Abstract
Background:
To explore the characteristics of resting-state functional connectivity in patients with different outcomes of prolonged disorders of consciousness (pDoC) by studying resting-state near-infrared imaging in patients with pDoC.
Methods:
60 patients with pDoC were processed with resting-state near-infrared imaging and divided into unresponsive wakefulness syndrome/vegetative state (UWS/VS) group, minimally conscious state (MCS) group and escape minimally conscious state (EMCS) group according to the post-treatment state of consciousness, to analyze the difference of resting-state functional connectivity in patients with different outcomes of patients with pDoC.
Results:
Functional connectivity (FC) between frontal lobe and left occipital lobe, frontal lobe and right occipital lobe, and left and right occipital lobes decreased in the UWS/VS group compared with the MCS group; functional connectivity between frontal lobe and left occipital lobe, frontal lobe and right occipital lobe, and left and right occipital lobes decreased in the UWS/VS group compared with the EMCS group; functional connectivity did not show any significant difference between the EMCS and MCS groups; and functional connectivity was more centralized in the MCS group and EMCS group.
Conclusion:
Different outcomes of patients with pDoC have different degrees of decline in functional connectivity between frontal lobe and occipital lobe and between occipital lobe, resting-state functional near-infrared spectroscopy has a certain reference significance for the prognosis of patients with pDoC, and it is helpful for exploring the exploration of the conscious residual brain areas.
1 Introduction
Over the past few decades, significant advances have been made in emergency procedures and techniques for the common causes of disorders of consciousness (DOC)-cardiac arrest, cerebral hemorrhage, massive cerebral infarction, and severe traumatic brain injury. However, the ensuing medical treatment of DOC patients from coma to recovery of consciousness is indeed a long and extremely costly process. Up to 40% of unresponsive brain-injured patients have their residual consciousness misjudged (1), leading to abandonment of subsequent treatment. Early identification or prediction of the prognosis and outcome of future awakenings in DOC patients is an appropriate pathway for rational treatment and rational control of healthcare costs.
According to the guidelines related to disorders of consciousness of the American Academy of Neurology and the implementation of the subcommittee system, the patient’s age, etiology, and duration of vegetative state are closely related to the recovery of consciousness (2). However, these are vague indicators, and in order to assess the value of treatment at an early stage, scholars have been trying to find more objective and accurate markers to assess the prognosis of patients with pDoC. In the study of consciousness in the brain, scientists have proposed the hypothesis of a “global neural workspace” based on a connectionist theoretical framework, and selective hypometabolism of the medial prefrontal cortex (mPFC), as the neural basis of consciousness, has been reported in a wide range of altered states of consciousness, such as sleep (3), drug-induced anesthesia (4), and acquired chronic pDoC states (5, 6). It can be used to assist in assessing the level of consciousness in DOC patients (7–9) and its functional connectivity strength is strongly associated with the prognosis of DOC patients (10). This suggests that enhanced functional connectivity of the mPFC predicts recovery of the neural network of consciousness. However, the recovery of the patient’s state of consciousness seems to be related not only to the internal cognitive network, but also to the external network of consciousness. For example, clinical means of awakening are often applied in terms of visual stimulation, and it has been observed that patients with earlier visual following, visual localization, and visual recognition seem to be more prone to awakening from the coma. Visual pursuit is considered to be one of the first signs that first appear during the recovery of consciousness (11). Studies have shown that moderate electroencephalography (EEG) frequencies dominated by alpha rhythms suggest a good prognosis for DOC patients (12). Some scholars have argued, based on their research, that the most representative α-rhythm is the occipital alpha rhythm from the visual cortex, which plays an important role in cognitive processes and sensory perception, and the occipital α-rhythm seems to reflect conscious perception and alertness in awake individuals (13–15). EEG studies have shown that loss of consciousness is associated with impaired information sharing over medium and long distances, reflecting the importance of long-distance cortical communication (16). Thus, long-range information transfer between the frontal-occipital lobes in the brains of DOC patients may reflect an internal orienting process of visual perception that is closely related to the patient’s awareness of the environment.
In recent years, techniques such as functional neuroimaging functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) and electrophysiology (EEG) have played a role in making prognostic judgments in patients with disorders of consciousness. Rundgree et al. found that patients with impaired consciousness after cardiac arrest had a correspondingly higher rate of poor prognosis as the severity of amplitude-integrated EEG increased (17). Moreover, a multicenter cohort study applying resting-state functional magnetic resonance imaging found that connectivity within the default mode network (DMN) in the patient’s brain was highly correlated with the probability of recovery (18). PET imaging studies in comatose patients following hypoxic or traumatic brain injury have demonstrated that early inflammatory components are predominantly located in key cortical and subcortical brain structures that are thought to be involved in the emergence of consciousness (19). The above functional brain imaging techniques have expanded the means of diagnosis and evaluation in the field of DOC, however, they also have certain limitations. fMRI and PET equipment are expensive, have high spatial resolution, and operate in a large space, which does not allow for bedside measurements. EEG is easy to operate at the bedside, but is susceptible to the magneto-electric environment and has low spatial resolution.
Function near-infrared spectroscopy (fNIRS) has become one of the key technologies to study the neural mechanism of brain processing because of its portable, inexpensive and non-invasive advantages (20). Molteni et al. (21) used three stimulation modalities to detect residual functional brain activity in two MCS, with passive motor stimulation having the strongest response, somatosensory stimulation the next strongest, and active stimulation the weakest, and Kempny et al. (22) found that patients with MCS possessed more of the typical fNIRS response (elevated HbO2 with a concomitant decrease in HbR) compared to patients with vegetative state. In relation to the prefrontal cortex, fNIRS showed a significant advantage due to no hair in detecting the cognitive tasks like mental arithmetic, music imagery, emotion induction, etc. (23). Therefore, fNIRS is more valuable in detecting changes in frontal and occipital functional connectivity in patients with pDoC.
Based on the above, we hypothesized that utilizing the functional network performance of fNIRS between the frontal-occipital lobes may reveal resting-state functional connectivity patterns in the pDoC. To the best of our knowledge, there are no studies that provide a complete and comprehensive assessment of this critical fronto-occipital lobe connection in coma, although this information may be valuable in the development of assessment tools for the neurologic prognosis of comatose patients. In the current work, we aimed to measure the residual integrity of the FC of frontal-occipital brain structures in a cohort of mixed-case coma patients to gain insights into the neural mechanisms underlying the recovery of consciousness and to explore the residual brain regions of consciousness.
2 Materials and methods
2.1 Participants
Sixty patients with chronic consciousness disorder who were hospitalized in the Department of Neurological Rehabilitation of Guangxi Jiangbin Hospital from January 2022 to January 2023 were included in this study. Inclusion criteria: (1) the patients met the internationally established diagnostic criteria for UWS/VS as assessed by a specialized physician (24); (2) the duration of the consciousness disorder was greater than 28 days; (3) the preservation of the brainstem reflexes and the sleep–wake cycle; (4) All participants were not on drugs that affected hemodynamics for the first 3 days of the experiment; (5) the patients’ family members signed the informed consent form. Exclusion criteria: (1) patients with previous neuropsychiatric disorders; (2) use of central stimulants or sedative drugs 1 week before the experiment; (3) unstable vital signs; (4) hospitalization less than 1 month.
2.2 Experimental method
Subjects were admitted to the hospital for resting state fNIRS scanning, and a comprehensive treatment plan was adopted during hospitalization, which included blood pressure control, blood glucose control, anti-infection and comprehensive rehabilitation. All subjects received the same combination of treatments during their hospitalization. Our experiments were conducted in a quiet, light-free stimulating environment. After 1 month then 2 professionally trained doctors assessed the level of consciousness of the subjects according to the modified Coma Recovery Scale (CRS-R), and the subjects were divided into UWS/VS group, MCS group and EMCS group according to their different levels of consciousness.
2.3 fNIRS data collection and processing
-
Resting-state fNIRS scanning: NirScan-6000C model fNIRS brain functional imaging device from Danyang Huichuang Medical Company (Figure 1) was used for resting-state fNIRS collection, with a total of 27 two-wavelength (730 nm, 850 nm) measurement channels in the bilateral brain, including 11 receivers and 11 light sources covering the frontal-occipital lobe region, with 19 channels (CH9~CH27) in the frontal lobe, and 8 channels (CH1–CH8) in the occipital lobe, the specific distribution and brain areas are shown in Figure 2 and Table 1, and all the signals were acquired at 12 Hz. Experimental data collection process time between 20 and 30 min.
-
fNIRS data processing: NirSpark software (HuiChuang, China) was used to analyze the fNIRS data. The steps were as follows: (1) correction for motion artifacts; (2) the initial light intensity data was converted to optical density; (3) removal of environmental and physiological noise with a bandpass filter of 0.01–0.20 Hz (25); (4) the filtered OD data was transmitted into the relative changes in the concentration of the HbO2 and HbR data based on the modified Beer–Lambert Law (26). Since HbO2 is a more reliable indicator of cortical blood flow changes (27) and exhibits a higher signal-to-noise ratio compared to HbR (28), it was selected for further analysis. (5) The Pearson’s correlation coefficient of the HbO2 concentration time series between each channel pair was calculated, and the coefficient was defined as the functional connectivity strength of the corresponding channel pair.
Figure 1

Functional near infrared acquisition device.
Figure 2

Channel arrangement of fNIRS brain functional imaging device. (A) The purple (S) and blue circles (D) represent the light source and the detector respectively, while the connecting lines between them represent the channels. (B) The layout diagram of 19 channels on the frontal lobe. (C) The layout diagram of 8 channels on the occipital lobe.
Table 1
| Label of channel | Aal area | Percentage |
|---|---|---|
| CH1 (S2-D2) | Frontal_Mid_Orb_R | 0.030612 |
| Frontal_Inf_Tri_R | 0.13605 | |
| Frontal_Inf_Orb_R | 0.83333 | |
| CH2 (S2-D7) | Frontal_Inf_Oper_R | 0.16667 |
| Frontal_Inf_Tri_R | 0.83333 | |
| CH3 (S3-D2) | Frontal_Sup_R | 0.0079681 |
| Frontal_Sup_Orb_R | 0.099602 | |
| Frontal_Mid_R | 0.23506 | |
| Frontal_Mid_Orb_R | 0.65737 | |
| CH4 (S3-D3) | Frontal_Sup_R | 0.33121 |
| Frontal_Sup_Orb_R | 0.42994 | |
| Frontal_Mid_Orb_R | 0.031847 | |
| Frontal_Sup_Medial_R | 0.1051 | |
| Frontal_Mid_Orb_R | 0.10191 | |
| CH5 (S3-D8) | Frontal_Sup_R | 0.83755 |
| Frontal_Mid_R | 0.16245 | |
| CH6 (S4-D3) | Frontal_Sup_L | 0.17822 |
| Frontal_Sup_Orb_L | 0.25413 | |
| Frontal_Sup_Medial_L | 0.21452 | |
| Frontal_Mid_Orb_L | 0.35314 | |
| CH7 (S4-D4) | Frontal_Sup_L | 0.26354 |
| Frontal_Sup_Orb_L | 0.12274 | |
| Frontal_Mid_L | 0.072202 | |
| Frontal_Mid_Orb_L | 0.54152 | |
| CH8 (S4-D9) | Frontal_Sup_L | 0.97122 |
| Frontal_Mid_L | 0.028777 | |
| CH9 (S5-D4) | Frontal_Mid_Orb_L | 0.092857 |
| Frontal_Inf_Tri_L | 0.12143 | |
| Frontal_Inf_Orb_L | 0.78571 | |
| CH10 (S5-D10) | Frontal_Inf_Oper_L | 0.022222 |
| Frontal_Inf_Tri_L | 0.97778 | |
| CH11 (S8-D2) | Frontal_Mid_R | 0.95094 |
| Frontal_Inf_Tri_R | 0.049057 | |
| CH12 (S8-D7) | Frontal_Mid_R | 0.5 |
| Frontal_Inf_Tri_R | 0.5 | |
| CH13 (S8-D8) | Frontal_Mid_R | 1 |
| CH14 (S9-D3) | Frontal_Sup_Medial_L | 0.48013 |
| Frontal_Sup_Medial_R | 0.51987 | |
| CH15 (S9-D8) | Frontal_Sup_R | 0.55303 |
| Frontal_Mid_R | 0.098485 | |
| Frontal_Sup_Medial_R | 0.34848 | |
| CH16 (S9-D9) | Frontal_Sup_L | 0.4359 |
| Frontal_Sup_Medial_L | 0.5641 | |
| CH17 (S10-D4) | Frontal_Mid_L | 0.88142 |
| Frontal_Inf_Tri_L | 0.11858 | |
| CH18 (S10-D9) | Frontal_Sup_L | 0.23377 |
| Frontal_Mid_L | 0.76623 | |
| CH19 (S10-D10) | Frontal_Mid_L | 0.65504 |
| Frontal_Inf_Tri_L | 0.34496 | |
| CH20 (S12-D13) | Lingual_R | 0.20599 |
| Occipital_Mid_R | 0.10112 | |
| Occipital_Inf_R | 0.69288 | |
| CH21 (S12-D14) | Occipital_Mid_R | 0.88618 |
| Occipital_Inf_R | 0.11382 | |
| CH22 (S13-D13) | Calcarine_R | 0.26756 |
| Cuneus_R | 0.10368 | |
| Lingual_R | 0.0033445 | |
| Occipital_Sup_R | 0.54181 | |
| Occipital_Mid_R | 0.076923 | |
| Occipital_Inf_R | 0.006689 | |
| CH23 (S13-D14) | Occipital_Sup_R | 0.42697 |
| Occipital_Mid_R | 0.57303 | |
| CH24 (S14-D15) | Occipital_Sup_L | 0.12044 |
| Occipital_Mid_L | 0.87956 | |
| CH25 (S14-D16) | Occipital_Sup_L | 0.052265 |
| Occipital_Mid_L | 0.94774 | |
| CH26 (S16-D15) | Occipital_Mid_L | 1 |
| CH27 (S16-D16) | Lingual_L | 0.19615 |
| Occipital_Mid_L | 0.58846 |
The corresponding brain regions of 27 fNIRS channels.
2.4 Statistical analysis
The gender differences of the three groups were compared using the chi-square test, and the age, education level, and disease duration of the three groups were analyzed using one-way ANOVA, with p < 0.05 being statistically significant; the fNIRS data were statistically analyzed using NirSpark software, and two-way ANOVA was used to compare the effects of different groups and types of connectivity (frontal lobe and left occipital lobe, frontal lobe and right occipital lobe, and left and right occipital lobe) on the functional connectivity. Comparisons of functional connectivity between connectivity channels were analyzed by one-way ANOVA with false discovery rate (FDR)-corrected p < 0.05.
3 Results
3.1 Comparison of general information
The differences between the three groups in terms of age, gender, education level, and disease duration were not significant (Table 2). It shows that there is no statistical significance between the general information of the subjects in the three groups and they are comparable.
Table 2
| Characteristics | UWS/VS group | MCS group | EMCS group | p-value |
|---|---|---|---|---|
| Gender (M/F) | 17/11 | 9/8 | 8/7 | 0.839 |
| Age (years) | 61.05 ± 11.31 | 64.15 ± 6.81 | 59.35 ± 7.91 | 0.232 |
| Education (years) | 10.35 ± 2.60 | 10.40 ± 2.58 | 10.30 ± 2.30 | 0.992 |
| Disease duration (months) | 6.30 ± 5.52 | 7.60 ± 6.68 | 6.50 ± 5.68 | 0.635 |
Demographic data.
3.2 Effects of different groups and connection types on functional connectivity
Functional connectivity between the frontal lobe and the left occipital lobe, the frontal lobe and the right occipital lobe, and the right and left occipital lobes decreased in the UWS/VS group when compared with the MCS group (p < 0.05). Functional connectivity between the frontal lobe and the left occipital lobe, the frontal lobe and the right occipital lobe, and the right and left occipital lobes decreased in the UWS/VS group compared with the EMCS group (p < 0.05). Compared with the EMCS group, no significant differences were observed in the functional connectivity between the frontal lobe and the left occipital lobe, the frontal lobe and the right occipital lobe, and the right and left occipital lobes in the MCS group (p > 0.05) (Table 3). The functional strengths of the different connection types in the three groups are shown in Table 4.
Table 3
| Groups | Connection type | T-Value | p-value |
|---|---|---|---|
| UWS/VS group VS MCS group | Frontal lobe and left occipital lobe | −3.7413 | 0.0034 |
| UWS/VS group VS MCS group | Frontal lobe and right occipital lobe | −2.8638 | 0.0080 |
| UWS/VS group VS MCS group | Left and right occipital lobes | −3.3691 | 0.0034 |
| UWS/VS group VS EMCS group | Frontal lobe and left occipital lobe | −6.3753 | 9.9295e-06 |
| UWS/VS group VS EMCS group | Frontal lobe and right occipital lobe | −5.3242 | 4.1184e-05 |
| UWS/VS group VS EMCS group | Left and right occipital lobes | −3.9125 | 0.0010 |
| MCS group VS EMCS group | Frontal lobe and left occipital lobe | −1.8881 | 0.1075 |
| MCS group VS EMCS group | Frontal lobe and right occipital lobe | −2.6459 | 0.0533 |
| MCS group VS EMCS group | Left and right occipital lobes | −1.1170 | 0.2755 |
Results of functional connection comparison among three groups with different connection types.
Table 4
| Connection type | UWS/VS group | MCS group | EMCS group |
|---|---|---|---|
| Frontal lobe and left occipital lobe | 0.0689 ± 0.0138 | 0.2654 ± 0.1267 | 0.3888 ± 0.1132 |
| Frontal lobe and right occipital lobe | 0.0745 ± 0.0205 | 0.2013 ± 0.1012 | 0.3657 ± 0.1445 |
| Left and right occipital lobes | 0.0892 ± 0.0256 | 0.2906 ± 0.1035 | 0.3928 ± 0.1436 |
Functional strength of three groups different connection types.
3.3 Functional connectivity diagrams of the three groups
Compared with the EMCS group, the channel connectivity strength of the MCS group and the UWS/VS group showed a decreasing trend in overall (Figures 3–5). The functional connectivity strengths of the MCS group and the EMCS group were large, distributed and more centralized, whereas the functional connectivity strengths of the UWS/VS group were small and discrete, and the histograms of mean functional connectivity strengths of the three groups are shown in Figure 6.
Figure 3

Functional connection of each channel in UWS/VS group. Connectivity matrix of 27 channels in UWS/VS group.
Figure 4

Functional connection of each channel in MCS group. Connectivity matrix of 27 channels in MCS group.
Figure 5
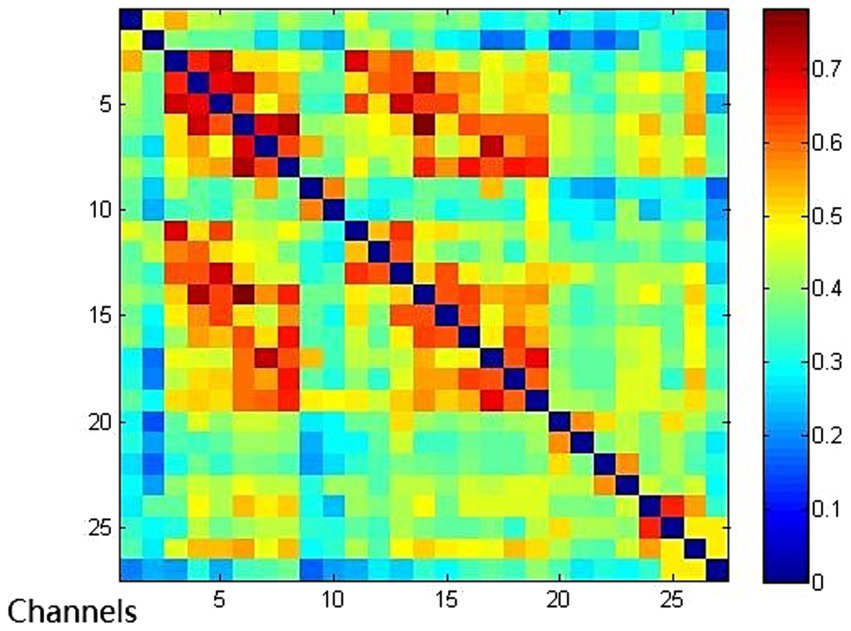
Functional connection of each channel in EMCS group. Connectivity matrix of 27 channels in EMCS group.
Figure 6

Three groups of average functional connection strength distribution. (A) Average functional connection strength distribution in UWS/VS group. (B) Average functional connection strength distribution in MCS group. (C) Average functional connection strength distribution in EMCS group. (D) Average functional connection strength distribution in three groups.
4 Discussion
In this study, we found that the UWS/VS group showed a significant decrease in functional connectivity between the frontal lobe and the left occipital lobe, the frontal lobe and the right occipital lobe, and the right and left occipital lobes compared with the EMCS group; however, no significant functional connectivity differences were demonstrated compared with the MCS group. There were significant differences in functional connectivity between the frontal and left occipital lobes, frontal and right occipital lobes, and left and right occipital lobes between the MCS and UWS/VS groups. Patients with different outcomes of pDoC had different degrees of functional connectivity alterations in resting-state fNIRS, which may be the direction of our future research on patients with pDoC prognosis.
We chose two brain regions, the frontal and occipital lobes, for the study of patients with disorders of consciousness, and both showed that patients with disorders of consciousness have differences in both inter- and intra-brain functional connectivity between these two brain regions, and that this difference may be evidence for us to determine prognosis. Decreased functional connectivity may be associated with reduced efficiency of information transfer between brain regions. It was shown that the prefrontal cortex, an important node of the consciousness pathway, exhibited significant functional connectivity abnormalities in patients with UWS/VS and MCS, which may reflect the critical role of the prefrontal lobe in the recovery of consciousness (29). Thibaut et al. (30) reported for the first time that tDCS stimulation of the left dorsolateral prefrontal cortical area showed improvement in consciousness in some MCS patients, confirming that the left dorsolateral prefrontal cortical area is a key node in the network of disorders of consciousness. In an experiment by Silva et al. (6), a significant correlation between patients’ CRS-R scores and posterior cingulate-medial prefrontal cortex activity in the resting state, in particular a reduction in functional connectivity between the medial prefrontal cortex and posterior cingulate cortex, predicted poor outcome in patients with disorders of consciousness. All of these studies demonstrate the importance of the frontal lobe in brain function in patients with disorders of consciousness.
Most previous studies on disorders of consciousness have ignored the role played by the occipital lobe in disorders of consciousness, but this paper found that the occipital lobe has different degrees of decreased functional connectivity in patients with disorders of consciousness. An fNIRS study of patients with impaired consciousness found that functional connectivity between prefrontal and occipital regions was significantly elevated in patients after transspinal stimulation, which demonstrates that increased connectivity strength between prefrontal and occipital regions is associated with improved consciousness (31). The results of the present study also support that the functional connectivity between the occipital lobes and between the occipital lobes and frontal lobes decreased in patients with minimally conscious state and persistent vegetative state compared to awake patients. Daniel Golkowski et al. (32) simultaneously applied fMRI, deoxyglucose positron emission tomography (FDG-PET), and EEG to assess the prognosis of patients with disorders of consciousness, and found that glucose metabolism in the occipital lobe was significantly higher in patients with MCS than in patients with VS as measured by FDG-PET, suggesting that the occipital lobe plays an important role in the recovery of consciousness, similar to the results in this study.
The present study did not observe the differences in functional connectivity between the MCS and EMCS groups in the three connectivity modality, which may be due to the fact that some of the MCS patients were very close to the EMCS group in the level of the conscious state and the FNIRS could not capture the small differences in the brain. Although the overall level of consciousness is higher in MCS patients than in UWS/VS group, both may have a similar degree of impairment of underlying network connections in the resting state due to similar widespread cortical damage (33). On the other hand, it is possible that the sample size limitation resulted in the failure to statistically reflect the differences between the two groups.
The tendency for functional connectivity strength to decrease with the gradient of level of consciousness (EMCS>MCS > UWS/VS) suggests that resting-state fNIRS metrics may serve as a quantitative complementary tool for consciousness assessment. Compared to clinical behavioral scales that are susceptible to motor functional limitations, functional connectivity parameters may be more sensitive to underlying neurological remodeling.
5 Limitations
The present study still has some limitations, as the lack of sample size led to the failure of subgrouping according to the etiology to observe the differences in brain functional connectivity between different causes of disorders of consciousness. In addition, due to the limited hospitalization period of some patients, it was not possible to follow up the fNIRS data for each group.
6 Conclusion
Overall, fNIRS can be used in the future as a new functional brain imaging technique, which is expected to assess the prognosis of patients with disorders of consciousness by detecting the strength of connectivity in functional brain regions. The resting-state-based fNIRS data increase the understanding of neuroimaging in patients with chronic disorders of consciousness, and the results of this study provide a theoretical basis for neuroimaging to study disease prognosis in patients with chronic disorders of consciousness.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangbin Hospital, Guangxi Zhuang Autonomous Region. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YaL: Conceptualization, Data curation, Formal Analysis, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. XL: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing. YiL: Data curation, Formal Analysis, Software, Writing – review & editing. CW: Investigation, Software, Supervision, Writing – review & editing. YB: Investigation, Software, Supervision, Writing – review & editing. YX: Investigation, Software, Writing – review & editing. WJ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the grant from the Qingxiu District Science and Technology Program Project of Nanning (Grant No. 2018037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor DW declared a past co-authorship with the author WJ.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fneur.2025.1642987.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Caroline S Audrey V Joseph G Ventura M Boly M Majerus S et al . Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. (2009) 9:35. doi: 10.1186/1471-2377-9-35
2.
Giacino JT Katz DI Schiff ND Whyte J Ashman EJ Ashwal S et al . Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology; the American congress of rehabilitation medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. (2018) 91:450–60. doi: 10.1212/WNL.0000000000005926
3.
Horovitz SG Braun AR Carr WS Picchioni D Balkin TJ Fukunaga M et al . Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci USA. (2009) 106:11376–81. doi: 10.1073/pnas.0901435106
4.
Enrico A Francisco G Carol PD Vanhaudenhuyse A Lesenfants D Boveroux P et al . Posterior cingulate cortex-related co-activation patterns: a resting state FMRI study in propofol-induced loss of consciousness. PLoS One. (2014) 9:e100012. doi: 10.1371/journal.pone.0100012
5.
Audrey V Quentin N Tshibanda LJ Bruno MA Boveroux P Schnakers C et al . Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain J Neurol. (2010) 133:161–71. doi: 10.1093/brain/awp313
6.
Stein S Francesco PD Corine V Riu B Loubinoux I Geeraerts T et al . Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology. (2015) 85:2036–44. doi: 10.1212/WNL.0000000000002196
7.
Koenig MA Holt JL Ernst T Buchthal SD Nakagawa K Stenger VA et al . MRI default mode network connectivity is associated with functional outcome after cardiopulmonary arrest. Neurocrit Care. (2014) 20:348–57. doi: 10.1007/s12028-014-9953-3
8.
Pengmin Q Xuehai W Zirui H Duncan NW Tang W Wolff A et al . How are different neural networks related to consciousness?Ann Neurol. (2015) 78:594–605. doi: 10.1002/ana.24479
9.
Xuehai W Qihong Z Jin H Tang W Mao Y Gao L et al . Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci. (2015) 35:12932–46. doi: 10.1523/JNEUROSCI.0415-15.2015
10.
Liu X Li J Gao J Zhou Z Meng F Pan G et al . Association of medial prefrontal cortex connectivity with consciousness level and its outcome in patients with acquired brain injury. J Clin Neurosci. (2017) 42:160–6. doi: 10.1016/j.jocn.2017.04.015
11.
Fins JJ . The minimally conscious state: definition and diagnostic criteria. Neurology. (2002) 59:1473. doi: 10.1212/WNL.59.9.1473
12.
Bagnato S Boccagni C Sant’Angelo A Prestandrea C Mazzilli R Galardi G . 2. EEG predictors of outcome in patients with disorders of consciousness. Clin Neurophysiol. (2015) 126:e1–1. doi: 10.1016/j.clinph.2014.10.021
13.
Cantero LJ Atienza M Salas MR . State-modulation of cortico-cortical connections underlying normal EEG alpha variants. Physiol Behav. (2000) 71:107–15. doi: 10.1016/S0031-9384(00)00334-6
14.
Lörincz ML Crunelli V Hughes SW . Cellular dynamics of cholinergically induced alpha (8-13 Hz) rhythms in sensory thalamic nuclei in vitro. Neuroscience. (2008) 28:660–71. doi: 10.1523/JNEUROSCI.4468-07.2008
15.
Hayashi K Mukai N Sawa T . Simultaneous bicoherence analysis of occipital and frontal electroencephalograms in awake and anesthetized subjects. Clin Neurophysiol. (2014) 125:194–201. doi: 10.1016/j.clinph.2013.06.024
16.
Bourdillon P Hermann B Guénot M Bastuji H Isnard J King JR et al . Brain-scale cortico-cortical functional connectivity in the delta-theta band is a robust signature of conscious states: an intracranial and scalp EEG study. Sci Rep. (2020) 10:14037–7. doi: 10.1038/s41598-020-70447-7
17.
Malin R Erik W Tobias C Rosén I Friberg H . Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. (2010) 38:1838–44. doi: 10.1097/CCM.0b013e3181eaa1e7
18.
Sair HI Hannawi Y Li S Kornbluth J Demertzi A Di Perri C et al . Early functional connectome integrity and 1-year recovery in comatose survivors of cardiac arrest. Radiology. (2018) 287:247–55. doi: 10.1148/radiol.2017162161
19.
Sarton B Tauber C Fridman E Péran P Riu B Vinour H et al . Neuroimmune activation is associated with neurological outcome in anoxic and traumatic coma. Brain J Neurol. (2024) 147:1321–30. doi: 10.1093/brain/awae045
20.
Scholkmann F Kleiser S Metz AJ Zimmermann R Mata Pavia J Wolf U et al . A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage. (2014) 85:6–27. doi: 10.1016/j.neuroimage.2013.05.004
21.
Molteni E Arrigoni F Bardoni A Galbiati S Villa F Colombo K et al . Bedside assessment of residual functional activation in minimally conscious state using NIRS and general linear models. Annu Int Conf IEEE Eng Med Biol Soc. (2013) 2013:3551–4. doi: 10.1109/EMBC.2013.6610309
22.
Kempny AM James L Yelden K Duport S Farmer S Playford ED et al . Functional near infrared spectroscopy as a probe of brain function in people with prolonged disorders of consciousness. Neuroimage Clin. (2016) 12:312–9. doi: 10.1016/j.nicl.2016.07.013
23.
Naseer N Hong KS . fNIRS-based brain-computer interfaces: a review. Front Hum Neurosci. (2015) 9:3. doi: 10.3389/fnhum.2015.00003
24.
Kondziella D Bender A Diserens K van Erp W Estraneo A Formisano R et al . European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. (2020) 27:741–56. doi: 10.1111/ene.14151
25.
Liu X Cheng F Hu S Wang B Hu C Zhu Z et al . Cortical activation and functional connectivity during the verbal fluency task for adolescent-onset depression: a multi-channel NIRS study. J Psychiatr Res. (2022) 147:254–61. doi: 10.1016/j.jpsychires.2022.01.040
26.
Keith DK Tatsuya Y Sato C Tagai K Dan I . Willingness-to-pay-associated right prefrontal activation during a single, real use of cosmetics as revealed by functional near-infrared spectroscopy. Front Hum Neurosci. (2019) 13:16. doi: 10.3389/fnhum.2019.00016
27.
Hoshi Y . Functional near-infrared spectroscopy: current status and future prospects. J Biomed Opt. (2007) 12:062106–09. doi: 10.1117/1.2804911
28.
Tong Y Frederick BD . Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. NeuroImage. (2010) 53:553–64. doi: 10.1016/j.neuroimage.2010.06.049
29.
Cui Y Song M Lipnicki DM Yang Y Ye C Fan L et al . Subdivisions of the posteromedial cortex in disorders of consciousness. NeuroImage. (2018) 20:260–6. doi: 10.1016/j.nicl.2018.07.025
30.
Ledoux D Thibaut A Bruno M-A Demertzi A Laureys S . tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology. (2014) 82:1112. doi: 10.1212/WNL.0000000000000260
31.
Si J Dang Y Zhang Y Li Y Zhang W Yang Y et al . Spinal cord stimulation frequency influences the hemodynamic response in patients with disorders of consciousness. Neurosci Bull. (2018) 34:659–67. doi: 10.1007/s12264-018-0252-4
32.
Golkowski D Merz K Mlynarcik C Kiel T Schorr B Lopez-Rolon A et al . Simultaneous EEG-PET-fMRI measurements in disorders of consciousness: an exploratory study on diagnosis and prognosis. J Neurol. (2017) 264:1986–95. doi: 10.1007/s00415-017-8591-z
33.
Wang Y Chen S Xia X Peng Y Wu B . Altered functional connectivity and regional brain activity in a triple-network model in minimally conscious state and vegetative-state/unresponsive wakefulness syndrome patients: a resting-state functional magnetic resonance imaging study. Front Behav Neurosci. (2022) 16:519. doi: 10.3389/fnbeh.2022.1001519
Summary
Keywords
resting-state, functional near-infrared spectroscopy, prolonged disorders of consciousness, outcomes, functional connectivity
Citation
Liang Y, Liang X, Li Y, Wang C, Bi Y, Xue Y and Jiang W (2025) A study on resting-state functional near-infrared spectroscopy in patients with different outcomes of prolonged disorders of consciousness. Front. Neurol. 16:1533853. doi: 10.3389/fneur.2025.1533853
Received
25 November 2024
Accepted
15 May 2025
Published
02 June 2025
Corrected
27 June 2025
Volume
16 - 2025
Edited by
Daifa Wang, Beihang University, China
Reviewed by
Murad Althobaiti, Imam Abdulrahman Bin Faisal University, Saudi Arabia
Ling Wang, Sichuan Normal University, China
Updates
Copyright
© 2025 Liang, Liang, Li, Wang, Bi, Xue and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyu Jiang, wenyu_jiang@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.