
Dasic D Morgan L Panezai A Syrmos N Ligarotti GKI Zaed I et al . A scoping review on the challenges, improvement programs, and relevant output metrics for neurotrauma services in major trauma centers. Surg Neurol Int. (2022) 13:171. 10.25259/SNI_203_2022
5.
Clark D Joannides A Adeleye AO Bajamal AH Bashford T Biluts H et al . Casemix, management, and mortality of patients rreseceiving emergency neurosurgery for traumatic brain injury in the Global Neurotrauma Outcomes Study: a prospective observational cohort study. Lancet Neurol. (2022) 21:438–49. 10.1016/S1474-4422(22)00037-0
6.
Corral L Javierre CF Ventura JL Marcos P Herrero JI Manez R . Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care. (2012) 16:R44. 10.1186/cc11243
7.
Pin-On Pin-On P Saringkarinkul A Punjasawadwong Y Kacha S Wilairat D . Serum electrolyte imbalance and prognostic factors of postoperative death in adult traumatic brain injury patients: a prospective cohort study. Medicine. (2018) 97:e13081. 10.1097/MD.0000000000013081
8.
Dey S Kumar R Tarat A . Evaluation of electrolyte imbalance in patients with traumatic brain injury admitted in the central ICU of a tertiary care centre: a prospective observational study. Cureus. (2021) 13:e17517. 10.7759/cureus.17517
9.
Rodríguez-Triviño CY Torres Castro I Dueñas Z . Hypochloremia in patients with severe traumatic brain injury: a possible risk factor for increased mortality. World Neurosurg. (2019) 124:e783–8. 10.1016/j.wneu.2019.01.025
10.
Jung E Ryu HH Ryu SJ Kong SY . Risk of pre-existing hyponatremia and mortality in patients with traumatic brain injury across age groups. Heliyon. (2022) 8:e10814. 10.1016/j.heliyon.2022.e10814
11.
Vedantam A Robertson CS Gopinath SP . Morbidity and mortality associated with hypernatremia in patients with severe traumatic brain injury. Neurosurg Focus. (2017) 43:E2. 10.3171/2017.7.FOCUS17418
12.
Ditch KL Flahive JM West AM Osgood ML Muehlschlegel S . Hyperchloremia, not concomitant hypernatremia, independently predicts early mortality in critically ill moderate-severe traumatic brain injury patients. Neurocrit Care. (2020) 33:533–41. 10.1007/s12028-020-00928-0
13.
Prisco L Iscra F Ganau M Berlot G . Early predictive factors on mortality in head injured patients: a retrospective analysis of 112 traumatic brain injured patients. J Neurosurg Sci. (2012) 56:131–6.
14.
Koumakis E Cormier C Roux C Briot K . The causes of hypo- and hyperphosphatemia in humans. Calcif Tissue Int. (2021) 108:41–73. 10.1007/s00223-020-00664-9
15.
Hernando N Wagner CA . Mechanisms and regulation of intestinal phosphate absorption. Compr Physiol. (2018) 8:1065–90. 10.1002/j.2040-4603.2018.tb00031.x
16.
Polderman KH Bloemers FW Peerdeman SM Girbes AR . Hypomagnesemia and hypophosphatemia at admission in patients with severe head injury. Crit Care Med. (2000) 28:2022–5. 10.1097/00003246-200006000-00057
17.
Blumsohn A . What have we learnt about the regulation of phosphate metabolism?Curr Opin Nephrol Hypertens. (2004) 13:397–401. 10.1097/01.mnh.0000133983.40182.c3
18.
Barak V Schwartz A Kalickman I Nisman B Gurman G Shoenfeld Y . Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med. (1998) 104:40–7. 10.1016/S0002-9343(97)00275-1
19.
Suzuki S Egi M Schneider AG Bellomo R Hart GK Hegarty C . Hypophosphatemia in critically ill patients. J Crit Care. (2013) 28:536.e9–19. 10.1016/j.jcrc.2012.10.011
20.
Haider DG Lindner G Wolzt M Ahmad SS Sauter T Leichtle AB et al . Hyperphosphatemia is an independent risk factor for mortality in critically ill patients: results from a cross-sectional study. PLoS ONE. (2015) 10:e0133426. 10.1371/journal.pone.0133426
21.
Lindsey KA Brown RO Maish GO Croce MA Minard G Dickerson RN . Influence of traumatic brain injury on potassium and phosphorus homeostasis in critically ill multiple trauma patients. Nutrition. (2010) 26:784–90. 10.1016/j.nut.2009.08.013
22.
Geerse DA Bindels AJ Kuiper MA Roos AN Spronk PE Schultz MJ . Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. (2010) 14:R147. 10.1186/cc9215
23.
Brautbar N Leibovici H Massry SG . On the mechanism of hypophosphatemia during acute hyperventilation: evidence for increased muscle glycolysis. Miner Electrolyte Metab. (1983) 9:45–50.
24.
Esnault P Roubin J Cardinale M D'Aranda E Montcriol A Cungi PJ et al . Spontaneous hyperventilation in severe traumatic brain injury: incidence and association with poor neurological outcome. Neurocrit Care. (2019) 30:405–13. 10.1007/s12028-018-0639-0
25.
Mohammed S Knoll S van Amburg A 3rd Mennes PA . Cefotetan-induced hemolytic anemia causing severe hypophosphatemia. Am J Hematol. (1994) 46:369–70. 10.1002/ajh.2830460422
26.
Stoff JS . Phosphate homeostasis and hypophosphatemia. Am J Med. (1982) 72:489–95. 10.1016/0002-9343(82)90520-4
27.
Knochel JP . The pathophysiology and clinical characteristics of severe hypophosphatemia. Arch Intern Med. (1977) 137:203–20. 10.1001/archinte.1977.03630140051013
28.
Jung SY Kwon J Park S Jhee JH Yun HR Kim H et al . Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS ONE. (2018) 13:e0191290. 10.1371/journal.pone.0191290
29.
Zhu GH Sun XP Liu Z Fan ZX Wang YL Tan J et al . The relation between serum phosphorus levels and long-term mortality in Chinese patients with ST-segment elevation myocardial infarction. J Geriatr Cardiol. (2019) 16:775–81. 10.11909/j.issn.1671-5411.2019.10.004
30.
Xu X Zhang L Liu W Li S Zhao Q Hua R et al . Analysis of the relationship between early serum phosphate levels and short-term mortality in septic patients: a retrospective study based on mimic-IV. Shock. (2023) 59:838–45. 10.1097/SHK.0000000000002119
31.
Al Harbi SA Al-Dorzi HM Al Meshari AM Tamim H Abdukahil SAI Sadat M et al . Association between phosphate disturbances and mortality among critically ill patients with sepsis or septic shock. BMC Pharmacol Toxicol. (2021) 22:30. 10.1186/s40360-021-00487-w
32.
Black LP Mohseni M Shirazi E Hartman K Smotherman C Hopson C et al . Association of early serum phosphate levels and mortality in patients with sepsis. West J Emerg Med. (2023) 24:416–23. 10.5811/WESTJEM.58959
33.
Kim DW Jung WJ Lee DK Lee KJ Choi HJ . Association between the initial serum phosphate level and 30-day mortality in blunt trauma patients. J Trauma Acute Care Surg. (2021) 91:507–13. 10.1097/TA.0000000000003271
34.
Hong Y Wang XH Xiong YT Li J Liu CF . Association between admission serum phosphate level and all-cause mortality among patients with spontaneous intracerebral hemorrhage. Risk Manag Healthc Policy. (2021) 14:3739–46. 10.2147/RMHP.S317615
35.
Yang Y Zhang P Cui Y Lang X Yuan J Jiang H et al . Hypophosphatemia during continuous veno-venous hemofiltration is associated with mortality in critically ill patients with acute kidney injury. Crit Care. (2013) 17:R205. 10.1186/cc12900
36.
Maniakhina LE Muir SM Tackett N Johnson D Mentzer CJ Mount MG . Significant hypophosphatemia is predictive of brain death in severe traumatic brain injury. Am Surg. (2023) 89:3278–80. 10.1177/00031348231160844
37.
Qiu SZ Zheng GR Chen B Huang JJ Shen J Mao W . Prognostic value of admission serum glucose-phosphate ratio in predicting the 6-month outcome of patients with severe traumatic brain injury: a retrospective study. Clin Chim Acta. (2020) 510:659–64. 10.1016/j.cca.2020.08.038
38.
Naffaa ME Mustafa M Azzam M Nasser R Andria N Azzam ZS et al . Serum inorganic phosphorus levels predict 30-day mortality in patients with community acquired pneumonia. BMC Infect Dis. (2015) 15:332. 10.1186/s12879-015-1094-6
39.
Zhong C You S Chen J Zhai G Du H Luo Y et al . Serum alkaline phosphatase, phosphate, and in-hospital mortality in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. (2018) 27:257–66. 10.1016/j.jstrokecerebrovasdis.2017.08.041
40.
Zhang JF Jing J Meng X Pan Y Wang YL Zhao XQ et al . Serum phosphate and 1-year outcome in patients with acute ischemic stroke and transient ischemic attack. Front Neurol. (2021) 12:652941. 10.3389/fneur.2021.652941
41.
Li Z Shen T Han Y . Effect of serum phosphate on the prognosis of septic patients: a retrospective study based on MIMIC-IV database. Front Med. (2022) 9:728887. 10.3389/fmed.2022.728887
42.
Barash Y Klang E Soffer S Zimlichman E Leibowitz A Grossman E et al . Normal-range emergency department serum phosphorus levels and all-cause mortality. Postgrad Med J. (2021) 97:83–8. 10.1136/postgradmedj-2019-137159
43.
Larsen VH Waldau T Gravesen H Siggaard-Andersen O . Erythrocyte 2,3-diphosphoglycerate depletion associated with hypophosphatemia detected by routine arterial blood gas analysis. Scand J Clin Lab Invest Suppl. (1996) 224:83–7. 10.3109/00365519609088626
44.
Gravelyn TR Brophy N Siegert C Peters-Golden M . Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am J Med. (1988) 84:870–6. 10.1016/0002-9343(88)90065-4
45.
Shuto E Taketani Y Tanaka R Harada N Isshiki M Sato M et al . Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. (2009) 20:1504–12. 10.1681/ASN.2008101106
46.
Nemer WF Teba L Schiebel F Lazzell VA . Cardiac arrest after acute hyperphosphatemia. South Med J. (1988) 81:1068–9.
Summary
Keywords
phosphate, hyperphosphatemia, hypophosphatemia, traumatic brain injury, mortality
Citation
Wang R, Yang X, Xu J and He M (2025) The U-shaped association between initial serum phosphate level and mortality of traumatic brain injury patients. Front. Neurol. 16:1474809. doi: 10.3389/fneur.2025.1474809
Received
02 August 2024
Accepted
19 May 2025
Published
18 June 2025
Volume
16 - 2025
Edited by
Zhuang Sha, The Affiliated Hospital of Xuzhou Medical University, China
Reviewed by
Mario Ganau, Oxford University Hospitals NHS Trust, United Kingdom
Luis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El Salvador
Updates
Copyright
© 2025 Wang, Yang, Xu and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianguo Xu xujg@scu.edu.cnMin He hemin19910306@wchscu.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.