- 1Department of Neurosurgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Neurovascular Centre, Changhai Hospital, Naval Medical University, Shanghai, China
Objective: To report a novel clinical entity, “Multi-regional Sequential Pain Syndrome” (MRSPS), and evaluate the relationship between hypoperfusion, cryptogenic vertebral artery dissection (CVAD), and MRSPS.
Methods: A 59-year-old female patient, with a chronic MRSPS condition spanning 10–30 years, underwent a comprehensive diagnostic evaluation including cranial CTA, MRI, DSA, and CT perfusion, culminating in the identification of CVAD via dynamic contrast-enhanced computed tomography (DCE-CT). The treatment strategy integrated repairing CVADs with stent implantation to address the hypoperfusion and MRSPS.
Results: Following intervention, hypoperfusion achieved complete improvement, and the patient achieved the complete resolution of pain and vertigo, with the modified Rankin Scale (mRS) score of 0 at the 1-year follow-up, signifying full neurological recovery.
Conclusion: CVADs, a key cause of brain tissue hypoperfusion in MRSPS, can be effectively treated by repair to alleviate the syndrome.
Introduction
Pain significantly impairs patients’ sleep (1–3), psychology (4), cognition (5), immune responses in the nervous system (6), and overall quality of life, including work and daily activities. Therefore, effective and timely pain control is essential. However, the pathophysiology of pain is complex and often unclear, resulting in palliative treatments being the primary option in most cases (4). It is noteworthy that a type of widespread, serial pain involving multiple areas in a top-down manner has not yet been named or reported. In this study, its etiologies, mechanisms, diagnosis, and treatment are being thoroughly investigated and discussed for the first time.
Materials and methods
Case presentation
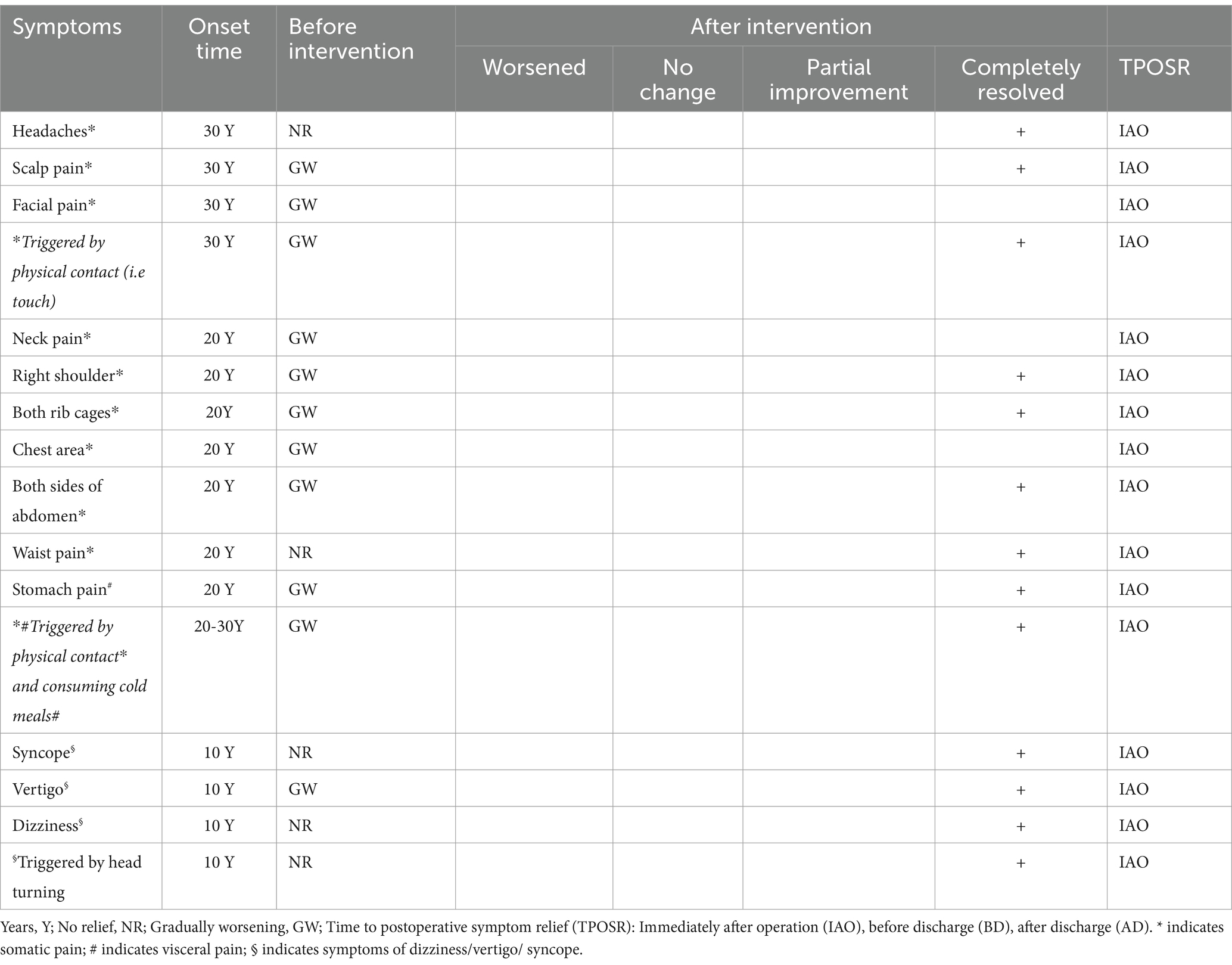
The patient, a middle-aged woman, has been enduring headaches, scalp pain, and facial pain for the past 36 years. Notably, these symptoms have been significantly aggravated by physical contact, particularly when her scalp and face are touched or during the process of washing. She has been taking over-the-counter pain medications for treatment. Twenty years ago, the patient developed additional pain in the neck, right shoulder, both rib cages, chest area, both sides of the abdomen, waist, and stomach. This pain, described as a series of linked sensations, could be triggered by touch, turning over, and consuming cold meals, milk, rice, and mixed congee, with poor relief from various pain medications and significant sleep disruption. In the last decade, the patient has also experienced persistent dizziness and vertigo that alternate, with head turning capable of inducing dizziness, sometimes leading to sudden falls. At night, during sleep, she often feels a spinning sensation or as if falling from a height. In the past 3 months, the series of linked pains have worsened, transitioning from intermittent to continuous, with pain medications becoming less effective. The patient has been treated for conditions such as neuralgia, disk herniation, and gastritis outside, with suboptimal outcomes.
Diagnosis
1. Chronic pain
2. Vertigo
3. Stomachache
4. Cryptogenic vertebral artery dissection
5. Multi-regional sequential pain syndrome
Test method
In this study, due to the patient’s presence of pain in multiple areas, the onset of pain occurred in a top-down sequence, with progressive exacerbation of the pain, and was accompanied by other intractable symptoms, such as stomachache, alternating episodes of dizziness and vertigo, and so on. Therefore, we firstly termed this clinical presentation as the “Multi-regional Sequential Pain Syndrome (MRSPS), as well confirmed in the following results.
Despite the absence of significant abnormalities in routine CT angiography (CTA, Figure 1A), magnetic resonance imaging (MRI), and digital subtraction angiography (DSA), cranial CT perfusion revealed hypoperfusion in the posterior circulation and bilateral temporal lobes (Figure 1B). Further examination with dynamic contrast enhanced computed tomography (DCE-CT) revealed CVAD in both vertebral arteries (Figures 1C–E) (7), with the right side being more severely affected (Figures 1C,D). The patient’s right vertebral artery exists a relatively smaller vascular diameter (Figure 1A), with relatively poor perfusion in the posterior circulation and the right temporal lobe (Figure 1B). Meanwhile, the right CVAD remains relatively longer. Especially, the most important characteristic of these dissections is that they are concealed within arteries that appear normal in morphology (7).
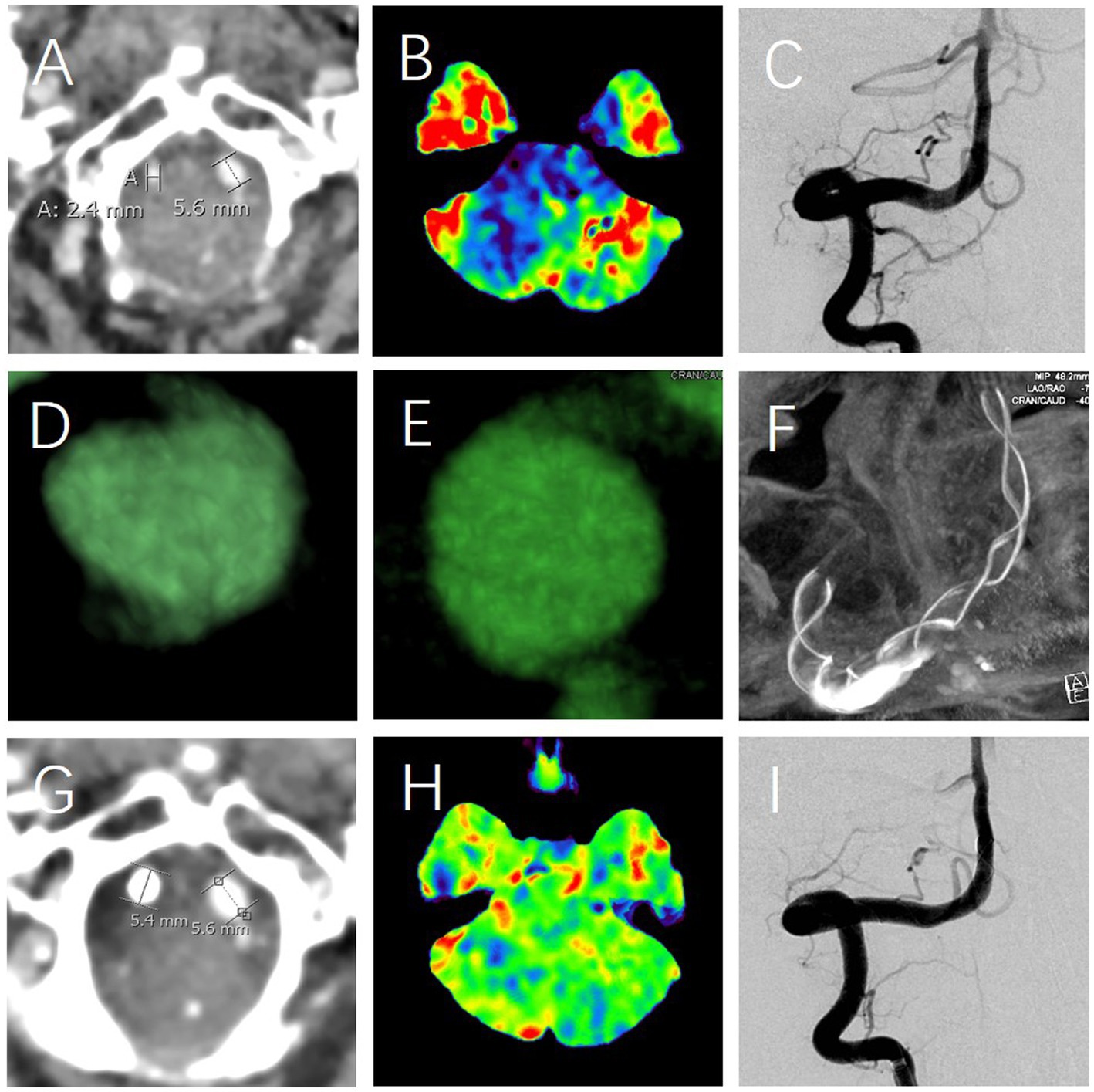
Figure 1. The diagnosis and treatment of multi-regional sequential pain syndrome. The first abnormality observed is a smaller right vertebral artery (A), coupled with a relatively better perfusion of posterior circulation (blue, B). DCE-CT identified the right CVAD, characterized by the normal vascular morphology (C) and abnormal intimal flaps (white arrow, D,E), which was subsequently addressed using a stent (F); The second significant abnormality observed was as follows: despite DSA showing no change in the morphological appearance of the vertebral artery (C vs. I), there were considerable changes detected in the vascular status (A vs. G) and perfusion (B vs. H) levels before and after the treatment. Specifically, the vascular status (A) and perfusion (B) exhibited marked differences when juxtaposed with their post-treatment counterparts in G and H, respectively.
Treatment and follow-up
The treatment strategy is to prioritize the elimination of the right CVAD. Therefore, on the basis of adequate dual antiplatelet medications, a stent reconstruction of the CVAD of the right vertebral artery was successfully performed under general anesthesia. Attentively, this form of dissection has also been effectively addressed in previous treatments (7). Throughout the perioperative period and during the postoperative 1-year clinical follow-up, the patient’s MRSPS has completely resolved (Table 1), allowing for the consumption of foods that previously triggered stomach pain without any issue. The patient’s symptoms of dizziness and vertigo have completely resolved, and the modified Rankin Scale (mRS) score was 0 at 12-month follow-up.
Discussion
The case presented in this study is a striking illustration of a previously uncharacterized clinical phenomenon, which we have termed MRSPS. The patient’s long-standing and progressively worsening pain, coupled with the alternating episodes of dizziness and vertigo, presented a complex diagnostic and therapeutic challenge. This discussion aims to explore the possible etiology, the rationale behind the treatment strategy, and the implications of outcomes.
Etiology
The etiology of MRSPS is likely multifactorial, with the patient’s CVADs playing a critical role. The dissections, particularly in the vertebral arteries, might be a cause of ischemia (7), leading to the hypoperfusion in the posterior circulation and bilateral temporal lobes (Figure 1B). This ischemia was related to the complex pain (8–10) and vertigo (11, 12), as seen in the patient following the successful therapy (Figure 1). The top-down progression of pain with vertigo might be attributed to the sequential involvement of the nervous system, starting from higher centers and progressively affecting lower regions, further supporting the mechanism of central pain (13).
Mechanisms
Ischemic CVADs can lead to the prolonged and chronic cerebral hypoperfusion (Figure 1B), potentially inducing pain and/or vertigo through the following mechanisms such as the demyelination (14), white matter degeneration (15), sustained abnormal activity of sensory neurons (16), lipid mediators represented by lysophosphatidic acid (17), inflammatory responses (18), neurotransmitter imbalances (19), neuronal dysfunction (20), cerebral autoregulatory disorders (21), and crosstalk between the immune system and nociceptive neurons (22), peripheral and central sensitization (23–25), and so on. When different neurons and nerve roots are affected, corresponding symptoms, such as pain and/or vertigo, occur. The physical triggers, such as touch and movement, in exacerbating the pain suggests a possible neurosensory component (26). This might be associated with the sensitization of both peripheral and central nociceptive pathways (27), a phenomenon commonly observed in chronic pain conditions. To a large extent, the success of peripheral nerve damage treatment is contingent upon brain plasticity during recovery (28), which was well exemplified in our study. With the recovery of hypoperfusion, the patient’s symptoms of pain and dizziness have completely disappeared (Figures 1B,H).
Treatment
The treatment strategy was guided by the principle of addressing the most significant pathology first. Given the relative severity and length of the right CVAD, it was identified as the primary target for therapy. The decision to take dual antiplatelets followed by stent implantation was based on the evidence supporting this approach in managing CVAD and improving perfusion (7, 29, 30). Following intervention, the diameter of the right vertebral artery has normalized (Figure 1A vs. Figure 1G), leading to an improvement in perfusion (Figure 1B vs. Figure 1H), and consequently, the patient’s symptoms have entirely resolved (Table 1). Therefore, stent reconstruction is especially important, as it targets not only the restoration of the dissecting artery integrity but also the potential mitigation of the ischemic brain insult (Figures 1F–H). This therapy aligns with the current viewpoint of the pathophysiology of MRSPS, where the improved perfusion is expected to alleviate its symptoms (Figure 1B vs. Figure 1H).
Outcomes
The complete resolution of the patient’s MRSPS post-therapy was a favorable outcome. In particular, it suggests that the identified CVADs were indeed the primary drivers of MRSPS. This study provides valuable insights into the therapy of complex pain syndromes with vascular etiologies. It underscores the importance of a comprehensive diagnostic approach, including advanced imaging techniques like DCE-CT, which can reveal cryptogenic but clinically significant vascular abnormalities (Figures 1C–E). Especially noteworthy is the fact that the persistent stomach pain, which had been resistant to treatment, has been resolved after the stent therapy (Figure 1). Moreover, foods that used to provoke the pain can now be eaten without issue. Despite the successful therapy in this case (Table 1), further study is required to understand the full spectrum of MRSPS, including identifying other etiologies, exploring the genetic and environmental factors, and developing standardized diagnostic and therapeutic criteria.
Conclusion
Hypoperfusion of brain tissue is one of the significant causes of MRSPS, and CVADs are a crucial pathological basis for this hypoperfusion. Repairing CVADs is an effective method for eliminating MRSPS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committees of Shanghai East Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XY: Conceptualization, Writing – original draft. K-JZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. J-ML: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Pudong New Area Health Commission (PW2022A-28) and Neuroscience Innovation Development Research Project (YXJL-2022-00351-0183).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haack, M, Simpson, N, Sethna, N, Kaur, S, and Mullington, J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. (2020) 45:205–16. doi: 10.1038/s41386-019-0439-z
2. Varallo, G, Giusti, EM, Manna, C, Castelnuovo, G, Pizza, F, Franceschini, C, et al. Sleep disturbances and sleep disorders as risk factors for chronic postsurgical pain: a systematic review and meta-analysis. Sleep Med Rev. (2022) 63:101630. doi: 10.1016/j.smrv.2022.101630
3. Albinni, B, de Zambotti, M, Iacovides, S, Baker, FC, and King, CD. The complexities of the sleep-pain relationship in adolescents: a critical review. Sleep Med Rev. (2023) 67:101715. doi: 10.1016/j.smrv.2022.101715
4. Cohen, SP, Vase, L, and Hooten, WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
5. Bushnell, MC, Ceko, M, and Low, LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. (2013) 14:502–11. doi: 10.1038/nrn3516
6. Fiore, NT, Debs, SR, Hayes, JP, Duffy, SS, and Moalem-Taylor, G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol. (2023) 19:199–220. doi: 10.1038/s41582-023-00777-3
7. Ma, J, Liu, Y, and Zhao, K. Microcephaly type 22 and autism spectrum disorder: a case report and review of literature. Dialogues Clin Neurosci. (2024) 26:24–7. doi: 10.1080/19585969.2024.2359918
8. Romanelli, MR, Thayer, JA, and Neumeister, MW. Ischemic pain. Clin Plast Surg. (2020) 47:261–5. doi: 10.1016/j.cps.2019.11.002
9. Hu, Q, Zheng, X, Chen, R, Liu, B, Tai, Y, Shao, X, et al. Chronic post-ischemia pain model for complex regional pain syndrome type-I in rats. J Vis Exp. (2020) 155:60562. doi: 10.3791/60562
10. O'Donnell, MJ, Diener, HC, Sacco, RL, Panju, AA, Vinisko, R, and Yusuf, S. Chronic pain syndromes after ischemic stroke: PRoFESS trial. Stroke. (2013) 44:1238–43. doi: 10.1161/STROKEAHA.111.671008
11. Paul, NL, Simoni, M, and Rothwell, PM. Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol. (2013) 12:65–71. doi: 10.1016/S1474-4422(12)70299-5
12. Tuna, MA, and Rothwell, PM. Diagnosis of non-consensus transient ischaemic attacks with focal, negative, and non-progressive symptoms: population-based validation by investigation and prognosis. Lancet. (2021) 397:902–12. doi: 10.1016/S0140-6736(20)31961-9
13. Phillips, K, and Clauw, DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. (2011) 25:141–54. doi: 10.1016/j.berh.2011.02.005
14. Mu, G, Li, Q, Lu, B, and Yu, X. Amelioration of nerve demyelination by hydrogen-producing silicon-based agent in neuropathic pain rats. Int Immunopharmacol. (2023) 117:110033. doi: 10.1016/j.intimp.2023.110033
15. Jakimovski, D, Topolski, M, Genovese, AV, Weinstock-Guttman, B, and Zivadinov, R. Vascular aspects of multiple sclerosis: emphasis on perfusion and cardiovascular comorbidities. Expert Rev Neurother. (2019) 19:445–58. doi: 10.1080/14737175.2019.1610394
16. Jones, MG, Rogers, ER, Harris, JP, Sullivan, A, Ackermann, DM, Russo, M, et al. Neuromodulation using ultra low frequency current waveform reversibly blocks axonal conduction and chronic pain. Sci Transl Med. (2021) 13:eabg9890. doi: 10.1126/scitranslmed.abg9890
17. Ueda, H. Pathogenic mechanisms of lipid mediator lysophosphatidic acid in chronic pain. Prog Lipid Res. (2021) 81:101079. doi: 10.1016/j.plipres.2020.101079
18. Sommer, C, Leinders, M, and Üçeyler, N. Inflammation in the pathophysiology of neuropathic pain. Pain. (2018) 159:595–602. doi: 10.1097/j.pain.0000000000001122
19. O'Hare, L, Tarasi, L, Asher, JM, Hibbard, PB, and Romei, V. Excitation-inhibition imbalance in migraine: from neurotransmitters to brain oscillations. Int J Mol Sci. (2023) 24:10093. doi: 10.3390/ijms241210093
20. Simms, BA, and Zamponi, GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. (2014) 82:24–45. doi: 10.1016/j.neuron.2014.03.016
21. Reinhard, M, Schork, J, Allignol, A, Weiller, C, and Kaube, H. Cerebellar and cerebral autoregulation in migraine. Stroke. (2012) 43:987–93. doi: 10.1161/STROKEAHA.111.644674
22. Conaghan, PG, Cook, AD, Hamilton, JA, and Tak, PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. (2019) 15:355–63. doi: 10.1038/s41584-019-0221-y
23. Ohashi, Y, Uchida, K, Fukushima, K, Inoue, G, and Takaso, M. Mechanisms of peripheral and central sensitization in osteoarthritis pain. Cureus. (2023) 15:e35331. doi: 10.7759/cureus.35331
24. Gustorff, B, Sycha, T, Lieba-Samal, D, Rolke, R, Treede, RD, and Magerl, W. The pattern and time course of somatosensory changes in the human UVB sunburn model reveal the presence of peripheral and central sensitization. Pain. (2013) 154:586–97. doi: 10.1016/j.pain.2012.12.020
25. Lang-Illievich, K, Klivinyi, C, Rumpold-Seitlinger, G, Dorn, C, and Bornemann-Cimenti, H. The effect of Palmitoylethanolamide on pain intensity, central and peripheral sensitization, and pain modulation in healthy volunteers-a randomized, double-blinded, placebo-controlled crossover trial. Nutrients. (2022) 14:4084. doi: 10.3390/nu14194084
26. Ashina, S, Robertson, CE, Srikiatkhachorn, A, et al. Trigeminal neuralgia. Nat Rev Dis Primers. (2024) 10:39. doi: 10.1038/s41572-024-00523-z
27. Li, C, Kim, HJ, Back, SK, and Na, HS. Common and discrete mechanisms underlying chronic pain and itch: peripheral and central sensitization. Pflugers Arch. (2021) 473:1603–15. doi: 10.1007/s00424-021-02599-y
28. Sunagawa, T, Ueda, A, Kurumadani, H, Zehry, HI, Date, S, and Ishii, Y. Transcranial direct current stimulation reduces ischemia-induced sensory disturbance in the hands of healthy subjects. Muscle Nerve. (2021) 64:610–3. doi: 10.1002/mus.27394
29. Zhao, K, Zhao, R, Yang, X, Guan, S, Liang, G, Wang, HL, et al. Predictors of unfavorable outcome in stent-assisted coiling for symptomatic unruptured intracranial spontaneous vertebral artery dissecting aneurysms (uis-VADAs): results from a multicenter study. J Neurointerv Surg. (2022) 14:1008–13. doi: 10.1136/neurintsurg-2021-017619
30. Zhao, KJ, Zhao, R, Huang, QH, Xu, Y, Hong, B, Fang, YB, et al. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with stent(s)-assisted coiling. Eur Radiol. (2014) 24:2088–96. doi: 10.1007/s00330-014-3225-7
Keywords: multi-regional sequential pain syndrome, hypoperfusion, cryptogenic vertebral artery dissection, pain, vertigo
Citation: Yang X, Zhao K-J and Liu J-M (2025) Multi-regional sequential pain syndrome. Front. Neurol. 16:1553455. doi: 10.3389/fneur.2025.1553455
Edited by:
Wangjun Qin, China-Japan Friendship Hospital, ChinaReviewed by:
Victor Mayoral, Bellvitge University Hospital, SpainXiangming Tang, Nanjing Medical University, China
Copyright © 2025 Yang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai-Jun Zhao, emtqd2NmendoQDE2My5jb20= Jian-Min Liu, Y2hzdHJva2VAMTYzLmNvbQ==
 Xiao Yang
Xiao Yang Kai-Jun Zhao
Kai-Jun Zhao Jian-Min Liu
Jian-Min Liu