Abstract
Background:
Benign paroxysmal positional vertigo (BPPV) is one of the most prevalent peripheral vestibular dysfunctions encountered in clinical practice, including dizziness and vertigo, which has a significant impact on people’s everyday lives and affects their quality of life in many ways. Researches indicate that individuals with recurrent benign paroxysmal positional vertigo (BPPV) may exhibit vitamin D insufficiency, and certain studies suggest that correcting severe vitamin D deficiency might effectively reduce BPPV recurrence; nevertheless, the findings have been inconsistent. As a result, we conducted the current Meta-analysis to investigate potential associations of vitamin D levels with the occurrence and recurrence of BPPV. In the meantime, the current study was done to evaluate the impact of vitamin D supplementation on the prevention of benign paroxysmal positional vertigo recurrence.
Methods:
Electronic databases (PubMed, EMBASE, SCOPUS and the Cochrane Library) were identified to search for relevant studies about (vitamin D or vitamin D supplementation) and (Benign paroxysmal positional vertigo incidence or recurrence) from inception to Dec 22, 2024. 60 studies with a total of 16,368 participants were included into this meta-analysis.
Results:
(1) The aggregated weighted mean difference (WMD) demonstrated that there was a significant reduction in vitamin D level in the BPPV cohort (WMD = −2.84; 95% CI −4.53 to −1.15) relative to the control cohort. Likewise, Recurrent BPPV groups had significantly lower levels of vitamin D compared to non-recurrent groups (WMD = −5.01; 95% CI −6.94 to −3.08). When the cupulolithiasis BPPV groups were compared to the canalolithiasis BPPV groups, the vitamin D level was lower in the cupulolithiasis groups (WMD = 5.09; 95% CI 2.05 to 8.12); (2) In this meta-analysis, the multivariable-adjusted relative risk (RR) indicated that increased vitamin D was inversely related to BPPV incidence (RR = 1.36; 95% CI 1.31, 1.41), but not significantly related to the recurrence (RR = 0.95, 95% CI 0.91, 0.99); (3) Vitamin D supplementation group had a lower recurrence rate than the control group which did not accepted vitamin D supplementation (RR =0.45, 95% CI = 0.36–0.55).
Conclusion:
The serum level of vitamin D is lower in patients with BPPV, especially recurrent BPPV, than in controls. There was a negative correlation between occurrence rate of BPPV episodes and vitamin D deficiency, which means that vitamin D deficiency may have a role in occurrence of BPPV. The present study indicates that vitamin D supplementation can significantly lower recurrence in benign paroxysmal positional vertigo. The level of vitamin D was lower in canalolithiasis than in cupulolithiasis BPPV groups.
Introduction
Benign paroxysmal positional vertigo (BPPV) is the predominant kind of peripheral vestibular dysfunction, produced by the displacement of otoconia from the otolith organs into the semicircular canals, resulting in clinical manifestations such as vertigo, vomiting, and dizziness (1). The diagnostic criteria for benign BPPV were formulated by the Committee for the Classification of Vestibular Disorders of the Bárány Society (2), which is identified by characteristic positioning nystagmus consistent with different semicircular canal otolith and clinical symptoms (3). In approximately 50% of BPPV patients, the condition arises from idiopathic origins (4), often linked to age-related degenerative processes. Among secondary factors, traumatic head injuries account for roughly 17% of BPPV cases (5), other reasons include serum vitamin D deficiency, osteoporosis, vascular risk factors and so on (6). In an episode of benign paroxysmal positional vertigo (BPPV), over 86% of patients have severe vertigo, which forces them to interrupt their work immediately, thus having a negative impact on their daily lives (7). BPPV typically recurs, with a recurrence rate of 15–56% during a 1–10 year period (6). This not only results in increasing medical expenses for individuals and their families but also imposes an additional burden on society in terms of healthcare resources utilization. Therefore, it is crucial to ascertain the variables that contribute to the onset and recurrence of benign paroxysmal positional vertigo (BPPV).
The primary components of otoconia are CaCO3 and glycoprotein crystals, which are connected to hair cells via protein fibers. The otoconia crystals are formed by the vestibular organ’s active calcium metabolism activities (8). Otoconia crystals possess a core nucleus predominantly made of organic glycoproteins with little calcium (Ca) content. The crystals are encircled by inorganic outer zones composed mostly of CaCO3, exhibiting elevated calcium concentrations (9). Vitamin D, a lipid-soluble prehormone, is essential for regulating calcium and phosphorus levels. Vitamin D can be gained by dietary intake, consumption of vitamin D-fortified foods, and skin exposure to UVB radiation (10). Adequate amounts of vitamin D are required for optimal calcium metabolism, which influences the function of the otoliths (11). Furthermore, vitamin D receptors are located in several cells of the inner ear, including those associated with the vestibular system. Insufficient vitamin D is considered to disrupt normal signaling pathways and cellular processes in these receptors, hence leading to vestibular dysfunction (12). According to a prior epidemiological investigation, the risk of BPPV recurrence rose from 15% in the first year following CRP therapy to 50% over the course of three years (6). Vitamin D affects calcium and bone metabolism, which has been linked to BPPV development.
Numerous investigations have demonstrated that low blood levels of 25-hydroxyvitamin D (25(OH)D) are associated with both the incidence and recurrence of BPPV (13–15), vitamin D insufficiency has been extensively studied as a risk factor for the development and recurrence of BPPV. However, the results are inconclusive. Talaat et al. (13) found that low levels of 25(OH)D were linked to the onset of BPPV and the recurrence of BPPV. Ding et al. (14) demonstrated 25(OH)D insufficiency was connected with BPPV incidence and recurrence, as well as negatively associated with the severity of BPPV. Some researchers have confirmed that vitamin D is not related to the pathogenesis of BPPV and its recurrence (16–18). The researchers reached contradictory conclusions.
Patients suffering from the various kinds of BPPV can be effectively treated with a suitable single canalith repositioning therapy in up to 85% of instances (19). Although the first therapy is effective, the 1-year recurrence rate of BPPV is reported to be between 13.7 and 48.0% (20). Several studies recently reported a greater frequency of vitamin D deficiency/insufficiency in individuals with BPPV than in controls (6, 12–26). Given these findings, the question of vitamin D supplementation arises. In reality, vitamin D treatment may reduce the recurrent episodes of BPPV, and curiously, a full remission was achieved in a large number of individuals after trials of vitamin D supplementation (27–30). Supplementation is intended to address the deficit and maybe improve the condition of BPPV sufferers. Some studies have also suggested that vitamin D intake can reduce recurrences of BPPV (13), but Rhim et al. (31)cannot get the same conclusion. So whether patients with benign paroxysmal positional vertigo (BPPV) can reduce the risk of BPPV by supplementing vitamin D remains controversial.
The primary purposes of this meta-analysis are to identify the relationship among vitamin D and BPPV occurrence, vitamin D and BPPV incidence, also summarize the evidence for supplementation of vitamin D on BPPV recurrences.
Materials
Search strategy and study selection
A thorough literature search was carried out from the beginning to Dec 22th, 2024, using MEDLINE, PubMed, the Cochrane Library, Web of Science, and the EMBASE databases, the following search phrases and keywords were linked by “and” or “or”: (Benign Paroxysmal positional vertigo or otolithiasis or BPPV) and (vitamin D or 25(OH) vitamin D or 25-hydroxylvitamin D or vitamin D supplementation). The study was done using the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) standard (32) and was preregistered in PROSPERO (CRD420251002488, see Supplementary file-PROSPERO).
Methods
Data extraction and study quality
Inclusion criteria
The search methodology was confined to publicly accessible data and articles in the English language. Publications were selected according to the following criteria: (1) The investigations were comparative studies carried out retrospectively or prospectively on adult patients. (2) examined the relationship between vitamin D, vitamin D supplementation and Benign Paroxysmal positional vertigo; (3) Vitamin D was measured using a recognized technique and represented as, or converted to, one international unit (ng/ml), providing relative risk (RR), odds ratio (OR), or weighted mean difference (WMD) with a 95% confidence interval. (4) Diagnosis of BPPV: The diagnostic criteria for benign BPPV were formulated by the Committee for the Classification of Vestibular Disorders of the Bárány Society (2). BPPV diagnosis was based on a characteristic history and observation of typical nystagmus during the Dix-Hallpike manoeuvre, supine roll, and cephalic hyperextension tests. For posterior semicircular canal BPPV, the Dix-Hallpike test was positive if nystagmus was recorded with appropriate positioning, latency, duration, and fatigability. Lateral semicircular canal BPPV (LC-BPPV) was diagnosed by positional nystagmus changing in the horizontal direction concurrent with vertigo triggered by the supine roll test. According to the direction of the nystagmus as horizontal geotropic and apogeotropic nystagmus, the LC-BPPV was classified as canalolithiasis or cupulolithiasis, respectively. A “recurrence” was defined as a new BPPV episode occurring at least 2 weeks after verifying the complete resolution of the previous one.
Exclusion criteria
Two reviewers (Peng Gao and Rui Ding) independently retrieved data from pertinent articles utilizing data extraction forms. Any discrepancies between the two reviewers were resolved through discussion with another author (YY Li). We excluded: (1) duplicate or irrelevant papers; (2) reviews, letters, or case reports, commentaries; (3) non-original research; (4) non-human subjects.
Data extraction
The impact estimates corrected for the maximum number of confounding factors, together with their 95% confidence intervals (CIs), for the comparison between the highest and lowest vitamin D levels, were obtained. Additionally, the vitamin D levels (mean ± standard deviation [SD]) were obtained for both BPPV and control patients. Conversely, the unit of circulating selenium was standardized to “ng/mL” throughout all trials.
Quality assessment
The two reviewers (Peng Gao and Rui Ding) independently assessed the quality and risk of bias of the included studies using the Newcastle-Ottawa score (NOS) (33); All disagreements between the two reviewers were fully discussed, and furthermore a third reviewer (YY Li) was consulted for unresolved discrepancies to reach a consensus. Researches that received scores of 0–4 and 5–9 were classified as high-quality and low-quality, respectively.
Statistical analysis
The meta-analysis was conducted using STATA version 17.0 and Review Manager software version 5.3. For continuous data, mean ± standard deviation is displayed, if the included studies provided the data using median and quartile values, we estimated the mean and standard deviation 17 using the Wan et al. Approach (34) Statistical significance was defined as p-values<0.05, and 95% confidence intervals were supplied. The weighted mean difference (WMD) was used as the metameter and the standard deviation (SD) was considered in evaluating the precision and significance of that point of estimate. Whereas heterogeneity across studies was evaluated using Cochrane-based Q and I (2) tests. Data followed by p < 0.05 or I (2) > 50% were considered to denote statistically significant heterogeneity, and were subjected to a randomized-effects model. Otherwise, a fixed-effects model was used. A funnel plot was used to determine publication bias.
Results
Study characteristics
Literature search and study characteristics
A total of 1,375 citations were located, and only the titles and abstracts passed muster. Full text of potentially relevant papers was read. Tables 1, 2 lists the features of the included studies, and Figure 1 displays the flow chart of the literature search. After removing the duplicates, 475 items were still present, 293 articles were excluded by screening the titles and abstracts. 12 reviews and 6 non-BPPV studies were removed. Eventually, the remaining sixty full-text papers were assessed based on the qualifying requirements (Figure 1).
Table 1
| Study and year | Country | Study type | N total | Case (umol/l) | Control (umol/l) | Vitamin D in recurrent | Vitamin D in non-recurrent | OR (incidence/recurrence) | Quality-NOS |
|---|---|---|---|---|---|---|---|---|---|
| Zhang, 2020 (41) | China | Case–control | 206 | 18.8 + −2.5 | 24 + −2.2 | NA | NA | 3.045 (1.467,4.638) | 9 |
| Yang, 2017 (3) | Korea | Case–control | 260 | 18.2 + −10.3 | 20 + −8.1 | NA | NA | NA | 8 |
| Yadav, 2021 (21) | India | Case–control | 46 | 26.7 + −16.09 | 15.74 + −5.78 | NA | NA | 0.28 (0.1,0.85) | 7 |
| Wu, 2018 (22) | China | Case–control | 152 | 20.99 + −6.76 | 23.17 + −6.49 | NA | NA | 3.8(1.25,11.73) | 9 |
| Wang, 2021 (23) | China | Case–control | 36 | 20.7 + −1.95 | 30.59 + −2.75 | NA | NA | 0.57 (0.13,1.03) | 9 |
| Wang, 2019 (26) | China | Case–control | 183 | 17.15 + −2.03 | 23.85 + −3.13 | NA | NA | 2.0 (0.94,3.33) | 6 |
| Thomas, 2022 (50) | India | Case–control | 98 | 21.26 + −0.57 | 17.59 + −0.06 | NA | NA | NA | 7 |
| Talaat, 2014 (13) | Egypt | Case–control | 180 | 16.04 + −10.26 | 19.53 + −8.45 | 11.93 + −7.57 | 16.04 + −10.26 | 4.54(1.41,14.58) | 7 |
| Song, 2020 (36) | China | Case–control | 320 | 16.89 + −7.45 | 15.95 + −8.06 | NA | NA | 1.57 (1.21,2.02) | 8 |
| Sarsitthithum, 2023 (35) | Thailand | Case–control | 137 | 21.5 + −5.3 | 26.3 + −6.8 | NA | NA | 1.51(1.32,1.72) | 8 |
| Saeed Al-Rawi, 2024 (43) | Ramadi | Case–control | 160 | 15.46 + −6.14 | 23.6 + −12.58 | 12.62 + −4.1 | 18.3 + −6.61 | 0.74 (0.50,1.10) | 8 |
| Resuli, 2022 (6) | İstanbul | Case–control | 358 | 18.8 + −6.1 | 30.74 + −8.53 | NA | NA | NA | 8 |
| Ren, 2023 (20) | China | Case–control | 364 | 17.17 + −2.29 | 19.26 + −2.39 | NA | NA | 1.643 (1.058,2.550) | 7 |
| Pecci, 2022 (15) | Italy | Case–control | 50 | 20.18 + −9.26 | 23.73 + −8.42 | NA | NA | 0.968 (0.914,1.026) | 8 |
| Parham, 2013 (25) | USA | Case–control | 29 | 10.32 + −0.92 | 9.98 + −0.96 | NA | NA | 0.50 (0.31, 0.80) | 8 |
| Melis, 2020 (40) | Italy | Case–control | 120 | 26.1 + −11.66 | 46.02 + −12.56 | NA | NA | 0.86 (0.76, 0.96) | 8 |
| Lee, 2017 (37) | Korea | Case–control | 184 | 34.2 + −14.3 | 30.3 + −18.6 | NA | NA | 2 (1.9,2.1) | 7 |
| Kim, 2020 (65) | Korea | Case–control | 78 | 17.54 + −8.93 | 15.61 + −9.76 | NA | NA | 3.2(0.8,12.1) | 7 |
| Karataş, 2017 (16) | Turkey | Case–control | 156 | 23 + −14.4 | 17 + −12.3 | NA | NA | 1.66 (0.87,3.17) | 9 |
| Kahraman, 2016 (39) | Turkey | Cohort | 74 | 9.73 + −8.77 | 19.08 + −5.92 | NA | NA | NA | 7 |
| Jeong, 2013 (24) | Korea | Case–control | 292 | 14.4 + −8.4 | 19.1 + −6.8 | NA | NA | 3.8(1.51,9.38) | 8 |
| Isik, 2017 (9) | Turkey | Case–control | 127 | 9.51 + −5.49 | 11.02 + −9.62 | NA | NA | 0.71(0.56,0.90) | 6 |
| Inan, 2021 (38) | Turkey | Case–control | 104 | 15.3 + −9.8 | 20.2 + −14.3 | NA | NA | 0.68 (0.56,0.83) | 8 |
| Hualan Yang, 2017 (12) | China | Case–control | 102 | 23.13 + −6.11 | 26.85 + −5.92 | NA | NA | NA | 8 |
| Han, 2018 (19) | China | Case–control | 165 | 19.1 + −5.2 | 22.5 + −5.8 | NA | NA | 2.1 (1.12, 3.95) | 8 |
| Goldschagg, 2021 (18) | Germany | Case–control | 379 | 23.4 + −9.4 | 24.9 + −10.1 | NA | NA | 0.62 (0.42, 0.92) | 7 |
| Ding, 2019 (14) | China | Case–control | 522 | 18.22 + −2.16 | 21.85 + −1.6 | NA | NA | 2.15(1.30,4.32) | 8 |
| Cobb, 2022 (48) | USA | Case–control | 6,135 | 31.4 + −16.5 | 26 + −11.2 | 29 + −12 | 37.6 + −18.3 | 0.47 (0.32, 0.63) | 8 |
| Cheng, 2021 (1) | China | Case–control | 640 | 23.2 + −4.09 | 25.8 + −3.43 | NA | NA | 0.88 (0.83,0.94) | 8 |
| Chauhan, 2023 (46) | India | Case–control | 88 | 27.9 + −15.89 | 39.05 + −21.15 | NA | NA | 0.71(0.53,0.93) | 9 |
| Bi, 2021 (47) | China | Case–control | 52 | 14.64 + −6.94 | 19.56 + −6.55 | NA | NA | 1.073 (0.964,1.194) | 8 |
| Bener, 2024 (55) | Turkey | Case–control | 833 | 19.04 + −8.37 | 21.19 + −9 | NA | NA | 1.32(0.59,2.96) | 9 |
| Bazoni, 2019 (44) | Brazil | Case–control | 109 | 27.8 + −10.1 | 23.8 + −11.28 | NA | NA | 0.57(0.,22,1.48) | 8 |
| Büki, 2013 (28) | Austria | Cohort | 18 | NA | NA | 13.41 + −1.9 | 28.77 + −11.13 | NA | 8 |
| Chen, 2017 (20) | China | Case–control | 249 | NA | NA | 18.1 + −6.6 | 19 + −7.6 | NA | 8 |
| Chu, 2024 (42) | Singapore | Case–control | 149 | NA | NA | 17.4 + −5.25 | 21.4 + −5.02 | 0.83(0.76,0.90) | 8 |
| Ding, 2019 (14) | China | Case–control | 174 | NA | NA | 11.85 + −3.29 | 18.77 + −2.02 | 5.16 (1.00,34.12) | 9 |
| Libonati, 2020 (56) | Italy | Rct | 109 | NA | NA | 18.2 + −6.6 | 36.9 + −5.7 | NA | 9 |
| Lin 2024 (49) | China | Case–control | 138 | NA | NA | 13.37 + −4.62 | 18.15 + −6.29 | NA | 8 |
| Maslovara, 2018 (17) | Croatia | Cohort | 31 | NA | NA | 22.88 + −13.77 | 19.11 + −6.27 | NA | 8 |
| Melis, 2020 (40) | Italy | Cohort | 73 | NA | NA | 19.53 + −15.33 | 25.85 + −14.1 | NA | 8 |
| Rhim, 2016 (29) | Korea | Cohort | 232 | NA | NA | 13.64 + −6.97 | 16.63 + −7.4 | NA | 7 |
| Rhim, 2020 (31) | Korea | Case–control | 332 | NA | NA | 14.1 + −13.5 | 14.9 + −13.9 | 0.976 (0.934, 1.020) | 8 |
| Shin, 2022 (45) | Republic of Korea | Cohort | 50 | NA | NA | 12.9 + −8 | 19.2 + −8.2 | NA | 8 |
| Yang, 2017 (3) | Korea | Case–control | 126 | NA | NA | 19.3 + −11.1 | 17.2 + −9.4 | NA | 8 |
| Zhang, 2018 (65) | China | Case–control | 78 | NA | NA | 17.15 + −6.04 | 18.45 + −4.28 | NA | 7 |
| Zhang, 2020 (41) | China | Case–control | 156 | NA | NA | 16.7 + −2.1 | 19.3 + −2.3 | NA | 9 |
Characteristics of the included studies.
NA, not available; NOS, Newcastle-Ottawa Scale; N, number; OR, odds ratio.
Table 2
| Study and year | case/control | intervention | Follow up (months) | vitamin D before treatment | vitamin D after treatment | P for recurrence difference |
|---|---|---|---|---|---|---|
| Büki, 2013 (28) | 4/14 | 8,000 IU cholecalciferol daily for two weeks, and daily 4,000 IU cholecalciferol for the next two weeks, then a weekly dose of 8,000 IU | 8 | 14 | 27 | p < 0.02 |
| Carneiro de Sousa, 2014 (29) | 71/57 | 5,000 IU of cholecalciferol vitamin D daily for 12 months | 12 | 14 | 30.6 | p = 0.001 |
| Califano, 2014 (30) | 68/68 | 10,000 IU to 50,000 IU of cholecalciferol Vitamin D weekly for 12 months | 12 | 18.2 + −10.43 | NA | p < 0.001 |
| Sheikhzadeh, 2016 (58) | 27/27 | 5,000 IU of cholecalciferol vitamin D daily for 2 months | 6 | 11.41 ± 1.9 | 34.2 ± 3.3 | P = 0.001 |
| Talaat, 2016 (13) | 28/65 | 50,000 IU oral vitamin D3, 3 times/week, for 1 month, followed by 50,000 IU oral vitamin D3, once every 2 weeks plus Calcium citrate 600 mg tablets twice daily | 18 | 6.7 + −2 | 28.3 + −5 | P < 0.001 |
| Rhim, 2020 (31) | 40/45 | Intramuscular injection 200,000 IU, three to four injections in the first year, The injection solution contains 200,000 IU (5 mg) of cholecalciferol | 24 | 6.06 + −3.5 | 31.1 + −6.8 | p = 0.883 |
| Jeong, 2020 (57) | 445/512 | Vitamin D 400 IU and 500 mg of calcium carbonate twice a day for 1 year. | 12 | 13.3 + −3.9 | 24.2 + −8.4 | p = 0.005 |
| Elmoursy, 2021 (53) | 20/20 | NA | 6–12 | NA | NA | p = 0.047 |
| Abdelmaksoud, 2021 (54) | 20/20 | Cholecalciferol 8,000 IU daily for 2 weeks followed by 4,000 IU daily for 2 weeks then 8,000 IU single dose weekly for 3 months | 6 | 12.4 ± 2 | 26.3 ± 4.1 | P = 0.000 |
| Pecci, 2022 (15) | 26/24 | 50.000 IU once a week for 2 weeks, then 25.000 IU once a week for 2 weeks, then 7.000 IU once a week for 2 months | 4–8 | 20.18 | 28.1 | p = 0.0003 |
| Sharma, 2022 (52) | 20/20 | Consisted of daily dose of 5,000 IU cholecalciferol for one month, then 5,000 IU cholecalciferol twice weekly for one month, then a weekly dose of 5,000 IU was given thereafter. | 6 | 12.3 | 27.2 | P = 0.001 |
| Sánchez, 2022 (27) | 17/18 | Colecalcifero 1,600 U once a week during 8–10 weeks | 6–13 | 18.5 + −6.8 | 26.2 + −4.9 | p = 0.017 |
| Kong, 2024 (51) | 20/18 | 7,000 IU of vitamin D weekly for a year | 6–12 | 12.0 ± 4.8 | 28.2 ± 6.5 | p = 0.003 |
Effects of vitamin D supplementation and BPPV recurrence observed in this review.
Subgroup of vitamin D supplementation and BPPV recurrence.
NA, not available.
Figure 1

Preferred reporting item for systematic reviews and meta-analysis (PRISMA) guideline.
Consequently, a total of 60 studies involving BPPV, vitamin D, vitamin D supplementation and the recurrence of BPPV, were examined qualitatively, and then a meta-analysis was conducted. (Table 1) 0.47 studies about vitamin D and incidence, recurrence of BPPV with a total of 14,654 patients, 13 studies about, vitamin D supplementation and the recurrence of BPPV, totaling 1714 participants, were analyzed qualitatively and meta-analyzed, details of the included studies are shown in Table 2. Seven to nine was the range of quality scores. Every record that was included was thought to be of high caliber.
The aggregated weighted mean difference difference (WMD) of the vitamin D level between BPPV and controls, non-recurrence BPPV and recurrence BPPV
The aggregated weighted mean difference (WMD) demonstrated that there was a significant reduction in vitamin D level in the BPPV cohort (WMD = −2.84; 95% CI −4.53 to −1.15) (Figure 2A) relative to the control cohort. Likewise, when the recurrent BPPV groups were compared with the non-recurrent BPPV groups, the statistical analysis showed significantly lower level of vitamin D among the recurrence BPPV groups (WMD = −5.01; 95% CI −6.94 to −3.08) (Figure 2B).
Figure 2

Vitamin D levels between BPPV and controls, non-recurrence BPPV, and recurrence BPPV (A) Vitamin D level between BPPV and controls (B) Vitamin D level between non-recurrence BPPV and recurrence BPPV (C) Vitamin D level between cupulolithiasis and canalolithiasis BPPV groups.
The aggregated mean difference (WMD) of the vitamin D level between cupulolithiasis and canalolithiasis BPPV groups
When the cupulolithiasis BPPV groups were compared with the canalolithiasis BPPV groups, the vitamin D level showed lower level of vitamin D in the cupulolithiasis groups (WMD = 5.09; 95% CI 2.05 to 8.12) (Figure 2C).
Multivariable-adjusted RR of BPPV for the highest compared with lowest circulating vitamin D level category
In this meta-analysis, the multivariable-adjusted relative risk (RR) indicated that increased circulating vitamin D was inversely related to BPPV incidence (RR = 1.36 95% CI 1.31, 1.41) (Figure 3A), but not significantly related to the recurrence (RR = 0.95, 95% CI 0.91, 0.99) (Figure 3B).
Figure 3

Association between vitamin D levels and BPPV (A) Association between vitamin D levels and BPPV risk (B) Association between vitamin D levels and recurrence of BPPV risk.
The connection between vitamin D supplementation and BPPV recurrence
Vitamin D supplementation group had a lower recurrence rate than the control group who did not accepted vitamin D supplementation (RR =0.45, 95% CI = 0.36–0.55) (Figure 4).
Figure 4

Vitamin D supplementation and BPPV recurrence.
Publication bias
The funnel plot was basically symmetrical, and there was no evidence of publication bias (Figure 5).
Figure 5
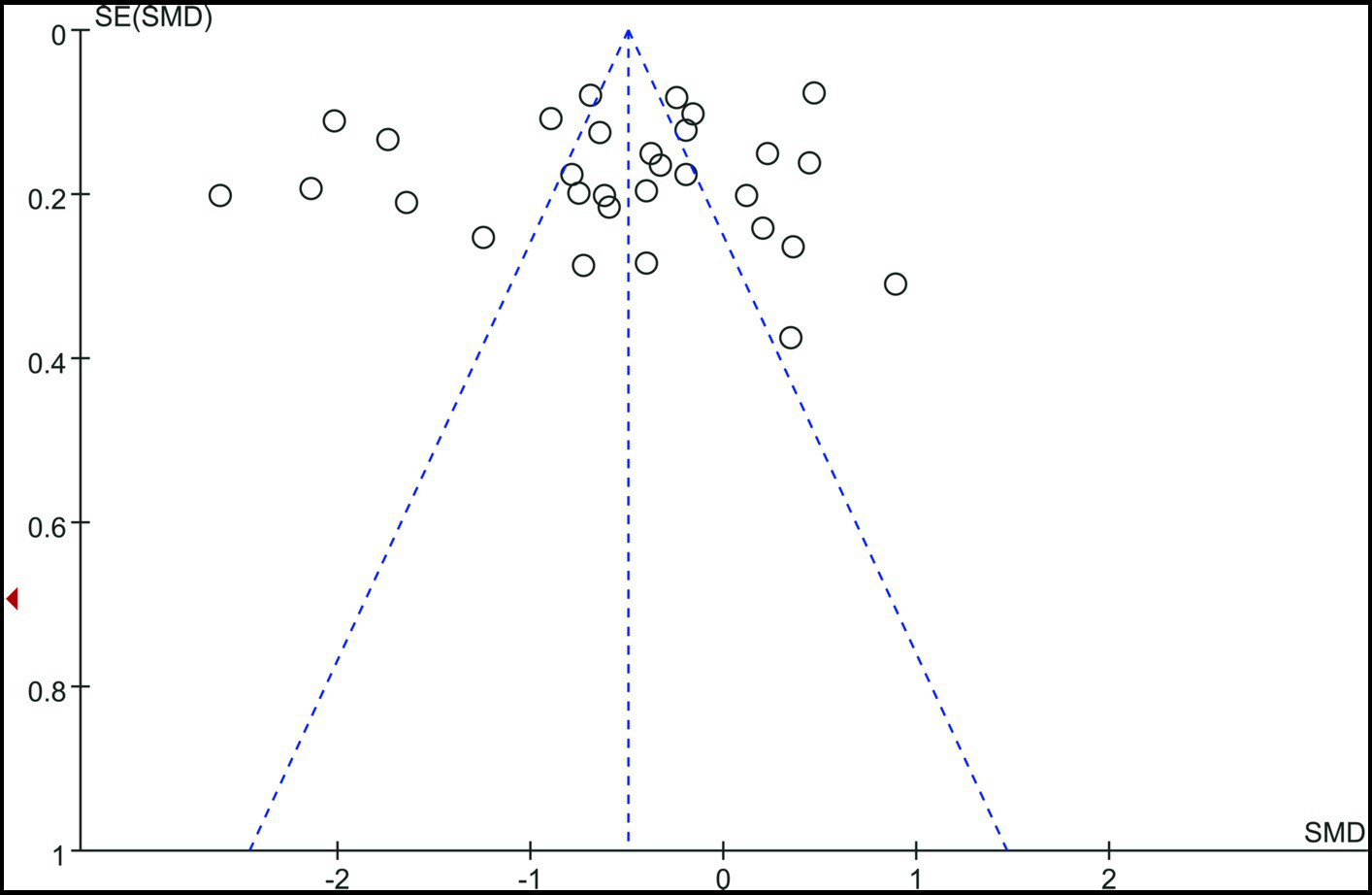
Funnel plot.
Discussion
Our meta-analysis indicates that blood vitamin D levels are lower in BPPV patients, particularly those with recurrent BPPV, than in controls. There was a negative association between the frequency of BPPV episodes and vitamin D deficit, indicating that vitamin D deficiency may play a role in the development of BPPV. Likewise, our current meta-analysis demonstrates that lower vitamin D levels are significantly related with BPPV incidence rather than recurrence. The current investigation found that vitamin D supplementation lowers BPPV recurrence in patients with vitamin D insufficiency. At the same time, we found that vitamin D levels were lower in cupulolithiasis than in canalolithiasis groups.
In vitamin D receptor null mice, otoconia exhibited degenerative characteristics such as fissures, fusion, and tiny particles. These data revealed a substantial link between BPPV and vitamin D insufficiency (18). Many studies (34–39) focus on the vitamin D level and BPPV populations, which included incidence and recurrence of BPPV, but got inconsistent conclusions. Melis et al. (40) suggested a relationship between vitamin D deficiency and BPPV onset. Jeong et al. (24) also shows lower level 25-OH vitamin D concentrations in patients with BPPV when compared with control group. Zhang et al. (41) conducted a prospective study found that vitamin D level is lower in BPPV groups. In Chu et al. (42), Saeed Al-Rawi et al. (43), and Ding (14) study, lower levels of vitamin D was associated with the recurrence of BPPV. On the contrary, Işık et al. (9) found no correlation between vitamin D levels and BPPV occurrence or recurrence. Bazoni (44) et al. and Kim et al. found that vitamin D levels are elevated in patients with BPPV. Shin et al. (45)and other researchers (46–48) found that lower serum vitamin D level is a risk factor for BPPV recurrence. Likewise, Lin et al. (49) indicated that 25(OH)-D levels were markedly decreased in recurrent BPPV group compared to a non-recurrent BPPV subgroup. Nevertheless, a Croatian investigation indicated that a diminished blood level of vitamin D3 did not elevate the probability of BPPV recurrence (17). Another investigation conducted in India revealed that there is no correlation between calcium and vitamin D levels and BPPV (50). Our meta-analysis were consistent with Talaat et al. (13) study, which showed that the BPPV population had a lower blood vitamin D levels than the controls. Meanwhile, our systematic review confirmed that, compared with non-recurrent BPPV population, patients with recurrent BPPV had lower vitamin D levels, which proves that BPPV and its recurrence are associated with lower serum vitamin D levels. Unfortunately, our systematic review cannot prove a causal relationship between the occurrence and recurrence of BPPV and vitamin D levels.
Although a causal relationship cannot be showed by a retrospective investigation, if lower vitamin D levels are associated with BPPV in this subgroup, it is fair to believe that supplementation may have a role in avoiding BPPV development. For example, Kong et al. (51) and other researchers (52–55) established a link between vitamin D supplementation and a reduction in BPPV recurrence. They revealed that daily supplementation with oral vitamin D and calcium carbonate over the year of therapy significantly reduced the recurrence of BPPV in people with lower blood vitamin D levels. Many studies (42, 55) established a negative correlation between the recurrence rate of BPPV and vitamin D deficit, suggested that vitamin D deficiency may contributes to BPPV recurrence. So, some researchers (56, 57) speculated that vitamin D therapy for people with vitamin D insufficiency may lessen the recurrence of BPPV. Rhim (31) et al. established that vitamin D supplementation would lower the recurrence rate throughout 6 months of follow-up, and he concluded that blood vitamin D concentrations significantly impact the recurrence of BPPV. Sheikhzadehi (58) et al. conducted a study demonstrate that oral nutritional therapy with vitamin D3 and antioxidants help decrease relapses in individuals suffering from high recurrence BPPV. The same result was reported in Sánchez et al (27) study, BPPV patients with lower blood vitamin D levels who received vitamin D supplementation showed a substantial decrease in vertigo episodes, which was consistent with our meta-analysis. Our current analysis found that vitamin D supplementation can dramatically minimize recurrence in BPPV patients who with vitamin D insufficiency.
Our meta-analysis showed that vitamin D levels were lower in cupulolithiasis than in canalolithiasis groups, which consistent with Nakada et al. (59) study, which stated the difference in serum vitamin D concentrations between canalolithiasis and cupulolithiasis. These results point to pathophysiological differences between canalolithiasis and cupulolithiasis in relation to vitamin D level. Further investigation is required to uncover the processes behind this phenomena in the future.
In conclusion, our meta-analysis reveals that vitamin D levels are quite important in BPPV patients. Vitamin D may influence calcium homeostasis and bone metabolism, which might impact the creation and function of otoconia in the inner ear. There is no unified theory regarding the mechanism between vitamin D levels and BPPV. The possible mechanisms are as follows: (1) Vitamin D may regulates calcium homeostasis by influencing calcium deposition and dissolution in the inner ear (otoconia) (25). (2) Immunomodulation: All of the body’s cells have the VDR, which plays a role in immunomodulation, cell division, and proliferation (48). It can regulate the function of immune cells, such as T lymphocytes and macrophages. Immune-mediated processes in the inner ear might be involved in the pathophysiology of BPPV. Vitamin D may control the inner ear’s immune response and stop autoimmune responses or excessive inflammation that might harm the vestibular system (48); (3) Antioxidant Activity: In the context of BPPV, oxidative stress could potentially affect the structure and function of otoconia and the vestibular sensory epithelium (60). Vitamin D may protect the inner ear from oxidative stress-induced damage while also maintaining proper vestibular function.
As we know, Vitamin D participates in several physiological processes, includes the regulation of arterial blood pressure, modulation of immune responses, regulation of insulin secretion, protection against certain cancers, renoprotection, and other beneficial effects (61). All elements of the epithelial Ca channel transport system are expressed as transcripts in the cochlea and semicircular canal duct, according to a recent experimental research. Vitamin D increases the otolith of utriculi, despite it being smaller than the bone (62). Similarly, calcium and 25(OH)-D help to assure the integrity of the support tissues’ hair cells. According to some researchers, the onset of recurrent otoconia dislocation may also be related to mechanisms of increased resorption and decreased fixation of calcium. Therefore, a reduction in calcium fixation may lead to deficiencies in the remodeling of the otoconia’s internal structure and their adhesion to the otoconial membrane (63–65). Thus, our research indicates that vitamin D supplementation may lower the recurrence risk of BPPV in individuals with vitamin D insufficiency. In conclusion, since the patients did not suffer from any illnesses that affected their vitamin D intake, conversion, or absorption, we suggest that doctors give BPPV patients the option to check their vitamin D levels and recommend either dietary or medication supplements if necessary.
Despite these findings, the meta-analysis has certain limitations. First, the majority of the papers considered were case–control studies, which are less reliable than randomized controlled trials. Second, because the studies were confined to those published in English, we cannot rule out the possibility of publication bias, despite the funnel plot being mostly symmetrical, indicating no significant danger. Third, future research should look at the potential links between vitamin D levels and BPPV subtypes, season, weather, skin color, lifestyle, nutrition, and vitamin assays. Furthermore, observational studies have reported more substantial effects of vitamin D supplementation than randomized controlled trials. In the future, more RCT (Randomized Controlled Trial) studies will be needed to confirm our conclusions.
Conclusion
The present meta-analysis evaluates vitamin D levels for BPPV and recurrent BPPV, which indicates that lower vitamin D levels are associated with the incidence and recurrence of BPPV, and vitamin D supplementation in BPPV patients with deficiency or insufficiency reduces recurrence of BPPV. Due to the limited quality and quantity of the listed studies, robust researches with sufficient sample sizes are necessary to validate the result.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
YL: Writing – original draft, Writing – review & editing, Data curation, Investigation. PG: Conceptualization, Writing – original draft. RD: Data curation, Writing – review & editing. YX: Methodology, Writing – review & editing. ZW: Data curation, Software, Writing – original draft. XP: Conceptualization, Writing – original draft. LL: Data curation, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1560616/full#supplementary-material
References
1.
Cheng Z Wang M Yu J . Benign Paroxysmal Positional vertigo and serum transthyretin in Chinese older adults. Asia Pac J Clin Nutr. (2021) 30:383–91. doi: 10.6133/apjcn.202109_30(3).0005
2.
von Brevern M Bertholon P Brandt T Fife T Imai T Nuti D et al . Benign paroxysmal positional vertigo: diagnostic criteria consensus document of the Committee for the Classification of vestibular disorders of the Bárány society. Acta Otorrinolaringol Esp Engl Ed. (2017) 68:349–60. doi: 10.1016/j.otorri.2017.02.007
3.
Yang CJ Kim Y Lee HS Park HJ . Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J Vestib Res. (2017) 27:287–94. doi: 10.3233/VES-170625
4.
Baloh RW Honubria V Jacobson K . Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. (1987) 37:371–8. doi: 10.1212/WNL.37.3.371
5.
Motin M Keren O Groswasser Z Gordon CR . Benign paroxysmal positional vertigo as the cause of dizziness in patients after severe traumatic brain injury: diagnosis and treatment. Brain Inj. (2005) 19:693–7. doi: 10.1080/02699050400013600
6.
Resuli AS Bedir A Özgür A . The relationship between benign paroxysmal positional vertigo and vitamin D, Cureus. Otolaryngol Head Neck Surg. (2022) 14:e26068. doi: 10.7759/cureus.26068
7.
von Brevern M Radtke A Lezius F . Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. (2007) 78:710–5. doi: 10.1136/jnnp.2006.100420
8.
Lundberg YW Zhao X Yamoah EN . Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res. (2006) 1091:47–57. doi: 10.1016/j.brainres.2006.02.083
9.
Işık GC Çevik Y Emektar E Çorbacıoğlu ŞK . Analysis of vitamin D and calcium levels in benign paroxysmal positional vertigo. Eurasian J Emerg Med. (2017) 16:128–32. doi: 10.5152/eajem.2017.58077
10.
Sfakianaki I Binos P Karkos P Dimas GG Psillas G . Risk factors for recurrence of benign paroxysmal positional vertigo. A Clinical Review J Clin Med. (2021) 10:4372. doi: 10.3390/jcm10194372
11.
Lundberg YW Xu Y Thiessen KD Kramer KL . Mechanisms of otoconia and otolith development. Dev Dyn. (2015) 244:239–53. doi: 10.1002/dvdy.24195
12.
Yang H Gu H Sun W Li Y Wu H Burnee M et al . Estradiol deficiency is a risk factor for idiopathic benign paroxysmal positional vertigo in postmenopausal female patients. Laryngoscope. (2018) 128:948–53. doi: 10.1002/lary.26628
13.
Talaat HS Kabel AM Khaliel LH Abuhadied G El-Naga HA Talaat AS . Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. (2016) 43:237–41. doi: 10.1016/j.anl.2015.08.009
14.
Ding J Liu L Kong WK Chen XB Liu X . Serum levels of 25-hydroxy vitamin D correlate with idiopathic benign paroxysmal positional vertigo. Biosci Rep. (2019) 39:BSR20190142. doi: 10.1042/BSR20190142
15.
Pecci R Mandalà M Marcari A . Vitamin D insufficiency/deficiency in patients with recurrent benign paroxysmal positional vertigo. J Int Adv Otol. (2022) 18:158–66. doi: 10.5152/iao.2022.21269
16.
Karataş A Yüceant GA Yüce T Hacı C Cebi IT Salviz M . Associationof benign paroxysmal positional Vertigo with osteoporosis and vitamin D Defi-ciency: a case controlled study. J Int Adv Otol. (2017) 13:259–65. doi: 10.5152/iao.2016.2640
17.
Maslovara S Butkovic Soldo S Sestak A Milinkovic K Rogic-Namacinski J Soldo A . 25 (OH) D3 levels, incidence and recurrence of different clinical forms of benig paroxysmal positional vertigo. Braz J Otorhinolaryngol. (2018) 84:453–9. doi: 10.1016/j.bjorl.2017.05.007
18.
Goldschagg N Teupser D Feil K . No evidence for a specific vitamin D deficit in benign paroxysmal positional vertigo. Eur J Neurol. (2021) 28:3182–6. doi: 10.1111/ene.14980
19.
Han W Fan Z Zhou M Guo X Yan W Lu XZ et al . Low 25-hydroxyvitamin D levels in postmenopausal female patients with benign paroxysmal positional vertigo. Acta Otolaryngol. (2018) 138:443–6. doi: 10.1080/00016489.2017.1416168
20.
Ren Y-Y Wang Y-J Li J-L Liu M Xia F . Low vitamin D and uric acid status in patients with benign paroxysmal positional vertigo. Sci Prog. (2023) 106:368504231205397. doi: 10.1177/00368504231205397
21.
Yadav H Irugu D Ramakrishanan L Singh A Abraham R Sikka K et al . An evaluation of serum Otolin-1 & vitamin-D in benign paroxysmal positional vertigo. J Vestib Res. (2021) 31:433–40. doi: 10.3233/VES-201601
22.
Wu Y Fan Z Jin H Guan Q Zhou M Lu X et al . Assessment of bone metabolism in male patients with benign paroxysmal positional vertigo. Front Neurol. (2018) 9:742. doi: 10.3389/fneur.2018.00742
23.
Wang L Liu J Fan Q Fan Z Xianrong X Li Z et al . Benign paroxysmal positional vertigo as a complication of 90-day head-down bed rest. Eur Arch Otorrinolaringol. (2021) 278:683–8. doi: 10.1007/s00405-020-06124-2
24.
Jeong SH Kim JS Shin JW . Decreased serum vitamin D in idiopathic benign paroxys mal positional vertigo. J Neurol. (2013) 260:832–8. doi: 10.1007/s00415-012-6712-2
25.
Parham K Leonard G Feinn RS Lafreniere D Kenny AM . Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: a pilot study. Laryngoscope. (2013) 123:2834–9. doi: 10.1002/lary.24162
26.
Wang Z Yao G Tao X Zhang J Zhang T Wu Z . Evaluation of bone mineral density and 25-(OH) vitamin D levels in middle-aged and elderly women with recurrent benign paroxysmal positional vertigo. Acta Otolaryngol. (2020) 140:89–93. doi: 10.1080/00016489.2019.1692146
27.
Sánchez JM Carlos J Leonardo H Kioko J Niembro I Lesser JC . Therapeutic effect of the correction of vitamin D deficiency in patients with benign paroxysmal positional Vertigo – a randomized clinical trial. Int Arch Otorhinolaryngol. (2022) 26:e666–70. doi: 10.1055/s-0041-1730992
28.
Büki B Ecker M Junger H Lundberg YW . Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypotheses. (2013) 80:201–4. doi: 10.1016/j.mehy.2012.11.029
29.
Carneiro de Sousa PJM Abreu Pereira DM Carneiro Melo Pereira de Magalhães P Duarte D R da S Trigueiros da Silva Cunha NM . Vitamin D deficiency and benign paroxysmal positioning vertigo. Hearing Balance Commun. (2019) 17:179–81. doi: 10.1080/21695717.2019.1590988
30.
Califano L Salafia F Melillo MG . Is hypovitaminosis D a risk factor for either the onset or the recurrence of benign paroxysmal positional Vertigo. Frontiera ORL. (2014) 144:138–45.
31.
Rhim GI . Effect of vitamin D injection in recurrent benign paroxysmal positional vertigo with vitamin D deficiency. Int Arch Otorhinolaryngol. (2020) 24:e423–8. doi: 10.1055/s-0039-3402431
32.
Liberati A Altman DG Tetzlaff J Mulrow C Gotzsche PC Ioannidis JPA et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
33.
Margulis V Pladevall M Riera-Guardia N Varas-Lorenzo C Hazell L Berkman ND et al . Quality assessment of observational studies in a rug-safety systematic review, comparison of two tools: the Newcastle-Ottawa scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
34.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
35.
Sarsitthithum K Wisupagan T Kiatthanabumrung S Jariengprasert C . The association between serum vitamin D levels and benign paroxysmal positional vertigo. Ear Nose Throat J. (2021) 102:473–7. doi: 10.1177/01455613211008561
36.
Song P Zhao X Xu Y Zhao Z Wang L Liu Y et al . Correlation between benign paroxysmal positional vertigo and 25-Hydroxyvitamin D. Front Neurol. (2020) 11:576. doi: 10.3389/fneur.2020.00576
37.
Lee SB Lee CH Kim YJ Kim HM . Biochemical markers of bone turnover in benign paroxysmal positional vertigo. PLoS One. (2017) 12:e0176011. doi: 10.1371/journal.pone.0176011
38.
Inan HC Mertoğlu C Erdur ZB . Investigation of serum calcium and 25-hydroxy vitamin D levels in benign paroxysmal positional vertigo patients. Ear Nose Throat J. (2021) 100:643–6. doi: 10.1177/0145561321989451
39.
Kahraman SS Ozcan O Arli C Ustun I Erduran R Akoglu E et al . Calcium homeostasis during attack and remission in patients with idiopathic benign paroxysmal positional vertigo. Otol Neurotol. (2016) 37:1388–92. doi: 10.1097/MAO.0000000000001167
40.
Melis A Rizzo D Gallus R Leo ME Turra N Masnaghetti D et al . Relationship between calcium metabolism and benign paroxysmal positional vertigo in North Sardinia population. J Vestib Res. (2020) 30:375–82. doi: 10.3233/VES-200025
41.
Zhang X Zhang Z Lv X . Predictive values of serum estradiol, calcium, and 25-Hydroxyvitamin D levels for recurrence of benign paroxysmal positional vertigo in postmenopausal women. Turk J Phys Med Rehabil. (2022) 68:30–6. doi: 10.5606/tftrd.2022.5964
42.
Chu C Chan YM Tang J . Clinical outcomes in patients with benign paroxysmal positional Vertigo and vitamin D deficiency: a Singaporean perspective. Cureus. (2024) 16:e60325. doi: 10.7759/cureus.60325
43.
Al-Rawi TSS Al-Ani RM Al-Rawi TS . Vitamin D deficiency and the risk of recurrent benign paroxysmal positional Vertigo. Cureus. (2024) 16:e52433. doi: 10.7759/cureus.52433
44.
Bazoni JA Soares D Iquinato A de Souza Marquez A de Souza Pinho Costa V de Moraes Marchiori G et al . Hypovitaminosis D, Low Bone Mineral Density, and Diabetes Mellitus as Probable Risk Factors for Benign Paroxysmal Positional Vertigo in the Elderly. Int Arch Otorhinolaryngol. (2020) 24:e272–7. doi: 10.1055/s-0039-1700583
45.
Shin H-I Park Y Lee HJ . Correlation between serum vitamin D level and benign paroxysmal positional vertigo recurrence. Auris Nasus Larynx. (2023) 50:700–7. doi: 10.1016/j.anl.2022.12.017
46.
Chauhan I Sidhu J Lal B Dhadwal M Azad R . Role of serum markers in benign paroxysmal positional Vertigo: are they useful?Indian J Otolaryngol Head Neck Surg. (2023) 75:1731–6. doi: 10.1007/s12070-023-03727-z
47.
Bi J Liu B Zhang Y Zhou Q . Study on the bone metabolism indices and Otoconin-90 in benign paroxysmal positional vertigo. Otol Neurotol. (2021) 42:e744–9. doi: 10.1097/MAO.0000000000003087
48.
Cobb LH Bailey VO Liu YF Teixido MT Rizk HG . Relationship of vitamin D levels with clinical presentation and recurrence of BPPV in a southeastern United States institution. Auris Nasus Larynx. (2023) 50:70–80. doi: 10.1016/j.anl.2022.05.011
49.
Lin T Changzhen W Zhang L Ding L . Association of Vitamin-D Deficiency with vestibular function in patients with idiopathic benign paroxysmal positional Vertigo. J Laryngol Otol. (2024) 139:40–7. doi: 10.1017/S0022215124001282
50.
Thomas RJ Goutham MK Bhat VS Kamath SD Aroor R Bhandary SK . Association of serum calcium and vitamin D with benign paroxysmal positional vertigo. Int Arch Otorhinolaryngol. (2022) 26:e365–9. doi: 10.1055/s-0041-1724093
51.
Kong TH Jung SY Seo YJ Shim DB . Vitamin D supplementation in preventing the recurrence of benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol. (2024) 9:e1225. doi: 10.1002/lio2.1225
52.
Sharma K Ojha T Dabaria R Chhabra B Trivedi BB Bansal M . Relation between Posterior Canal benign paroxysmal positional Vertigo and vitamin D deficiency Indian. J Otolaryngol Head Neck Surg. (2022) 74:4405–S4408. doi: 10.1007/s12070-021-03070-1
53.
Elmoursy MM Abbas AS . The role of low levels of vitamin D as a co-factor in the relapse of benign paroxysmal positional vertigo (BPPV). Am J Otolaryngol. (2021) 42:103134. doi: 10.1016/j.amjoto.2021.103134
54.
Abdelmaksoud AA Fahim DFM Bazeed SES Alemam MF Aref ZF . Relation between vitamin D defciency and benign paroxysmal positional vertigo. Scientifc Reports. (2021) 11:1685. doi: 10.1038/s41598-021-96445-x
55.
Bener A Erdoğan A Üstündağ ÜV . The impact of serums calcium 25-Hydroxy vitamin D, ferritin, uric acid, and sleeping disorders on benign paroxysmal positional Vertigo patients. Audiol Res. (2024) 14:640–8. doi: 10.3390/audiolres14040054
56.
Libonati GA Leone A Martellucci S . Prevention of recurrent benign paroxysmal positional vertigo: the role of combined supplementation with vitamin D and antioxidants. Audiol Res. (2022) 12:445–56. doi: 10.3390/audiolres12040045
57.
Jeong S Kim J-S Kim H-J Choi JY Koo JW Choi KD et al . Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: a randomized trial. Neurology. (2020) 95:e1117–25. doi: 10.1212/WNL.0000000000010343
58.
Sheikhzadeh M Lotfi Y Mousavi A Heidari B Bakhshi E . The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: a case-control study. Caspian J Intern Med. (2016) 7:173–7. PMID:
59.
Nakada T Sugiura S Uchida Y . Difference in serum levels of vitamin D between canalolithiasis and cupulolithiasis of the horizontal semicircular canal in benign paroxysmal positional vertigo. Front Neurol. (2019) 10:176. doi: 10.3389/fneur.2019.00176
60.
Chang S Lee H . Vitamin D and health - the missing vitamin in humans. Pediatr Neonatol. (2019) 60:237–44. doi: 10.1016/j.pedneo.2019.04.007
61.
Büki B Jünger H Zhang Y . The price of immune responses and the role of vitamin D in the inner ear. Otol Neurotol. (2019) 40:701–9. doi: 10.1097/MAO.0000000000002258
62.
Yamauchi D Nakaya K Raveendran NN Harbidge DG Singh R Wangemann P et al . Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol. (2010) 10:1. doi: 10.1186/1472-6793-10-1
63.
Vibert D Sans A Kompis M Travo C Mühlbauer RC Tschudi I et al . Ultrastructural changes in Otoconia of osteoporotic rats. Audiol Neurotol. (2008) 13:293–301. doi: 10.1159/000124277
64.
Zhang S Xing J Gong Y Li P Wang B Xu L . Downregulation of VDR in benign paroxysmal positional vertigo patients inhibits otolith-associated protein expression levels. Mol Med Rep. (2021) 24:591. doi: 10.3892/mmr.2021.12230
65.
Kim SY Han SH Kim YH Park MH . Clinical features of recurrence and osteoporotic changes in benign paroxysmal positional vertigo. Auris Nasus Larynx. (2017) 44:156–61. doi: 10.1016/j.anl.2016.06.006
Summary
Keywords
BPPV, 25-hydroxy vitamin D, recurrence, vitamin D supplementation, canalolithiasis, cupulolithiasis
Citation
Li Y, Gao P, Ding R, Xu Y, Wang Z, Pei X and Li L (2025) Association between vitamin D, vitamin D supplementation and benign paroxysmal positional vertigo: a systematic review and meta-analysis. Front. Neurol. 16:1560616. doi: 10.3389/fneur.2025.1560616
Received
15 January 2025
Accepted
10 March 2025
Published
16 April 2025
Volume
16 - 2025
Edited by
Hubertus Axer, Jena University Hospital, Germany
Reviewed by
Andrea Migliorelli, University Hospital of Ferrara, Italy
Frank Thömke, Johannes Gutenberg University Mainz, Germany
Updates
Copyright
© 2025 Li, Gao, Ding, Xu, Wang, Pei and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianhe Li, lilianhe2025@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.