Abstract
Objective:
This study aims to explore the correlation between glioma location in the limbic system and the risk of secondary epilepsy.
Methods:
This retrospective study included 170 cases of lower-grade gliomas treated with initial surgery from July 2007 to July 2019, sourced from the Chinese Glioma Genome Atlas (CGGA) database (http://www.cgga.org.cn). Patients were categorized into epilepsy and non-epilepsy groups based on postoperative symptoms. Imaging data were obtained from the Imaging Center of Beijing Tiantan Hospital, and postoperative epilepsy episodes were collected through follow-up. T2-weighted (T2WI) DICOM raw image data were converted into NII images, and tumor-susceptible regions were delineated using MRIcro software. Standardized T2WI gliomas and regions of interest (ROI) were analyzed using voxel-based lesion-symptom mapping (VLSM) software (https://crl.ucsd.edu/?). Overlapping ROIs were mapped for each patient. For voxel analysis in epilepsy-susceptible regions, the voxel with the highest t-value was defined as the peak voxel (PV). If the ROI overlapped with this voxel, the glioma was considered to confer a higher risk of epilepsy at that location. Statistical analysis was conducted using SPSS to compare glioma involvement in limbic system regions identified in imaging reports with patients’ postoperative epilepsy history.
Results:
Voxels significantly associated with tumor-related epilepsy were primarily located in the medial frontal lobe’s supplementary motor area of the left hemisphere. The peak voxel at this location was X = 88, Y = 155, Z = 134 (tmax = 4.69, p = 0.041), indicating the highest correlation with tumor-related epilepsy. Conversely, voxels less sensitive to epilepsy were mainly in the upper anterior cingulate gyrus. The peak voxel for this region was X = 91, Y = 166, Z = 79 (tmax = 3.70, p = 0.857), indicating the lowest correlation with tumor-related epilepsy.
Conclusion:
Epilepsy-susceptible regions of tumor-related epilepsy are located in the supplementary motor area of the medial frontal lobe in the left hemisphere. Regions not susceptible to epilepsy could primarily be in the anterior upper cingulate gyrus. Gliomas involving the anterior cingulate gyrus in the limbic system are associated with a lower postoperative risk of tumor-related epilepsy.
1 Introduction
Gliomas are the most common primary brain tumors (1), and epilepsy is one of the most frequent postoperative symptoms associated with gliomas (2–5). Histopathological studies suggest that tumor-related epilepsy is linked to changes in the tumor microenvironment, such as elevated concentrations of excitatory neurotransmitters like glutamate, which can promote seizure activity (6, 7). Additionally, tumor-related epilepsy is more commonly observed in lower-grade gliomas (5, 8, 9). The occurrence of preoperative tumor-related epilepsy has been found to correlate with tumor location, with tumors involving the temporal or frontal lobes being more prone to inducing seizures (9–11).
For postoperative tumor-related epilepsy, a meta-analysis revealed that favorable outcomes are associated with factors such as patient age over 45 years, focal seizures, complete tumor resection, and a preoperative epilepsy duration of less than 1 year. However, no significant correlation was found between tumors involving the temporal lobe and the risk of postoperative epilepsy (12). Other studies, however, have identified supratentorial tumors involving the temporal lobe, parietal lobe, and thalamus as independent risk factors for postoperative epilepsy (13). Thus, the relationship between tumor-involved regions and postoperative tumor-related epilepsy remains unclear.
Voxel-based lesion-symptom mapping (VLSM) enables precise voxel-level analysis, facilitating the identification of correlations between lesion location and specific tumor-related manifestations (14–16). VLSM has been widely applied in glioma-related research, revealing associations between tumor location and various aspects such as cognitive function (17–24), molecular pathology (25–30), genomics (31, 32), and prognosis (33–35). Regarding tumor-related epilepsy, previous studies have primarily focused on preoperative epilepsy. VLSM studies on glioblastoma have shown that preoperative epilepsy correlates with lesions in the superior and posterior frontal lobes, insula, and superior temporal lobe, while postoperative epilepsy is associated with the superior frontal gyrus, supplementary motor area, medial frontal region, anterior superior corona radiata, inferior medial occipital region, and caudate nucleus (33, 36).
In the limbic system, tumors involving the cingulate gyrus are associated with tumor-related epilepsy, whether preoperative or postoperative, while postoperative epilepsy is linked to the body of the corpus callosum. In our previous research using VLSM, preoperative tumor-related epilepsy in lower-grade gliomas was found to be associated with lesions in the left premotor area, part of which includes the cingulate gyrus (37). However, quantitative studies investigating the relationship between postoperative tumor-related epilepsy and tumor-involved regions in lower-grade gliomas (WHO grades II and III) remain limited.
To address this gap, this study retrospectively analyzes MRI scans of patients with lower-grade gliomas, utilizing VLSM technology to map voxel-level correlations between postoperative tumor-related epilepsy and non-epileptic outcomes. By elucidating the relationship between tumor location and epilepsy occurrence, this study aims to inform epilepsy management strategies for patients with lower-grade gliomas.
2 Materials and methods
2.1 Study participants
This study included 170 patients with lower-grade gliomas treated with initial surgery between July 2007 and July 2019. The patients were selected from the Chinese Glioma Genome Atlas (CGGA) database1 and the external set from the First Affiliated Hospital of Zhengzhou University (38). Inclusion criteria were as follows:
-
Pathological confirmation of lower-grade glioma (WHO grade II or III) according to the 2016 WHO classification of central nervous system tumors (39).
-
Preoperative magnetic resonance imaging (MRI) with at least T2-weighted imaging (T2WI) sequences available.
-
Patients aged 18 years or older.
-
No prior radiotherapy, chemotherapy, biopsy, or other invasive procedures or adjuvant treatments before surgery.
-
Availability of complete clinical data.
The study was approved by the Ethics Committee of Beijing Tiantan Hospital and adhered to the principles of the Helsinki Declaration. Informed consent was waived, as patients consented to the use of their clinical data for medical research at the time of admission.
2.2 Magnetic resonance imaging and preprocessing
All 170 included patients underwent preoperative MRI examinations performed on a Siemens MAGNETOM Prisma 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with an external set taken from the First Affiliated Hospital of Zhengzhou University. The MRI protocol and parameters included T2-weighted imaging (T2WI) sequences, with the following acquisition parameters:
-
Axial acquisition.
-
Repetition time (TR): 5,800 ms.
-
Echo time (TE): 10 ms.
-
Flip angle: 150 degrees.
-
Number of slices: 24.
-
Field of view: 240 × 188 mm2.
-
Voxel size: 0.6 × 0.6 × 5 mm3.
-
Matrix: 384 × 300.
The raw MRI data were saved in DICOM (Digital Imaging and Communications in Medicine) format. To facilitate image preprocessing, the files were converted to NIFTI (Neuroimaging Informatics Technology Initiative, “.nii”) format using the DICOM-to-NIFTI conversion tool in MRICRON software (https://www.nitrc.org/projects/mricron, McCausland Center for Brain Imaging, University of South Carolina). The converted files were indexed and stored in a database as backup data for subsequent analyses.
To delineate the tumor location and signal intensity, tumor-related information was extracted from the MRI scans. Tumor segmentation was performed based on the T2WI sequence to define regions of interest (ROI). Areas with abnormal signal intensities on T2WI were considered indicative of lower-grade gliomas (40–43). Tumor ROIs were segmented using MRIcro software.2 The ROI boundaries for each patient were independently delineated by two experienced neurosurgeons (with over 5 years of neuroimaging experience). Consistency analysis was conducted, and if the difference between their delineated ROIs exceeded 5%, a senior radiologist (with more than 20 years of neuroimaging experience) reviewed the images and defined the final tumor boundaries. All three physicians were blinded to the clinical data of the lower-grade glioma patients.
The T2WI images and segmented ROIs were then registered to the standard brain space (Montreal Neurological Institute, MNI-152 space). The registration process employed the standard nonlinear spatial normalization algorithm implemented in SPM8.3 Each patient’s images were resampled to 1 mm × 1 mm × 1 mm resolution for higher spatial accuracy (44–47).
2.3 Assessment of tumor-related epilepsy
Postoperative epilepsy was assessed through annual telephone follow-ups, starting 1 year after surgery. Patients and their family members provided detailed accounts of postoperative epilepsy, including the presence of seizures, the timing of seizures, seizure types, and postoperative antiepileptic treatments. Seizure types were classified based on the 2017 International League Against Epilepsy (ILAE) classification (48), categorizing seizures by their origin (focal onset or focal to bilateral tonic-clonic), level of awareness (impaired or retained awareness), motor or non-motor features, and more specific seizure subtypes.
2.4 Voxel-based lesion-symptom mapping
Standardized lower-grade glioma T2WI and ROI images were analyzed using voxel-based lesion-symptom mapping (VLSM) software4 to map the overlapping ROI regions across patients.
VLSM analysis employs a General Linear Model (GLM), which is widely used in medical statistics and can be adapted for various analyses, including ANOVA, regression, ANCOVA, and multilevel regression. The general relationship between the dependent variable Y and independent variables X in a GLM is expressed as follows in Equation 1:
where:
-
Y is the vector of observed dependent variable values.
-
X is the design matrix of independent variables.
-
β is the vector of regression coefficients.
-
e is the vector of independent random errors.
In this study:
-
Y: indicates tumor involvement at a voxel (1 = involved, 0 = not involved).
-
X: symptom matrix, where X1 is a constant symptom (1 = seizure, 0 = seizure-free), and X2, X3, … represent variables like gender and age etc.
The GLM computes the coefficient β1 for X1 and its corresponding t-value to test its significance (via p-value). A voxel is retained if its t-value exceeds a threshold determined through permutation testing (permutation testing, n = 1,000) (49). The threshold corresponds to the top 5% of t-values in the permutation distribution (α = 0.05, power >0.8).
The final seizure-prone region is defined by the retained voxels. Conversely, if seizure-free status is defined as X1 = 1 and seizure as X1 = 0, the analysis identifies the seizure-free sensitive region.
The voxel with the highest t-value in a sensitive region is defined as the peak voxel (PV). If an ROI involves the PV, the patient has a higher risk of experiencing the corresponding symptom.
2.5 Statistical analysis
All VLSM-related statistical analyses were conducted using MATLAB 2014a (The MathWorks Inc., MA, United States), while clinical variables were analyzed using R (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant, and all tests were two-sided.
Categorical variables were described as proportions, while continuous variables were evaluated for normality using the Shapiro–Wilk test (S–W test).
-
For normally distributed variables (p > 0.05), results were presented as mean ± standard deviation (SD).
-
For non-normally distributed variables (p < 0.05), results were presented as median ± interquartile range (IQR).
Statistical tests included
-
Categorical variables: Pearson’s chi-square test or Fisher’s exact test, depending on cell frequencies (chi-square if theoretical frequencies ≥5, otherwise Fisher’s exact test).
-
Continuous variables:
-
For normally distributed variables: unpaired t-test or analysis of variance (ANOVA).
-
For non-normally distributed variables: rank-sum tests such as Kruskal–Wallis (K–W test) or Mann–Whitney (M–W test).
-
3 Results
3.1 Clinical characteristics of patients
The primary clinical and pathological characteristics of the 170 patients are summarized in Table 1. Among the cohort, 119 patients (70%) experienced postoperative epilepsy, while 51 patients (30%) remained seizure-free.
Table 1
| Clinical information | Postoperative seizures | No postoperative seizures | Total | p-value |
|---|---|---|---|---|
| Total number (%) | 119 (70) | 51 (30) | 170 | — |
| Gender distribution (male, %) | 64 (53.78) | 33 (64.71) | 97 (57.06) | 0.25 |
| Age (mean ± SD) | 40.03 ± 9.92 | 39.88 ± 11.35 | — | 0.934 |
| Lobar involvement | ||||
| Parietal lobe | 15 | 10 | 25 | 0.001 |
| Frontal lobe | 44 | 86 | 130 | 0.041 |
| Temporal lobe | 19 | 55 | 74 | 0.278 |
| Insula | 14 | 39 | 53 | 0.489 |
| Occipital lobe | 1 | 1 | 2 | 0.552 |
| Limbic system | ||||
| Hippocampus | 0 | 7 | 7 | 0.024 |
| Amygdala | 0 | 2 | 2 | 0.231 |
| Thalamus | 1 | 5 | 6 | 0.444 |
| Corpus callosum | 5 | 16 | 21 | 0.500 |
| Cingulate gyrus | 2 | 4 | 6 | 0.857 |
| Mammillary body | 0 | 0 | 0 | — |
| Fornix | 0 | 0 | 0 | — |
| Hypothalamus | 0 | 0 | 0 | — |
Association of demographic factors and brain region involvement with postoperative seizure occurrence (N = 170).
Among the 170 patients, 119 (53.78%) experienced postoperative epilepsy. Statistical analysis showed that age and gender had no significant influence on postoperative seizures (p > 0.05).
-
Lobar involvement: seizures were significantly more associated with parietal lobe involvement (p = 0.001) and less frequent with frontal lobe involvement (p = 0.041 and p = 0.041). No significant associations were observed for the temporal, insular, or occipital lobes (p > 0.05).
-
Limbic system involvement: hippocampal involvement was significantly associated with postoperative seizures (p = 0.024), while other regions, such as the amygdala, thalamus, corpus callosum, and cingulate gyrus, showed no significant associations (p > 0.05).
Completely excluding the preoperative seizures from the no postoperative seizures group, that is the non-epilepsy group we had in all 44 patients (Supplementary Table 1).
3.2 Relationship between glioma location and risk of secondary epilepsy
Based on the tumor locations of 170 patients, an overlay map was created to summarize all lesions in these glioma patients (Figure 1). This map shows that many brain regions, including the bilateral frontal lobes, temporal lobes, and insular cortices, exhibit significant tumor overlap.
Figure 1

Tumor overlay map of glioma locations in 170 patients.
Additionally, to identify voxel distributions with sufficient statistical power, a power map based on p-values was constructed to detect voxels with adequate reliability in the VLSM analysis (p < 0.05). In this study, only results within regions with high reliability (>0.8) were included in the final analysis.
The power map demonstrates that the majority of regions in both hemispheres provide highly reliable statistical analyses (Figure 2).
Figure 2

Power map highlighting brain regions with sufficient statistical power for voxel-based lesion-symptom mapping (p < 0.05, power >0.8).
The VLSM analysis of this study, performed using MATLAB, identified the peak voxel (PV) coordinates as X = 88, Y = 155, Z = 134, with a t-value of tmax = 4.69. Mapping these results onto the MNI brain structure template and analyzing with MRIcron software revealed that the voxels significantly associated with tumor-related epilepsy were primarily located in Brodmann Area 8, specifically in the supplementary motor area (SMA) of the medial left frontal lobe. Based on this finding, we conclude that the SMA of the medial left frontal lobe demonstrates the strongest association with tumor-related epilepsy (Figures 3, 4).
Figure 3

VLSM analysis highlights the medial supplementary motor area of the left frontal lobe as the region most strongly associated with tumor-related epilepsy.
Figure 4

MRIcron Brodmann Area 8.
Additionally, we calculated the regions insensitive to tumor-related epilepsy. Following the VLSM analysis using MATLAB, the peak voxel (PV) coordinates for this region were identified as X = 91, Y = 166, Z = 79, with a t-value of tmax = 3.70, corresponding to MRIcron Brodmann Area 32. Mapping this voxel onto the MNI brain structure template revealed that the voxels insensitive to tumor-related epilepsy were primarily located in the superior anterior cingulate cortex. Based on this finding, we conclude that the superior anterior cingulate cortex has the lowest association with tumor-related epilepsy after surgery (see Figures 5,6).
Figure 5
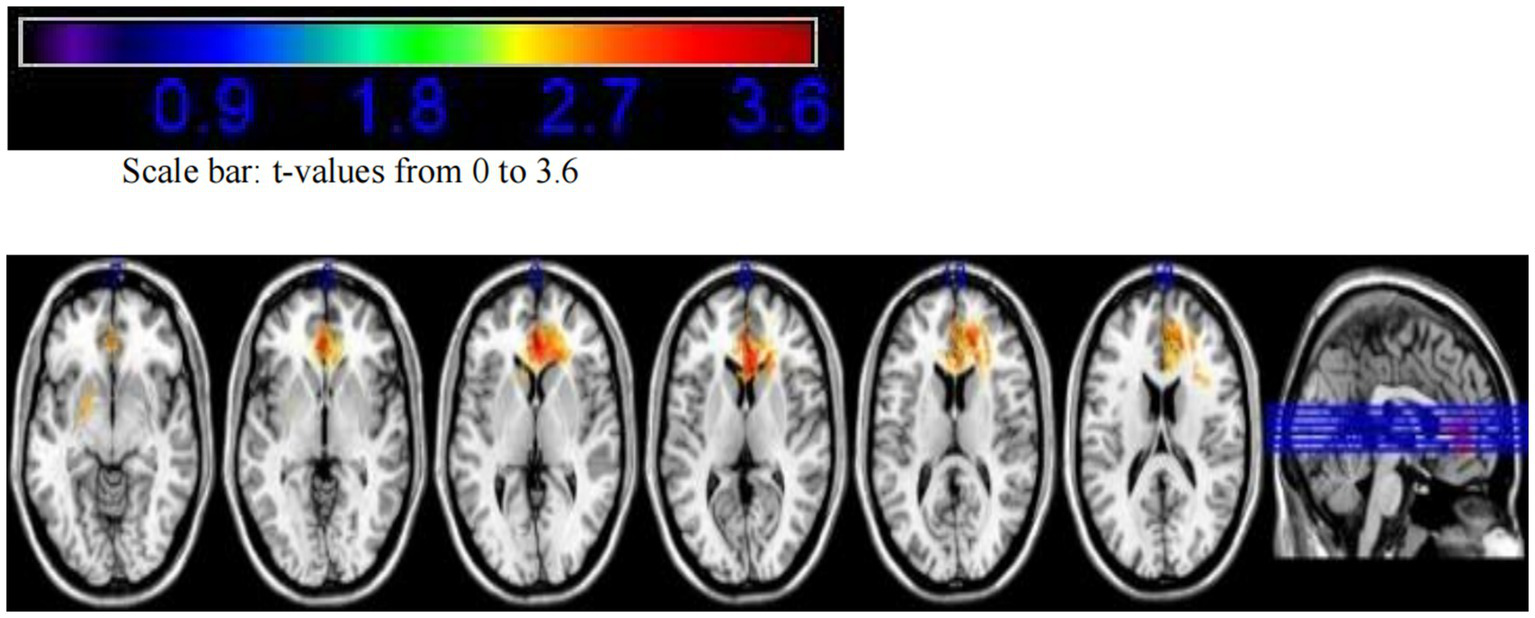
VLSM analysis indicates that the superior anterior cingulate cortex has the lowest association with tumor-related epilepsy.
Figure 6

MRIcron Brodmann Area 32.
3.3 Validation of epilepsy-susceptible regions identified by VLSM
Statistical analysis confirmed that the cingulate gyrus within the limbic system has the weakest correlation with postoperative epilepsy (p = 0.857). In contrast, the frontal lobe exhibits a certain degree of correlation. Additionally, compared to other brain lobes, the frontal lobe had the largest sample size in this study (p = 0.041) (Table 1).
4 Discussion
Tumor-related epilepsy is one of the most common symptoms following glioma surgery. This study aimed to determine the correlation between tumor-involved regions and postoperative tumor-related epilepsy at the voxel level and further explore whether low-grade gliomas involving the limbic system increase the risk of postoperative epilepsy. Retrospective analysis was conducted on 170 cases of low-grade gliomas (WHO grade II and III), employing VLSM (voxel-based lesion-symptom mapping) to identify epilepsy-prone regions. The epilepsy-prone area was found to be in the left medial frontal lobe’s supplementary motor area, while the non-epilepsy-prone region was primarily located in the anterior cingulate gyrus. Tumors involving the limbic system were associated with a lower risk of postoperative tumor-related epilepsy. These findings highlight the correlation between tumor-involved regions and postoperative epilepsy, offering valuable insights for predicting prognosis and developing anti-epileptic strategies for low-grade glioma patients. Statistical analysis revealed that the parietal and frontal lobes were more susceptible to postoperative epilepsy. Within the limbic system, gliomas involving the hippocampus showed a higher likelihood of causing epilepsy, likely due to its proximity to the temporal lobe.
4.1 Tumor-related epilepsy
Gliomas are the most common primary intracranial tumors (50). Epilepsy is one of the most frequent symptoms both preoperatively and postoperatively in glioma patients (51). In low-grade gliomas, preoperative tumor-related epilepsy occurs in approximately 65–90% of cases, with 70–90% of gliomas presenting with epilepsy as the initial symptom. Focal seizures progressing to bilateral tonic-clonic seizures account for approximately 67% of cases. In glioblastomas, epilepsy occurs in about 30–62% of patients, with two-thirds presenting with epilepsy as the initial symptom. Among these, 40% experience focal seizures progressing to bilateral tonic-clonic seizures, and 12% experience status epilepticus. Uncontrolled tumor-related epilepsy can severely impact patients’ quality of life and impose economic and emotional burdens on both patients and caregivers (52, 79). Conversely, preoperative tumor-related epilepsy is considered a favorable prognostic factor, as glioma patients with seizures generally have better outcomes (51).
The mechanism of tumor-related epilepsy is distinct from that of primary epilepsy. The epileptogenic focus is often located in the peritumoral cortex (53) rather than within the tumor core (80). Two primary mechanisms have been proposed: (1) Epileptic activity originates in peritumoral tissues due to mechanical compression, mass effect, local ischemia, hypoxia, and a reduction in pH, leading to the formation of epileptogenic foci (9, 53, 81, 82). The slow growth and infiltration of the tumor cause nerve conduction block in subcortical local and distant networks (83, 84). (2) Epileptic activity originates from the tumor itself, which secretes excitatory neurotransmitters such as glutamate, altering the microenvironment and disrupting the balance between excitatory and inhibitory mechanisms (9, 11, 54–58). Additionally, early postoperative epilepsy (within the first week) may be influenced by tumor location, histology, volume, edema, and postoperative complications (85–88). In high-grade gliomas, ischemia or hypoxia may contribute to early postoperative seizures (59, 60).
According to the 2016 WHO classification of central nervous system tumors (39), IDH mutations are one of the most critical molecular markers for glioma classification and prognosis. Several studies have identified a significant correlation between IDH mutations and preoperative tumor-related epilepsy (61–64). IDH mutations may contribute to postoperative epilepsy by producing D-2-hydroxyglutarate (D2HG), a structural analog of glutamate that mimics excitatory neurotransmitter activity at NMDA receptors, leading to hyperactive neuronal circuits and increased susceptibility to seizures (63). Exogenous D2HG also can increase the discharge rate of rat cortical cells (89–91). However, some studies suggest no significant association between IDH1 mutations and epilepsy in anaplastic gliomas (65). Another meta-analysis study found that in low-grade gliomas (WHO grade II), IDH mutation is associated with preoperative tumor-related epilepsy, with an odds ratio (OR) of 2.47 (95% confidence interval [CI], 1.70-3.57), however in higher-grade gliomas (WHO grades III-IV) there was no significant correlation (92).
Surgical resection, particularly removal of more than 90% of the tumor volume, is one of the most effective methods for controlling tumor-related epilepsy (11, 66–69). While some studies indicate that the extent of resection is unrelated to seizure control (93, 94), intraoperative electrocorticography (ECoG) may reduce postoperative epilepsy incidence (70, 71). Adjuvant treatments, including radiotherapy (96) and chemotherapy (95), have been shown to reduce seizure frequency by 50–60%, with 20–40% of patients achieving seizure freedom. The mechanisms may involve altering the microenvironment of epileptogenic foci or disrupting seizure-conducting pathways (72). Recurrent seizures after long-term seizure control in postoperative patients may be associated with tumor recurrence, particularly in glioblastoma (97).
A meta-analysis of postoperative epilepsy in glioma patients highlighted the association between postoperative epilepsy and factors such as age, preoperative seizure duration, extent of resection, and seizure type. Patients with focal seizures had a 32% higher risk of postoperative epilepsy, while those with generalized seizures had a 23% lower risk. Gross total resection increased the likelihood of seizure control by 47%. Patients aged ≥45 years and those with a preoperative seizure history of <1 year were more likely to achieve seizure control (12).
Evidence-based antiepileptic therapy is encouraged for managing tumor-related epilepsy (98–101). Levetiracetam is considered a first-line treatment due to its efficacy and safety profile (73). Lacosamide has also shown efficacy but is associated with side effects such as dizziness (74). This study underscores the importance of understanding the mechanisms, risk factors, and management strategies for tumor-related epilepsy, providing guidance for improving patient outcomes and quality of life (79).
4.2 Tumor location and tumor-related epilepsy
Low-grade gliomas involving the perilesional system, especially the insular lobe and frontal motor areas, are associated with tumor-related epilepsy (75). A meta-analysis including 16 studies with a total of 4,323 patients showed that tumor location is the strongest predictor of tumor-related epilepsy. Gliomas involving the occipital lobe were least likely to cause preoperative tumor-related epilepsy, while those involving the frontal lobe had the highest likelihood. In contrast, the parietal, occipital, and temporal lobes showed no significant correlation with tumor-related epilepsy (76). The mechanism behind the higher epilepsy risk associated with gliomas involving the frontal lobe remains unclear. On one hand, the frontal lobe is the largest among all brain lobes, and gliomas are most likely to form in this region, thus increasing the incidence of epilepsy related to gliomas in this area (77). On the other hand, the frontal lobe has widespread connections with surrounding brain regions, including the thalamus, basal ganglia, and brainstem. Abnormal discharges from the frontal lobe can spread to these areas, triggering corresponding seizures. Tumors in the frontal lobe primarily affect the cortex and superficial brain tissues, with minimal involvement of deep white matter fibers, which helps facilitate the spread of abnormal discharges (78).
In a VLSM study on glioblastomas, preoperative tumor-related epilepsy was found to be associated with the right superior, middle, and inferior temporal gyri, the right fusiform gyrus, the parahippocampal gyrus, the right anterior and posterior central gyri, the right middle frontal gyrus, the left posterior central gyrus, the left superior frontal gyrus, and the bilateral cingulate gyri. The voxel distribution related to epilepsy was extensive, with the largest voxel clusters found in the right anterior and posterior central gyri, the left posterior central gyrus, and the right temporal lobe and parahippocampal gyrus, indicating that tumors affecting these regions have the highest value for analyzing tumor-related epilepsy (33).
In our study, the epilepsy-prone area was found to be in the left medial frontal gyrus in the supplementary motor area, while the non-epilepsy-prone area was primarily in the anterior part of the cingulate gyrus. Therefore, tumors involving the limbic system have a lower risk of postoperative tumor-related epilepsy. Combining the findings from the above VLSM-based studies, the pre-motor area appears to be the most important epilepsy-sensitive area, likely due to the fact that motor seizures are more easily observed. Regarding the limbic system, the relationship with tumor-related epilepsy is contradictory. Tumors involving the parahippocampal gyrus and cingulate gyrus are associated with preoperative epilepsy in high-grade gliomas, and those involving the anterior medial part of the cingulate gyrus, the knee and body of the corpus callosum are related to postoperative epilepsy. However, lesions in the posterior hippocampus, parahippocampal gyrus, fornix, and amygdala in high-grade gliomas showed no association with postoperative epilepsy, and lesions in the left cingulate gyrus were linked to preoperative epilepsy in low-grade gliomas. Our study, however, found no association between cingulate gyrus involvement and postoperative seizures in low-grade gliomas. This suggests that further large-scale prospective studies, combined with video EEG for epilepsy classification might be needed to better differentiate the correlation between different locations in the limbic system and epilepsy.
4.3 Limitations
This study is a single-center retrospective study, and its findings require further validation through large-sample, multi-center studies. The follow-up of postoperative epilepsy symptoms was mainly conducted through phone follow-ups, where descriptions from patients and their families were used to determine the presence and specific types of postoperative seizures. The lack of auxiliary examination methods, such as video-EEG, means that the diagnosis and classification of seizures may be biased.
5 Conclusion
This retrospective study included 170 patients with low-grade gliomas (WHO grade II and III). Using the VLSM method, we identified the epilepsy-prone area for tumor-related epilepsy, located in the left medial frontal gyrus in the supplementary motor area. The non-epilepsy-prone area was primarily located in the anterior part of the cingulate gyrus. Therefore, tumors involving the limbic system (such as the cingulate gyrus, corpus callosum, hypothalamus, and amygdala) have a lower risk of postoperative tumor-related epilepsy. This study clarifies the correlation between tumor involvement and postoperative tumor-related epilepsy, thus providing helpful guidance for prognostic prediction and the development of antiepileptic strategies for low-grade gliomas after surgery.
Statements
Data availability statement
The datasets presented in this article are available from the first author upon reasonable request. Requests should be directed to NM, mandelawilliam@163.com.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Tiantan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NM: Writing – original draft, Writing – review & editing. LW: Writing – review & editing. LX: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to thank Doctors Yinyan Wang, Ziwen Fan, Yuchao Liang, Mingzhe Han and Mateus Tamba N’Dende Macho for their help in analyzing the data and bringing out the final draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1556286/full#supplementary-material
Footnotes
References
1.
Muster RH Young JS Woo PYM Morshed RA Warrier G Kakaizada S et al . The relationship between stimulation current and functional site localization during brain mapping. Neurosurgery. (2020) 88:1043–50. doi: 10.1093/neuros/nyaa364,
2.
Lynam LM Lyons MK Drazkowski JF Sirven JI Noe KH Zimmerman RS et al . Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. (2007) 109:634–8. doi: 10.1016/j.clineuro.2007.05.017
3.
Scott GM Gibberd FB . Epilepsy and other factors in the prognosis of gliomas. Acta Neurol Scand. (1980) 61:227–39. doi: 10.1111/j.1600-0404.1980.tb01487.x,
4.
Vertosick FT Jr Selker RG Arena VC . Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery. (1991) 28:496–501. doi: 10.1227/00006123-199104000-00002,
5.
Taphoorn MJB . Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. (2003) 30:45–8. doi: 10.1053/j.seminoncol.2003.11.023,
6.
Huang L You G Jiang T Li G Li S Wang Z . Correlation between tumor-related seizures and molecular genetic profile in 103 Chinese patients with low-grade gliomas: a preliminary study. J Neurol Sci. (2011) 302:63–7. doi: 10.1016/j.jns.2010.11.024,
7.
de Groot M Reijneveld JC Aronica E Heimans JJ . Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. (2012) 135:1002–16. doi: 10.1093/brain/awr310,
8.
Lee JW Wen PY Hurwitz S Black P Kesari S Drappatz J et al . Morphological characteristics of brain tumors causing seizures. Arch Neurol. (2010) 67:336–42. doi: 10.1001/archneurol.2010.2,
9.
van Breemen MSM Wilms EB Vecht CJ . Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. (2007) 6:421–30. doi: 10.1016/S1474-4422(07)70103-5,
10.
Duffau H Capelle L Denvil D Sichez N Gatignol P Taillandier STL et al . Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. (2003) 98:764–78. doi: 10.3171/jns.2003.98.4.0764,
11.
Chang EF Potts MB Evren Keles G Lamborn KR Chang SM Barbaro NM et al . Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. (2008) 108:227–35. doi: 10.3171/JNS/2008/108/2/0227,
12.
Shan X Fan X Liu X Zhao Z Wang Y Jiang T . Clinical characteristics associated with postoperative seizure control in adult low-grade gliomas: a systematic review and meta-analysis. Neuro-Oncol. (2018) 20:324–31. doi: 10.1093/neuonc/nox130,
13.
Saadeh FS Melamed EF Rea ND Krieger MD . Seizure outcomes of supratentorial brain tumor resection in pediatric patients. Neuro-Oncol. (2018) 20:1272–81. doi: 10.1093/neuonc/noy026,
14.
Dronkers NF Wilkins DP Van Valin RD Jr Redfern BB Jaeger JJ . Lesion analysis of the brain areas involved in language comprehension. Cognition. (2004) 92:145–77. doi: 10.1016/j.cognition.2003.11.002,
15.
Bates E Wilson SM Saygin AP Dick F Sereno MI Knight RT et al . Voxel-based lesion-symptom mapping. Nat Neurosci. (2003) 6:448–50. doi: 10.1038/nn1050
16.
Verdon V Schwartz S Lovblad KO Hauert CA Vuilleumier P . Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. (2010) 133:880–94. doi: 10.1093/brain/awp305,
17.
Niki C Kumada T Maruyama T Tamura M Kawamata T Muragaki Y . Primary cognitive factors impaired after glioma surgery and associated brain regions. Behav Neurol. (2020) 2020:1–9. doi: 10.1155/2020/7941689,
18.
Habets EJJ Hendriks EJ Taphoorn MJB Douw L Zwinderman AH Vandertop WP et al . Association between tumor location and neurocognitive functioning using tumor localization maps. J Neuro-Oncol. (2019) 144:573–82. doi: 10.1007/s11060-019-03259-z,
19.
Pisoni A Mattavelli G Casarotti A Comi A Riva M Bello L et al . The neural correlates of auditory-verbal short-term memory: a voxel-based lesion-symptom mapping study on 103 patients after glioma removal. Brain Struct Funct. (2019) 224:2199–211. doi: 10.1007/s00429-019-01902-z,
20.
Campanella F Del Missier F Shallice T Skrap M . Localizing memory functions in brain tumor patients: anatomical hotspots over 260 patients. World Neurosurg. (2018) 120:e690–709. doi: 10.1016/j.wneu.2018.08.145,
21.
Pisoni A Mattavelli G Casarotti A Comi A Riva M Bello L et al . Object-action dissociation: a voxel-based lesion-symptom mapping study on 102 patients after glioma removal. NeuroImage Clin. (2018) 18:986–95. doi: 10.1016/j.nicl.2018.03.022,
22.
Nakajima R Kinoshita M Okita H Yahata T Matsui M Nakada M . Neural networks mediating high-level mentalizing in patients with right cerebral hemispheric gliomas. Front Behav Neurosci. (2018) 12:33. doi: 10.3389/fnbeh.2018.00033,
23.
Kinoshita M Nakajima R Shinohara H Miyashita K Tanaka S Okita H et al . Chronic spatial working memory deficit associated with the superior longitudinal fasciculus: a study using voxel-based lesion-symptom mapping and intraoperative direct stimulation in right prefrontal glioma surgery. J Neurosurg. (2016) 125:1024–32. doi: 10.3171/2015.10.JNS1591,
24.
Banerjee P Leu K Harris RJ Cloughesy TF Lai A Nghiemphu PL et al . Association between lesion location and language function in adult glioma using voxel-based lesion-symptom mapping. NeuroImage Clin. (2015) 9:617–24. doi: 10.1016/j.nicl.2015.10.010,
25.
Wang K Wang Y Fan X Li Y Liu X Wang J et al . Regional specificity of 1p/19q co-deletion combined with radiological features for predicting the survival outcomes of anaplastic oligodendroglial tumor patients. J Neuro-Oncol. (2018) 136:523–31. doi: 10.1007/s11060-017-2673-8,
26.
Darlix A Deverdun J Menjot N de Champfleur F Castan SZ Rigau V et al . IDH mutation and 1p19q codeletion distinguish two radiological patterns of diffuse low-grade gliomas. J Neuro-Oncol. (2017) 133:37–45. doi: 10.1007/s11060-017-2421-0,
27.
Wang Y Zhang T Li S Fan X Ma J Wang L et al . Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas. Eur J Neurol. (2015) 22:348–54. doi: 10.1111/ene.12578,
28.
Zhang T Wang Y Fan X Ma J Li S Jiang T et al . Anatomical localization of p53 mutated tumors: a radiographic study of human glioblastomas. J Neurol Sci. (2014) 346:94–8. doi: 10.1016/j.jns.2014.07.066,
29.
Wang YY Zhang T Li SW Qian TY Fan X Peng XX et al . Mapping p53 mutations in low-grade glioma: a voxel-based neuroimaging analysis. AJNR Am J Neuroradiol. (2015) 36:70–6. doi: 10.3174/ajnr.A4065,
30.
Wang Y Fan X Zhang C Zhang T Peng X Li S et al . Anatomical specificity of O6-methylguanine DNA methyltransferase protein expression in glioblastomas. J Neuro-Oncol. (2014) 120:331–7. doi: 10.1007/s11060-014-1555-6,
31.
Tejada Neyra MA Neuberger U Reinhardt A Brugnara G Bonekamp D Sill M et al . Voxel-wise radiogenomic mapping of tumor location with key molecular alterations in patients with glioma. Neuro-Oncol. (2018) 20:1517–24. doi: 10.1093/neuonc/noy134,
32.
Yuan Y Yunhe M Xiang W Yanhui L Ruofei L Jiewen L et al . Mapping genetic factors in high-grade glioma patients. Clin Neurol Neurosurg. (2016) 150:159–63. doi: 10.1016/j.clineuro.2016.09.012
33.
Roux A Roca P Edjlali M Sato K Zanello M Dezamis E et al . MRI atlas of IDH wild-type supratentorial glioblastoma: probabilistic maps of phenotype, management, and outcomes. Radiology. (2019) 293:633–43. doi: 10.1148/radiol.2019190491,
34.
Nakajima R Kinoshita M Yahata T Nakada M . Recovery time from supplementary motor area syndrome: relationship to postoperative day 7 paralysis and damage of the cingulum. J Neurosurg. (2019) 132:865–74. doi: 10.3171/2018.10.JNS182391,
35.
Sagberg LM Iversen DH Fyllingen EH Jakola AS Reinertsen I Solheim O . Brain atlas for assessing the impact of tumor location on perioperative quality of life in patients with high-grade glioma: a prospective population-based cohort study. NeuroImage Clin. (2019) 21:101658. doi: 10.1016/j.nicl.2019.101658,
36.
Cayuela N Simó M Majós C Rifà-Ros X Gállego Pérez-Larraya J Ripollés P et al . Seizure-susceptible brain regions in glioblastoma: identification of patients at risk. Eur J Neurol. (2018) 25:387–94. doi: 10.1111/ene.13518,
37.
Wang Y Qian T You G Peng X Chen C You Y et al . Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro-Oncol. (2015) 17:282–8. doi: 10.1093/neuonc/nou130,
38.
Zhao Z Zhang K Wang Q Li G Zeng F Zhang Y et al . Chinese Glioma Genome Atlas (CGGA): a comprehensive resource with functional genomic data from Chinese glioma patients. Genomics Proteomics Bioinformatics. (2020) 19:1–12. doi: 10.1016/j.gpb.2020.10.005
39.
Louis DN Perry A Reifenberger G von Deimling A Figarella-Branger D Cavenee WK et al . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1,
40.
Li Y Qian Z Xu K Wang K Fan X Li S et al . MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. NeuroImage Clin. (2018) 17:306–11. doi: 10.1016/j.nicl.2017.10.030,
41.
Wang Y Fan X Li H Lin Z Bao H Li S et al . Tumor border sharpness correlates with HLA-G expression in low-grade gliomas. J Neuroimmunol. (2015) 282:1–6. doi: 10.1016/j.jneuroim.2015.02.013,
42.
Kinoshita M Sakai M Arita H Shofuda T Chiba Y Kagawa N et al . Introduction of high throughput magnetic resonance T2-weighted image texture analysis for WHO grade 2 and 3 gliomas. PLoS One. (2016) 11:e0164268. doi: 10.1371/journal.pone.0164268,
43.
Mandonnet E Delattre J-Y Tanguy M-L Swanson KR Carpentier AF Duffau H et al . Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. (2003) 53:524–8. doi: 10.1002/ana.10528,
44.
Louis Collins D Neelin P Peters TM Evans AC . Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. (1994) 18:192–205. doi: 10.1097/00004728-199403000-00005,
45.
Evans AC . An MRI-based stereotactic atlas from 250 young normal subjects. Soc Neurosci Abstr. (1992) 18:408.
46.
Evans AC Marrett S Neelin P Collins L Worsley K Dai W et al . Anatomical mapping of functional activation in stereotactic coordinate space. NeuroImage. (1992) 1:43–53. doi: 10.1016/1053-8119(92)90006-9,
47.
Evans AC Collins DL Mills SR Brown ED Kelly RL Peters TM . (1993). 3D statistical neuroanatomical models from 305 MRI volumes. 1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference. 1813–1817
48.
Fisher RS Helen Cross J French JA Higurashi N Hirsch E Jansen FE et al . Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670,
49.
Medina J Kimberg DY Anjan Chatterjee H Coslett B . Inappropriate usage of the Brunner–Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. (2010) 48:341–3. doi: 10.1016/j.neuropsychologia.2009.09.016,
50.
Tao Jiang GF Tang YL Peng XX Xiao Zhang XW Zhai XP Yang JQ et al . Prevalence estimates for primary brain tumors in China: a multi-center cross-sectional study. Chin Med J. (2011) 124:2578–83. doi: 10.3760/cma.j.issn.0366-6999.2011.17.003
51.
Fan X Li Y Shan X You G Wu Z Li Z et al . Seizures at presentation are correlated with better survival outcomes in adult diffuse glioma: a systematic review and meta-analysis. Seizure. (2018) 59:16–23. doi: 10.1016/j.seizure.2018.04.018,
52.
Hixson JD . Stopping antiepileptic drugs: when and why?Curr Treat Options Neurol. (2010) 12:434–42. doi: 10.1007/s11940-010-0083-8,
53.
Habela CW Ernest NJ Swindall AF Sontheimer H . Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J Neurophysiol. (2009) 101:750–7. doi: 10.1152/jn.90840.2008,
54.
Ricci R Bacci A Tugnoli V Battaglia S Maffei M Agati R et al . Metabolic findings on 3T 1H-MR spectroscopy in peritumoral brain edema. AJNR Am J Neuroradiol. (2007) 28:1287–91. doi: 10.3174/ajnr.A0564,
55.
Ye ZC Sontheimer H . Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. (1999) 59:4383–91.
56.
Bordey A Sontheimer H . Electrophysiological properties of human astrocytic tumor cells in situ: enigma of spiking glial cells. J Neurophysiol. (1998) 79:2782–93. doi: 10.1152/jn.1998.79.5.2782,
57.
Bordey A Sontheimer H . Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. (1998) 32:286–303. doi: 10.1016/S0920-1211(98)00059-X,
58.
Armstrong TS Grant R Gilbert MR Lee JW Norden AD . Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro-Oncol. (2016) 18:779–89. doi: 10.1093/neuonc/nov269,
59.
Chassoux F Landre E . Prevention and management of postoperative seizures in neuro-oncology. Neurochirurgie. (2017) 63:197–203. doi: 10.1016/j.neuchi.2016.10.013,
60.
Yuen TI Morokoff AP Bjorksten A D'Abaco G Paradiso L Finch S et al . Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology. (2012) 79:883–9. doi: 10.1212/WNL.0b013e318266fa89,
61.
Liubinas SV D'Abaco GM Moffat BM Gonzales M Feleppa F Nowell CJ et al . IDH1 mutation is associated with seizures and protoplasmic subtype in patients with low-grade gliomas. Epilepsia. (2014) 55:1438–43. doi: 10.1111/epi.12662
62.
Stockhammer F Misch M Helms HJ Lengler U Prall F von Deimling A et al . IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. (2012) 21:194–7. doi: 10.1016/j.seizure.2011.12.007,
63.
Chen H Judkins J Thomas C Wu M Khoury L Benjamin CG et al . Mutant IDH1 and seizures in patients with glioma. Neurology. (2017) 88:1805–13. doi: 10.1212/WNL.0000000000003911,
64.
Toledo M Sarria-Estrada S Quintana M Maldonado X Martinez-Ricarte F Rodon J et al . Epileptic features and survival in glioblastomas presenting with seizures. Epilepsy Res. (2017) 130:1–6. doi: 10.1016/j.eplepsyres.2016.12.013,
65.
Yang P You G Zhang W Wang Y Wang Y Yao K et al . Correlation of preoperative seizures with clinicopathological factors and prognosis in anaplastic gliomas: a report of 198 patients from China. Seizure. (2014) 23:844–51. doi: 10.1016/j.seizure.2014.07.003,
66.
Hildebrand J Lecaille C Perennes J Delattre JY . Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. (2005) 65:212–5. doi: 10.1212/01.wnl.0000168903.09277.8f,
67.
Zaatreh MM Firlik KS Spencer DD Spencer SS . Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. (2003) 61:636–41. doi: 10.1212/01.WNL.0000079374.78589.1B
68.
Simon M Neuloh G von Lehe M Meyer B Schramm J . Insular gliomas: the case for surgical management. J Neurosurg. (2009) 110:685–95. doi: 10.3171/2008.7.JNS17639,
69.
Duffau H Capelle L Lopes M Bitar A Sichez JP van Effenterre R . Medically intractable epilepsy from insular low-grade gliomas: improvement after an extended lesionectomy. Acta Neurochir. (2002) 144:563–73. doi: 10.1007/s00701-002-0941-6,
70.
Fallah A Weil AG Sur S Miller I Jayakar P Morrison G et al . Epilepsy surgery related to pediatric brain tumors: Miami Children’s hospital experience. J Neurosurg Pediatr. (2015) 16:675–80. doi: 10.3171/2015.4.PEDS14476,
71.
Yao P-S Zheng S-F Wang F Kang D-Z Lin Y-X . Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J Neurosurg. (2017) 128:840–5. doi: 10.3171/2016.11.JNS161296,
72.
Pace A Vidiri A Galiè E Carosi M Telera S Cianciulli AM et al . Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. (2003) 14:1722–6. doi: 10.1093/annonc/mdg502
73.
Nasr ZG Paravattil B Wilby KJ . Levetiracetam for seizure prevention in brain tumor patients: a systematic review. J Neuro-Oncol. (2016) 129:1–13. doi: 10.1007/s11060-016-2146-5,
74.
Villanueva V Saiz-Diaz R Toledo M Piera A Mauri JA Rodriguez-Uranga JJ et al . NEOPLASM study: real-life use of lacosamide in patients with brain tumor-related epilepsy. Epilepsy Behav. (2016) 65:25–32. doi: 10.1016/j.yebeh.2016.09.033,
75.
Pallud J McKhann GM . Diffuse low-grade glioma-related epilepsy. Neurosurg Clin N Am. (2019) 30:43–54. doi: 10.1016/j.nec.2018.09.001,
76.
Zhang J Yao L Peng S Fang Y Tang R Liu J . Correlation between glioma location and preoperative seizures: a systematic review and meta-analysis. Neurosurg Rev. (2019) 42:603–18. doi: 10.1007/s10143-018-1014-5
77.
Chaichana KL Parker SL Olivi A Quiñones-Hinojosa A . Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. J Neurosurg. (2009) 111:282–92. doi: 10.3171/2009.2.JNS081132,
78.
Leone MA Ivashynka AV Tonini MC Bogliun G Montano V Ravetti C et al . Risk factors for a first epileptic seizure symptomatic of brain tumour or brain vascular malformation. A case control study. Swiss Med Wkly. (2011) 141:w13155. doi: 10.4414/smw.2011.13155,
79.
Pace A Dirven L Koekkoek JAF Golla H Fleming J Rudà R et al . European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. (2017) 18:e330–40. doi: 10.1016/S1470-2045(17)30345-5,
80.
Pallud J Le Van Quyen M Bielle F Pellegrino C Varlet P Cresto N et al . Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med. (2014) 6:244ra289. doi: 10.1126/scitranslmed.3008065
81.
Schaller B . Influences of brain tumor-associated pH changes and hypoxia on epileptogenesis. Acta Neurol Scand. (2005) 111:75–83. doi: 10.1111/j.1600-0404.2004.00355.x,
82.
Wolf HK Roos D Blümcke I Pietsch T Wiestler OD . Perilesional neurochemical changes in focal epilepsies. Acta Neuropathol. (1996) 91:376–84. doi: 10.1007/s004010050439
83.
Beaumont A Whittle IR . The pathogenesis of tumour associated epilepsy. Acta Neurochir. (2000) 142:1–15. doi: 10.1007/s007010050001,
84.
Shamji MF Fric-Shamji EC Benoit BG . Brain tumors and epilepsy: pathophysiology of peritumoral changes. Neurosurg Rev. (2009) 32:275–85. doi: 10.1007/s10143-009-0191-7,
85.
Manaka S Ishijima B Mayanagi Y . Postoperative seizures: epidemiology, pathology, and prophylaxis. Neurol Med Chir. (2003) 43:589–600. doi: 10.2176/nmc.43.589,
86.
Yu Sokolova E Savin IA Kadasheva AB Gavryushin AV Pitskhelauri DI Kozlov AV et al . The management of patients with new epileptic seizures in the early period after resection of hemispheric tumors: two case reports and a literature review. Zh Vopr Neirokhir Im NN Burdenko. (2017) 81:96–103. doi: 10.17116/neiro201781596-103,
87.
Tigaran S Cascino GD McClelland RL So EL Marsh WR . Acute postoperative seizures after frontal lobe cortical resection for intractable partial epilepsy. Epilepsia. (2003) 44:831–5. doi: 10.1046/j.1528-1157.2003.56402.x,
88.
Skardelly M Brendle E Noell S Behling F Wuttke TV Schittenhelm J et al . Predictors of preoperative and early postoperative seizures in patients with intra-axial primary and metastatic brain tumors: a retrospective observational single center study. Ann Neurol. (2015) 78:917–28. doi: 10.1002/ana.24522,
89.
Dang L White DW Gross S Bennett BD Bittinger MA Driggers EM et al . Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. (2010) 465:966. doi: 10.1038/nature09132,
90.
Moussawi K Riegel A Nair S Kalivas PW . Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci. (2011) 5:94. doi: 10.3389/fnsys.2011.00094,
91.
Kalinina J Ahn J Devi NS Wang L Li Y Olson JJ et al . Selective detection of the D-enantiomer of 2-hydroxyglutarate in the CSF of glioma patients with mutated isocitrate dehydrogenase. Clin Cancer Res. (2016) 22:6256–65. doi: 10.1158/1078-0432.CCR-15-2965,
92.
Phan K Ng W Lu VM McDonald KL Fairhall J Reddy R et al . Association between IDH1 and IDH2 mutations and preoperative seizures in patients with low-grade versus high-grade glioma: a systematic review and meta-analysis. World Neurosurg. (2018) 111:e539–45. doi: 10.1016/j.wneu.2017.12.112,
93.
Michelucci R Pasini E Meletti S Fallica E Rizzi R Florindo I et al . Epilepsy in primary cerebral tumors: the characteristics of epilepsy at the onset (results from the PERNO study—Project of Emilia Romagna Region on Neuro-Oncology). Epilepsia. (2013) 54:86–91. doi: 10.1111/epi.12314
94.
Rosati A Tomassini A Pollo B Ambrosi C Schwarz A Padovani A et al . Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neuro-Oncol. (2009) 93:395–400. doi: 10.1007/s11060-009-9796-5
95.
You G Sha ZY Yan W Zhang W Wang YZ Li SW et al . Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro-Oncol. (2012) 14:230–41. doi: 10.1093/neuonc/nor205,
96.
Chalifoux R Elisevich K . Effect of ionizing radiation on partial seizures attributable to malignant cerebral tumors. Stereotact Funct Neurosurg. (1996) 67:169–82. doi: 10.1159/000099446,
97.
Vecht CJ Kerkhof M Duran-Pena A . Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. (2014) 19:751–9. doi: 10.1634/theoncologist.2014-0060,
98.
Kerrigan S Grant R . Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev. (2011) 8:CD008586. doi: 10.1002/14651858.CD008586,
99.
Bähr O Hermisson M Rona S Rieger J Nussbaum S Körtvelyessy P et al . Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trial. Acta Neurochir. (2012) 154:229–35. doi: 10.1007/s00701-011-1144-9,
100.
Usery JB Madison Michael L Sills AK Finch CK . A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J Neuro-Oncol. (2010) 99:251–60. doi: 10.1007/s11060-010-0126-8,
101.
Liang S Fan X Zhao M Shan X Li W Ding P et al . Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med. (2019) 8:4527–35. doi: 10.1002/cam4.2362,
Summary
Keywords
glioma, epilepsy, VLSM, limbic system, frontal lobe
Citation
William NKM, Wang L and Xianzhi L (2026) Voxel-based mapping of postoperative epilepsy risk in glioma patients: supplementary motor area and limbic system correlations. Front. Neurol. 16:1556286. doi: 10.3389/fneur.2025.1556286
Received
06 January 2025
Accepted
06 May 2025
Published
05 January 2026
Volume
16 - 2025
Edited by
Marta Maschio, Hospital Physiotherapy Institutes (IRCCS), Italy
Reviewed by
Kapil Gururangan, Northwestern University, United States
Jinghui Wang, University of Maryland School of Medicine, United States
Updates
Copyright
© 2026 William, Wang and Xianzhi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Xianzhi, fccliuxz@zzu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.