Abstract
Introduction:
Eye movements have been proposed as biomarkers to track disease progression and treatment effects in neurological diseases. Before such variables are used in the clinic or in drug trials, properties such as measurement error must be documented. In this study, we assessed repeatability, reliability, and stability of fixation, smooth pursuit, and saccade measurements in patients with Parkinson’s disease, cerebellar ataxia, and healthy adults.
Methods:
Fixation, smooth pursuit, and saccade metrics were measured in 16 patients with Parkinson’s disease, 16 patients with ataxia, and 25 healthy adults with an eye tracker (BulbiCam). The same operator repeated the measurements six times over 2 days in the patient group and two times the same day in the healthy adults. Reliability, repeatability, and stability were assessed with the intraclass correlation coefficient (ICC), Bland–Altman plots with the Agreement Index, and the Stability Index, respectively.
Results:
Mean pupil size in the fixation test and latency, accuracy and peak velocity in the pro-saccade test were found reliable, repeatable, and stable. Mean and max fixation in the fixation test were found reliable and stable. Smooth pursuit measurements were found repeatable within patients and stable, but not reliable.
Conclusion:
The saccade and pupil variables may be used both on a population level and for individual patient follow-up. Mean and max fixation duration may be used on the population level but used in the clinical evaluation on individual patients they need to be repeated.
Introduction
Diseases affecting the nervous system are the leading cause of global disability, and the research effort to ease this burden is formidable (1). Finding effective treatment also involves the search for biomarkers. In Parkinson’s disease (PD), there are promising imaging and fluid biomarkers for early diagnosis and risk stratification (2). For cerebellar ataxias, the continual identification of novel genetic causes enhances diagnostic accuracy. However, there is a lack of biomarkers to track disease progression objectively in many neurological diseases.
Eye movement measurements are candidate biomarkers in neurological diseases as they give valuable information about the healthy and pathological brain. Eye movements can be objectively measured and quantified, and oculomotor abnormalities are well described in diseases such as Huntington’s disease (3), Alzheimer’s dementia (4), multiple sclerosis (5), stroke (6), PD (7–9), and cerebellar ataxia (10).
When searching for biomarkers and clinical variables that can track disease progression or monitor effect of interventions, the degree of measurement error is crucial. Repeatability and reliability studies provide information about this variable and strengthens the validity of the results. Repeatability is the degree to which repeated scores or ratings are identical, or the agreement, within subjects (11). Reliability is a term often used in measurements with clinical scales and questionnaires. It extends beyond repeatability within subjects to encompass repeatability both between and within subjects, but mainly between subjects. A reliable variable must be both repeatable between and within subjects. Reliability of a measurement depends on the variation between subjects and thus relates to the population of which the subjects can be considered a random sample. The intraclass correlation coefficient (ICC) is mostly used to express the reliability of a measurement on population level. The Bland–Altman plots and calculation of agreement limits are recommended for evaluating repeatability within subjects. Stability is a term used for repeatability of measurements over several time points and can reveal practice effects that may be relevant in longitudinal studies or clinical trials. A reliable and repeatable biomarker can be used both on a population level and for follow up of an individual subject. If the biomarker is reliable and stable in terms of high ICC, but not repeatable within patient, it can still be used on a population level but would need to be repeated for use on individual subjects. A within-subjects repeatable and stable but not reliable biomarker is not applicable in clinical studies on a population level but can be used in the follow-up of individual subjects.
Studies on reliability of fixation, smooth pursuit, and saccadic eye movements have been done in healthy individuals (12–16) with different eye-tracking devices. Except for a study of presymptomatic Huntington’s disease gene carriers (17), to our knowledge, there exist few studies on reliability of eye movement measurements in neurological patients. Reliability and repeatability of a measurement technique are not fixed, but a result of interactions between the equipment, the subjects measured, and the context of assessment. As neurological patients frequently have difficulties with fixating and limitations in their eye movements, calibration can be challenging. It is therefore important to know the reliability of these eye movements not only in the healthy population but also in the relevant patient population. We chose to examine patients with PD and ataxia as they have well described eye movement pathologies (18) and there is a lack of objective methods to monitor disease progression. We also examined healthy adults of the same age to evaluate whether the reliability and repeatability were disease specific and for comparison with previous studies in healthy adults.
To assess reliability and repeatability of fixation, smooth pursuit, and saccade measurements in these participants, we use ICC and Bland–Altman analysis, and we propose a novel Stability Index for repeated measurements.
Methods
The study population consists of patients previously diagnosed with PD or hereditary or sporadic cerebellar ataxia of both genders, and healthy adults, passed the age of 18 years, without any eye disease and other known serious diseases. For complete inclusion and exclusion criteria, see Supplementary material.
The study subjects were recruited from the outpatient clinic of the Department of Neurology, Oslo University Hospital. All participants gave written informed consent. The study was approved by the institutions data protection officer of Oslo University Hospital and considered by the Regional Ethics committee to be exempt (REK Nord, application number 401897). The study followed the tenets of the Helsinki Declaration with ClinicalTrial.gov number NCT 05449041 and EudraCT number 2021-006250-31.
The PD subjects consisted of seven women and nine men with the mean age of 65 years (range: 45–80) and disease duration from diagnosis of 7 years (range: 0.5–23), while the cerebellar ataxia subjects consisted of eight women and eight men with the mean age of 56 years (range: 34–75) and mean disease duration of 10 years (range: 0.5–33). The PD patients and cerebellar ataxia subjects were analyzed as one group. The healthy adult group consisted of 12 women and 13 men with a mean age of 61 years (range: 33–80).
On clinical examination five of the ataxia patients had nystagmus, 12 saccadic smooth pursuit, and three noted as having hypometric saccades. Among the PD patients, seven were categorized as having saccadic smooth pursuit and six had hypometric saccades on clinical examination. All the healthy adults underwent normal clinical neuroophthalmological examinations.
Study design
The study was performed as a controlled, but non-randomized stratified parallel group trial with six repeated measurements in the two patient groups and two measurements in the healthy group. The clinical diagnosis was used as a stratification factor.
Equipment
The BulbiCam produced by BulbiTech (Trondheim, Norway) was used for eye movement recordings. The apparatus uses dark pupil/bright pupil and corneal reflex technique video-oculography with a frequency of 400 frames per second to produce gaze direction data. It contains two screens and one infrared eye-tracking camera. BulbiCam can show stimuli to one or both eyes and track one or both eyes depending on the test chosen. Further details about the apparatus and software are given in the Supplementary material. BulbiHub software versions 221,031 and 221,216 were used in the study.
Clinical procedure
Participants were placed in a comfortable chair with backrest and armrest. The BulbiCam was suspended from the ceiling with a wire and the participants attached to the camera with a headband (for a detailed setup, see the Supplementary material). BulbiCam registrations were done three times a day on two consecutive days for the patients, and two registrations on 1 day for the healthy participants. Each registration took approximately 10 min and was repeated after a 50-min break. Calibration was done automatically by software of the machine with an 8-point saccade test for the fixation and smooth pursuit test and the saccade and prosaccades test has in-test calibration (more details in the Supplementary material). All registrations were done by the same operator. Participants were given standardized instructions in Norwegian before each task (Supplementary material). The BulbiCam tasks used in this study are as follows:
Fixation
A central green cross in a dot target recommended by Thaler et al. (19) was illuminated for 11 s and repeated four times with a 4-s break in between. BulbiHub software denotes any horizontal eye movement above a threshold of 30 degrees per second as a saccade. The time between these saccadic intrusions are given as mean fixation duration and max fixation duration in milliseconds. The mean pupil size during the task with dark grey screens is given in millimeters (mm). As the BulbiCam is attached to the participant, no light from the room affects the pupils during the task.
Prosaccades
A pro-saccadic step task with 20 trials designed according to the standardized anti-saccade protocol (20). A green cross in dot target moves 10 degrees horizontal from the center with randomized direction and duration of the foreperiod. The variables obtained from the prosaccade test were latency (ms), accuracy (%), and peak velocity (degrees/s).
Smooth pursuit
A green dot moves sinusoidally 8 degrees left and right of the center, first at a frequency of 0.2 Hz, and then at 0.5 Hz and then follows a sequence when the target moves one half cycle at 0.25 Hz, one half cycle at 0.33 Hz, two half cycles at 0.5 Hz, and then five half cycles at 1 Hz. The variables obtained from the smooth pursuit test were first gain (0.2 Hz), second gain (0.5 Hz), and third gain from the right eye given in percent.
Saccade
A prosaccadic step task that consists of five trials of horizontal 20 degrees, five trials of horizontal 7.5 degrees saccades and 5 trials of vertical 9 degrees prosaccades. Each of the three variables, latency, accuracy, and peak velocity, was obtained for horizontal 20-degree, horizontal 7.5-degree, and vertical 9-degree saccades.
Eye movement analysis
The analysis was done using BulbiHub software with automatic blink removal and interpolation of missing glints and saccade detection. For the fixation task filter settings were set to moving average 1 (smoothing averaging one frame before and after the present frame) and velocity calculation using the smoothed gaze position compared with three frames before (N-3) and velocity threshold 30 degrees/s in BulbiHub software before export. Information on frequency filter in the software is not available from the manufacturer. For the smooth pursuit task, the results from the software were analyzed for the right eye. Software assigns 9 points for the first stimulus frequency, 5 points for the second stimulus, and 7 points for the third stimulus frequency and trough cross-correlation analysis calculates time-shift between phase of visual stimulus and phase of the eye movement, showing values for the gain in percent. With the step saccade task, we did not expect express saccades and therefore discharged trials with latencies <100 ms as technical errors or anticipatory saccades. The velocity threshold for saccade detection was 30 degrees/s. Trials with velocities above 700 degrees/s were regarded as technical errors and removed. The amplitude was assessed with amplitude accuracy, the measured saccade amplitude in relation to the target amplitude in percent. Trials with amplitude accuracy less than 10% were considered peristimulus fixation instability, instead of voluntary saccades, and therefore removed. The BulbiHub denotes trials with in-test calibration errors as amplitude gain of 120% instead of Na, and these trials were also removed.
Statistical analysis
Repeatability and Reliability
A reliable variable on a population level needs to be repeatable both between and within participants. A reliable variable on an individual participant level needs to be repeatable and stable within participants. The performed reliability and repeatability analysis in this study includes both the intraclass correlation coefficient (3,1) (ICC) (21) and the Bland–Altman model (22, 23). The ICC was calculated with the two-way mixed-effect absolute agreement model, mainly focusing on repeatability between patients (24). The Bland–Altman model was used to express the repeatability or agreement within participants. This model is expressed graphically by the Bland–Altman plot, given as the mean difference between two measurements of the same object with a 95% prediction interval calculated as mean difference ± 2 times the standard deviation of the difference between the two measurements (SDdiff). These intervals are referred to as Agreement limits. In addition, an Agreement Index (AI) (25) defined as 1 minus the ratio between the half width between the agreement limits and the measurement mean level is used. AI = 1–2*SDdiff/Mean of the measurements. We used the following categorization of both AI and ICC (24):
-
< 0.50—poor.
-
[0.50, 0.75 > —moderate.
-
[0.75, 0.90 > —good.
-
[0.90, 1.00]—excellent.
Stability
Let SDb and SDw denote the standard deviation between and within participants, respectively. The ratio SDw/SDb is considered a good indicator for stability. A low ratio indicates good stability and must be below 1 to claim stability. To obtain a stability index which increases with increased stability, we introduce the stability index as SI=1−SDw/SDb. In contrast to other classification indices where the classification limits are set without scientific justification, such as for ICC and AI, here it is desired to base the limits on probabilities. Let n denote the number of repeated observations within patients and m the number of patients. The ratio between two chi-squared distributions with (n-1) and (m-1) degrees of freedom (df) multiplied with the inverse df-ratio is Fisher-distributed with (n-1) and (m-1) df (26). Consequently, if you calculate the ratio between the sum of squares within patients () and the sum of squares between patients () and multiply this ratio by ((m–1)/(n–1)), the result follows an F-distribution with (n–1) and (m–1) degrees of freedom.
is Fisher-distributed with (n–1) and (m–1) df.
and P (SI ≤ = α. Different choice of α gives the classification limits of the stability index SI.
In this study, we have n = 6 repeated observations and m = 32 patients which gives [SDw/SDb]2 F-distributed with 5 and 31 df for each patient. This gives the possibility to calculate and classify the stability for each patient (SIi; i = 1 to 32) on a given variable. SIi may vary between patients, and the mean of the SIs may be given with confidence intervals for the total material. The α-values 0.05, 0.10, 0.20, and 0.40 provide the following classification limits:
-
Excellent stability (α=0.05) gives SI ≥ =0.53
-
Very good stability (α=0.10) gives I< SI0.44
-
Good stability (α=0.20) gives I < SI 0.34
-
Acceptable stability (α=0.40) gives I 1 – < SI 0.14
-
Poor stability (α>0.40) gives SI < SI < 0.14
Statistical analyses were performed in SAS version 9.4.
Results
Fixation
The pupil size variable in the fixation task was reliable, repeatable, and stable (Tables 1, 2; Figures 1A, 2A). Mean and max fixation duration were reliable for both patients and healthy controls. However, these fixation duration variables were not found repeatable but stable in both groups (Tables 1, 2).
Table 1
| Test | Variable | Patients (n = 32) | Healthy controls (n = 25) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Second | ICC | AI | First | Second | ICC | AI | ||
| Fixation | Mean fixations (ms) | 1,311 | 1,378 | 0.63 | −0.50 | 1,431 | 1,690 | 0.78 | −018 |
| Max fixations (ms) | 5,476 | 5,074 | 0.71 | 0.15 | 5,955 | 6,088 | 0.85 | 0.47 | |
| Mean pupil (mm) | 2.9 | 2.9 | 0.94 | 0.86 | 2.83 | 2.78 | 0.96 | 0.91 | |
| Smooth pursuit | First gain (%) | 92.7 | 94.8 | 0.07 | 0.51 | 98.3 | 98.7 | 0.32 | 0.95 |
| Second gain (%) | 91.2 | 91.7 | 0.18 | 0.80 | 93.1 | 95.7 | 0.31 | 0.86 | |
| Third gain (%) | 82.6 | 81.9 | 0.30 | 0.78 | 89.1 | 88.1 | 0.20 | 0.75 | |
| Pro saccade | Latency (ms) | 283.7 | 292.0 | 0.50 | 0,57 | 277.0 | 285.7 | 0.45 | 0.66 |
| Accuracy (%) | 83.8 | 87.5 | 0.75 | 0,72 | 91.1 | 94.3 | 0.28 | 0.73 | |
| Peak velocity (deg/s) | 312.8 | 313.6 | 0.80 | 0,64 | 352.2 | 370.4 | 0.54 | 0.66 | |
| Saccade latency (ms) | Horizontal 20 deg. | 301.6 | 310.5 | 0.41 | 0.53 | 277.0 | 278.5 | 0.42 | 0.70 |
| Horizontal 7.5 deg | 236.3 | 230.3 | 0.76 | 0.73 | 228.6 | 219.8 | 0.79 | 0.81 | |
| Vertical | 251.2 | 270.3 | 0.46 | 0.59 | 260.5 | 261.2 | 0.89 | 0.82 | |
| Saccade accuracy (%) | Horizontal 20 deg. | 76.9 | 82.4 | 0.00 | 0.58 | 82.8 | 85.8 | 0.23 | 0.67 |
| Horizontal 7.5 deg. | 81.7 | 90.3 | 0.36 | 0.56 | 85.9 | 84.6 | 0.36 | 0.66 | |
| Vertical | 89.5 | 84.2 | 0.10 | 0.44 | 97.8 | 97.8 | 0.15 | 0.65 | |
| Saccade peak velocity (deg/s) | Horizontal 20 deg. | 334.8 | 347.1 | 0.71 | 0.55 | 410.7 | 406.5 | 0.61 | 0.63 |
| Horizontal 7.5 deg. | 293.6 | 320.7 | 0.39 | 0.55 | 344.6 | 336.6 | 0.28 | 0.67 | |
| Vertical | 318.8 | 295.8 | 0.59 | 0.52 | 377.8 | 372.5 | 0.61 | 0.71 | |
Reliability and repeatability of the BulbiCam tests.
Reliability related to population is expressed by the intraclass correlation (ICC) and the repeatability within patients by the Agreement Index (AI). The results are expressed by mean value. Ms, milliseconds; mm, millimeters; Hz, Hertz; %, percent; deg, degrees; deg/s, degrees per second.
Table 2
| Test | Variable | M1 | M2 | M3 | M4 | M5 | M6 | ICC | SI (95%CI) | Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixation | Mean fixations (ms) | 1,311 | 1,378 | 1,486 | 1,612 | 1,397 | 1708 | 0.56 | 0.33 (0.08–0.57) | Good |
| Max fixations (ms) | 5,476 | 5,074 | 5,549 | 5,673 | 5,357 | 5,286 | 0.62 | 0.48 (0.37–0.59) | Very good | |
| Mean pupil (mm) | 2.9 | 2.9 | 2.9 | 2.8 | 2.8 | 2.8 | 0.94 | 0.78 (0.73–0.83) | Excellent | |
| Smooth pursuit | First gain (%) | 92.7 | 94.8 | 96.5 | 96.9 | 97.4 | 94.8 | 0.24 | 0.80 (0.64–0.95) | Excellent |
| Second gain (%) | 91.2 | 91.7 | 89.6 | 89.6 | 91.7 | 91.4 | 0.18 | 0.27 (−0.05–0.58) | Acceptable | |
| Third gain (%) | 82.6 | 81.9 | 82.2 | 79.9 | 79.9 | 80.1 | 0.31 | −0.04 (−0.34–0.27) | Not acceptable | |
| Prosaccade | Latency (ms) | 283.7 | 292.0 | 292.5 | 295.2 | 290.7 | 292.5 | 0.56 | 0.52 (0.36–0.68) | Very good |
| Accuracy (%) | 83.8 | 87.5 | 89.4 | 86.7 | 84.3 | 86.0 | 0.61 | 0.53 (0.40–0.67) | Excellent | |
| Peak velocity (deg/s) | 312.8 | 313.6 | 337.8 | 320.3 | 318.6 | 320.2 | 0.69 | 0.54 (0.42–0.66) | Excellent | |
| Saccade latency (ms) | Horizontal 20 deg | 301.6 | 310.5 | 287.0 | 294.3 | 294.1 | 329.3 | 0.40 | 0.31 (0.05–0.57) | Acceptable |
| Horizontal 7.5 deg. | 236.3 | 230.3 | 240.6 | 238.7 | 229.9 | 230.9 | 0.50 | 0.44 (0.29–0.59) | Very good | |
| Vertical | 251.2 | 270.3 | 252.9 | 252.7 | 256.5 | 265.9 | 0.47 | 0.18 (−0.11–0.47) | Acceptable | |
| Saccade accuracy (%) | Horizontal 20 deg. | 76.9 | 82.4 | 79.5 | 79.2 | 78.5 | 80.2 | 0.23 | 0.31(0.15–0.47) | Acceptable |
| Horizontal 7.5 deg. | 81.7 | 90.3 | 84.1 | 82.8 | 81.6 | 77.4 | 0.35 | 0.37(0.20–0.54) | Good | |
| Vertical | 89.5 | 84.2 | 83.5 | 81.1 | 82.9 | 89.7 | 0.18 | 0.08(−0.08–0.24) | Not acceptable | |
| Saccade peak velocity | Horizontal 20 deg. | 334.8 | 347.1 | 343.8 | 357.5 | 329.7 | 338.1 | 0.73 | 0.51(0.42–0.61) | Very good |
| Horizontal 7.5 deg. | 293.6 | 320.7 | 308.4 | 296.0 | 288.4 | 286.6 | 0.51 | 0.34(0.18–0.49) | Good | |
| Vertical | 318.8 | 295.8 | 300.8 | 296.0 | 289.5 | 315.1 | 0.53 | 0.28 (0.17–0.39) | Acceptable |
Stability of the BulbiCam tests.
The six measurements are denoted as M1 to M6 and expressed by mean values. Stability is expressed by the Stability Index (SI) with 95% Confidence Intervals (CI). The classification is based on SI. Ms, milliseconds; mm, millimetre; Hz, Hertz; deg, degrees; deg/s, degrees per second.
Figure 1

Bland–Altman plot of some selected variables from the fixation, smooth ursuit, and prosaccade test in the merged patient (n = 32) and healthy control (n = 25) material. (A) Mean pupil diameter in millimeters from the fixation task. (B) Smooth pursuit gain of first (0.2 Hz) stimuli. (C) Saccade peak velocity in degrees per second in the prosaccade task. The full horizontal line shows the mean difference between the two measurements, and the dotted horizontal lines indicate the agreement limits. The blue circles (○) shows healthy controls, the red plus (+) Parkinson patients, and the green cross (X) ataxia patients.
Figure 2
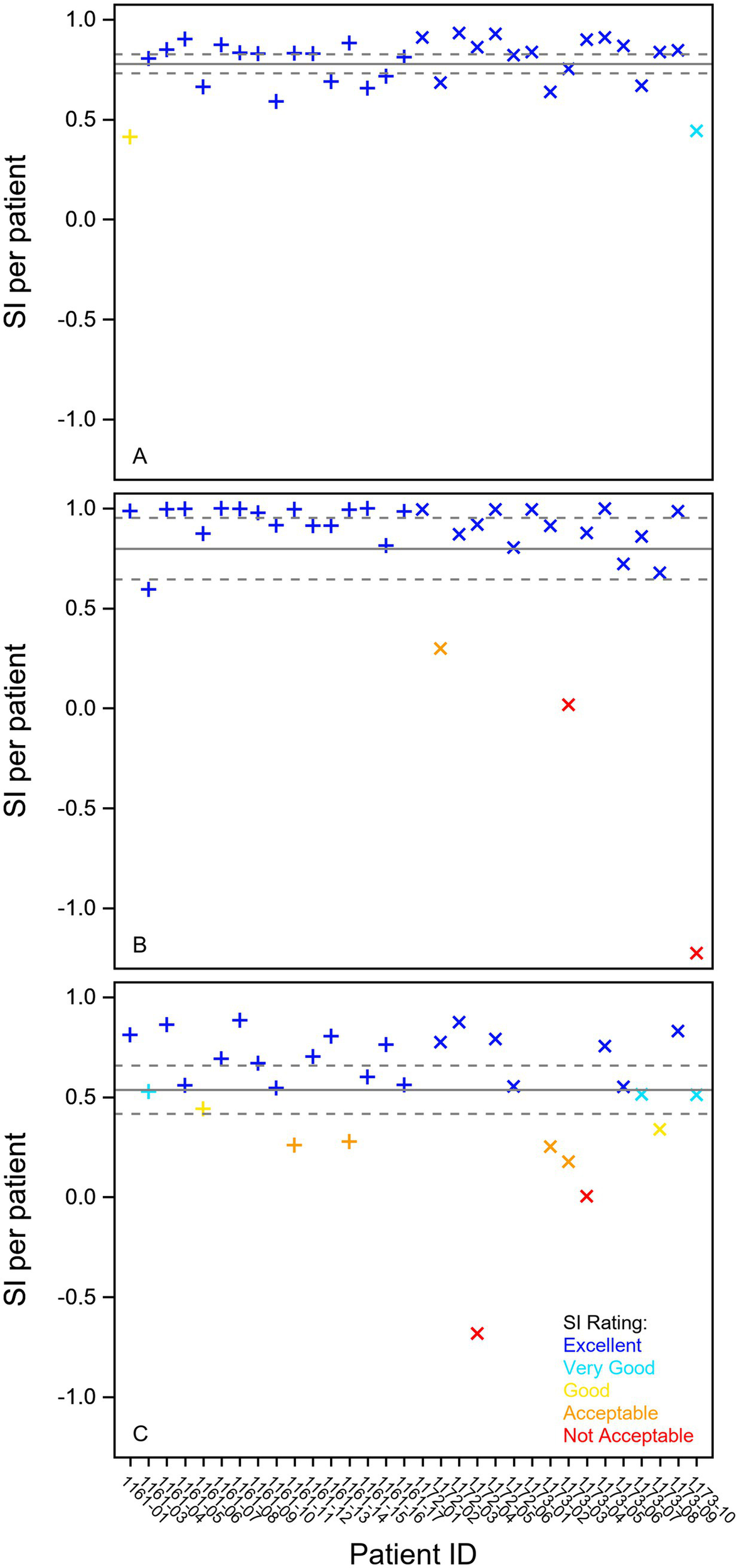
Stability plot of individual results for some selected variables from the fixation, smooth pursuit, and prosaccade test in 16 Parkinson patients (+) and 16 ataxia patients (X). (A) Mean pupil diameter in millimeters from the fixation task. (B) Smooth pursuit gain in percent (%) of first (0.2 Hz) stimuli. (C) Saccade peak velocity in degrees per second in the Prosaccade task. The full line shows the mean stability index, and the dotted lines indicate the 95% confidence interval. The individual stability index is given on the x-axis and the classification in different colors (blue—excellent, cyan—very good, yellow—good, orange—acceptable, red—not acceptable). SI Stability Index.
Smooth pursuit
None of the three smooth pursuit variables were found reliable neither in the patients nor the controls (Table 1). However, all these variables were repeatable (Table 1, Figure 1B) and 0.2 Hz and 0.5 Hz gain were stable (Table 2; Figure 2B).
Prosaccades
Latency, accuracy, and peak velocity in the prosaccades task were all found reliable, repeatable, and stable in the patient group (Tables 1, 2; Figure 1B; 2C). In the healthy group, only the peak velocity was reliable, but all three variables were repeatable.
Saccades
In the saccade task with vertical, short, and long horizontal saccades, the peak velocity of the short and vertical saccades as well as the latency of the short saccades were found reliable, repeatable, and stable (Tables 1, 2). Latency of the long saccades and the accuracy of both short and long horizontal saccades were found repeatable (Table 1) and stable (Table 2). Additional Bland–Altman plots and stability plots are found in the Supplementary material.
Classification
Seven variables were classified as reliable, repeatable, and stable, seven as repeatable and stable, but not reliable (Table 3).
Table 3
| Test | Variables | Parkinson and ataxia patients | Healthy controls | Merged classification | |||
|---|---|---|---|---|---|---|---|
| ICC | AI | SI | ICC | AI | |||
| Fixation | Mean fixations | + | − | + | + | − | Reliable + stable |
| Max fixations | + | − | + | + | − | Reliable + stable | |
| Mean pupil | + | + | + | + | + | Reliable + repeatable + stable | |
| Smooth pursuit | First gain | − | + | + | − | + | Repeatable + stable |
| Second gain | − | + | + | − | + | Repeatable + stable | |
| Third gain | − | + | − | − | + | Repeatable | |
| Pro Saccade | Latency | + | + | + | − | + | Reliable + repeatable + stable |
| Accuracy | + | + | + | − | + | Reliable + repeatable + stable | |
| Peak velocity | + | + | + | + | + | Reliable + repeatable + stable | |
| Saccade latency | Horizontal 20 | − | + | + | − | + | Repeatable + stable |
| Horizontal 7.5 | + | + | + | + | + | Reliable + repeatable + stable | |
| Vertical | − | + | + | + | + | Repeatable + stable | |
| Saccade accuracy | Horizontal 20 | − | + | + | − | + | Repeatable + stable |
| Horizontal 7.5 | − | + | + | − | + | Repeatable + stable | |
| Vertical | − | − | − | − | + | Not acceptable | |
| Saccade peak velocity | Horizontal 20 | + | + | + | + | + | Reliable + repeatable + stable |
| Horizontal 7.5 | − | + | + | − | + | Repeatable + stable | |
| Vertical | + | + | + | + | + | Reliable + repeatable + stable | |
Merged summary of reliability, repeatability, and stability.
Significant values of reliability (ICC), repeatability (AI), and stability (SI) for each test variable are given with (+).
Discussion
Eye movement measurements have been proposed as clinical biomarkers in neurological diseases. The validity of such biomarkers depends on the amount of measurement error and should be investigated in a relevant sample. In this study, we have assessed test–retest reliability, repeatability, and stability of fixation, smooth pursuit, and saccade measurements in a group of patients with suspected oculomotor abnormalities and healthy adults.
The fixation task variable pupil size was the most reliable, repeatable, and stable of the analyzed variables. The reliability and repeatability of a measurement is a product of interactions between the recording equipment, the subjects, and the measurement setting. The fixation task is undemanding to the participant and the illumination presented to the pupil from the apparatus and the task is constant, and we therefore expect the pupil to be of stable size during this short task. With recording equipment sampling at 400 frames per second, this variable offers a robust data set less susceptible to outliers. We interpret the good results in pupil measurements as low technical measurement error by the equipment and excellent reliability and stability of this variable means that it could be used in both cross-sectional trials and longitudinal trials.
The mean and max fixation duration were reliable on a group level assessed with ICC. However, the repeatability within patients was judged as poor by the Agreement Index, indicating greater variability within the individual than between individuals. The Bland–Altman plot shows that some participants have great variation between repeated measurements, and this is seen in both healthy individuals and patients. This means that fixation duration could be used to categorize or differentiate between groups in cross-sectional studies, but this test should be used with caution when following up the individual patient or in longitudinal trials where repeatability is crucial. The stability of all six measurements in the patients were however very good, indicating that increased recording time or number of cycles would likely improve the repeatability.
The smooth pursuit measurements showed poor reliability for all three stimuli speeds. This task design is prone to measurement error and susceptible to outliers because of few data points as it only includes nine, five, and seven data points (first, second, and third stimulus, respectively) to calculate the gain value. However, the repeatability was good and the stability excellent for the 0.2 Hz and acceptable for the 0.5 Hz gain. This means that for follow-up of the individual patient in the clinic or in longitudinal trials this task has the potential to detect changes from one visit to another, but the current task is not suitable for studies on a population level.
The saccade measurements in the prosaccade task showed good to excellent reliability and repeatability in the patient group. This test is applicable both on a population level and for the individual patient in clinical follow-up. In the healthy group, the latency and accuracy variables showed poor reliability. This discrepancy could be because subject variability can cause unreasonably low or high ICC values when measurement errors are fixed. Six of the ataxia patients, three of the PD patients, and none of the healthy adults had hypometric saccades on clinical examination. The higher ICC in patient saccade amplitude accuracy versus the healthy group is likely due to the lack of variability within the healthy participants and the great heterogeneity in the patient group. We note that limitation of software of saccade amplitude accuracy of 120% warrants caution when analyzing cohorts with hypermetric saccades, and preferably, raw data should be analyzed in this setting. The saccade task with short, long, and vertical saccades were overall repeatable and stable. However, the reliability varied with parameter and saccade length and direction, probably due to the low number of trials.
Comparison between studies is difficult, as differences in tasks, recording equipment, statistical approach, and participants influence the results and conclusions. Our prosaccade task was designed with stimulus size and presentation following the internationally standardized antisaccade protocol (20), but with fewer trials. This protocol was also used in a reliability study of saccade measurements in healthy adults by Plomecka et al. (12), which reported ICC values between 0.58 and 0.87 in the older group of participants, with lower ICCs in the younger participants. In our study, we observed higher ICCs in the patient group than the healthy group. This disparity might stem from the variability in the groups as we expect more saccade abnormalities and hence variability with older age and neurological disease (27). Ettinger et al. (15) also found that saccade and fixation measurements are reliable, but with different task designs. While the ICCs reported in both Plomecka et al. (12) and Ettinger et al. (15) are comparable, they are not identical to those we observed. The eye-tracking device differed across these studies: Plomecka et al. utilized the EyeLink 1000Plus (SR-Research, Ottawa, Canada), while Ettinger et al. employed an IRIS model 6,500 (Skalar Medical BV, Delft, Netherlands). Although BulbiCam has similar technical capabilities, there are clear differences in the technical setup (head-mounted/table mounted) as well as hardware and software configurations. As BulbiCam is equipped with preprogrammed tests and built-in analysis, assessing the variations in eye-movement analysis is challenging.
Standardized task protocols have been published like “The Internationally Standardized Antisaccade protocol” (20) and the DEMoNS protocol for saccade measurements (28). The Ataxia Global Initiative Working Group on Digital-Motor Biomarkers published in 2023 recommendations on task design for eye tracking studies in ataxia patients. Standardization of eye tracking protocols and reporting is crucial for exploiting the full potential of eye movements as a biomarker. In addition to these task protocols (10, 20, 28), Dunn et al. have published guidelines for reporting eye-tracking research across disciplines (29). We recommend implementing standardized task designs and transparent protocols in future studies. However, the trade-off between enough trials for reliability and repeatability on the one hand must be weighed against the feasibility in the relevant patient population in terms of cooperation and fatigue.
A strength of this study is the various statistical approaches to judge the measurement’s reliability, repeatability, and stability. Most reliability studies use ICC (12, 15), but other statistical methods (13–16) have also been used. As ICC is a measure of correlation, it can show excellent reliability even though the repeated measurements do not show repeatability. In longitudinal trials and in individual patient follow-up, a biomarker without repeatability is of little value. We have supplemented the ICC with a repeatability analysis both graphically with Bland–Altman plots and calculated an Agreement Index. ICC can also be used as a measure of stability. However, the limits of categorization into poor, moderate, good, or excellent are only arbitrary and not based on statistical probability. ICC does not give information about the stability of the measurements within an individual. We therefore suggested the Stability Index with categorization based on probabilities.
Our study has some limitations
The sample size is moderate and could limit some of the analysis. The patient cohort is very heterogeneous as the ataxia group includes participants with different genetic and sporadic aetiologies. Furthermore, PD patients were in different stages of the disease and used different treatments that may alter eye movements. The reliability, repeatability, and stability of the studied parameters may be different in a more homogeneous sample, but our results give an implication of what parameters to study further. The study is also limited to a single operator, and future research would benefit from evaluating inter-rater reliability, particularly for application in multicenter trials. This study does face limitations due to the constraints of the eye-tracking system used. The calibration setup is non-customizable, and detailed operational information is not available, presenting challenges particularly for patients with nystagmus or fixation instability, as standard calibration may not account for these conditions effectively. Moreover, filter and analysis settings of software are not under full operator control, limiting comprehensive understanding and replication of findings with other devices. These limitations should be weighed against the benefits of using an eye-tracking system that is designed for ease of use in clinical settings.
Conclusion
Eye movement measurements have repeatedly been promoted as promising biomarkers but lack the standardization and thorough validation to be utilized in randomized clinical trials and in the clinic. Our study has evaluated test–retest reliability and repeatability and has proposed a new method for evaluating stability with the Stability Index. We find that the saccade and pupil measurements in our study are reliable, repeatable, and stable in PD and ataxia patients. We recommend choosing oculomotor parameters appropriate to the study design with reliable parameters in cross-sectional studies and parameters with good repeatability and stability for longitudinal study designs and in the clinic for individual patient follow-up.
Statements
Data availability statement
The datasets presented in this article are not readily available due to legal restrictions, as they contain information that could compromise the privacy of research participants. Requests to access the datasets should be directed to soejac@ous-hf.no.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Data curation, Investigation, Writing – original draft, Writing – review & editing. AE: Investigation, Supervision, Writing – review & editing. SR: Investigation, Writing – review & editing. MT: Investigation, Supervision, Writing – review & editing. EK: Conceptualization, Investigation, Supervision, Writing – review & editing. SL: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Research Council of Norway/Norges forskningsråd (project number 327785) as part of a Research and Development project applied for by BulbiTech. Oslo University Hospital and Meddoc are contractual partners in this project. The funder had no role in the design, data collection, data analysis, or reporting of this study.
Acknowledgments
We want to thank all our participants for their willingness to contribute to research and colleagues at the Department of Neurology, Oslo University Hospital for help with recruitment.
Conflict of interest
MT has received research grants from the South-Eastern Norway Regional Health Authority. SL is employed by Meddoc who is a contractual partner of BulbiTech in this project.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1556314/full#supplementary-material
References
1.
Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. (2017) 16:877–97. doi: 10.1016/S1474-4422(17)30299-5
2.
Zarkali A Thomas GEC Zetterberg H Weil RS . Neuroimaging and fluid biomarkers in Parkinson's disease in an era of targeted interventions. Nat Commun. (2024) 15:5661. doi: 10.1038/s41467-024-49949-9
3.
Blekher T Johnson SA Marshall J White K Hui S Weaver M et al . Saccades in presymptomatic and early stages of Huntington disease. Neurology. (2006) 67:394–9. doi: 10.1212/01.wnl.0000227890.87398.c1
4.
Hannonen S Andberg S Kärkkäinen V Rusanen M Lehtola JM Saari T et al . Shortening of saccades as a possible easy-to-use biomarker to detect risk of Alzheimer's disease. J Alzheimers Dis. (2022) 88:609–18. doi: 10.3233/JAD-215551
5.
Gehrig J Bergmann HJ Fadai L Soydaş D Buschenlange C Naumer MJ et al . Visual search in naturalistic scenes reveals impaired cognitive processing speed in multiple sclerosis. Front Neurol. (2022) 13:838178. doi: 10.3389/fneur.2022.838178
6.
Delazer M Sojer M Ellmerer P Boehme C Benke T . Eye-tracking provides a sensitive measure of exploration deficits after acute right MCA stroke. Front Neurol. (2018) 9:359. doi: 10.3389/fneur.2018.00359
7.
Terao Y Fukuda H Yugeta A Hikosaka O Nomura Y Segawa M et al . Initiation and inhibitory control of saccades with the progression of Parkinson's disease—changes in three major drives converging on the superior colliculus. Neuropsychologia. (2011) 49:1794–806. doi: 10.1016/j.neuropsychologia.2011.03.002
8.
Frei K . Abnormalities of smooth pursuit in Parkinson's disease: a systematic review. Clin Park Relat Disord. (2021) 4:100085. doi: 10.1016/j.prdoa.2020.100085
9.
Tsitsi P Benfatto MN Seimyr G Larsson O Svenningsson P Markaki I . Fixation duration and pupil size as diagnostic tools in Parkinson's disease. J Parkinsons Dis. (2021) 11:865–75. doi: 10.3233/JPD-202427
10.
Garces P Antoniades CA Sobanska A Kovacs N Ying SH Gupta AS et al . Quantitative oculomotor assessment in hereditary Ataxia: systematic review and consensus by the Ataxia global initiative working group on digital-motor biomarkers. Cerebellum. (2024) 23:896–911. doi: 10.1007/s12311-023-01559-9
11.
Kottner J Audigé L Brorson S Donner A Gajewski BJ Hróbjartsson A et al . Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol. (2011) 64:96–106. doi: 10.1016/j.jclinepi.2010.03.002
12.
Płomecka MB Barańczuk-Turska Z Pfeiffer C Langer N . Aging effects and test-retest reliability of inhibitory control for saccadic eye movements. eNeuro. (2020) 7):ENEURO.0459-19.2020. doi: 10.1523/ENEURO.0459-19.2020
13.
Klein C Fischer B . Instrumental and test-retest reliability of saccadic measures. Biol Psychol. (2005) 68:201–13. doi: 10.1016/j.biopsycho.2004.06.005
14.
Klein C Berg P . Four-week test-retest stability of individual differences in the saccadic CNV, two saccadic task parameters, and selected neuropsychological tests. Psychophysiology. (2001) 38:704–11. PMID:
15.
Ettinger U Kumari V Crawford TJ Davis RE Sharma T Corr PJ . Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology. (2003) 40:620–8. doi: 10.1111/1469-8986.00063
16.
Roy-Byrne P Radant A Wingerson D Cowley DS . Human oculomotor function: reliability and diurnal variation. Biol Psychiatry. (1995) 38:92–7. doi: 10.1016/0006-3223(94)00225-R
17.
Blekher T Weaver MR Cai X Hui S Marshall J Jackson JG et al . Test-retest reliability of saccadic measures in subjects at risk for Huntington disease. Invest Ophthalmol Vis Sci. (2009) 50:5707–11. doi: 10.1167/iovs.09-3538
18.
Leigh RJ David SZ . The Neurology of Eye Movements, 5 edn, Contemporary Neurology Series (New York: Oxford Academic) (2015) online edn, 1 June 2015. doi:10.1093/med/9780199969289.001.0001 (Accessed November 2, 2024).
19.
Thaler L Schütz AC Goodale MA Gegenfurtner KR . What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vision Res. (2013) 76:31–42. doi: 10.1016/j.visres.2012.10.012
20.
Antoniades C Ettinger U Gaymard B Gilchrist I Kristjansson A Kennard C et al . An internationally standardised antisaccade protocol. Vis Res. (2013) 84:1–5. doi: 10.1016/j.visres.2013.02.007
21.
McGraw KO Wong SP . Forming inferences about some intraclass correlation coefficients. Psychol Methods. (1996) 1:30–46. doi: 10.1037/1082-989X.1.1.30
22.
Bland JM Altman DG . Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. (2003) 22:85–93. doi: 10.1002/uog.122
23.
Bland JM Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. (1986) 1:307–10. PMID:
24.
Koo TK Li MY . A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
25.
Suther KR Hopp E Smevik B Fiane AE Lindberg HL Larsen S et al . Can visual analogue scale be used in radiologic subjective image quality assessment?Pediatr Radiol. (2018) 48:1567–75. doi: 10.1007/s00247-018-4187-8
26.
Bland M . An introduction to medical statistics. 3rd ed. Oxford: Oxford University Press (2000).
27.
Ma W Zhang M . The effects of age and sex on the incidence of multiple step saccades and corrective saccades. Front Aging Neurosci. (2022) 14:963557. doi: 10.3389/fnagi.2022.963557
28.
Nij Bijvank JA Petzold A Balk LJ Tan HS Uitdehaag BMJ Theodorou M et al . A standardized protocol for quantification of saccadic eye movements: DEMoNS. PLoS One. (2018) 13:e0200695. doi: 10.1371/journal.pone.0200695
29.
Dunn MJ Alexander RG Amiebenomo OM Arblaster G Atan D Erichsen JT et al . Minimal reporting guideline for research involving eye tracking. Behav Res Methods. (2024) 56:4351–7. doi: 10.3758/s13428-023-02187-1
Summary
Keywords
eye movement, saccades, smooth pursuit, cerebellar ataxia, Parkinson’s disease
Citation
Dalbro SEJ, Elsais A, Rydning SL, Toft M, Kerty E and Larsen SE (2025) Repeatability, reliability, and stability of eye movement measurements in Parkinson’s disease, cerebellar ataxia, and healthy adults. Front. Neurol. 16:1556314. doi: 10.3389/fneur.2025.1556314
Received
06 January 2025
Accepted
24 March 2025
Published
28 April 2025
Volume
16 - 2025
Edited by
Owen B. White, Monash University, Australia
Reviewed by
Larry Allen Abel, The University of Melbourne, Australia
Amanda Douglass, The University of Melbourne, Australia
Updates
Copyright
© 2025 Dalbro, Elsais, Rydning, Toft, Kerty and Larsen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Solveig E. J. Dalbro, soejac@ous-hf.no
†ORCID: Solveig E. J. Dalbro, orcid.org/0009-0001-8548-060X
Ahmed Elsais, orcid.org/0009-0006-6190-3969
Siri Lynne Rydning, orcid.org/0000-0001-9584-0325
Mathias Toft, orcid.org/0000-0002-6723-6865
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.