- 1Department of Biomedical and Dental Sciences and Morphofunctional Imaging, Policlinico Universitario, University of Messina, Messina, Italy
- 2Unit of Neurology and Neuromuscular Disorders, Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
- 3Williams College, Williamstown, MA, United States
- 4Department of Medical Sciences, Hackensack Meridian School of Medicine, Nutley, NJ, United States
- 5Department of Neurology, Hackensack University Medical Center, Hackensack Meridian School of Medicine, Nutley, NJ, United States
Background: The relationship between psychological comorbidity and functional impairment in multiple sclerosis (MS) remains to be thoroughly investigated. This study examined the associations between temperament traits, psychological comorbidities, and disability as measured by the World Health Organization Disability Assessment Schedule (WHODAS) 2.0 in persons with Relapsing–Remitting Multiple Sclerosis (RRMS).
Methods: In this cross-sectional study, persons with RRMS underwent a comprehensive assessment of temperament profiles, psychological status, and functional disability. Assessment tools included the Depression Anxiety Stress Scale (DASS-21) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Autoquestionnaire (TEMPS-A) short form. Functional status was evaluated using the 36-item WHODAS 2.0. Multivariate regression analyses were performed to evaluate relationships between variables.
Results: The study cohort comprised 105 persons with RRMS Hierarchical regression models showed that age and disease duration were significant predictors, with age positively associated with D02 and D06 dimensions, and disease duration linked to D02, D05, and D06 WHODAS 2.0 dimensions. Among temperament traits, hyperthymic temperament showed negative associations across multiple dimensions. Anxiety had strong positive association with disability. Model fit improved significantly with each step, with Step 3 explaining additional variance.
Conclusion: These findings demonstrate significant associations between temperamental characteristics, particularly hyperthymic traits, −anxiety, and functional disability in persons with RRMS. Future research should investigate these relationships over time to understand causal links and create better treatments to slow disability progression. These findings could help improve how we assess and treat patients.
1 Introduction
Multiple Sclerosis (MS) causes progressive neurological dysfunction through inflammation-mediated demyelination in the central nervous system. Persons with – Relapsing–Remitting Multiple Sclerosis (RRMS) often display multisystem deficits that impair functional capacity and impact their quality of life (1, 2). Thus, clinical assessment tools are essential for quantifying disease progression and functional impairment (3). In RRMS, the Expanded Disability Status Scale (EDSS) is a typical standardized assessment tool to quantify and track longitudinal changes across various neurological domains, such as pyramidal, cerebellar, brainstem, sensory, bowel/bladder, visual, and cognitive functions. Addressing the methodological limitations of the EDSS is crucial for improving patient care (4, 5). Given the subjectivity of clinical evaluation, there are concerns about the EDSS inter-rater reliability and its sensitivity in detecting slight changes, particularly in cognitive non-motor symptoms (6, 7). The Multiple Sclerosis Functional Composite (MSFC) was developed to address the limitations of traditional ordinal scales like the EDSS (8). This tool has proven effective in quantifying upper limb function, ambulation, and cognitive functions (8). However, the scale has significant limitations and requires proper training and a labor-intensive interpretation of results (Z-score) (9). More recently, the Multiple Sclerosis Performance Test (MSPT), a tablet-based assessment tool, has been introduced to evaluate multiple functional domains, including walking speed, manual dexterity, processing speed, contrast sensitivity, and patient-reported outcomes (10). Although the MSPT addresses several of the MSFC’s limitations, further validation studies are required to establish its reliability and clinical utility (11). Hence, current clinical tools for assessing MS disability have significant limitations. As our understanding of MS deepens and new therapies emerge, we need additional instruments to evaluate patients fully.
The World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) assesses functional disability across six key domains: understanding and communicating, mobility, self-care, getting along with people, life activities, and participation in society (12). The World Health Organization (WHO) developed the scale to keep with the International Classification of Functioning, Disability, and Health (ICF) framework parameters. This assessment tool is reliable and suitable across different cultures for clinical care and research (12). WHODAS 2.0 has been shown to be effective in evaluating disability in RRMS. However, the factors influencing functional disability in this group are not yet well-defined (13–17). Previous studies in patients with neurological and psychiatric disorders have confirmed a strong link between disability and psychological comorbidities, including anxiety, depression, and stress, highlighting the importance of investigating this association in RRMS (18–20). For instance, depression in RRMS can decrease motivation and interest in daily activities, increasing, in this way, perceived disability. Anxiety is often characterized by distress and fear, and prolonged stress can intensify physical symptoms and reduce coping abilities, further impacting disability (21). The connection between mental health and functional disability is further reinforced by evidence that personality traits, which increase susceptibility to psychiatric disorders, are linked to a reduced quality of life in persons with RRMS (22). Furthermore, there is a lack of research on the influence of temperament—innate, stable emotional traits that form the foundation of personality in persons with MS. Temperament traits, particularly those related to emotional reactivity, inhibitory control, and activity level, play a crucial role in psychiatric predisposition and reactivity throughout development. Temperament is defined as early-appearing, biologically rooted variations in emotional and behavioral responses, exhibiting moderate stability and influenced by genetic and environmental factors (23). Core dimensions such as activity level, mood, adaptability, and persistence have been linked to vulnerability for various psychiatric conditions including anxiety, depression, bipolar disorder, and substance use disorders (24). The Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Autoquestionnaire (TEMPS-A) identifies five affective temperament types—cyclothymic, depressive, hyperthymic, irritable, and anxious—that serve as subsyndromal phenotypes predictive of mood disorder onset and severity (25). For instance, cyclothymic temperament, characterized by rapid mood shifts and affective instability, is strongly associated with bipolar spectrum disorders and heightened emotional reactivity to stressors (26), while anxious temperament correlates with increased stress sensitivity and anxiety disorders (27). Conversely, hyperthymic temperament, marked by elevated energy and resilience, may modulate mood regulation differently but still influence psychiatric outcomes under stress (28). These temperament traits interact dynamically with neurobiological systems—such as those governing impulsivity, sensation seeking, and inhibitory control—and are influenced by genes affecting synaptic plasticity and associative learning (29, 30). Emerging evidence suggests that, persons with MS score higher in depressive, cyclothymic, irritable, and anxious temperaments compared to healthy controls (31). Similarly, research based on Cloninger’s model suggests that people with MS show higher levels of harm avoidance and lower levels of reward dependence (32). Thus, it is conceivable that specific temperament traits heighten the vulnerability to mood and anxiety disorders in MS, which in turn exacerbate cognitive dysfunction and functional impairment, factors directly linked to disability measured by the WHODAS.
To address this gap in the literature, the present observational, cross-sectional study aims to investigate the following research questions: (1) to what extent do demographic variables and disease duration predict functional disability (WHODAS 2.0) in persons with RRMS? (2) Do temperament traits (TEMPS-A: cyclothymic, depressive, hyperthymic, irritable, and anxious) significantly improve the prediction of functional disability beyond demographic and disease-related factors? (3) Does the addition of psychological symptoms (DASS-21: depression, anxiety, and stress) provide significant incremental validity in predicting functional disability assesses with WHODAS 2.0 in persons with RRMS? We hypothesize that (1) demographic variables and longer disease duration will be associated with greater functional disability, (2) temperament traits will significantly improve disability prediction beyond Step 1 variables, (3) DASS-21 symptoms will provide significant incremental validity beyond Steps 1 and 2.
2 Methods
2.1 Participants
This observational, cross-sectional study was conducted at the University of Messina, Italy. A total of 105 participants with RRMS defined with McDonald criteria (2017 revision) (33) and with Lublin criteria (34) were recruited at the Multiple Sclerosis Center, Department of Neurology, from September 2023 to December 2023. All patients who were older than 18 years old and on disease-modifying therapy were informed about the study by their treating physician during their regular follow-up visits. We excluded individuals with a history of severe psychiatric and neurologic disorders, active exacerbation of MS requiring steroid treatment in the last 30 days before the enrollment into our study, or significant cognitive impairment. The Montreal Cognitive Assessment (MoCA) Italian version was used to evaluate cognitive function. The MoCA assesses various cognitive domains, including attention, executive function, memory, language, visuospatial skills, abstract thinking, calculation, and orientation. Higher scores indicate better performance (0–30 range). Based on a previous Italian validation study (35), a cut-off of 17.36 was recommended for screening purposes for detecting cognitive impairment in the Italian population. Nonetheless, in our study we have used a more conservative cut off and we have included in the study only subjects with a MoCA score > 24. The study was approved by the local IRB al performed following the Declaration of Helsinki. Written informed consent was obtained before inclusion.
2.2 Assessment
During a regular follow-up visit we assessed the participants’ sociodemographic characteristics (age, gender, education, living status). The educational level was categorized in 4 groups according to the years at school (not attended school, 0 years; Primary school, 5 years; Secondary school, 8 years; High school, 13 years; University degree ≥17 years). We then ascertained the date and type of diagnosis (RRMS).
The Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Auto-questionnaire (TEMPS-A) short version was administered in its validated Italian form to assess affective temperaments. This 39-item self-report questionnaire evaluates five temperamental dimensions: depressive, cyclothymic, hyperthymic, irritable, and anxious. Higher scores indicate a stronger expression of that temperamental trait. The Italian version shows robust psychometric properties (36). In our study, the Cronbach’s α was >0.73 for each domain.
The DASS-21 is a commonly used self-report questionnaire that evaluates the intensity of depression, anxiety, and stress symptoms. It is a reliable and efficient measure of emotional well-being and has been validated across different populations. Depression subscale indicates feelings of sadness, hopelessness, worthlessness, detachment, disinterest, lack of pleasure, and sluggishness. The anxiety subscale measures symptoms associated with physical arousal, tension, situational stress, and anxious feelings. The stress subscale assesses difficulty in relaxing, nervousness, irritability, and impatience. Participants rate their symptoms on a Likert scale over the past week. Higher scores suggest more severe depression, anxiety, or stress. In this study, the Cronbach’s α for this questionnaire was >0.76 for each domain (37).
The WHODAS 2.0 is a 36-item assessment evaluating disability across diverse cultures in six areas: understanding and communicating (cognition), mobility, self-care, social relationships, daily activities, and community participation. Participants rate their task difficulties over the past 30 days from 1 (none) to 5 (extreme/unable). Examining specific activities provides detailed insights into daily functions. An item response theory algorithm converts responses to 0–100 scores, with higher numbers indicating greater disability. The assessment showed strong reliability in our study (Cronbach’s α: total score = 0.81, all dimensions > 7.4) (38).
2.3 Statistical analysis
Data (continuous and categorical variables) were reported as mean (standard deviation, SD) and (%). Descriptive statistics were calculated for all variables. We have used Cronbach’s alphas to assess the internal consistencies of the psychological variables (temperament traits, anxiety, depression, and stress) and the WHODAS 2.0 (total score and different dimensions). Next, we performed a multiple correlation analysis to assess whether the psychological variables were associated with disability. Furthermore, we employed hierarchical regression models to examine predictors of disability (WHODAS 2.0 total and subdomain scores) in three sequential steps: (1) demographic/clinical factors (sex, age, education, relationship status, disease duration), (2) temperament traits (TEMPS-A subscales), and (3) psychiatric symptoms (DASS-21 subscales), reporting standardized beta coefficients (β) with significance levels (*p < 0.05, **p < 0.01) and assessing model fit through R2 change (ΔR2) and F-tests, while controlling for multicollinearity All analyses were performed with SPSS Statistics, version 23 (IBM Corp., Armonk, NY, United States).
3 Results
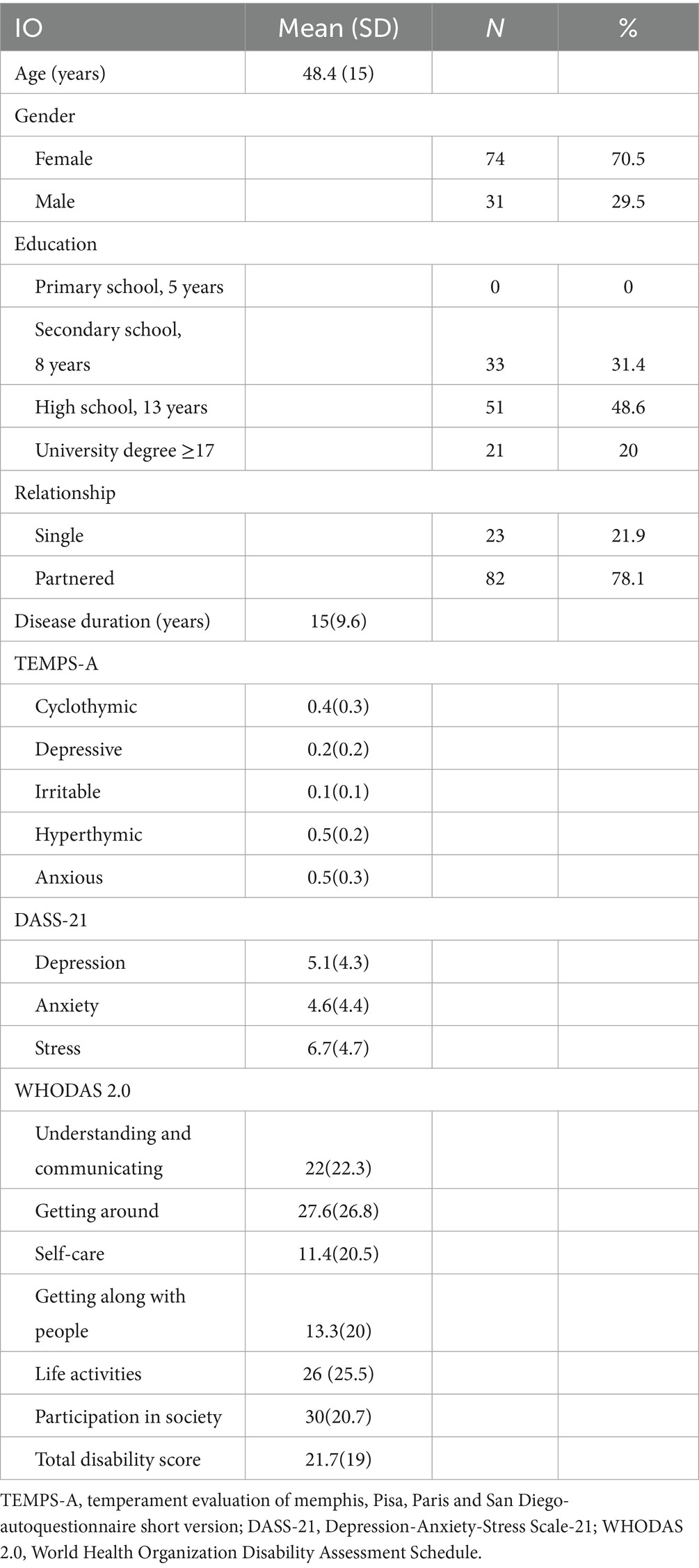
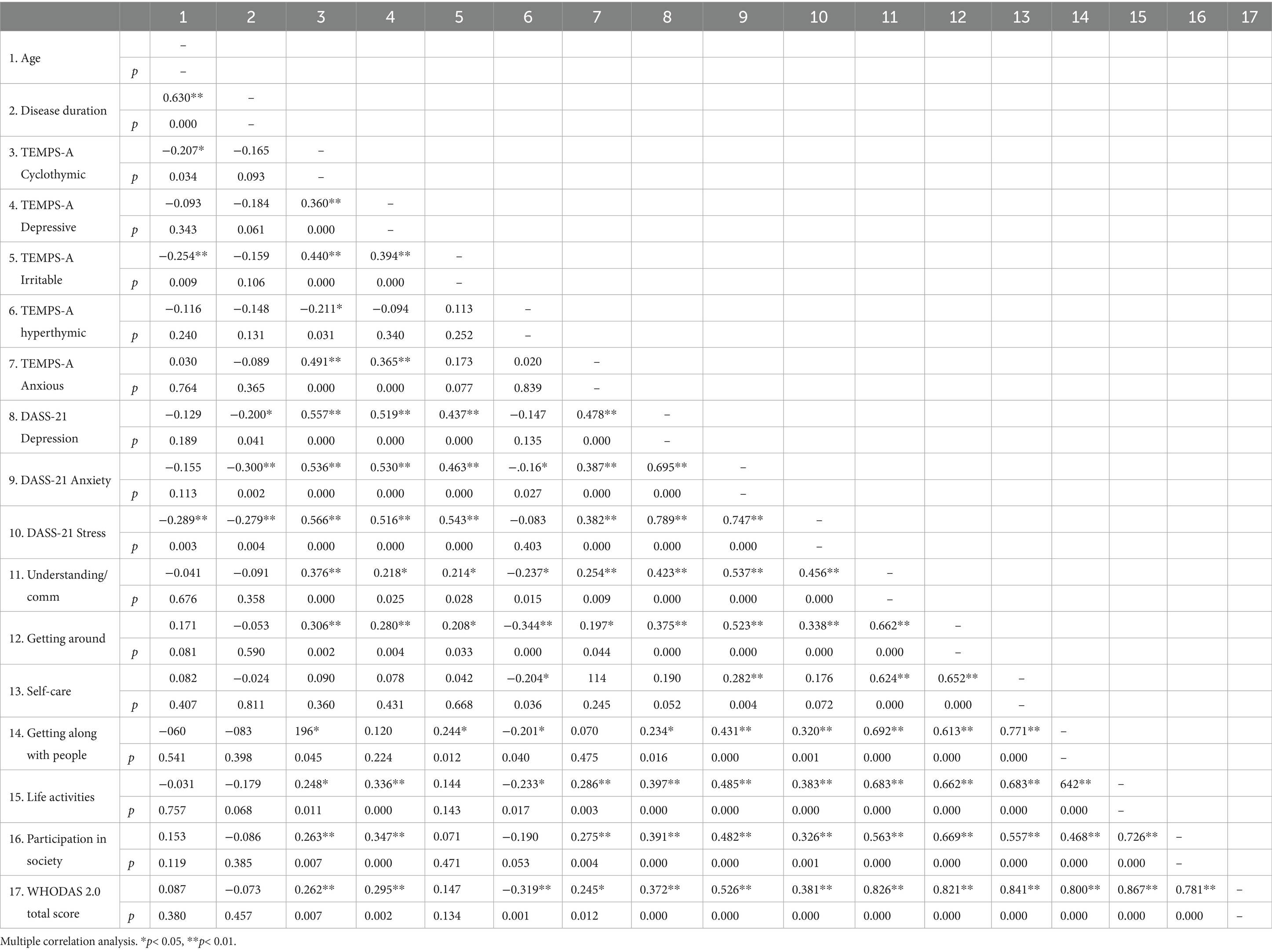
The study included 105 person with RRMS, with a mean age of 48.4 years (SD = 15), predominantly female (70.5%, n = 74), and mostly partnered (78.1%, n = 82). Education levels were varied, with high school being the most common (48.6%, n = 51). The mean disease duration was 15 years (SD = 9.6), and temperament assessments (TEMPS-A) revealed higher scores for hyperthymic (0.5, SD = 0.2) and anxious (0.5, SD = 0.3) traits. Psychological symptoms (DASS-21) indicated moderate stress (6.7, SD = 4.7), depression (5.1, SD = 4.3), and anxiety (4.6, SD = 4.4), while disability assessments (WHODAS 2.0) showed significant challenges in participation in society (mean = 30, SD = 20.7) and mobility (mean = 27.6, SD = 26.8), with a total disability score of 21.7 (SD = 19), reflecting moderate overall disability in the study population. Data are reported in Table 1. The intercorrelation matrix results of the variables are shown in Table 2. The correlation analysis revealed significant associations between clinical variables and the total WHODAS 2.0 disability score in persons with RRMS. Psychological distress (DASS-21) showed strong positive correlations, with stress (r = 0.381, p < 0.001), anxiety (r = 0.526, p < 0.001), and depression (r = 0.372, p < 0.001) all linked to higher overall disability. Temperament traits (TEMPS-A) also played a role, with cyclothymic (r = 0.262, p = 0.007), depressive (r = 0.295, p = 0.002), and anxious (r = 0.245, p = 0.012) temperaments positively associated with greater disability. Conversely, hyperthymic temperament correlated negatively (r = −0.319, p = 0.001), suggesting a protective effect. Age and disease duration did not significantly correlate with total disability (p > 0.05). We performed seven hierarchical multiple linear regression models employing as dependent variables the WHODAS 2.0 dimensions score (D01–D06) and the total sum score. The predictors were entered in steps: step 1 (age, sex, education, relationship, and disease duration), step 2 (temperament dimensions scores), and step 3 (DASS-21-dimension). Model 7 (WHODAS Total Score) demonstrated a progressive increase in explanatory power across the three steps. The initial Step 1 was non-significant [R2 = 0.038, F(5,99) = 0.782, p = 0.565], with no individual predictors reaching significance. The addition of affective temperaments in Step 2 substantially improved model fit [ΔR2 = 0.217, total R2 = 0.255, F(10,94) = 3.211, p = 0.001], with hyperthymic temperament as significant predictor (β = −0.350, p = 0.001), indicating a strong protective effect against overall functional disability. The final model incorporating DASS-21 scales further enhanced predictive validity [ΔR2 = 0.125, total R2 = 0.379, F(13,91) = 4.278, p < 0.001], with both hyperthymic temperament (β = −0.257, p = 0.010) and anxiety (β = 0.409, p = 0.006) emerging as significant predictors, demonstrating opposing effects on functional disability.
Model 1 (D01-Understanding and Communicating) followed a similar pattern, beginning with a non-significant Step 1 [R2 = 0.015, F(5,99) = 0.294, p = 0.915]. The introduction of affective temperaments significantly improved the model [ΔR2 = 0.188, total R2 = 0.202, F(10,94) = 2.383, p = 0.015], with hyperthymic temperament as the only significant predictor (β = −0.231, p = 0.030). The final step incorporating psychological distress measures yielded the most robust model [ΔR2 = 0.148, total R2 = 0.350, F(13,91) = 3.774, p < 0.001], where anxiety became the main predictor (β = 0.398, p = 0.008). Model 2 (D02-Getting Around) was unique in showing significant predictors in Step 1 (R2 = 0.086), with age associated (β = 0.275, p = 0.044) and disease duration (β = 0.259, p = 0.039) positively associated with mobility difficulties. The addition of temperamental variables produced the largest R2 change across all models [ΔR2 = 0.245, total R2 = 0.330, F(10,94) = 4.640, p < 0.001], with hyperthymic temperament showing the strongest protective effect (β = −0.344, p = 0.001). The final model achieved the highest explanatory power among all domains [total R2 = 0.422, F(13,91) = 5.118, p < 0.001], with both hyperthymic temperament (β = −0.258, p = 0.007) and anxiety (β = 0.394, p = 0.006) maintaining significance, indicating that mobility functioning is influenced by both temperamental resilience and psychological distress.
Model 3 (D03-Self-Care) did not achieve statistical significance at any step. The Step 1 was non-significant [R2 = 0.037, F(5,99) = 0.753, p = 0.586], and the addition of temperamental variables provided minimal improvement [ΔR2 = 0.056, total R2 = 0.092, F(10,94) = 0.955, p = 0.488]. The complete model including DASS-21 scales remained non-significant [total R2 = 0.155, F(13,91) = 1.288, p = 0.235], suggesting that self-care functioning may be relatively independent of the affective temperaments and psychological distress variables examined in this study. Model 4 (D04-Getting Along with People) exhibited a unique pattern where neither Step 1 variables [R2 = 0.011, F(5,99) = 0.215, p = 0.956] nor the addition of temperamental variables [total R2 = 0.128, F(10,94) = 1.377, p = 0.203] were significant. However, with the incorporation of DASS-21 scales the model reached statistical significance [total R2 = 0.246, F(13,91) = 2.287, p = 0.012], with anxiety as a highly significant predictor (β = 0.462, p = 0.005). This pattern suggests that interpersonal difficulties are primarily driven by current psychological distress rather than stable temperamental characteristics. Model 5 (D05-Life Activities) showed Step 1 significance (R2 = 0.050) with disease duration as a weak predictor (β = −0.259, p = 0.043). The temperamental variables significantly enhanced the model [ΔR2 = 0.176, total R2 = 0.226, F(10,94) = 2.741, p = 0.005], with hyperthymic temperament as the primary predictor (β = −0.264, p = 0.012). The final Step 3L maintained significance [total R2 = 0.305, F(13,91) = 3.076, p = 0.001], with anxiety becoming the sole significant predictor (β = 0.307, p = 0.047), indicating a shift from temperamental to symptom-based prediction of life activity limitations. Model 6 (D06-Participation in Society) began with significant Step 1 predictors (R2 = 0.080), including positive associations with age (β = 0.321, p = 0.019) and with disease duration (β = 0.300, p = 0.018). The temperamental variables substantially improved the model [ΔR2 = 0.186, total R2 = 0.266, F(10,94) = 3.414, p = 0.001], with depressive temperament as a significant positive predictor (β = 0.261, p = 0.018). The final Step 3 achieved strong predictive validity [total R2 = 0.369, F(13,91) = 4.100, p < 0.001], with anxiety becoming the main predictor (β = 0.385, p = 0.010).
Across all models, anxiety consistently emerged as the most robust predictor in final models, showing significant positive associations with six of the seven functional domains, while hyperthymic temperament demonstrated consistent protective. Results are shown in Table 3.
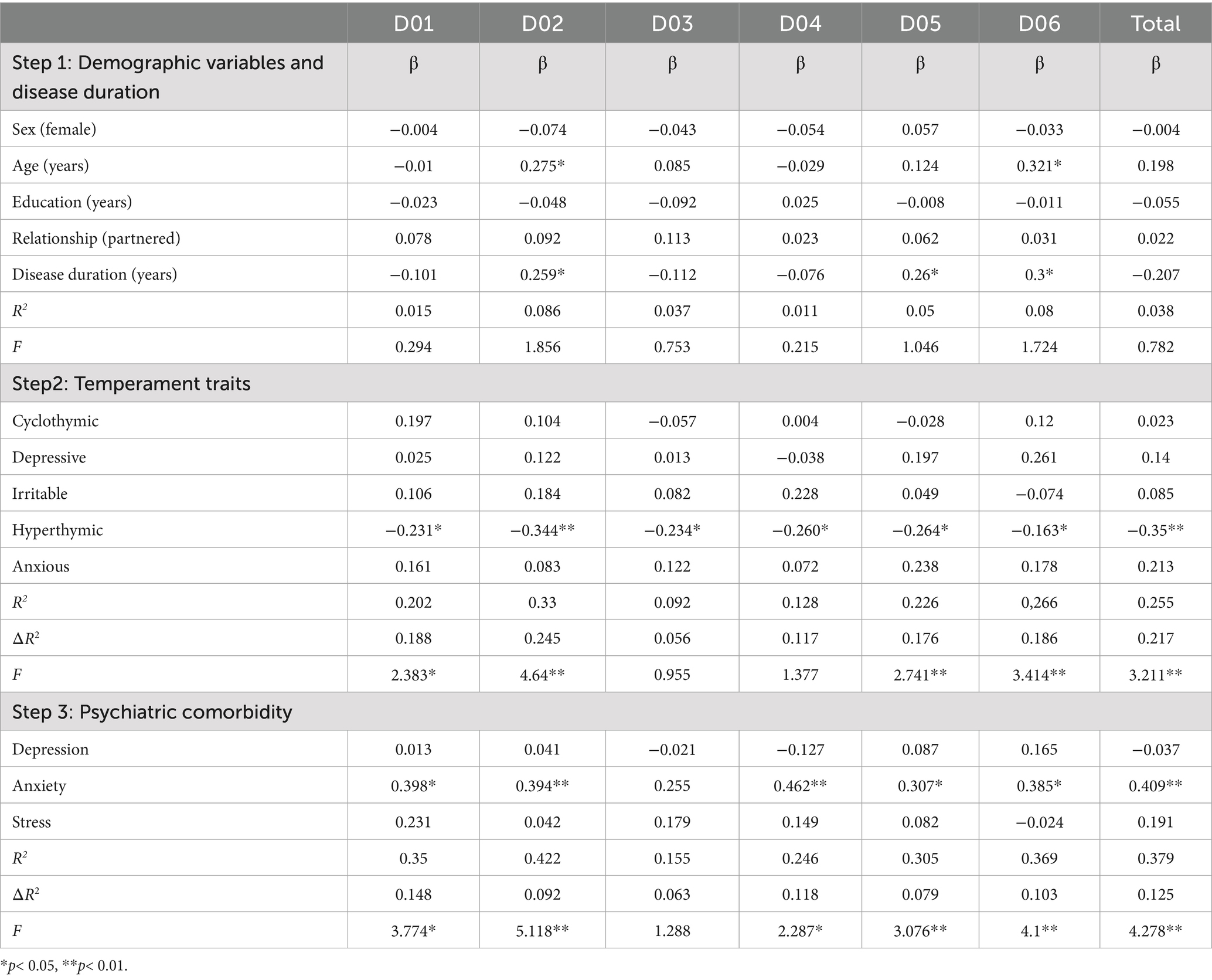
Table 3. Hierarchical regression models predicting WHODAS 2.0 disability domains (D01: Cognition, D02: Mobility, D03: Self-care, D04: Getting along, D05: Life activities, D06: Participation) and total disability scores. Step 1 (Demographics variables/Disease duration); step 2 (Temperament traits assessed with the TEMPS-A); step 3 (psychiatric comorbidities, assessed with the DASS-21). WHODAS 2.0 = World Health Organization Disability Assessment Schedule 2.0; TEMPS-A = Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Auto-questionnaire; DASS-21 = Depression, Anxiety, and Stress Scales-21.
4 Discussion
In this study, we investigated disability assessed with WHODAS 2.0 in RRMS by analyzing its connection to psychological distress and emotional temperament traits. Age was significantly associated with increased mobility deficits and greater impairment in social participation. Our findings demonstrate that psychological factors, particularly temperament traits and psychiatric comorbidities (anxiety), significantly contribute to disability prediction beyond traditional demographic and clinical variables.
The initial model (Step 1) examining demographic variables and disease duration revealed limited predictive value, with age and disease duration emerging as the most consistent predictors across disability domains. Age has been consistently identified as a significant predictor of long-term disability in RRMS, which aligns with our findings showing positive associations between age and several disability dimensions (D02, D06). The significant positive correlation between disease duration and disability measures (D02, D05, D06) corroborates existing literature demonstrating the progressive nature of MS-related impairment over time impacting their ability to participate in social activities (39, 40). Also, studies of the MSFC which evaluates neurological impairment through arm function, walking ability, and cognitive processing, revealed that age-related factors increase mobility impairments, leading to social isolation (3). Both the EDSS and MSFC scores typically worsen with age, leading to reduced physical mobility, decreased social interactions, and increased dependency on support systems (41). Our results are consistent with studies that have described a correlation between age and mobility using WHODAS 2.0 (42, 43). On the contrary, sex, education level, and relationship status showed minimal predictive value in our cohort, suggesting that these demographic factors may have less influence on disability outcomes assessed with the WHODAS than previously assumed in some studies (44).
Furthermore, specific temperamental dimensions tested with the TEMPS-A have been associated with psychological distress, depression, anxiety, and stress associated with neuropsychiatric diseases (45). These e temperaments assessment reveals distinct correlations with diseases outcomes. The hyperthymic temperamental profile demonstrates significant associations with favorable clinical trajectories (46, 47). The underlying mechanisms appear to involve enhanced stress-response and psychological resilience (48). Conversely, cyclothymic, anxious, and depressive temperamental profiles correlate with increased disability and demonstrate strong associations with affective symptomatology (49) and impaired stress-response mechanisms (50). Previous studies investigating affective temperaments assessed with the TEMPS-A in RRMS are limited (31) and have highlighted their association with quality of life (51). Our findings in person with RRMS expand these observations, further demonstrating that hyperthymic temperament traits are associated with improved mobility, enhanced social engagement, and reduced overall disability levels, as measured by the WHODAS. This inverse correlation suggests that hyperthymic temperament may serve as a protective neurobiological mechanism in MS, modulating disease progression, functional capacity, and social participation across multiple domains.
Our study indicated that the anxiety score strongly predicted disability in RRMS. Previous epidemiological research has identified a prevalence of anxiety disorders in RRMS ranging from 15.8 to 57%, which is higher than rates found in the general population (52, 53). Among the various anxiety disorders present in MS patients, Generalized Anxiety Disorder is the most common, with a prevalence of 18.6%. Specific phobias and panic disorder occur at lower rates of 10.8 and 10.0%, respectively (54). Research shows anxiety and depression are often comorbid, with each condition increasing the risk of developing the other over time (55, 56). Our findings align with previous studies demonstrating how anxiety contributes to disability. For instance, anxiety may contribute to disease progression through stress-induced immunological activation (57–62). In our study, we found that anxiety s, rather than depression, were more significant predictors of disability as measured by the WHODAS. While previous research using the EDSS found links between depression and disability in persons with MS, the results vary across studies. This may be due to differences between the WHODAS and EDSS assessments, since EDSS primarily emphasizes physical impairment and mobility while WHODAS assesses multiple domains of functioning (58, 63, 64). Consequently, the greater predictive strength of anxiety and stress observed in our research may indicate WHODAS’s enhanced sensitivity to the psychological factors influencing daily functioning and social participation. This aligns with previous literature indicating that traditional disability measures like the EDSS may underestimate the impact of psychological symptoms on the overall functioning of persons with MS. On the contrary, a longitudinal study assessing disability progression using the WHODAS 2.0 in persons with MS showed that depression was associated with higher disability (65). Although the study included a large population of persons with MS, potential methodological limitations and the significant variability in baseline disability values make it necessary to confirm the findings in future studies. Thus, the heterogeneity of results previously observed in studies using the EDSS is also evident when using the WHODAS. The heterogeneity across studies may be linked to several factors: (1) differences in sample characteristics, particularly regarding depression severity and disease duration; (2) varying adjustment for potential mediators such as fatigue, cognitive impairment, or pain that may account for depression’s apparent effects; and (3) measurement differences in both depression (diagnostic criteria vs. symptom scales) and disability (WHODAS total score vs. specific subdomains). It is also possible that these conflicting results may be due to the contribution of multiple variables, both cognitive and psychological (65). In our study, the use of a homogeneous population without significant cognitive deficits represents a novelty in the literature. A multicenter study with a larger population is currently being planned to confirm our results.
Our study has a few limitations. First, it utilizes a cross-sectional design, which does not allow for discussing causal links or temporal sequences between psychological symptoms and functional outcomes. In addition, the research was performed at a single site. Moreover, we did not include in our cohort MS participants with a significant cognitive impairment. We used a screening cognitive assessment tool (MoCA) to minimize participant burden and avoid a lengthy study. Although MoCA is an effective screening instrument, it may overlook minimal or domain-specific cognitive deficits. Thus, future studies should employ more comprehensive cognitive batteries to better evaluate the full spectrum of cognitive function and their association with disability assessed with the WHODAS 2.0 scale. In addition, this study’s sample size of 105 participants, while reasonable (66), may limit the detection of smaller effects given the predictors analyzed. The risk of overfitting and unmeasured confounding variables should be considered when interpreting results. Future studies with larger, more diverse samples could strengthen these observations. While this study originally planned to analyze only the total WHODAS score, we expanded our model to include all WHODAS dimensions due to their consistent alignment with existing literature and recurrent predictor patterns. However, these additional findings should be interpreted cautiously as exploratory, serving as pilot data for future larger-scale validation.
5 Conclusion
This study found significant associations between psychological factors (particularly anxiety) and higher disability assessed with the WHODAS 2.0 in RRMS, while hyperthymic temperament correlated with lower disability. As a cross-sectional analysis, these findings suggest clinical relevance of psychological assessment in MS care, but causal relationships remain unclear. Future longitudinal studies should investigate these interactions over time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the University of Messina, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CI: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. MB: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. AG: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. EM: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. RW: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. SG: Conceptualization, Formal analysis, Writing – original draft. OY: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. AB: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. FI: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. FT: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. FB: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuhlmann, T, Moccia, M, Coetzee, T, Cohen, JA, Correale, J, Graves, J, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. (2023) 22:78–88. doi: 10.1016/s1474-4422(22)00289-7
2. Tur, C, Carbonell-Mirabent, P, Cobo-Calvo, Á, Otero-Romero, S, Arrambide, G, Midaglia, L, et al. Association of Early Progression Independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. (2023) 80:151–60. doi: 10.1001/jamaneurol.2022.4655
3. Lublin, FD, Häring, DA, Ganjgahi, H, Ocampo, A, Hatami, F, Čuklina, J, et al. How patients with multiple sclerosis acquire disability. Brain. (2022) 145:3147–61. doi: 10.1093/brain/awac016
4. Kurtzke, JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/wnl.33.11.1444
5. Hobart, J, Freeman, J, and Thompson, A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain. (2000) 123:1027–40. doi: 10.1093/brain/123.5.1027
6. Meyer-Moock, S, Feng, YS, Maeurer, M, Dippel, FW, and Kohlmann, T. Systematic literature review and validity evaluation of the expanded disability status scale (Edss) and the multiple sclerosis functional composite (Msfc) in patients with multiple sclerosis. BMC Neurol. (2014) 14:58. doi: 10.1186/1471-2377-14-58
7. van Munster, CE, and Uitdehaag, BM. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. (2017) 31:217–36. doi: 10.1007/s40263-017-0412-5
8. Fischer, JS, Rudick, RA, Cutter, GR, and Reingold, SC. The multiple sclerosis functional composite measure (Msfc): an integrated approach to Ms clinical outcome assessment. National Ms Society Clinical Outcomes Assessment Task Force. Mult Scler. (1999) 5:244–50. doi: 10.1177/135245859900500409
9. Uitdehaag, BMJ. Disability outcome measures in phase iii clinical trials in multiple sclerosis. CNS Drugs. (2018) 32:543–58. doi: 10.1007/s40263-018-0530-8
10. Rudick, RA, Miller, D, Bethoux, F, Rao, SM, Lee, JC, Stough, D, et al. The multiple sclerosis performance test (Mspt): an Ipad-based disability assessment tool. J Visual Exper. (2014) 88:e51318. doi: 10.3791/51318
11. Rhodes, JK, Schindler, D, Rao, SM, Venegas, F, Bruzik, ET, Gabel, W, et al. Multiple sclerosis performance test: technical development and usability. Adv Ther. (2019) 36:1741–55. doi: 10.1007/s12325-019-00958-x
12. World Health Organization. Measuring health and disability: manual for World Health Organization (who) disability assessment schedule 2.0 (Whodas 2.0). (2010)
13. Giovannetti, AM, Schiavolin, S, Brenna, G, Brambilla, L, Confalonieri, P, Cortese, F, et al. Cognitive function alone is a poor predictor of health-related quality of life in employed patients with Ms: results from a cross-sectional study. Clin Neuropsychol. (2016) 30:201–15. doi: 10.1080/13854046.2016.1142614
14. Raggi, A, Giovannetti, AM, Schiavolin, S, Confalonieri, P, Brambilla, L, Brenna, G, et al. Development and validation of the multiple sclerosis questionnaire for the evaluation of job difficulties (Msq-job). Acta Neurol Scand. (2015) 132:226–34. doi: 10.1111/ane.12387
15. Salehi, R, Negahban, H, Khiavi, FF, Saboor, S, Majdinasab, N, and Shakhi, K. Validity and reliability of the World Health Organization disability assessment schedule 2.0 36-item Persian version for persons with multiple sclerosis. Korean J Family Med. (2020) 41:195–201. doi: 10.4082/kjfm.18.0155
16. Pokryszko-Dragan, A, Marschollek, K, Chojko, A, Karasek, M, Kardyś, A, Marschollek, P, et al. Social participation of patients with multiple sclerosis. Adv Clin Exper Med. (2020) 29:469–73. doi: 10.17219/acem/115237
17. Young, CA, Rog, DJ, Sharrack, B, Constantinescu, C, Kalra, S, Harrower, T, et al. Measuring disability in multiple sclerosis: the Whodas 2.0. Qual Life Res. (2023) 32:3235–46. doi: 10.1007/s11136-023-03470-6
18. Herrold, AA, Kletzel, SL, Mallinson, T, Pape, TLB, Weaver, JA, Guernon, A, et al. Psychometric measurement properties of the World Health Organization disability assessment schedule 2.0 (Whodas) evaluated among veterans with mild traumatic brain injury and behavioral health conditions. Disabil Rehabil. (2021) 43:1313–22. doi: 10.1080/09638288.2019.1660914
19. Yuliana, S, Yu, E, Rias, YA, Atikah, N, Chang, HJ, and Tsai, HT. Associations among disability, depression, anxiety, stress, and quality of life between stroke survivors and their family caregivers: an actor-partner interdependence model. J Adv Nurs. (2023) 79:135–48. doi: 10.1111/jan.15465
20. Hudson, M, Thombs, BD, Steele, R, Watterson, R, Taillefer, S, and Baron, M. Clinical correlates of quality of life in systemic sclerosis measured with the World Health Organization disability assessment schedule ii. Arthritis Rheum. (2008) 59:279–84. doi: 10.1002/art.23344
21. Krause, N, Derad, C, von Glasenapp, B, Riemann-Lorenz, K, Temmes, H, van de Loo, M, et al. Association of Health Behaviour and Clinical Manifestation in early multiple sclerosis in Germany – baseline characteristics of the power@Ms1 randomised controlled trial. Mult Scler Relat Disord. (2023) 79:105043. doi: 10.1016/j.msard.2023.105043
22. Yeni K, TM. Relationship of personality traits with stigmatization, depression, and quality of life in patients with multiple sclerosis. Neurol Asia. (2023) 28:1031–40. doi: 10.54029/2023mkx
23. Chess, S, and Thomas, A. Temperamental individuality from childhood to adolescence. J Am Acad Child Psychiatry. (1977) 16:218–26. doi: 10.1016/s0002-7138(09)60038-8
24. Benazzi, F, and Akiskal, HS. A downscaled practical measure of mood lability as a screening tool for bipolar ii. J Affect Disord. (2005) 84:225–32. doi: 10.1016/j.jad.2003.09.010
25. Akiskal, HS, and Benazzi, F. Psychopathologic correlates of suicidal ideation in major depressive outpatients: is it all due to unrecognized (bipolar) depressive mixed states? Psychopathology. (2005) 38:273–80. doi: 10.1159/000088445
26. Rihmer, A, Rozsa, S, Rihmer, Z, Gonda, X, Akiskal, KK, and Akiskal, HS. Affective temperaments, as measured by Temps-a, among nonviolent suicide attempters. J Affect Disord. (2009) 116:18–22. doi: 10.1016/j.jad.2008.10.024
27. Alci, D, Eroglu, M, and Asik, M. Temperament traits as key modulators of depression and anxiety in hyperthyroidism: implications for personalized treatment. Cureus. (2025) 17:e81503. doi: 10.7759/cureus.81503
28. Gonda, X, Eszlari, N, Torok, D, Gal, Z, Bokor, J, Millinghoffer, A, et al. Genetic underpinnings of affective temperaments: a pilot Gwas investigation identifies a new genome-wide significant Snp for anxious temperament in Adgrb3 gene. Transl Psychiatry. (2021) 11:337. doi: 10.1038/s41398-021-01436-1
29. Qiu, F, Akiskal, HS, Kelsoe, JR, and Greenwood, TA. Factor analysis of temperament and personality traits in bipolar patients: correlates with comorbidity and disorder severity. J Affect Disord. (2017) 207:282–90. doi: 10.1016/j.jad.2016.08.031
30. Rihmer, Z, Akiskal, KK, Rihmer, A, and Akiskal, HS. Current research on affective temperaments. Curr Opin Psychiatry. (2010) 23:12–8. doi: 10.1097/YCO.0b013e32833299d4
31. Özkan, A, Altinbaş, K, Koç, ER, Şen, HM, and Özişik Karaman, HI. Affective temperament profiles in patients with multiple sclerosis: association with mood disorders. Noro Psikiyatr Ars. (2016) 53:311–6. doi: 10.5152/npa.2015.12393
32. Gazioglu, S, Cakmak, VA, Ozkorumak, E, Usta, NC, Ates, C, and Boz, C. Personality traits of patients with multiple sclerosis and their relationship with clinical characteristics. J Nerv Ment Dis. (2014) 202:408–11. doi: 10.1097/nmd.0000000000000114
33. Thompson, AJ, Banwell, BL, Barkhof, F, Carroll, WM, Coetzee, T, Comi, G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the Mcdonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/s1474-4422(17)30470-2
34. Lublin, FD, Reingold, SC, Cohen, JA, Cutter, GR, Sørensen, PS, Thompson, AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2014) 83:278–86. doi: 10.1212/wnl.0000000000000560
35. Conti, S, Bonazzi, S, Laiacona, M, Masina, M, and Coralli, MV. Montreal cognitive assessment (Moca)-Italian version: regression based norms and equivalent scores. Neurol Sci. (2015) 36:209–14. doi: 10.1007/s10072-014-1921-3
36. Preti, A, Vellante, M, Zucca, G, Tondo, L, Akiskal, K, and Akiskal, H. The Italian version of the validated short Temps-a: the temperament evaluation of Memphis, Pisa, Paris and San Diego. J Affect Disord. (2010) 120:207–12. doi: 10.1016/j.jad.2009.02.025
37. Lovibond, PF, and Lovibond, SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (Dass) with the Beck depression and anxiety inventories. Behav Res Ther. (1995) 33:335–43. doi: 10.1016/0005-7967(94)00075-u
38. Reise, SP, and Waller, NG. Item response theory and clinical measurement. Annu Rev Clin Psychol. (2009) 5:27–48. doi: 10.1146/annurev.clinpsy.032408.153553
39. Finlayson, ML, and Cho, CC. A profile of support group use and need among middle-aged and older adults with multiple sclerosis. J Gerontol Soc Work. (2011) 54:475–93. doi: 10.1080/01634372.2011.575446
40. Wecker, S, Freudenstein, D, Ganser, I, Angstwurm, K, Lee, DH, and Linker, RA. The impact of different lifestyle factors on disability in multiple sclerosis at older ages: a monocentric retrospective study. Ther Adv Neurol Disord. (2024) 17:17562864241284166. doi: 10.1177/17562864241284166
41. Marrie, RA, Cohen, J, Stuve, O, Trojano, M, Sørensen, PS, Reingold, S, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. (2015) 21:263–81. doi: 10.1177/1352458514564491
42. Ferrer, MLP, Perracini, MR, Rebustini, F, and Buchalla, CM. Whodas 2.0-Bo: normative data for the assessment of disability in older adults. Rev Saude Publica. (2019) 53:19. doi: 10.11606/s1518-8787.2019053000586
43. Norén, P, Karlsson, J, Ohlsson-Nevo, E, Möller, M, and Hermansson, L. Psychometric evaluation of the Whodas 2.0 and prevalence of disability in a Swedish general population. J Patient Report Outcomes. (2023) 7:36. doi: 10.1186/s41687-023-00580-0
44. Bove, R, Musallam, A, Healy, BC, Raghavan, K, Glanz, BI, Bakshi, R, et al. Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler. (2014) 20:1584–92. doi: 10.1177/1352458514527864
45. Favaretto, E, Bedani, F, Brancati, GE, De Berardis, D, Giovannini, S, Scarcella, L, et al. Synthesising 30 years of clinical experience and scientific insight on affective temperaments in psychiatric disorders: state of the art. J Affect Disord. (2024) 362:406–15. doi: 10.1016/j.jad.2024.07.011
46. Rybakowski, JK. Factors associated with Lithium efficacy in bipolar disorder. Harv Rev Psychiatry. (2014) 22:353–7. doi: 10.1097/hrp.0000000000000006
47. Silva, RAD, Mograbi, DC, Camelo, EVM, Amadeo, LN, Santana, CMT, Landeira-Fernandez, J, et al. The relationship between insight and affective temperament in bipolar disorder: an exploratory study. Trends Psychiatry Psychother. (2018) 40:210–5. doi: 10.1590/2237-6089-2017-0073
48. Luciano, M, Steardo, L Jr, Sampogna, G, Caivano, V, Ciampi, C, Del Vecchio, V, et al. Affective temperaments and illness severity in patients with bipolar disorder. Medicina. (2021) 57:54. doi: 10.3390/medicina57010054
49. Vázquez, GH, Tondo, L, Mazzarini, L, and Gonda, X. Affective temperaments in general population: a review and combined Analysis from National Studies. J Affect Disord. (2012) 139:18–22. doi: 10.1016/j.jad.2011.06.032
50. DeGeorge, DP, Walsh, MA, Barrantes-Vidal, N, and Kwapil, TR. A three-year longitudinal study of affective temperaments and risk for psychopathology. J Affect Disord. (2014) 164:94–100. doi: 10.1016/j.jad.2014.04.006
51. Salhofer-Polanyi, S, Friedrich, F, Löffler, S, Rommer, PS, Gleiss, A, Engelmaier, R, et al. Health-related quality of life in multiple sclerosis: temperament outweighs Edss. BMC Psychiatry. (2018) 18:143. doi: 10.1186/s12888-018-1719-6
52. Karimi, S, Andayeshgar, B, and Khatony, A. Prevalence of anxiety, depression, and stress in patients with multiple sclerosis in Kermanshah-Iran: a cross-sectional study. BMC Psychiatry. (2020) 20:166. doi: 10.1186/s12888-020-02579-z
53. Peres, DS, Rodrigues, P, Viero, FT, Frare, JM, Kudsi, SQ, Meira, GM, et al. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: a systematic review and Meta-analysis. Brain Beh Immun Health. (2022) 24:100484. doi: 10.1016/j.bbih.2022.100484
54. Korostil, M, and Feinstein, A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. (2007) 13:67–72. doi: 10.1177/1352458506071161
55. Marrie, RA, Reingold, S, Cohen, J, Stuve, O, Trojano, M, Sorensen, PS, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. (2015) 21:305–17. doi: 10.1177/1352458514564487
56. Smith, SJ, and Young, CA. The role of affect on the perception of disability in multiple sclerosis. Clin Rehabil. (2000) 14:50–4. doi: 10.1191/026921500676724210
57. Garfield, AC, and Lincoln, NB. Factors affecting anxiety in multiple sclerosis. Disabil Rehabil. (2012) 34:2047–52. doi: 10.3109/09638288.2012.667503
58. Tsivgoulis, G, Triantafyllou, N, Papageorgiou, C, Evangelopoulos, ME, Kararizou, E, Sfagos, C, et al. Associations of the expanded disability status scale with anxiety and depression in multiple sclerosis outpatients. Acta Neurol Scand. (2007) 115:67–72. doi: 10.1111/j.1600-0404.2006.00736.x
59. Janssens, AC, Buljevac, D, van der Doorn, PA, Meché, FG, Polman, CH, Passchier, J, et al. Prediction of anxiety and distress following diagnosis of multiple sclerosis: a two-year longitudinal study. Mult Scler. (2006) 12:794–801. doi: 10.1177/1352458506070935
60. Goodin, DS, Ebers, GC, Johnson, KP, Rodriguez, M, Sibley, WA, and Wolinsky, JS. The relationship of Ms to physical trauma and psychological stress: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. (1999) 52:1737–45. doi: 10.1212/wnl.52.9.1737
61. Rogić Vidaković, M, Šimić, N, Poljičanin, A, Nikolić Ivanišević, M, Ana, J, and Đogaš, Z. Psychometric properties of the Croatian version of the depression, anxiety, and stress Scale-21 and multiple sclerosis impact Scale-29 in multiple sclerosis patients. Mult Scler Relat Disord. (2021) 50:102850. doi: 10.1016/j.msard.2021.102850
62. Francalancia, J, Mavrogiorgou, P, Juckel, G, Mitrovic, T, Kuhle, J, Naegelin, Y, et al. Death anxiety and attitudes towards death in patients with multiple sclerosis: an exploratory study. Brain Sci. (2021) 11:964. doi: 10.3390/brainsci11080964
63. Gay, MC, Bungener, C, Thomas, S, Vrignaud, P, Thomas, PW, Baker, R, et al. Anxiety, emotional processing and depression in people with multiple sclerosis. BMC Neurol. (2017) 17:43. doi: 10.1186/s12883-017-0803-8
64. Frank, HA, Chao, M, Tremlett, H, Marrie, RA, Lix, LM, McKay, KA, et al. Comorbidities and their association with outcomes in the multiple sclerosis population: a rapid review. Mult Scler Relat Disord. (2024) 92:105943. doi: 10.1016/j.msard.2024.105943
65. Parker, RMA, Tilling, K, Mills, R, Tennant, A, Ben-Shlomo, Y, Constantinescu, CS, et al. Assessing disability progression using the WHODAS 2.0 in multiple sclerosis: investigating clinical and socio-demographic factors in a large longitudinal cohort study (tonic-Ms). Mult Scler Relat Disord. (2024) 93:106228. doi: 10.1016/j.msard.2024.106228
Keywords: multiple sclerosis, WHODAS 2.0, temperament traits, anxiety, depression, stress
Citation: Infortuna C, Buccafusca M, Graceffa AMS, Maiorana E, Wang R, Ganesh S, Yedidia O, Bruno A, Iannuzzo F, Thomas FP and Battaglia F (2025) Exploring the relationship between temperament traits, psychological symptoms, and functional disability assessed with the WHODAS 2.0 in persons with multiple sclerosis. Front. Neurol. 16:1561995. doi: 10.3389/fneur.2025.1561995
Edited by:
Eduardo Fernández-Jiménez, European University of Madrid, SpainReviewed by:
Omri Zveik, Hadassah Medical Center, IsraelJelena Brasanac, Charité University Medicine Berlin, Germany
Copyright © 2025 Infortuna, Buccafusca, Graceffa, Maiorana, Wang, Ganesh, Yedidia, Bruno, Iannuzzo, Thomas and Battaglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fortunato Battaglia, Zm9ydHVuYXRvLmJhdHRhZ2xpYUBobWhuLm9yZw==
 Carmenrita Infortuna1
Carmenrita Infortuna1 Orion Yedidia
Orion Yedidia Antonio Bruno
Antonio Bruno Florian P. Thomas
Florian P. Thomas Fortunato Battaglia
Fortunato Battaglia