- 1School of Preclinical Medicine, Zunyi Medical University, Zunyi, China
- 2Department of Neurosurgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Gastrointestinal Surgery, Chongqing Jiulongpo People's Hospital, Chongqing, China
- 4Department of General Surgery, Xipeng Town Health Center of Jiulongpo District, Chongqing, China
Background and purpose: Serum ferritin, a well-established biomarker of iron status, has been inconsistently linked to stroke risk in previous studies. This meta-analysis aims to systematically evaluate the association between serum ferritin levels and the risk of stroke.
Methods: We conducted a systematic literature search across the PubMed and Embase databases to identify relevant studies. Studies meeting predefined eligibility criteria were selected, and relevant data were extracted. Statistical analysis was performed using Stata 12.0.
Results: Ten studies, including eight longitudinal and three cross-sectional, were included in our meta-analysis. Cross-sectional studies showed that stroke patients had significantly higher serum ferritin levels than controls. Longitudinal studies suggested a 22% increase in stroke risk in individuals with higher serum ferritin. Subgroup analysis indicated that further high-quality population-based cohort studies are warranted to validate these findings. Dose–response meta-analysis confirmed a positive association between serum ferritin levels and stroke risk.
Conclusion: This meta-analysis provides evidence of a positive association between increased serum ferritin levels and stroke incidence. While these results are promising, definitive conclusions cannot be drawn at this time. Therefore, additional robust, prospective cohort studies are imperative to substantiate this relationship.
Introduction
Stroke is a serious cerebrovascular disease. Current data indicates that approximately 12 million people suffer strokes annually, making it one of the most lethal diseases worldwide (1, 2). Although advances in active thrombolytic therapy and surgical interventions have substantially decreased stroke-related mortality, the persistently high incidence of disability and long-term sequelae renders stroke a profoundly debilitating disorder. As a result, preventive strategies have emerged as a paramount focus in stroke management.
Iron is a vital element for human health; however, high concentrations of iron can induce severe cytotoxicity via the Fenton reaction. Furthermore, iron status is closely linked to cardiovascular disease (3–7). Serum ferritin, a key indicator of iron storage/iron level, indirectly reflects whether an individual is experiencing iron deficiency or overload (8–10). This suggests that serum ferritin levels can serve as a proxy for overall health to some extent. However, observational studies have yielded inconsistent findings regarding the association between serum ferritin and stroke risk (11–19). To clarify this relationship, a meta-analysis was conducted aimed at evaluating the quantitative association between serum ferritin levels and the risk of stroke.
Methods
The review was conducted in accordance with the PRISMA guidelines and was prospectively registered with the PROSPERO database of systematic reviews (CRD42024628065).
Literature search
Literature concerning ferritin and stroke was systematically searched using the PubMed and Embase databases. Two thematic categories were combined with the Boolean operator “AND”. The search incorporated the following terms: Ferritins, Iron, Stroke, Cerebrovascular Disorders, Cerebral Hemorrhage, Cerebral Infarction, Subarachnoid Hemorrhage.
Inclusion and exclusion criteria
Literature was included as the following criteria: (1) Studies were included if they provided data on or allowed for the estimation of the association between serum ferritin levels and stroke. (2) Studies were included if they reported risk ratios (RRs) or odds ratios (ORs) with their corresponding 95% confidence intervals (CIs), or if these could be calculated from the reported data. (3) In the longitudinal studies, serum ferritin levels were at minimum categorized into high-level and low-level groups. Literature met following criteria were excluded: (1) Meeting abstracts were excluded due to insufficient data available online. (2) In cases where studies utilized the same participant population cohort, the study with the largest sample size was included, and other studies from this population cohort were excluded. (3) Studies were excluded if they presented stroke data as part of total cardiovascular disease data, without providing sufficient detailed information specific to stroke.
Literature quality assessment
Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess the literature quality. Literature with score equal to 9 were regarded as high quality, otherwise, a score lower than 9 is low quality. The detailed data are supplied in Supplementary Table S1.
Data abstraction
Data were independently extracted by two authors, Zheng and Wang. Any disagreements were resolved through discussion. The data extraction contents contain the following points: The first author’s name, year of publication, sample size, number of cases, mean age at baseline, time of mean follow up, adjusted factors, type of stroke, gender, serum ferritin level and the RRs/ORs. Note on Serum Ferritin Levels: When serum ferritin levels were reported as a range, the average value was estimated assuming a normal distribution.
Statistical analysis
Stata 12.0 was used as statistical analysis software. A fixed effects model was the prior, if met I2 > 50%, indicating a large heterogeneity, use the random effect model instead. Then category analysis was performed by the characteristics (Quality of original literature, adjust risk factors, Sex, mean age at baseline, Race) provides in these literatures. For dose response meta-analysis, linear and a cubic spline model were both used to assess the relationship between serum ferritin and stroke risk. And sensitivity analysis was also used to evaluate the reliability of our results. Due to the results are positive or not often affects the publication of the literature, then the Begg test was used for assessment of publication bias.
Statistical analyses were performed using Stata 12.0. A fixed-effects model was initially employed for meta-analysis. However, if significant heterogeneity was detected (I2 > 50%), a random-effects model was used instead. Subgroup analyses were conducted based on characteristics reported in the included studies, such as study quality, adjusted risk factors, sex, mean age at baseline, and race. For dose–response meta-analysis, a linear and a cubic spline model were applied to investigate the relationship between serum ferritin levels and stroke risk. Sensitivity analyses were performed to evaluate the reliability of our findings. Publication bias was assessed using Begg’s test and Egger’s test, given that the likelihood of publication can be influenced by the direction of the results.
Results
Literature search and study characteristics
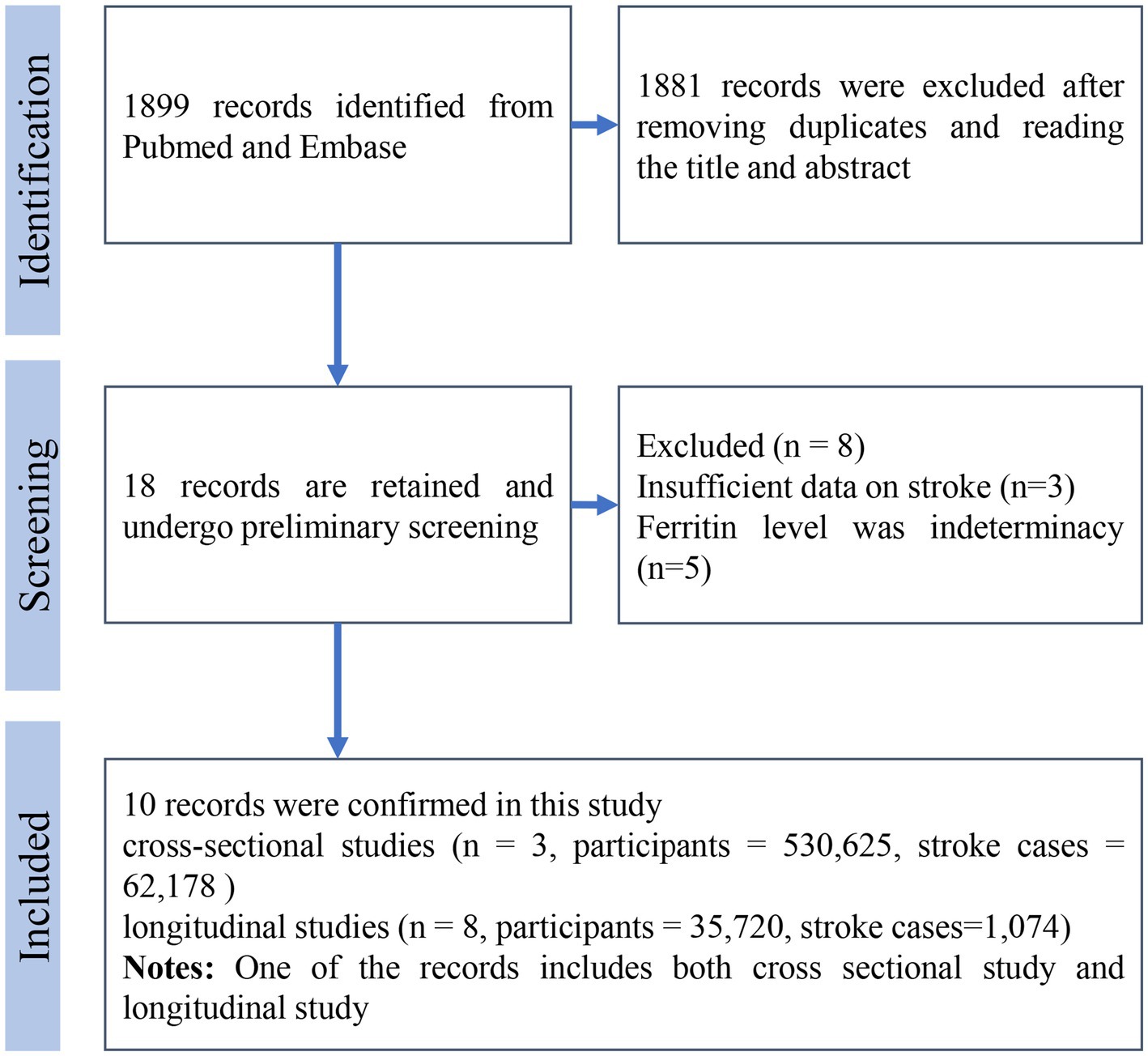
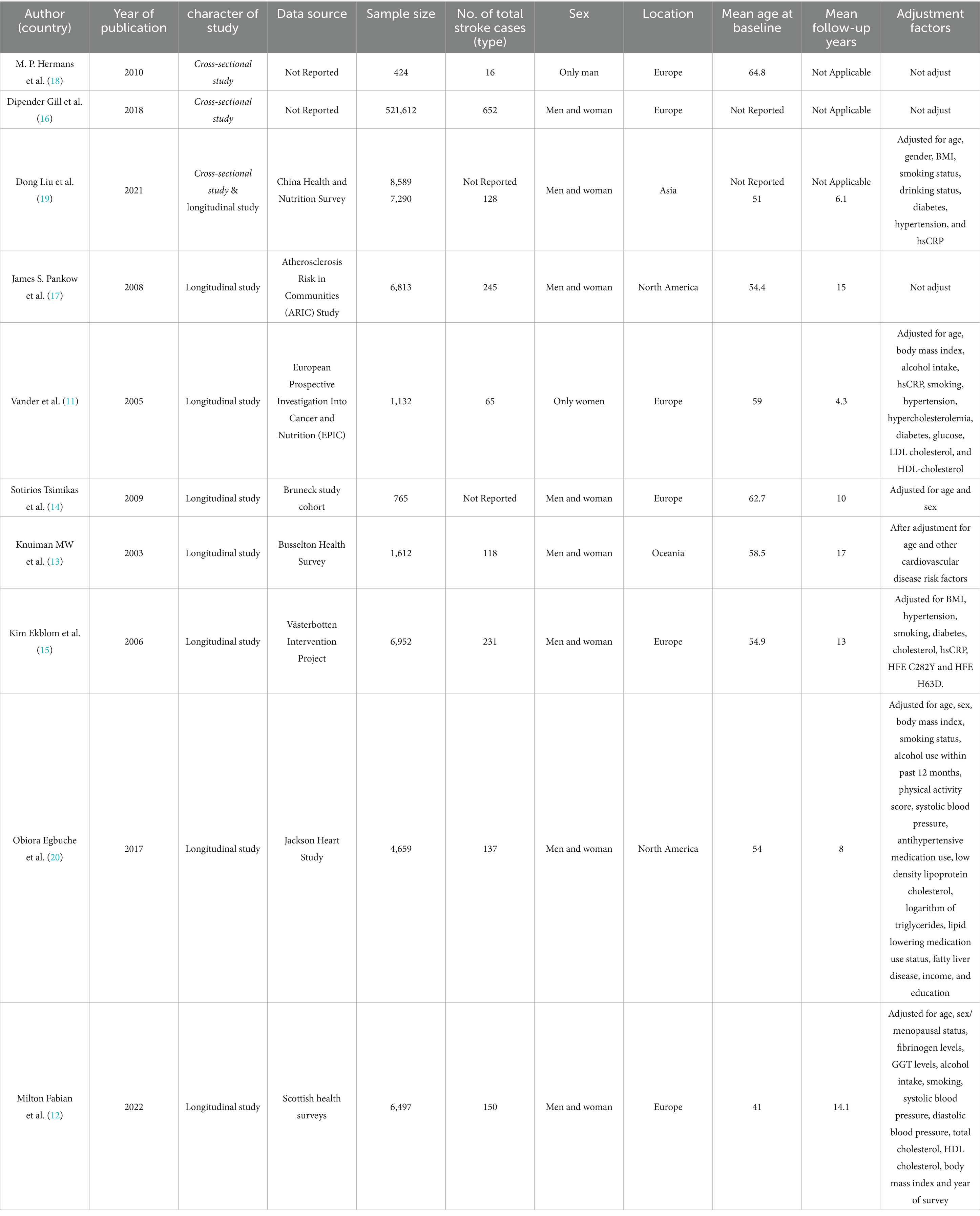
The literature search process is illustrated in Figure 1. Initially, 1899 records were identified through database searching. After two rounds of meticulous screening, 10 literatures were ultimately included in the meta-analysis (11–18, 20, 21). Among these literatures, three were cross-sectional studies and eight were longitudinal studies. The cross-sectional studies involved 530,625 participants, while the longitudinal studies included 35,720 participants. In the cross-sectional studies, there were 62,178 stroke patients, although one study did not report the number of stroke cases. For longitudinal studies, 1,074 cases were reported. The mean age of participants at baseline ranged from 41 to 64.8 years. The mean follow-up time for longitudinal studies varied from 4.3 to 17 years. Seven studies were conducted in Europe, two in North America, and one in Asia. All detailed information is summarized in Table 1.
Results of qualitative analysis
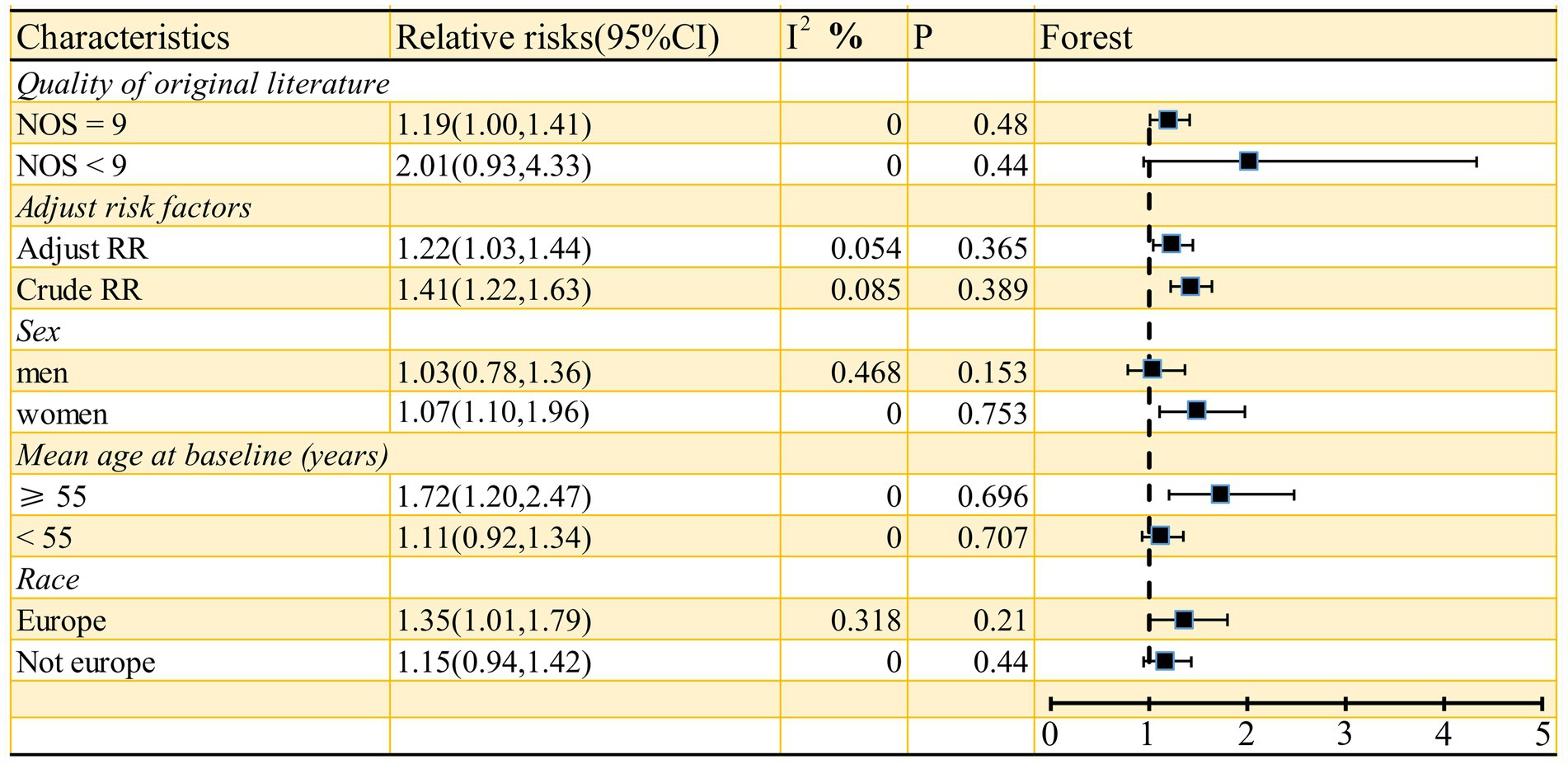
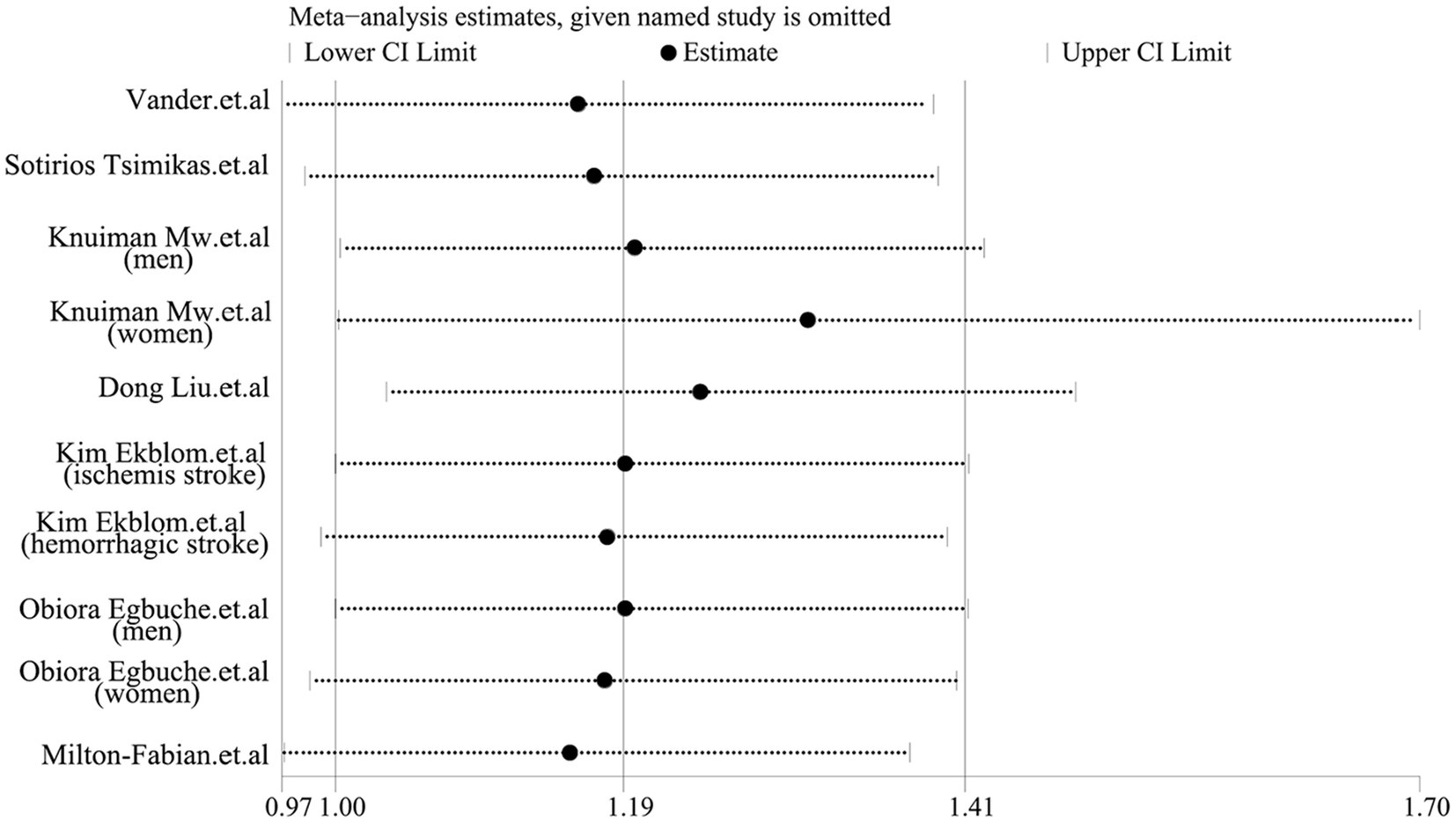
As shown in Figure 2, the pooled odds ratio (OR) for cross-sectional studies was 1.25 (95% CI: 1.11, 1.40), with low heterogeneity (I2 = 29.9%, p = 0.24). A qualitative analysis of longitudinal studies indicated a 22% increased risk of stroke incidence in individuals with high serum ferritin levels (RR = 1.22, 95% CI: 1.03–1.44, I2 = 0%, p = 0.44). Further categorical analyses of longitudinal studies, stratified by study characteristics (quality, adjustment for risk factors, sex, mean age at baseline, and race), yielded inconsistent results, as detailed in Figure 3. Sensitivity analysis (Figure 4) revealed that excluding certain studies resulted in non-significant findings.
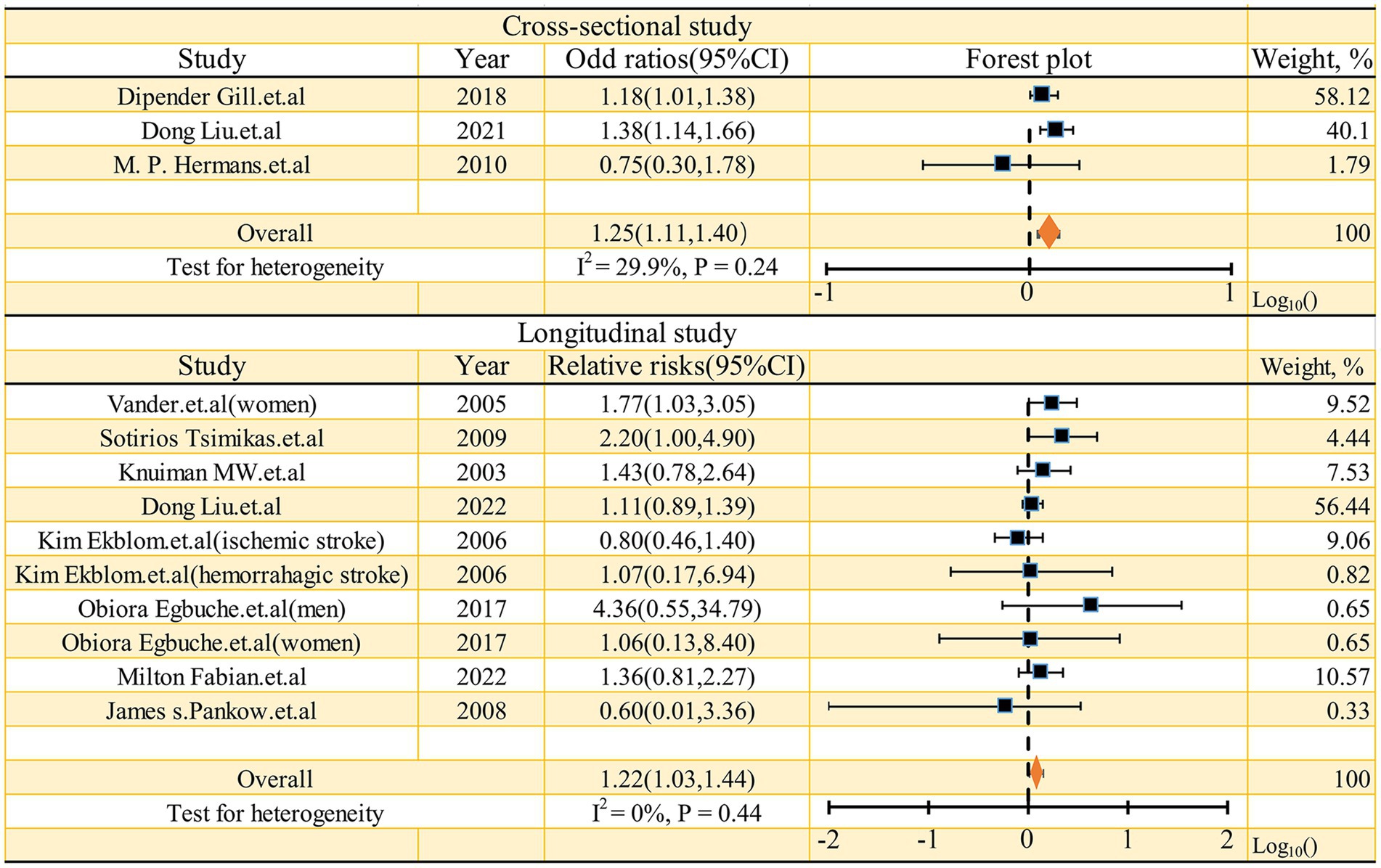
Figure 2. Forest plot of studies examining the association between serum ferritin and risk of stroke.
Dose response meta-analysis
Based on the data provided in the referenced literature, four studies (11–13, 20) were utilized for dose–response meta-analysis. Figure 5 illustrates the quantitative results from longitudinal studies. The cubic spline model analysis suggests an approximate positive correlation between serum ferritin levels and the incidence of stroke (p = 0.1053). The linear quantitative model analysis further revealed that for every 50 ng/mL increase in serum ferritin, the risk of stroke increases by 8% (RR = 1.08, 95% CI 1.01–1.16, p = 0.02). Specifically: A 100 ng/mL increase in serum ferritin corresponds to a 17% increased risk of stroke (RR = 1.17, 95% CI 1.02–1.35); A 150 ng/mL increase corresponds to a 27% increased risk (RR = 1.27, 95% CI 1.03–1.57); A 200 ng/mL increase corresponds to a 38% increased risk (RR = 1.38, 95% CI 1.04–1.83). These findings underscore the potential stroke risk associated with elevated serum ferritin levels and advocate for monitoring and management strategies in clinical settings.
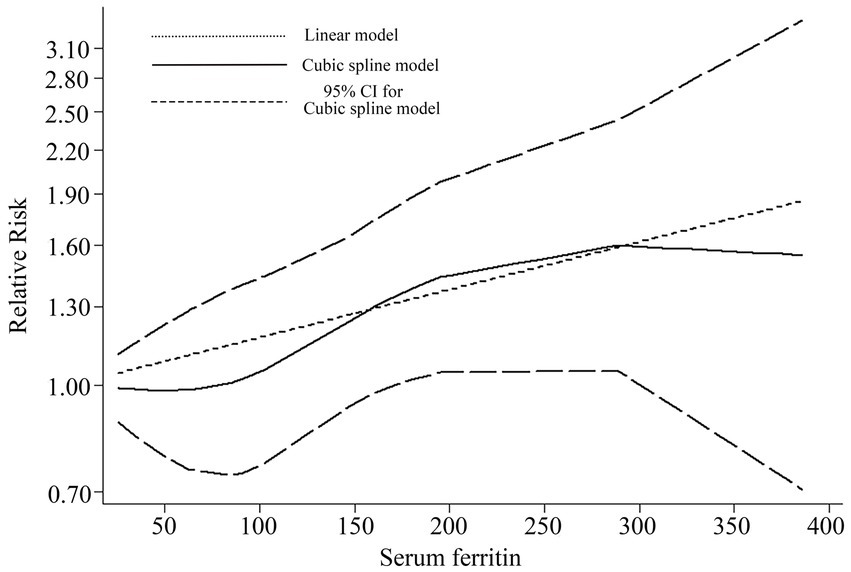
Figure 5. Dose–response for relationship between serum ferritin and risk of stroke by linear and a cubic spline model.
Publication bias
The Begg’s test (p = 0.353 > 0.05) and Egger’s test (p = 0.248 > 0.05) found no obvious publication bias for this meta-analysis (Supplementary Figure S1).
Discussion
As we know, this is the first time that we have evaluated the relationship between serum ferritin and stroke risk through meta-analysis. In this meta-analysis, the results indicated that individuals with high serum ferritin levels may have a higher risk of stroke compared to those with low serum ferritin levels. Furthermore, a dose–response meta-analysis also revealed a positive correlation between serum ferritin and stroke risk. Additionally, the meta-analysis of cross-sectional studies suggested that there may be a close association between serum ferritin and stroke.
Several potential mechanisms underlie this phenomenon. Firstly, numerous clinical studies have demonstrated that serum ferritin levels are closely associated with carotid atherosclerosis, hypertension, diabetes, steatotic liver and other conditions, these factors have been established as risk factors for stroke onset. Therefore, we hypothesize that serum ferritin may indirectly reflect stroke occurrence through its association with carotid atherosclerosis, hypertension, diabetes, steatotic liver and other conditions (22–26). Secondly, as a critical indicator of iron homeostasis, serum ferritin levels are markedly elevated in the context of pathological iron excess. Iron overload can generate reactive oxygen species via the Fenton reaction (27, 28), further triggering inflammatory responses, damaging vascular endothelial cells, and increasing the risk of cardiovascular and cerebrovascular disease (29). Additionally, research has shown that high red meat consumption is linked to cardiovascular diseases (30), and individuals with higher red meat intake typically have elevated serum ferritin levels (31).
Previous meta-analyses have suggested a positive association between ferritin levels and the risk of coronary artery disease (32), given the shared pathophysiology between coronary artery disease and stroke to some extent (As mentioned previously, diseases such as carotid atherosclerosis, hypertension, diabetes, steatotic liver and other conditions), this finding further implies that serum ferritin could serve as a predictive risk factor for stroke occurrence. In our meta-analysis, we pooled three cross-sectional studies, yielding a pooled odds ratio (OR) of 1.25 (95% CI: 1.11, 1.40), with a low level of heterogeneity (I2 = 29.9%, p = 0.24). While cross-sectional studies cannot establish causality, they do suggest a close relationship between serum ferritin levels and stroke. Moreover, the longitudinal studies included in our meta-analysis offer more robust evidence supporting the role of serum ferritin as a predictive risk factor. Meta-analysis of these longitudinal studies demonstrated a positive correlation between serum ferritin levels and stroke risk (high level serum ferritin vs. low level ferritin, RR = 1.22, 95% CI: 1.03–1.44, I2 = 0, p = 0.44). Consequently, we have reason to believe that serum ferritin can be considered as a valuable indicator for predicting the occurrence of stroke.
While this meta-analysis yielded a positive overall result, certain limitations must be acknowledged. Although the total qualitative analysis was positive, the categorical analysis (based on the quality of original literature, adjustment of risk factors, sex, mean age at baseline, and race) for longitudinal studies revealed several negative findings (Figure 3). Moreover, the sensitivity analysis also produced some negative results. These findings suggest that the robustness of our results should be carefully reconsidered, despite the possibility that the negative results stem from the limited number of studies included in the subgroup analysis. Second, we used the highest and lowest serum ferritin groups in the article to conduct the qualitative analysis, but in some literature, the exact serum ferritin level is indeed unclear (14, 15, 19), potentially contributing to a false positive result. Thirdly, according to the cubic spline model dose–response meta-analysis results, the population with extremely low serum ferritin levels (within the 0 to 100 μg/L range) demonstrated a little higher incidence of stroke. Although this finding did not reach statistical significance, several potential explanations warrant consideration. Supporting this, some studies have identified excessively low serum ferritin as a potential risk factor for stroke (11), and a link between iron-deficiency anemia and increased stroke risk has also been reported (33, 34). Therefore, more high-quality cohort studies are needed to determine whether excessively low serum ferritin levels can be used as a reliable predictor of stroke.
Conclusion
This meta-analysis revealed a positive correlation trend between serum ferritin levels and stroke risk. However, the categorical analysis and sensitivity analysis provided less conclusive results. Therefore, further studies are needed to validate and strengthen our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Writing – original draft, Data curation, Formal analysis. YW: Data curation, Methodology, Writing – original draft. ZX: Supervision, Writing – review & editing, Project administration. DL: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1539407/full#supplementary-material
References
1. Zhou, M, Wang, H, Zeng, X, Yin, P, Zhu, J, Chen, W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
3. Cornelissen, A, Guo, L, Sakamoto, A, Virmani, R, and Finn, AV. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine. (2019) 47:598–606. doi: 10.1016/j.ebiom.2019.08.014
4. Wunderer, F, Traeger, L, Sigurslid, HH, Meybohm, P, Bloch, DB, and Malhotra, R. The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacol Res. (2020) 153:104664. doi: 10.1016/j.phrs.2020.104664
5. Winterbourn, CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. (1995) 82–83:969–74. doi: 10.1016/0378-4274(95)03532-x
6. Gillum, RF, Sempos, CT, Makuc, DM, Looker, AC, Chien, CY, and Ingram, DD. Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I epidemiologic Followup study. National Health and nutrition examination survey. Am J Epidemiol. (1996) 144:59–68. doi: 10.1093/oxfordjournals.aje.a008855
7. Corti, MC, Gaziano, M, and Hennekens, CH. Iron status and risk of cardiovascular disease. Ann Epidemiol. (1997) 7:62–8. doi: 10.1016/s1047-2797(96)00112-3
8. Daru, J, Colman, K, Stanworth, SJ, De La Salle, B, Wood, EM, and Pasricha, SR. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr. (2017) 106:1634S–9S. doi: 10.3945/ajcn.117.155960
9. Knovich, MA, Storey, JA, Coffman, LG, Torti, SV, and Torti, FM. Ferritin for the clinician. Blood Rev. (2009) 23:95–104. doi: 10.1016/j.blre.2008.08.001
10. Garcia-Casal, MN, Pasricha, SR, Martinez, RX, Lopez-Perez, L, and Pena-Rosas, JP. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst Rev. (2021) 2021:CD011817. doi: 10.1002/14651858.CD011817.pub2
11. van der, AD, Grobbee, DE, Roest, M, Marx, JJ, Voorbij, HA, and van der Schouw, YT. Serum ferritin is a risk factor for stroke in postmenopausal women. Stroke. (2005) 36:1637–41. doi: 10.1161/01.STR.0000173172.82880.72
12. Suarez-Ortegon, MF, McLachlan, S, Fernandez-Real, JM, Tuomainen, TP, Aregbesola, A, and Wild, SH. Serum ferritin and incident cardiometabolic diseases in Scottish adults. Cardiovasc Diabetol. (2022) 21:26. doi: 10.1186/s12933-022-01450-7
13. Knuiman, MW, Divitini, ML, Olynyk, JK, Cullen, DJ, and Bartholomew, HC. Serum ferritin and cardiovascular disease: a 17-year follow-up study in Busselton, Western Australia. Am J Epidemiol. (2003) 158:144–9. doi: 10.1093/aje/kwg121
14. Tsimikas, S, Willeit, J, Knoflach, M, Mayr, M, Egger, G, Notdurfter, M, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J. (2009) 30:107–15. doi: 10.1093/eurheartj/ehn502
15. Ekblom, K, Hultdin, J, Stegmayr, B, Johansson, I, Van Guelpen, B, Hallmans, G, et al. Iron stores and HFE genotypes are not related to increased risk of ischemic stroke. A prospective nested case-referent study. Cerebrovasc Dis. (2007) 24:405–11. doi: 10.1159/000108429
16. Gill, D, Monori, G, Tzoulaki, I, and Dehghan, A. Iron status and risk of stroke. Stroke. (2018) 49:2815–21. doi: 10.1161/STROKEAHA.118.022701
17. Pankow, JS, Boerwinkle, E, Adams, PC, Guallar, E, Leiendecker-Foster, C, Rogowski, J, et al. HFE C282Y homozygotes have reduced low-density lipoprotein cholesterol: the atherosclerosis risk in communities (ARIC) study. Transl Res. (2008) 152:3–10. doi: 10.1016/j.trsl.2008.05.005
18. Hermans, MP, Ahn, SA, Amoussou-Guenou, KD, Balde, NM, and Rousseau, MF. Do high ferritin levels confer lower cardiovascular risk in men with type 2 diabetes? Diabet Med. (2010) 27:417–22. doi: 10.1111/j.1464-5491.2010.02979.x
19. Liu, D, Zhang, Y, Wang, CC, Xiao-Hong, E, and Zuo, H. Markers of iron metabolism and stroke risk: cross-sectional and longitudinal findings from the China health and nutrition survey (CHNS). Iran J Public Health. (2022) 51:115–23. doi: 10.18502/ijph.v51i1.8302
20. Egbuche, O, Millard, HR, Renelus, B, Maihemuti, A, Musani, SK, Fox, ER, et al. Serum ferritin levels in blacks without known cardiovascular disease (from the Jackson heart study). Am J Cardiol. (2017) 120:1533–40. doi: 10.1016/j.amjcard.2017.07.045
21. Luo, D, Zhong, Z, Qiu, Y, Wang, Y, Li, H, Lin, J, et al. Abnormal iron status is associated with an increased risk of mortality in patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. (2021) 31:1148–55. doi: 10.1016/j.numecd.2020.12.018
22. Xu, H, Song, Y, Xu, J, Gu, Y, Zhang, Q, Liu, L, et al. Increased serum ferritin levels are independently associated with carotid atherosclerosis in women. Br J Nutr. (2017) 117:1623–30. doi: 10.1017/S0007114517001544
23. Ryoo, JH, Kim, SY, Oh, CM, Park, SK, Kim, E, Park, SJ, et al. The incidental relationship between serum ferritin levels and hypertension. Int J Cardiol. (2015) 183:258–62. doi: 10.1016/j.ijcard.2014.10.152
24. Choi, B, Yeum, KJ, Park, SJ, Kim, KN, and Joo, NS. Elevated serum ferritin and mercury concentrations are associated with hypertension; analysis of the fourth and fifth Korea national health and nutrition examination survey (KNHANES IV-2, 3, 2008-2009 and V-1, 2010). Environ Toxicol. (2015) 30:101–8. doi: 10.1002/tox.21899
25. Diaz-Lopez, A, Iglesias-Vazquez, L, Palleja-Millan, M, Rey Renones, C, Flores Mateo, G, and Arija, V. Association between iron status and incident type 2 diabetes: a population-based cohort study. Nutrients. (2020) 12:3249. doi: 10.3390/nu12113249
26. Yan, J, Guan, T, Guo, M, and Liu, J. Serum ferritin and non-alcoholic fatty liver disease: a meta-analysis and systematic review. Turk J Gastroenterol. (2023) 34:952–60. doi: 10.5152/tjg.2023.22453
27. Grant, ES, Clucas, DB, McColl, G, Hall, LT, and Simpson, DA. Re-examining ferritin-bound iron: current and developing clinical tools. Clin Chem Lab Med. (2021) 59:459–71. doi: 10.1515/cclm-2020-1095
28. Liu, K, Huang, L, Qi, S, Liu, S, Xie, W, Du, L, et al. Ferroptosis: the entanglement between traditional drugs and Nanodrugs in tumor therapy. Adv Healthc Mater. (2023) 12:e2203085. doi: 10.1002/adhm.202203085
29. Petrucci, G, Rizzi, A, Hatem, D, Tosti, G, Rocca, B, and Pitocco, D. Role of oxidative stress in the pathogenesis of atherothrombotic diseases. Antioxidants (Basel). (2022) 11:1408. doi: 10.3390/antiox11071408
30. Kaluza, J, Wolk, A, and Larsson, SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke. (2012) 43:2556–60. doi: 10.1161/STROKEAHA.112.663286
31. Quintana Pacheco, DA, Sookthai, D, Wittenbecher, C, Graf, ME, Schubel, R, Johnson, T, et al. Red meat consumption and risk of cardiovascular diseases-is increased iron load a possible link? Am J Clin Nutr. (2018) 107:113–9. doi: 10.1093/ajcn/nqx014
32. Zhou, Y, Liu, T, Tian, C, Kang, P, and Jia, C. Association of serum ferritin with coronary artery disease. Clin Biochem. (2012) 45:1336–41. doi: 10.1016/j.clinbiochem.2012.06.013
33. Chang, YL, Hung, SH, Ling, W, Lin, HC, Li, HC, and Chung, SD. Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS One. (2013) 8:e82952. doi: 10.1371/journal.pone.0082952
Keywords: serum ferritin, stroke, meta-analysis, cerebrovascular accident, iron
Citation: Zheng W, Wang Y, Xia Z and Liu D (2025) Serum ferritin and risk of stroke: a meta-analysis of observation studies. Front. Neurol. 16:1539407. doi: 10.3389/fneur.2025.1539407
Edited by:
Anna Bersano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Umesh Kumar, University of Innsbruck, AustriaAbhilasha Shiv Chandra Singh, Massachusetts General Hospital and Harvard Medical School, United States
Shihua Shi, ETH Zürich, Switzerland
Shravan Sivakumar, Beth Israel Lahey Health, United States
Copyright © 2025 Zheng, Wang, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuwei Xia, OTM1MzA3MzMzQHFxLmNvbQ==; Dengliang Liu, MTMzNDAyMTA0ODJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wen Zheng1†
Wen Zheng1† Yangyang Wang
Yangyang Wang