- 1Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
Objective: This study investigates the prevalence of negative emotions and sleep disturbances in gastric cancer patients, explores their relationship, and suggests targeted interventions to enhance their physical and mental well-being.
Methods: A total of 650 gastric cancer patients from the First Affiliated Hospital of Soochow University (March 2020 to March 2023) were included. Negative emotions, including anxiety and depression, were assessed using the Positive and Negative Affect Schedule (PANAS), while sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI). Descriptive statistics and Pearson correlation analysis were employed to analyze the data and explore the relationship between negative emotions and sleep quality.
Results: Of the 650 patients, 533 (82%) exhibited negative emotions, and 560 (86.15%) experienced sleep disturbances. A significant positive correlation was found between negative emotion scores and sleep quality (r = 0.682, p < 0.05). Patients with poor sleep quality had significantly higher negative emotion scores (p < 0.05). Factors such as gender, age, tumor stage, and education level influenced negative emotion scores, while room type significantly impacted sleep quality (p < 0.05).
Conclusion: Negative emotions and sleep disturbances are common and interrelated in gastric cancer patients. Addressing psychological factors, particularly anxiety and depression, is crucial for improving sleep quality and overall recovery. Integrated psychological and sleep management interventions should be incorporated into routine care to improve patients’ quality of life and treatment outcomes.
1 Introduction
Gastric cancer, a prevalent malignancy of the digestive system, continues to be a major global health challenge, with increasing incidence and mortality rates. In particular, gastric cancer has become one of the leading causes of cancer-related deaths in China (1). Despite advancements in treatment methods, which have led to a significant prolongation of survival, the overall quality of life for gastric cancer patients has not seen corresponding improvements. Clinical research indicates that, during the course of treatment—including surgery, chemotherapy, and other therapeutic interventions—gastric cancer patients often experience various degrees of negative emotions (such as anxiety, depression, and fear) and sleep disturbances (2). These psychological issues not only compromise the patients’ physical and mental well-being, but they also adversely affect treatment adherence, which in turn can impact treatment outcomes and survival prognosis (3).
Negative emotions and sleep quality are closely interrelated. Research has shown that chronic negative emotional states can significantly impair sleep quality, while poor sleep can exacerbate negative emotions, creating a vicious cycle (4). For gastric cancer patients, emotional responses during treatment are often influenced by the burden of the disease and the uncertainties associated with its treatment, whereas poor sleep quality further aggravates both the psychological and physical burden. Patients with impaired sleep quality are more likely to exhibit mood swings, cognitive decline, and a negative outlook on treatment. Therefore, understanding the current status of negative emotions and sleep quality in gastric cancer patients, and implementing effective interventions, has become an essential component of comprehensive cancer care (5, 6).
Currently, research examining the relationship between negative emotions and sleep quality in gastric cancer patients is limited, particularly with respect to Chinese populations. Most existing studies focus on individual psychological or sleep interventions for cancer patients, but comprehensive, empirically tested intervention strategies remain scarce (7). Thus, the present study aims to conduct a systematic investigation of gastric cancer patients treated at the First Affiliated Hospital of Soochow University between March 2020 and March 2023, to assess their emotional and sleep status, explore the correlation between these two factors, and provide clinical evidence for improving their physical and mental health. The goal is to propose targeted intervention strategies to enhance patients’ well-being and overall quality of life (8, 9).
Gastric cancer patients face distinct physiological and psychological challenges compared to those with other malignancies (10). The high prevalence of nutritional deficiencies, gastrointestinal discomfort, and aggressive treatments, such as gastrectomy, contribute significantly to emotional distress and sleep disturbances (11, 12). Despite these challenges, research on the interplay between negative emotions and sleep quality in this population remains limited. This study aims to bridge this gap by systematically quantifying the relationship between negative emotions and sleep quality, evaluating the impact of emotional distress on sleep disturbances, and providing scientific evidence to support the development of personalized psychological interventions and sleep management strategies. Ultimately, these findings seek to inform targeted interventions that enhance patient well-being and improve overall quality of life.
2 Materials and methods
2.1 Study population
This retrospective cohort study included 650 patients with primary gastric cancer who were treated at the First Affiliated Hospital of Soochow University between March 2020 and March 2023. All patients had been clinically diagnosed with gastric cancer and met the inclusion criteria outlined below. The demographic characteristics of the study population were as follows: 370 males and 280 females; age range from 28 to 88 years, with a mean age of (56.73 ± 8.56) years. Occupational status: 312 employed, 204 retired, and 134 unemployed; Education level: 222 patients with middle school or lower education, and 428 with high school or higher education. Tumor staging: Stage I (76 patients), Stage II (146 patients), Stage III (291 patients), and Stage IV (137 patients). Type of hospital accommodation: 88 patients in single rooms, 258 in double rooms, and 304 in triple rooms. Length of hospitalization: 442 patients were hospitalized for ≤7 days, 128 for 8–15 days, and 80 for ≥16 days. Time Since Last Chemotherapy/Radiotherapy: 468 patients (72.0%) had ≤4 weeks since their last chemotherapy/radiotherapy, and 182 patients (28.0%) had >4 weeks. Insurance type: 533 patients had medical insurance (180 with urban employee insurance, 148 with urban resident insurance, 205 with new rural cooperative insurance), and 117 were self-paying. Among the enrolled patients, 124 (19.1%) had controlled hypertension, 98 (15.1%) had type 2 diabetes, and 76 (11.7%) had a history of coronary artery disease. These comorbidities were recorded but were not the primary focus of this study.
This study adhered to ethical guidelines and received approval from the Ethics Committee of the First Affiliated Hospital of Soochow University. Informed consent was obtained from all participants prior to enrollment. Statistical analysis revealed no significant differences in the demographic characteristics of the study participants (p > 0.05), ensuring comparability across the groups.
2.2 Diagnostic criteria
All patients included in the study were diagnosed with primary gastric cancer based on clinical symptoms, auxiliary examinations (such as CT, MRI, and endoscopy), and pathological confirmation. Tumor staging was determined according to the 8th edition of the TNM staging system by the Union for International Cancer Control (UICC). Staging was verified by two independent oncology specialists at the time of patient enrollment.
2.3 Inclusion criteria
(1) Diagnosis of primary gastric cancer;
(2) Age ≥18 years;
(3) Ability to communicate verbally and willingness to participate in the survey;
(4) Written informed consent obtained from the patient and their family members, with a full understanding of the study and agreement to participate.
2.4 Exclusion criteria
(1) Concurrent autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus);
(2) Severe chronic diseases (e.g., HIV/AIDS, hepatitis B or C);
(3) Severe organ dysfunction (e.g., heart failure, renal failure);
(4) Uncontrolled diabetes or hypertension;
(5) Pregnant or breastfeeding women;
(6) Severe psychiatric disorders (e.g., depression, schizophrenia).
(7) Thyroid disorders affecting metabolic and neuropsychiatric function (e.g., hypothyroidism, hyperthyroidism).
(8) Severe vitamin deficiencies (e.g., Vitamin D or B12 deficiency) that could contribute to sleep disturbances or mood disorders.
2.5 Dropout and exclusion criteria
(1) Patient death during the study period;
(2) Patient voluntary withdrawal from the study.
2.6 Research methodology
2.6.1 Study design and data collection
This study utilized a descriptive research design to evaluate the multidimensional aspects of 650 gastric cancer patients through clinical interviews and standardized psychological assessment scales. After signing informed consent, patients participated in a 7-day clinical interview and survey, which included assessments of general demographic data, medical history, negative emotions, and sleep quality. Patients were enrolled at different treatment stages, with 72% having received their last chemotherapy or radiotherapy session within the past 4 weeks. Treatment timing was recorded and considered in subgroup analyses. All data collection was conducted during hospitalization to ensure consistency in reporting. Data were collected by trained research staff to ensure consistency and accuracy.
2.6.2 Assessment of negative emotions
Negative emotions were assessed using the Positive and Negative Affect Schedule (PANAS). This scale includes 20 adjectives reflecting emotions, with 10 items related to positive affect and 10 to negative affect. Negative emotions were assessed by the items: 2, 4, 6, 7, 8, 11, 13, 15, 18, and 20. A 5-point Likert scale (1 = very slightly or not at all, 5 = extremely) was used (13). Higher scores indicate stronger negative emotions. The PANAS scale has shown good internal consistency with a Cronbach’s α coefficient of 0.752.
Patients with a prior diagnosis of major depressive disorder (MDD) were excluded based on their medical records. However, no formal depression screening tool (e.g., Beck Depression Inventory-II, Hamilton Depression Rating Scale) was administered, which may be a limitation of this study.
2.6.3 Assessment of sleep quality
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). The PSQI consists of 19 self-rated items and 5 clinician-rated items, covering seven dimensions: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, hypnotic drug use, and daytime dysfunction. Each dimension is scored on a 0–3 scale, with a total score ≥7 indicating poor sleep quality, and a score <7 indicating normal sleep (14).
2.6.4 Quality control
To ensure data integrity and scientific rigor, the following quality control measures were implemented:
(1) Pre-study preparation: Expert consultations, survey design, and pilot testing were conducted to ensure the relevance and scientific validity of the research tools.
(2) Standardization: Unified inclusion/exclusion criteria and standardized assessment tools were employed to minimize bias and ensure the reliability of the data.
(3) Training and supervision: All research staff were thoroughly trained to ensure uniform data collection procedures.
(4) Data management: Data were entered into the Epidata database, with double-entry verification to ensure data accuracy and consistency.
2.7 Statistical analysis
All statistical analyses were conducted using SPSS 19.0 statistical software.
Continuous variables were expressed as mean ± standard deviation (M ± SD) and analyzed using independent-sample t-tests or t’-tests (when variance heterogeneity was detected).
Multiple-group comparisons were performed using one-way analysis of variance (ANOVA), followed by post-hoc Tukey tests where significant differences were found.
Effect sizes were calculated to assess the magnitude of differences:
(1) Cohen’s d for t-tests: Small (d = 0.2), Moderate (d = 0.5), Large (d ≥ 0.8).
(2) Eta-squared (η2) for ANOVA: Small (η2 = 0.01), Moderate (η2 = 0.06), Large (η2 ≥ 0.14).
Pearson correlation analysis was used to evaluate the relationship between negative emotions (PANAS scores) and sleep quality (PSQI scores): Small (r = 0.10–0.29), Moderate (r = 0.30–0.49), Large (r ≥ 0.50).
Categorical variables were analyzed using chi-square (χ2) tests, where applicable.
A two-tailed p-value of <0.05 was considered statistically significant for all tests.
3 Results
3.1 Comparison of negative emotion scores among gastric cancer patients with different clinical characteristics
Among the 650 patients, 533 (82%) exhibited varying degrees of negative emotions, while 117 (18%) reported no significant emotional distress.
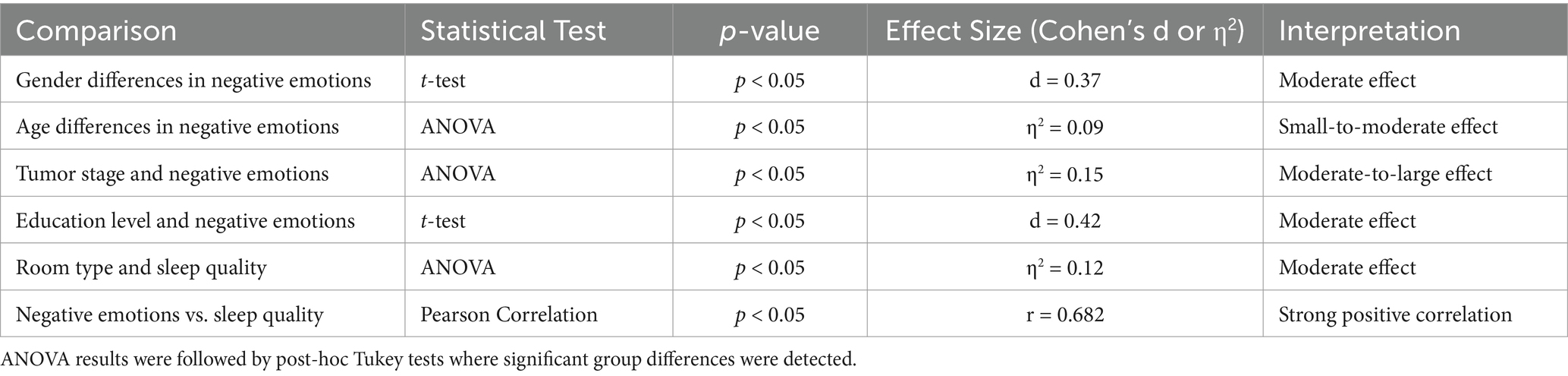
Negative emotion scores, as measured by the Positive and Negative Affect Schedule (PANAS), varied significantly based on gender, age, tumor stage, and education level (p < 0.05). Female patients exhibited significantly higher PANAS negative emotion scores (M = 13.12 ± 7.98) than male patients (M = 15.85 ± 8.12, p < 0.05, Cohen’s d = 0.37), indicating a moderate effect size. This difference may be influenced by hormonal variations, coping strategies, and social expectations (Figure 1). Patients aged >60 years had significantly higher PANAS negative emotion scores (M = 30.2 ± 6.7) compared to those aged ≤60 years (Age≤45:M = 17.22 ± 7.43, Age 46–60: M = 14.15 ± 6.29,p < 0.05, η2 = 0.09), suggesting a small-to-moderate effect. Advanced-stage gastric cancer patients (Stages III-IV) exhibited significantly higher PANAS negative emotion scores (Stages III:M = 18.12 ± 5.91,Stages IV:M = 23.67 ± 7.01) than early-stage patients (Stages I-II) (Stages I:M = 9.78 ± 5.66,Stages II:M = 12.45 ± 6.44, p < 0.05, η2 = 0.15), indicating a moderate-to-large effect. Patients with lower educational attainment reported significantly higher PANAS negative emotion scores (M = 19.87 ± 6.52) than those with higher education levels (M = 13.21 ± 5.79, p < 0.05, Cohen’s d = 0.42), demonstrating a moderate effect size.
A detailed comparison of PANAS scores across different patient subgroups is provided in Table 1.
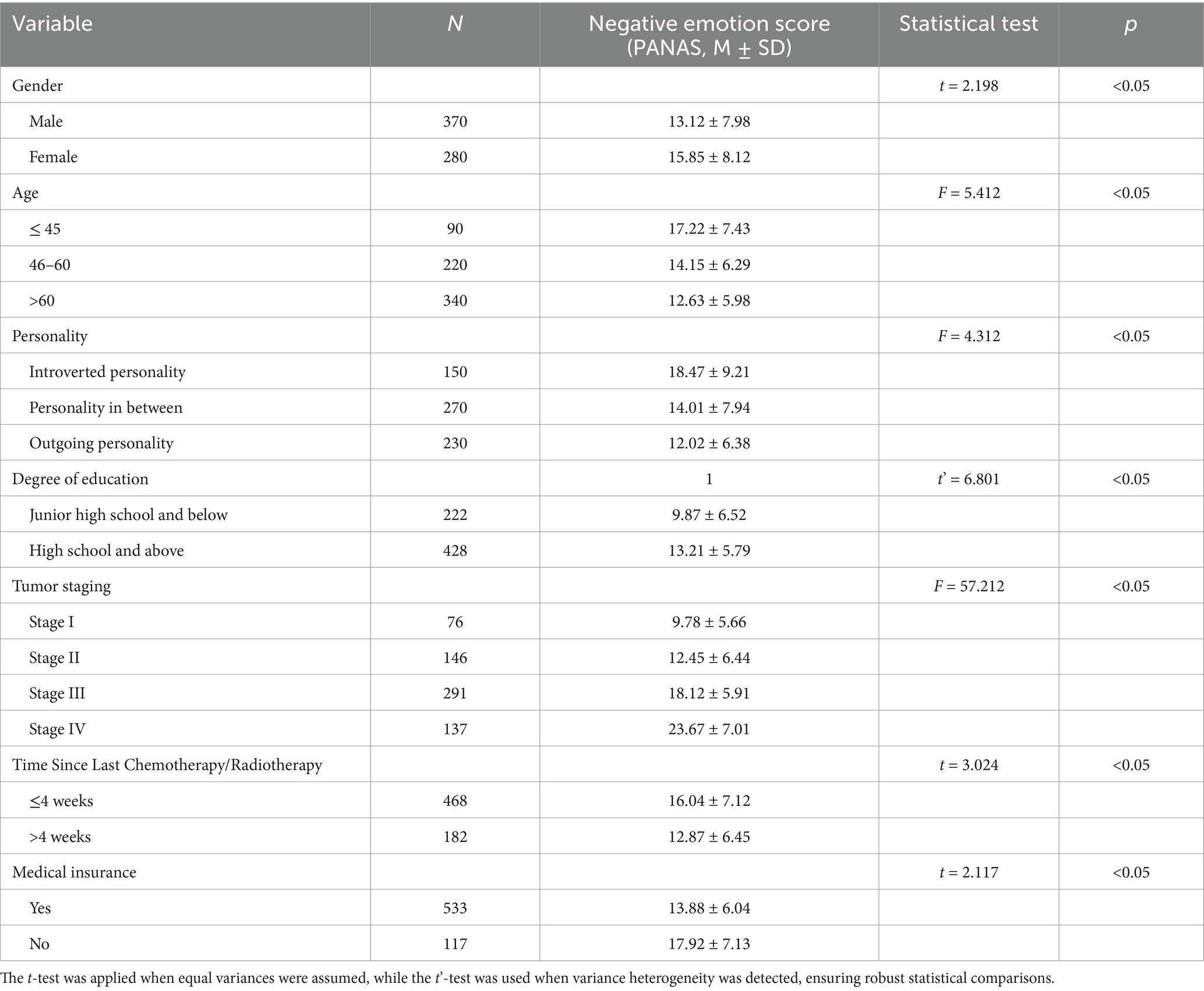
Table 1. Comparison of negative emotion scores of gastric cancer patients with different clinical characteristics (Mean ± SD).
3.2 Comparison of sleep quality scores among gastric cancer patients with different clinical characteristics
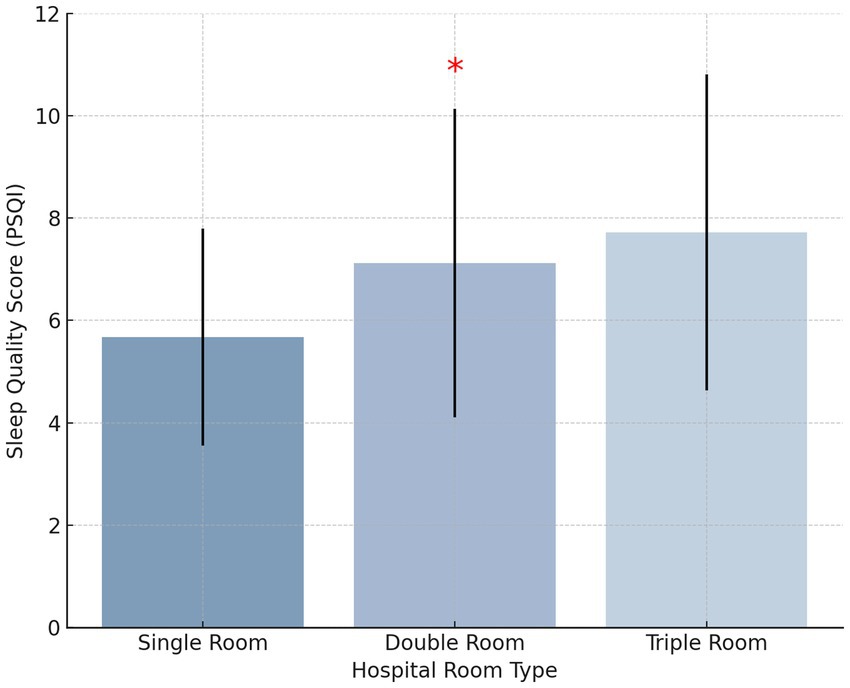
Among the 650 patients, 560 (86.15%) reported sleep disturbances, while only 90 (13.85%) had normal sleep quality. Definition of Sleep Disturbances: In this study, sleep disturbances were defined as a Pittsburgh Sleep Quality Index (PSQI) score ≥7, while normal sleep quality was defined as a PSQI score <7. This threshold is consistent with established research on clinical sleep assessment. No significant differences in sleep quality scores were observed based on gender, age, tumor stage, or education level (p > 0.05, η2 < 0.02 for all comparisons). However, hospital room type was found to significantly impact sleep quality (p < 0.05, η2 = 0.12). Patients in single-occupancy rooms reported significantly better sleep quality than those in double- or triple-occupancy rooms (p < 0.05, Cohen’s d = 0.58), indicating a moderate-to-large effect (Figure 2). Effect of Length of Hospital Stay: Further analysis showed that length of hospital stay did not have a statistically significant impact on sleep quality (p > 0.05, η2 < 0.02). However, patients hospitalized for ≥16 days exhibited a trend toward poorer sleep quality compared to those hospitalized for ≤7 days, suggesting that prolonged hospitalization may contribute to worsening sleep, though additional factors likely mediate this relationship.
A detailed comparison is provided in Table 2.
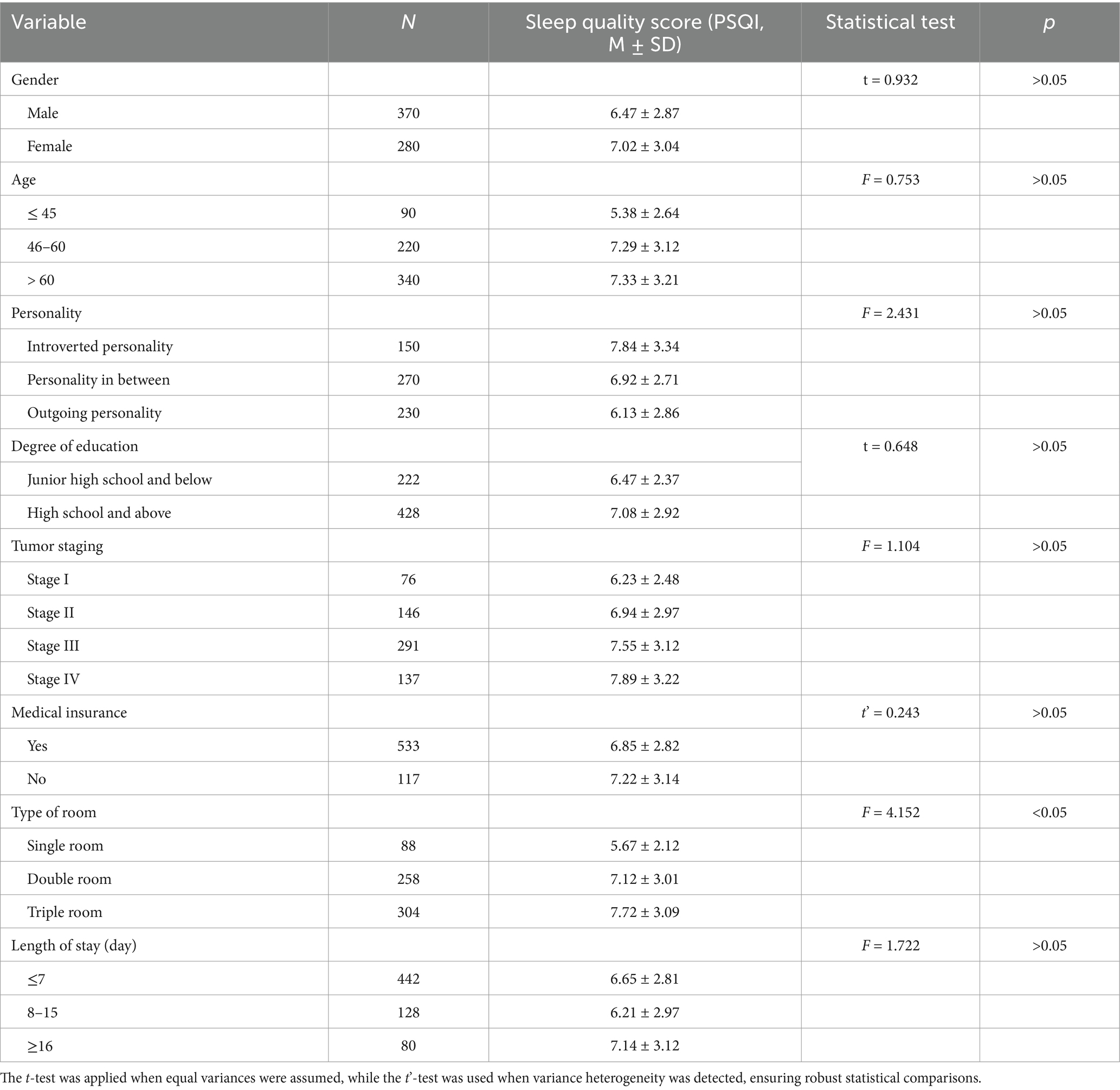
Table 2. Comparison of sleep quality scores of gastric cancer patients with different clinical characteristics (Mean ± SD).
3.3 Correlation between negative emotions and sleep quality
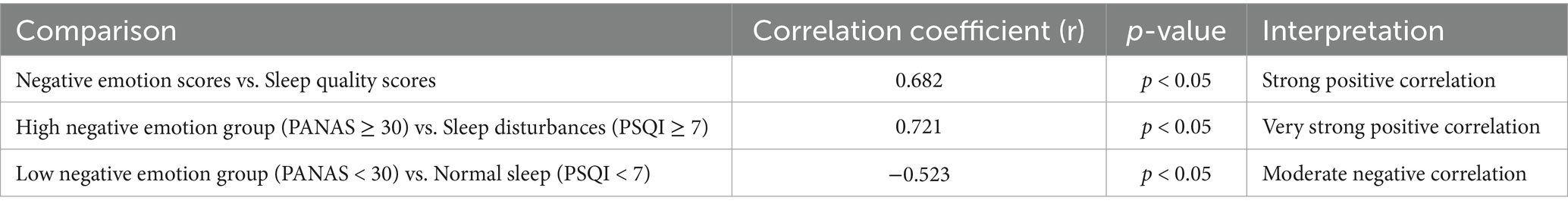
Further analysis showed that patients with sleep disturbances (PSQI ≥7) had significantly higher PANAS negative emotion scores (M = 31.5 ± 7.0) than those with normal sleep quality (PSQI <7) (M = 24.7 ± 6.3, p < 0.05, Cohen’s d = 0.58), indicating a moderate-to-large effect size. As illustrated in Figure 3, Pearson correlation analysis indicated a strong positive correlation between negative emotions and sleep quality (r = 0.682, p < 0.05). This finding confirms that higher levels of emotional distress are associated with poorer sleep quality, emphasizing the need for integrated psychological and sleep management interventions in clinical care. However, it is important to note that correlation does not imply causation. While this study confirms a strong association, future longitudinal research is needed to establish whether negative emotions directly contribute to sleep disturbances or whether other confounding factors, such as disease progression and treatment effects, mediate this relationship.
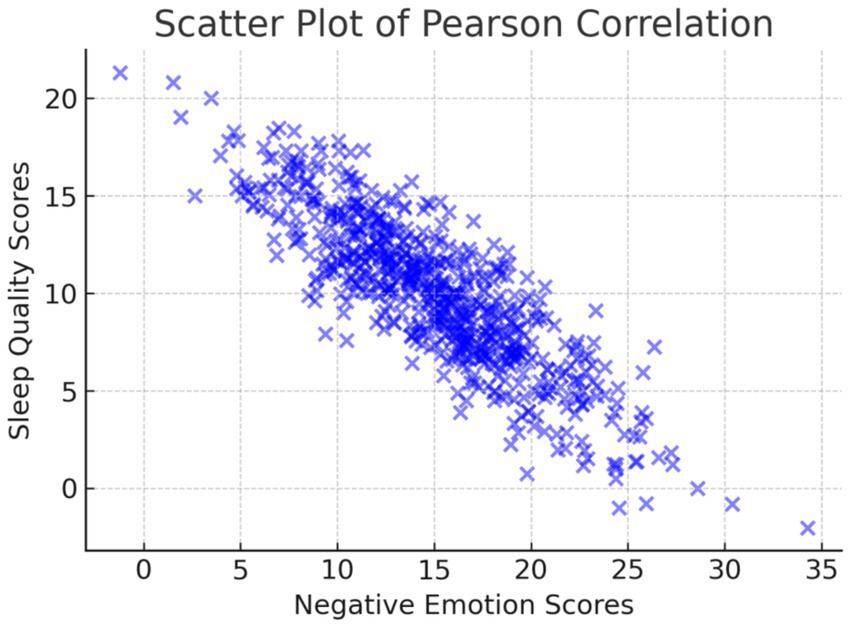
Figure 3. Scatter plot illustrating the Pearson correlation between negative emotion scores and sleep quality scores.
A summary of PANAS score distributions across different patient characteristics is presented in Table 3.
3.4 Summary of key statistical results
To strengthen our statistical analysis, we have also included an additional table summarizing key statistical findings (Table 4).
4 Discussion
4.1 Characteristics and influencing factors of negative emotions in gastric cancer patients
The findings of this study indicate that negative emotions, particularly anxiety and depression, are prevalent among gastric cancer patients. Several clinical and demographic factors influence the severity of emotional distress.
First, female patients exhibited significantly higher negative emotion scores than male patients (p < 0.05). This difference may be influenced by a combination of biopsychosocial factors, including hormonal variations, gender-specific coping strategies, and societal expectations (15). Previous studies suggest that women tend to experience greater affective responses to stress and illness, which may contribute to increased negative emotions (15, 16).
Second, older patients (≥70 years) had significantly higher negative emotion scores than younger patients (≤60 years) (p < 0.05). This may be attributed to physical decline, loss of independence, and existential anxiety regarding mortality, which exacerbate emotional distress (17).
Third, advanced-stage gastric cancer patients (Stages III-IV) exhibited significantly higher negative emotion scores than early-stage patients (Stages I-II) (p < 0.05). This is likely due to concerns about prognosis, disease progression, and aggressive treatment regimens (18, 19).
Finally, education level and social support play crucial roles in psychological well-being. Patients with lower educational attainment often exhibit higher anxiety and fear due to limited disease-related knowledge, while stronger social support networks have been associated with reduced psychological distress (20–22).
4.2 Current status and influencing factors of sleep quality in gastric cancer patients
Sleep disturbances are highly prevalent among gastric cancer patients, with 86.15% of patients in this study reporting impaired sleep quality. This aligns with prior research demonstrating that factors such as cancer-related pain, chemotherapy-induced side effects, and psychological distress contribute to sleep disturbances (23–25).
Although age, gender, and tumor stage did not significantly impact sleep quality in this study (p > 0.05), hospital room type had a statistically significant effect (p < 0.05). Specifically, patients in single-occupancy rooms reported significantly better sleep quality than those in shared rooms (p < 0.05). This finding underscores the importance of hospital environment optimization, as private and quiet spaces may promote better rest and recovery (26–28).
However, hospitalization conditions encompass multiple factors beyond room type, such as ward noise levels, nighttime medical interruptions, and hospital care routines (29, 30). These were not explicitly controlled in this study, necessitating further investigation into their impact on sleep quality.
4.3 The relationship between negative emotions and sleep quality
A key finding of this study is the significant positive correlation between negative emotion scores and sleep quality scores (r = 0.682, p < 0.05), indicating that higher levels of negative emotions are associated with poorer sleep quality.
This relationship can be explained through physiological and psychological mechanisms (31–33). Anxiety and depression activate the body’s stress response system, leading to increased sympathetic nervous activity and elevated secretion of stress hormones (e.g., cortisol and adrenaline), which disrupt sleep architecture (34–36). Additionally, prolonged sleep disturbances exacerbate psychological distress, creating a vicious cycle (37–39).
These findings highlight the bidirectional relationship between negative emotions and sleep disturbances, reinforcing the need for integrated psychological and sleep management interventions (39–41).
4.4 Influence of social support, treatment regimens, and hospitalizations
While this study establishes a strong correlation between negative emotions and sleep disturbances, several potential confounding factors should be considered:
4.4.1 Social support
Social support is a key determinant of emotional resilience and psychological well-being. Patients receiving strong emotional and practical support from family, friends, and healthcare providers tend to experience lower anxiety and depression levels, which may, in turn, improve sleep quality (42, 43). Although social support was not directly assessed in this study, it is likely that differences in support networks contributed to variations in psychological and sleep outcomes. Future studies should incorporate validated instruments, such as the Multidimensional Scale of Perceived Social Support (MSPSS), to better quantify this relationship (44, 45).
4.4.2 Treatment regimens
Gastric cancer treatments—including surgery, chemotherapy, radiotherapy, and targeted therapies—can significantly affect both emotional and sleep health (46). Chemotherapy-induced side effects, such as fatigue, nausea, and neuropathy, may lead to sleep disturbances, while the psychological burden of aggressive treatment may exacerbate anxiety and depression (47, 48). However, this study did not stratify patients based on their specific treatment regimens, which may have introduced variability in the observed associations. Future research should account for treatment-related factors and their influence on psychological and sleep parameters (49, 50).
4.4.3 Hospital setting and length of stay
Beyond room type, multiple environmental factors—such as ward noise levels, nighttime medical interventions, and staff interruptions—may influence sleep quality (51). Our findings suggest that single-occupancy rooms promote better sleep, yet additional research using objective sleep assessments (e.g., actigraphy or polysomnography) is needed to comprehensively evaluate environmental influences on sleep quality.
Although length of hospitalization has been proposed as a factor influencing sleep, our analysis did not reveal a significant association (p > 0.05, η2 < 0.02). However, patients hospitalized for ≥16 days exhibited a trend toward poorer sleep quality, possibly due to long-term exposure to hospital-related disruptions, increased medical interventions, and prolonged psychological distress (52, 53).
4.5 Study limitations and future research directions
4.5.1 This study has several limitations
(1) Cross-Sectional Design—The study’s cross-sectional nature limits causal inference. Future longitudinal studies should track changes over time to clarify the directionality of the relationship between negative emotions and sleep disturbances.
(2) Self-Reported Measures—A key limitation of this study is the reliance on self-reported sleep data, specifically using the Pittsburgh Sleep Quality Index (PSQI). While the PSQI is a validated tool, it is subject to recall bias and does not provide objective sleep parameters, such as sleep architecture or respiratory disturbances. To enhance data accuracy and provide a more comprehensive understanding of sleep disturbances in gastric cancer patients, future research should incorporate objective measures, such as actigraphy or polysomnography (PSG), which would allow for a more detailed assessment of sleep patterns and related disruptions.
(3) Lack of Intervention Testing—While this study highlights the link between psychological distress and sleep disturbances, it does not assess intervention efficacy. Future studies should evaluate structured interventions, including cognitive-behavioral therapy (CBT), pharmacological approaches, and hospital-based sleep improvement strategies.
(4) Potential Confounding Factors—Variables such as social support, treatment regimens, and hospitalization environment were not fully controlled. Future research should use multivariate analyses to adjust for these confounders and explore their impact on emotional well-being and sleep quality.
(5) Generalizability—As this study was conducted at a single medical center, findings may not be fully generalizable. Multi-center studies with larger, more diverse populations are needed to validate results.
(6) Comorbidities—Although patients with uncontrolled metabolic or cardiovascular conditions were excluded, controlled hypertension and diabetes were present in a subset of participants. Future studies should examine the potential impact of these comorbidities on sleep and emotional well-being.
By addressing these limitations, future research can provide stronger evidence for targeted interventions that improve psychological health and sleep quality in gastric cancer patients.
4.5.2 Future research directions and interventions
To maximize clinical impact, future research should prioritize testing evidence-based interventions through rigorous study designs rather than solely proposing theoretical approaches. Key areas for future investigation include:
(1) Psychological Interventions.
Randomized controlled trials (RCTs) should evaluate the effectiveness of cognitive-behavioral therapy (CBT), mindfulness-based stress reduction (MBSR), and other psychological interventions in alleviating emotional distress and improving sleep quality in gastric cancer patients.
(2) Hospital-Based Environmental Modifications.
Research should investigate whether optimizing hospital environments, such as noise reduction protocols, single-room accommodations, and improved lighting conditions, can enhance sleep quality and overall well-being.
(3) Pharmacological and Integrative Approaches.
Comparative studies should assess the efficacy of melatonin, sedatives, anti-inflammatory agents, and other pharmacological treatments for managing sleep disturbances while minimizing adverse effects.
(4) Longitudinal Studies on Prognosis and Treatment Outcomes.
Prospective cohort studies should examine the long-term bidirectional relationship between negative emotions and sleep disturbances and its impact on cancer progression, treatment adherence, and overall survival rates.
(5) Digital Health and Real-Time Monitoring.
The integration of mobile health (mHealth) applications and wearable technology could facilitate continuous real-time monitoring of emotional distress and sleep patterns, enabling early detection and timely intervention.
(6) Non-Invasive Sleep Monitoring Technologies.
Future studies should incorporate objective sleep assessments using actigraphy or wearable sleep tracking devices to enhance data reliability while reducing patient burden compared to polysomnography.
By advancing research in these areas, future studies can contribute to the development of personalized, multidisciplinary intervention strategies, ultimately improving the mental health, sleep quality, and overall quality of life for gastric cancer patients.
4.6 Clinical implications
The findings of this study emphasize the need for a multidisciplinary approach to managing psychological distress and sleep disturbances in gastric cancer patients. Clinical recommendations include:
(1) Routine psychological screening to identify patients at risk for severe anxiety and depression.
(2) Personalized sleep management strategies, including environmental modifications, sleep hygiene education, and pharmacological treatments where appropriate.
(3) Strengthening social support networks, integrating family-based interventions into routine cancer care.
(4) Optimizing hospitalization conditions, such as reducing nighttime disturbances and improving ward design to promote better sleep.
By addressing these factors, future clinical strategies can enhance both mental well-being and sleep quality, ultimately improving patient outcomes and quality of life.
5 Conclusion
This study highlights the prevalence of negative emotions and sleep disturbances in gastric cancer patients, with a significant negative correlation between the two. Key factors such as gender, age, and tumor stage were found to influence emotional distress and sleep quality, with female patients, older individuals, and those in advanced stages experiencing greater challenges. The hospital environment, particularly single-occupancy rooms, also contributed to improved sleep quality.
Given the bidirectional relationship between negative emotions and sleep, integrated interventions addressing both psychological and sleep health are essential. Clinical strategies should include routine psychological screenings, personalized sleep management, and optimizing the hospital environment. Future research should focus on longitudinal studies and intervention evaluations to further understand and improve the care of gastric cancer patients.
In conclusion, addressing both emotional and sleep-related issues is crucial for improving the well-being and clinical outcomes of gastric cancer patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Human Ethics Committee of the Medical Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GW: Conceptualization, Writing – original draft, Supervision, Writing – review & editing. QQZ: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. SP: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 23KJB320016), titled “To explore the impact of oligodendrocyte progenitor cells (OPCs) intracerebroventricular injection on vascular cognitive impairment caused by white matter damage”, covering the period from July 2023 to June 2025.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu, H, Xu, J, Yao, Q, Zhang, Z, Guo, Q, and Lin, J. Rab7 is associated with poor prognosis of gastric cancer and promotes proliferation, invasion, and migration of gastric cancer cells. Med Sci Monit. (2020) 26:e922217. doi: 10.12659/MSM.922217
2. Zhou, T, Wu, Y, Meng, Q, and Kang, J. Influence of the acoustic environment in hospital wards on patient physiological and psychological indices. Front Psychol. (2020) 11:1600. doi: 10.3389/fpsyg.2020.01600
3. Fang, Z, Li, T, Chen, W, Wu, D, Qin, Y, Liu, M, et al. Gab2 promotes cancer stem cell like properties and metastatic growth of ovarian cancer via downregulation of miR-200c. Exp Cell Res. (2019) 382:111462. doi: 10.1016/j.yexcr.2019.06.007
4. Young, E, Philpott, H, and Singh, R. Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: current evidence and what the future may hold. World J Gastroenterol. (2021) 27:5126–51. doi: 10.3748/wjg.v27.i31.5126
5. Gao, Q, Li, H, Zou, Y, Hou, B, and Liu, L. Effectiveness of a comprehensive post-operative health education program in improving quality of life after gastric cancer surgery. Annal Palliat Med. (2020) 9:921–6. doi: 10.21037/apm.2020.04.14
6. Cao, XL, Wang, X, Li, P, and Ju, W. Psychological effects of advanced care on patients received endoscopic gastric cancer resection. Medicine (Baltimore). (2019) 98:e17497. doi: 10.1097/MD.0000000000017497
7. Chéour, S, Chéour, C, Gendreau, T, Bouazizi, M, Singh, KP, Saeidi, A, et al. Remediation of cognitive and motor functions in Tunisian elderly patients with mild Alzheimer’s disease: implications of music therapy and/or physical rehabilitation. Front Aging Neurosci. (2023) 15:1216052. doi: 10.3389/fnagi.2023.1216052
8. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
9. Zamanifar, S, Bagheri-Saveh, MI, Nezakati, A, Mohammadi, R, and Seidi, J. The effect of music therapy and aromatherapy with chamomile-lavender essential oil on the anxiety of clinical nurses: a randomized and double-blind clinical trial. J Med Life. (2020) 13:87–93. doi: 10.25122/jml-2019-0105
10. Kunz, D, Hempfling, W, Hoepig, N, Maass, H, Steffen, S, Herrmann, T, et al. Sleep disturbances in patients with gastrointestinal cancer: a longitudinal study. Support Care Cancer. (2012) 20:2131–2138. doi: 10.1007/s00520-011-1337-1
11. Mystakidou, K, Tsilika, E, Parpa, E, Katsouda, E, Galanos, A, and Vlahos, L. Assessment of anxiety and depression in advanced cancer patients and their relationship with quality of life. Qual Life Res. (2005) 14:1825–33. doi: 10.1007/s11136-005-4324-3
12. Palesh, OG, Roscoe, JA, Mustian, KM, Roth, T, Savard, J, Ancoli-Israel, S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center–Community Clinical Oncology Program. J Clin Oncol. (2010) 28:292–8. doi: 10.1200/JCO.2009.22.5011
13. Crawford, JR, and Henry, JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. (2004) 43:245–65. doi: 10.1348/0144665031752934
14. Hartmann, JA, Carney, CE, Lachowski, A, and Edinger, JD. Exploring the construct of subjective sleep quality in patients with insomnia. J Clin Psychiatry. (2015) 76:e768–73. doi: 10.4088/JCP.14m09066
15. Deng, Y, Chang, L, Yang, M, Huo, M, and Zhou, R. Gender differences in emotional response: inconsistency between experience and expressivity. PLoS One. (2016) 11:e0158666. doi: 10.1371/journal.pone.0158666
16. Sayilan, AA, Kulakaç, N, and Sayılan, S. The effects of noise levels on pain, anxiety, and sleep in patients. Nurs Crit Care. (2021) 26:79–85. doi: 10.1111/nicc.12525
17. Smyth, EC, Nilsson, M, Grabsch, HI, van Grieken, NCT, and Lordick, F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
18. Ye, Z, Zhang, Y, Du, M, Lu, S, Zhao, Q, and Yang, S. The correlation between probiotics and anxiety and depression levels in Cancer patients: a retrospective cohort study. Front Psychol. (2022) 13:830081. doi: 10.3389/fpsyt.2022.830081
19. Büttner-Teleagă, A, Kim, YT, Osel, T, and Richter, K. Sleep disorders in cancer-a systematic review. Int J Environ Res Public Health. (2021) 18:696. doi: 10.3390/ijerph182111696
20. Li, Y, Ni, N, Zhou, Z, Dong, J, Fu, Y, Li, J, et al. Hope and symptom burden of women with breast cancer undergoing chemotherapy: a cross-sectional study. J Clin Nurs. (2021) 30:2293–300. doi: 10.1111/jocn.15759
21. Llamas-Ramos, I, Llamas-Ramos, R, Carrillo-González, GM, Sepúlveda-Ramírez, J, and Vargas-Rosero, E. Symptom prevalence in Spanish and Colombian oncology patients measured with the MSAS. Cancers (Basel). (2022) 14:14. doi: 10.3390/cancers14071624
22. Abd-ElGawad, M, Abdelsattar, NK, Kamel, MA, Sabri, YA, Fathy, EM, el-Moez, NA, et al. The effect of music intervention in decreasing pain and anxiety during outpatient hysteroscopy procedure: a systematic review and meta-analysis of randomized control trials. BMC Womens Health. (2023) 23:360. doi: 10.1186/s12905-023-02489-8
23. Wang, L, Huang, YE, Zhang, H, Sun, Y, Liu, Y, Zhao, L, et al. Individualized whole-course management in prostatic puncture biopsy: application and efficiency. Zhonghua Nan Ke Xue. (2022) 28:608–11.
24. Alashram, AR, Janada, Q, Ghrear, T, and Annino, G Role of music therapy in improving cognitive function post-traumatic brain injury: a systematic review. Appl Neuropsychol Adult (2023). 1–10, doi: 10.1080/23279095.2023.2228951 [Epub ahead of print].
25. Zhu, X, Han, S, Chu, H, Wang, M, and Chen, S. Influence of self-management exercise intervention on the cancer related fatigue severity and self-management efficacy of patients with non-small cell lung cancer after operation. J Pak Med Assoc. (2020) 70 [Special Issue]:88–93.
26. Kenwood, MM, Kalin, NH, and Barbas, H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology. (2022) 47:260–75. doi: 10.1038/s41386-021-01109-z
27. Qian, X, Gong, L, Zhou, F, Zhang, Y, and Wang, H. High-quality nursing combined with the whole-course responsibility nursing intervention reduces the incidence of complications in severe aneurysmal subarachnoid hemorrhage. Evid Based Complement Alternat Med. (2022) 2022:1–7. doi: 10.1155/2022/3252718
28. Gong, H, Chu, Y, Hu, Q, and Song, Q. Preoperative radiotherapy is associated with significant survival benefits for patients with gastric signet ring cell carcinoma: a SEER-based approach. Technol Cancer Res Treat. (2020) 19:1533033820960746. doi: 10.1177/1533033820960746
29. Chai, Y, Li, L, Wu, YL, Wang, T, Jia, YM, Lin, XL, et al. The effects of case management for breast cancer patients: a protocol for systematic review and meta-analysis. Medicine (Baltimore). (2022) 101:e28960. doi: 10.1097/MD.0000000000028960
30. Kundes, MF, Kement, M, Yegen, F, Alkan, M, Kaya, S, and Kaptanoglu, L. Effects of clinical factors on quality of life following curative gastrectomy for gastric cancer. Niger J Clin Pract. (2019) 22:661–8. doi: 10.4103/njcp.njcp_181_18
31. Tao, F, Gong, L, and Dong, Q. Effect of negative emotions on patients with advanced gastric cancer receiving systemic chemotherapy: a prospective study. J Gastrointest Oncol. (2023) 14:952–62. doi: 10.21037/jgo-23-248
32. Shi, X, Gong, L, Liu, Y, Hou, K, Fan, Y, Li, C, et al. 4-phenylbutyric acid promotes migration of gastric cancer cells by histone deacetylase inhibition-mediated IL-8 upregulation. Epigenetics. (2020) 15:632–45. doi: 10.1080/15592294.2019.1700032
33. Aalero-Cantero, I, Casals, C, Espinar-Toledo, M, Barón-López, FJ, García-Agua Soler, N, and Vázquez-Sánchez, MÁ. Effects of music on the quality of life of family caregivers of terminal cancer patients: a randomised controlled trial. Healthcare (Basel). (2023) 11:1985. doi: 10.3390/healthcare11141985
34. Symon, A, McFadden, A, White, M, Fraser, K, and Cummins, A. Using a quality care framework to evaluate user and provider experiences of maternity care: a comparative study. Midwifery. (2019) 73:17–25. doi: 10.1016/j.midw.2019.03.001
35. Yin, L, Zhang, W, Liu, L, Guo, L, Guo, M, He, X, et al. Application of nursing intervention based on the IKAP model in self-management of patients with gastric cancer. Am J Transl Res. (2022) 14:6389–98.
36. Shukla, A, Kaushik, N, Hemlata, H, Verma, R, Gautam, S, and Singh, GP. Improvement in patient satisfaction and anxiety with perioperative music therapy in patients undergoing total abdominal hysterectomy: a single-blind prospective study. Cureus. (2023) 15:e39519. doi: 10.7759/cureus.39519
37. Thrift, AP, and El-Serag, HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. (2020) 18:534–42. doi: 10.1016/j.cgh.2019.07.045
38. Zhang, Q, Wan, R, and Liu, C. The impact of intense nursing care in improving anxiety, depression, and quality of life in patients with liver cancer: a systematic review and meta-analysis. Medicine (Baltimore). (2020) 99:e21677. doi: 10.1097/MD.0000000000021677
39. Hamzeh, S, Safari-Faramani, R, and Khatony, A. Effects of aromatherapy with lavender and peppermint essential oils on the sleep quality of Cancer patients: a randomized controlled trial. Evid Based Complement Alternat Med. (2020) 2020:7480204. doi: 10.1155/2020/7480204
40. Huda, N, Banda, KJ, Liu, AI, and Huang, TW. Effects of music therapy on spiritual well-being among patients with advanced cancer in palliative care: a meta-analysis of randomized controlled trials. Semin Oncol Nurs. (2023) 39:151481. doi: 10.1016/j.soncn.2023.151481
41. Zhang, S, Chen, H, Zhang, M, Sun, X, and Liu, X. Reduction of depression symptoms in laryngeal cancer patients receiving psychology services. Am J Transl Res. (2020) 12:6637–45.
42. Zimet, GD, Powell, SS, Farley, GK, Werkman, S, and Berkoff, K. The multidimensional scale of perceived social support. J Pers Assess. (1990) 55:610–7. doi: 10.1207/s15327752jpa5503&4_17
43. Zimet, GD, Dahlem, NW, Zimet, SG, and Farley, GK. The multidimensional scale of perceived social support. J Pers Assess. (1988) 52:30–41. doi: 10.1207/s15327752jpa5201_2
44. Green, Z, Çiçek, I, and Yıldırım, M. Social support and mental health: the mediating role of perceived stress. Front Psychol. (2024) 15:1330720. doi: 10.3389/fpsyg.2024.1330720
45. Savard, J, and Morin, CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. (2001) 19:895–908. doi: 10.1200/JCO.2001.19.3.895
46. Morgenthaler, T, Kramer, M, Alessi, C, Friedman, L, Boehlecke, B, Brown, T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of sleep medicine report. Sleep. (2006) 29:1415–9.
47. Edinger, JD, Wohlgemuth, WK, Radtke, RA, Marsh, GR, and Quillian, RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. (2001) 24:391–8. doi: 10.1001/jama.285.14.1856
48. Becker, PM. Pharmacologic and nonpharmacologic treatments of insomnia. Neurol Clin. (2005) 23:1149–63. doi: 10.1016/j.ncl.2005.05.002
49. Cheng, SY, Wang, Y, Zhang, H, van de Klundert, J, Zhu, Y, Gou, Z, et al. Hospitalized patients’ sleep quality compared between multioccupancy rooms and single-patient rooms. Int J Environ Res Public Health. (2022) 19:2148. doi: 10.3390/ijerph19042148
50. Sloane, PD, Rufolo, M, Bull, M, Jayasundera, T, Mitchell, CM, Zimmerman, S, et al. Single-occupancy patient rooms: a systematic review of the literature since 2006. J Patient Safety. (2018) 14:1–8. doi: 10.1097/PTS.0000000000000120
51. Zhang, L, Li, Z, Wang, Y, Wang, J, Wang, Y, Wang, Y, et al. Sleep quality of hospitalized patients, contributing factors, and prevalence of associated disorders. Front Psychol. (2019) 10:1–8. doi: 10.3389/fpsyg.2019.00195
52. Koh, D, Seow, E, Tan, M, Lee, VJ, Chan, G, Lim, D, et al. The impact of hospitalization on sleep: a review of the literature. J Clin Nurs. (2018) 27:553–563. doi: 10.1111/jocn.14107
Keywords: gastric cancer, negative emotions, sleep quality, psychological intervention, intervention strategies
Citation: Wang G, Zhang Q and Pan S (2025) Investigation of negative emotions and sleep quality in gastric cancer patients and intervention strategies. Front. Neurol. 16:1536736. doi: 10.3389/fneur.2025.1536736
Edited by:
Iván Pérez-Neri, National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra, MexicoReviewed by:
Ibrahim ACIR, Bakırköy Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi, TürkiyeRenato Garcia Gonzalez, Facultad de Medicina, Benemérita Universidad Autónoma de Puebla, Mexico
Shujing Zhang, University of California, Los Angeles, United States
Copyright © 2025 Wang, Zhang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengjie Pan, NDQ4MzMxODEyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Gang Wang
Gang Wang Quanquan Zhang2†
Quanquan Zhang2† Shengjie Pan
Shengjie Pan