- 1Public Health School, Zunyi Medical University, Zunyi, China
- 2School of Nursing, Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 3School of Nursing, Zunyi Medical University, Zunyi, China
- 4NHC Key Laboratory of Pulmonary Immune-related Diseases, Guizhou Provincial People’s Hospital, Guiyang, China
- 5Department of Nursing, Guizhou Provincial People’s Hospital, Guiyang, China
Background: Neuroinflammation is linked to cognitive function. However, epidemiological research on two emerging inflammation markers—the systemic immune-inflammation index (SII) and the systemic inflammation response index (SIRI)—remains limited in the context of cognitive performance. This study investigates the relationship between SII, SIRI, and cognitive performance in older adults.
Methods: This cross-sectional analysis included 2,194 participants from the 2011–2014 National Health and Nutrition Examination Survey (NHANES) who met eligibility criteria. Logistic regression, subgroup analysis, and restricted cubic spline modeling were used to assess the associations between cognitive performance and inflammation markers, specifically SII and SIRI.
Results: After adjusting for population weights, participants with low cognitive function had an SII of 541.54 (95% CI: 360.00–796.50, p = 0.037) and an SIRI of 1.28 (95% CI: 0.82–2.18, p = 0.031). In fully adjusted models, higher levels of both SII (OR = 0.858, 95% CI: 0.856–0.859) and SIRI (OR = 0.891, 95% CI: 0.889–0.892) were significantly associated with lower odds of normal cognitive function, indicating an increased risk of cognitive impairment. Neutrophil-related markers (NC, NLR, SIRI) exhibited the strongest inverse associations. Subgroup analysis showed more consistent associations for SIRI across demographic and behavioral factors, while SII displayed fewer. RCS analysis indicated a stronger non-linear relationship for SIRI (p = 0.005) compared to SII (p = 0.018) after full adjustment.
Conclusion: This study suggests a positive association between SII, SIRI, and cognitive function, with a more pronounced relationship for SIRI. These findings highlight the potential of SIRI as a novel, accessible marker for predicting cognitive impairment risk.
1 Introduction
Cognitive impairment (CI) refers to a decline in cognitive functions, including memory, language, attention, problem-solving, and executive function. CI is linked to diminished daily functioning, increased comorbidity risks, and long-term care dependency, placing substantial medical and societal burdens (1). With global aging, CI has become an escalating health concern among older adults, underscoring the importance of identifying and mitigating its risk factors to reduce prevalence.
The systemic immune-inflammation index (SII) and the systemic inflammation response index (SIRI) are composite inflammatory markers developed in recent years, derived from neutrophil, lymphocyte, monocyte, and platelet counts (2–4). Neutrophil count (NC) reflects acute inflammatory responses, lymphocyte count (LC) plays a central role in immune regulation, platelet count (PC) signals both coagulative and inflammatory activities, and monocyte count (MC) is involved in immune surveillance. Initially proposed for liver cancer prognosis (5), SII has since been explored in various contexts, while SIRI was introduced to predict post-chemotherapy survival in patients with cancers (6), with subsequent research linking elevated SIRI to lymphovascular invasion (7). Given the critical role of inflammation in chronic diseases, easily obtainable hematological indices from routine blood tests (8, 9) are widely used to assess systemic inflammation (10, 11). Common markers include the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR). However, limited research has examined the relationship between emerging inflammatory markers like SII and SIRI and cognitive function. Investigating these associations may provide early diagnostic insights, elucidate inflammation-related neurobiological mechanisms, and offer practical biomarkers for cognitive performance assessment.
2 Methods
2.1 Data source
This study employed cross-sectional data from the NHANES, spanning three consecutive cycles: 2011–2012 and 2013–2014. Conducted by the National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention (CDC), NHANES assesses the health and nutritional status of individuals across various age groups in the U.S., from children to older adults. All NHANES protocols were approved by the NCHS Ethics Review Board, and informed consent was obtained from each participant.
2.2 Study population
Data from two NHANES cycles (2011–2012 and 2013–2014) were retrieved, including information from 19,931 participants. A total of 3,153 participants aged ≥ 60 years were included as they completed all cognitive function assessments. Participants with missing data for SII or SIRI measurements (N = 326) or covariates (N = 633) were excluded. Ultimately, 2,194 participants were included in the analysis (Figure 1).

Figure 1. Flowchart of participant selection from NHANES 2011–2014. PC, Platelet count; MC, Monocyte count; LC, Lymphocyte count; NC, Neutrophil count; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PPN, Product of platelet count and neutrophil count; SII, Systemic immune inflammation index; SIRI, System inflammation response index.
2.3 Immune-inflammation index
SII and SIRI were calculated based on complete blood count (CBC) laboratory results from the NHANES database. These calculations utilized separate measurements of PC, NC, MC, and LC, reported in units of 1,000 cells per μL (4, 12, 13). The indices were derived as follows:
SII = (platelet count × neutrophil count)/lymphocyte count;
SIRI = (neutrophil count × monocyte count)/lymphocyte count.
To further explore the relationship between inflammation indices and cognitive performance, additional markers such as PLR, NLR, the product of platelet and neutrophil count (PPN), and MLR were also analyzed (14, 15). Given the skewed distribution of these biomarkers, Log2 transformations were applied to PC, MC, LC, NC, NLR, PLR, MLR, PPN, SII, and SIRI for regression analysis, with the transformed values used in subsequent statistical evaluations (16–18).
2.4 Cognitive function assessment
Cognitive performance in participants aged ≥ 60 years was evaluated using three standardized tests: (1) the Consortium to Establish a Registry for Alzheimer’s Disease Test, comprising the Immediate Recall Test (CERAD-IR) and Delayed Recall Test (CERAD-DR); (2) the Animal Fluency Test (AFT); and (3) the Digit Symbol Substitution Test (DSST). These instruments are extensively used in cohort studies on cognitive function and its risk factors (19–21). A composite Z-score, referred to as global cognitive performance (GCP), was calculated by averaging the standardized scores from the CERAD, AFT, and DSST tests (22, 23). Participants were classified into two groups—normal cognitive ability and low cognitive ability—using the median of the total Z-score as a threshold.
2.5 Data covariates
Continuous covariates included in this study were age, diastolic blood pressure (DBP, mmHg), systolic blood pressure (SBP, mmHg), total cholesterol (mg/dL), white blood cell count (WBC, 1000 cells/μL), and red blood cell count (RBC, 1000 cells/μL). Categorical covariates comprised sex (male, female); age categories (60–69, 70–79, ≥ 80); race/ethnicity (Mexican American, Non-Hispanic Black, Non-Hispanic White, Other Hispanic, and other races including multiracial); marital status (married/living with partner, widowed/divorced/separated, never married); education level (below high school, high school graduate, above high school); poverty-income ratio (PIR; low < 2.23, middle 2.24–4.28, high > 4.29); body mass index (BMI) categories (underweight < 18.5, normal 18.5–24.9, overweight 25–29.9, obese ≥ 30); smoking status (ever smoked at least 100 cigarettes in lifetime); alcohol consumption (defined as drinking at least 12 alcoholic drinks per year); stroke history; diabetes status based on multiple criteria, including a confirmed diagnosis, medication or insulin use, HbA1c ≥ 6.5%, fasting blood glucose ≥ 7.0 mmol/L, or a two-hour post-OGTT blood glucose ≥ 11.1 mmol/L; depression (PHQ-9 score > 10), as per previous validations (24); sleep disorder; and overall health status (categorized as excellent/very good/good or fair/poor) (25–28).
2.6 Statistical analysis
Statistical analyses were performed with adjustments for the complex survey design, incorporating sample weights as per CDC guidelines. The study weight was derived by halving the current year’s weight. Analyses initially included only participants with complete data on exposures and outcomes. Continuous variables were summarized as means with standard deviations, and categorical variables as percentages. Independent t-tests assessed continuous variables, while chi-square tests evaluated categorical variables. A multivariable logistic regression model was then constructed to examine the independent associations between SII, SIRI, other inflammatory markers, and low cognitive performance across three models. Subgroup analyses were conducted based on sociodemographic and lifestyle factors. The restricted cubic spline (RCS) method was employed to explore the potential non-linear relationship between SII, SIRI, and cognitive performance. All analyses were executed using R and SPSS software, with a significance level set at p < 0.05.
3 Results
3.1 General characteristics of the study population
A total of 2,194 participants were included in this study, categorized into two groups based on Global Cognitive Performance (GCP): low cognitive performance (n = 238) and normal cognitive performance (n = 1,956). Demographic and clinical characteristics were compared between the groups, revealing significant differences (p < 0.05) in age, race, marital status, education level, PIR, stroke history, diabetes, depression, general health status, SBP, RBC, WBC, NC, NLR, MLR, and SII. Of the 2,194 participants, 1,105 were male (50.36%) and 1,089 were female (49.64%). The majority of participants were aged 60–69 years (54.38%), though a notably higher proportion of those aged ≥ 80 years was observed in the low cognitive performance group (30.67%). These results indicate that cognitive decline is strongly linked to socioeconomic and health-related factors, with systemic inflammation potentially playing a significant role. Detailed results are shown in Table 1.

Table 1. Weighted baseline characteristics of the study participants categorized by cognitive performance status.
3.2 Association between SII, SIRI, and cognitive performance
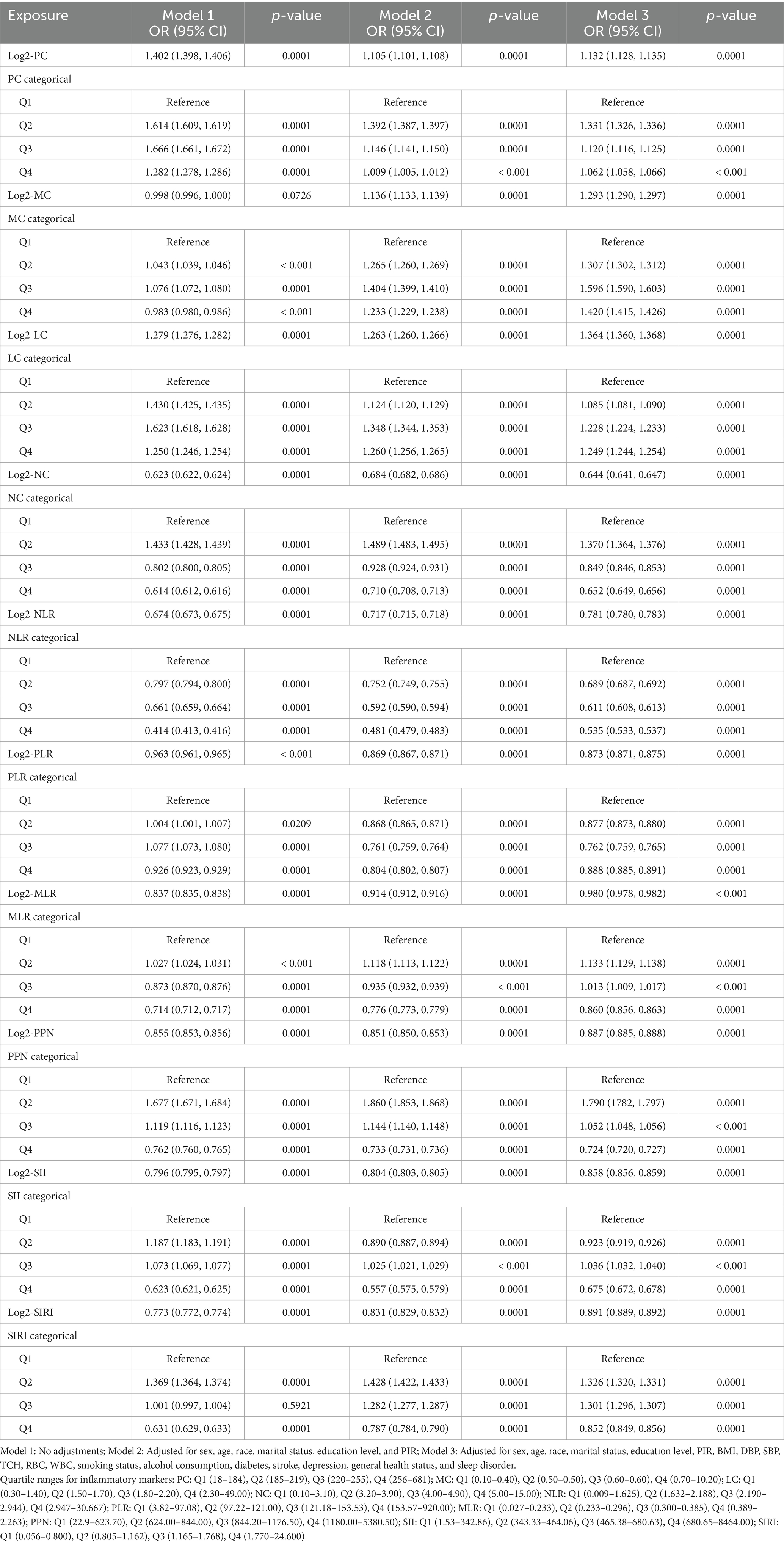
Table 2 presents the associations between Log2-transformed SII, SIRI, and other inflammatory markers and cognitive performance. Several inflammatory indices, when treated as continuous variables, were significantly associated with cognitive outcomes. Log2-PC was positively correlated with cognitive performance across all models (Model 1: OR = 1.402; Model 3: OR = 1.132, both p < 0.0001), suggesting a protective effect of higher platelet counts. Similarly, Log2-LC showed a positive association (Model 3: OR = 1.293, p < 0.0001), indicating a potential beneficial impact of lymphocytes. In contrast, higher levels of Log2-NC and Log2-NLR were consistently linked to lower odds of normal cognitive performance (Model 3: OR for NC = 0.644; OR for NLR = 0.781; both p < 0.0001), suggesting that neutrophil-driven inflammation may have a detrimental effect. Elevated levels of SII and SIRI were also significantly associated with poorer cognitive performance (Model 3: OR for SII = 0.858; OR for SIRI = 0.891; both p < 0.0001). Quartile analyses confirmed these observations, with participants in the highest quartiles of SII and SIRI exhibiting significantly higher odds of cognitive impairment compared to those in the lowest quartiles.
3.3 Subgroup analyses of the association between SII, SIRI, and cognitive performance
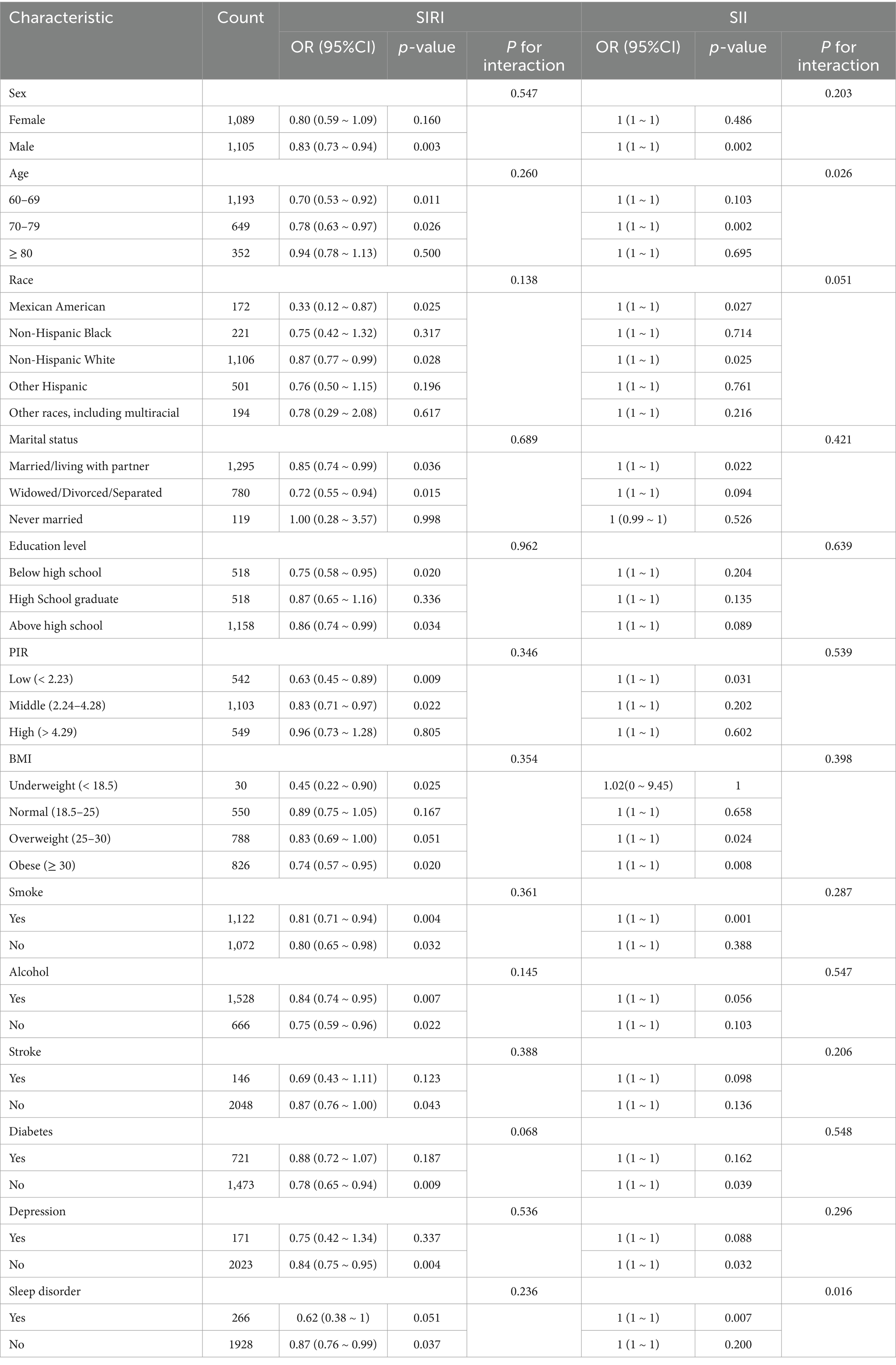
Subgroup analyses indicated a particularly strong association between SIRI and cognitive performance in males (OR = 0.83, p = 0.003), individuals aged 60–79, and those with lower PIR (OR = 0.63, p = 0.009), suggesting that biological sex, age, and socioeconomic status may influence the impact of systemic inflammation. While SIRI exhibited consistent associations across subgroups, SII also exhibited significant effects in certain groups, such as males and individuals aged 70–79, with evidence of an age-related interaction (P-interaction = 0.026). These results highlight the role of social and biological factors in modulating inflammation-driven cognitive decline (Table 3).
3.4 SII, SIRI, and cognitive performance: restricted cubic spline plots analysis
The RCS plots in Figure 2 demonstrate significant non-linear relationships between SII, SIRI, and cognitive performance. In the unadjusted Model 0, both SII and SIRI exhibited notable non-linear associations with cognitive performance (SII: P for overall < 0.001, P for nonlinearity = 0.010; SIRI: P for overall < 0.001, P for nonlinearity = 0.009), indicating a complex, potentially threshold-dependent relationship between systemic inflammation and cognitive health. In Model 1, adjusted for demographic and socioeconomic factors, these associations remained significant (SII: p = 0.005; SIRI: p = 0.002), although the non-linear patterns were less pronounced, suggesting that some of the variation could be explained by these baseline confounders. In the fully adjusted Model 2, which controlled for health-related factors such as diabetes, stroke, sleep disorders, smoking, and alcohol use, the association between SIRI and cognitive performance remained statistically significant (P-overall = 0.005), though the non-linearity was less marked. The persistence of this relationship, particularly for SIRI, after comprehensive adjustments, underscores its potential as a robust biomarker for cognitive risk, emphasizing the role of systemic inflammation in cognitive decline beyond traditional demographic and clinical factors.

Figure 2. Non-linear relationship between SII, SIRI, and cognitive performance, as assessed using RCS. Panel (A) represents Model 0, with no adjustments for confounding factors. Panel (B) represents Model 1, with adjustments for age, gender, PIR, BMI, race, education level, and marital status. Panel (C) represents Model 2, with adjustments for age, gender, PIR, BMI, race, education level, marital status, diabetes, stroke, sleep disorder, smoking, alcohol use, and depression.
4 Discussion
This study provides novel evidence on the relationship between two composite inflammatory indices—SII and SIRI—and cognitive function in a nationally representative population. Both markers were positively associated with cognitive performance, with SIRI demonstrating a stronger and more consistent correlation. These findings suggest that SIRI may be a more sensitive indicator of cognitive impairment risk, highlighting the role of systemic inflammation in cognitive decline. This association remained robust across various demographic subgroups, as confirmed by subgroup and interaction analyses.
SIRI, SII, and other composite inflammatory markers offer a more refined representation of peripheral inflammation and have been closely linked to central nervous system inflammation and cognitive decline (29, 30). The connection between SII, SIRI, and cognitive function may be explained by mechanisms involving chronic systemic inflammation. Such inflammation has been shown to compromise the blood–brain barrier, triggering neuroinflammation that leads to synaptic dysfunction and neuronal loss, ultimately resulting in cognitive impairment (31, 32). SIRI, which integrates neutrophil and lymphocyte counts, may more accurately reflect this chronic inflammatory response. Elevated neutrophil levels are associated with increased oxidative stress, which may further exacerbate neurodegeneration and cognitive dysfunction (33). In contrast, SII may be more indicative of an acute inflammatory state.
Our findings are consistent with previous studies reporting associations between systemic inflammation and cognitive impairment (34, 35), further supporting the role of inflammation in cognitive decline. The study also underscores the differential impact of SII and SIRI across demographic and socioeconomic subgroups. Notably, SIRI exhibited stronger associations in specific populations, such as males and individuals with lower income levels, suggesting that both biological and social determinants may modulate the effects of inflammation on cognitive function. These results align with the work of David Furman and colleagues, who identified chronic systemic inflammation—driven by lifestyle-related factors—as a key contributor to various diseases, including autoimmune and neurodegenerative disorders (36).
Recent clinical studies highlight the significant role of immuno-bone regulation in neuroinflammation and cognitive decline, in addition to systemic inflammation. This mechanism operates through various levels, including bone-derived factors such as Sclerostin and osteocalcin (OCN), the Wnt signaling pathway, and neuroinflammatory axes like cGAS/STING. For example, in patients with osteoporosis, osteocyte-secreted Sclerostin can cross the blood–brain barrier, inhibit neuronal Wnt/β-catenin signaling, and promote β-amyloid (Aβ) accumulation, thus accelerating cognitive decline (37). In response to these pathological mechanisms, novel bioengineering and nanotechnology-based interventions have been developed to modulate the bone microenvironment and regulate bone metabolism for osteoporosis treatment. Bioinspired nanovesicles (BNVs) have been utilized to reprogram the secretory phenotype of bone endothelial cells (38), while extracellular vesicle-based delivery systems derived from mesenchymal stem cells (MSCs) induced from human induced pluripotent stem cells (iPSCs) have been designed for siRNA transport in therapeutic applications (39). Additionally, a engineered cell-membrane-coated nanogels PNG @mR&C, constructed using bone mesenchymal stem cell (BMSC) membranes overexpressing RANK and CXCR4, enables the targeted clearance of nuclear factor-𝜿B ligand (RANKL) within the bone microenvironment and controlled release of PTH 1–34, effectively inhibiting bone resorption and restoring metabolic homeostasis (40).
OCN, a crucial osteoblast-secreted protein, has been shown to alleviate cognitive impairment by reducing amyloid burden and enhancing glycolysis in glial cells (41). Moreover, microglial activation in the central nervous system driven by the cGAS-STING pathway has been identified as a key factor in aging-related chronic inflammation and functional decline (42). Systemic inflammatory markers SIRI and SII may indirectly reflect a broader immunoregulatory mechanism linking bone metabolism and central nervous system function.
Sleep quality plays a pivotal role in cognitive function. Although no statistically significant association between sleep disorders and cognitive performance was identified in this study, residual confounding may account for this lack of association. Nonetheless, prior research has indicated that sleep may serve as a key modulator in the relationship between inflammation and cognition. Sleep deprivation activates brain microglia, triggering the release of pro-inflammatory cytokines that initiate neuroinflammation and accelerate cognitive decline (43–45). Chronic sleep deprivation further promotes systemic inflammation, oxidative stress, and cellular damage, exacerbating neural dysfunction and cognitive impairment (46, 47). A recent study involving both human participants and mouse models found that insufficient sleep activates oxidative stress and integrated stress response pathways in GABAergic neurons, potentially contributing to the onset and progression of neurological disorders (48). As GABAergic neurons are integral to sleep regulation, memory consolidation, and stress responses (49), these findings highlight the critical role of sleep quality in preserving cognitive health.
This study has several limitations. Its observational design prevents causal inference, and unmeasured confounders may be present. While SII and SIRI reflect systemic inflammation, they do not encompass all pathways associated with cognitive decline. Lifestyle factors such as diet and stress were not considered. Future longitudinal studies should track inflammatory markers over time and incorporate broader behavioral and biological variables, including those related to immuno-bone regulation. The observed non-linear relationship between SIRI and cognition warrants further mechanistic exploration.
5 Conclusion
This study demonstrates that elevated SII and SIRI levels are significantly associated with an increased risk of cognitive impairment, with SIRI exhibiting greater sensitivity and consistency across various models and subgroups. As an inflammation-based biomarker derived from routine blood tests, SIRI shows practical potential for early identification of individuals at high risk for cognitive decline.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
XW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft. QW: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft. YL: Investigation, Resources, Supervision, Writing – original draft. HZ: Data curation, Resources, Visualization, Writing – original draft. FZ: Data curation, Formal analysis, Resources, Visualization, Writing – original draft. SL: Data curation, Formal analysis, Resources, Writing – original draft. LZ: Supervision, Writing – review & editing. JL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Natural Science Foundation of China (NSFC, No. 72364005 and 82160633), and the GZPH-NSFC-2021-17.
Acknowledgments
We appreciate the NHANES participants and the National Center for Health Statistics at the CDC for making this data available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vaz, M, and Silvestre, S. Alzheimer's disease: recent treatment strategies. Eur J Pharmacol. (2020) 887:173554. doi: 10.1016/j.ejphar.2020.173554
2. Mangalesh, S, Dudani, S, and Mahesh, NK. Development of a novel inflammatory index to predict coronary artery disease severity in patients with acute coronary syndrome. Angiology. (2024) 75:231–9. doi: 10.1177/00033197231151564
3. Xia, Y, Xia, C, Wu, L, Li, Z, Li, H, and Zhang, J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:1128. doi: 10.3390/jcm12031128
4. Zhao, S, Dong, S, Qin, Y, Wang, Y, Zhang, B, and Liu, A. Inflammation index SIRI is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. (2022) 9:1066219. doi: 10.3389/fcvm.2022.1066219
5. Hu, B, Yang, XR, Xu, Y, Sun, YF, Sun, C, Guo, W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.Ccr-14-0442
6. Qi, Q, Zhuang, L, Shen, Y, Geng, Y, Yu, S, Chen, H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
7. Chao, B, Ju, X, Zhang, L, Xu, X, and Zhao, Y. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical Cancer patients. Front Oncol. (2020) 10:766. doi: 10.3389/fonc.2020.00766
8. Abbate, V, Barone, S, Troise, S, Laface, C, Bonavolontà, P, Pacella, D, et al. The combination of inflammatory biomarkers as prognostic Indicator in salivary gland malignancy. Cancers (Basel). (2022) 14:5934. doi: 10.3390/cancers14235934
9. Wang, RH, Wen, WX, Jiang, ZP, Du, ZP, Ma, ZH, Lu, AL, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
10. Gasparyan, AY, Ayvazyan, L, Mukanova, U, Yessirkepov, M, and Kitas, GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. (2019) 39:345–57. doi: 10.3343/alm.2019.39.4.345
11. Wang, Q, Ma, J, Jiang, Z, and Ming, L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. (2018) 37:4–11. doi: 10.23736/s0392-9590.17.03848-2
12. Jin, Z, Wu, Q, Chen, S, Gao, J, Li, X, Zhang, X, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. (2021) 14:131–40. doi: 10.2147/jir.S283835
13. Li, H, Wu, X, Bai, Y, Wei, W, Li, G, Fu, M, et al. Physical activity attenuates the associations of systemic immune-inflammation index with total and cause-specific mortality among middle-aged and older populations. Sci Rep. (2021) 11:12532. doi: 10.1038/s41598-021-91324-x
14. Hua, Y, Sun, JY, Lou, YX, Sun, W, and Kong, XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. (2023) 379:118–26. doi: 10.1016/j.ijcard.2023.03.016
15. Zuo, P, Xu, R, Hu, L, Hu, W, and Tong, S. Association between monocyte lymphocyte ratio and abdominal aortic calcification in US adults: a cross-sectional study. Clinics (Sao Paulo). (2023) 78:100232. doi: 10.1016/j.clinsp.2023.100232
16. Atalay, F, Kars, A, Topal, K, and Yavuz, Z. Systemic immune inflammation index in patients with recurrent aphthous stomatitis. Braz J Otorhinolaryngol. (2022) 88:621–4. doi: 10.1016/j.bjorl.2022.02.007
17. Kubota, K, Ito, R, Narita, N, Tanaka, Y, Furudate, K, Akiyama, N, et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer. (2022) 22:368. doi: 10.1186/s12885-022-09439-x
18. Tang, Y, Peng, B, Liu, J, Liu, Z, Xia, Y, and Geng, B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400
19. Botelho, J, Leira, Y, Viana, J, Machado, V, Lyra, P, Aldrey, JM, et al. The role of inflammatory diet and vitamin D on the link between periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. (2021) 13:924. doi: 10.3390/nu13030924
20. Lee, S, Min, JY, Kim, B, Ha, SW, Han, JH, and Min, KB. Serum sodium in relation to various domains of cognitive function in the elderly US population. BMC Geriatr. (2021) 21:328. doi: 10.1186/s12877-021-02260-4
21. Pang, K, Liu, C, Tong, J, Ouyang, W, Hu, S, and Tang, Y. Higher Total cholesterol concentration may be associated with better cognitive performance among elderly females. Nutrients. (2022) 14:4198. doi: 10.3390/nu14194198
22. Lim, CR, Harris, K, Dawson, J, Beard, DJ, Fitzpatrick, R, and Price, AJ. Floor and ceiling effects in the OHS: an analysis of the NHS PROMs data set. BMJ Open. (2015) 5:e007765. doi: 10.1136/bmjopen-2015-007765
23. Scherr, M, Kunz, A, Doll, A, Mutzenbach, JS, Broussalis, E, Bergmann, HJ, et al. Ignoring floor and ceiling effects may underestimate the effect of carotid artery stenting on cognitive performance. J Neurointerv Surg. (2016) 8:747–51. doi: 10.1136/neurintsurg-2014-011612
24. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
25. Guo, W, Song, Y, Sun, Y, Du, H, Cai, Y, You, Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol. (2022) 13:1071465. doi: 10.3389/fendo.2022.1071465
26. Li, Q, Wang, SS, Liu, GD, Wang, JH, Zhao, YL, Liu, M, et al. Mediating role of inflammatory indicators in the association between sleep status and blood pressure in centenarians: evidence from China Hainan centenarian cohort study. J Geriatr Cardiol. (2024) 21:874–83. doi: 10.26599/1671-5411.2024.09.009
27. Xu, J-P, Zeng, R-X, Zhang, Y-Z, Lin, S-S, Tan, J-W, Zhu, H-Y, et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens Res. (2023) 46:1009–19. doi: 10.1038/s41440-023-01195-0
28. Yun, JA, Jeong, KS, Ahn, YS, Han, Y, and Choi, KS. The interaction of inflammatory markers and alcohol-use on cognitive function in Korean male firefighters. Psychiatry Investig. (2021) 18:205–13. doi: 10.30773/pi.2020.0101
29. Jia, S, Yang, H, Huang, F, and Fan, W. Systemic inflammation, neuroinflammation and perioperative neurocognitive disorders. Inflamm Res. (2023) 72:1895–907. doi: 10.1007/s00011-023-01792-2
30. Moran, GW, and Thapaliya, G. The gut-brain Axis and its role in controlling eating behavior in intestinal inflammation. Nutrients. (2021) 13:981. doi: 10.3390/nu13030981
31. Haruwaka, K, Ikegami, A, Tachibana, Y, Ohno, N, Konishi, H, Hashimoto, A, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. (2019) 10:5816. doi: 10.1038/s41467-019-13812-z
32. Varatharaj, A, and Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. (2017) 60:1–12. doi: 10.1016/j.bbi.2016.03.010
33. Vitte, J, Michel, BF, Bongrand, P, and Gastaut, JL. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J Clin Immunol. (2004) 24:683–92. doi: 10.1007/s10875-004-6243-4
34. Geng, C, and Chen, C. Association between elevated systemic inflammatory markers and the risk of cognitive decline progression: a longitudinal study. Neurol Sci. (2024) 45:5253–9. doi: 10.1007/s10072-024-07654-x
35. Guo, Z, Zheng, Y, Geng, J, Wu, Z, Wei, T, Shan, G, et al. Response to comments on "unveiling the link between systemic inflammation markers and cognitive performance among older adults in the US: a population-based study using NHANES 2011-2014 data". J Clin Neurosci. (2024) 130:110891. doi: 10.1016/j.jocn.2024.110891
36. Furman, D, Campisi, J, Verdin, E, Carrera-Bastos, P, Targ, S, Franceschi, C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
37. Shi, T, Shen, S, Shi, Y, Wang, Q, Zhang, G, Lin, J, et al. Osteocyte-derived sclerostin impairs cognitive function during ageing and Alzheimer’s disease progression. Nat Metab. (2024) 6:531–49. doi: 10.1038/s42255-024-00989-x
38. Cui, Y, Li, Z, Guo, Y, Qi, X, Yang, Y, Jia, X, et al. Bioinspired Nanovesicles convert the skeletal endothelium-associated secretory phenotype to treat osteoporosis. ACS Nano. (2022) 16:11076–91. doi: 10.1021/acsnano.2c03781
39. Cui, Y, Guo, Y, Kong, L, Shi, J, Liu, P, Li, R, et al. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. (2022) 10:207–21. doi: 10.1016/j.bioactmat.2021.09.015
40. Cui, Y, Lv, B, Li, Z, Ma, C, Gui, Z, Geng, Y, et al. Bone-targeted biomimetic Nanogels re-establish osteoblast/osteoclast balance to treat postmenopausal osteoporosis. Small. (2024) 20:2303494. doi: 10.1002/smll.202303494
41. Shan, C, Zhang, D, Ma, DN, Hou, YF, Zhuang, QQ, Gong, YL, et al. Osteocalcin ameliorates cognitive dysfunctions in a mouse model of Alzheimer's disease by reducing amyloid β burden and upregulating glycolysis in neuroglia. Cell Death Discov. (2023) 9:46. doi: 10.1038/s41420-023-01343-y
42. Gulen, MF, Samson, N, Keller, A, Schwabenland, M, Liu, C, Glück, S, et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature. (2023) 620:374–80. doi: 10.1038/s41586-023-06373-1
43. Atrooz, F, and Salim, S. Chapter eight - sleep deprivation, oxidative stress and inflammation In: R Donev, editor. Advances in protein chemistry and structural biology : Academic Press (2020). 309–36.
44. Gentry, NW, McMahon, T, Yamazaki, M, Webb, J, Arnold, TD, Rosi, S, et al. Microglia are involved in the protection of memories formed during sleep deprivation. Neurobiol Sleep Circadian Rhyth. (2022) 12:100073. doi: 10.1016/j.nbscr.2021.100073
45. Wadhwa, M, Prabhakar, A, Ray, K, Roy, K, Kumari, P, Jha, PK, et al. RETRACTED ARTICLE: inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. (2017) 14:222. doi: 10.1186/s12974-017-0998-z
46. Kröller-Schön, S, Daiber, A, Steven, S, Oelze, M, Frenis, K, Kalinovic, S, et al. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Euro Heart J. (2018) 39:3528–39. doi: 10.1093/eurheartj/ehy333
47. Periasamy, S, Hsu, DZ, Fu, YH, and Liu, MY. Sleep deprivation-induced multi-organ injury: role of oxidative stress and inflammation. EXCLI J. (2015) 14:672–83. doi: 10.17179/excli2015-245
48. You, Y, Li, J, Zhang, Y, Li, X, Li, X, and Ma, X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: insights from population-based and mice studies. CNS Neurosci Ther. (2024) 30:e14783. doi: 10.1111/cns.14783
Keywords: cognitive performance, inflammatory markers, systemic immune-inflammation index, system-inflammation response index, NHANES
Citation: Wang X, Wen Q, Li Y, Zhu H, Zhang F, Li S, Zhan L and Li J (2025) Systemic inflammation markers (SII and SIRI) as predictors of cognitive performance: evidence from NHANES 2011–2014. Front. Neurol. 16:1527302. doi: 10.3389/fneur.2025.1527302
Edited by:
Paolo Ragonese, University of Palermo, ItalyReviewed by:
Sagar Vyavahare, Augusta University, United StatesYongzhi Cui, Shanghai Jiao Tong University, China
Copyright © 2025 Wang, Wen, Li, Zhu, Zhang, Li, Zhan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, Njk0ODA3MDU1QHFxLmNvbQ==; Lin Zhan, emhhbmxpbjMwMEBob3RtYWlsLmNvbQ==
 Xiaoyue Wang
Xiaoyue Wang Qinghua Wen2
Qinghua Wen2 Yujie Li
Yujie Li