- Department of Neurology, The Affiliated Chaohu Hospital of Anhui Medical University, Hefei, China
Background: Parkinson’s disease (PD) is a prevalent neurodegenerative condition that has significant effects on public health. This study examines the relationship between the family poverty income ratio (PIR) and the prevalence of PD among American adults aged ≥40 years.
Methods: This study leverages data from eight consecutive cycles (2003–2004 to 2017–2020) of the National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional surveillance program that employs stratified multistage probability sampling. The analytical cohort comprises 30,039 U.S. adults aged ≥40 years after applying exclusion criteria. To investigate the relationships between PD, PIR, and other covariates, weighted univariable logistic regression is utilized. The association between PIR and PD is then further assessed using weighted multivariable logistic regression. The possible linear or nonlinear character of this association is investigated using smoothed curve fitting. To evaluate the consistency of the association between PIR and PD across different clinical and demographic subgroups, subgroup analyses are conducted.
Results: A total of the 30,039 participants in the study, 14,743 are men (49.08%) and 15,296 are women (50.92%). With an odds ratio of 0.83 (95% confidence interval: 0.75–0.91, p = 0.0003), PIR was found to be negatively associated with PD after controlling for all other variables. Subgroup analyses are stratified by gender, marital status, body mass index, diabetes, hypertension, stroke, and smoking status. These analyses reveal no statistically significant inverse association between the PIR and PD. However, race, age, and educational attainment exhibit significant interaction effects (p for interaction < 0.05), suggesting these variables may influence the PIR-PD relationship.
Conclusion: Among American adults aged ≥40, this study shows a significant inverse relationship between PIR and the prevalence of PD. The results highlight how socioeconomic status may have an impact on the emergence of neurodegenerative diseases. To fully understand the intricate relationship between socioeconomic factors and PD, more extensive prospective studies are necessary.
1 Introduction
Parkinson’s disease (PD) is a common neurodegenerative condition that mainly affects people over 65 and whose incidence gradually rises with age (1). It is projected that more than 12 million people worldwide will have PD by 2040 (2). Significantly, PD is not just found in the elderly; a sizable portion of people under 50 are also diagnosed with the illness (2). Degeneration of dopaminergic neurons in the substantia nigra and the buildup of misfolded proteins in Lewy bodies and Lewy neurites are hallmarks of PD pathophysiology (3). Both motor and non-motor symptoms can be seen in the clinical presentation of PD. Resting tremor, bradykinesia, and muscle rigidity are examples of motor symptoms, whereas sleep disturbances, autonomic dysfunction, neuropsychological and cognitive impairments, gastrointestinal problems, visual disturbances, and exhaustion are examples of non-motor symptoms (4).
PD has become more common as the world’s population ages, placing a heavy financial strain on families and society as a whole. PD is acknowledged as a significant public health concern in lower-income populations, despite the fact that it is frequently thought to be more common in populations with higher socioeconomic status (SES) (5). SES and disease risk are strongly correlated, with those with lower SES typically having higher risk because of their lifetime exposure to harmful health determinants (6). Cardiometabolic, respiratory, psychiatric, and musculoskeletal disorders are more common in people with lower socioeconomic status (7). Household income is frequently used to measure SES and has a significant impact on health outcomes. Research has indicated that neurological development may be negatively impacted by lower socioeconomic status and limited income (8).
In addition, family income has been linked to neurological condition susceptibility: people from high-income households are less likely to suffer from ischemic stroke and Alzheimer’s disease, but there are no significant correlations between income and the risk of cerebral aneurysm, epilepsy, intracranial hematoma, or subarachnoid hemorrhage (9). PD has a complex etiology that involves interactions between lifestyle, environmental, and genetic factors. Unhealthy eating habits, insufficient nutritional intake, obesity, physical inactivity, and lifestyle choices like smoking may all contribute to the high burden of PD-related disability in less developed nations (10, 11). Higher education levels seemed to lower the risk, while diabetes, thyroid dysfunction, and gender were linked to increased risk in a survey of 24,000 Italian citizens looking into risk factors for PD. Age, coffee intake, daily physical activity, and hypertension are just a few of the numerous influencing factors that may have complex or nonlinear effects on the onset of PD (12). We used data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2020 to examine the relationship between PD and the PIR in a sizable, representative sample of American adults aged 40 and over, considering the paucity of cross-sectional studies evaluating this relationship.
2 Method
2.1 Sources of data
The NHANES, a program created to evaluate the dietary patterns and physical health of the American people through physical examinations and interviews conducted every two years, provided the data for this study. The National Center for Health Statistics’ (NCHS) Research Ethics Review Board authorized the NHANES study, and all participants gave their informed consent in compliance with the Declaration of Helsinki’s principles.
2.2 Study and design
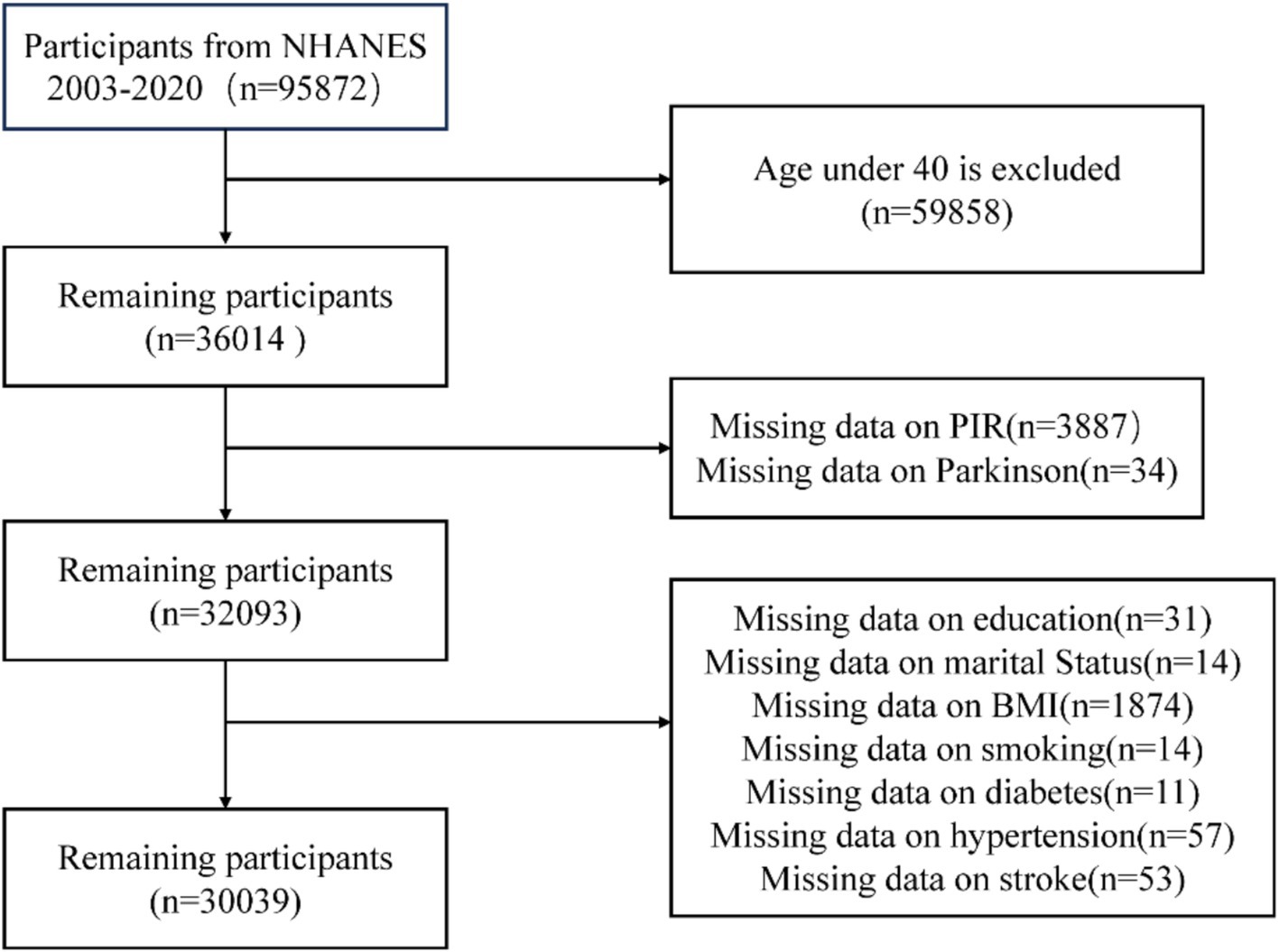
NHANES data from 2003 to 2020, which included an initial sample of 95,872 participants, were used in this cross-sectional study. Participants under 40 (n = 59,858), those with missing PIR data (n = 3,887), those with missing information about the diagnosis of PD (n = 34), those without educational records (n = 31), those without marital status records (n = 14), those without BMI records (n = 1,874), those without smoking history records (n = 14), and those without diabetes (n = 11), hypertension (n = 57), and stroke (n = 54) were among the exclusion criteria. 30,039 participants were left for the final analysis after these exclusions were applied (Figure 1).
2.3 Study variables
The use of “anti-Parkinsonian drugs” recorded in the prescription data was used to determine whether PD was present (13, 14). Only those receiving anti-Parkinsonian medication treatment were classified as having PD; those not receiving such treatment were classified as not having the disease. This identification method is limited by the specific medications and code available in the NHANES dataset. Calculated by dividing family (or individual) income by the appropriate poverty threshold for the survey year, the family income to poverty ratio (PIR) served as a gauge of socioeconomic status. Low income (PIR < 1), middle income (PIR 1–4), and high income (PIR > 4) were the three PIR levels (15).
2.4 Covariates
Gender, age, marital status (divorced, cohabiting, married, unmarried, separated, or widowed), ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other ethnicities), and educational attainment (less than 9th grade, 9th to 11th grade, high school graduate, college, or postsecondary education) were among the covariates in this study. Weight in kilograms divided by height in meters squared (kg/m2) was used to calculate the body mass index (BMI). Self-reported answers to the question, “Have you smoked at least 100 cigarettes in your lifetime?” were used to determine smoking status. Self-reports of having been informed by a healthcare professional that the respondent had diabetes, hypertension, or stroke were used to identify diagnoses.
2.5 Statistical analysis
Categorical variables were reported as frequency and percentage, and continuous variables as mean ± standard deviation. The relationship between PIR and PD was examined using a weighted multivariate logistic regression model, which included smoothed curve fitting to examine any possible nonlinear relationships. Three models were created: Model 1 was left unaltered, Model 2 was modified to account for age, gender, and race, and Model 3 was further modified to account for diabetes, hypertension, stroke, education, BMI, marital status, and smoking status. Likelihood ratio tests were used to analyze interactions. The formula for the base model was PD ~ PIR + demographic characteristics + health indicators + age + interaction term. Sex, race, marital status, and education level were among the demographic variables, and BMI, smoking, stroke, diabetes, and hypertension status were among the health indicators. To evaluate possible effect modification across age, sex, BMI, diabetes, hypertension, stroke, and smoking status categories, subgroup analyses were then conducted. R software (version 4.4.1) and EmpowerStats (version 4.0) were used for all statistical analyses, and a p value < 0.05 was deemed statistically significant.
3 Results
3.1 Demographic characteristics of participants
A total of the 30,039 participants in the statistical analysis, 389 had a diagnosis of PD, while 29,650 were categorized as participants without PD. PD patients were more likely to be older, female, have a higher body mass index, be non-Hispanic White, and have a lower family income than non-PD patients (Table 1).
3.2 Univariate regression analysis between PIR and PD
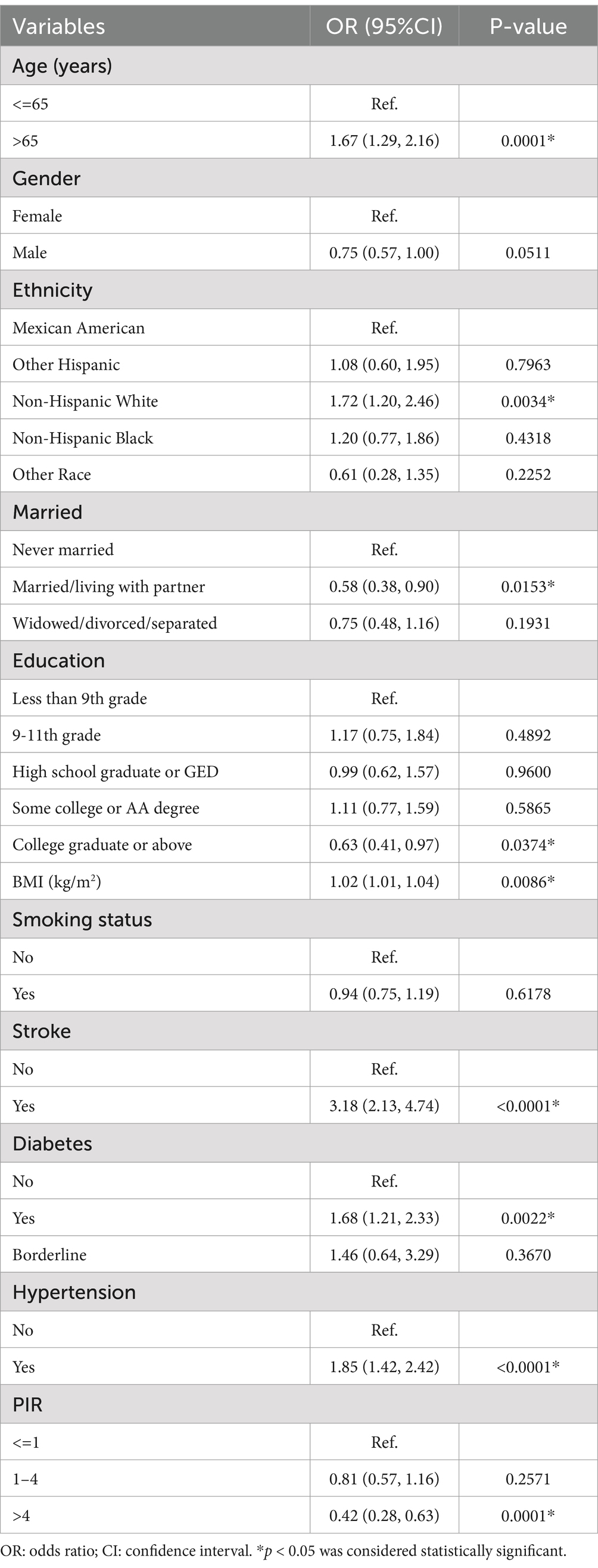
Significant correlations were found between PD and a number of variables in the entire study population, including age, non-Hispanic White ethnicity, marital status (married or cohabiting), higher educational attainment (college and above), BMI, stroke, diabetes mellitus, hypertension, and income level (p < 0.05). The prevalence of PD was positively correlated with age [OR = 1.67, 95% CI: (1.29, 2.16)]. The odds of PD were significantly higher for patients with a history of stroke or diabetes than for those without these conditions [OR = 3.18, 95% CI: (2.13, 4.74) and OR = 1.68, 95% CI: (1.21, 2.33), respectively]. Likewise, PD risk was higher in hypertensive individuals than in non-hypertensive individuals [OR = 1.85, 95% CI: (1.42, 2.42)]. On the other hand, those with greater incomes had a noticeably lower risk of PD [OR = 0.42, 95% CI: (0.28, 0.63)]. (Table 2).
3.3 Multivariate regression analysis of the relationship between PIR and PD
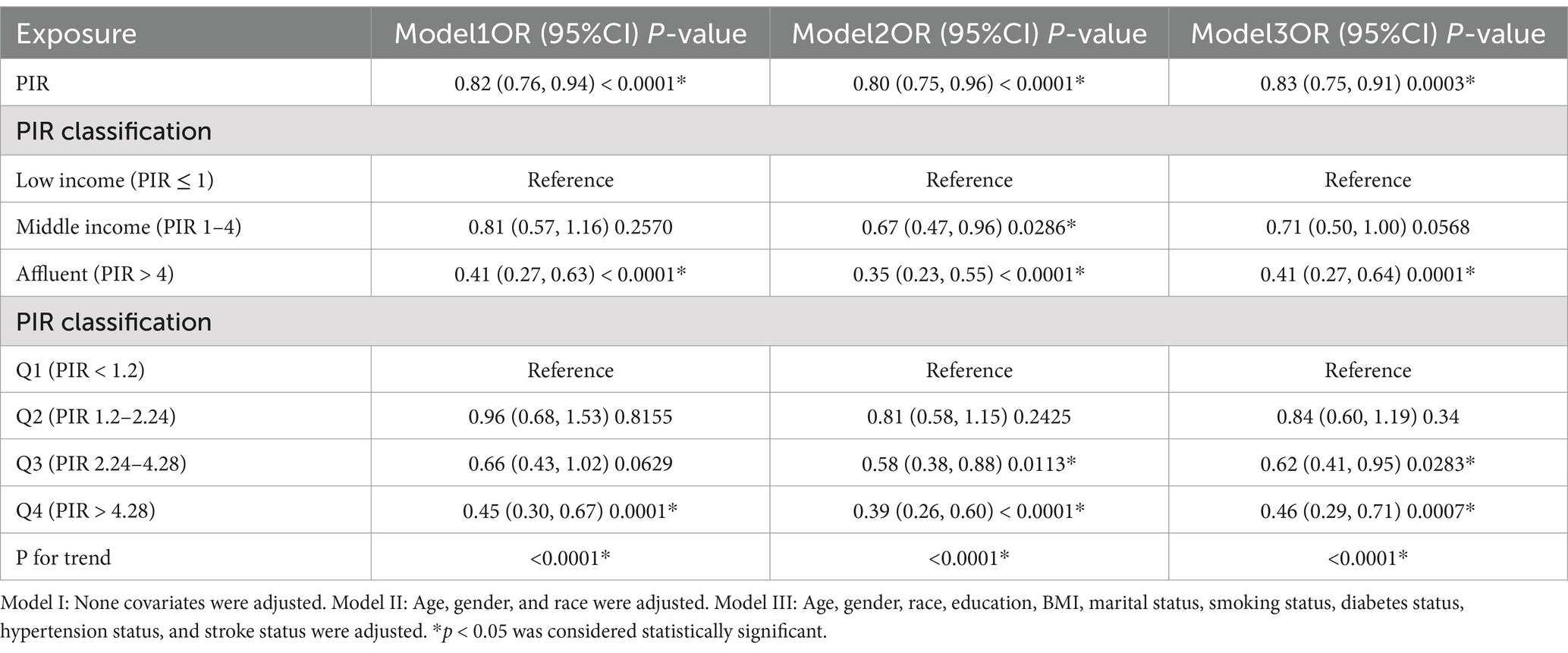
All three models weighted multivariate logistic regression analysis showed a consistent negative correlation between PIR and PD. The odds ratio was 0.82 [95% CI: (0.76–0.94)] in Model 1 (unadjusted); 0.80 [95% CI: (0.75–0.96)] in Model 2 (adjusted for age, gender, and race); and 0.83 [95% CI: (0.75–0.91)] in Model 3 (fully adjusted for age, gender, race, education, BMI, marital status, smoking, diabetes, hypertension, and stroke). The high-income group had a significantly lower risk of PD according to additional analysis that treated PIR as a categorical variable (PIR > 4). Participants in Q2 (PIR 1.2–2.24), Q3 (PIR 2.24–4.28), and Q4 (PIR > 4.28) all displayed a statistically significant inverse association with PD when PIR was split into quartiles, in contrast to those in Q1 (PIR < 1.2) (Table 3).
3.4 Subgroup analysis
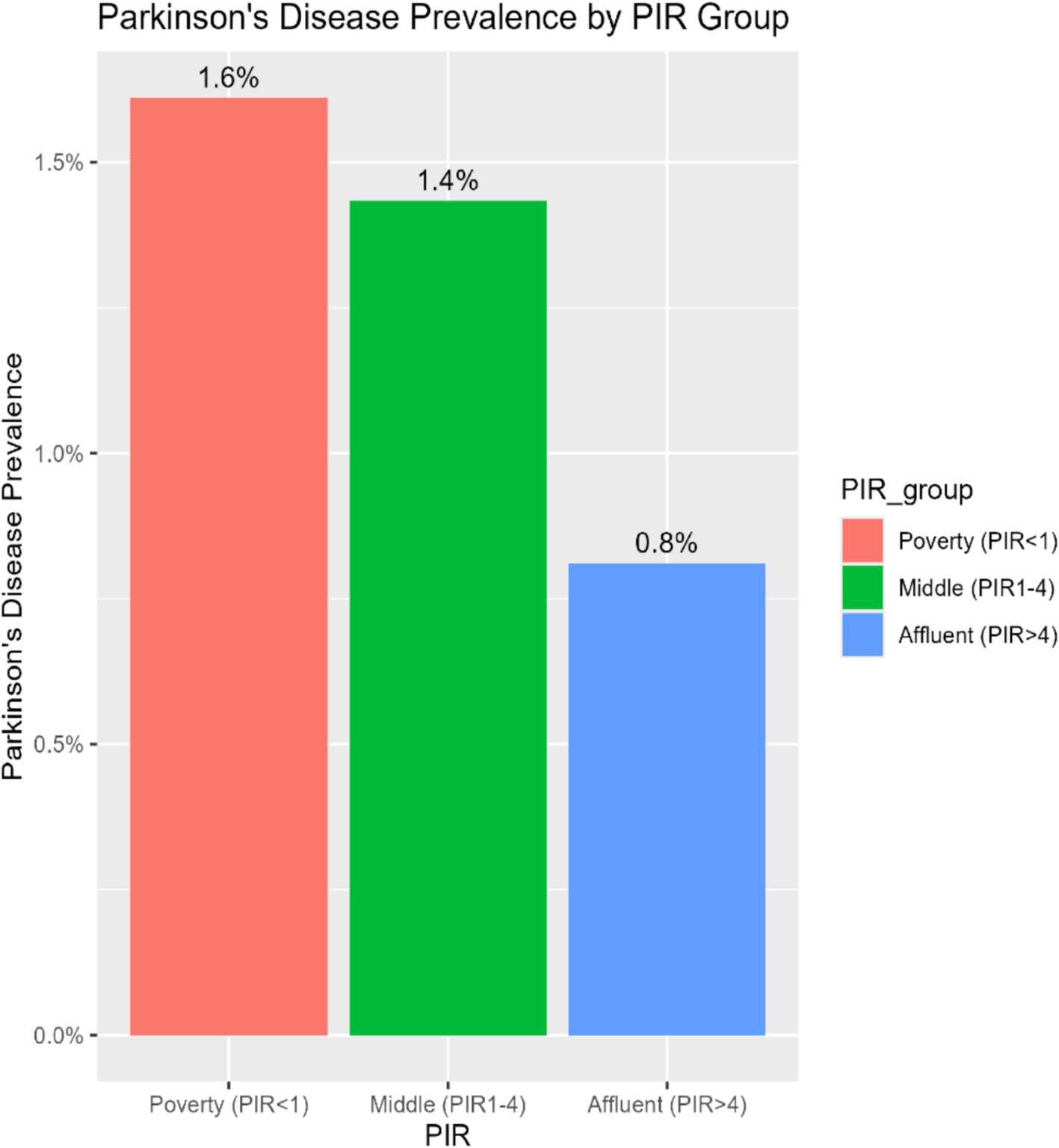
To investigate the consistency of the relationship between PIR and PD across various demographic and clinical contexts, subgroup stratification was carried out based on gender, age, race, education level, marital status, BMI, diabetes, hypertension, stroke, and smoking status. Within the subgroups based on gender, marital status, BMI, diabetes, hypertension, stroke, and smoking, the inverse relationship between PIR and PD was not statistically significant. However, there were notable interactions with age, education level, and race (p for interaction < 0.05). Among age-stratified groups, middle-aged adults (40–65 years) and older adults (>65 years) had a 16 and 14% lower risk of PD, respectively, for every unit increase in PIR. The middle-aged subgroup showed a stronger negative correlation between PIR and PD (p = 0.017) (Figure 2). A nonlinear inverse relationship between PIR and PD was also discovered through the application of a smoothed curve-fitting analysis (Figure 3). Finally, Figure 4 shows the prevalence of PD by socioeconomic group according to PIR: the highest prevalence (1.6%) was found in the lowest income group (PIR < 1), followed by the middle-income group (PIR 1–4) at 1.5%, and the lowest prevalence (0.5%) was found in the highest income group (PIR > 4).
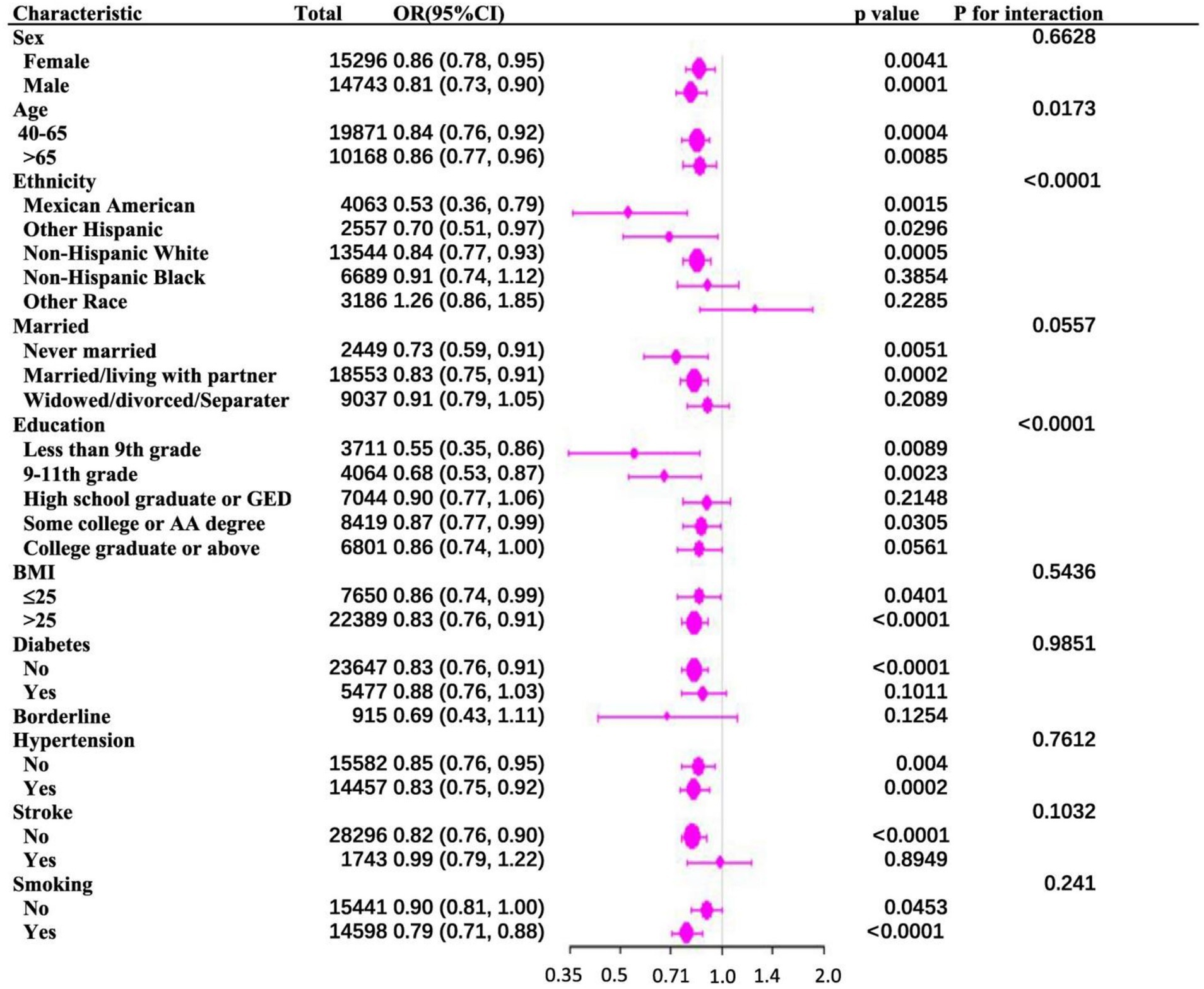
Figure 2. (A) Subgroup analysis of the association between family PIR and Parkinson’s disease. (B) The above model adjusted for age, gender, race, education, BMI, marital status, smoking status, diabetes status, hypertension status, and stroke status.

Figure 3. (A) Smooth curve fittings of the association between PIR and Parkinson’ disease. (B) The associations were adjusted for age, gender, race, education, BMI, marital status, smoking status, diabetes status, hypertension status, and stroke status.
4 Discussion
This study aimed to investigate the correlation between the PIR and the incidence of PD in individuals aged 40 and above in the United States. This cross-sectional analysis of 30,039 participants revealed that individuals with elevated PIR values were significantly less likely to develop PD. A strong inverse correlation between PIR and PD was observed, remaining stable across fully adjusted models. Subgroup analyses indicated significant interactions between PIR and PD concerning race, age, and educational attainment; however, no significant interaction effects were found for gender, marital status, BMI, diabetes, hypertension, stroke, or smoking status.
PD is affected by various environmental and biological factors. Epidemiological studies have shown that exposure to insecticides, low-frequency magnetic fields, solvents, heavy metals, chemical agents, and air pollutants represents significant risk factors for PD, especially in developing and middle-income nations. These exposures may elicit pathological alterations in alpha-synuclein proteins within the olfactory and gastrointestinal systems, which subsequently disseminate through parasympathetic and sympathetic neural pathways, ultimately resulting in the onset of Lewy body disorders and PD (16, 17). In addition, the advancement of PD can be intensified by risk factors including inadequate access to medical resources, inequities in healthcare provision, detrimental lifestyle choices, and psychosocial stressors (18). Socioeconomic disparities may induce chronic psychological stress, which can impair 5-hydroxytryptaminergic transmission and noradrenergic activity in brain areas such as the prefrontal cortex, striatum, and hippocampus, thus influencing the pathophysiology of PD (19).
Socioeconomic disparities can profoundly influence disease risk among populations. Individuals from affluent households may experience advantages from prompt identification and management of disease symptoms, whereas those from economically disadvantaged groups may encounter increased risk due to restricted awareness, healthcare accessibility, and preventive resources (20). However, previous research regarding the correlation between household income and the prevalence of PD has yielded inconsistent results (10). One study indicated a reduced prevalence of PD among individuals with lower household incomes, in contrast to another investigation that found a significantly lower PIR in individuals with PD compared to those without the condition (2.68 ± 0.12), implying a negative correlation between PIR and PD. Multivariable regression analyses, controlled for potential confounders, further corroborated this inverse relationship. Lisa et al. documented analogous results in a Canadian cohort, indicating that lower SES correlated with elevated prevalence and incidence rates of PD. Urban low-income populations demonstrated a greater disease burden than their high-income counterparts (21). A Korean national cohort study similarly revealed that diminished individual-level socioeconomic status, characterized by income and health insurance type, was inversely correlated with PD mortality, while regional socioeconomic indicators, such as residential area, showed no significant association with PD mortality (22).
The stratified analysis of this study revealed the association between educational attainment and PD risk. The data demonstrated a statistically significant reduction in PD prevalence among individuals with university-level or higher education compared to those with lower educational levels (p < 0.05). Existing literature suggests that higher education may influence disease progression through dual neuroprotective mechanisms: first, cognitive reserve acquired through education may delay clinical manifestation by enhancing neural compensatory functions (23); second, neuroimaging evidence indicates that prolonged educational experience can induce structural neuroplasticity, while epigenomic studies suggest its potential regulatory effect on CGG repeat expansion in fragile X messenger ribonucleoprotein 1 (FMR1) gene, thereby enhancing neuronal resistance to degenerative processes (24, 25). Moreover, education may exert indirect neuroprotective effects by modulating lifestyle and socioeconomic status – individuals with higher educational attainment exhibit less severe motor dysfunction. This enhanced clinical resilience to PD pathology-induced motor impairments likely originates from the synergistic effects of multiple education-associated mechanisms, including strengthened synaptic plasticity, optimized brain network efficiency, and activation of neural regenerative mechanisms (26). However, the precise dose–response relationship between educational attainment and PD risk, along with its mechanistic pathways, requires validation through multicenter prospective cohort studies. In age-stratified analysis, the incidence rate showed a progressive increase among patients aged over 65 years (27), potentially associated with age-related blood–brain barrier dysfunction and cumulative mitochondrial impairment (28). No statistically significant interactions were observed in subgroups stratified by sex, BMI, marital status, diabetes, hypertension, stroke, or smoking status, possibly due to insufficient statistical power to detect moderate effect sizes in current analysis, as well as subgroup heterogeneity and confounding factors. Longitudinal studies have indicated that smoking, alcohol consumption, and high cholesterol exhibit protective effects against PD, while no association was observed between BMI and PD risk (29). The relationship between diabetes and PD remains controversial. Diabetes and PD may share common cellular mechanisms, including mitochondrial dysfunction and underexpression of the transcriptional regulator PPARγ coactivator 1α (PGC1α), which impairs mitochondrial biogenesis and respiratory function (29).
This research possesses multiple limitations. First, as a cross-sectional study, the study precludes causal inference regarding the observed association between PIR and PD. The temporality between socioeconomic exposure and PD onset cannot be ascertained, and potential mechanistic pathways remain speculative. Additionally, participants were diagnosed with PD solely based on whether they were taking Parkinson’s medication. This approach lacks diagnostic specificity, as antiparkinsonian drugs are prescribed for other movement disorders, potentially inflating false-positive rates. Conversely, untreated early-stage PD cases or those with atypical presentations may be systematically excluded, leading to outcome misclassification and attenuated effect estimates. Finally, as an independent socioeconomic indicator, the PIR has limitations in accurately reflecting an individual’s specific likelihood of disease prevalence. PIR does not account for regional cost-of-living variations or temporal income fluctuations, limiting its granularity in characterizing individual-level socioeconomic vulnerability. Our findings reveal a notable nonlinear inverse relationship between PIR and PD, indicating that PIR may serve as a predictive tool for identifying populations at elevated risk for the condition.
5 Conclusion
PIR is strongly correlated with the occurrence of PD in individuals aged over 40 in the United States. A diminished PIR is associated with a heightened probability of developing PD, suggesting that PIR may function as a significant predictive indicator for the risk of disease onset. These findings underscore the potential benefits of enacting proactive measures to alleviate disease progression, including elevating income levels in economically disadvantaged areas and augmenting early screening and detection initiatives for PD.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CL: Writing – original draft, Writing – review & editing, Data curation, Methodology, Project administration, Software. JH: Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We express our gratitude to the Foundation of Chaohu Hospital Affiliated to Anhui Medical University (ZQN202301) for its support of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PIR, Poverty income ratio; PD, Parkinson’s disease; NHANES, National Health and Nutrition Examination Survey; BMI, Body mass index; SES, Socioeconomic status.
References
1. Chhetri, JK, Mei, S, Wang, C, and Chan, P. New horizons in Parkinson’s disease in older populations. Age Ageing. (2023) 52:1–10. doi: 10.1093/ageing/afad186
2. Darweesh, SKL, Raphael, KG, Brundin, P, Matthews, H, Wyse, RK, Chen, H, et al. Parkinson Matters. J Parkinsons Dis. (2018) 8:495–8. doi: 10.3233/jpd-181374
3. Chu, Y, Hirst, WD, Federoff, HJ, Harms, AS, Stoessl, AJ, and Kordower, JH. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain. (2024) 147:444–57. doi: 10.1093/brain/awad388
4. Dovonou, A, Bolduc, C, Soto Linan, V, Gora, C, PeraltaIii, MR, and Lévesque, M. Animal models of Parkinson’s disease: bridging the gap between disease hallmarks and research questions. Transl Neurodegener. (2023) 12:12–36. doi: 10.1186/s40035-023-00368-8
5. Ou, Z, Pan, J, Tang, S, Duan, D, Yu, D, Nong, H, et al. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories from 1990 to 2019. Front Public Health. (1990) 9:9. doi: 10.3389/fpubh.2021.776847
6. Ben-Shlomo, Y, Darweesh, S, Llibre-Guerra, J, Marras, C, San Luciano, M, and Tanner, C. The epidemiology of Parkinson’s disease. Lancet. (2024) 403:283–92. doi: 10.1016/s0140-6736(23)01419-8
7. Álvarez-Gálvez, J, Ortega-Martín, E, Carretero-Bravo, J, Pérez-Muñoz, C, Suárez-Lledó, V, and Ramos-Fiol, B. Social determinants of multimorbidity patterns: a systematic review. Front Public Health. (2023) 11:1–13. doi: 10.3389/fpubh.2023.1081518
8. Kivimäki, M, Batty, GD, Pentti, J, Shipley, MJ, Sipilä, PN, Nyberg, ST, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. (2020) 5:e140–9. doi: 10.1016/s2468-2667(19)30248-8
9. Nong, W, Mo, G, and Luo, C. Exploring the bidirectional causal link between household income status and genetic susceptibility to neurological diseases: findings from a Mendelian randomization study. Front Public Health. (2023) 11:1–9. doi: 10.3389/fpubh.2023.1202747
10. Yang, F, Johansson, ALV, Pedersen, NL, Fang, F, Gatz, M, and Wirdefeldt, K. Socioeconomic status in relation to Parkinson’s disease risk and mortality. Medicine. (2016) 95:e4337. doi: 10.1097/md.0000000000004337
11. Duda, J, and Ongun, N. Does nutritional status affect Parkinson’s disease features and quality of life? PLoS One. (2018) 13:1–14. doi: 10.1371/journal.pone.0205100
12. Gialluisi, A, De Bartolo, MI, Costanzo, S, Belvisi, D, Falciglia, S, Ricci, M, et al. Risk and protective factors in Parkinson’s disease: a simultaneous and prospective study with classical statistical and novel machine learning models. J Neurol. (2023) 270:4487–97. doi: 10.1007/s00415-023-11803-1
13. Zeng, Z, Cen, Y, Xiong, L, Hong, G, Luo, Y, and Luo, X. Dietary copper intake and risk of Parkinson’s disease: a cross-sectional study. Biol Trace Elem Res. (2023) 202:955–64. doi: 10.1007/s12011-023-03750-9
14. Xu, S, Li, W, and Di, Q. Association of Dietary Patterns with Parkinson’s disease: a cross-sectional study based on the United States National Health and nutritional examination survey database. Eur Neurol. (2023) 86:63–72. doi: 10.1159/000527537
15. Zhao, Y, Zhao, J, Xie, R, Zhang, Y, Xu, Y, Mao, J, et al. Association between family income to poverty ratio and HPV infection status among U.S. women aged 20 years and older: a study from NHANES 2003-2016. Front Oncol. (2023) 13:1265356. doi: 10.3389/fonc.2023.1265356
16. Akhmedullin, R, Supiyev, A, Kaiyrzhanov, R, Issanov, A, Gaipov, A, Sarria-Santamera, A, et al. Burden of Parkinson’s disease in Central Asia from 1990 to 2021: findings from the global burden of disease study. BMC Neurol. (2024) 24:444. doi: 10.1186/s12883-024-03949-w
17. Dorsey, ER, De Miranda, BR, Horsager, J, and Borghammer, P. The body, the brain, the environment, and Parkinson’s disease. J Parkinsons Dis. (2024) 14:363–81. doi: 10.3233/jpd-240019
18. Ren, Z, Xu, Y, Sun, J, Han, Y, An, L, and Liu, J. Chronic diseases and multimorbidity patterns, their recent onset, and risk of new-onset Parkinson’s disease and related functional degeneration in older adults: a prospective cohort study. eClinicalMedicine. (2023) 65:102265. doi: 10.1016/j.eclinm.2023.102265
19. Dallé, E, and Mabandla, MV. Early life stress, depression and Parkinson’s disease: a new approach. Mol Brain. (2018) 11:18. doi: 10.1186/s13041-018-0356-9
20. Curtis, LJ. An economic perspective on the causal explanations for the socioeconomic inequalities in health. Rev Panam Salud Publica. (2018) 42:42. doi: 10.26633/rpsp.2018.53
21. Lix, LM, Hobson, DE, Azimaee, M, Leslie, WD, Burchill, C, and Hobson, S. Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: a population-based analysis. J Epidemiol Community Health. (2009) 64:335–40. doi: 10.1136/jech.2008.084954
22. Yoon, SY, Shin, J, Chang, JS, Lee, SC, and Kim, YW. Effects of socioeconomic status on mortality after Parkinson’s disease: a nationwide population-based matched cohort study in Korean populations. Parkinsonism Relat Disord. (2020) 80:206–11. doi: 10.1016/j.parkreldis.2020.10.017
23. Kotagal, V, Bohnen, NI, Müller, MLTM, Koeppe, RA, Frey, KA, Langa, KM, et al. Educational attainment and motor burden in Parkinson’s disease. Mov Disord. (2015) 30:1143–7. doi: 10.1002/mds.26272
24. Hong, J, Dembo, RS, DaWalt, LS, Baker, MW, Berry-Kravis, E, and Mailick, MR. Mortality in women across the FMR1 CGG repeat range: the neuroprotective effect of higher education. Cells. (2023) 12:1–22. doi: 10.3390/cells12172137
25. Rosselli, M, Uribe, IV, Ahne, E, and Shihadeh, L. Culture, ethnicity, and level of education in Alzheimer’s disease. Neurotherapeutics. (2022) 19:26–54. doi: 10.1007/s13311-022-01193-z
26. Blume, J, Rothenfusser, E, Schlaier, J, Bogdahn, U, and Lange, M. Educational attainment and motor burden in advanced Parkinson’s disease – the emerging role of education in motor reserve. J Neurol Sci. (2017) 381:141–3. doi: 10.1016/j.jns.2017.08.3241
27. Pringsheim, T, Jette, N, Frolkis, A, and Steeves, TDL. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945
28. Palmer, JE, Wilson, N, Son, SM, Obrocki, P, Wrobel, L, Rob, M, et al. Autophagy, aging, and age-related neurodegeneration. Neuron. (2025) 113:29–48. doi: 10.1016/j.neuron.2024.09.015
Keywords: Parkinson, NHANES, family poverty income ratio, socio-economic status, neurodegenerative disease
Citation: Li C, Huang J and Zhang Y (2025) Association between family poverty income ratio and Parkinson’s disease in adults aged 40 and older: a study from NHANES 2003–2020. Front. Neurol. 16:1552139. doi: 10.3389/fneur.2025.1552139
Edited by:
Jiahao Cai, Guangzhou Medical University, ChinaReviewed by:
Muthu Raj Salaikumaran, Baylor College of Medicine, United StatesHongkun Zhao, Liaoning University, China
Yan Zhu, Guangdong Second Provincial General Hospital, China
Copyright © 2025 Li, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yemin Zhang, MTU1MTY5MTE3QHFxLmNvbQ==
 Cheng Li
Cheng Li Jun Huang
Jun Huang Yemin Zhang
Yemin Zhang