Abstract
Background:
Epilepsy is a common chronic neurological disease, and identifying modifiable risk factors for epilepsy and seizure is extremely important. Currently, the relationship between tobacco exposure and epilepsy or seizure is controversy.
Objective:
The objective of this study is to test the relationship between tobacco smoke exposures and epilepsy in United States (US) participants of the National Health and Nutrition Examination Survey (NHANES).
Methods:
This is a cross-sectional study using data from NHANES 2013–2018. We included all participants in these cycles and excluded those with missing variables. Weighted logistic regression models, weighted sensitivity analysis and weighted subgroup analysis were conducted to estimate the association between active and passive tobacco smoke exposures and epilepsy.
Results:
We included 15,277 participants in NHANES, of whom 131 reported with epilepsy [taking at least one antiseizure medication (ASM) for epilepsy and recurrent seizures]. The weighted mean age of individuals is 42.35 years, 49.08% (95% confidence interval [CI] 48.03–50.12) were male, 64.56% (95%CI 63.70–65.41) were Non-Hispanic White, and 59.95% (95%CI 58.98–60.92) were private insurance. The weighted prevalence of epilepsy was 0.82% (95%CI 0.60–1.11) and 0.60% (95%CI 0.42–0.86) in those with and without tobacco smoke exposures, respectively. After adjusting for covariates, active and passive tobacco smoke exposure was not associated with epilepsy [weighted adjusted odd ratio (OR) 1.16, 95% CI 0.68–1.98, p-value = 0.576] and the results remained in multiple sensitivity analyses. However, we found that tobacco exposure was a protective factor for epilepsy in those aged 40–50 (OR 0.23, 95%CI 0.10–0.53, p-value < 0.001).
Conclusion:
In summary, tobacco exposure was not associated with epilepsy in the US population and this result remained after adjusting for confounding factors, and the sensitivity analysis was robust. However, in stratified analysis, tobacco exposure was a protective factor for epilepsy patients aged 40–50.
1 Introduction
Epilepsy is one of the most common chronic neurological disorders, affecting approximately 70 million persons all around the world and posing significant challenges and burden to public health and quality of life (1). According to the global burden of epilepsy in 2016, epilepsy accounted for 13.5 million disability-adjusted life-years (DALYs) and was responsible for 0.56% of total DALYs globally (2, 3). The prevalence of epilepsy is higher in low-income countries than in high-income countries and males demonstrating a marginally elevated risk relative to females (1, 4). The incidence has a bimodal distribution, with two distinct peaks: in infants under 1 year of age and in people aged 50 years and older (1). Within the older adult population (>50 years), the incidence rate increases with increasing age, and the incidence rate of people over 70 years old is the highest (1). Epilepsy is complex and multifactorial, with both genetic and environmental factors playing crucial roles. The risk factors vary among different age groups. Brain developmental abnormalities typically occur in those with onset before adulthood, while epilepsy related to head trauma, infection, and tumors can occur at any age and cerebrovascular disease usually occur in older people (1).
The smoke generated during smoking consists of a complex mixture of compounds, with different effects on human health, such as nicotine, tar, carbon monoxide, and polycyclic aromatic hydrocarbons. Tobacco exposure has negative effect to some diseases such as stroke, coronary heart disease, and lung cancer, while in others such as inflammatory bowel diseases, it has positive effect (5–8).
Tobacco smoke exposure is complex in epilepsy because some studies considered proconvulsant effects while others suggested anticonvulsant effects (9, 10). A prospective study, using data from the Nurses’ Health Study II, included 116,363 women aged 25–42 years. They found that after adjusting for stroke and other factors, current cigarette smoking was associated with increased risk of seizure (RR 2.60, 95% CI 1.53–4.42) and past smoking was associated with increased risk of epilepsy (RR 1.46, 95% CI 1.01–2.12) compared with never smoking (10). A meta-analysis of Mendelian randomization (MR) studies assessing the causal association of smoking in a variety of diseases and a two-sample MR study of risk factors for epilepsy identified that genetic liability to smoking was associated with increased risk of epilepsy (11, 12). For individuals in older age, the Framingham Heart Study showed that smoking was not associated with an increasing risk of epilepsy, but the Atherosclerosis Risk in Communities (ARIC) study indicated that smoking increased the risk of late-onset epilepsy (13, 14). A small sample study found that smokers with epilepsy were four times more likely to experience seizures than non-smokers with epilepsy, and the risk of refractory epilepsy did not increase (15). Furthermore, smoking and nicotine have been known to decrease the serum levels and anticonvulsive effect of lamotrigine (16–18). However, a retrospective study on Chinese males with epilepsy found a beneficial effect of smoking on seizure control (9).
In recent years, the prevalence of both active and passive smoking has been steadily increasing, particularly among individuals with poorer overall health (19–21). This trend highlights the need to pay closer attention to the impact of tobacco exposure on these vulnerable populations. The prevalence of smoking among people with epilepsy ranges from 20 to 48%, with most studies indicating that this rate is higher compared to those without epilepsy (22–29). However, the impact of tobacco exposure on epilepsy remains controversial. This study aimed to investigate the relationship between tobacco smoke exposure and epilepsy.
2 Materials and methods
2.1 Data sources and study population
This is a retrospective cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) 2013–2018. The NHANES, a nationally representative survey, is conducted by the National Center for Health Statistics (NCHS) of Centers for Disease Control and Prevention (CDC) for assessing the physical and nutritional status of US population. We included all participants from 2013–2014, 2015–2016, 2017–2018 cycles of NHANES across different age groups (N = 29,400) and excluded those epilepsy status, tobacco exposure status or covariates was unavailable (N = 24, 7,629 and 4,019, respectively) in this analysis. Ultimately, the study included 17,728 participants (Figure 1). The datasets used in this analysis can be obtained from the NHANES official website1. Moreover, all participants in NHANES had signed informed consent forms.
Figure 1

Flow chart.
2.2 Definition of tobacco smoke exposures
In our study, tobacco exposures were divided into active and passive tobacco exposures. Participants who answered yes to the question “During the past 5 days, including today, did you smoke cigarettes, pipes, cigars, little cigars or cigarillos, water pipes, hookahs, or e-cigarettes?” or the concentrations of cotinine in serum greater than 10 μg/L were identified as having active tobacco exposures. Passive tobacco exposures were defined as those who did not smoke, had at least one household smoker, or had the concentrations of cotinine in serum greater than 0.05 μg/L. Cotinine is one of the main metabolites of nicotine and the half-life of cotinine is longer than nicotine (15–20 h vs. 0.5–3 h). Therefore, the concentrations of cotinine in body fluids (such as serum, urine, and saliva) can be used as biomarkers for active smoking or secondhand smoke exposures.
Tobacco exposure was classified as active or passive. Due to the lack of detailed information in NHANES, factors such as smoking frequency, duration, and intensity were not included in the analysis. We acknowledge this as a limitation, and future studies should incorporate these aspects to better understand their impact on epilepsy risk.
2.3 Definition of epilepsy
All participants in the NHANES were required to respond to the following question: “In the past 30 days, have you used or taken medication for which a prescription is needed? Do not include prescription vitamins or minerals you may have already told me about.” Participants were then asked to provide the complete name and main reasons of each prescription medication they used. In our analysis, we defined participants as patients with epilepsy if they responded taking at least one prescription medication for the reason of “epilepsy and recurrent seizures” (ICD-10-CM codes: G40). We observed that some medications were reported for “epilepsy and recurrent seizures” but were not standard anti-seizure medications (ASMs). Therefore, we did not take such medications into consideration (Supplementary Table 1). The definition of epilepsy is according to previous studies (30, 31).
2.4 Other covariates
Other covariates included in our study were age, sex, race-ethnicity, BMI, family poverty-income ratio, insurance status, the concentrations of cotinine and the survey cycle. The age of participants is recorded by year, and individuals 80 or over 80 are recorded as 80 years of age. The race-ethnicity was categorized as Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race. Body Mass Index (BMI) was regarded as a continuous variable. We used family poverty-income ratio to represent the economic situation. It was calculated as the ratio of family (or individual) income to the poverty guidelines specific to the survey year. We obtained the insurance information from the Health Insurance questionnaire. Insurance status was classified into the following: without any health insurance, covered by private insurance, and covered by public insurance only. Public insurance includes Medicare, Medicaid, Children’s Health Insurance Program (CHIP), Indian Health Service, military health plan (Tricare/VA/Champ-VA), state-sponsored health plan, and other government insurance.
2.5 Statistical analysis
All analyses used sampling weights to indicate complex sampling designs according to the data analysis tutorials of NHANES. We excluded participants with missing variables from the analysis, which could potentially introduce selection bias. While this approach is a common practice to ensure data consistency and reliability, we acknowledge that it may limit the generalizability of our findings. The exclusion of missing data could introduce some bias, especially if the missing data are not missing at random. In future studies, more advanced statistical methods, such as multiple imputations or propensity score matching, could be employed to address missing data and better assess their impact on the results.
To describe continuous variables, the survey-weighted mean and SD were used if the data conforms to normal distribution, and median and interquartile ranges were reported otherwise. For categorical variables, we reported survey-weighted proportions and 95% confidence intervals (CIs). Weighted multiple logistic regression was used to estimate crude and adjusted odds ratios (ORs) and 95% CIs of the association between tobacco smoke exposures and epilepsy. In our analyses, we established three models. The first model was not adjusted, the second model was adjusted for age, gender, and race-ethnicity, and the third model was adjusted for age, sex, race-ethnicity, BMI, family poverty-income ratio category, insurance status, and survey cycle. However, we acknowledge the importance of verifying all assumptions, including checking for outliers and testing for independence of errors. Future studies should consider conducting diagnostic tests such as the Durbin-Watson test for autocorrelation and the use of leverage statistics for detecting influential outliers.
To further test our findings, we conduct two sensitivity analyses. Firstly, the limit of detection of cotinine is used as a cutoff for tobacco exposure (cotinine level <0.05 μg/L vs. cotinine level ≥0.05 μg/L). Secondly, the smoking status is categorized as none, passive tobacco smoke exposures, and active tobacco smoke exposures. Then weighted multiple logistic regression was performed. In sub-group analysis, we stratified by age group, sex, race-ethnicity, family poverty-income ratio category, and insurance status. Age subgroups were classified into ≤20 years old, 20 ~ 30 years old, 30 ~ 40 years old, 40 ~ 50 years old, 50 ~ 60 years old, and >60 years old. The variables used in the analysis are classified according to their measurement scales: continuous variables: Age, BMI, Cotinine; dichotomous variables: Epilepsy, Tobacco exposure; ordinal variables: Poverty-income ratio categories; nominal variables: Sex, Race/ethnicity; Interval/ratio variables: Insurance status; This classification is crucial for understanding the nature of the data and ensuring appropriate statistical methods are used. All data handling was conducted with RStudio software version 4.0.5 and statistical analyses were performed using StataMP version 18. p-values < 0.05 were deemed significant.
3 Results
3.1 Demographics characteristics of study participants
There were 29,400 individuals participated in NHANES survey between 2013 and 2018. Of these, 17,728 individuals were included in our analysis (as presented in Figure 1). Table 1 demonstrates the distribution of age, sex, race-ethnicity, poverty-income ratio, insurance status, BMI, serum cotinine, and epilepsy in those with and without tobacco smoke exposure. The weighted mean age of all individuals in our study is 42.35 years, and 47.03 years of those without tobacco smoke exposure and 37.65 years of those with exposure. Male participants are more likely to experience tobacco smoke exposure. The Non-Hispanic White group accounted for the most weighted proportion in total population, non-exposure group, and exposure group (64.56% [95%CI 63.70–65.41%] vs. 68.21% [95%CI 67.01%-69.38] vs. 60.89% [95%CI 59.67–62.10%]). More than 50% of study participants who have a poverty index more than 180% of the federal poverty guideline (66.11% [95%CI 65.23–66.98%] in total, 77.40% [95%CI 76.32–78.44%] in non-exposure group, 54.79% [95%CI 53.46–56.12%] in exposure group). No matter in which groups, study participants tended to have private insurance (59.95% [95%CI 58.98–60.92%] in total, 70.93% [95%CI 69.61–72.20%] in non-exposure group, 48.94% [95%CI 47.56–50.34%] in exposure group). The survey-weighted prevalence of participants who had epilepsy was 0.71% (95%CI 0.56–0.90%), and it is consistent with previous researches (1, 2). The proportions of epilepsy in participants with and without tobacco smoke exposures was 0.82% (95%CI 0.60–1.11%) and 0.60% (95%CI 0.42–0.86%), respectively.
Table 1
| Characteristics | % (95%CI) | VIF | ||
|---|---|---|---|---|
| Total (N = 17,728) | Tobacco smoke exposure | |||
| None (N = 7,919) | Any (n = 9,809) | |||
| Age, mean (SD), y | 42.35 (0.19) | 47.03 (0.27) | 37.65 (0.26) | 1.33 |
| Sex | 1.00 | |||
| Male | 49.08 (48.03–50.12) | 45.49 (43.93–47.04) | 52.68 (51.30–54.05) | |
| Female | 50.92 (49.88–51.97) | 54.51 (52.96–56.07) | 47.32 (45.95–48.70) | |
| Race/ethnicity | 1.03 | |||
| Mexican American | 9.44 (9.03–09.86) | 10.40 (9.79–11.04) | 8.47 (7.94–9.04) | |
| Other Hispanic | 5.94 (5.62–6.29) | 6.33 (5.86–6.84) | 5.55 (5.12–6.03) | |
| Non-Hispanic White | 64.56 (63.70–65.41) | 68.21 (67.01–69.38) | 60.89 (59.67–62.10) | |
| Non-Hispanic Black | 11.05 (10.65–11.46) | 6.50 (6.07–6.96) | 15.61 (14.94–16.31) | |
| Other Race | 9.01 (8.55–9.49) | 8.56 (7.95–9.21) | 9.46 (8.79–10.18) | |
| Family Poverty-income ratio | 1.03 | |||
| FPIR < 1.3 | 23.89 (23.17–24.64) | 14.39 (13.58–15.24) | 33.43 (32.27–34.61) | |
| 1.3 ≤ FPIR ≤ 1.8 | 9.99 (9.49–10.51) | 8.22 (7.57–8.91) | 11.77 (11.03–12.56) | |
| FPIR > 1.8 | 66.11 (65.23–66.98) | 77.40 (76.32–78.44) | 54.79 (53.46–56.12) | |
| Insurance | 1.06 | |||
| None | 13.51 (12.89–14.15) | 8.90 (8.18–9.67) | 18.13 (17.17–19.15) | |
| Private | 59.95 (58.98–60.92) | 70.92 (69.61–72.20) | 48.94 (47.56–50.34) | |
| Public (only) | 26.54 (25.72–27.377) | 20.18 (19.08–21.33) | 32.92 (31.73–34.13) | |
| BMI, median (IQR), kg/㎡ | 26.70 (22.30–31.80) | 27.50 (23.60–32.20) | 26.00 (21.10–31.40) | 1.32 |
| Serum cotinine, median (IQR), μg/L | 0.05 (0.01–1.51) | 0.01 (0.011–0.20) | 0.74 (0.09–126.00) | 1.06 |
| Epilepsy | 0.71 (0.56–0.90) | 0.60 (0.42–0.86) | 0.82 (0.60–1.11) | — |
Demographic characteristics of participants in NHANES 2013–2018.
To address potential multicollinearity issues, we checked the variance inflation factors (VIFs) for the covariates included in the regression models. None of the VIFs exceeded the commonly accepted threshold of 10, suggesting that multicollinearity is not a significant concern in our analysis.
3.2 Association between tobacco smoke exposures and epilepsy
We verified the association between tobacco exposure and epilepsy in Table 2. In our analysis, tobacco smoke exposures are not significantly associated with epilepsy. In the unadjusted analysis, the OR for epilepsy in participants with tobacco exposure is 1.37(95%CI 0.85–2.21, p-value = 0.196) compared to those without exposure. But the result is not statistically significant. When adjusting for age, sex, and race-ethnicity and adjusting for full covariates, the results remain not statistically significant (OR 1.57, 95%CI 0.95–2.59, p-value = 0.078 and OR 1.16, 95%CI 0.68–1.98, p-value = 0.576).
Table 2
| Exposure | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Any tobacco exposure | 1.37 (0.85–2.21) | 0.196 | 1.57 (0.95–2.59) | 0.078 | 1.16(0.68–1.98) | 0.576 |
| Sensitivity analyses | ||||||
| Lower cutoff of cotinine (<0.015 μg/L) | 1.01 (0.61–1.66) | 0.972 | 1.12 (0.65–1.91) | 0.688 | 0.80 (0.45–1.42) | 0.454 |
| Passive tobacco exposure | 1.14 (0.61–2.12) | 0.690 | 1.37 (0.72–2.59) | 0.333 | 1.05 (0.53–2.06) | 0.892 |
| Active smoking | 1.64 (0.97–2.77) | 0.066 | 1.76 (1.01–3.08) | 0.046 | 1.28 (0.72–2.25) | 0.399 |
Association between active and passive tobacco smoke exposures and epilepsy.
Model 1: unadjusted.
Model 2: adjusted for age, sex, and race-ethnicity.
Model 3: adjusted for age, sex, race-ethnicity, BMI, family poverty-income ratio category, insurance status, and survey cycle.
To further explore the relationship between tobacco smoke exposure and epilepsy, we conducted a sensitivity analysis. Firstly, we used cotinine cutoff (0.015 μg/L) as the boundary value to define tobacco smoke exposure status, and obtained similar results (OR 0.80, 95% CI 0.45–1.42). Furthermore, we performed another sensitivity analysis by dividing tobacco smoke exposure into none, active and passive. When adjusting for age, sex, and race-ethnicity, we observed the positive correlation between tobacco exposure and epilepsy (OR 1.76, 95% CI [1.01–3.08]). But after full adjustment, we found results similar to that of the main analyses (OR 1.28, 95%CI [0.72–2.25]). Thus, there were no statistically significant relationship between tobacco smoke exposure and epilepsy (Table 2). We tested the linearity assumption between continuous predictors and the logit of the outcome using the Box-Tidwell procedure, and found no significant violations.
3.3 Weighted subgroup analysis
Moreover, we conducted weighted subgroup analyses to assess whether the association between tobacco smoke exposures and epilepsy was influenced by age group, sex, race-ethnicity, family poverty-income ratio category, and insurance status. We divided age into multiple groups and adjusted for all covariates, it was found that a significant negative correlation between tobacco smoke exposures and epilepsy was observed only in participants aged 40 ~ 50 years (OR 0.23, 95%CI 0.10–0.53). Conversely, the relationship was not statistically significant for those who were in other age groups or stratified by sex, race-ethnicity, family poverty-income ratio category, and insurance status. This finding was consistent with our main results (Figure 2).
Figure 2
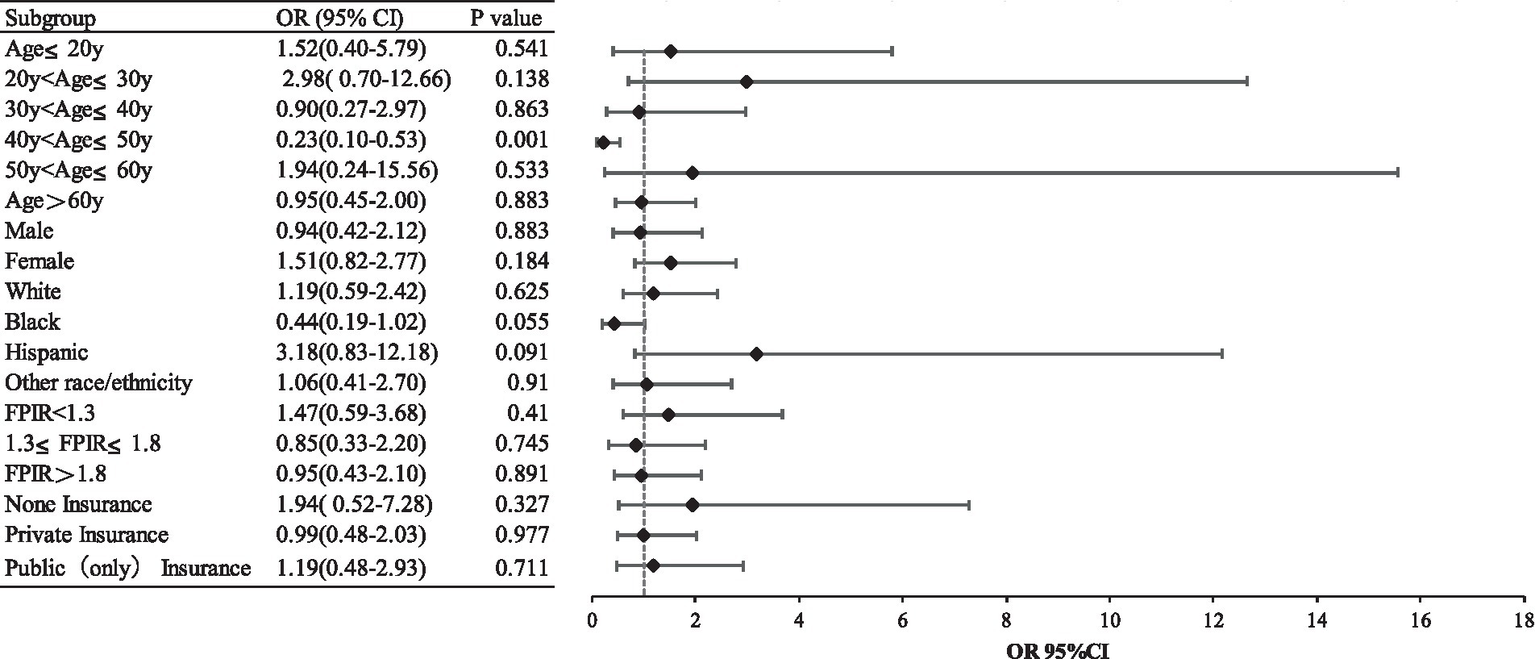
Subgroup analysis.
4 Discussion
In this nationally representative study, 17,728 survey participants were included and the prevalence of epilepsy was 7‰, which is consistent with previous studies (4). We found no statistical association between tobacco exposure and epilepsy and the results persisted after adjusting for age, sex, race/ethnicity, body mass index category, poverty-income ratio category, insurance status, and survey year. In different subgroups of age, sex, and race/ethnicity, we only found a statistically significant association in the age-group of 40–50 years. The results were similar when using different definitions of tobacco exposure and in the group of active tobacco exposure or passive tobacco exposure.
In current literature, data on the relationship between tobacco smoke exposures and epilepsy are limited, and the association remains inconclusive. While it is widely accepted that tobacco exposure is a detrimental factor for individuals with epilepsy, our findings suggest that both active and passive tobacco smoke exposure may act as a protective factor for epilepsy in individuals aged 40–50 years. This result is similar to the research reported by Gao et al., but contrasts with other studies that identify tobacco exposure as a risk factor (9–14). These discrepancies may be attributed to differences in study populations, definitions of tobacco smoke exposure, and criteria for diagnosing epilepsy. First, many studies have been limited by small sample sizes, and none have utilized the NHANES database to explore the relationship between tobacco exposure and epilepsy. In contrast, our study employed data from the NHANES database (2013–2018) with weighted statistical analysis, providing national representativeness and a large sample size. Second, while previous studies typically relied on self-reported tobacco use, we assessed tobacco exposure through both self-reports and biomarkers. Additionally, we included participants exposed to both active and passive tobacco smoke. Finally, compared to earlier studies, we defined epilepsy based on the use of antiepileptic medications and the occurrence of recurrent seizures in the past 30 days.
The components of tobacco smoke are complex, and current studies have shown that some of these constituents, including nicotine, have anticonvulsant effects. Nicotine, a major alkaloid in tobacco, is rapidly absorbed into the bloodstream through the lungs and transported to the brain when tobacco products are smoked or used. In animal studies, low-dose nicotine has been shown to exert protective effects on seizure activity, suggesting a potential therapeutic role (32). The mechanisms through which nicotine influences epilepsy are multifaceted, and understanding these pathways is crucial for elucidating its clinical implications (33, 34). First, nicotine acts primarily through neuronal nicotinic acetylcholine receptors (nAChRs), which are ligand-gated ion channels widely distributed in both pre-and post-synaptic regions of the brain (33, 34). nAChRs modulate ion flux across cell membranes, influencing neuronal excitability and the release of various neurotransmitters (33, 34). This action impacts synaptic plasticity and other physiological and behavioral processes (33, 34). Dysregulation of nAChRs has been implicated in the pathophysiology of epilepsy, and nAChRs are considered potential therapeutic targets (33, 34). For instance, autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) is a rare form of epilepsy caused by mutations in genes encoding nAChRs (35). Given that nicotine is an agonist of nAChRs, its chronic exposure may alter receptor sensitivity, potentially offering therapeutic benefits for epilepsy linked to nAChR mutations (34, 36–38). Additionally, studies have demonstrated that nicotine and its metabolite, cotinine, can rapidly cross the blood–brain barrier and bind directly to MD2 proteins on microglial membranes (39, 40). This binding induces conformational changes in MD2, which may influence its function and reduce neuroinflammation caused by microglial activation (39, 40). Neuroinflammation is a key contributor to the onset and progression of epilepsy, and by modulating this pathway, nicotine may reduce seizure susceptibility and protect against long-term neurological damage (41). This anti-inflammatory effect presents a novel avenue for epilepsy treatment, although the dual effects of nicotine (i.e., both potential benefits and risks) must be carefully balanced in clinical practice. Furthermore, other tobacco smoke constituents, such as selenium, zinc, carbon dioxide, toluene, acetone, and nickel chloride, have also been shown to possess anticonvulsant properties, suggesting that nicotine may not be the only active component in tobacco with potential therapeutic effects (42–47). However, the complex nature of tobacco smoke and its varying effects on the brain necessitate further investigation to better understand its overall impact on epilepsy management. While nicotine has demonstrated potential anticonvulsant effects in certain studies, these findings should be interpreted with caution, given the potential limitations of sample size, study design, and other confounding factors. It is important to note that our results may be influenced by random variation or residual confounding, and additional studies with larger sample sizes and longitudinal designs are required to validate these findings. Further research is required to fully understand the biological mechanisms underlying these effects and their clinical implications for epilepsy treatment. This finding could lead to the development of novel therapies for drug-resistant epilepsy, although the risks associated with nicotine exposure, including addiction and adverse health effects, must be considered in clinical decision-making.
Our study has several limitations that should be noted. First, our definition of epilepsy was based on medication intake, which may not have captured all individuals with epilepsy, as some patients might have refused medication or discontinued it due to good control of their condition. However, multiple previous studies had used this definition, and there was evidence that the existence of ASM can greatly improve the detection of epilepsy in research datasets (30, 31, 48, 49). Second, the NHANES database does not provide detailed information on key characteristics of epilepsy, such as seizure frequency, duration, or classification. This limits our ability to fully assess the relationship between tobacco exposure and different subtypes of epilepsy. Third, there is a risk of residual confounding due to the lack of certain covariates, such as family history of epilepsy, history of head trauma, and past encephalitis or meningitis, which could influence the risk of epilepsy. These factors were not available in the NHANES dataset and could have affected the results. Fourth, due to the lack of detailed information in NHANES, factors such as smoking frequency, duration, and intensity were not included in the analysis. Finally, it is important to emphasize that this study is cross-sectional in nature and therefore cannot establish causality. The dataset does not provide information on the temporal relationship between tobacco exposure and the onset of epilepsy. Given the potential for random variation and the limitations in sample size, further studies are needed to validate our findings. There should be some better design, more confounders considered and longitudinal studies to be performed to investigate how tobacco smoke exposure affects the epilepsy network. In the future, extract from cigarette as adjunctive medication or nicotine therapy may help obtain better seizure control in patients.
5 Conclusion
In summary, tobacco exposure was not associated with epilepsy in the US population and this result remained after adjusting for confounding factors, and the sensitivity analysis was robust. However, in stratified analysis, tobacco exposure was a protective factor for epilepsy patients aged 40–50. More studies are need to investigate how smoking affects the epilepsy network in the brain, as well as a prospective study that considers more confounding factors to increase evidence of the association between tobacco exposure and epilepsy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
TS: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – original draft. CJ: Data curation, Formal analysis, Writing – original draft. QW: Data curation, Formal analysis, Resources, Writing – original draft. JM: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the staff at the National Center for Health Statistics of the Centers for Disease Control for designing and creating the NHANES database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1502894/full#supplementary-material
Footnotes
References
1.
Thijs RD Surges R O’brien TJ Sander JW . Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
2.
Beghi E Giussani G Nichols E Abd-Allah F Abdela J Abdelalim A et al . Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:357–75. doi: 10.1016/S1474-4422(18)30454-X
3.
Feigin VL Nichols E Alam T Bannick MS Beghi E Blake N et al . Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X
4.
Beghi E . The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
5.
Lin MP Ovbiagele B Markovic D Towfighi A . Association of Secondhand Smoke with Stroke Outcomes. Stroke. (2016) 47:2828–35. doi: 10.1161/STROKEAHA.116.014099
6.
Steenland K Thun M Lally C Heath C . Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation. (1996) 94:622–8. doi: 10.1161/01.CIR.94.4.622
7.
Wang GZ Cheng X Li XC Liu YQ Wang XQ Shi X et al . Tobacco smoke induces production of chemokine CCL20 to promote lung cancer. Cancer Lett. (2015) 363:60–70. doi: 10.1016/j.canlet.2015.04.005
8.
Cosnes J Carbonnel F Carrat F Beaugerie L Cattan S Gendre J . Effects of current and former cigarette smoking on the clinical course of Crohn’s disease. Aliment Pharmacol Ther. (1999) 13:1403–11. doi: 10.1046/j.1365-2036.1999.00630.x
9.
Gao H Sander JW Du X Chen J Zhu C Zhou D . Smoking prevalence and seizure control in Chinese males with epilepsy. Epilepsy Behav. (2017) 73:268–72. doi: 10.1016/j.yebeh.2017.04.008
10.
Dworetzky BA Bromfield EB Townsend MK Kang JH . A prospective study of smoking, caffeine, and alcohol as risk factors for seizures or epilepsy in young adult women: data from the Nurses’ health study II. Epilepsia. (2010) 51:198–205. doi: 10.1111/j.1528-1167.2009.02268.x
11.
Yuan S Tomson T Larsson SC . Modifiable risk factors for epilepsy: a two-sample Mendelian randomization study. Brain Behav. (2021) 11:e02098. doi: 10.1002/brb3.2098
12.
Larsson SC Burgess S . Appraising the causal role of smoking in multiple diseases: a systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine. (2022) 82:104154. doi: 10.1016/j.ebiom.2022.104154
13.
Johnson EL Krauss GL Lee AK Schneider ALC Dearborn JL Kucharska-Newton AM et al . Association between midlife risk factors and late-onset epilepsy: results from the atherosclerosis risk in communities study. JAMA Neurol. (2018) 75:1375–82. doi: 10.1001/jamaneurol.2018.1935
14.
Stefanidou M Himali JJ Devinsky O Romero JR Ikram MA Beiser AS et al . Vascular risk factors as predictors of epilepsy in older age: the Framingham heart study. Epilepsia. (2022) 63:237–43. doi: 10.1111/epi.17108
15.
Johnson AL Mcleish AC Shear PK Sheth A Privitera M . The role of cigarette smoking in epilepsy severity and epilepsy-related quality of life. Epilepsy Behav. (2019) 93:38–42. doi: 10.1016/j.yebeh.2019.01.041
16.
Reinsberger C Dorn T Krämer G . Smoking reduces serum levels of lamotrigine. Seizure. (2008) 17:651–3. doi: 10.1016/j.seizure.2008.05.009
17.
Milosheska D Lorber B Vovk T Kastelic M Dolžan V Grabnar I . Pharmacokinetics of lamotrigine and its metabolite N-2-glucuronide: influence of polymorphism of UDP-glucuronosyltransferases and drug transporters. Br J Clin Pharmacol. (2016) 82:399–411. doi: 10.1111/bcp.12984
18.
Czuczwar M Kiś J Czuczwar P Wielosz M Turski W . Nicotine diminishes anticonvulsant activity of antiepileptic drugs in mice. Pol J Pharmacol. (2003) 55:799–802. PMID:
19.
Alkan Ö Ünver Ş . Secondhand smoke exposure for different education levels: findings from a large, nationally representative survey in Turkey. BMJ Open. (2022) 12:e057360. doi: 10.1136/bmjopen-2021-057360
20.
Ünver Ş Tekmanli HH Alkan Ö . Passive smoking as a risk factor among older adults: an ordered probability approach for Türkiye. Front Public Health. (2023) 11:1142635. doi: 10.3389/fpubh.2023.1142635
21.
Alkan Ö Ünver Ş . Tobacco smoke exposure among women in Turkey and determinants. J Subst Abus. (2021) 27:43–9. doi: 10.1080/14659891.2021.1885518
22.
Sapkota S Kobau R Croft JB King BA Thomas C Zack MM . Prevalence and trends in cigarette smoking among adults with epilepsy – United States, 2010-2017. MMWR Morb Mortal Wkly Rep. (2020) 69:1792–6. doi: 10.15585/mmwr.mm6947a5
23.
Yeni N Tumay F Tonguç Ö Azaroğlu E Bozok N . Survey on smoking, consuming alcohol, and using illicit drugs in patients with epilepsy. Noro Psikiyatr Ars. (2015) 52:354–8. doi: 10.5152/npa.2015.8772
24.
Roberts JI Patten SB Wiebe S Hemmelgarn BR Pringsheim T Jetté N . Health-related behaviors and comorbidities in people with epilepsy: changes in the past decade. Epilepsia. (2015) 56:1973–81. doi: 10.1111/epi.13207
25.
Cui W Zack MM Kobau R Helmers SL . Health behaviors among people with epilepsy--results from the 2010 National Health Interview Survey. Epilepsy Behav. (2015) 44:121–6. doi: 10.1016/j.yebeh.2015.01.011
26.
Hinnell C Williams J Metcalfe A Patten SB Parker R Wiebe S et al . Health status and health-related behaviors in epilepsy compared to other chronic conditions--a national population-based study. Epilepsia. (2010) 51:853–61. doi: 10.1111/j.1528-1167.2009.02477.x
27.
Konda K Ablah E Konda KS Liow K . Health behaviors and conditions of persons with epilepsy: a bivariate analysis of 2006 BRFSS data. Epilepsy Behav. (2009) 16:120–7. doi: 10.1016/j.yebeh.2009.07.010
28.
Elliott JO Lu B Moore JL Mcauley JW Long L . Exercise, diet, health behaviors, and risk factors among persons with epilepsy based on the California health interview survey, 2005. Epilepsy Behav. (2008) 13:307–15. doi: 10.1016/j.yebeh.2008.04.003
29.
Zhong R Li Z Zhang X Chen Q Lin W . Current cigarette smoking is associated with a high seizure frequency and anxiety symptoms in people with epilepsy. Front Neurol. (2022) 13:834694. doi: 10.3389/fneur.2022.834694
30.
Terman SW Aubert CE Hill CE Maust DT Betjemann JP Boyd CM et al . Polypharmacy in patients with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. (2020) 111:107261. doi: 10.1016/j.yebeh.2020.107261
31.
Terman SW Hill CE Burke JF . Disability in people with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. (2020) 112:107429. doi: 10.1016/j.yebeh.2020.107429
32.
De Fiebre CM Collins AC . Decreased sensitivity to nicotine-induced seizures as a consequence of nicotine pretreatment in long-sleep and short-sleep mice. Alcohol. (1988) 5:55–61. doi: 10.1016/0741-8329(88)90044-4
33.
Terry AV Jones K Bertrand D . Nicotinic acetylcholine receptors in neurological and psychiatric diseases. Pharmacol Res. (2023) 191:106764. doi: 10.1016/j.phrs.2023.106764
34.
Fox J Thodeson DM Dolce AM . Nicotine: a targeted therapy for epilepsy due to nAChR gene variants. J Child Neurol. (2021) 36:371–7. doi: 10.1177/0883073820974851
35.
Itier V Bertrand D . Mutations of the neuronal nicotinic acetylcholine receptors and their association with ADNFLE. Neurophysiol Clin. (2002) 32:99–107. doi: 10.1016/S0987-7053(02)00294-0
36.
Nguyen SM Deering L Nelson GT Mcdaniel SS . Nicotine patch improved autosomal dominant sleep-related hypermotor epilepsy. Pediatr Neurol. (2021) 123:41–2. doi: 10.1016/j.pediatrneurol.2021.07.006
37.
Brodtkorb E Picard F . Tobacco habits modulate autosomal dominant nocturnal frontal lobe epilepsy. Epilepsy Behav. (2006) 9:515–20. doi: 10.1016/j.yebeh.2006.07.008
38.
Willoughby JO Pope KJ Eaton V . Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study. Epilepsia. (2003) 44:1238–40. doi: 10.1046/j.1528-1157.2003.58102.x-i1
39.
Li H Peng Y Lin C Zhang X Zhang T Wang Y et al . Nicotine and its metabolite cotinine target MD2 and inhibit TLR4 signaling. Innovation. (2021) 2:100111. doi: 10.1016/j.xinn.2021.100111
40.
Zhang W Lin H Zou M Yuan Q Huang Z Pan X et al . Nicotine in inflammatory diseases: anti-inflammatory and pro-inflammatory effects. Front Immunol. (2022) 13:826889. doi: 10.3389/fimmu.2022.826889
41.
Ravizza T Scheper M Di Sapia R Gorter J Aronica E Vezzani A . mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment. Nat Rev Neurosci. (2024) 25:334–50. doi: 10.1038/s41583-024-00805-1
42.
Rong L Frontera AT Jr Benbadis SR . Tobacco smoking, epilepsy, and seizures. Epilepsy Behav. (2014) 31:210–8. doi: 10.1016/j.yebeh.2013.11.022
43.
Seven M Basaran SY Cengiz M Unal S Yuksel A . Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. (2013) 104:35–9. doi: 10.1016/j.eplepsyres.2012.09.013
44.
Tolner EA Hochman DW Hassinen P Otáhal J Gaily E Haglund MM et al . Five percent CO₂ is a potent, fast-acting inhalation anticonvulsant. Epilepsia. (2011) 52:104–14. doi: 10.1111/j.1528-1167.2010.02731.x
45.
Cruz SL Gauthereau MY Camacho-Muñoz C López-Rubalcava C Balster RL . Effects of inhaled toluene and 1,1,1-trichloroethane on seizures and death produced by N-methyl-D-aspartic acid in mice. Behav Brain Res. (2003) 140:195–202. doi: 10.1016/S0166-4328(02)00323-6
46.
Likhodii S Nylen K Burnham WM . Acetone as an anticonvulsant. Epilepsia. (2008) 49:83–6. doi: 10.1111/j.1528-1167.2008.01844.x
47.
Rehni AK Singh N . Reversal of pentylenetetrazole-induced seizure activity in mice by nickel chloride. Indian J Pharmacol. (2009) 41:15–8. doi: 10.4103/0253-7613.48885
48.
Terman SW Aubert CE Hill CE Skvarce J Burke JF Mintzer S . Cardiovascular disease risk, awareness, and treatment in people with epilepsy. Epilepsy Behav. (2021) 117:107878. doi: 10.1016/j.yebeh.2021.107878
49.
Holden EW Grossman E Nguyen HT Gunter MJ Grebosky B Von Worley A et al . Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. (2005) 8:1–14. doi: 10.1089/dis.2005.8.1
Summary
Keywords
epilepsy, seizure, tobacco smoke exposures, cotinine, nicotine, NHANES
Citation
Song T, Jia C, Wang Q and Mu J (2025) The association between active and passive tobacco smoke exposures and epilepsy in United States participants of the National Health and Nutrition Examination Survey (2013–2018). Front. Neurol. 16:1502894. doi: 10.3389/fneur.2025.1502894
Received
27 September 2024
Accepted
28 April 2025
Published
09 May 2025
Volume
16 - 2025
Edited by
Lécio Figueira Pinto, University of São Paulo, Brazil
Reviewed by
Yanfeng Gong, Chinese Center for Disease Control and Prevention, China
Erkan Oktay, Atatürk University, Türkiye
Updates
Copyright
© 2025 Song, Jia, Wang and Mu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Mu, mujie2010@foxmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.