- 1Department of Neurology, The People’s Hospital of Pengzhou, Pengzhou, China
- 2Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, China
The treatment of wide-neck intracranial aneurysms (WNIAs) is a challenge in interventional therapy. Intrasaccular therapy, including intrasaccular flow disruption or formation of a neck bridge within the saccular, has been extensively used recently. The Medina® Embolisation Device, Luna™ Aneurysm Embolisation System, Artisse™ Aneurysm Embolisation System, Contour Neurovascular System™, Neqstent™ Coil Assisted Flow Diverter, Woven EndoBridge (WEB) device, Nautilus™ Intrasaccular System, and Trenza Embolization Device™ have obtained Conformité Européene Mark certification. However, only WEB devices have been approved by the US Food and Drug Administration. The SEAL™ Endovascular Embolization System is approved for sale only in New Zealand. Angiographic results post-procedure and in the follow-up are the main indices used to measure the efficacy of intrasaccular therapy. Complications include ischaemia, thromboembolism, and related haemorrhage. This review summarizes and discusses the efficacy and safety of intrasaccular devices in treating WNIAs. Despite this, complete and satisfactory occlusions are accomplished in most cases of intrasaccular therapy for WNIAs, and the associated complications are typically regarded as acceptable. However, it is important to note that the occlusion rate with intrasaccular therapy is generally lower than that achieved through surgical clipping. Long-term follow-up of intrasaccular therapy and recurrence and retreatment of WNIAs is limited.
1 Introduction
Intrasaccular treatment of intracranial aneurysms (IAs) involves the insertion of a flow disruptor or forming a neck bridge to offer favourable stability compared to coiling alone. Balloon- or stent-assisted coiling and flow diversion are the primary endovascular treatments for wide-neck intracranial aneurysms (WNIAs). However, complex stent configurations, the risk of covering bifurcation branches or obstructing parent vessels, and antiplatelet therapy have led to the innovation of new intrasaccular therapies for IAs. The Woven EndoBridge (WEB) device (Sequent Medical, Aliso Viejo, CA, USA, now Terumo Neuro, Aliso Viejo, CA, USA), an intrasaccular flow disruption device, was the first intrasaccular technique used to treat WNIAs and was first utilised in 2010 (1). Intrasaccular therapy does not involve metal stents within the parent vessel or antiplatelet therapy, thus reducing the risk of ischaemia, thromboembolism, and haemorrhage.
Scholars discussed the definition of a WNIA, and the most prevalent definition is a neck diameter of ≥4 mm or a dome-to-neck ratio of <2 (2). Angiographic results are assessed using the Raymond–Roy or modified Raymond–Roy occlusion classification, an angiographic classification tool for grading the occlusion of aneurysms treated with embolisation (3). Classes I and II are complete obliteration and residual neck, respectively. Complete occlusion refers to class I, and adequate occlusion refers to classes I and II.
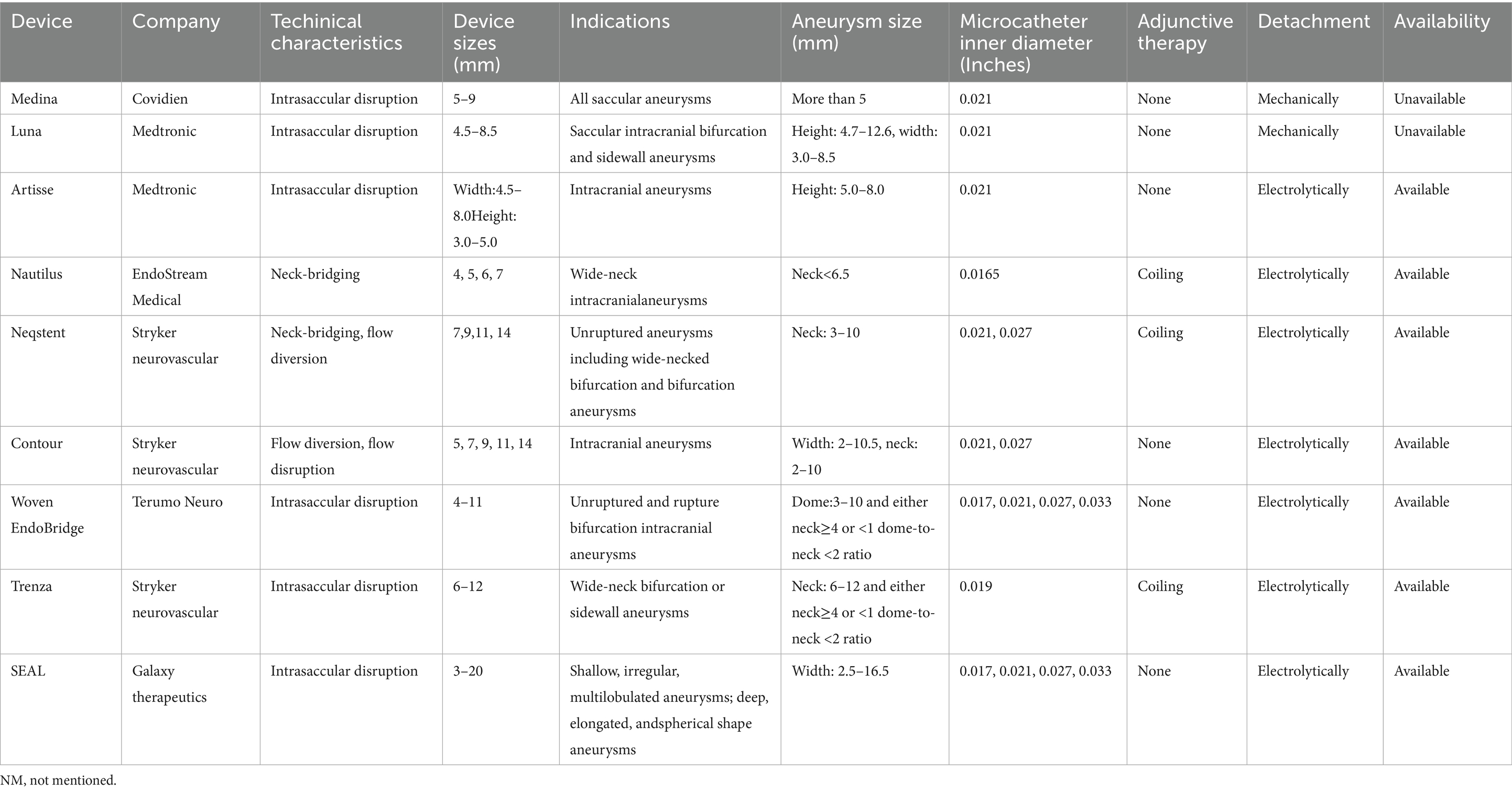
To date, eight devices have received Conformité Européene (CE) Mark certification: Medina® Embolisation Device (Covidien, Irvine, CA, USA), Luna™ Aneurysm Embolisation System (Medtronic, Minneapolis, MN, USA), Artisse™ Aneurysm Embolisation System (Medtronic, Minneapolis, MN, USA), Neqstent™ Coil Assisted Flow Diverter (Cerus Endovascular, Fremont, CA, USA, now Stryker neurovascular, Kalamazoo, MI, USA), Contour Neurovascular System™ (Cerus Endovascular, now Stryker neurovascular, Kalamazoo, MI, USA), WEB device, Nautilus™ Intrasaccular System (EndoStream, Or Akiva, Israel), and Trenza embolization device™ (Stryker neurovascular, Kalamazoo, MI, USA) (4, 5). However, only the WEB device has been approved by the US Food and Drug Administration (FDA) (6). Additionally, the The Saccular Endovascular Aneurysm Lattice (SEAL™) Endovascular Embolization System (Galaxy Therapeutics Inc., Milpitas, CA, USA) is approved for sale only in New Zealand (7). This review summarises and discusses the efficacy and safety of intrasaccular therapy for WNIAs.
2 Methods
We searched Web of Science, Medline, Cochrane, and Embase using the following terms: intracranial aneurysm, wide-neck, endovascular procedure, and intrasaccular device from database inception to May 1, 2025. The following criteria were used to determine the suitability of studies for inclusion: (1) Randomized controlled trials (RCTs), as well as cohort studies, case reports, and case series. (2) Studies that reported data on patients with both ruptured and unruptured WNIAs treated with intrasaccular therapy, including information on angiographic classification. (3) The definition for WNIAs included a neck diameter of≥4 mm or a dome-to-neck ratio of <2. Patients who were admitted for other medical issues, those who had undergone surgical craniotomy, individuals with severe dysfunction of other organs or systems, and patients with malignant tumors were excluded from the study. Additionally, studies involving pregnant subjects were not considered. We reviewed all retrieved literature’s abstracts to identify suitable trials. We also retrieved the references from all the literature to minimise error and bias. This review is based on previous studies and contains no new studies with human participants or animals performed by any authors.
3 Results
3.1 Medina embolisation device
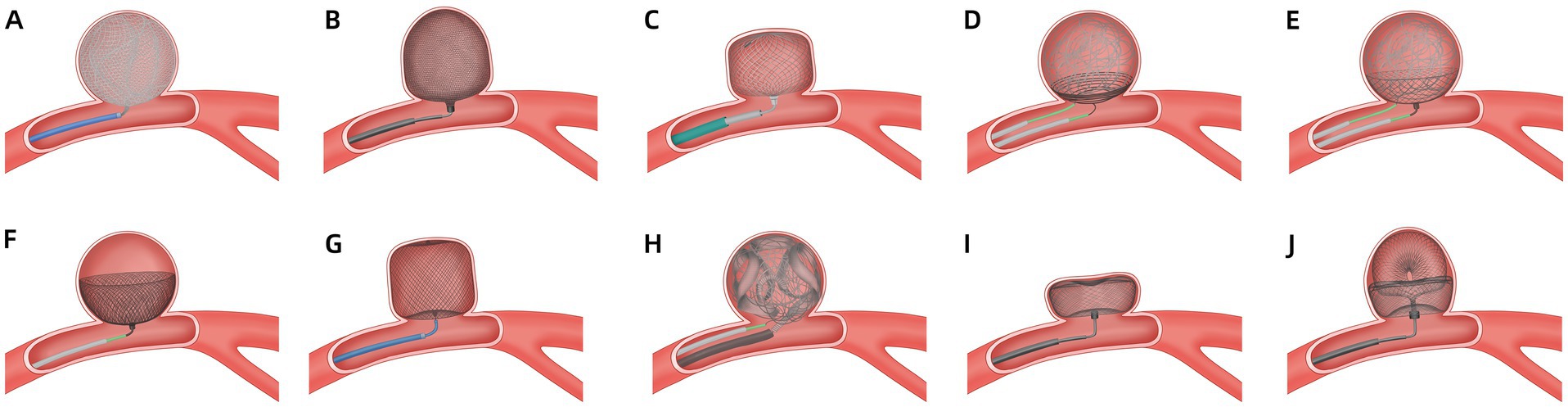
The Medina is a mechanically detached, braided, self-expanding device comprising a radiopaque three-dimensional set core wire and a wing-like shape memory alloy filament that forms a low-porosity metal mesh (Figure 1A) (8). The Medina is applied via a 0.021-inch diameter microcatheter and can be re-sheathed and redeployed, as with standard coils. Aguilar Perez et al. (9) successfully applied a Medina device in 14 patients with unruptured aneurysms. Immediate and 6–12 months angiography results showed complete and adequate occlusion rates of 6.7, 42.9, 36.4, and 54.5%, respectively. No complications occurred. However, adjunctive devices are required in wide-neck cases. Sourour et al. (10) evaluated Medina’s safety and 6–9-month efficacy in 12 patients with 13 WNIAs. In 15% of cases, Medina was used alone. However, 85 and 31% of cases used additional coils or adjunctive balloons, respectively. Postprocedure and 6-month complete occlusion rates were 61.5 and 83%, respectively. One patient with no clinical consequences of thromboembolic complications was observed. Bhogal et al. (11) reported 14 aneurysms in 13 patients treated with Medina. The aneurysm neck width ranged from 1.9 to 6.9 mm. A complete occlusion rate was observed in 15.4% of the cases, and an adequate occlusion rate in 23.1%. Five months after angiography, the complete and adequate occlusion rates were 38.5 and 76.9%, respectively. Seventy-five percent of cases treated with two or more Medina had complete occlusion at 6 months, and three patients experienced temporary complications. A study reported angiographic outcomes in 19 patients with 20 WNIAs treated with Medina (12). The post-procedure, 6-month, and long-term follow-up complete occlusion rates were 61, 75, and 80%, respectively. Angiographic recanalisation was documented in 11% of the cases at mid-term follow-up. Three cases (15%) of thromboembolic complications were observed; however, only one was responsible for clinical sequelae. No recanalisation occurred during the long-term follow-up. Compared with the other devices, the Medina had relatively poor immediate, mid-term, and long-term efficacy, and outcomes highly depended upon the number of devices or adjunctive devices. Due to the high cost of using multiple devices and the difficulty of reoperation, the device was not used clinically.
3.2 Luna aneurysm embolisation system
The Luna is a pre-shaped, ovoid, self-expanding, mechanically detachable endovascular device made from a double layer of wire mesh designed to provide coverage across the aneurysm neck and isolate the saccular artery from the parent artery (Figure 1B). Its size (4.5–8.5 mm) is based on the aneurysm width, shape, and dome-to-neck ratio. The device can be inserted via a microcatheter with a 0.027-inch lumen. Piotin et al. (13) evaluated the efficacy and safety in 63 bifurcation and sidewall IAs, of which 95.2% were unruptured. The neck sizes were 1.9–8.7 mm, and the immediate adequate occlusion rate was 18%. However, adequate occlusion rates were achieved in 78.0 and 79.2% of patients at 12 and 36 months, respectively. Two major strokes (3.2%), one minor stroke (1.6%), and three incidents of haemorrhage in two subjects (3.2%) were observed. No morbidities were observed after 12 months of treatment. Morbidity was 1.8% at 36 months, and one case of mortality (1.6%) was observed. Kwon et al. (14) demonstrated that the Luna may not be suitable for fusiform, wide-neck, or complex aneurysms, as few WNIAs were included in the study reported by Piotin (13). Due to the appearance of the Artisse, Luna is no longer in clinical use.
3.3 Artisse aneurysm embolisation system
The Artisse is a second-generation Luna device. It is a flex-to-fit intrasaccular device that conforms to the aneurysm’s shape (Figure 1C) (15). This device is intended for the endovascular embolization of saccular intracranial aneurysms. Its width and height diameter range from 4.5 to 8 mm and 3.0 to 5.0 mm, respectively. The device has 20 sizes. The device can be inserted via a microcatheter with a 0.021-inch lumen. The device detaches through an electrolytic, handheld detachment system. Piotin et al. (16) reported the outcomes of patients with unruptured bifurcation aneurysms treated with Artisse. The mean aneurysm neck size was 4.0 ± 0.8 mm (range 3.2–5.8 mm), and the dome-to-neck ratio was 1.82:1. Adequate occlusion was achieved in 66.7 and 57.1% of patients at 6 and 36 months of follow-up, respectively. Of the 9 participants, 22.2% experienced major strokes, including one procedure-related parent vessel occlusion and one haemorrhagic stroke. Due to the small sample size of the study, further research is necessary to confirm the results. Besides, more research is needed to guide the selection of appropriate device sizes, as a great variety of sizes is available.
3.4 Nautilus intrasaccular system
The Nautilus is a novel device designed for neck bridging, a technique used to enhance coil stability and reduce the risk of coil protrusion in WNIAs (Figure 1D) (5). The device is a nitinol-based detachable disk-like implant with flexible layers that can be delivered via a 0.017-inch microcatheter. The device is fully retrievable and can be repositioned at any time. The coils are delivered to the aneurysm using another microcatheter. The suitable device size is at least 0.5 mm larger than the width of the neck. Several case reports have described the successful application of the Nautilus in IAs (17–19). Recently, Sirakov et al. (20) reported the outcomes in 41 patients with WNIAs treated with the Nautilus. Immediate and follow-up complete occlusion rates were 73.1 and 94.5%, respectively. No procedure-related deaths or morbidities were reported. For immediate aneurysm dome protection, the adjunctive coiling technique enhances its application in treating acutely ruptured aneurysms. The results of the Nautilus are promising, but further long-term studies are required. These studies have limitations such as small sample size, being a single-center series, retrospective analysis without a comparator group, and a short follow-up period.
3.5 Neqstent coil assisted flow diverter
The Neqstent is a neck-bridging, electrolytically detachable, self-expanding device used in conjunction with coiling or other embolisation products to facilitate the occlusion of WNIAs (Figure 1E) (21). It treats a wide range of unruptured aneurysm morphologies including wide-necked bifurcation and bifurcation aneurysms. The concave-shaped device has 64 wires with a diameter of 0.0015 inches and can pass through a 0.017-inch microcatheter for coiling. The Neqstent is a derivative of the Contour that may be applied to ruptured IAs. Four sizes (7, 9, 11, and 14 mm) are suitable for 3–10 mm neck-width IAs. The device is delivered via a 0.021–0.027-inch microcatheter. The Neqstent was implanted in 36/38 unruptured WNIAs in a prospective multicentre study (22). The median neck length of the aneurysm was 5.2 mm (range 2.1–11.4 mm). The immediate adequate occlusion rate was 25%, which increased to 77.8% at 6 months. Complete occlusion was achieved in 80.6% of patients at the last angiography. Related complications were reported in 10.5% of the patients, including one haemorrhagic event and three thromboembolic events. The primary limitations include the sample size, lack of randomization, and the inclusion of patients with previous aneurysm treatment. The Neqstent remains within the neck of the aneurysm, allowing the coil to be fixed without needing a stent. The additional flow-directing characteristics may enhance the new endothelialization. Although 0.017-inch microcatheters can pass through the reticular structure on the side of the Neqstent, the best technique for locating coil microcatheters into aneurysms is jailing.
3.6 Contour neurovascular system
The Contour is easily re-sheathable and re-deployable, and is a neck-bridging device (Figure 1F) (23, 24). The device expands to a cup-like shape to fill the proximal half of the IA. The Contour, which has a dual-layer conformation with 144 wires, has both the effect of flow diversion and disruption. Size selection (5, 7, 9, 11, and 14 mm) is based on the IA width and neck width, which range from 2 mm to 10.5 mm and 2 mm to 10 mm, respectively. The Contour can be deployed via a 0.021-inch or 0.027-inch microcatheter. A review reported three patients with three unruptured aneurysms treated with the Contour (25). The neck widths of 2 IAs were >4 mm. One patient had incomplete occlusion post-procedure, and 6 months later. One patient had complete occlusion at 3 and 15 months. Akhunbay-Fudge et al. (26) reported outcomes of 11 patients with WNIAs treated with the Contour. The complete and adequate occlusion rates at 1 year were 55.56 and 100%, respectively. Two patients had thromboembolic events but without permanent neurological disability or death. Liebig et al. (27) reported the safety and efficacy outcomes of 32 patients with unruptured intracranial bifurcation aneurysms who received treatment with the Contour. The median aneurysm neck width was 4.3 ± 1.4 mm (range 2.4–7.4 mm) and the dome-to-neck ratio was 1.4 ± 0.4. Complete occlusion was observed in 44 and 69% of the patients at 6 and 12 months, respectively. The adequate occlusion rate was 84% at the final follow-up. Ghozy et al. (28) reported that 131 patients with WNIAs were treated using a Contour. The occlusion rate was 84.21%, and the thromboembolic event rate was 8.53%. Mostafa et al. (29) reported that complete and adequate occlusion rates were 20 and 100%, respectively, at 6 months in 5 patients with wide-neck basilar tip aneurysms treated with a Contour device. Diana et al. (30) studied the safety and feasibility of Contour and Neqstent in 15 cases of IAs. The neck width in 6 cases was >4 mm. In one case, deployment failed because of the parent artery’s stenosis; in another case, balloon-assisted coiling was selected to protect against coil protrusion. One ischaemic event (6.7%) was observed, and no haemorrhagic complications, mortality, or mobility were reported. Overall, the Contour is a safe and effective treatment option for WNIAs. Sizing is simpler than the WEB, which offers only five sizes. The slightly oversized design guarantees secure placement without any unintended movement after deployment.
3.7 Woven EndoBridge device
The WEB device, introduced in 2010, is the first and only FDA-approved device for intrasaccular flow disruption in ruptured and unruptured aneurysms (Figure 1G) (31). The WEB device is indicated for use at the internal carotid artery terminus, middle cerebral artery bifurcation, anterior communicating artery complex, or basilar artery apex for endovascular treatment with saccular, wide-neck bifurcation ruptured and unruptured IAs with a dome diameter of 3–10 mm and either neck size ≥4 mm or a dome-to-neck ratio 1–2. During treatment, the physician selects the appropriate device size based on the size, shape and location of the IA to be occluded. The WEB is an electrothermally detached braided nitinol cage-like device, which includes three types: dual-layer (WEB-DL), single-layer (WEB-SL), and single-layer spherical lower-profile (WEB-SLS). However, WEB-DL is no longer commercially available. The evolution from WEB-DL to WEB-SL balances porosity, radial force, and thrombogenicity (32). The device can be delivered using 0.017-, 0.027-, or 0.033-inch microcatheters. The WEB device causes rapid thrombosis and occlusion of the aneurysm, leading to immediate alteration of haemodynamics. The WEB device has become widely used in wide-neck bifurcation aneurysms following the WEB-Intrasaccular Therapy (WEB-IT) prospective study (33). A 10-year study on WEB application post-procedure and long-term angiographic efficacy reported no variations in complications or mortality (34). Fujii et al. (35) reported the WEB use in 29 patients with WNIAs. The mean neck width was 3.7 ± 0.6 mm, and the adequate occlusion rate was 75.9% after 6 months. WEB shape modification was a contributing factor for adequate occlusion, as identified by multivariate analysis. One patient had a renal artery injury, and another had a minor cerebral infarction. Mantilla et al. (36) conducted a systematic review of 22 articles, including 1705 patients with 1,224 aneurysms, and discussed the application of WEB devices in IAs, predominantly WNIAs. The immediate and follow-up adequate occlusion rates were 33.3 and 49.7%, respectively. A total of 6.5 and 3.1% of patients had thromboembolic complications and other complications, respectively. The mortality rate was approximately 1%. However, Morioka et al. (37) reported persistent contrast-filling in the WEB in 50% (10/20) of IAs after 3 months. Persistent contrast filling was associated with postoperative dual antiplatelet therapy for at least a month, a wide neck (median 4.5 mm), and a lower deviation of the aneurysm axis from the inlet flow line.
The current data suggest that the WEB device is most suitable for treating typical wide-necked and bifurcated aneurysms due to its compact structure and variable size. However, it may not be effective for aneurysms with a shallow depth, very irregular shapes, or those requiring secondary treatment. Additionally, the rigidity of the delivery system and the device itself may pose challenges in accurately placing it. Selecting the appropriate size is a significant issue. Compared with Contour, which only relies on the maximum width diameter for selection, correctly choosing the WEB size is more challenging. The WEB is more likely to be too small, explaining some recurrence and retreatment cases.
3.8 Trenza embolization device
The Trenza embolization device is a frame coil implant that creates a stable ω-shaped basket filled with coils with flow-disruption properties to treat challenging mid-to-large-sized broad-neck bifurcation or sidewall aneurysms (Figure 1H) (38). It is available in 6- to 12-mm sizes, each with a fixed length (eg, 6–11 mm, 7–13 mm, 8–15 mm, and so forth). The device is attached to a flexible delivery wire, and the detachment of the device is electrothermal. An observational single-center retrospective study reported the experience of 12 patients (10 unruptured and 2 ruptured) with WNIAs treated by the Trenza device (38). The median dome-to-neck ratio was 1.8 (interquartile range, 1.6–1.9). Complete and adequate occlusion was 67 and 83% at the 6.5-months follow-up. There were 3 (25%) major ischemic complications, leading to 2 (17%) permanent and 1 (8%) transient neurologic deficit. Two (17%) patients were retreated. One (8%) treated aneurysm ruptured 1.6 months after follow-up and resulted in death. A retrospective multicenter analysis reported the results of a 6-month follow-up involving 25 aneurysms (3 ruptured) in 25 patients who received the Trenza device (39). Twenty-one cases met the criteria for WNIAs. The rates of complete and adequate occlusion were 42.1 and 89.5% after 6 months. Additionally, 10.5% of cases required retreatment. There was one reported symptomatic thrombotic event, and two cases received stent assistance. The limitations of these two studies, due to the retrospective design and a relatively small number of included patients. More studies are needed.
3.9 The SEAL endovascular embolization system
The SEAL Embolization System is a novel intrasaccular aneurysm low-disruptor (7). The SEAL is a self-expanding, dual-layer, nitinol, and core platinum wire mesh braided implant. The SEAL has two configurations: one configuration has only the base portion (Figure 1I) (SEAL Base), and the second configuration includes an ovoid upper loop with a base bridging component (Figure 1J) (SEAL Arc). Pabon et al. (7) present the first case report of SEAL demonstrated in a patient with acute hemorrhage from a ruptured, complex, left middle cerebral artery trilobed shallow wide-necked bifurcation aneurysm. Immediate and a 1-year angiographic follow-up demonstrated complete occlusion with no safety concerns. The SEAL device is only approved for marketing and commercialization in New Zealand. This case report is part of the Pre-SEALIT ethics committee-approved early feasibility clinical study in Colombia. The SEAL device may be a promising novel technology that can potentially treat very shallow aneurysms with limited height and irregular, multilobulated aneurysms.
4 Discussion
The characteristics of intrasaccular devices are summarized in Table 1. The available research indicates that intrasaccular devices are suitable for typical WNIAs because of their variable size and compact nature. However, deploying intrasaccular devices within WNIAs with shallow depths, irregular shapes, and multilobular or pretreated aneurysms presents some challenges (40). The SEAL device may be a promising device to treat very shallow aneurysms (7). Trimboli et al. (41) studied the safety and efficacy of balloon-assisted WEB deployment in 33 IAs, of which 25 aneurysms (75.8%) were classified as WNIAs. At the mid-term follow-up, complete and adequate occlusion rates were 85.2 and 92%, respectively. One (3.0%) patient died of procedure-related complications. Goertz et al. (42) reported the outcomes of 178 IAs treated with WEB or stent-assisted WEB devices. Stent implantation was performed in 15 patients (8.4%). The thromboembolic complication rate was higher in the stent-associated WEB group (33.3% vs. 8.0%), whereas ischaemic stroke rates were comparable between the groups (0% vs. 1.8%). At the 6-month follow-up, the complete and adequate occlusion rates were 73.4 and 92.8% after WEB and 66.7 and 86.7% after stent-associated WEB, respectively.
Appropriate sizing selection is a major issue in all intrasaccular therapies. Studies associated with the Medina device have demonstrated that using more than one device is associated with an improved occlusion rate. However, the method is expensive. Compared with the Contour, which only requires the largest diameter, the sizing of WEB devices is difficult. Therefore, the WEB is more prone to undersizing, which may influence the recurrence and retreatment rates of IAs. Goertz et al. (43) performed a retrospective multicentre study of 247 patients with 251 IAs treated using WEB device oversizing. Of 237 aneurysms, 94.4% were WNIAs, with median neck width and dome-to-neck ratios of 4.6 ± 1.4 mm (range 1.2–10.3 mm) and 1.4 ± 0.4 mm (0.6–3.4 mm). Ruptured aneurysms were present in 25.5% of cases (n = 64). The WEB device was successfully applied in 98.8% of patients, achieving a mean WEB/dome ratio of 1.2 ± 0.1. The midterm complete and adequate occlusion rates were 66.3 and 88.4%, respectively. The thromboembolic complication rate was 7.2%. Complications were reported in eight procedures (3.2%), the morbidity rate was 0.8%, and no mortalities were observed in this study. Oversizing the WEB device may yield promising complete and adequate mid-term occlusion rates. One study (n = 120) demonstrated that an increased width-to-height ratio and a lower aneurysm height/neck diameter ratio were significant risk factors for complications in WEB therapy (44). Due to the vary sizes, it’s difficult to determine the correct size without any prior experience. In an animal model, Sim&Cure software was used to run various simulations and assist to select the right size of Artisse (45). Jadhav et al. (46) already introduced a machine learning model to help predict the outcome of treatment with a WEB device for WNIAs. Artificial intelligence could play a crucial role in helping us select more suitable device types and sizes in the future.
Previous studies have reported that surgical clipping has a complete occlusion rate of up to 96% (47). Comparatively, the rate of residual aneurysm or recurrence ranges from 60 to 95% with endovascular treatment. Recurrence and retreatment rates are higher in patients with larger or wide-neck aneurysms. Initial partial aneurysm thrombosis, aneurysm size, and simultaneous treatment with WEB and a coil were associated with higher recurrence and retreatment rates (48). Cagnazzo et al. (49) reported that aneurysm rupture, undersized WEB, WEB shape change, neck diameter, and angle between the parent artery and aneurysm dome were associated with a lower adequate occlusion rate. However, the results were insignificant. A recent study on the retreatment of residual and recurrent aneurysms treated with WEB suggested that a neck remnant might not be adequate, and cases must be followed up closely (50). Although thrombotic events also exist, the rates of residual and recurrent aneurysms are significantly higher than those of thrombotic events. The surface coating of the device that encourages thrombosis or endothelialization may be beneficial in improving the rates of complete or adequate occlusion.
The different intrasaccular devices used in WNIAs demonstrate varying degrees of efficacy and safety. Hecker et al. (51) compared a Contour device (34 patients with 40 aneurysms) with a WEB device (30 patients with 30 aneurysms) for the treatment of WNIAs. In the Contour cohort, the complete and adequate occlusion rates at the last follow-up were 75 and 90%, respectively. One patient (2.5%) required retreatment, and one patient experienced a symptomatic thromboembolic event. Three adjunctive stents (10%) were used for branch occlusion. In the WEB cohort, the complete and adequate occlusion rates were 63.3 and 90%, respectively, with a retreatment rate of 20%. Four (13.3%) patients required an additional stent because of device protrusion into a branch. Two asymptomatic thromboembolic events (6.7%) and one major ischaemic event (3.3%) were reported. The Contour cohort outcomes were promising, although a more extended follow-up period is necessary. Several factors can influence the occlusion rate of WNIAs when an embolization device is implanted. These factors include the availability, morphology, structure, and material properties of the devices, as well as the location and shape of the WNIAs. Consequently, different studies may report varying occlusion rates. However, further RCTs are needed to validate these findings.
The outcomes of microsurgical clipping and endovascular techniques in WNIAs vary. A review found that lipping is linked to a higher rate of occlusion and lower rates of residual and recurrent aneurysms. In contrast, coiling results in lower morbidity and mortality, along with a more favorable postoperative outcome (47). In comparing the efficacy and safety of WEB (63 patients) and clipping (103 patients), microsurgical clipping was associated with a higher complete occlusion rate. In contrast, the WEB technique demonstrated a lower complication rate (52). This study included unruptured anterior circulation aneurysms. Complications occurred in 13% of patients in the clipping group and 6% in the WEB group. A retrospective study that included 84 patients with 89 unruptured WNIAs compared the efficacy and safety of WEB with those of microsurgical clipping (53). Of the WNIAs, 99% were completely occluded with clipping compared with a WEB occlusion rate of 53% at 1 year follow-up. The morbidity rates in the microsurgical clipping and WEB groups were 1 and 3%, respectively. This retrospective study involved patients with unruptured WNIAs, as per the criteria for WEB. Fusiform, multilobulated, and dissecting aneurysms were excluded. The inclusion criteria for these studies varied, making it impossible to demonstrate the superiority of WEB intrasaccular therapy over microsurgical clipping. Therapeutic decisions should be made based on individual preferences. In addition, compared with traditional coil or coil-assisted therapy, the higher costs of intrasaccular treatment might limit its use as the first choice of treatment for WNIAs.
5 Conclusion
With innovations in intrasaccular devices, the safety and occlusion rate of intrasaccular treatments for WNIAs are promising. However, most current research focuses primarily on unruptured WNIAs; long-term follow-up and outcome data are lacking. Future studies on ruptured WNIAs, the comparison of intrasaccular therapy with microsurgical clipping, and long-term outcomes are needed.
Author contributions
KZ: Data curation, Writing – original draft. HW: Writing – original draft, Data curation, Methodology. ZG: Methodology, Software, Writing – original draft. CZ: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all the people who participated in the primary trials and the teams who did them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
WNIAs, wide-neck intracranial aneurysms; IAs, intracranial aneurysms; WEB, Woven EndoBridge; CE, Conformité Européene; FDA, the US Food and Drug Administration.
References
1. Klisch, J, Sychra, V, Strasilla, C, Liebig, T, and Fiorella, D. The woven EndoBridge cerebral aneurysm embolization device (WEB II): initial clinical experience. Neuroradiology. (2011) 53:599–607. doi: 10.1007/s00234-011-0891-x
2. Hendricks, BK, Yoon, JS, Yaeger, K, Kellner, CP, Mocco, J, De Leacy, RA, et al. Wide-neck aneurysms: systematic review of the neurosurgical literature with a focus on definition and clinical implications. J Neurosurg. (2019) 133:159–65. doi: 10.3171/2019.3.JNS183160
3. Mascitelli, JR, Moyle, H, Oermann, EK, Polykarpou, MF, Patel, AA, Doshi, AH, et al. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. (2015) 7:496–502. doi: 10.1136/neurintsurg-2014-011258
4. Hecker, C, Broussalis, E, Griessenauer, CJ, and Killer-Oberpfalzer, M. A mini-review of intrasaccular flow diverters. J Neurointerv Surg. (2023) 15:70–4. doi: 10.1136/neurintsurg-2021-018426
5. EndoStream. (2025). NAUTILUS™, A-traumatic, self-conforming intrasaccular flow diverter. Available online at: https://endostream.com/ (Accessed May 1, 2025).
6. Mouchtouris, N, Hasan, D, Samaniego, EA, Saiegh, FA, Sweid, A, Abbas, R, et al. The woven EndoBridge (WEB) device: feasibility, techniques, and outcomes after FDA approval. J Neurosurg. (2021) 136:1266–72. doi: 10.3171/2021.5.JNS21889
7. Pabon, B, Torres, V, Woodward, K, Cardozo, M, Tan, B, Chaubal, V, et al. Treatment of a ruptured shallow trilobed cerebral aneurysm with the novel saccular endovascular aneurysm lattice (SEAL) device: a case report with one year follow-up. Interv Neuroradiol. (2023) 29:481–7. doi: 10.1177/15910199231187048
8. Turk, AS, Maia, O, Ferreira, CC, Freitas, D, Mocco, J, and Hanel, R. Periprocedural safety of aneurysm embolization with the Medina coil system: the early human experience. J Neurointerv Surg. (2016) 8:168–72. doi: 10.1136/neurintsurg-2014-011585
9. Aguilar Perez, M, Bhogal, P, Martinez Moreno, R, Bäzner, H, Ganslandt, O, and Henkes, H. The Medina embolic device: early clinical experience from a single center. J Neurointerv Surg. (2017) 9:77–87. doi: 10.1136/neurintsurg-2016-012539
10. Sourour, NA, Vande Perre, S, Maria, FD, Papagiannaki, C, Gabrieli, J, Pistocchi, S, et al. Medina® embolization device for the treatment of intracranial aneurysms: safety and angiographic effectiveness at 6 months. Neurosurgery. (2018) 82:155–62. doi: 10.1093/neuros/nyx161
11. Bhogal, P, Brouwer, PA, Yeo, L, Svensson, M, and Söderman, M. The Medina embolic device: Karolinska experience. Interv Neuroradiol. (2018) 24:4–13. doi: 10.1177/1591019917733125
12. Haffaf, I, Clarençon, F, Shotar, E, Rolla-Bigliani, C, Vande Perre, S, Mathon, B, et al. Medina embolization device for the treatment of intracranial aneurysms: 18 months’ angiographic results. J Neurointerv Surg. (2019) 11:516–22. doi: 10.1136/neurintsurg-2018-014110
13. Piotin, M, Biondi, A, Sourour, N, Mounayer, C, Jaworski, M, Mangiafico, S, et al. The LUNA aneurysm embolization system for intracranial aneurysm treatment: short-term, mid-term and long-term clinical and angiographic results. J Neurointerv Surg. (2018) 10:e34. doi: 10.1136/neurintsurg-2018-013767
14. Kwon, SC, Ding, YH, Dai, D, Kadirvel, R, Lewis, DA, and Kallmes, DF. Preliminary results of the Luna aneurysm embolization system in a rabbit model: a new intrasaccular aneurysm occlusion device. AJNR Am J Neuroradiol. (2011) 32:602–6. doi: 10.3174/ajnr.A2314
15. Medtronic. (2023). Artisse™ Intrasaccular device. Available online at: https://europe.medtronic.com/xd-en/healthcare-professionals/products/neurological/aneurysm-treatment/artisse.html (Accessed May 1, 2025).
16. Piotin, M, Fahed, R, Redjem, H, Smajda, S, Desilles, JP, Escalard, S, et al. The ARTISSE intrasaccular device for intracranial aneurysm treatment: short-term, mid-term and long-term clinical and angiographic results. J Neurointerv Surg. (2022) 14:957–61. doi: 10.1136/neurintsurg-2021-017806
17. Matanov, S, Sirakov, A, Sirakova, K, and Sirakov, S. Nautilus-assisted coiling of an Unruptured wide-necked aneurysm of the posterior communicating artery. World Neurosurg. (2021) 151:117. doi: 10.1016/j.wneu.2021.04.139
18. Sirakov, A, Matanov, S, Bhogal, P, and Sirakov, S. Nautilus-assisted coil embolization for a complex AcomA wide-necked aneurysm in the setting of acute subarachnoid hemorrhage. J Neurointerv Surg. (2022) 14:310. doi: 10.1136/neurintsurg-2021-017670
19. Sirakov, A, Mocco, J, and Sirakov, S. Nautilus-assisted coil occlusion for treatment of a ruptured aneurysm at the bifurcation of the right middle cerebral artery: complete occlusion achieved in one session with a good clinical outcome In: H Henkes, P Lylyk, and O Ganslandt, editors. The aneurysm casebook. Cham: Springer (2022)
20. Sirakov, A, Bhogal, P, Sirakova, K, Penkov, M, Minkin, K, Ninov, K, et al. Endovascular treatment of wide-necked intracranial aneurysms using the Nautilus Intrasaccular system: initial case series of 41 patients at a single center. J Neurointerv Surg. (2023) 15:989–94. doi: 10.1136/jnis-2022-019295
21. Stryker neurovascular. (2020). Neqstent™ Coil Assisted Flow Diverter. Available online at: https://www.stryker.com/gb/en/neurovascular/products/neqstent--coil-assisted-flow-diverter.html (Accessed May 1, 2025).
22. Liebig, T, Gal, G, O’Kelly, C, Wodarg, F, Killer-Oberpfalzer, M, Ozpeynirci, Y, et al. Neqstent coil-assisted flow diverter (NQS) for the treatment of bifurcation aneurysms: the coil-assisted flow diversion safety and performance study (CAFI). J Neurointerv Surg. (2024) 16:721–5. doi: 10.1136/jnis-2022-020056
23. Stryker Neurovascular. (2020). Contour Neurovascular System™. Size. Place. Done. Available online at: https://www.stryker.com/gb/en/neurovascular/products/contour-neurovascular-system.html (Accessed May 1, 2025).
24. Biondi, A, Primikiris, P, Vitale, G, and Charbonnier, G. Endosaccular flow disruption with the contour neurovascular system: angiographic and clinical results in a single-center study of 60 unruptured intracranial aneurysms. J Neurointerv Surg. (2023) 15:838–43. doi: 10.1136/jnis-2022-019271
25. Bhogal, P, Lylyk, I, Chudyk, J, Perez, N, Bleise, C, and Lylyk, P. The contour-early human experience of a novel aneurysm occlusion device. Clin Neuroradiol. (2021) 31:147–54. doi: 10.1007/s00062-020-00876-4
26. Akhunbay-Fudge, CY, Deniz, K, Tyagi, AK, and Patankar, T. Endovascular treatment of wide-necked intracranial aneurysms using the novel contour neurovascular system: a single-center safety and feasibility study. J Neurointerv Surg. (2020) 12:987–92. doi: 10.1136/neurintsurg-2019-015628
27. Liebig, T, Killer-Oberpfalzer, M, Gal, G, Schramm, P, Berlis, A, Dorn, F, et al. The safety and effectiveness of the contour neurovascular system (contour) for the treatment of bifurcation aneurysms: the CERUS study. Neurosurgery. (2022) 90:270–7. doi: 10.1227/NEU.0000000000001783
28. Ghozy, S, Lashin, BI, Elfil, M, Bilgin, C, Kobeissi, H, Shehata, M, et al. The safety and effectiveness of the contour neurovascular system for the treatment of wide-necked aneurysms: a systematic review and meta-analysis of early experience. Interv Neuroradiol. (2024) 30:496–505. doi: 10.1177/15910199221139546
29. Mostafa, K, Bueno Neves, F, Gärtner, F, Peters, S, Hensler, J, Larsen, N, et al. Contour device implantation for treatment of intracranial aneurysms in the basilar tip. Interv Neuroradiol. (2023) 10:15910199231219018. doi: 10.1177/15910199231219018
30. Diana, F, de Dios Lascuevas, M, Peschillo, S, Raz, E, Yoshimura, S, Requena Ruiz, M, et al. Intrasaccular flow disruptor-assisted coiling of intracranial aneurysms using the novel contour neurovascular systems and NEQSTENT: a single-center safety and feasibility study. Brain Sci. (2022) 12:991. doi: 10.3390/brainsci12080991
31. Terumo Neuro. (2022). WEB™ Embolization System Indications, Safety, and Warnings. Available online at: https://www.terumoneuro.com/chapla/products/web-family/additional-information/indications-for-use (Accessed May 1, 2025).
32. Mine, B, Pierot, L, and Lubicz, B. Intrasaccular flow-diversion for treatment of intracranial aneurysms: the woven EndoBridge. Expert Rev Med Devices. (2014) 11:315–25. doi: 10.1586/17434440.2014.907741
33. Arthur, AS, Molyneux, A, Coon, AL, Saatci, I, Szikora, I, Baltacioglu, F, et al. The safety and effectiveness of the woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB Intrasaccular therapy (WEB-IT) study. J Neurointerv Surg. (2019) 11:924–30. doi: 10.1136/neurintsurg-2019-014815
34. Dmytriw, AA, Dibas, M, Ghozy, S, Adeeb, N, Diestro, JDB, Phan, K, et al. The woven EndoBridge (WEB) device for the treatment of intracranial aneurysms: ten years of lessons learned and adjustments in practice from the world wide WEB consortium. Transl Stroke Res. (2023) 14:455–64. doi: 10.1007/s12975-022-01072-x
35. Fujii, T, Teranishi, K, Kitamura, T, Mitome-Mishima, Y, Kondo, A, and Oishi, H. Woven Endo bridge shape modification contributes to decrease in adequate occlusion rate after woven Endo bridge implantation for wide-neck bifurcation intracranial aneurysms. Neuroradiol J. (2024) 37:54–9. doi: 10.1177/19714009231212363
36. Mantilla, DE, D, D, Ortiz, AF, Nicoud, F, and Costalat, V. The woven EndoBridge device, an effective and safe alternative endovascular treatment of intracranial aneurysm-systematic review. Interv Neuroradiol. (2023) 11:15910199231201544. doi: 10.1177/15910199231201544
37. Morioka, J, Nakahara, I, Matsumoto, S, Hasebe, A, Tanabe, J, Suyama, K, et al. Persistent contrast-filling in the woven endobridge device three months after its implantation for cerebral aneurysm: incidence, predictive factors, and outcome. Clin Neurol Neurosurg. (2023) 231:107837. doi: 10.1016/j.clineuro.2023.107837
38. Raj, R, and Numminen, J. Initial experiences with the Trenza embolization device for the treatment of wide-neck intracranial aneurysms: a 12-patient case series. AJNR Am J Neuroradiol. (2024) 45:418–23. doi: 10.3174/ajnr.A8153
39. Wolf, MN, Valvassori, L, Gioppo, A, Rautio, R, Hoeltje, J, Schramm, P, et al. Retrospective multicenter analysis of the Trenza embolization device for endovascular therapy of intracranial aneurysms: initial results and short-term follow-up. J Neurointerv Surg. (2025):22314. doi: 10.1136/jnis-2024-022314
40. Goyal, N, Hoit, D, DiNitto, J, Elijovich, L, Fiorella, D, Pierot, L, et al. How to WEB: a practical review of methodology for the use of the woven EndoBridge. J Neurointerv Surg. (2020) 12:512–20. doi: 10.1136/neurintsurg-2019-015506
41. Trimboli, A, Wenderoth, JD, Cheung, AK, Whitley, J, McQuinn, A, Williams, C, et al. Balloon assisted woven endobridge deployment (BAWD): a safety and efficacy study. Interv Neuroradiol. (2023) 7:15910199231185632. doi: 10.1177/15910199231185632
42. Goertz, L, Liebig, T, Siebert, E, Pflaeging, M, Forbrig, R, Pennig, L, et al. Stent-assisted WEB embolization: aneurysm characteristics, outcome and case report of a WEB delivered through a stent. Acta Neurochir. (2022) 164:2181–90. doi: 10.1007/s00701-022-05115-y
43. Goertz, L, Liebig, T, Siebert, E, Zopfs, D, Pennig, L, Schlamann, M, et al. Oversizing of the woven EndoBridge for treatment of intracranial aneurysms improves angiographic results (WEBINAR). World Neurosurg. (2024) 181:e182–91. doi: 10.1016/j.wneu.2023.09.090
44. Tsutsumi, K, Ueki, K, Morita, A, Usui, M, and Kirino, T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. (2001) 32:1191–4. doi: 10.1161/01.str.32.5.1191
45. Hecker, C, Hufnagl, C, Oellerer, A, Griessenauer, CJ, and Killer-Oberpfalzer, M. The Artisse Intrasaccular device: a new Intrasaccular flow diverter for the treatment of cerebral aneurysms. AJNR Am J Neuroradiol. (2025) 46:84–9. doi: 10.3174/ajnr.A8478
46. Jadhav, A, Kashyap, S, Bulu, H, Dholakia, R, Liu, AY, Syeda-Mahmood, T, et al. Towards automatic prediction of outcome in treatment of cerebral aneurysms. AMIA Annu Symp Proc. (2023) 2022:570–9. Available at: https://knowledge.amia.org/eventdata/esearch?q=Ashutosh&size=n_20_n
47. Belavadi, R, Gudigopuram, SVR, Raguthu, CC, Gajjela, H, Kela, I, Kakarala, CL, et al. Surgical clipping versus endovascular coiling in the Management of Intracranial Aneurysms. Cureus. (2021) 13:e20478. doi: 10.7759/cureus.20478
48. Kabbasch, C, Goertz, L, Siebert, E, Herzberg, M, Borggrefe, J, Dorn, F, et al. Factors that determine aneurysm occlusion after embolization with the woven EndoBridge (WEB). J Neurointerv Surg. (2019) 11:503–10. doi: 10.1136/neurintsurg-2018-014361
49. Cagnazzo, F, Cloft, HJ, Lanzino, G, and Brinjikji, W. WEB (woven EndoBridge) device for intracranial aneurysm treatment: technical, radiological, and clinical findings in a consecutive north American cohort. Acta Neurochir. (2023) 165:2077–86. doi: 10.1007/s00701-023-05668-6
50. Srinivasan, VM, Dmytriw, AA, Regenhardt, RW, Vicenty-Padilla, J, Alotaibi, NM, Levy, E, et al. Retreatment of residual and recurrent aneurysms after embolization with the woven EndoBridge device: multicenter case series. Neurosurgery. (2022) 90:569–80. doi: 10.1227/neu.0000000000001883
51. Hecker, C, Broussalis, E, Pfaff, JAR, Pikija, S, Griessenauer, CJ, and Killer-Oberpfalzer, M. Comparison of the contour neurovascular system and woven EndoBridge device for treatment of wide-necked cerebral aneurysms at a bifurcation or sidewall. J Neurosurg. (2023) 139:563–72. doi: 10.3171/2022.12.JNS222268
52. Goertz, L, Liebig, T, Siebert, E, Pennig, L, Laukamp, KR, Celik, E, et al. Woven Endobridge embolization versus microsurgical clipping for Unruptured anterior circulation aneurysms: a propensity score analysis. Neurosurgery. (2021) 88:779–84. doi: 10.1093/neuros/nyaa539
Keywords: intracranial aneurysm, wide-neck aneurysms, endovascular procedure, intrasaccular device, interventional therapy
Citation: Zhuo K, Wu H, Gou Z and Zhang C (2025) Intrasaccular therapy in wide-neck intracranial aneurysms: a narrative review. Front. Neurol. 16:1552848. doi: 10.3389/fneur.2025.1552848
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Bowen Sun, First Affiliated Hospital of Harbin Medical University, ChinaShail Thanki, Ochsner Lafayette General Hospital, United States
Copyright © 2025 Zhuo, Wu, Gou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changwei Zhang, Njg2NDYwNzVAcXEuY29t
†These authors have contributed equally to this work
 Kaiquan Zhuo
Kaiquan Zhuo Hongxia Wu
Hongxia Wu Zhongji Gou1
Zhongji Gou1