Abstract
Background:
Epilepsy affects approximately 70 million people globally, with a prevalence in Mexico of 10.8 to 20 cases per thousand. Antiseizure Medications (ASM) are the first line of treatment for people with epilepsy (PWE), aiming to achieve early seizure control while minimizing adverse effects that could impact quality of life.
Materials and methods:
This retrospective cohort study analyzed data from 2020 to 2024 collected from medical records, clinical histories, and electronic systems, using REDCAP® and SPSSV21®. It included all epilepsy patients treated at the National Institute of Neurology and Neurosurgery “MVS” in Mexico City. Descriptive statistics were reported as means ± standard deviations for quantitative variables and percentages for categorical variables. Bivariate analysis used the Q Cochran test for dichotomous variables and the chi-square or Fisher’s exact test for qualitative variables.
Results:
Of 1,192 prescriptions, third-generation ASMs accounted for the majority (53.7%), led by levetiracetam (24.1%), lamotrigine (14%), and lacosamide (6%). Second-generation ASMs comprised 42.4%, including valproate (21.5%), carbamazepine (11.3%), and clonazepam (5.5%). First-generation ASMs were less frequently prescribed (3.9%), primarily phenytoin (2.3%), primidone (1.0%), and phenobarbital (0.3%). Third-generation ASMs were the most prescribed for focal seizures (38.6%), generalized seizures (13.3%), and seizures of unknown (1.9%) or unclassified types (2.1%).
Discussion:
Compared to a 2012 study in the same population, which showed second-generation ASM as dominant, this study highlights a significant shift toward third-generation ASM, now representing over half of prescriptions. While valproate and carbamazepine remain versatile second-generation options, newer ASMs, such as levetiracetam, are increasingly favored.
Conclusion:
These findings demonstrate a preference for second- and third-generation ASMs in tertiary hospitals in Latin America, which is concordant with global trends. First-generation ASMs are still prescribed but at lower rates. These results provide insights into changing prescription practices and access to newer medications, informing future research and hospital policies.
Highlights
-
At present, the NINNMVS prescribes third (53.7%) and second-generation (42.4%) ASM principally.
-
Levetiracetam, Valproate, and Lamotrigine account for the most prescribed ASM in PWE at our center.
-
Despite the availability of newer medications, first-generation ASMs remain in clinical use, accounting for 3.9% of prescriptions.
-
These trends in the prescription of new-generation ASMs in LATAM align with their worldwide use in PWE.
Introduction
Epilepsy is a common neurological disorder; it affects around 70 million people worldwide and is a common cause of disability and increased healthcare costs (1). Latin America (LATAM) is not the exception; around 6·3 million people in this continent have active epilepsy (2). Specifically in Mexico, there is a prevalence of epilepsy of over 10.8 to 20 cases per thousand people (3). The main objective of epilepsy treatment is to have early seizure control by starting medication as soon as the patient has been diagnosed while avoiding adverse effects that could diminish the quality of life. The first line of treatment for people with epilepsy (PWE) consists of antiseizure medications (ASM) worldwide; there are 25 ASM available (4). Treatment selection depends on the individual characteristics of the patient, such as age, sex, the desire to get pregnant, comorbidities, and tolerability, as well as the characteristics of the disease, such as seizure type or the diagnosis of an epileptic syndrome (1).
Until 1990, there were only six ASMs in existence, which are now called “First Generation” ASMs and include, in alphabetical order: benzodiazepines (BZD), carbamazepine (CBZ), phenobarbital (PB), phenytoin (PTH), primidone (PRM), and valproate (VPA). Throughout the years, new medications have been introduced in the market, “Second Generation” ASM include: Lamotrigine (LTG), Levetiracetam (LEV), Oxcarbazepine (OXC), and Topiramate (TPM) (4). And newer “Third Generation” ASMs are now available: Brivaracetam (BRV), Eslicarbazepine (ESL), and Lacosamide (LCS) (4). The use of first-generation ASMs, such as CBZ or VPA, as the first line of treatment in PWE is still a practice worldwide. However, the availability of new ASMs has led physicians to expand their treatment options (5). Although the SANAD II study has shown similar efficacies between first—and second-generation ASM, the later ones have fewer adverse effects, which can improve treatment adherence and patient’s quality of life (6).
The Epilepsy Priority Program (EPP) in Mexico established a Multicenter Epilepsy Registry from March 2021 to December 2022, it consisted of 6,653 patients all over the country. The Epilepsy Clinic, along with the Clinical Epileptology Fellowship at NINNMVS, participated in the project and facilitated the recollection of sociodemographic and clinical data from PWE (7).
The following study described the use of new-generation ASM in the subgroup of PWE in the EPP Multicenter Epilepsy Registry who attended the Epilepsy Clinic at NINNMVS in Mexico City and contrasts it with the use of previous ASM in the same population. In Mexico, health access is divided into three groups. Government health insurance is available for Government employees through the Institute for Social Security and Services for State Workers (ISSSTE), general employees through the Mexican Institute of Social Security (IMSS), and the uninsured population through the Ministry of Health (SSA - Secretaría de Salud). The rest of the population pays for health insurance through private companies. (8) Private health insurance companies have access to all ASMs, including newer generations (except cannabidiol and cenobamate, which are not available in the country). Government-run health institutes have a more limited availability, especially in primary care centers, where available ASMs are included annually in a “basic catalog of medication.” In 2024, for example, it included VPA, LEV, PHT, PB, CBZ, TPM, and OXC. Newer generation ASMs are only available in the tertiary care level, such as the NINNMVS, which includes BVC, BZD, LTG and LCM (9).
Materials and methods
Study design
This study aimed to determine the use of new-generation ASM in a referral hospital in LATAM. An observational retrospective cohort from 2021 through 2024 was obtained from the EPP Multicenter Epilepsy Registry. The study had the approval of NINN Bioethics and Research Committees No. 68/21.
Patients
The study included PWE, a subgroup of the EPP registry, who were evaluated by one Epileptologist (I.E.M.J.) and Clinical Epileptology Fellows in the Epilepsy Clinic at the NINNMVS. Data were collected from patients’ medical records up to their most recent follow-up and entered into REDCap® for organization and storage. The data were then analyzed using SPSS® version 21. Clinical and sociodemographic data of PWE were obtained. Seizure types were classified according to the 2017 ILAE classification system (10).
Use of antiseizure medications
Current and previous ASM use in PWE was analyzed. Previous ASMs refer to those prescribed by other institutions or physicians before the patient’s first visit to our clinic, based on their prior treatment regimen. Antiseizure medications were divided into three classes: (I) First generation, (II) Second generation, and (III) Third generation, based on Gunasekera’s classification (9) (see Figure 1).
Figure 1

Classification of ASM generation according to Gunasekera. ASM, Anti-seizure medications. Figure modified from Gunasekera’s classification (22).
Sample and statistical analysis
Descriptive analysis was performed for quantitative variables with means and standard deviation, while percentages and proportions were used for qualitative variables. Bivariate analysis was conducted using the Q Cochran test for dichotomous dependent variables and either the Chi-square test or Fisher’s exact test for qualitative variables, and Student’s t test or its equivalent non-parametric were used for quantitative variables, all analysis was done using SPSS® version 21.
Results
Sociodemographic and clinical characteristics
A group of 635 patients from the EPP Multicenter Epilepsy Registry who attended the NINNMVS Epilepsy Clinic were included. Among them, 378 were female (59.5%), and 257 were male (40.5%). The patients’ mean age was 36.88 ± 13.4 (15–79). The mean time since epilepsy diagnosis to inclusion in the registry was 12.95 ± 6.9 years (6 months - 25 years). The majority of patients were on monotherapy (253, 39.8%), while 271 (42.7%) were prescribed multiple ASMs. The sociodemographic and clinical characteristics of the PWE, including education level, past medical history, seizure type, epilepsy syndrome and or epilepsy etiologies, and epilepsy surgery are summarized in Table 1.
Table 1
| Patients characteristics n = 635 | ||
|---|---|---|
| Sociodemographic data | ||
| n (%) | ||
| Sex | Female | 378 (59.5%) |
| Male | 257 (40.5%) | |
| Age | In years (mean ± SD) | 36.88 ± 13.4 |
| Highest education level | None | 62 (9.8%) |
| Elementary school | 87 (13.7%) | |
| Secondary school | 143 (22.5%) | |
| High school | 163 (25.7%) | |
| University | 122 (19.2%) | |
| Postgraduate degree | 11 (1.7%) | |
| Special education | 21 (3.3%) | |
| Clinical data | ||
| Time with the diagnosis of epilepsy | Years (mean ± SD) | 12.95 ± 6.9 |
| History | Febrile seizures | 58 (9.1%) |
| Family history of epilepsy | 84 (13.2%) | |
| Seizure type | Focal onset | 429 (67.6%) |
| Generalized onset | 174 (27.4%) | |
| Unknown onset | 29 (4.6%) | |
| Unclassified seizures | 3 (0.5%) | |
| Etiology | Structural | 368 (58%) |
| Genetic | 98 (15.4%) | |
| Infectious | 13 (2%) | |
| Metabolic | 2 (0.3%) | |
| Inmune | 25 (3.9%) | |
| Unknown | 161 (25.4%) | |
| Epilepsy surgery | Corpus Callosotomy | 8 (1.3%) |
| Vagal Nerve Stimulator (VNS) | 1 (0.2%) | |
| Hemispherectomy | 5 (0.8%) | |
| Lesionectomy | 21 (3.3%) | |
| Temporal lobectomy | 38 (6%) | |
| Extra Temporal Resection | 3 (0.5%) | |
| Transsphenoidal Resection | 8 (1.3%) | |
| Electroencephalogram | Total available | 410 (54.6%) |
| Normal | 166 (26.1%) | |
| Abnormal | 244 (38.44%) | |
| Seizure frequency | Seizure free | 249 (39.2%) |
| 1–3 seizures per month | 310 (48.8%) | |
| 4–6 seizures per month | 50 (7.9%) | |
| >10 seizures per month | 26 (4.1%) | |
| Number of ASM | Monotherapy; n (%) | 253 (39.8%) |
| Polytherapy | 271 (42.7%) | |
| 2 ASMs | 160 (25.2%) | |
| 3 ASMs | 83 (13.1%) | |
| 4 ASMs | 23 (3.6%) | |
| 5 ASMs | 5 (0.8%) | |
Characteristics of patients with epilepsy included in this study.
Current use of antiseizure medications
A total of 1,192 prescriptions were provided at the NINNMVS Epilepsy Clinic throughout the study. Of these, 47 (3.9%) prescriptions were for first-generation ASM, with PHT being the most commonly prescribed (28, 2.3%), followed by PRM (12, 1.0%) and PB (4, 0.3%). Meanwhile, 505 prescriptions (42.4%) were for second-generation ASM, primarily VPA (256, 21.5%), CBZ (135, 11.3%), and CZP (65, 5.5%). Finally, third-generation ASM accounted for 640 prescriptions (53.7%), including LEV (287, 24.1%), LTG (167, 14%), and LCM (72, 6%) (Figure 2).
Figure 2
Current trends in prescriptions of anti-seizure medications drugs at NINNMVS Epilepsy Clinic. ASM, Anti-seizure medication.
Previous use of antiseizure medications
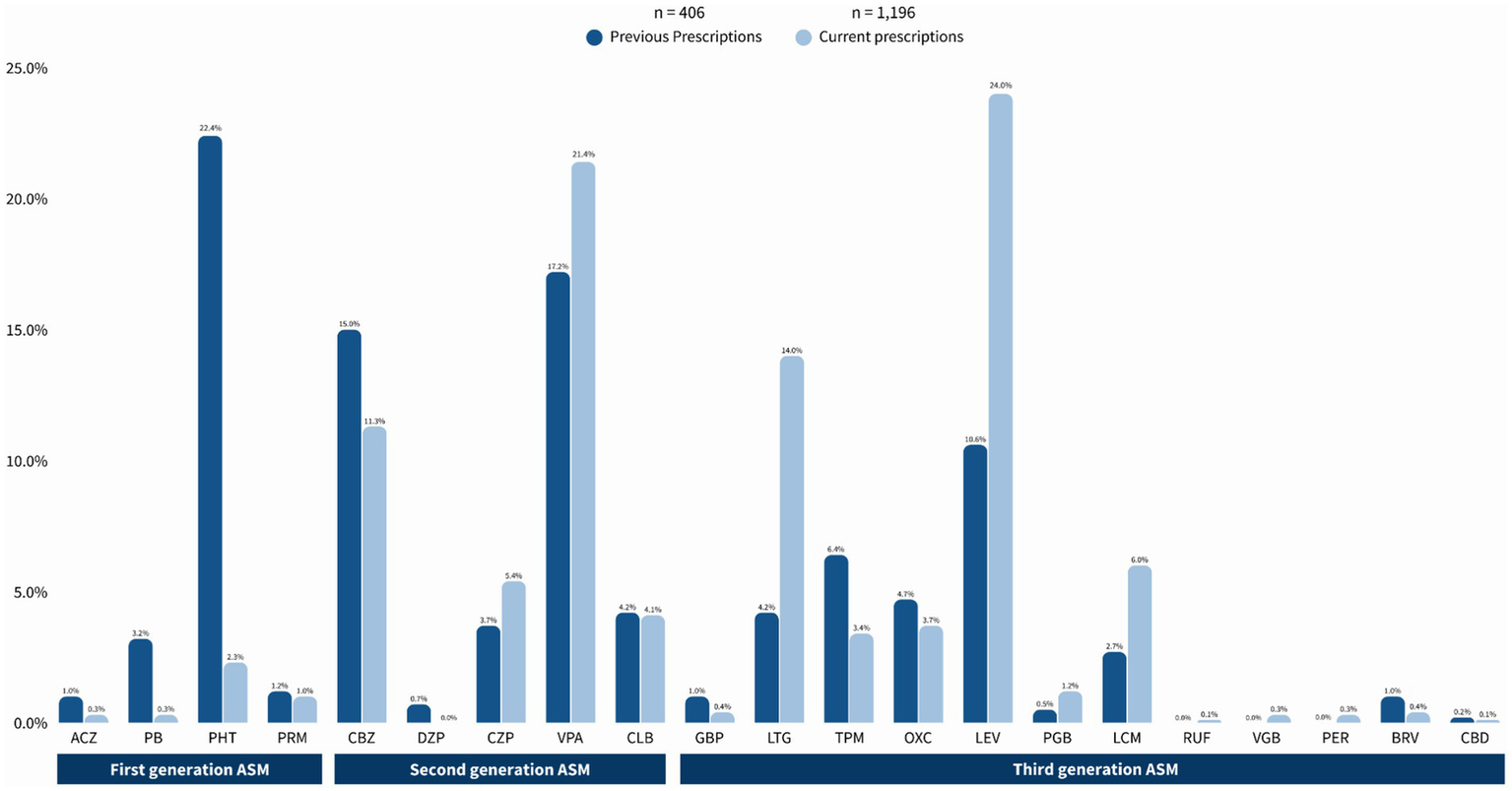
Table 2 compares previous ASM use before attending our center with current use, where applicable, in the same population of PWE. Of a total of 406 prescriptions, 113 (27.8%) were First-Generation ASM, primarily PHT (91, 22.4%) and PB (12, 3.2%). Second-generation ASMs accounted for 166 (40.9%) of the prescriptions, with VPA (70, 17.2%) and CBZ (61, 15%) being the most common. Finally, Third-Generation ASM constituted 127 (31.3%) of the total prescriptions, with the most prevalent being LEV (43, 10.6%), LTG (17, 4.2%), and LCM (11, 2.7%).
Table 2
| Prescription of antiseizure medications | |||||
|---|---|---|---|---|---|
| Class of ASM (International abbreviation) | Previous prescriptions of ASM n = 406 n (%) | Current prescriptions of ASM n = 1,196 n (%) | p | ||
| First Generation | Acetazolamide (ACZ) | 4 (1.0%) | 3 (0.3%) | 0.705 | |
| Phenobarbital (PB) | 13 (3.2%) | 4 (0.3%) | 0.020 | ||
| Phenytoin (PHT) | 91 (22.4%) | 28 (2.3%) | <0.001 | ||
| Primidone (PRM) | 5 (1.2%) | 12 (1.0%) | 0.071 | ||
| Second generation | Carbamazepine (CBZ) | 61 (15%) | 135 (11.3%) | <0.001 | |
| Diazepam (DZP) | 3 (0.7%) | 0 (0%) | - | ||
| Clonazepam (CZP) | 15 (3.7%) | 65 (5.4%) | <0.001 | ||
| Valproate (VPA) | 70 (17.2%) | 256 (21.4%) | <0.001 | ||
| Clobazam (CLB) | 17 (4.2%) | 49 (4.1%) | |||
| Third generation | 1990 | Gabapentin (GBP) | 4 (1.0%) | 5 (0.4%) | 0.705 |
| Lamotrigine (LTG) | 17 (4.2%) | 167 (14.0%) | <0.001 | ||
| Topiramate (TPM) | 26 (6.4%) | 41 (3.4%) | 0.063 | ||
| Oxcarbazepine (OXC) | 19 (4.7%) | 44 (3.7%) | 0.001 | ||
| Levetiracetam (LEV) | 43 (10.6%) | 287 (24.0%) | <0.001 | ||
| 2000 | Pregabalin (PGB) | 2 (0.5%) | 14 (1.2%) | 0.003 | |
| Lacosamide (LCM) | 11 (2.7%) | 72 (6.0%) | <0.001 | ||
| Rufinamide (RUF) | 0 (0%) | 1 (0.1%) | - | ||
| Vigabatrin (VGB) | 0 (0%) | 3 (0.3%) | - | ||
| 2010 | Perampanel (PER) | 0 (0%) | 4 (0.3%) | - | |
| Brivaracetam (BRV) | 4 (1.0%) | 5 (0.4%) | 0.739 | ||
| Cannabidiol (CBD) | 1 (0.2%) | 1 (0.1%) | 1.000 | ||
Previous and current use of antiseizure medications in PWE attended at NINNMVS.
As for first-generation ASM, a significant reduction was observed in the prescription of PB (13 vs. 4, p = 0.020) and PHT (91 vs. 18, p < 0.001), while others, such as ACZ and ESM, did not show statistically significant differences. In second-generation ASM, there was a notable increase in the use of CBZ (61 vs. 135, p < 0.001), CZP (15 vs. 65, p < 0.001), VPA (70 vs. 256, p < 0.001), and CLB (17 vs. 49, p < 0.001). Meanwhile, for third-generation ASM, drugs such as LTG (17 vs. 167, p < 0.001) and LEV (43 vs. 287, p < 0.001) showed significant increases, whereas TPM presented a non-significant increase (26 vs. 41, p = 0.063).
Of the 635 patients, 379 (59.7%) had not received any treatment before their assessment at the Epilepsy Clinic. During follow-up in the NINNMVS, only 22 patients (3.5%) remained untreated due to the patient’s desire not to take ASM or due to non-compliance with treatment.
Table 3 shows a comparison between the prescription of different generations of ASM by seizure type. For focal seizures, third-generation ASMs were the most prescribed, with 462 (38.6%) prescriptions, primarily LEV (192, 16.1%) and LTG (124, 10.4%). This was followed by second-generation ASM with 355 (29.7%) prescriptions, mainly VPA (151, 12.6%) and CBZ (116, 9.7%). First-generation ASMs were the least prescribed, with 34 (3%) prescriptions, namely PHT (21, 1.8%). For generalized seizures, third-generation ASM also dominated, with 157 (13.3%) prescriptions, primarily LEV (82, 6.9%), followed by LTG (36, 3%) and TPM (13, 1.1%). Second-generation ASM followed with 135 (11.2%) prescriptions, mainly VPA (96, 8%) and CBZ (16, 1.3%). First-generation ASMs accounted for only 11 (0.9%) prescriptions, mainly for PHT (6, 0.5%) and PRM (5, 0.4%). For seizures of unknown type, third-generation ASMs were again the most prescribed, with 23 (1.9%) prescriptions, primarily LEV (11, 0.9%) and LTG (7, 0.6%). Second-generation ASM followed with 15 (1.5%) prescriptions, mainly VPA (9, 0.9%). First-generation ASMs had only 1 (0.1%) prescription. Finally, for unclassified seizures, only LEV was prescribed, with 2 (0.2%) prescriptions.
Table 3
| Antiseizure medications prescription | |||||
|---|---|---|---|---|---|
| n = 1,196 n (%) | Total n (%) | p | |||
| Focal seizures | First generation | Acetazolamide | 3 (0.3%) | 34 (3%) | 0.488 |
| Phenobarbital | 3 (0.3%) | ||||
| Phenytoin | 21 (1.8%) | ||||
| Primidone | 7 (0.6%) | ||||
| Second generation | Carbamazepine | 116 (9.7%) | 355 (29.7%) | ||
| Clonazepam | 50 (4.2%) | ||||
| Valproate | 151 (12.6%) | ||||
| Clobazam | 38 (3.2%) | ||||
| Third generation | Gabapentin | 5 (0.4%) | 462 (38.6%) | ||
| Lamotrigine | 124 (10.4%) | ||||
| Topiramate | 27 (2.3%) | ||||
| Oxcarbazepine | 36 (3.0%) | ||||
| Levetiracetam | 192 (16.1%) | ||||
| Pregabalin | 11 (0.9%) | ||||
| Lacosamide | 58 (4.8%) | ||||
| Vigabatrin | 1 (0.1%) | ||||
| Perampanel | 4 (0.3%) | ||||
| Brivaracetam | 4 (0.3%) | ||||
| Generalized | First generation | Phenytoin | 6 (0.5%) | 11 (0.9%) | 0.539 |
| Primidone | 5 (0.4%) | ||||
| Second generation | Carbamazepine | 16 (1.3%) | 135 (11.2%) | ||
| Clonazepam | 13 (1.1%) | ||||
| Valproate | 96 (8.0%) | ||||
| Clobazam | 10 (0.8%) | ||||
| Third generation | Lamotrigine | 36 (3.0%) | 157 (13.3%) | ||
| Topiramate | 13 (1.1%) | ||||
| Oxcarbazepine | 8 (0.7%) | ||||
| Levetiracetam | 82 (6.9%) | ||||
| Pregabalin | 3 (0.3%) | ||||
| Lacosamide | 10 (0.8%) | ||||
| Rufinamide | 1 (0.1%) | ||||
| Vigabatrin | 2 (0.2%) | ||||
| Brivaracetam | 1 (0.1%) | ||||
| Cannabidiol | 1 (0.1%) | ||||
| Unknown | First generation | Phenobarbital | 1 (0.1%) | 1 (0.1%) | 0.830 |
| Second generation | Carbamazepine | 3 (0.3%) | 15 (1.5%) | ||
| Clonazepam | 2 (0.2%) | ||||
| Valproate | 9 (0.9%) | ||||
| Clobazam | 1 (0.1%) | ||||
| Third generation | Lamotrigine | 7 (0.6%) | 23 (1.9%) | ||
| Topiramate | 1 (0.1%) | ||||
| Levetiracetam | 11 (0.9%) | ||||
| Lacosamide | 4 (0.3%) | ||||
| Unclassified | Third generation | Levetiracetam | 2 (0.2%) | 2 (0.2%) | - |
| No treatment | None | 22 (3.5%) | 22 (3.5%) | - | |
Antiseizure medications prescription according to seizure type based on the 2017 ILAE seizure classification.
Seizure freedom and use of ASMs
Table 4 summarizes de baseline seizure frequency patients presented before initiating treatment in our clinic, and seizure frequency after starting treatment. Before attending, 310 (48.8%) of the patients had between one and three seizures per month, and 249 (39.2%) were seizure-free at the time of first visit. After starting treatment in our clinic, 403 (63.5%) patients achieved seizure freedom, while 227 (35.7%) persisted with seizures.
Table 4
| Seizure frequency and use of ASMs | ||
|---|---|---|
| n = 635 n (%) |
Total n (%) |
|
| Seizure frequency before treatment in Epilepsy Clinic | Seizure free; n (%) | 249 (39.2%) |
| 1–3 seizures per month | 310 (48.8%) | |
| 4–6 seizures per month | 50 (7.9%) | |
| >10 seizures per month | 26 (4.1%) | |
| Seizure frequency after treatment in Epilepsy Clinic | Seizure freedom | 403 (63.5%) |
| Persistent seizures | 227 (35.7%) | |
| NS | 5 (0.8%) | |
Baseline and current seizure frequency of studied patients.
Table 5 summarizes seizure freedom and persistence for each prescribed ASM, with percentages representing the proportion of patients within each ASM group experiencing either outcome.
Table 5
| Seizure freedom/persistence and use of ASMs | |||||
|---|---|---|---|---|---|
| n = total of prescriptions of each ASM | n (%) | Total n (%) | p | ||
| Seizure freedom | First generation | ACZ: 3 prescriptions | 1 (33.3%) | 27 (57.4%) | 0.004 |
| PB: 4 prescriptions | 1 (25.0%) | ||||
| PHT: 28 prescriptions | 20 (71.4%) | ||||
| PRM: 12 prescriptions | 5 (41.6%) | ||||
| Second generation | CBZ: 135 prescriptions | 74 (54.8%) | 277 (54.9%) | <0.001 | |
| DZP: 0 prescriptions | - | ||||
| CZP: 65 prescriptions | 31 (47.7%) | ||||
| VPA: 256 prescriptions | 152 (59.4%) | ||||
| CLB: 49 prescriptions | 20 (40.8%) | ||||
| Third generation | GBP: 5 prescriptions | 4 (80.0%) | 369 (57.3%) | 0.035 | |
| LTG: 167 prescriptions | 91 (54.5%) | ||||
| TPM: 41 prescriptions | 15 (36.6%) | ||||
| OXC: 44 prescriptions | 29 (65.0%) | ||||
| LEV: 287 prescriptions | 175 (61.0%) | ||||
| PGB: 14 prescriptions | 9 (64.3%) | ||||
| LCM: 72 prescriptions | 40 (55.6%) | ||||
| RUF: 1 prescriptions | 1 (100%) | ||||
| VGB: 3 prescriptions | 1 (33.3%) | ||||
| PER: 4 prescriptions | 0 (0.0%) | ||||
| BRV: 5 prescriptions | 3 (60.0%) | ||||
| CBD: 1 prescriptions | 1 (100%) | ||||
| Persistent seizures | First generation | ACZ: 3 prescriptions | 2 (66.7%) | 20 (4.3%) | 0.565 |
| PB: 4 prescriptions | 3 (75.0%) | ||||
| PHT: 28 prescriptions | 8 (28.6%) | ||||
| PRM: 12 prescriptions | 7 (58.3%) | ||||
| Second generation | CBZ: 135 prescriptions | 61 (45.2%) | 225 (44.6%) | <0.001 | |
| DZP: 0 prescriptions | - | ||||
| CZP: 65 prescriptions | 34 (52.3%) | ||||
| VPA: 256 prescriptions | 101 (39.5%) | ||||
| CLB: 49 prescriptions | 29 (59.2%) | ||||
| Third generation | GBP: 5 prescriptions | 1 (20.0%) | 271 (42.1%) | 0.015 | |
| LTG: 167 prescriptions | 74 (44.3%) | ||||
| TPM: 41 prescriptions | 26 (63.4%) | ||||
| OXC: 44 prescriptions | 15 (34.1%) | ||||
| LEV: 287 prescriptions | 110 (38.3%) | ||||
| PGB: 14 prescriptions | 5 (35.7%) | ||||
| LCM: 72 prescriptions | 32 (44.4%) | ||||
| RUF: 1 prescriptions | 0 (0.0%) | ||||
| VGB: 3 prescriptions | 2 (66.7%) | ||||
| PER: 4 prescriptions | 4 (100%) | ||||
| BRV: 5 prescriptions | 2 (40.0%) | ||||
| CBD: 1 prescriptions | 0 (0.0%) | ||||
Seizure freedom and persistence according to prescribed ASM.
The analysis revealed significant differences in seizure freedom based on the generation of ASMs. First-generation (p = 0.004) and second-generation (p < 0.001) drugs showed statistically significant seizure freedom rates. In terms of percentage, first- and third-generation ASMs had the highest seizure freedom rates among the prescribed medications (57.4 and 57.3%, respectively), with LEV (61.0%), VPA (59.4%), and PHT (71.4%) standing out.
Regarding seizure persistence, significant differences were found for second-generation ASMs (p < 0.001), with a higher frequency of persistent seizures in users of PB, CLB, TPM, and PER. No significant differences were observed for first- and third-generation drugs (p = 0.565 and p = 0.015, respectively).
Discussion
Historical prescription trends
This study represents a new update on ASM prescription trends in a real-life setting in a LATAM country in this millennium. A study conducted in Mexico at NINNMVS by Martínez-Juárez et al. in 2012 (11) examined 206 patients to evaluate the frequency of drug-resistant epilepsy and the ASM prescribed in this population. The most commonly used ASMs were second-generation drugs such as VPA and CBZ either in mono or polytherapy, with a very slight tendency towards using third-generation ASMs as adjunctive therapy.
Study key findings
In our study, we compared patients’ previous treatment regimens before attending our clinic with those prescribed there to identify prescribing trends at our national third-level referral center. Third-generation ASMs represented 53.7% of the prescriptions, indicating their significant prevalence in clinical practice and widespread use compared to first and second-generation ASMs. Levetiracetam (LEV) and lamotrigine (LTG) demonstrated a statistically significant increase in their use in PWE attended at NINN; this may be due to their efficacy, safety profiles, and clinical acceptance. The remaining prescriptions were for second-generation ASM, suggesting a high prevalence and potential preference for these medications in the studied population. Among this group, VPA, CZP, CLB, and CBZ were the most commonly used with a statistical significance, where VPA stood out as the most versatile medication overall. Its broad-spectrum and efficacy makes it suitable for treating almost all types of seizures and epilepsy syndromes, despite its association with a risk of congenital malformations (12). This shows a trend to use newer medications; however, as they may not be as widely available or due to physician preference for medications with a longer and well-documented history of use, second-generation ASM still accounts for a wide amount of prescriptions.
Furthermore, our analysis revealed that first- and second-generation drugs were associated with statistically significant seizure freedom rates. However, when considering percentages, first- and third-generation ASMs demonstrated the highest seizure freedom rates, with LEV (61.0%), VPA (59.4%), and PHT (71.4%) standing out. In contrast, second-generation ASMs showed statistically significant rates of persistent seizures, particularly among patients prescribed PB, CLB, TPM, and PER. These outcomes may be influenced by various factors, including the different combinations of ASMs, patient adherence to treatment, drug-resistant epilepsy, and other variables that can affect the efficacy of the medications. Additionally, some medications show higher rates of both seizure freedom and persistent seizures, which could be attributed to their lower prescription frequency or their enhanced therapeutic effect when combined with other ASMs.
Global perspectives on antiseizure medications use
To our knowledge, few studies in LATAM address the current trends in the prescription of ASM in PWE. Comparable findings were observed by Assis et al. in Brazil, where there was a significant rise in the adoption of new-generation ASMs, such as LTG and LEV, and a drop in suboptimal ASM prescriptions from 73.3 to 51.5% (13). A study in another developing country by Joshi et al. in 2020 found that VPA and CZP were preferred for generalized seizures, while CLB, CBZ, OZC, and LCM were more commonly prescribed for focal seizures in a population from India (14). In our study, third-generation ASMs, mainly LEV and LTG, were prescribed for both focal and generalized seizures. Second-generation ASMs, such as VPA and CBZ, played a significant role in the treatment of both seizure types.
In developed countries, a study by Bolin et al. (15) in Sweden revealed a significant increase in the use of third-generation ASMs; LEV was the most commonly prescribed medication for initial treatment, with its use rising from 10% in 2010 to 55% in 2022. Lamotrigine (LTG) also demonstrated strong adherence rates, with the highest number of patients remaining on their initial therapy. Meanwhile, CBZ and VPA experienced a marked decline in use, dropping from 35 to 5% and 20 to 5%, respectively.
Another study conducted in Canada by Leong et al. reported a dramatic rise in the use of newer ASMs from 0.3 to 15 per 1,000 prescriptions, with LTG playing a significant role, while older ASMs declined from 7.5 to 6.4 per 1,000 prescriptions (16). Similarly, research by Bensken and Sánchez Fernández (17) in the United States highlighted a decreased use of first-generation ASMs and a substantial rise in the prescription of LCM, similar to the findings described in this study.
Other studies corroborate these trends. For instance, Powell et al. in the UK reported a reduced use of CBZ and increased use of LEV and LTG over time (18). Jin et al. in Japan also reported a statistically significant increase in newer ASMs and a decline in the older ones, with the prescription of LEV rising from 15.6 to 22.6%. However, VPA remained the most prescribed ASM in that study (19).
In contrast, a study by Lavu et al. (20) in Canada reported only a 0.09% increase in the use of new-generation ASMs following the COVID-19 pandemic, alongside a 5.11% decrease in overall ASM prescriptions, excluding GBP and CZP. Interestingly, our study observed a significant increase in the prescription of GBP and CZP.
These findings suggest that Mexico is leaning towards global trends. Our study revealed a high prevalence and increasing use of LEV and LTG, with VPA remaining the most widely used ASM overall. Several situations could explain this trend towards the use of newer ASMs in Mexico. As explained previously, the healthcare system does not have all the ASMs available, and in primary care centers or rural areas, the use of PHT and VPA is still frequent. However, since NINNMVS is a tertiary care center, newer ASMs have become available for prescription, especially for people with drug-resistant epilepsy. The population cared for in our center corresponds to low and middle income, these people usually cannot afford private health insurance or buying new ASMs such as BVC, LTG, and LCM. Therefore, the inclusion of newer ASMs in the hospital’s medication catalog has probably enabled physicians to lean toward global prescription trends and increase the use of third-generation ASMs. However, in order to confirm this assumption, further studies should be conducted and include our patients and physicians.
Current and future directions in the use of antiseizure medications
Epilepsy has a significant socio-sanitary impact, as it reduces the patient’s quality of life and life expectancy by 2 to 10 years and increases the mortality rate by 2 to 3 times compared to individuals without the disease. Despite the availability of newer medications, first-generation ASMs remain in clinical use, accounting for 3.9% of prescriptions, with both PB and PHT showing a significant reduction compared to previous decades.
According to these findings, and after analyzing the ASM market in the United States, it was observed that first-generation ASMs continue to be prescribed despite the increasing use of second and third-generation alternatives. This trend highlights a shift away from these first-generation medications due to safety concerns and proven effectiveness. However, their continued use may reflect limitations in accessing newer ASMs, such as economic constraints or availability issues.
The World Health Organization’s Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders emphasizes addressing these challenges to ensure quality treatment for PWE. The plan proposes strategies to reduce the treatment gap by improving access to ASMs making them more available and affordable, ensuring their safety and their high quality (21).
Limitations
This study does not specifically address differences between brand-name and generic ASMs, nor does it distinguish between regular and extended-release formulations. Additionally, it does not emphasize seizure freedom or the duration of treatment and retention for each ASM. Instead, its primary aim is to describe current prescription trends within our clinic in a LATAM country.
Conclusion
The study indicates that the use of second- and third-generation ASM is preferred in a tertiary hospital in LATAM, aligning with trends observed in other developing and developed countries. First-generation ASMs are still prescribed, although at a lower rate. The high prevalence of second-generation ASMs, particularly LEV and LTG, suggests a shift towards the newer medications, but first-generation ASMs, such as VPA, are still prescribed. This evidence highlights statistically significant changes in prescription trends within the same population and may inform future research and prescription policies at the hospital, as well as provide insights into the factors influencing treatment choices and access to the latest-generation medications.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of The National Institute of Neurology and Neurosurgery MVS Mexico City, Mexico. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JC-M: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JG-S: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. IF-C: Investigation, Supervision, Writing – original draft. BV-C: Writing – original draft. FV-L: Writing – original draft. PR-L: Writing – review & editing. AS-V: Writing – original draft. LM-C: Data curation, Formal analysis, Methodology, Writing – review & editing. MR-C: Methodology, Writing – review & editing. DB-G: Data curation, Investigation, Methodology, Writing – original draft. EV-M: Writing – review & editing. FS-D: Writing – review & editing. GM-S: Writing – review & editing. SM-M: Supervision, Writing – review & editing. DM-P: Data curation, Writing – review & editing. LG-R: Writing – original draft. AJ-P: Validation, Writing – review & editing. AO-M: Validation, Writing – review & editing. JG-C: Writing – review & editing. MB-Y: Validation, Writing – review & editing. MH-N: Writing – review & editing. JR-A: Supervision, Writing – review & editing. MS-D: Data curation, Formal analysis, Methodology, Writing – review & editing. IM-J: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article was published with an Educational Grant provided by SunPharma Mexico. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We extend our sincere gratitude to the Priority Epilepsy Program (PEP) for their invaluable support in establishing a database of our center for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Thijs RD Surges R O’Brien TJ Sander JW . Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
2.
Pacheco-Barrios K Navarro-Flores A Cardenas-Rojas A de Melo PS Uygur-Kucukseymen E Alva-Diaz C et al . Burden of epilepsy in Latin America and the Caribbean: a trend analysis of the global burden of disease study 1990 - 2019. Lancet Reg Health Am. (2022) 8:100140. doi: 10.1016/j.lana.2021.100140
3.
Valdes-Galvan RE Gonzalez-Calderon G Castro-Martinez E . Acute seizure epidemiology in a neurological emergency department. Rev Neurol. (2019) 68:321–5. doi: 10.33588/rn.6808.2018218
4.
Kanner AM Bicchi MM . Antiseizure medications for adults with epilepsy: a review. JAMA. (2022) 327:1269–81. doi: 10.1001/jama.2022.3880
5.
Perucca P Scheffer IE Kiley M . The management of epilepsy in children and adults. Med J Aust. (2018) 208:226–33. doi: 10.5694/mja17.00951
6.
Marson A Burnside G Appleton R Smith D Leach JP Sills G et al . The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. (2021) 397:1375–86. doi: 10.1016/S0140-6736(21)00246-4
7.
Reséndiz-Aparicio JC Ruiz-García M Castro-Martínez E . Registro multicéntrico de epilepsia en México. Rev Neurol. (2024) 78:9–15. doi: 10.33588/rn.7801.2023296
8.
González Block MÁ Reyes Morales H Hurtado LC Balandrán A Méndez E . Mexico: health system review. Health Syst Transit. (2020) 22:1–222. PMID:
9.
Instituto de Salud para el Bienestar . Medicamentos 2023–2024 (298 Claves) (2025) Available online at: https://www.gob.mx/insabi/documentos/medicamentos-2023-2024-298-claves (Accesed February 2, 2025).
10.
Scheffer IE Berkovic S Capovilla G Connolly MB French J Guilhoto L et al . ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
11.
Martínez-Juárez IE López-Zapata R Gómez-Arias B Bravo-Armenta E Romero-Ocampo L Estévez-Cruz Z et al . Refractory epilepsy: use of the new definition and related risk factors. A study in the Mexican population of a third-level Centre. Rev Neurol. (2012) 54:159–66.
12.
Tomson T Battino D Perucca E . Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. (2016) 15:210–8. doi: 10.1016/S1474-4422(15)00314-2
13.
Assis T Bacellar A Cortes L Santana S Costa G Nascimento O . Trends in prescribing patterns of antiepileptic drugs among older adult inpatients in a Brazilian tertiary center. Arq Neuropsiquiatr. (2021) 79:22–9. doi: 10.1590/0004-282x-anp-2020-0012
14.
Joshi R Tripathi M Gupta P Gulati S Gupta YK . Prescription pattern of antiepileptic drugs in a tertiary care center of India. Indian J Pharmacol. (2020) 52:283–9. doi: 10.4103/ijp.IJP_507_17
15.
Bolin K Patric B Tomson T . Trends in Antiseizure medication initiation, switch, or termination in patients with newly diagnosed epilepsy. Neurol Int. (2024) 103:e209500. doi: 10.1212/WNL.0000000000209500
16.
Leong C Mamdani MM Gomes T Juurlink DN Macdonald EM Yogendran M . Antiepileptic use for epilepsy and nonepilepsy disorders: a population-based study (1998-2013). Neurology. (2016) 86:939–46. doi: 10.1212/WNL.0000000000002446
17.
Bensken WP Sánchez FI . Trends in Antiseizure medication use: implications for practice and clinical care. Neurology. (2022) 99:319–20. doi: 10.1212/WNL.0000000000200852
18.
Powell G Logan J Kiri V Borghs S . Trends in antiepileptic drug treatment and effectiveness in clinical practice in England from 2003 to 2016: a retrospective cohort study using electronic medical records. BMJ Open. (2019) 9:e032551. doi: 10.1136/bmjopen-2019-032551
19.
Jin K Obara T Hirano K Hirai D Kiuchi M Tanaka T et al . Prescription trends in anti-seizure medications for adult patients with epilepsy in Japan: a retrospective cohort study using the database of health insurance claims between 2015 and 2019. Epilepsy Behav. (2022) 134:108841. doi: 10.1016/j.yebeh.2022.108841
20.
Lavu A Janzen D Aboulatta L Peymani P Haidar L Desrochers B et al . Prescription trends of antiseizure medications before and during the COVID-19 pandemic. Front Neurol. (2023) 14:1135962. doi: 10.3389/fneur.2023.1135962
21.
World Health Organization Intersectoral global action plan on epilepsy and other neurological disorders [internet]. (2023). Available online at: https://www.who.int/publications/i/item/9789240076624 (Accessed January 14, 2025).
22.
Gunasekera CL Sirven JI Feyissa AM . The evolution of antiseizure medication therapy selection in adults: is artificial intelligence -assisted antiseizure medication selection ready for prime time?J Cent Nerv Syst Dis. (2023) 15:11795735231209209. doi: 10.1177/11795735231209209
Summary
Keywords
antiseizure medications, prescription, epilepsy, epileptic syndromes, LATAM
Citation
Colado-Martinez J, Gonzalez-Salido J, Fuentes-Calvo I, Vázquez-Cruz BC, Vasquez-Lopez F, Robles-Lomelin P, Solis-Velázquez AM, Marin-Castañeda LA, Rivas-Cruz MA, Barrios-González DA, Valenzuela-Mendivil E, Sotelo-Díaz F, Mendez-Suarez G, Martínez-Medina S, Martínez-Piña DA, Gómez-Rodríguez LJ, Jara-Prado A, Ochoa-Morales A, Guerrero-Camacho J, Breda-Yepes ML, Herrera-Noguera MN, Reséndiz-Aparicio JC, Sebastián-Díaz MA and Martínez-Juárez IE (2025) Trends in prescription of new antiseizure medications in a single center in Latin America: evidence of clinical practice. Front. Neurol. 16:1562079. doi: 10.3389/fneur.2025.1562079
Received
16 January 2025
Accepted
06 May 2025
Published
21 May 2025
Volume
16 - 2025
Edited by
Daichi Sone, Jikei University School of Medicine, Japan
Reviewed by
Rajarshi Mazumder, University of California, Los Angeles, United States
Kapil Gururangan, Northwestern University, United States
Updates
Copyright
© 2025 Colado-Martinez, Gonzalez-Salido, Fuentes-Calvo, Vázquez-Cruz, Vasquez-Lopez, Robles-Lomelin, Solis-Velázquez, Marin-Castañeda, Rivas-Cruz, Barrios-González, Valenzuela-Mendivil, Sotelo-Díaz, Mendez-Suarez, Martínez-Medina, Martínez-Piña, Gómez-Rodríguez, Jara-Prado, Ochoa-Morales, Guerrero-Camacho, Breda-Yepes, Herrera-Noguera, Reséndiz-Aparicio, Sebastián-Díaz and Martínez-Juárez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iris E. Martínez-Juárez, imartinez@innn.edu.mx; iris.martinezju@comunidad.unam.mx
†These authors share first authorship
†ORCID: Iris E. Martínez-Juárez, orcid.org/0000-0001-6512-5312
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.