Abstract
Background:
Intracranial infection is a severe complication following intracranial aneurysm surgery, associated with higher rates of morbidity and mortality. This study aimed to develop and validate a nomogram to predict the risk for intracranial infection after intracranial aneurysm surgery. This nomogram was designed to assist clinicians in identifying high-risk patients and implementing targeted preventive measures, ultimately improving postoperative outcomes.
Methods:
This retrospective cohort study included patients who underwent intracranial aneurysm surgery at a single center. Data regarding potential predictors, including clinical characteristics, surgical details, and laboratory test results, were collected. Independent risk factors for intracranial infection were identified using univariate and multivariate logistic regression analyses. A nomogram was constructed on the basis of these predictors. Nomogram performance was evaluated using the area under the receiver operating characteristic curve (AUC) for discrimination, calibration plots for predictive accuracy, and decision curve analysis (DCA) for clinical utility.
Results:
Data from 612 patients who underwent intracranial aneurysm surgery were analyzed, with 428 and 184 patients in the training and validation cohorts, respectively. Multivariate logistic regression analysis identified pneumonia, external ventricular drainage, tracheotomy, procalcitonin, C-reactive protein, and albumin levels as independent risk factors for intracranial infections (p < 0.05). A nomogram, constructed on the basis of these predictors, exhibited excellent discrimination, with an AUC of 0.91 (95% confidence interval [CI] 0.88–0.93) in the training cohort and 0.89 (95% CI 0.84–0.93) in the validation cohort. DCA demonstrated that the nomogram provided a significant net clinical benefit across a range of risk thresholds, supporting its utility in clinical decision making.
Conclusion:
The nomogram developed was a robust and practical tool for predicting the risk for intracranial infection after intracranial aneurysm surgery. It demonstrated strong predictive accuracy and calibration, with potential applications in identifying high-risk patients and guiding individualized preventive strategies. However, validation using a broader and more diverse population is recommended to enhance the generalizability of the model.
Introduction
Intracranial aneurysm is an aneurysm formed by abnormal expansion of arterial vessels inside the brain caused by congenital anomalies or acquired injuries (1). It is characterized by insidious onset and a high rate of disability and death (2). Surgery is often used to treat intracranial aneurysms by blocking the blood supply of the aneurysm to avoiding intracranial hemorrhage (3), and maintaining blood flow in the brain tissues (4). However, postoperative complications are also important factors affecting prognosis in this patient population (5), such as intracranial infections (ICIs) (6). Epidemiological investigations have shown that the incidence of ICIs complicating intracranial aneurysms after surgery ranges from 2.6 to 30.0%, and the mortality rate is > 30.0% (7). The occurrence of ICI (s) increases the difficulty of treatment, hospitalization costs, prolongs hospital stay, leads to readmission and/or reoperation, has serious neurological sequelae (8), and increases the risk for death (9). Therefore, early identification of high-risk factors for ICI after intracranial aneurysm surgery plays an important role in patient prognosis and reducing the risk of death (10).
Postoperatively, patients who undergo procedures for intracranial aneurysm experience more manifestations of noninfectious meningitis due to neurological impairment, fever, and cerebrospinal fluid (CSF) changes caused by underlying diseases. Furthermore, the clinical manifestations of postoperative ICIs are easily masked, making accurate diagnosis of ICI more difficult (11). Although a previous study investigated risk factors for ICI after intracranial aneurysm surgery (12), few have addressed the prediction and assessment of risk factors for ICI after aneurysm surgery using multi-index combined risk assessment. Most previous studies used logistic regression analysis and lacked effective predictive models. The nomogram presents the results of regression analysis using intuitive graphics and data to predict the probability of clinical events. Nomograms offer several advantages for predicting ICIs. By integrating multiple patient-specific and clinical variables, nomograms enable personalized risk assessment, facilitating tailored interventions for high-risk individuals (13). Their graphical representation ensures user-friendliness, enabling clinicians to easily calculate risk without requiring advanced statistical expertise. Additionally, nomograms often demonstrate superior predictive accuracy and calibration compared with conventional risk scores, enhancing their reliability in clinical practice. Accordingly, the present study aimed to develop and validate a nomogram for predicting ICI after intracranial aneurysm surgery, offering a tool to enhance risk stratification and clinical decision-making.
Methods
Participants
A retrospective analysis of data from 612 patients, who were admitted to First People’s Hospital of Changde City (Hunan, China) between July 2020 and January 2024, was performed. The inclusion criteria were as follows: underwent intracranial aneurysm clipping surgery; confirmed diagnosis of postoperative ICI; and complete medical records, examination data, and treatment information. Exclusion criteria were as follows: preoperative diagnosis of ICI; concurrent severe organ failure; discharge, transfer, withdrawal from treatment, or death during the operation; and incomplete medical record data.
Data collection
In this study, basic patient information was collected within 24 h after admission, while clinical laboratory investigations were retrieved from the hospital’s electronic medical system and obtained at the time of ICI diagnosis. The collected clinical characteristics included age, length of hospital stay, sex, pneumonia, hypertension, diabetes, hyperlipidemia, coronary heart disease (CHD), external ventricular drainage (EVD), tracheotomy, and laboratory tests, including procalcitonin (PCT), Blood Lactic Acid (LaC), C-reactive protein (CRP), Bacterial Endotoxin Test (BET), albumin (Alb), blood urea nitrogen (BUN), creatinine (Cr), red blood cells (RBC), white bold cells (WBC), hemoglobin (Hb), neutrophil elastase (NE), and lymphocytes (LYM). Data entry was performed independently by 2 researchers. The dataset was validated and cleaned to ensure its accuracy and consistency. Once finalized, it was locked to prevent further modifications before statistical analysis.
Definition of ICI
A positive CSF culture is the gold standard for diagnosing ICI. When a CSF culture is negative, an ICI can be diagnosed if the following criteria are fulfilled: fever (postoperative body temperature > 38.5°C), with other systemic infections ruled out; neurological symptoms (exclusion of other causes accompanied by symptoms such as decreased consciousness, nausea, vomiting, positive signs of meningeal irritation) and positive pathological reflexes; laboratory findings (peripheral WBC count > 10 × 109/L; CSF findings include WBC count > 10 × 106/L, glucose < 2.25 mmol/L, protein > 0.45 g/L, and chloride, < 120 mmol/L) (14, 15).
Statistical analysis
Data were entered into a spreadsheet (Excel 2010, Microsoft Corp., Redmond, WA, USA), and statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria)1. Categorical data are expressed as percentage (%) and analyzed using either the chi-squared test or Fisher’s exact test. For continuous data, normally distributed variables are expressed as mean ± standard deviation (SD) and compared between groups using the t-test, while non-normally distributed variables are expressed as median (interquartile range [IQR], i.e., P25−P75) and analyzed using the Mann–Whitney U test. Logistic regression was used to identify independent risk factors for ICI following intracranial aneurysm surgery, with the results expressed odds ratio (OR) and corresponding 95% confidence interval (CI). The sample was randomly split in a 7: 3 ratio, with 70% used as the modeling group and 30% as the validation group. A nomogram was generated based on the predictive model formula for visual prediction. The predictive accuracy of the model for postoperative ICI was evaluated using receiver operating characteristic (ROC) curves. The discriminative ability of the model was assessed using the area under the ROC curve (AUC) and concordance index (C-index). Model fit, calibration, and clinical utility were evaluated using the Hosmer-Lemeshow goodness-of-fit test, calibration curves, and decision curve analysis (DCA), respectively.
Ethics approval
The study was approved by the Ethics Committee of the First People’s Hospital of Changde City (2024–025).
Results
Demographic data, univariate analysis, and Pearson correlation analysis
A total of 618 patients met the inclusion criteria. We excluded 2 patients with organ failure, and 4 patients with incomplete medical record data. Ultimately, a total of 612 patients were included (Figure 1). Among 612 patients who underwent surgery for intracranial aneurysms, 68 developed postoperative ICI, corresponding to an incidence rate of 11.1%. Most patients in the study population were elderly (mean [± SD] age, 60.55 ± 9.51 years) and female (55.4%). In terms of clinical characteristics, there was a statistically significant difference between the non-ICI group and the ICI group in terms of length of stay, sex, pneumonia, hypertension, hyperlipidemia, CHD, EVD, and tracheotomy (p < 0.05). The differences in age and diabetes status were not statistically significant (p > 0.05) (Table 1). According to Pearson correlation analysis, there was no significant correlation of ICI with Age, Diabetes, Cr, RBC, Hb, NE, and LYM. Regarding laboratory investigations comparing the non-ICI and ICI groups, univariate analysis revealed that PCT (p < 0.001), LaC (p < 0.001), CRP (p < 0.001), BET (p < 0.001), Alb (p < 0.001), BUN (p < 0.001), and WBC (p < 0.001) were associated with an increased odds of ICIs. However, there were no statistical differences between the 2 groups in terms of Cr, RBC, Hb, NE, or LYM (p > 0.05) (Table 1).
Figure 1

Study flow chart.
Table 1
| Variables | Total (n = 612) | No infection group (n = 544) | Infection group (n = 68) | χ 2/t/Z | Pearson value | p |
|---|---|---|---|---|---|---|
| Age | 60.55 ± 9.51 | 60.50 ± 9.67 | 60.99 ± 8.21 | −0.51 | 0.016 | 0.607 |
| Length of stay | 19.00 (13.00, 29.00) | 18.00 (12.00, 28.00) | 25.50 (20.75, 40.25) | −4.68 | 0.135** | <0.001 |
| Gender | 21.61 | 0.317** | <0.001 | |||
| Male | 273 (44.61) | 273 (50.18) | 40 (58.82) | |||
| Female | 339 (55.39) | 271 (49.82) | 28 (41.18) | |||
| Pneumonia | 71.12 | 0.341** | <0.001 | |||
| No | 377 (61.60) | 367 (67.46) | 10 (14.71) | |||
| Yes | 235 (38.40) | 177 (32.54) | 58 (85.29) | |||
| Hypertension | 20.51 | 0.183** | <0.001 | |||
| No | 265 (43.30) | 253 (46.51) | 12 (17.65) | |||
| Yes | 347 (56.70) | 291 (53.49) | 56 (82.35) | |||
| Diabetes | 2.01 | −0.057 | 0.156 | |||
| No | 523 (85.46) | 461 (84.74) | 62 (91.18) | |||
| Yes | 89 (14.54) | 83 (15.26) | 6 (8.82) | |||
| Hyperlipidemia | 11.38 | 0.136** | <0.001 | |||
| No | 504 (82.35) | 458 (84.19) | 46 (67.65) | |||
| Yes | 108 (17.65) | 86 (15.81) | 22 (32.35) | |||
| CHD | 7.81 | 0.113** | 0.005 | |||
| No | 559 (91.34) | 503 (92.46) | 56 (82.35) | |||
| Yes | 53 (8.66) | 41 (7.54) | 12 (17.65) | |||
| EVD | 24.47 | 0.200** | <0.001 | |||
| No | 508 (83.01) | 466 (85.66) | 42 (61.76) | |||
| Yes | 104 (16.99) | 78 (14.34) | 26 (38.24) | |||
| Tracheotomy | 20.09 | 0.181** | <0.001 | |||
| No | 480 (78.43) | 441 (81.07) | 39 (57.35) | |||
| Yes | 132 (21.57) | 103 (18.93) | 29 (42.65) | |||
| PCT | 0.41 (0.19, 0.64) | 0.37 (0.17, 0.58) | 0.89 (0.62, 1.08) | −10.39 | 0.509** | <0.001 |
| LaC | 8.89 (5.47, 11.81) | 9.69 (6.49, 12.15) | 1.12 (0.75, 1.46) | −13.45 | −0.650** | <0.001 |
| CRP | 33.73 (19.13, 48.38) | 33.29 (18.56, 47.33) | 43.27 (23.57, 57.29) | −3.32 | 0.153** | <0.001 |
| BET | 0.84 (0.34, 1.37) | 0.90 (0.52, 1.43) | 0.16 (0.14, 0.23) | −11.92 | −0.436** | <0.001 |
| Alb | 37.40 (32.53, 43.79) | 39.06 (34.36, 44.61) | 30.27 (27.96, 31.73) | −11.01 | −0.427** | <0.001 |
| BUN | 7.43 (5.26, 9.63) | 7.66 (5.41, 10.16) | 6.35 (3.94, 7.82) | −4.61 | −0.194** | <0.001 |
| Cr | 63.00 (47.00, 79.10) | 64.00 (47.00, 81.00) | 59.82 (52.07, 69.24) | −1.18 | −0.060 | 0.238 |
| RBC | 3.64 (3.02, 4.24) | 3.65 (2.99, 4.34) | 3.50 (3.22, 3.93) | −1.02 | −0.052 | 0.307 |
| WBC | 10.48 (8.24, 12.87) | 10.40 (8.00, 12.83) | 11.34 (9.84, 13.93) | −3.51 | 0.176** | <0.001 |
| Hb | 94.32 (87.03, 102.72) | 94.22 (86.65, 102.49) | 96.00 (89.00, 103.00) | −1.15 | 0.048 | 0.250 |
| NE | 8.57 (6.22, 11.01) | 8.48 (6.16, 11.00) | 9.06 (6.85, 11.12) | −1.19 | 0.047 | 0.235 |
| LYM | 1.38 (0.88, 1.90) | 1.39 (0.89, 1.91) | 1.32 (0.87, 1.78) | −0.96 | −0.041 | 0.335 |
Univariate analysis and Pearson correlation analysis results of the ICI following intracranial aneurysm surgery.
CHD, coronary heart disease; CRP, C-reactive protein; BET, Bacterial Endotoxin Test; Alb, albumin; PCT, Procalcitonin; LaC, Blood Lactic Acid; BUN, Blood Urea Nitrogen; Cr, creatinine; RBC, red blood cell; WBC, white blood cell; Hb, Hemoglobin; NE, neutrophilic granulocyte; LYM, lymphocyte.
χ 2, Chi-square test value; t, t-test value; Z, Mann–Whitney U test value.
** p < 0.01.
Multivariate analysis
All factors that were statistically significant (i.e., p < 0.05) in the univariate analysis were included in the logistic multivariate analysis, including length of hospital stay, sex, pneumonia, hypertension, hyperlipidemia, CHD, EVD, tracheotomy, PCT, LaC, CRP, BET, Alb, BUN, and WBC. A stepwise method was used to select the variables. Results revealed that pneumonia (OR 4.904 [95% CI 3.410–6.397]), EVD (OR 5.883 [95% CI 4.513–7.253]), tracheotomy (OR 5.983 [95% CI 4.619–7.348]), PCT (OR 4.332 [95% CI 3.048–5.616]), CRP (OR 6.862 [95% CI 5.127–8.597]), and Alb (OR 4.679 [95% CI 3.610–5.747]) were independent risk factors (Table 2). A risk prediction model was established for ICI based on these 6 predictors, which were independently associated with the odds of ICI according to logistic regression analysis.
Table 2
| Variable | β | SE | Wald χ2 | p | OR (95% CI) |
|---|---|---|---|---|---|
| Pneumonia | 5.942 | 2.323 | 2.561 | 0.010 | 4.904 (3.410 ~ 6.397) |
| EVD | 4.112 | 2.095 | 1.973 | 0.049 | 5.883 (4.513 ~ 7.253) |
| Tracheotomy | 1.789 | 0.669 | 6.607 | 0.002 | 5.983 (4.619 ~ 7.348) |
| PCT | 7.966 | 3.303 | 2.414 | 0.016 | 4.332 (3.048 ~ 5.616) |
| CRP | 0.096 | 0.047 | 2.242 | 0.025 | 6.862 (5.127 ~ 8.597) |
| Alb | −1.052 | 0.501 | −2.090 | 0.037 | 4.679 (3.610 ~ 5.747) |
Multivariate logistic regression analysis for ICI after intracranial aneurysm surgery.
β, regression coefficient; CI, confidence interval.
Nomogram establishment and evaluation
Based on the results of multivariate logistic regression analysis, 6 independent risk factors were identified as predictors of ICI after intracranial aneurysm surgery. A nomogram was developed using these predictors to provide a visual, user-friendly tool for risk prediction (Figure 2). Each predictor was assigned a score according to its regression coefficient, with higher scores reflecting a greater contribution to ICI risk. The total score was calculated by summing the individual scores for all predictors and mapping to the corresponding probability of ICI.
Figure 2
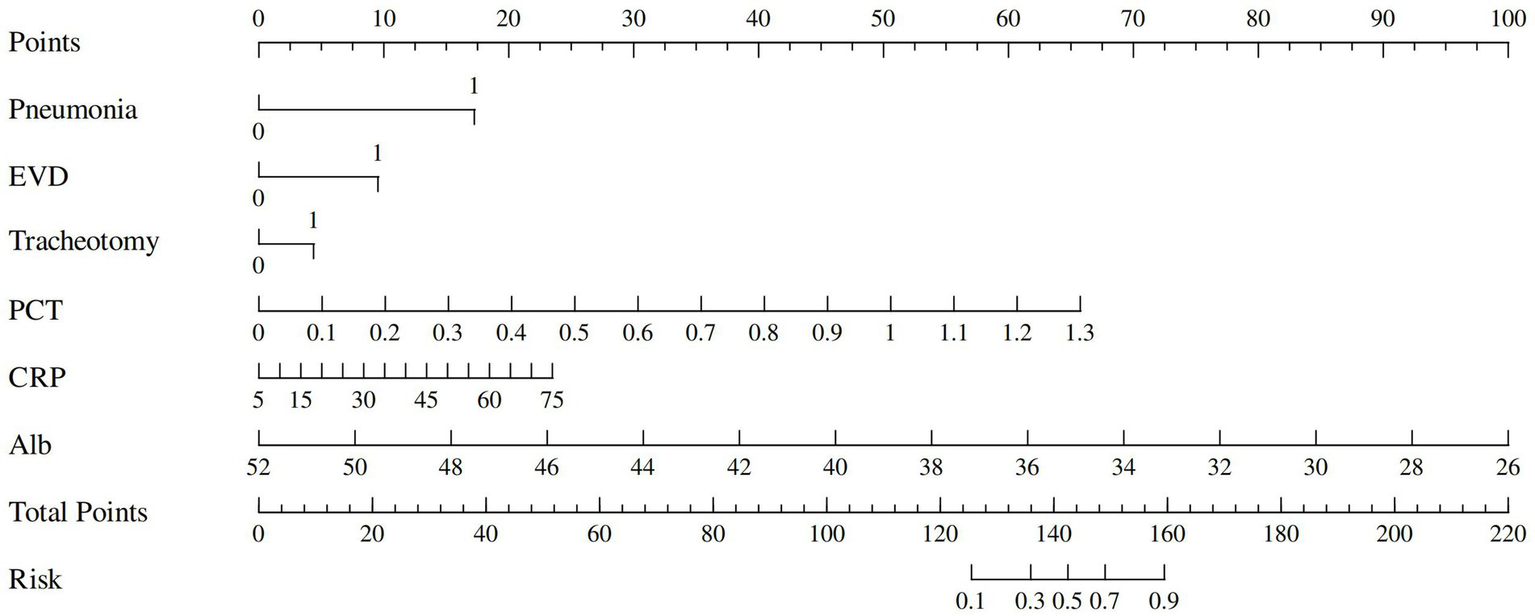
Nomogram for predicting risk of ICI.
The predictive performance of the nomogram was evaluated using the following metrics: discrimination, the discriminative ability of the nomogram was assessed according to the AUC and the concordance index (C-index). In the training cohort, the nomogram achieved an AUC of 0.91 (95% CI 0.88–0.93) (Figure 3A), indicating excellent discriminative ability. The validation cohort demonstrated similar performance, with an AUC of 0.89 (95% CI 0.84–0.93) (Figure 3B). Calibration was evaluated by plotting calibration curves that compared the predicted probability of ICI with the observed outcomes. Both the training (Figure 4A) and validation (Figure 4B) cohorts demonstrated good agreement between the predicted and actual probabilities, as reflected by the non-significant Hosmer-Lemeshow goodness-of-fit test results (p > 0.05). The clinical utility of the nomogram was analyzed using DCA (Figures 5A,B), which calculated the net benefit of using the model across a range of threshold probabilities.
Figure 3
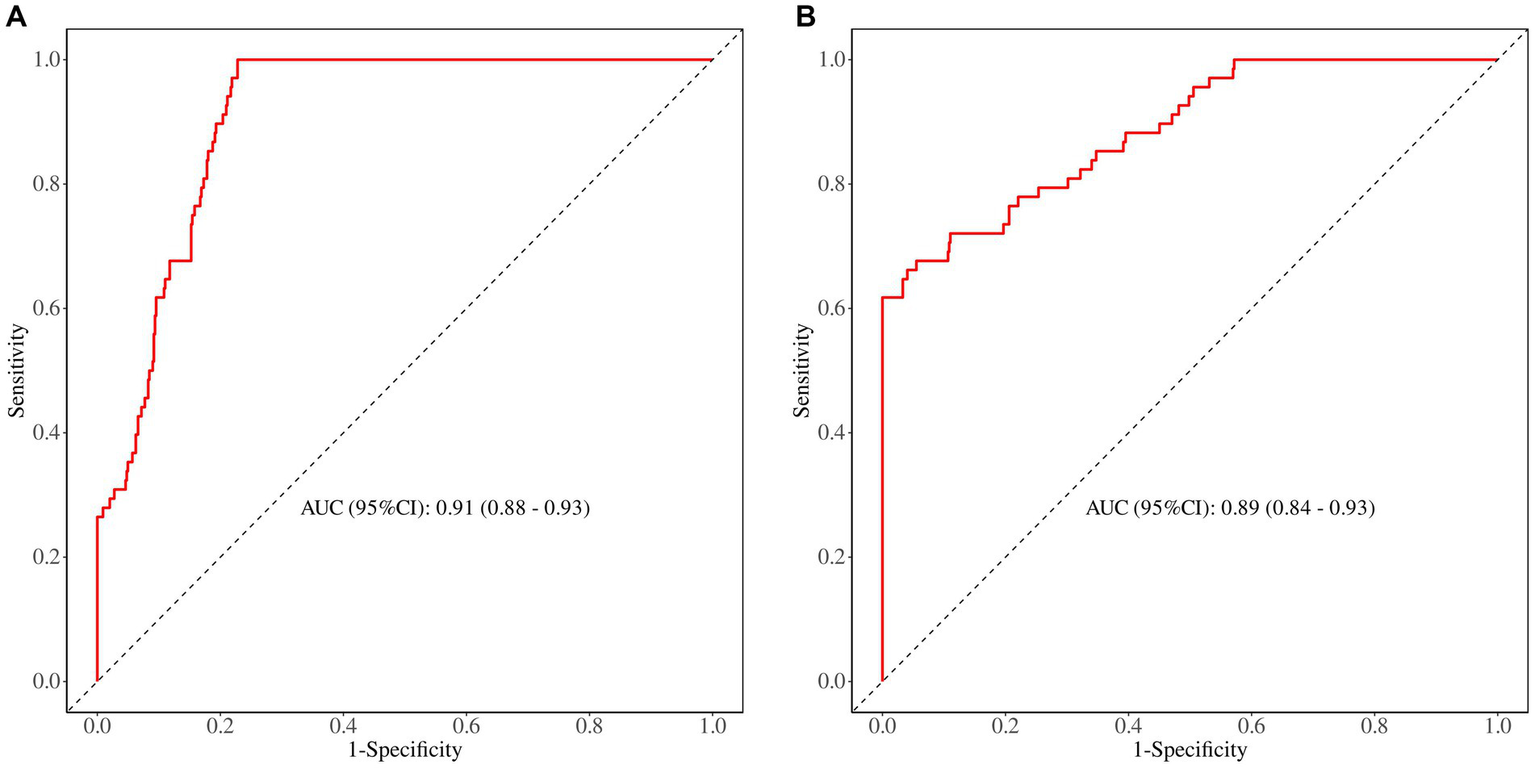
(A) The ROC curve in the training set. (B) The ROC curve in the validation set.
Figure 4
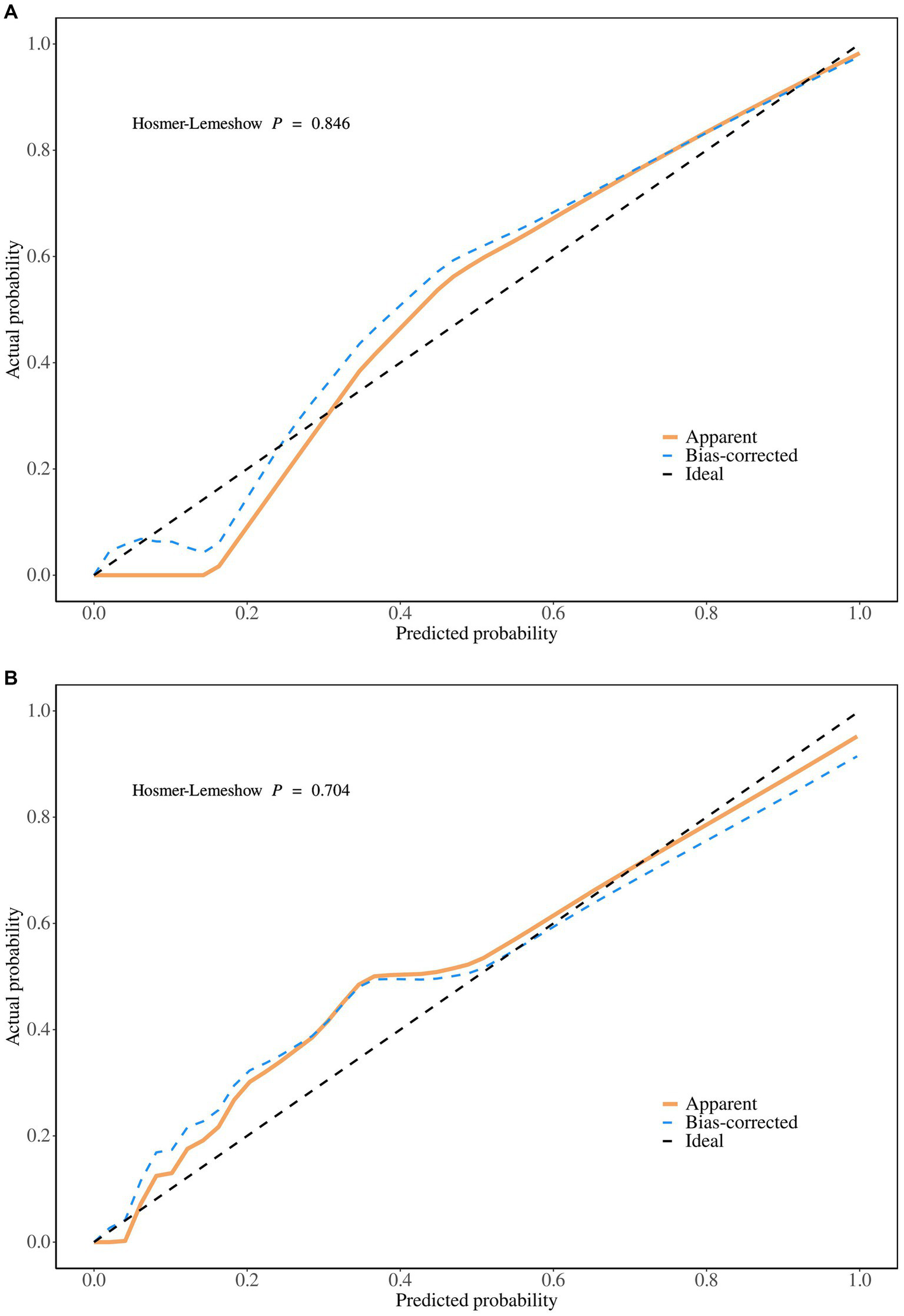
(A) The calibration plot in the training set. (B) The calibration plot in the validation set.
Figure 5
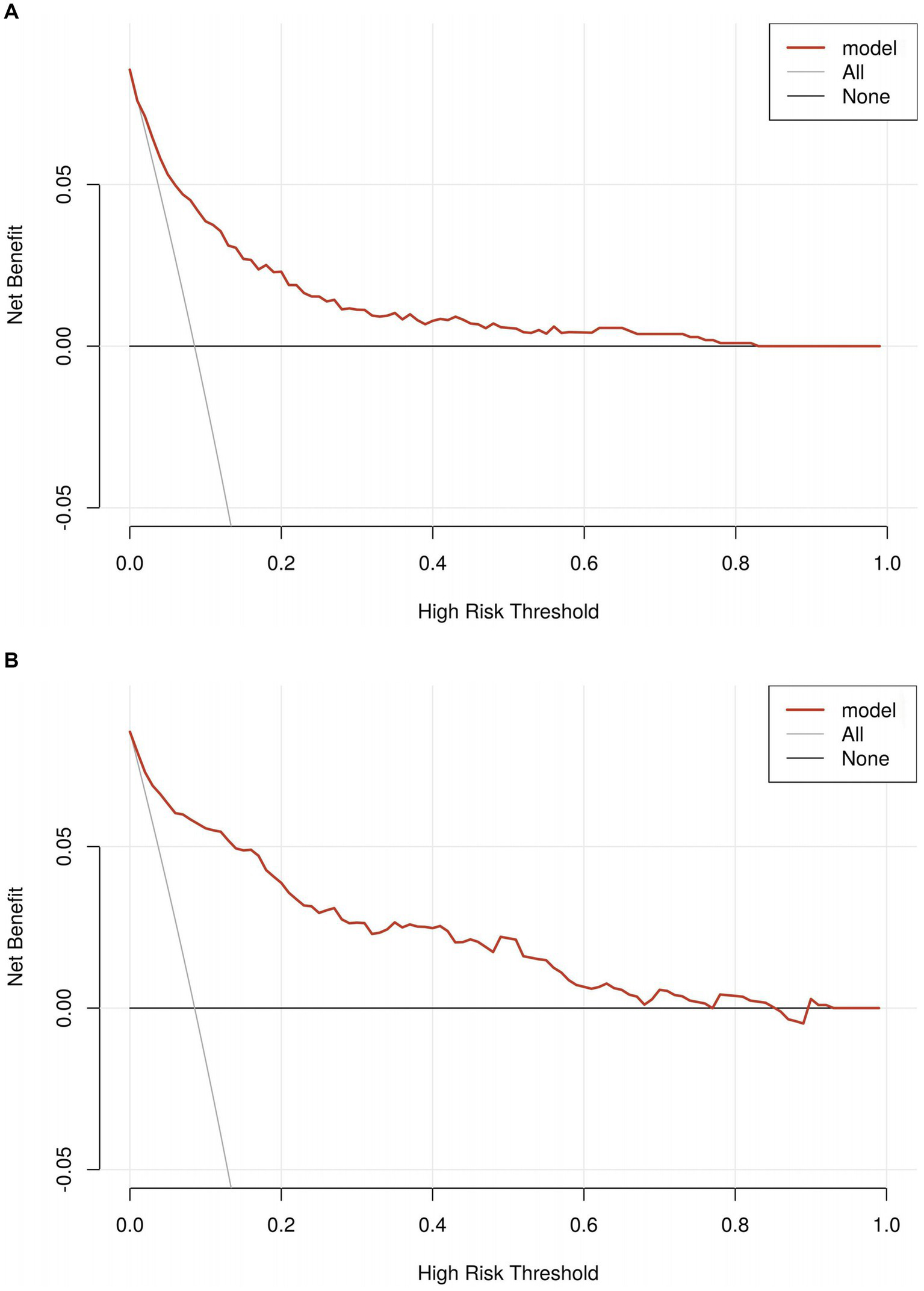
(A) The decision curve analyses in the training set. (B) The decision curve analyses in the validation set.
Discussion
ICI (s) significantly increase the risk for persistent complications in patients undergoing cerebral aneurysm surgery (16). These infections impose substantial burdens on patients and their families. Identifying and understanding the relevant risk factors are essential for effective prevention and management. To the best of our knowledge, this is the first nomogram model developed to predict ICI after intracranial aneurysm surgery, demonstrating excellent predictive performance. To validate the accuracy and reliability of the model, multiple indicators were used to comprehensively evaluate its predictive capability for ICI after intracranial aneurysm surgery. In our study, 6 independent risk factors for postsurgical ICI were identified using multivariate logistic regression analysis. These included EVD, pneumonia, tracheotomy, and PCT, CRP, and Alb levels. Based on these factors, a nomogram prediction model was developed. This model serves as a practical and accessible tool for clinicians, aiding in risk stratification and guiding targeted interventions to mitigate the likelihood of ICI (s).
Consistent with previous studies, our results indicated that the EVD was a significant risk factor for postoperative ICI in patients undergoing intracranial aneurysm surgery. EVD disrupts the natural barriers of the central nervous system, creating a direct pathway for pathogens to enter the CSF (17). Furthermore, the presence of an indwelling catheter in the sterile environment of the brain provides a surface for biofilm formation, which enhances bacterial adherence and resistance to antimicrobial treatment (s) (18). To mitigate the risk for ICI, strict adherence to aseptic techniques during EVD placement and maintenance is essential (19). Routine monitoring for early signs of infection, timely removal of the EVD once it is no longer necessary, and considering prophylactic antibiotic regimens should also be prioritized. By implementing these measures, the likelihood of postoperative ICI can be significantly reduced, thereby improving the outcomes of patients with intracranial aneurysms (20).
Pneumonia was a significant risk factor for postoperative ICI in patients undergoing intracranial aneurysm surgery, underscoring its crucial role as an independent predictor in the postoperative period. Pneumonia is often associated with prolonged intubation, mechanical ventilation, and/or impaired cough reflex due to neurological deficits, and can lead to systemic inflammation and hematogenous dissemination of pathogens (21). These mechanisms contribute to the seeding of infectious agents in the CSF or surgical site, increasing the likelihood of ICI (22). To address this, vigilant respiratory management in the perioperative and postoperative phases is critical. Strategies such as early weaning from mechanical ventilation, effective pulmonary hygiene, and prevention of aspiration are paramount (23). Additionally, timely identification and treatment of pneumonia through clinical and radiological assessment, along with the judicious use of antibiotics, can help mitigate the cascade of infections leading to ICI. Recognizing and managing pneumonia as a modifiable risk factor is vital for improving surgical outcomes in this patient population (24).
Tracheotomy is one of the most effective interventions for maintaining airway patency in neurocritical patients with coma, airway obstruction, and/or respiratory impairment (25). However, this procedure disrupts the physiological barrier of the airway mucosa, directly exposing the previously sterile lower airway to external pathogens. This creates a direct pathway for bacteria to enter the airways, thereby increasing the risk for infection. In patients requiring mechanical ventilation, procedures such as tracheal intubation or tracheotomy inherently compromise the airway’s protective barriers (26). Prolonged pressure exerted by the tubing and the endotracheal cuff can physically damage the airway mucosa, leading to edema, congestion, erosion, and even necrosis (27). The risk for infection increases with the duration of mechanical ventilation. Tracheotomy further increases the risk for respiratory infections due to increased air exposure, which may also contribute to severe complications, including ICI. These risks underscore the need for meticulous care and monitoring of tracheotomized patients to minimize infection-related complications.
Aside from clinical characteristics, laboratory investigations in patients with intracranial aneurysms also indicate that ICIs may be more severe. This study found that CRP, PCT, and Alb levels were associated with the occurrence of ICI. Elevated CRP levels often reflect an acute-phase response to tissue injury, systemic inflammation (28), or infection, and persistently high postoperative CRP levels can indicate an increased risk for the development of ICI. CRP promotes the recruitment of immune cells to sites of injury or infection, contributing to the local inflammatory response (29). However, excessive or prolonged inflammation can compromise the blood–brain barrier, facilitating the entry of pathogens into the central nervous system. Additionally, elevated CRP levels are often associated with systemic conditions, such as pneumonia or urinary tract infections, which can further predispose patients to secondary intracranial infections. Elevated postoperative PCT levels are strongly associated with an increased likelihood of ICI because they reflect the presence of bacterial pathogens and the activation of systemic immune defenses (30). Unlike other inflammatory markers, such as CRP, PCT levels rise more rapidly in response to bacterial infections and decline quickly after effective treatment, providing a dynamic measure of infection status (31). Persistently elevated or rising PCT levels in the postoperative period may indicate ongoing infection or inadequate response to treatment, necessitating further diagnostic and therapeutic interventions. Alb level has been identified as a significant risk factor for postoperative ICI in patients undergoing surgery for intracranial aneurysms, underscoring its pivotal role in immune competence and physiological stability. Hypoalbuminemia, commonly observed in surgical and critically ill patients, is strongly associated with increased susceptibility to infections, including those caused by ICI. Low serum Alb levels may reflect poor nutritional status, systemic inflammation, or severe physiological stress, all of which compromise the body’s ability to mount an effective immune response (32). Hypoalbuminemia has also been linked to impaired wound healing and disruption of the blood–brain barrier, increasing the likelihood of pathogen invasion into the central nervous system. In the postoperative period, hypoalbuminemia may arise from surgical stress, fluid shifts, or inadequate nutritional intake (33). Patients with low Alb levels often experience prolonged recovery times, higher rates of complications, and an increased risk for secondary infections. Therefore, assessing and optimizing Alb levels is crucial for reducing the risk associated with ICI.
To the best of our knowledge, this study is the first to comprehensively explore risk factors and develop a predictive model for ICIs following intracranial aneurysm surgery. Notably, we constructed a robust nomogram that accurately estimated the likelihood of postoperative ICIs, offering an essential tool for early risk assessment and targeted clinical management. The nomogram demonstrated robust predictive accuracy, good calibration, and significant clinical utility, making it a valuable tool for personalized risk assessment of ICI following intracranial aneurysm surgery. It supports individualized risk evaluation and enhances clinical decision-making, ultimately improving patient outcomes.
Limitations
This study has several limitations. First, the nomogram prediction model lacked external validation. To enhance reliability and generalizability, multicenter studies should be conducted to gather external datasets for validation, which would significantly bolster the credibility of the model. Second, findings from this investigation may have limited applicability to broader populations. Increasing the sample size and incorporating data from multiple centers would improve the generalizability of the model. Additionally, prolonged operative duration can contribute to higher infection risk due to extended tissue exposure, increased blood loss, and prolonged mechanical ventilation. We acknowledge that larger or more complex aneurysms may necessitate prolonged surgical duration, extensive tissue manipulation, and increased use of surgical adjuncts, all of which could indirectly elevate ICI risk. While we did not include these variables in our final predictive model, future studies should explore their role in infection risk stratification to enhance the accuracy and clinical applicability of ICI prediction. Furthermore, a key limitation is that all variables in the current nomogram were obtained postoperatively, limiting its value for preoperative risk assessment. Future studies should incorporate preoperative factors such as HH grade, aneurysm size and location, surgical duration, intraoperative rupture, and antibiotic use to improve early prediction and clinical utility. As such, to refine and validate the model further, a multicenter prospective study with a larger sample size is essential. Such efforts would help address potential confounding biases, enable identification of additional relevant risk factors and ultimately produce a more accurate and reliable predictive tool.
Conclusion
The prediction nomogram model, based on 6 common clinical and laboratory test variables (pneumonia, EVD, tracheotomy, PCT, CRP, and Alb), was able to easily and accurately predict ICI after intracranial aneurysm surgery. The nomogram developed was a robust and practical tool for predicting the risk for ICI after intracranial aneurysm surgery. It demonstrated strong predictive accuracy and calibration with potential applications in identifying high-risk patients and guiding individualized preventive strategies. However, validation in a broader population is recommended to enhance the generalizability of the model.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by First People’s Hospital of Changde City. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Informed consent was waived due to the retrospective nature of the study, and patient data were anonymized for analysis.
Author contributions
YY: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. YT: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. YG: Writing – original draft, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Hunan Provincial Natural Science Foundation Of China (2024JJ7006, 2025JJ80420).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1.
Nafees Ahmed S Prakasam P . A systematic review on intracranial aneurysm and hemorrhage detection using machine learning and deep learning techniques. Prog Biophys Mol Biol. (2023) 183:1–16. doi: 10.1016/j.pbiomolbio.2023.07.001
2.
Goldberg J Schoeni D Mordasini P Z'Graggen W Gralla J Raabe A et al . Survival and outcome after poor-grade aneurysmal subarachnoid hemorrhage in elderly patients. Stroke. (2018) 49:2883–9. doi: 10.1161/strokeaha.118.022869
3.
Lv X Wu Z Qu RB Jin H . Endovascular treatment of cerebral aneurysms during acute (<72 hours) subarachnoid hemorrhage. J Neurol Sci. [Turkish] (2012) 29:535–541.
4.
Tawk RG Hasan TF D'Souza CE Peel JB Freeman WD . Diagnosis and treatment of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. Mayo Clin Proc. (2021) 96:1970–2000. doi: 10.1016/j.mayocp.2021.01.005
5.
Grasso G Torregrossa F Cohen-Gadol AA . Avoiding complications in aneurysm ligation: operative tips and tricks. World Neurosurg. (2022) 159:259–65. doi: 10.1016/j.wneu.2021.10.181
6.
Salmanov AG Shchehlov DV Mamonova M Svyrydiuk OE Bortnik IM Chabanovych NB et al . Healthcare-associated infections in postoperative patients with intracranial aneurysm in Ukraine. Pol Merkur Lekarski. (2024) 52:137–44. doi: 10.36740/Merkur202402101
7.
Schwartz C Lenski M Romagna A Schichor C Tonn JC Brueckmann H et al . Diffusion-weighted magnetic resonance imaging for detection of postoperative intracranial pyogenic abscesses in neurosurgery. Acta Neurochir. (2019) 161:985–93. doi: 10.1007/s00701-019-03875-8
8.
Alawieh A Chaudry MI Turner RD Turk AS Spiotta AM . Infectious intracranial aneurysms: a systematic review of epidemiology, management, and outcomes. J Neurointerv Surg. (2018) 10:713–21. doi: 10.1136/neurintsurg-2017-013603
9.
Radwan W Sawaya R . Intracranial haemorrhage associated with cerebral infections: a review. Scand J Infect Dis. (2011) 43:675–82. doi: 10.3109/00365548.2011.581304
10.
Tehli GY Kirmizigoz S Durmaz MO Ezgu MC Tehli O . Risk factors and surgical treatment options for intracranial infections. Turk Neurosurg. (2023) 33:308–17. doi: 10.5137/1019-5149.Jtn.40387-22.4
11.
Abdalkader M Xie J Cervantes-Arslanian A Takahashi C Mian AZ . Imaging of intracranial infections. Semin Neurol. (2019) 39:322–33. doi: 10.1055/s-0039-1693161
12.
Guo X Fang J Wu Y . Risk factors of intracranial infection in patients after intracranial aneurysm surgery: implication for treatment strategies. Medicine. (2021) 100:e27946. doi: 10.1097/md.0000000000027946
13.
Balachandran VP Gonen M Smith JJ DeMatteo RP . Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/s1470-2045(14)71116-7
14.
Shi ZH Xu M Wang YZ Luo XY Chen GQ Wang X et al . Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. (2017) 31:5–9. doi: 10.1080/02688697.2016.1253827
15.
Kannoth S Thomas SV . Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care. (2009) 11:120–9. doi: 10.1007/s12028-009-9208-x
16.
Nonaka S Oishi H Tsutsumi S Teranishi K Tanoue S Yasumoto Y et al . Endovascular therapy for infectious intracranial aneurysm: a report of four cases. J Stroke Cerebrovasc Dis. (2016) 25:e33–7. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.033
17.
Sorinola A Buki A Sandor J Czeiter E . Risk factors of external ventricular drain infection: proposing a model for future studies. Front Neurol. (2019) 10:226. doi: 10.3389/fneur.2019.00226
18.
Li S Wang P Tian S Zhang J . Risk factors and cerebrospinal fluid indexes analysis of intracranial infection by Acinetobacter baumannii after neurosurgery. Heliyon. (2023) 9:e18525. doi: 10.1016/j.heliyon.2023.e18525
19.
Huang TF Su YK Su IC Yeh YK Liu HW Kan IH et al . Risk, predictive, and preventive factors for noninfectious Ventriculitis and external ventricular drain infection. Neurocrit Care. (2024) 41:109–18. doi: 10.1007/s12028-023-01925-9
20.
Kelemen J Sztermen M Dakos E Agocs G Budai J Katona J et al . Risk assessment and recommended approaches to optimize infection control and antibiotic stewardship to reduce external ventricular drain infection: a single-center study. Antibiotics. (2024) 13:1093. doi: 10.3390/antibiotics13111093
21.
Lusquinhos J Tavares M Abelha F . Postoperative pulmonary complications and perioperative strategies: a systematic review. Cureus. (2023) 15:e38786. doi: 10.7759/cureus.38786
22.
Martin-Loeches I Rodriguez AH Torres A . New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr Opin Crit Care. (2018) 24:347–52. doi: 10.1097/mcc.0000000000000535
23.
Croke L . Preventing postoperative pulmonary complications. AORN J. (2022) 116:P7–p10. doi: 10.1002/aorn.13840
24.
Haines KL Agarwal S . Postoperative pulmonary complications-a multifactorial outcome. JAMA Surg. (2017) 152:166–7. doi: 10.1001/jamasurg.2016.4102
25.
Zhu H Das P Brereton J Roberson D Shah RK . Surveillance and management practices in tracheotomy patients. Laryngoscope. (2012) 122:46–50. doi: 10.1002/lary.22375
26.
Ilan O Gross M Zaltzman Y Sasson A Marcus EL . Diagnosis and conservative management of late tracheotomy complications in chronic ventilator-dependent patients. Head Neck. (2015) 37:716–21. doi: 10.1002/hed.23665
27.
Hosokawa K Nishimura M Egi M Vincent JL . Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. (2015) 19:424. doi: 10.1186/s13054-015-1138-8
28.
Rizo-Téllez SA Sekheri M Filep JG . C-reactive protein: a target for therapy to reduce inflammation. Front Immunol. (2023) 14:1237729. doi: 10.3389/fimmu.2023.1237729
29.
Yin P Fan Y Dong W Shao S Zhu J Zhu X et al . The value of CD64 in the early diagnosis for intracranial infection after Craniocerebral surgery. World Neurosurg. (2023) 176:e1–7. doi: 10.1016/j.wneu.2022.11.007
30.
Yu Y Li HJ . Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz J Med Biol Res. (2017) 50:e6021. doi: 10.1590/1414-431x20176021
31.
Wang H Zhou C Fu Y . Factors influencing procalcitonin in the cerebrospinal fluid of patients after neurosurgery and its diagnostic value for intracranial infection. BMC Neurol. (2023) 23:288. doi: 10.1186/s12883-023-03339-8
32.
Fu P Zhang Y Zhang J Hu J Sun Y . Prediction of intracranial infection in patients under external ventricular drainage and neurological intensive care: a multicenter retrospective cohort study. J Clin Med. (2022) 11:3973. doi: 10.3390/jcm11143973
33.
Sun M Gu L Li Y Luo F Tao Y Shen W et al . Procalcitonin/albumin ratio predicts the outcome after severe traumatic brain injury: a propensity score-matched analysis. Neurocrit Care. (2024) 40:664–73. doi: 10.1007/s12028-023-01792-4
Summary
Keywords
nomogram, predict, infect, intracranial, intracranial aneurysm surgery
Citation
Yang Y, Tang Y and Gong Y (2025) Development and validation of a nomogram for predicting intracranial infection after intracranial aneurysm surgery. Front. Neurol. 16:1563848. doi: 10.3389/fneur.2025.1563848
Received
22 January 2025
Accepted
19 May 2025
Published
02 June 2025
Volume
16 - 2025
Edited by
Michael L. James, Duke University, United States
Reviewed by
Xianli Lv, Tsinghua University, China
Sricharan Veeturi, University at Buffalo, United States
Updates
Copyright
© 2025 Yang, Tang and Gong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youwen Gong, 1755144149@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.