- 1Department of Neurology, The Fourth People's Hospital Affiliated to Tongji University, Shanghai, China
- 2Department of Neurology, Center of Cerebrovascular Disorders, Changhai Hospital, Second Military Medical University, Shanghai, China
- 3Department of Neurosurgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
Objective: The safety and effectiveness of thrombolysis in patients with intracranial artery dissection (IAD) are still controversial. This study aims to assess the safety and efficacy of intravenous thrombolysis (IVT) in patients with intracranial vertebrobasilar artery dissection (i-VBAD) related acute ischemic stroke (AIS).
Methods: A retrospective review of 32 patients admitted to our Neurovascular Center between January 2016 and June 2021 with AIS due to i-VBAD was conducted. Patients were identified and divided into IVT group (n = 8) and non-IVT group (n = 24) receiving standard antithrombotic therapy.
Results: The mean age of the 32 patients was 49.28 ± 15.6 years, with a male predominance (87.5%). All patients presented with clinical manifestations consistent with posterior circulation infarct. Patients in the IVT group were significantly older than those in non-IVT group (58.88 vs. 46.08 years, p = 0.043) and had a higher prevalence of diabetes mellitus (50.0% vs. 8.3%, p = 0.023). No intracranial hemorrhage was observed in of the eight patients in IVT group. An excellent functional outcome, defined as an modified Rankin Scale score of 0–1, was achieved in all eight patients in the IVT group (100%) compared to 15 of the 24 patients in the non-IVT group (62.5%, p = 0.070). Although the difference did not reach statistical significance, the trend suggested a potential benefit of IVT in this patient population.
Conclusion: IVT appears safe with no hemorrhagic complications in i-VBAD patients. It may offer better functional outcomes compared to standard therapy. Larger, prospective, multicenter studies are needed for definitive validation.
Introduction
Stroke is a leading cause of disability and death in China (1), with a tendency to affect younger populations. Cerebral vascular dissection is a significant cause of stroke in young people, accounting for approximately 10–25% of cases (2). In Asian populations, the incidence of intracranial artery dissection (IAD) is higher than that of extracranial artery dissection (EAD) (3, 4), with the most common type of IAD being intracranial vertebrobasilar artery dissection (i-VBAD) (5, 6). Unruptured i-VBAD can lead to severe and potentially fatal ischemic events. However, as previous long-term follow-up studies have shown no occurrence of subarachnoid hemorrhage (SAH) in patients with unruptured i-VBAD (3, 7), it is generally accepted that treatment for unruptured i-VBAD should focus on managing ischemic stroke rather than preventing bleeding.
Two international multicenter studies have demonstrated that IVT treatment of acute ischemic stroke (AIS) within 4.5 h after symptom onset is both safe and effective (8, 9). Most randomized controlled trials investigating the efficacy of IVT did not specifically exclude patients with AIS caused by IAD. However, the safety and effectiveness of IVT in patients with IAD remain controversial. In fact, IVT was also not recommended for these patients by 6 out of 9 experts in the 2021 edition of European Stroke Organization (ESO) guidelines on IVT for AIS (10). Additionally, an increasing number of hospitals emergency rooms are utilizing multimodality imaging to evaluate brain vessels in suspected stroke patients. This comprehensive imaging can make it challenging for physicians to decide whether to administer thrombolysis to AIS patients within the treatment time window if i-VBAD is detected. In this study, we present the detailed processes and outcomes of IVT and non-IVT treatments in patients with AIS caused by i-VBAD, with the hope to help clinicians deal with similar cases more effectively.
Patients and methods
All VBAD patients admitted to our hospital between January 2016 and June 2021 were reviewed retrospectively. Patients with extracranial vertebral artery dissection, asymptomatic vertebrobasilar dissection, and i-VBAD presenting as SAH were excluded from the study. Written informed consent was obtained from the patients or their legal representatives, and the study protocol was approved by the Ethics Committee of our Hospital. No vulnerable patients were included in this study. The study was conducted in accordance with the provisions of the Declaration of Helsinki, and this retrospective study did not cause any harm to the patients. The aim of the present study was to investigate the safety and effectiveness of IVT alone in i-VBAD patients in clinical practice. Patients who received endovascular therapy during the acute phase of stroke or within 3 months were excluded from the study. In our series, i-VBAD was diagnosed through a combination of multimodal CT scans and MRI, ensuring a comprehensive assessment for all patients. The diagnosis was established based on several criteria (11–13): the presence of clinical symptoms consistent with posterior circulation ischemia, such as dizziness, ataxia, and dysphagia; visualization of an intramural hematoma on cranial MRI, which is the gold standard for dissection diagnosis; detection of abnormal perfusion in the posterior circulation or occipital lobe via CT perfusion imaging; and characteristic findings on cerebral CT angiography (CTA), including a beaded appearance or tapering of the vertebral artery, indicating potential dissection.
Prior to IVT, a multimodal CT scan, including non-contrast CT brain imaging, CT perfusion, and cerebral CT CTA, was performed. The decision to administer IVT was based on several key factors (9): adherence to the 4.5-h therapeutic time window from symptom onset to treatment initiation, the presence of sudden neurological deficits, the results of multimodal CT imaging to exclude significant intracranial hemorrhage, and the absence of any contraindications. Patients received alteplase at a dose of 0.9 mg per kilogram of recent body weight, with 10% administered as a bolus, followed by micro-pump delivery of the remaining 90% as a constant infusion over a period of 60 min (8). Eight patients showed no signs of intracerebral hemorrhage 24 h after thrombolysis, and were subsequently administered antithrombotic therapy.
Brain MRI was performed in all patients. MRI examination included T1-weighted (T1WI), T2-weighted (T2WI), fluid attenuated inversion recovery sequence imaging and DWI. Additionally, partial patients underwent high-resolution MRI (HR-MRI) to assess for the presence of vessel dissection when necessary. The clinical data of patients, including age, gender, vascular risk factors, the National Institute of Health stroke scale (NIHSS) score, pre-hospital time, and 90-day modified Rankin Scale (mRS) score, were collected. Pre-hospital time was defined as the interval from symptom onset to the documented time of hospital arrival.
Statistical analyses were performed with SPSS 21.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY). Continuous variables were summarized as mean (standard deviation, SD) or median (interquartile range, IQR). Categorical variables were presented as percentages. Categorical variables were analyzed and compared using Fisher’s exact test, and continuous variables were compared by using the independent samples t-test. NIHSS score and pre-hospital time that did not fit a normal distribution were compared by Mann-Whiney U test.
Results
Clinical features
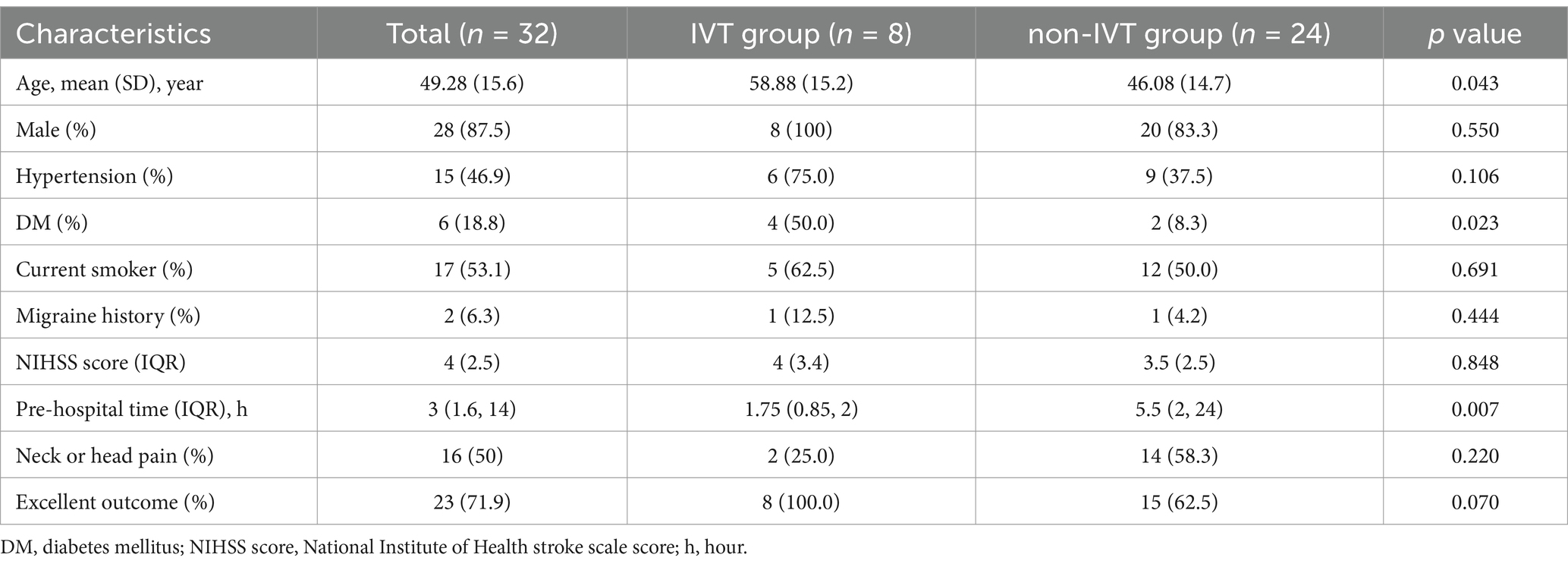
The mean age of the 32 patients was 49.28 ± 15.6 years, with a male predominance (n = 28). Demographics, risk factors, and outcomes are summarized in Table 1. Patients in the IVT group were older than those in the non-IVT group (58.88 vs. 46.08 years, p = 0.043) and had a higher incidence of diabetes (50.0 vs. 8.3%, p = 0.023). There was no significant difference in the distribution of gender, hypertension, current smoker, migraine history, and NIHSS score between the two groups (p > 0.05). The pre-hospital time in the IVT group was shorter than that in the non-IVT group (1.75 vs. 5.5 h, p = 0.007). Excellent outcome (mRS ≤ 1) at 90-day follow-up were achieved in all eight patients in the IVT group and in 15 patients (62.5%) in the non-IVT group (Figure 1). The lack of a significant difference in outcomes between the two groups (p = 0.07) may be attributed to the small sample size.
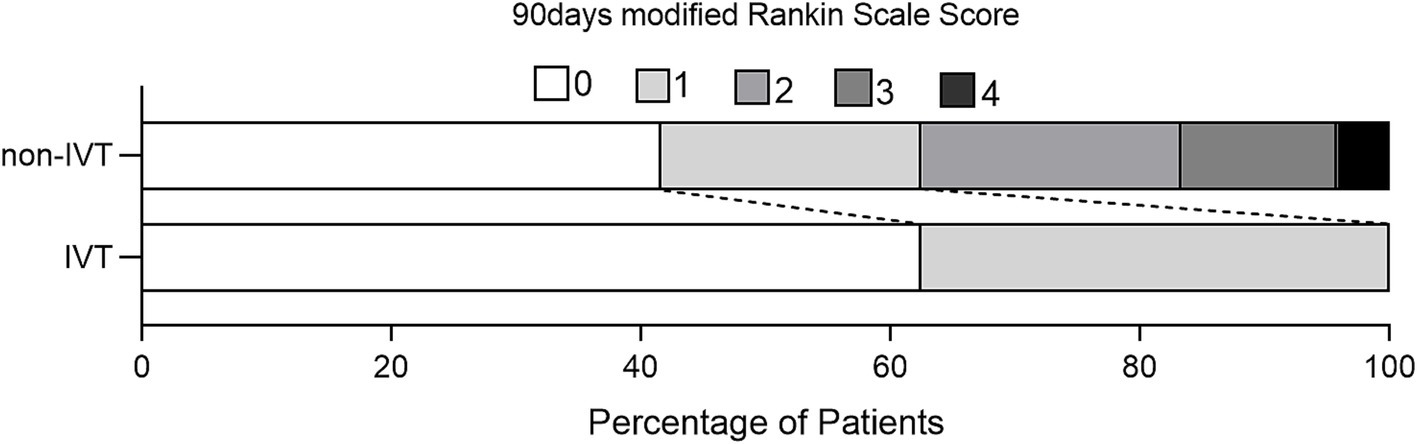
Figure 1. Distribution of scores on the modified Rankin Scale at 90 days for patients without and with IVT.
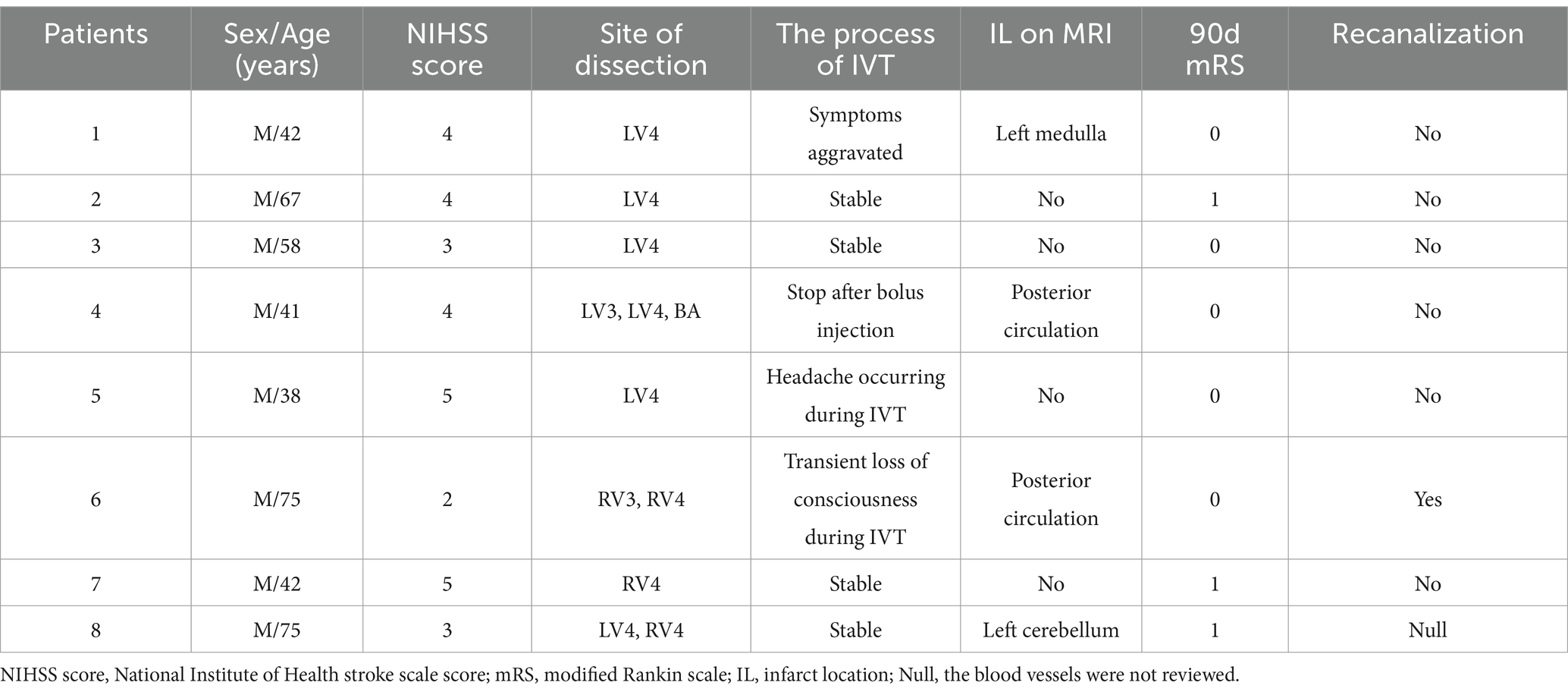
Patient 5 discontinued IVT at 23 min (total dose of alteplase: 24.5 mg) due to the complaint of a headache, although the dysarthria improved and repeat MRA showed no enlargement of the intramural hematoma and no expansion of the dissection-flap. Patient 4 discontinued thrombolysis after a 6 mg bolus of alteplase due to fear of serious hemorrhage as subsequent brain CTA revealed a dissecting aneurysm proximal to the basilar artery (Figure 2). Patient 6 lost consciousness at 20 min after thrombolysis, and no bleeding was found in the head CT. Thrombolysis was continued, and the patient regained consciousness 15 min later. The symptoms in Patient 1 were significantly aggravated 55 min after IVT, but head CT showed no bleeding, and therefore IVT continued until the end, with the significant relief of dizziness and dysphagia. The thrombolytic process was successful, and symptoms improved after IVT in the remaining four patients (shown in Table 2).
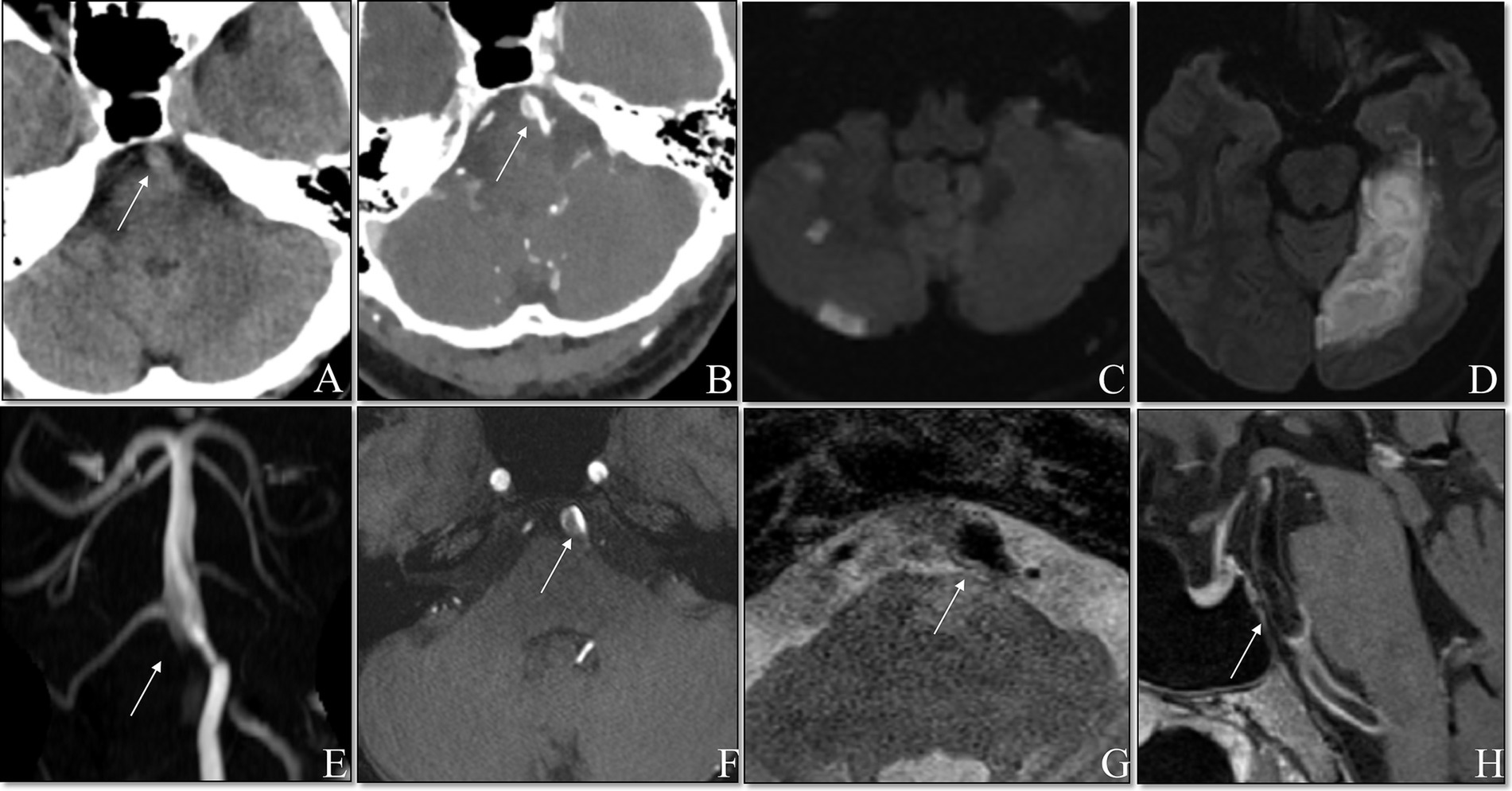
Figure 2. Non-contrasted cranial CT showed suspicious double lumen and intimal flap (A). Contrasted cranial CT showed the mural hematoma (B). DWI showed fresh posterior circulation infracts (C,D). TOF MRA demonstrated the basilar artery with a dissecting aneurysm (E). TOF sources showed the mural hematoma (F). Axial T2WI showed the dilated lumen of basilar artery (G). Contrast-enhanced T1WI showed an enhancement along the surface of the mural hematoma (H).
Neuroimaging results
In the IVT group, V4 dissection was observed in seven patients, and one patient had combined V4 and basilar artery (BA) dissection. In the non-IVT group, V4 dissection was present in 22 patients, BA dissection in one patient, and V4 combined with bilateral V1 dissection in one patient.
Of the eight patients in the IVT group, one had cerebellar infarction, two had brainstem infarction, two had multi-territory areas of infarction, and three had no fresh infarction. Of the 24 patients in non-IVT group, 13 had brainstem infarction, three had cerebellar infarction, seven had multi-territory areas of infarction, and one had thalamic infarction on MRI-DWI. No bleeding was observed in any of the patients in the IVT group, as confirmed by multiple head CT examinations.
Three months later, brain vessel examinations showed no vascular change in five patients (83.3%) in the IVT group compared to eight patients (44.4%) in the non-IVT group. Recanalization was observed in one patient (16.7%) in the IVT group and 10 patients (55.6%) in the non-IVT group. Two patients in the IVT group and 6 patients in the non-IVT group did not undergo follow-up brain vessel examinations.
Discussion
Arterial dissections are caused by a tear in the intima or media of the vessel wall, resulting in bleeding within the arterial wall and leading to intramural hematoma formation (6, 14, 15). Patients with IAD may manifest as SAH, AIS or local compression symptoms, with acute cerebral infarction being the most common form (3, 16). In our series, symptoms were relieved following aggravation during or after IVT, and all eight patients in the IVT group recovered well, indicating that thrombolytic therapy for AIS caused by i-VBAD was effective. Subsequent imaging examinations, including CT and/or MRI, confirmed no hemorrhage, indicating that thrombolytic therapy is also safe for i-VBAD patients. However, as symptom fluctuation may cause distress to the patient, adequate communication prior to IVT is essential.
Arterial dissection is increasingly recognized as a cause of stroke due to growing familiarity with its clinical features and advancements in neurovascular imaging. In our study, patients in the IVT group were older and had a higher proportion of diabetes, suggesting that clinicians might be hesitant to diagnose AIS in younger individuals with posterior circulation ischemia who lack vascular risk factors. Emergency multimodal imaging is valuable for diagnosing VBAD-related cerebral infarction, and our findings may assist clinicians in managing similar cases.
Although many studies have demonstrated the safety of thrombolysis for patients with EAD (17, 18), the risk of intracranial hemorrhage can be higher in IAD, especially when the lesion is located in the posterior circulation (15, 19). Thrombolysis may increase the risk of intramural hematoma enlargement, dissection flap expansion, and bleeding (19, 20). Consequently, European stroke experts generally do not recommend IVT for patients with IAD (10). Moreover, some authors believe that thrombolysis could lyse the thrombus within the arterial wall, which may potentially increase the risk of dissection expansion due to the added shear force on the damaged vessel wall (21). The media layer, which is crucial for vessel strength, is thinner in intracranial arteries (22, 23). Unlike the local compression symptoms caused by the rupture of an EAD, a rupture of an IAD can result in SAH, which is more dangerous. These factors may also contribute to why clinicians are reluctant to consider thrombolysis for patients with IAD. There is limited literature about the treatment of IAD with IVT. Some individual studies report the risk of symptomatic intracranial hemorrhage (sICH) and other serious adverse events of thrombolysis was not increased in IAD patients (24, 25). It is well-known that trauma, mechanical stress, or ever sudden neck movements or stretching is a common mechanisms causing arterial tears (14). Arterial dissections almost always occur in regions where arteries are mobile and not anchored to bony structures or other arteries (6, 26). In contrast, intracranial arteries are fixed to the brain’s surface, making them less susceptible to external forces. Therefore, the risk of hematoma enlargement and dissection rupture caused by thrombolytics alone may primarily be a theoretical concern.
In our study, no instances of ICH following IVT were observed in patients with i-VBAD. Tsivgoulis et al. reported a relatively low incidence of sICH in similar populations, with an sICH rate of 2.5% in a multicenter study involving 122 patients with dissection-related ischemic stroke treated with IVT (25). These findings suggest a favorable safety profile for thrombolysis in such cases. Similarly, Bernardo et al. found no sICH in a single-center series of 15 patients (27). Additionally, a meta-analysis by Vergouwen et al. reported a pooled sICH rate of 3.3% for cervical artery dissection, further supporting the notion that thrombolysis may not significantly elevate the risk of hemorrhagic complications in patients with arterial dissection (28). The CADISS trial highlights the diagnostic complexities inherent to dissection-related strokes, indicating variability in patient outcomes (29).
Most patients with i-VBAD in our series recovered well by the 90-day follow-up, which aligns with previous findings that those with ischemic presentations often had favorable outcomes (3, 7). Lower initial NIHSS scores and posterior circulation lesions might contribute to these positive outcomes (25). Dual antiplatelet therapy (DAPT) is a viable treatment option for patients with minor ischemic stroke (NIHSS < 3–5 points) and has been shown to be at least as effective as single antiplatelet therapy in preventing recurrent stroke (30–32). This is particularly relevant for patients with i-VBAD without distal vessel occlusion. Dissection can lead to ischemic stroke either through thromboembolism or, less commonly, from hemodynamic insufficiency due to severe arterial stenosis or occlusion (33, 34). Since the propagation and embolization of red (erythrocyte–fibrin) thrombi is the main mechanism (6), Professor Caplan suggested that thrombolysis could be an ideal treatment for patients with IAD, provided it is administered within the appropriate time window and without large area infarction. Symptom fluctuations during thrombolytic treatment may be attributed to the thrombolysis procedures.
The optimal secondary stroke prevention regimen for patients with IAD remains unclear. Patients in the IVT group were treated with IVT alone and then started on antithrombotic therapy, considering that imaging studies show spontaneous recanalization in 20–58% of patients with IAD (35). Ischemic lesions are generally managed with medical therapy. Endovascular therapy should only be considered if the patient experiences recurrent strokes despite medical treatment (36).
A retrospective study of unruptured spontaneous intracranial VAD suggested that female gender, the nonsmoking status, and the absence of posteroinferior cerebellar artery involvement might be associated with spontaneous vascular normalization (7). The proportions of male and current smokers were similar between the two groups; however, the proportion of spontaneous recanalization in the non-IVT group is consistent with previous reports and is higher than that in IVT group. Whether alteplase adversely affects the healing of artery dissection requires further investigation. Quicker treatment improves AIS outcomes by restoring blood flow sooner and reducing ischemia duration, possibly explaining the better outcomes in the IVT group. But further studies are needed to confirm this. Furthermore, the absence of randomization and the small sample size, which may introduce biases and reduce statistical power. It’s important to note that the small number of patients from a single institution may not be representative. Prospective, multicenter studies of patients with AIS secondary to IAD are needed to determine the optimal treatment regimen.
Conclusion
In conclusion, this preliminary study suggests that IVT may be safe and effective for AIS caused by i-VBAD, with no observed hemorrhage. However, the small sample size and lack of randomization limit the strength of this conclusion. The trend toward better outcomes at 90 days should be interpreted with caution. Larger, prospective, multicenter studies are needed to confirm the safety and efficacy of IVT in i-VBAD patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Changhai Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NW: Data curation, Writing – original draft. WL: Conceptualization, Investigation, Writing – original draft. HL: Methodology, Visualization, Writing – original draft. BD: Investigation, Methodology, Writing – review & editing. KZ: Writing – review & editing. TW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Research Initiation Grant from the Shanghai Fourth People’s Hospital, Affiliated to Tongji University (sykyqd07501); Pudong New Area Health Commission (PW2022A-28) and Neuroscience Innovation Development Research Project (YXJL-2022-00351-0183).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou, M, Wang, H, Zeng, X, Yin, P, Zhu, J, Chen, W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
2. Schievink, WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. (2001) 344:898–906. doi: 10.1056/NEJM200103223441206
3. Kim, BM, Kim, SH, Kim, DI, Shin, YS, Suh, SH, Kim, DJ, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology. (2011) 76:1735–41. doi: 10.1212/WNL.0b013e31821a7d94
4. Tsukahara, T, and Minematsu, K. Overview of spontaneous cervicocephalic arterial dissection in Japan. Acta Neurochir Suppl. (2010) 107:35–40. doi: 10.1007/978-3-211-99373-6_5
5. Redekop, GJ. Extracranial carotid and vertebral artery dissection: a review. Can J Neurol Sci. (2008) 35:146–52. doi: 10.1017/S0317167100008556
6. Caplan, LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. (2008) 4:34–42. doi: 10.1038/ncpneuro0683
7. Kim, MK, and Lim, YC. Conservative Management of Unruptured Spontaneous Intracranial Vertebral Artery Dissection. World Neurosurg. (2019) 126:e402–9. doi: 10.1016/j.wneu.2019.02.063
8. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
9. Hacke, W, Kaste, M, Bluhmki, E, Brozman, M, Dávalos, A, Guidetti, D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
10. Berge, E, Whiteley, W, Audebert, H, de Marchis, GM, Fonseca, AC, Padiglioni, C, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I–LXII. doi: 10.1177/2396987321989865
11. Debette, S, and Leys, D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. (2009) 8:668–78. doi: 10.1016/S1474-4422(09)70084-5
12. Montalvan, V, Ulrich, A, Wahlster, S, and Galindo, D. Arterial dissection as a cause of intracranial stenosis: a narrative review. Clin Neurol Neurosurg. (2020) 190:105653. doi: 10.1016/j.clineuro.2019.105653
13. Teasdale, E, Zampakis, P, Santosh, C, and Razvi, S. Multidetector computed tomography angiography: application in vertebral artery dissection. Ann Indian Acad Neurol. (2011) 14:35–41. doi: 10.4103/0972-2327.78048
14. Park, KW, Park, JS, Hwang, SC, Im, SB, Shin, WH, and Kim, BT. Vertebral artery dissection: natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. (2008) 44:109–15. doi: 10.3340/jkns.2008.44.3.109
15. Engelter, ST, Lyrer, P, and Traenka, C. Cervical and intracranial artery dissections. Ther Adv Neurol Disord. (2021) 14:17562864211037238. doi: 10.1177/17562864211037238
16. Traenka, C, Grond-Ginsbach, C, Goeggel Simonetti, B, Metso, TM, Debette, S, Pezzini, A, et al. Artery occlusion independently predicts unfavorable outcome in cervical artery dissection. Neurology. (2020) 94:e170–80. doi: 10.1212/WNL.0000000000008654
17. Engelter, ST, Dallongeville, J, Kloss, M, Metso, TM, Leys, D, Brandt, T, et al. Thrombolysis in cervical artery dissection – data from the cervical artery dissection and Ischaemic stroke patients (CADISP) database. Eur J Neurol. (2012) 19:1199–206. doi: 10.1111/j.1468-1331.2012.03704.x
18. Peng, J, Liu, Z, Luo, C, Chen, L, Hou, X, Xiao, L, et al. Treatment of cervical artery dissection: Antithrombotics, thrombolysis, and endovascular therapy. Biomed Res Int. (2017) 2017:1–6. doi: 10.1155/2017/3072098
19. Engelter, ST, Rutgers, MP, Hatz, F, Georgiadis, D, Fluri, F, Sekoranja, L, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. (2009) 40:3772–6. doi: 10.1161/STROKEAHA.109.555953
20. Vergouwen, MD. Intravenous thrombolysis in ischaemic stroke secondary to cervical artery dissection: safe but not effective. Eur J Neurol. (2012) 19:1155–6. doi: 10.1111/j.1468-1331.2012.03731.x
21. Qureshi, AI, Chaudhry, SA, Hassan, AE, Zacharatos, H, Rodriguez, GJ, Suri, MF, et al. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the United States. Arch Neurol. (2011) 68:1536–42. doi: 10.1001/archneurol.2011.213
22. Peltier, J, Toussaint, P, Deramond, H, Gondry, C, Bruniau, A, Gontier, MF, et al. The dural crossing of the vertebral artery. Surg Radiol Anat. (2003) 25:305–10. doi: 10.1007/s00276-003-0139-5
23. Farrell, MA, Gilbert, JJ, and Kaufmann, JC. Fatal intracranial arterial dissection: clinical pathological correlation. J Neurol Neurosurg Psychiatry. (1985) 48:111–21. doi: 10.1136/jnnp.48.2.111
24. Bernardo, F, Nannoni, S, Strambo, D, Bartolini, B, Michel, P, and Sirimarco, G. Intravenous thrombolysis in acute ischemic stroke due to intracranial artery dissection: a single-center case series and a review of literature. J Thromb Thrombolysis. (2019) 48:679–84. doi: 10.1007/s11239-019-01918-6
25. Tsivgoulis, G, Zand, R, Katsanos, AH, Sharma, VK, Goyal, N, Krogias, C, et al. Safety and outcomes of intravenous thrombolysis in dissection-related ischemic stroke: an international multicenter study and comprehensive meta-analysis of reported case series. J Neurol. (2015) 262:2135–43. doi: 10.1007/s00415-015-7829-x
26. Reap, VJ. Spontaneous vertebral artery dissection in a healthy 26 year old female patient: a case study. Adv Emerg Nurs J. (2018) 40:21–6. doi: 10.1097/TME.0000000000000174
27. Bernardo, F, Nannoni, S, Strambo, D, Puccinelli, F, Saliou, G, Michel, P, et al. Efficacy and safety of endovascular treatment in acute ischemic stroke due to cervical artery dissection: a 15-year consecutive case series. Int J Stroke. (2019) 14:381–9. doi: 10.1177/1747493018823161
28. Markus, HS, Levi, C, King, A, Madigan, J, and Norris, J. Cervical artery dissection in stroke study (CADISS) investigators. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the cervical artery dissection in stroke study (CADISS) randomized clinical trial final results. JAMA Neurol. (2019) 76:657–64. doi: 10.1001/jamaneurol.2019.0072
29. Zinkstok, SM, Vergouwen, MD, Engelter, ST, Lyrer, PA, Bonati, LH, Arnold, M, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. (2011) 42:2515–20. doi: 10.1161/STROKEAHA.111.617282
30. Dong, J, Wang, F, and Sundararajan, S. Use of dual antiplatelet therapy following ischemic stroke. Stroke. (2020) 51:e78–78e80. doi: 10.1161/STROKEAHA.119.028400
31. Chen, HS, Cui, Y, Zhou, ZH, Zhang, H, Wang, LX, Wang, WZ, et al. Dual antiplatelet therapy vs Alteplase for patients with minor nondisabling acute ischemic stroke: the ARAMIS randomized clinical trial. JAMA. (2023) 329:2135–44. doi: 10.1001/jama.2023.7827
32. Bhatia, K, Jain, V, Aggarwal, D, Vaduganathan, M, Arora, S, Hussain, Z, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: Meta-analysis of randomized controlled trials. Stroke. (2021) 52:e217–217e223. doi: 10.1161/STROKEAHA.120.033033
33. Engelter, ST, Traenka, C, Von Hessling, A, and Lyrer, PA. Diagnosis and treatment of cervical artery dissection. Neurol Clin. (2015) 33:421–41. doi: 10.1016/j.ncl.2014.12.002
34. Lucas, C, Moulin, T, Deplanque, D, Tatu, L, and Chavot, D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. (1998) 29:2646–8. doi: 10.1161/01.STR.29.12.2646
35. Ahn, SS, Kim, BM, Suh, SH, Kim, DJ, Kim, DI, Shin, YS, et al. Spontaneous symptomatic intracranial vertebrobasilar dissection: initial and follow-up imaging findings. Radiology. (2012) 264:196–202. doi: 10.1148/radiol.12112331
Keywords: acute ischemic stroke, vertebrobasilar artery dissection, intravenous thrombolysis, HR-MRI, safety and efficacy
Citation: Wang N, Liu W, Lin H, Deng B, Zhao K and Wu T (2025) Safety and efficacy of intravenous thrombolysis for acute ischemic stroke secondary to intracranial vertebrobasilar artery dissection. Front. Neurol. 16:1528168. doi: 10.3389/fneur.2025.1528168
Edited by:
Manoj K. Mahata, University of Göttingen, GermanyReviewed by:
Johann Pelz, University Hospital Leipzig, GermanyQazi Zeeshan, University of Pittsburgh Medical Center, United States
Copyright © 2025 Wang, Liu, Lin, Deng, Zhao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijun Zhao, emtqd2NmendoQDE2My5jb20=; Tao Wu, dHd1MTYzQDE2My5jb20=
†These authors have contributed equally to this work
 Nuo Wang
Nuo Wang Wei Liu1†
Wei Liu1† Kaijun Zhao
Kaijun Zhao Tao Wu
Tao Wu