Abstract
Objective:
To elucidate the contrast-enhanced ultrasound (CEUS) features of carotid artery plaques in patients who have experienced an ischemic stroke (IS).
Methods:
A computerized search was conducted in databases such as Pub-Med, EMSCO, and Ovid to identify studies reporting CEUS findings of carotid artery plaques. Patients were categorized as IS and non-IS based on clinical and radiological diagnosis, and the quantitative and semi-quantitative CEUS data were analyzed for differences between the two groups.
Results:
After the computerized search, a total of 13 eligible studies, comprising 3,092 participants (1,953 with stroke), were included for analysis. IS patients exhibited significantly higher plaque enhancement intensity versus control group (SMD = 0.71, 95% CI: 0.32, 1.11). The positive rate of plaque enhancement within the plaques was significantly higher in IS patients versus non-IS patients (OR = 3.25, 95% CI: 1.86, 5.68). The sensitivity of hyperintense lesion-based diagnosis of stroke was 0.68 (95% CI: 0.54, 0.80), and the specificity was 0.61 (95% CI: 0.47, 0.73), with an area under the curve (AUC) of 0.697.
Conclusion:
There are significant differences in CEUS characteristics of carotid artery plaques between IS and non-IS patients. IS patients display markedly augmented plaque enhancement intensity and a higher rate of positive enhancement compared to non-stroke individuals. These noteworthy findings have critical implications in enhancing the accuracy of IS diagnosis and improving the stratification of stroke risk for patients.
Systematic review registration:
This study is registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY), 202540006.
1 Introduction
Ischemic stroke (IS) stands as a prominent worldwide contributor to both mortality and disability, presenting a substantial risk to public health and welfare (1). Atherosclerosis, a persistent inflammatory, metabolic, and multifaceted condition impacting the inner lining of medium and large arteries, emerges as a primary causative factor of IS, with carotid artery atherosclerotic plaque playing a significant role in its development (2). Traditionally, the assessment of stroke risk has focused on the degree of carotid stenosis. While severe carotid artery narrowing remains an important risk factor, emerging evidence suggests that patients with non-severe stenosis may also experience ischemic events (3, 4). Recent studies indicate that the stability of carotid artery atherosclerotic plaque, rather than just the degree of stenosis, is closely linked to the occurrence of ischemic stroke (IS). In fact, 25–50% of IS events are associated with the rupture of vulnerable plaques (5–7).
The distinctive features of unstable carotid artery plaques can be detected and measured using a range of non-invasive imaging techniques, including ultrasonography (US), computed tomography (CT), high-resolution magnetic resonance imaging (MRI), and nuclear imaging methods (8, 9). These advanced imaging modalities provide valuable information beyond just the degree of arterial narrowing, allowing for improved risk stratification and targeted management of patients at risk of ischemic stroke. Ultrasonography is an excellent screening tool for carotid artery atherosclerosis, as it is cost-effective, rapid, and widely accessible, enabling frequent follow-up examinations (10, 11). Contrast-enhanced ultrasound (CEUS) is a novel non-invasive technique that employs contrast agents containing gas microbubbles, which generate strong echo signals under ultrasound, enhancing image contrast and allowing for clearer visualization of tissue perfusion and structural features (12). As a “tracer” of the vascular system, CEUS can clearly delineate the contours of the vascular intima and carotid artery atherosclerotic plaques, including ulcerated plaques, enabling the assessment of plaque stability based on morphological characteristics (13, 14).
To date, however, there has been a paucity of multi-center, large-scale studies investigating the CEUS features of carotid artery plaques in the ischemic stroke population. To address this gap, we evaluate the CEUS characteristics of carotid artery plaques in patients with ischemic stroke.
2 Methodology
2.1 Literature search strategy
We conducted a literature search in the Embase, PubMed, and Ovid electronic databases to screen studies that reported the CEUS features of carotid artery plaques in patients with IS. The search terms used included “stroke,” “carotid plaque,” and “contrast-enhanced ultrasound.” The search was limited to publications up to June 1, 2024, without any language restrictions. Next, we conducted a thorough manual search through the bibliographies of the chosen articles to uncover any supplementary studies that could bear relevance to the subject matter.
2.2 Study selection criteria
The following predefined eligibility criteria were utilized for study inclusion: (1) Participants: Patients diagnosed with carotid artery atherosclerotic plaques were enrolled. All patients underwent CEUS prior to carotid endarterectomy. Based on the North American Symptomatic Endarterectomy Trial criteria (NASCET), the patients were categorized into two groups: those with asymptomatic and those with symptomatic internal carotid artery stenosis attributable to plaques. Symptomatic internal carotid artery stenosis was defined as the onset of neurological manifestations associated with the ipsilateral carotid artery within the preceding 120 - day period. Other potential etiologies of stroke, such as cardioembolism, were strictly excluded. Notably, none of the patients with asymptomatic internal carotid artery stenosis due to plaques had a history of ischemic events resulting from carotid artery stenosis. (2) Intervention: All participants underwent CEUS examination. (3) Outcome measures: Quantitative or semi-quantitative CEUS characteristics of carotid artery plaques, with clinical and imaging diagnoses of IS and non-ischemic stroke (non-IS). (4) Study design: No restrictions on study type. Research with fewer than 10 participants, along with individual case reports and case series, were omitted from consideration. Two separate evaluators screened the collected articles independently, with any disparities being reconciled by a third reviewer.
2.3 CEUS plaque enhancement
CEUS plaque enhancement, plaque enhanced intensity was calculated by subtracting baseline from peak intensities in the core, plaque shoulder, and vessel lumen.
2.4 Data extraction
A systematic data extraction process was carried out using an Excel spreadsheet to collect the following information from the included studies: publication year, first author, study design, participant count, and outcomes. Two reviewers independently extracted the data and verified the information, with any disagreements addressed with a third reviewer.
2.5 Heterogeneity assessment
The heterogeneity across the included studies was evaluated utilizing the corrected p-value and the I-squared (I2) statistic. Studies were deemed to exhibit negligible heterogeneity when the I2 statistic was below 50%, prompting the use of a fixed-effects meta-analytic model. Conversely, an I2 value of 50% or greater was interpreted as indicative of substantial heterogeneity, leading the authors to employ a random-effects approach to provide a more conservative statistical description of the effect sizes.
2.6 Statistical analysis
The analysis of the data was conducted utilizing the meta package in the R programming language, and figures were generated accordingly. For quantitative data on plaque enhancement, the pooled effect size was reported as the standardized mean difference (SMD) with its 95% confidence interval (CI). For qualitative data, such as the presence or absence of plaque enhancement or intraplaque neovascularization (IPN), the pooled effect size was represented by the odds ratio (OR) with its 95% CI. Statistical significance was determined by whether the 95% CI of the SMD or OR contained 0 or 1, respectively. The diagnostic accuracy was analyzed using the R package meta4diag.
3 Results
3.1 Literature screening and selection
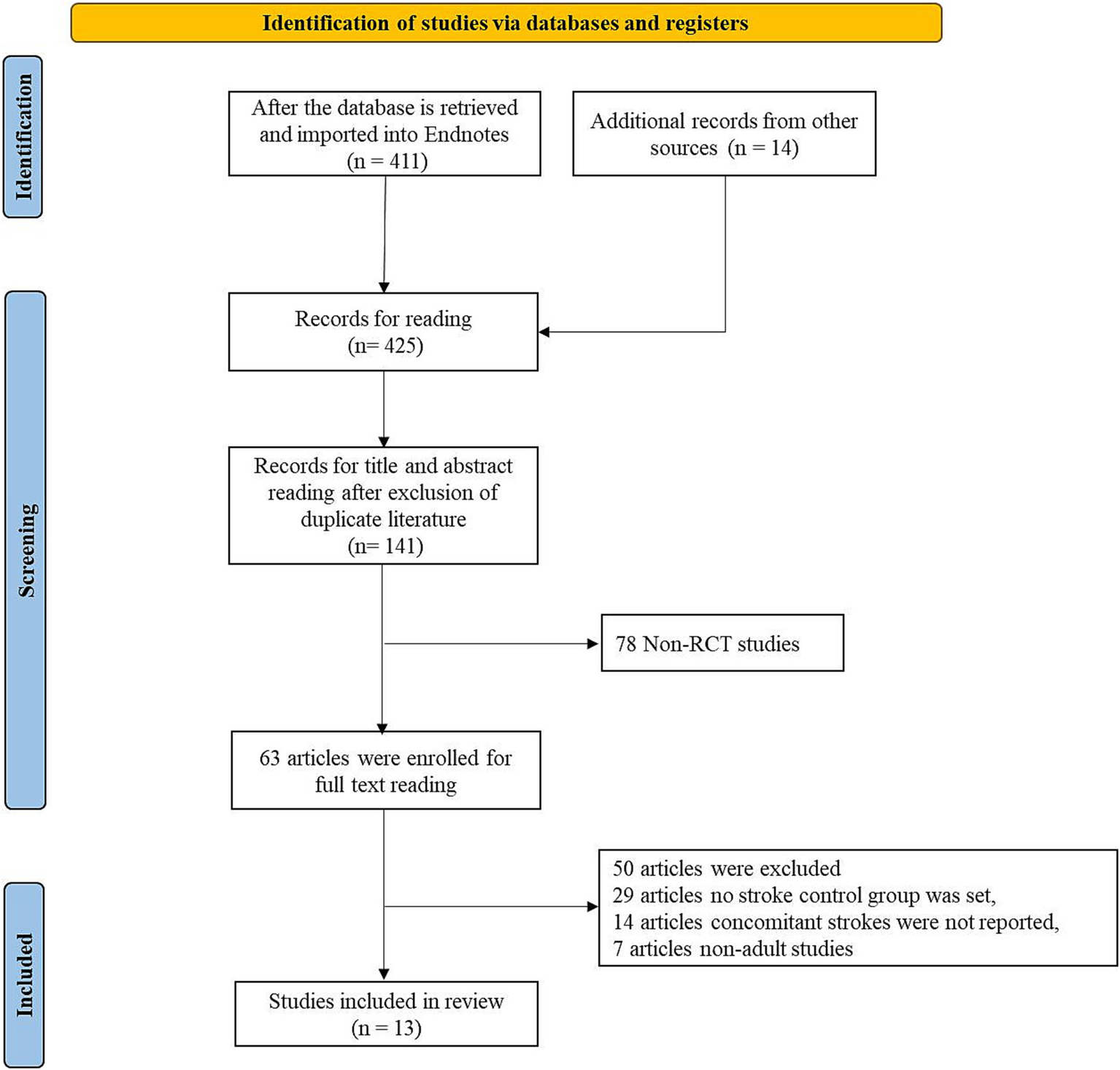
Initially, the literature search yielded 425 articles, and after eliminating duplicates, 141 articles remained. Subsequently, a screening of titles and abstracts produced the exclusion of 78 non-clinical studies, resulting in 63 full-text articles for further evaluation of their eligibility. Among these, 14 articles were excluded due to the inability to extract the specified data, 29 articles did not have a stroke control group, and 7 articles focused only on pediatric populations. Ultimately, 13 studies were included in the analysis.
3.2 Characteristics of included studies
The 13 included studies involved a total of 3,092 participants, of whom 1,953 had ischemic stroke (IS). The most commonly used contrast agents were SonoVue, Sonazoid, and Optison, with SonoVue being the most frequently employed. The regions of interest (ROI) included the plaque, plaque surface, and plaque shoulder (Table 1).
Table 1
| First author | Year | Enrollments | With stroke | Agent | Region of interest |
|---|---|---|---|---|---|
| Xiong (28) | 2009 | 71 | 35 | SonoVue | Entire plaque |
| Saito (29) | 2014 | 50 | 19 | Sonazoid | Entire plaque and shoulders |
| Luo (30) | 2019 | 116 | 62 | SonoVue | Entire plaque |
| Jain (31) | 2020 | 60 | 32 | SonoVue | Entire plaque and surface |
| Li (14) | 2023 | 660 | 349 | SonoVue | Entire plaque |
| Li (32) | 2024 | 61 | 32 | SonoVue | Entire plaque |
| Tan (33) | 2022 | 188 | 72 | SonoVue | Entire plaque and surface |
| Huang (34) | 2021 | 24 | 161 | Optison | Entire plaque |
| Li (35) | 2018 | 116 | 62 | SonoVue | Entire plaque |
| Cui (36) | 2023 | 321 | 162 | SonoVue | Entire plaque |
| Huang (37) | 2010 | 176 | 81 | SonoVue | Entire plaque |
| Zhao (38) | 2022 | 60 | 60 | SonoVue | Entire plaque |
| Cui (13) | 2022 | 50 | 12 | SonoVue | Entire plaque |
Included studies characteristics.
3.3 Meta-analysis of quantitative CEUS plaque enhancement
Three studies reported quantitative analysis of CEUS plaque enhancement, comprising 116 IS patients and 121 non-IS controls. Considerable diversity was evident across the studies, indicating substantial heterogeneity (I2 = 51%, p = 0.13), and a random-effects model was employed. The results demonstrated a higher plaque enhancement intensity in IS patients versus the control group (SMD = 0.71, 95% CI: 0.32, 1.11) (Figure 1).
Figure 1

The flow chart for study retrieval and selection.
3.4 Diagnostic value of semi-quantitative lesion analysis for stroke
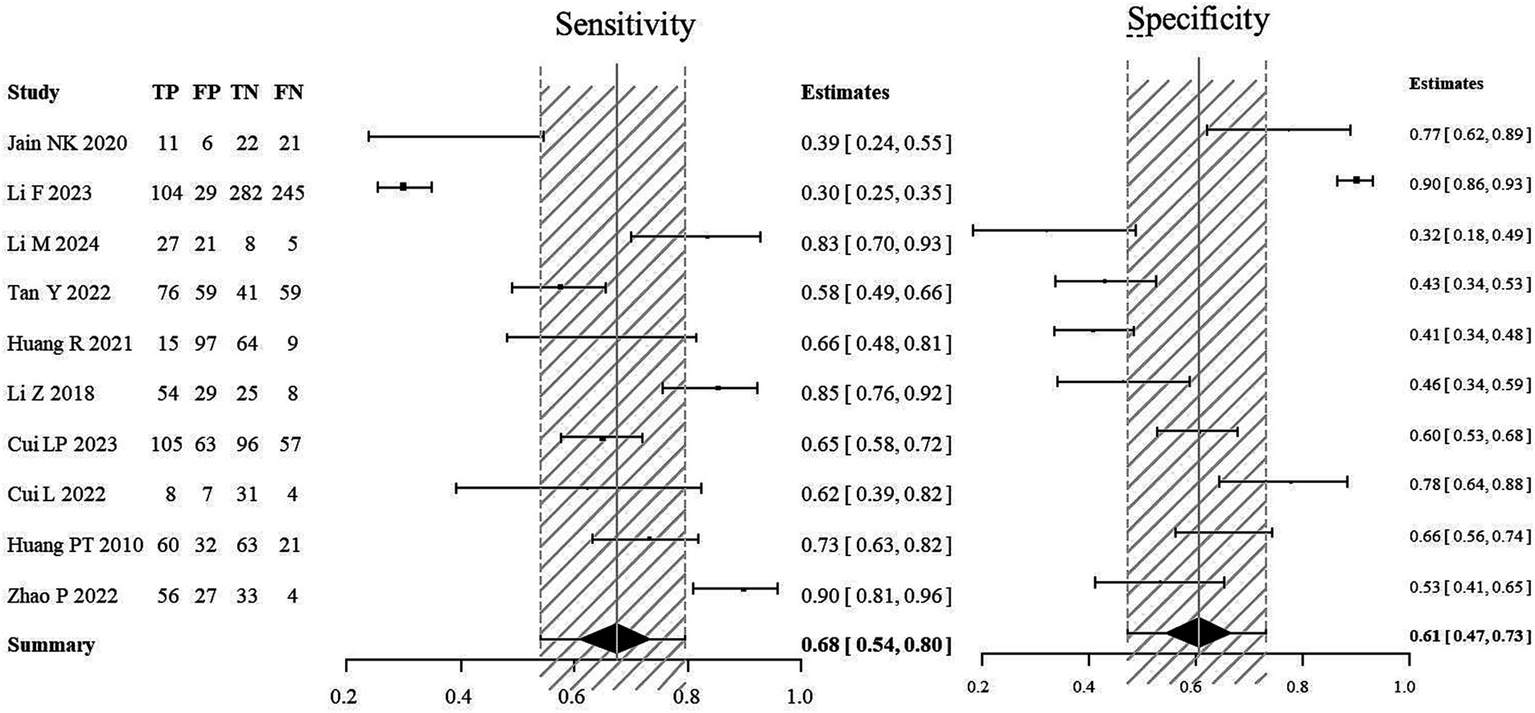
For the stroke group, semi-quantitative positivity was considered as true positive (TP), and semi-quantitative negativity as false negative (FN). In the non-stroke group, semi-quantitative negativity was considered as true negative (TN), and semi-quantitative positivity as false positive (FP). A meta-analysis was performed to evaluate the diagnostic value of semi-quantitative positivity for stroke. The SROC scatter plot did not show a clear “shoulder-arm” pattern, and the Spearman correlation coefficient was 0.633 (p = 0.076), suggesting no threshold effect. The pooled sensitivity of semi-quantitative positivity for diagnosing stroke was 0.68 (95% CI: 0.54, 0.80), the pooled specificity was 0.61 (95% CI: 0.47, 0.73), and the AUC was 0.697 (Figures 2A,B).
Figure 2
The forest plot of enhanced intensity in plaque by quantitative analysis. Significant variation was apparent among the studies, reflecting considerable heterogeneity (I2 = 51%, p = 0.13), prompting the application of a random-effects model. Findings revealed a notably elevated intensity of plaque enhancement in patients with ischemic stroke compared to the control group (SMD = 0.71, 95% CI: 0.32, 1.11).
3.5 Meta-analysis of semi-quantitative CEUS plaque enhancement
Ten research studies presented semi-quantitative evaluations of plaque enhancement. In this analysis, the absence of enhancement or localized enhancement restricted to the plaque’s edge was deemed as negative, while linear and widespread enhancement were regarded as positive outcomes. The rate of positive plaque enhancement was notably higher in IS when compared to those without IS. Considerable variability was observed among the studies (I2 = 80%, p < 0.001), leading to the adoption of a random-effects model. The findings pointed towards a significantly elevated positive rate of CEUS plaque enhancement in IS versus non-IS patients (OR = 3.25, 95% CI: 1.86, 5.68) (Figure 3).
Figure 3

Forest plot of the sensitivity and specificity of semi-quantitative positivity for the diagnosis of stroke. The combined sensitivity of semi-quantitative positivity for stroke diagnosis was 0.68 (95% CI: 0.54, 0.80), while the combined specificity stood at 0.61 (95% CI: 0.47, 0.73), with an AUC of 0.697.
3.6 Publication bias assessment
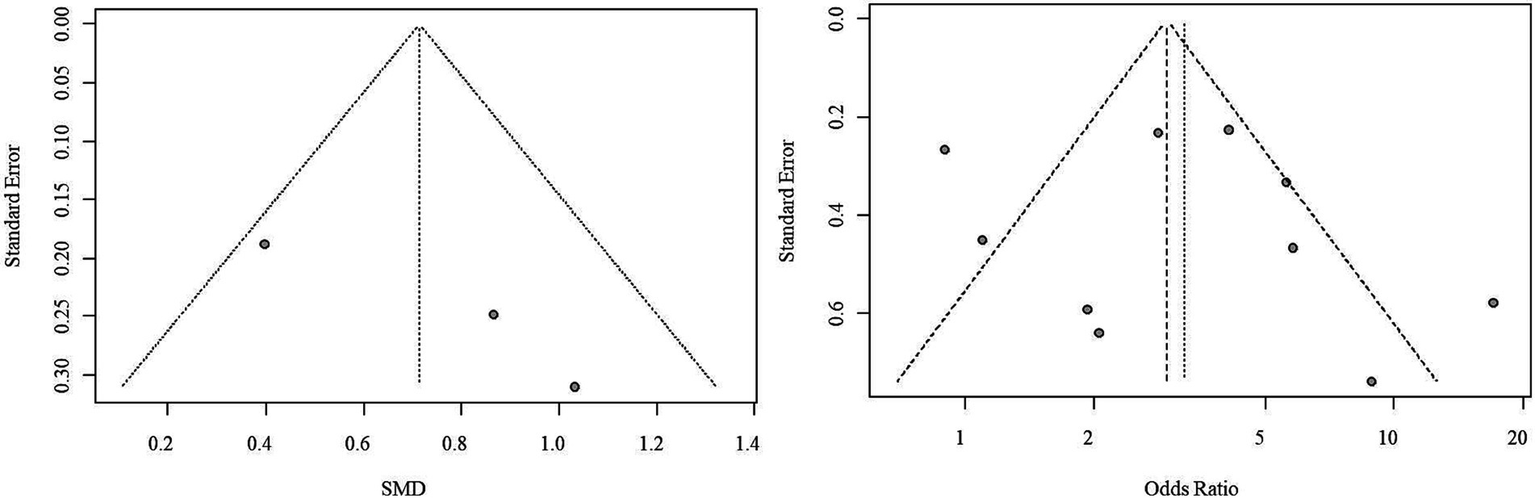
Due to the limited number of studies available for each outcome, the feasibility of conducting Begg’s test and Egger’s test to assess potential publication bias was restricted. Nonetheless, upon visually inspecting the funnel plots, indications of potential publication bias for both outcome measures were noted (Figure 4).
Figure 4

SROC curve of semi-quantitative positivity for the diagnosis of stroke. The scatter plot of the SROC did not exhibit a distinct “shoulder-arm” pattern, and the Spearman correlation coefficient was 0.633 (p = 0.076), indicating the absence of a threshold effect.
4 Discussion
CEUS has gained popularity in its application for quantifying the structural characteristics of carotid atherosclerotic plaques, evaluating plaque stability, and assessing neovascularization. This analysis revealed that patients with a history of stroke exhibited significantly greater intensity of plaque enhancement versus the control group. This finding highlights the potential of CEUS as a valuable tool in distinguishing carotid plaques between stroke patients and non-stroke individuals. CEUS has the capability to detect neo-angiogenesis or enhanced inflammatory activity within the plaque of patients with IS. These processes are typically associated with increased plaque instability and rupture risk. Therefore, an increase in contrast enhancement intensity may serve as a potential biomarker of plaque vulnerability and tendency of stroke. Moreover, the rate of positive plaque enhancement was higher in IS patients versus non-stroke patients. The presence of enhanced contrast within the plaque, as detected by CEUS, further emphasizes the value of this modality in identifying high-risk atherosclerotic plaques. The existence of intra-plaque enhancement may indicate an active, unstable plaque state, which is associated with a higher risk of plaque rupture and consequently, an increased likelihood of stroke occurrence (Figure 5).
Figure 5
The forest plot of enhanced intensity in plaque by semi-quantitative analysis. Considerable heterogeneity was observed among the studies (I2 = 80%, p < 0.001), leading to the adoption of a random-effects model. The outcomes highlighted a significantly higher rate of positive CEUS plaque enhancement in ischemic stroke patients compared to non-ischemic stroke patients (OR = 3.25, 95% CI: 1.86, 5.68).
The sensitivity of CEUS in the diagnosis of IS indicates that it can correctly identify approximately 68% of patients who have experienced an IS event. While this value is not exceptionally high, it still maintains clinical utility, given the complex and multifactorial nature of stroke diagnosis. In non-IS patients, CEUS can correctly exclude approximately 61% of individuals. The relatively low specificity may reflect the limitations of CEUS in distinguishing plaque characteristics not associated with stroke, or the presence of similar plaque enhancement features in some non-stroke patients. The AUC was 0.697, indicating a moderate overall diagnostic performance of CEUS in differentiating IS patients from non-IS individuals. Although the AUC value did not reach a very high level, it still suggests the diagnostic potential of CEUS, particularly when combined with other clinical information and imaging modalities (Figure 6).
Figure 6
Funnel plot of publication bias. Observations from the funnel plots suggest the potential presence of publication bias for both outcome measures.
Vulnerable carotid plaques are a significant contributor to ischemic stroke, and the formation of new blood vessels (neovascularization) within the plaque plays a crucial role in increasing plaque vulnerability (15). The neomicrovasculature that develops within the plaque lacks the normal connective tissue support, making it prone to rupture and bleeding. This can lead to plaque instability and intraplaque hemorrhage (16). The presence of this neomicrovasculature within the carotid plaque serves as an independent risk factor for plaque rupture, hemorrhage, and a strong predictor of future cardiovascular and cerebrovascular events (17). The development of these delicate new blood vessels disrupts the structural integrity of the plaque, rendering it more unstable and prone to potentially devastating consequences, such as ischemic stroke.
CEUS, as a technique that reflects the microvascular system of the plaque, employs contrast agent microbubbles with similar characteristics to red blood cells, remaining within the vascular lumen (18). A number of studies have established a connection between the enhancement observed in CEUS and the histological vascular density of carotid plaques (19, 20). In patients undergoing carotid endarterectomy, pre-operative CEUS and CT examinations were conducted, followed by quantitative analysis of plaque enhancement and subsequent histopathological analysis. The results indicated a significantly higher CEUS plaque enhancement intensity in patients with CT-diagnosed IS compared to asymptomatic patients. Moreover, higher CEUS enhancement intensity correlated with a thinner fibrous cap and increased inflammatory infiltration on histopathology (21).
Quantitative analysis is an emerging trend in radiology, aiming to minimize subjectivity and enhance inter-observer consistency (22). The evaluation of neovascularization in atherosclerotic plaques using CEUS involves both semi-quantitative visual assessment and software-based quantitative assessment (23). The semi-quantitative assessment employs a scoring system proposed by Meng et al. (39), which categorizes plaque neovascularization into three levels: mild, with detectable microbubble flow only in the plaque’s adventitia; moderate, with detectable microbubble flow in the plaque shoulders and within the plaque; and severe, with detectable microbubble flow throughout the entire plaque, including the plaque tip. Quantitative assessment utilizes dedicated analysis software to quantify the plaque enhancement intensity and neovascularization within the plaque on CEUS. The contrast quantification software adjusts the ROI frame-by-frame based on plaque size and shape, and establishes another ROI in the center of the carotid lumen near the plaque’s proximal end as a reference (24). The software then automatically generates time-intensity curves for the plaque and lumen, calculating the enhancement intensity values and enhancement density (25). Schmidt et al. (26) confirmed a significant correlation between CEUS-detected neovascular quantity and histologically determined plaque vascular density, indicating good consistency between CEUS and histopathology in assessing plaque neovascularization. In patients scheduled for carotid endarterectomy, it is feasible to perform quantitative and volumetric imaging of the carotid artery and neovascularization within the plaque using CEUS (27). In this meta-analysis, both quantitative and semi-quantitative assessments supported the value of CEUS in ischemic stroke. The results of this meta-analysis underscore the potential role of CEUS in the assessment of carotid plaque stability and stroke risk. However, to establish CEUS as a routine clinical examination tool, further research is needed to optimize its diagnostic performance, determine the optimal examination protocol, and delineate its applicability across different patient populations. Additionally, exploring the combined use of CEUS with other imaging modalities, such as CT and MRI, may improve the accuracy and reliability of stroke risk assessment. While this meta-analysis provides new insights and evidence to support the application of CEUS in stroke prevention and management, future studies will need to address the current challenges and drive the further development of this technology.
However, this study has certain limitations. The included studies were primarily observational or cross-sectional in design, resulting in lower methodological quality. Additionally, the criteria for semi-quantitative plaque enhancement grading were not completely consistent, leading to unavoidable heterogeneity. Furthermore, the dichotomization of semi-quantitative plaque enhancement data may have reduced the interpretability of the results.
5 Conclusion
The CEUS characteristics of carotid plaques in IS significantly differ from those in patients without stroke, demonstrating higher plaque enhancement intensity and positive enhancement rate. These findings emphasize the prominent role of CEUS in the diagnosis and risk stratification of IS.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, it does not require the approval of the independent ethics committee.
Author contributions
SZ: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. PH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all authors who provided data for this network meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Pu L Wang L Zhang R Zhao T Jiang Y Han L . Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke. (2023) 54:1330–9. doi: 10.1161/STROKEAHA.122.040073
2.
Conti P Shaik-Dasthagirisaeb Y . Atherosclerosis: a chronic inflammatory disease mediated by mast cells. Cent Eur J Immunol. (2015) 3:380–6. doi: 10.5114/ceji.2015.54603
3.
Arasu R Arasu A Muller J . Carotid artery stenosis: an approach to its diagnosis and management. Aust J Gen Pract. (2021) 50:821–5. doi: 10.31128/AJGP-10-20-5664
4.
Chang RW Tucker LY Rothenberg KA Lancaster E Faruqi RM Kuang HC et al . Incidence of ischemic stroke in patients with asymptomatic severe carotid stenosis without surgical intervention. JAMA. (2022) 327:1974–82. doi: 10.1001/jama.2022.4835
5.
Porambo ME Demarco JK . MR imaging of vulnerable carotid plaque. Cardiovasc Diagn Ther. (2020) 10:1019–31. doi: 10.21037/cdt.2020.03.12
6.
Tang Y Zhang J Liu W Jin W Li S Qian Z et al . Analysis of carotid vulnerable plaque MRI high-risk features and clinical risk factors associated with concomitant acute cerebral infarction. BMC Cardiovasc Disord. (2023) 23:173. doi: 10.1186/s12872-023-03199-7
7.
Zhang Y Bai Y Xie J Wang J He L Huang M et al . Carotid plaque components and other carotid artery features associated with risk of stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2022) 31:106857. doi: 10.1016/j.jstrokecerebrovasdis.2022.106857
8.
Fabiani I Palombo C Caramella D Nilsson J De Caterina R . Imaging of the vulnerable carotid plaque: role of imaging techniques and a research agenda. Neurology. (2020) 94:922–32. doi: 10.1212/WNL.0000000000009480
9.
Huibers A De Borst GJ Wan S Kennedy F Giannopoulos A Moll FL et al . Non-invasive carotid artery imaging to identify the vulnerable plaque: current status and future goals. Eur J Vasc Endovasc Surg. (2015) 50:563–72. doi: 10.1016/j.ejvs.2015.06.113
10.
Johri AM Nambi V Naqvi TZ Feinstein SB Kim ESH Park MM et al . Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. (2020) 33:917–33. doi: 10.1016/j.echo.2020.04.021
11.
Kablak-Ziembicka A Przewlocki T . Clinical significance of carotid intima-media complex and carotid plaque assessment by ultrasound for the prediction of adverse cardiovascular events in primary and secondary care patients. J Clin Med. (2021) 10:10. doi: 10.3390/jcm10204628
12.
Baud JM Stanciu D Yeung J Maurizot A Chabay S De Malherbe M et al . Contrast enhanced ultrasound of carotid plaque in acute ischemic stroke (CUSCAS study). Rev Neurol (Paris). (2021) 177:115–23. doi: 10.1016/j.neurol.2020.03.023
13.
Cui L Xing Y Wang L Liu K Chen H Li C et al . Carotid Intraplaque neovascularization and future vascular events in patients with asymptomatic carotid stenosis. Front Pharmacol. (2022) 13:804810. doi: 10.3389/fphar.2022.804810
14.
Li F Gu SY Zhang LN Chen J Yao MH Wu TT et al . Carotid plaque score and ischemic stroke risk stratification through a combination of B-mode and contrast-enhanced ultrasound in patients with low and intermediate carotid stenosis. Front Cardiovasc Med. (2023) 10:1209855. doi: 10.3389/fcvm.2023.1209855
15.
Gu SY Zhang LN Chen J Li F Yao MH Jia CX et al . Associations of plaque morphology and location with Intraplaque neovascularization in the carotid artery by contrast-enhanced ultrasound imaging. Front Neurol. (2023) 14:1097070. doi: 10.3389/fneur.2023.1097070
16.
Aarli SJ Thomassen L Waje-Andreassen U Logallo N Kvistad CE Naess H et al . The course of carotid plaque vulnerability assessed by advanced Neurosonology. Front Neurol. (2021) 12:702657. doi: 10.3389/fneur.2021.702657
17.
Saba L Nardi V Cau R Gupta A Kamel H Suri JS et al . Carotid artery plaque calcifications: lessons from histopathology to diagnostic imaging. Stroke. (2022) 53:290–7. doi: 10.1161/STROKEAHA.121.035692
18.
Brinjikji W Huston J 3rd Rabinstein AA Kim GM Lerman A Lanzino G . Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. (2016) 124:27–42. doi: 10.3171/2015.1.JNS142452
19.
Amamoto T Sakata N Ogata T Shimada H Inoue T . Intra-plaque vessels on contrast-enhanced ultrasound sonography predict carotid plaque histology. Cerebrovasc Dis. (2018) 46:265–9. doi: 10.1159/000495299
20.
Cantisani V Di Leo N David E Clevert DA . Role of CEUS in vascular pathology. Ultraschall Med. (2021) 42:348–66. doi: 10.1055/a-1403-2400
21.
Vavuranakis M Sigala F Vrachatis DA Papaioannou TG Filis K Kavantzas N et al . Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa. (2013) 42:184–95. doi: 10.1024/0301-1526/a000267
22.
Deng PZ Zhao BG Huang XH Xu TF Chen ZJ Wei QF et al . Preoperative contrast-enhanced computed tomography-based radiomics model for overall survival prediction in hepatocellular carcinoma. World J Gastroenterol. (2022) 28:4376–89. doi: 10.3748/wjg.v28.i31.4376
23.
Zeng P Zhang Q Liang X Zhang M Luo D Chen Z . Progress of ultrasound techniques in the evaluation of carotid vulnerable plaque neovascularization. Cerebrovasc Dis. (2024) 53:479–87. doi: 10.1159/000534372
24.
Song Y Xing H Zhang Z Felix LO . Detection of carotid atherosclerotic Intraplaque neovascularization using superb microvascular imaging: a Meta-analysis. J Ultrasound Med. (2021) 40:2629–38. doi: 10.1002/jum.15652
25.
Oura K Kato T Ohba H Terayama Y . Evaluation of Intraplaque neovascularization using superb microvascular imaging and contrast-enhanced ultrasonography. J Stroke Cerebrovasc Dis. (2018) 27:2348–53. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.023
26.
Schmidt C Fischer T Ruckert RI Oberwahrenbrock T Harms L Kronenberg G et al . Identification of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS One. (2017) 12:e0175331. doi: 10.1371/journal.pone.0175331
27.
Venu G Sandeep S Kunlin C Jing J Aaron MD David MM . 3D contrast-enhanced ultrasound (CEUS) imaging of carotid artery plaques and intra-plaque angiogenesis. Circulation. (2014) 130. doi: 10.1161/circ.130
28.
Xiong L Deng YB Zhu Y Liu YN Bi XJ . Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. (2009) 251:583–9. doi: 10.1148/radiol.2512081829
29.
Saito K Nagatsuka K Ishibashi-Ueda H Watanabe A Kannki H Iihara K . Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke. (2014) 45:3073–5. doi: 10.1161/STROKEAHA.114.006483
30.
Luo X Li W Bai Y Du L Wu R Li Z . Relation between carotid vulnerable plaques and peripheral leukocyte: a case-control study of comparison utilizing multi-parametric contrast-enhanced ultrasound. BMC Med Imaging. (2019) 19:74. doi: 10.1186/s12880-019-0374-9
31.
Jain NK Singh G Muralidharan CG Gupta A Chatterjee S Rajesh U . Assessment of plaque vulnerability in carotid atherosclerotic plaques using contrast-enhanced ultrasound. Med J Armed Forces India. (2022) 78:422–9. doi: 10.1016/j.mjafi.2020.09.013
32.
Li M Guo R . Study on the consistency of angiogenesis in carotid plaque evaluated by contrast-enhanced ultrasound and superb microvascular imaging and its correlation with stroke occurrence. J Ultrasound Med. (2024) 43:771–9. doi: 10.1002/jum.16409
33.
Tan Y Nie F Wu G Guo F Wang Y Wang L . Impact of H-type hypertension on Intraplaque neovascularization assessed by contrast-enhanced ultrasound. J Atheroscler Thromb. (2022) 29:492–501. doi: 10.5551/jat.61275
34.
Huang R Demarco JK Ota H Macedo TA Abdelmoneim SS Huston J 3rd et al . Prognostic value of Intraplaque neovascularization detected by carotid contrast-enhanced ultrasound in patients undergoing stress echocardiography. J Am Soc Echocardiogr. (2021) 34:614–24. doi: 10.1016/j.echo.2020.12.016
35.
Li Z Bai Y Li W Gao F Kuang Y Du L et al . Carotid vulnerable plaques are associated with circulating leukocytes in acute ischemic stroke patients: an clinical study based on contrast-enhanced ultrasound. Sci Rep. (2018) 8:8849. doi: 10.1038/s41598-018-27260-0
36.
Cui L Liu R Zhou F Liu Y Tian B Chen Y et al . Added clinical value of Intraplaque neovascularization detection to color Doppler ultrasound for assessing ischemic stroke risk. Neuropsychiatr Dis Treat. (2024) 20:899–909. doi: 10.2147/NDT.S456872
37.
Huang PT Chen CC Aronow WS Wang XT Nair CK Xue NY et al . Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol. (2010) 2:89–97. doi: 10.4330/wjc.v2.i4.89
38.
Zhao P Xu E Yuan R Zhou R Pan J . The predictive value of contrast-enhanced ultrasound combined with serum miR-124 level in acute cerebral infarction and their correlation with the contrast enhancement of carotid atherosclerotic plaque. Neuropsychiatr Dis Treat. (2022) 18:1397–403. doi: 10.2147/NDT.S372557
39.
Meng Q Xie X Li L Jiang C Zhao K Bai Z et al . Assessment of neovascularization of carotid artery atherosclerotic plaques using superb microvascular imaging: a comparison with contrast-enhanced ultrasound imaging and histology. Quant Imaging Med Surg. (2021) 11:1958–69. doi: 10.21037/qims-20-933
Summary
Keywords
carotid artery plaque, contrast-enhanced ultrasound, ischemic stroke, carotid stenosis, meta-analysis
Citation
Zhou S and Hui P (2025) Predictive value of contrast-enhanced carotid ultrasound features for stroke risk: a systematic review and meta-analysis. Front. Neurol. 16:1487850. doi: 10.3389/fneur.2025.1487850
Received
28 August 2024
Accepted
25 March 2025
Published
16 May 2025
Volume
16 - 2025
Edited by
Patricia Martínez Sánchez, Torrecárdenas University Hospital, Spain
Reviewed by
Antonio Arjona-Padillo, Torrecárdenas University Hospital, Spain
Andrzej Fedak, Jagiellonian University Medical College, Poland
Updates
Copyright
© 2025 Zhou and Hui.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pinjing Hui, pinjing-hui@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.