- 1Second Clinical Medical College, Yunnan University of Chinese Medicine, Kunming, Yunnan, China
- 2School of Basic Medical Sciences, Yunnan University of Chinese Medicine, Kunming, Yunnan, China
Introduction: Tourette Syndrome (TS), a complex neurodevelopmental disorder, has seen a substantial increase in research activity, yet a systematic bibliometric analysis elucidating the global research landscape remains lacking. This study therefore employs bibliometric methods to comprehensively examine the evolution of TS research trends, international collaboration patterns, core contributors, and research hotspots, thereby providing a scientific foundation for future research directions and policy development.
Methods: Based on the Web of Science Core Collection, a topic-based search strategy yielded 4,011 records (1960–2024). Bibliometric analyses were performed using R software and VOSviewer, incorporating annual publication trends, geographical distribution, journal impact metrics (impact factor and H-index), core author collaboration networks, and keyword co-occurrence mapping to assess the structure and dynamics of the research ecosystem.
Results: The bibliometric analysis encompassed 4,011 publications involving 12,860 authors and 5,524 keywords. TS research exhibited a phased growth pattern. Psychiatry, psychology, and neurosciences & neurology emerged as the dominant research domains. While the United States remained the primary contributor, European countries—particularly the United Kingdom, Germany, and Denmark—demonstrated superior international collaboration. Movement Disorders proved the most productive journal, whereas JAMA Psychiatry held the greatest impact. Leading contributors such as Dr. James F. Leckman and institutions including Yale University showed exceptional research productivity. Over time, research themes have shifted from early emphases on genetics and neuroimaging to recent focuses on patient quality of life and precision interventions, reflecting a trend toward interdisciplinary integration and clinical translation.
Conclusion: Tourette syndrome (TS) research has evolved from descriptive analyses to multidisciplinary integration, yet requires enhanced cross-regional collaboration and application of emerging technologies. Future efforts should prioritize elucidating gene–environment interaction mechanisms, advancing AI-assisted diagnostics, and refining personalized treatment strategies. Concurrently, bridging regional research disparities through global alliances and standardized data platforms is imperative to ensure that scientific discoveries are translated into clinical and societal benefits. Study limitations regarding potential language and database biases underscore the importance of inclusive methodologies in subsequent investigations.
1 Introduction
Tourette Syndrome (TS) is a neurodevelopmental disorder characterized by persistent motor and vocal tics for at least 1 year, often accompanied by comorbid neuropsychiatric conditions such as Attention Deficit/Hyperactivity Disorder (ADHD) and Obsessive-Compulsive Disorder (OCD), which complicate diagnosis and management (1–4). The disorder typically manifests between the ages of 2 and 21, with symptom severity peaking in early adolescence. The epidemiology of TS is more complex than previously believed; it was once considered a rare disorder, and some even labeled it as “psychogenic” (5). The prevalence of TS varies depending on its definition, diagnostic criteria, and the methods used in epidemiological studies. While many individuals experience a reduction in tic severity during late adolescence, a significant proportion continue to face persistent tics and associated psychosocial challenges into adulthood (6, 7). TS has a profound impact on academic performance, employment prospects, and quality of life, leading to substantial social and economic burdens, which underscores the need for continued research in this field (8, 9).
Bibliometrics, first introduced by Pritchard in 1969 (10), has become a crucial tool for evaluating interdisciplinary research trends and academic impact. This quantitative approach analyzes publications, citations, and collaboration networks to reveal the structure and dynamics of research fields. Bibliometric tools such as VOSviewer (11) and R-bibliometrix (12) enable the visualization of key themes, influential authors, and emerging trends, offering valuable insights into the evolution of scientific knowledge. In the fields of neuroscience and psychiatry, bibliometric analyses have successfully elucidated the research landscapes of disorders such as autism spectrum disorder (ASD) (13) and Attention Deficit/Hyperactivity Disorder (ADHD) (14), guiding future research directions.
Although research on TS has grown significantly, comprehensive bibliometric analyses that provide an integrated overview of the global research landscape remain limited (15). Most existing studies focus on specific areas, such as pharmacological interventions or genetic research, without offering a synthesis of broader research trends and knowledge gaps (16, 17). Given the importance of bibliometric analyses in mapping research landscapes and identifying emerging themes (18, 19), there is a clear need for a comprehensive bibliometric study on TS to guide future research directions and foster collaboration. To address this gap, this study conducts a bibliometric analysis of TS-related publications spanning 1960 through 2024, with particular emphasis on publication trends, leading contributors, and emerging research hotspots. By offering a detailed overview of the current state of TS research, this paper aims to highlight opportunities for innovation and guide future scientific exploration.
2 Methods
2.1 Literature retrieval strategy
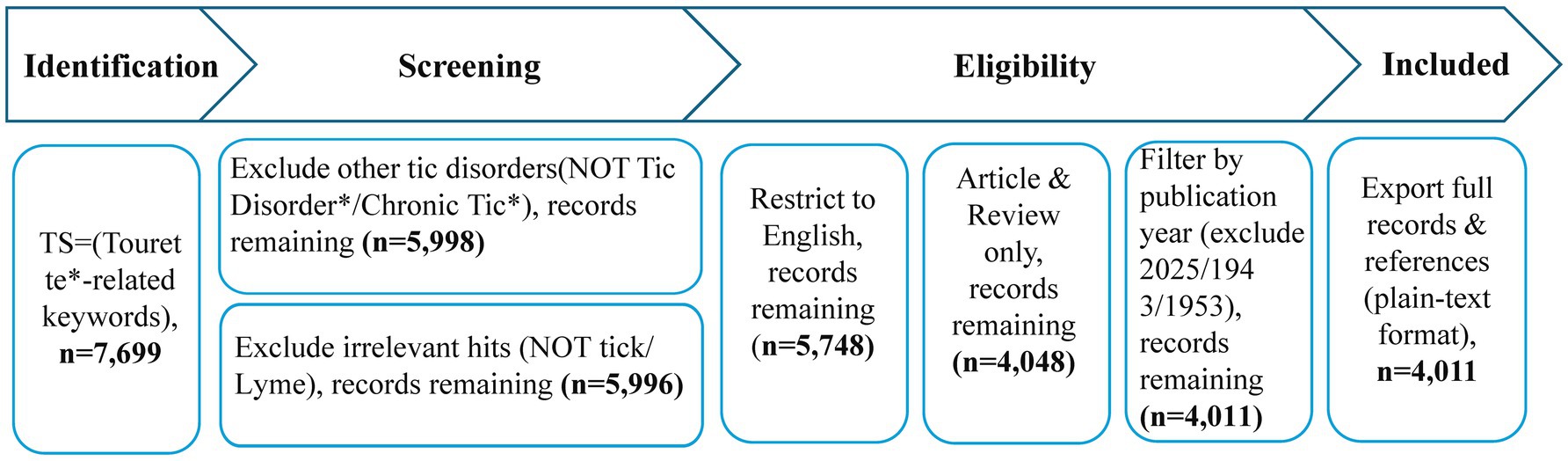
Selecting an appropriate data source is critical for bibliometric analysis. The Web of Science (WOS) database is widely recognized as a premier choice for bibliometric studies due to its extensive coverage, high-quality indexing, and robust citation analysis capabilities (20). This study employs bibliometric methods to map the global TS literature. On April 30, 2025, we retrieved 7,699 records from the Web of Science Core Collection using a targeted query—TS = (“Tourette* Syndrome” OR “Gilles de la Tourette” OR “motor tic*” OR “vocal tic*” OR “coprolalia”)—to focus on core TS terminology and avoid dilution by broader “tic disorder” entries. We then excluded non-TS tic disorders (“Tic Disorder,” “Chronic Tic”), applied a filter to remove misleading terms (“tick,” “Lyme”), and restricted results to English-language articles and review articles to ensure consistency and peer-reviewed quality. Next, we discarded 35 records from the incomplete year 2025 and two isolated entries from 1943 and 1953 (Data verification revealed that the literature records from 1943 and 1953 exhibited a 100% missing rate for the following fields: Abstract, Corresponding Author, Author Keywords, and Keywords Plus). By including publications from 1960 to 2024, the bibliometric analysis offers a comprehensive, continuous, and systematic view of the long-term trends and evolutionary phases in TS research.
Through multiple iterations of search strategy optimization, a balance between recall and precision was ensured. All data cleaning steps (such as criteria for record exclusion) were transparently presented in a flowchart (Figure 1), enhancing methodological reproducibility. The refined dataset comprised 4,011 records, all exported in “Plain Text” format with “Full Records and Cited References” for downstream bibliometric analysis.
2.2 Bibliometric analysis
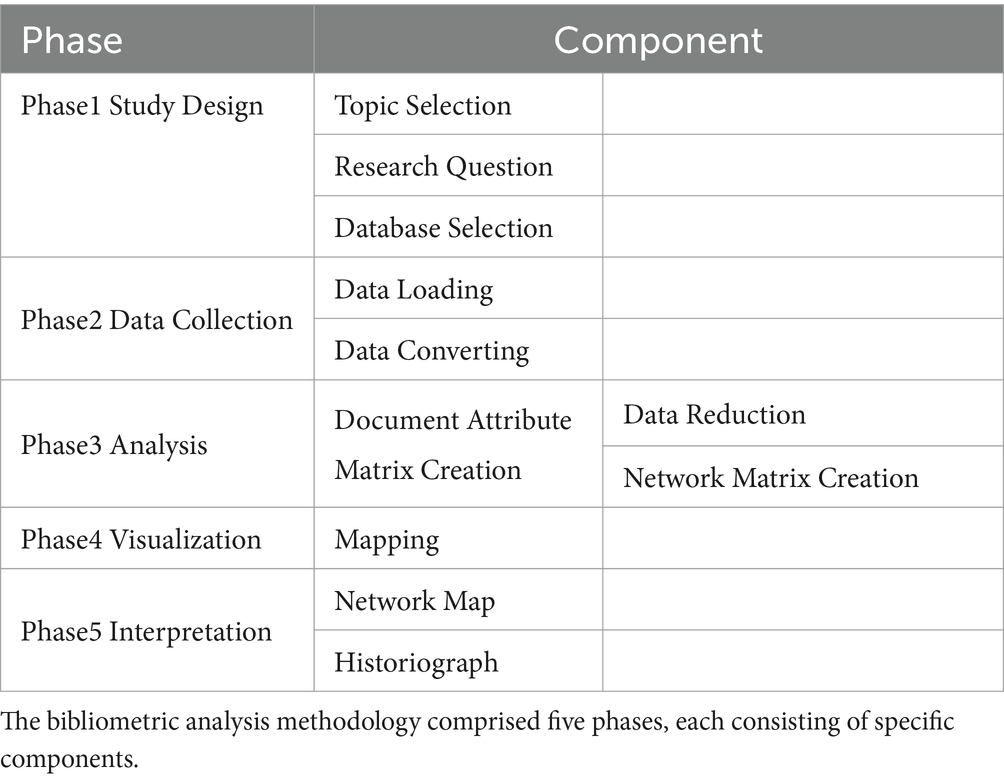
Bibliometric analysis typically follows a structured five-step approach, including study design, data collection, analysis, visualization, and interpretation (12). Table 1 illustrates this methodological framework, which ensures a systematic and comprehensive analysis of research data.
In the study design phase, we selected TS as the research topic and chose the Web of Science (WOS) Core Collection as the data source. During the data collection phase, we performed a comprehensive and precise search using a specific search formula in WOS, ensuring scientific rigor and reliability by selecting peer-reviewed articles and reviews, which yielded 4,011 documents.
No duplicates were identified due to the high-quality indexing of the Web of Science Core Collection, which ensures unique records. All records were imported using Biblioshiny Web and converted into Bibliometrix R data and Excel formats for further analysis. Missing values in the exported Excel spreadsheet (including cited references, author affiliations and countries, DOIs, and journal impact factors) were filled, and information on countries, institutions, and journals was extracted to assess their impact. Additionally, key bibliometric indicators such as publication year, publication count, author count, and keyword count were extracted using Biblioshiny, which facilitated the generation of descriptive statistics. Moreover, data on national publication output, collaboration patterns, and high-frequency keyword trends were exported for subsequent visualization of relevant thematic maps. Tidyverse (ggplot2) was employed to create high-quality graphics and visualizations for the temporal evolution and key themes in TS research. Additionally, VOSviewer was used to construct co-citation networks, keyword co-occurrence networks, and collaboration networks, revealing key themes and collaboration patterns within the research field.
We applied Bradford’s law to classify journals in the TS field into “core” and “dispersed” zones (21). Journals were ranked in descending order by their number of published articles, and the cumulative article count (4,011 articles across 991 journals) was calculated. The total corpus was then partitioned into three equal segments of approximately 1,337 articles each. The smallest set of journals whose combined output reached the first segment was defined as the core zone (Zone 1); the next set of journals accounting for the second segment comprised Zone 2; and the remaining journals constituted the peripheral zone (Zone 3). In our analysis, the core zone comprised 36 journals, which is consistent with the minimal core size predicted by Bradford’s distribution. This threshold-based approach allowed us to identify a small set of highly productive core journals and to quantify the contributions of the larger body of less-productive journals.
The results of our data analysis and visualization, including insights from Bradford’s law, will be detailed in the subsequent sections. This study employed a robust methodology to ensure the validity of bibliometric insights and enhance our understanding of global trends in TS research.
3 Results
The preliminary findings of the bibliometric analysis offer a detailed overview of the current landscape in TS research. After applying document type filters and excluding irrelevant records, a total of 4,011 papers published between 1960 and 2024 were included in the study. The analysis examined publication trends, geographical distribution, journal contributions, author influence, and keyword patterns, providing insights into the developmental trajectory, global distribution, and key research areas within TS. Detailed discussions of these aspects, including the methods and tools used for analysis, are provided in the following sections.
3.1 Descriptive bibliometric analysis
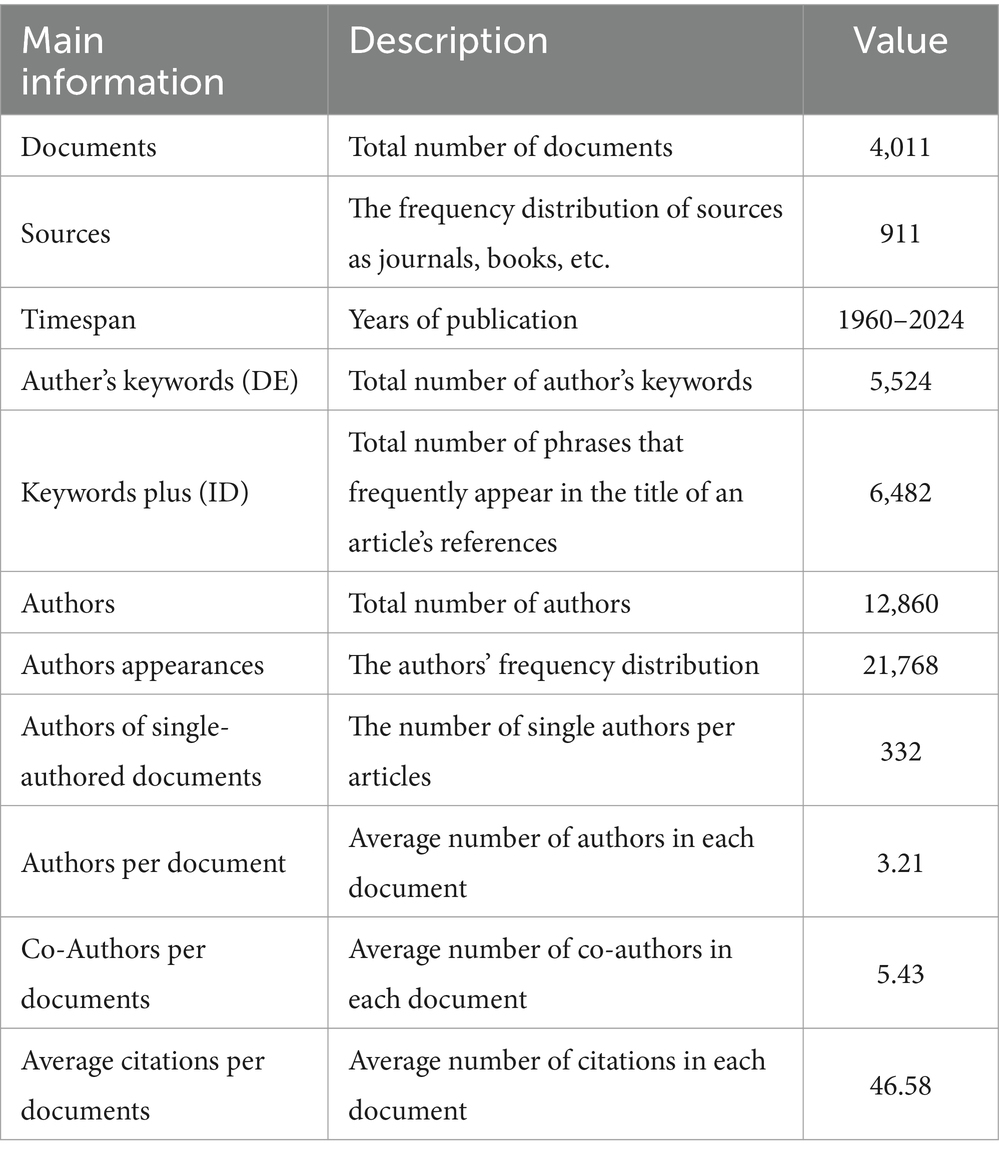
The descriptive analysis provides a comprehensive overview of the research landscape on TS (Table 2), encompassing 4,011 documents published between 1960 and 2024, reflecting a mature yet evolving field. These studies are disseminated across 911 diverse sources, indicating a broad and interdisciplinary approach. The analysis identifies 5,524 author keywords and 6,482 Keywords Plus from references, highlighting the wide range of subtopics and methodologies explored in TS research. The collaborative nature of the field is evident, with an average of 3.21 authors and 5.43 co-authors per document, suggesting a robust and interconnected research community. Furthermore, the average citation rate of 46.58 citations per document underscores the significant influence and recognition of TS research within the academic community.
3.2 Publication trends
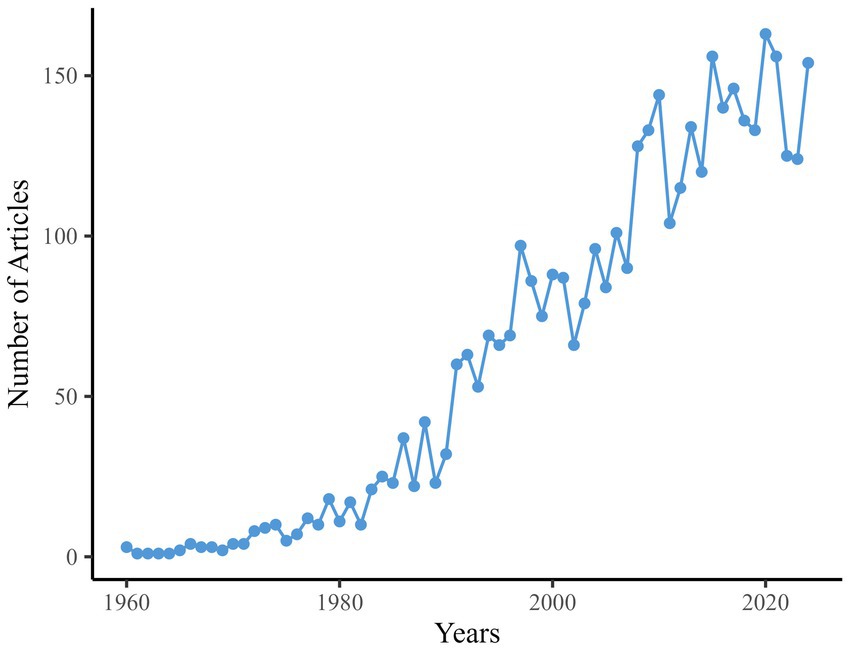
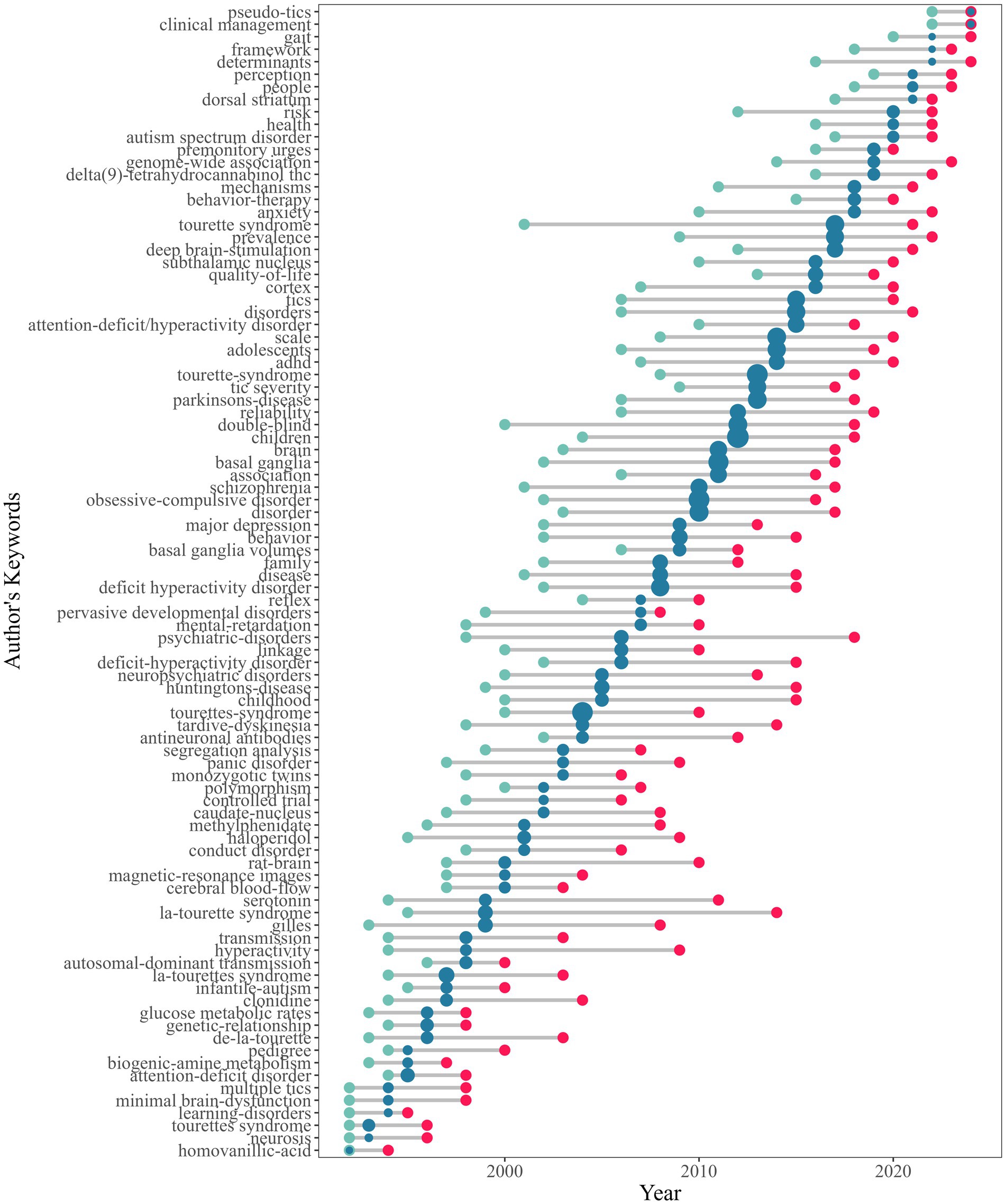
Figure 2 delineates the annual publication trends in TS research. Output remained minimal (≤4 articles/year) throughout the 1960s, first reaching double digits in 1974 (10 articles). Steady growth characterized the 1980s, peaking at 42 articles in 1988. A pronounced acceleration occurred during the 1990s, culminating in 97 articles by 1997. The early 2000s saw fluctuations between 66 and 101 annual publications, followed by sustained expansion from 2008 onward, with output exceeding 120 articles/year after 2013 and reaching its zenith in 2020 (163 articles). A transient decline to 124 articles in 2023 preceded a rebound to 154 in 2024. This pattern of escalating output, punctuated by periodic milestones, aligns with the developmental trajectory of an evolving research domain.
3.3 Research areas
Following the classification framework of Clarivate Analytics, each paper in the Web of Science (WOS) database is allocated to one or more research areas. Figure 3A illustrates the temporal evolution of TS research areas, showing an expansion from 2 fields in 1960 to 45 fields in 2024. Figure 3B presents the top 10 most productive research domains in TS research, including behavioral sciences, biochemistry and molecular biology, general and internal medicine, genetics and heredity, neurosciences and neurology, pediatrics, pharmacology and pharmacy, psychiatry, psychology, and surgery. These fields constitute 91.25% of the total TS-related publications (3,660 out of 4,011). Over the period from 1960 to 2024, psychiatry, psychology, and neurosciences and neurology consistently emerged as the leading research areas, with neurosciences and neurology achieving its highest publication volume in 2015 (n = 91).
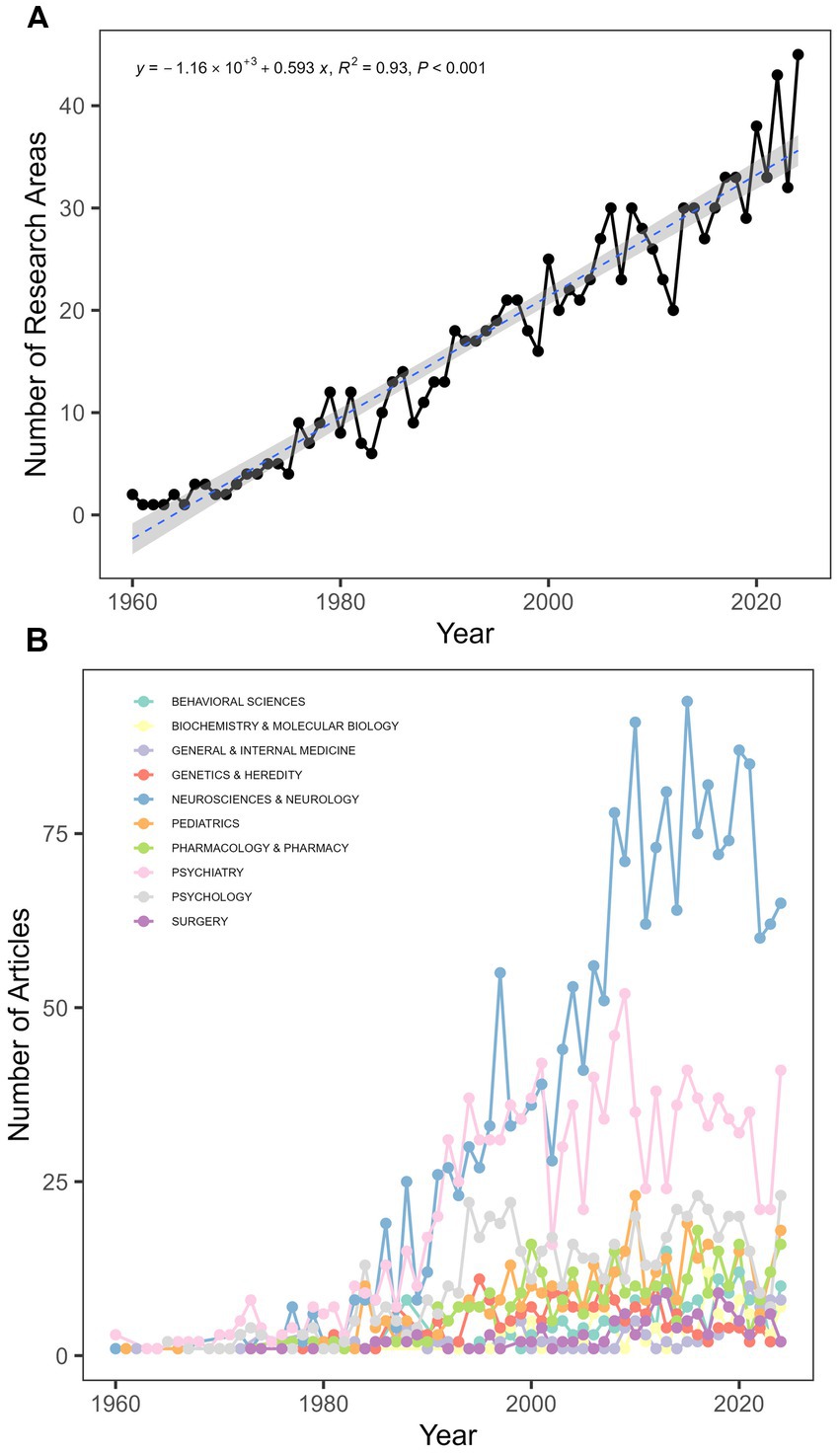
Figure 3. Temporal evolution and productivity of TS research areas. (A) Temporal evolution of research areas; (B) Top 10 most productive research areas.
3.4 Research countries
The research findings indicate that since 1960, a total of 61 countries have participated in studies on TS, with the United States consistently leading in publication numbers and maintaining its dominant position (Figure 4). The top five countries in terms of scientific output are the United States (n = 1,597), the United Kingdom (n = 330), Germany (n = 272), China (n = 224), and Canada (n = 193). After analyzing the data, we assessed the research performance of the top 10 countries, including their research cooperation models, total citations, and average citations per article (Figure 5). The first part presents the research cooperation types of each country, including SCP (Single Country Publication) and MCP (Multinational Cooperative Publication). The United States, with the highest number of publications (n = 1,597), primarily consists of single-country collaborations (n = 1,408, 88.2%). In contrast, European nations exhibit higher proportions of multinational collaborations (MCP), with the United Kingdom (32.4%), Germany (34.6%), Italy (33%), France (33%), and particularly the Netherlands (35%) demonstrating the most prominent engagement patterns. These elevated MCP rates collectively signify stronger intra-regional academic collaboration trends within Europe. The average citation count per article reflects the quality and influence of each country’s research outcomes. United States has the highest average citation count (n = 62.6), followed by the Netherlands (n = 53.6) and the United Kingdom (n = 52.9). The United States leads in total citations with 99,894, demonstrating its absolute advantage in research. The United Kingdom and Germany rank second and third with 17,446 and 11,259 citations, respectively. Notably, despite China’s high publication output of 224 articles, its total citation count is relatively low at 3,722, with an average of 16.6 citations per article, highlighting a gap in international influence compared to research powerhouses in Europe and America. In terms of global collaboration (Figure 6), the United States has the highest number of international collaborations with 54 connections. The United Kingdom follows closely with 48 connections, while Germany ranks third at 43. Canada and the Netherlands each demonstrate 39 collaborations, and Italy completes the network with 37 connections.
Figure 4. Annual publication trends by country. Publication counts are based on author nationality; multi-country collaborations are counted for each contributing nation.
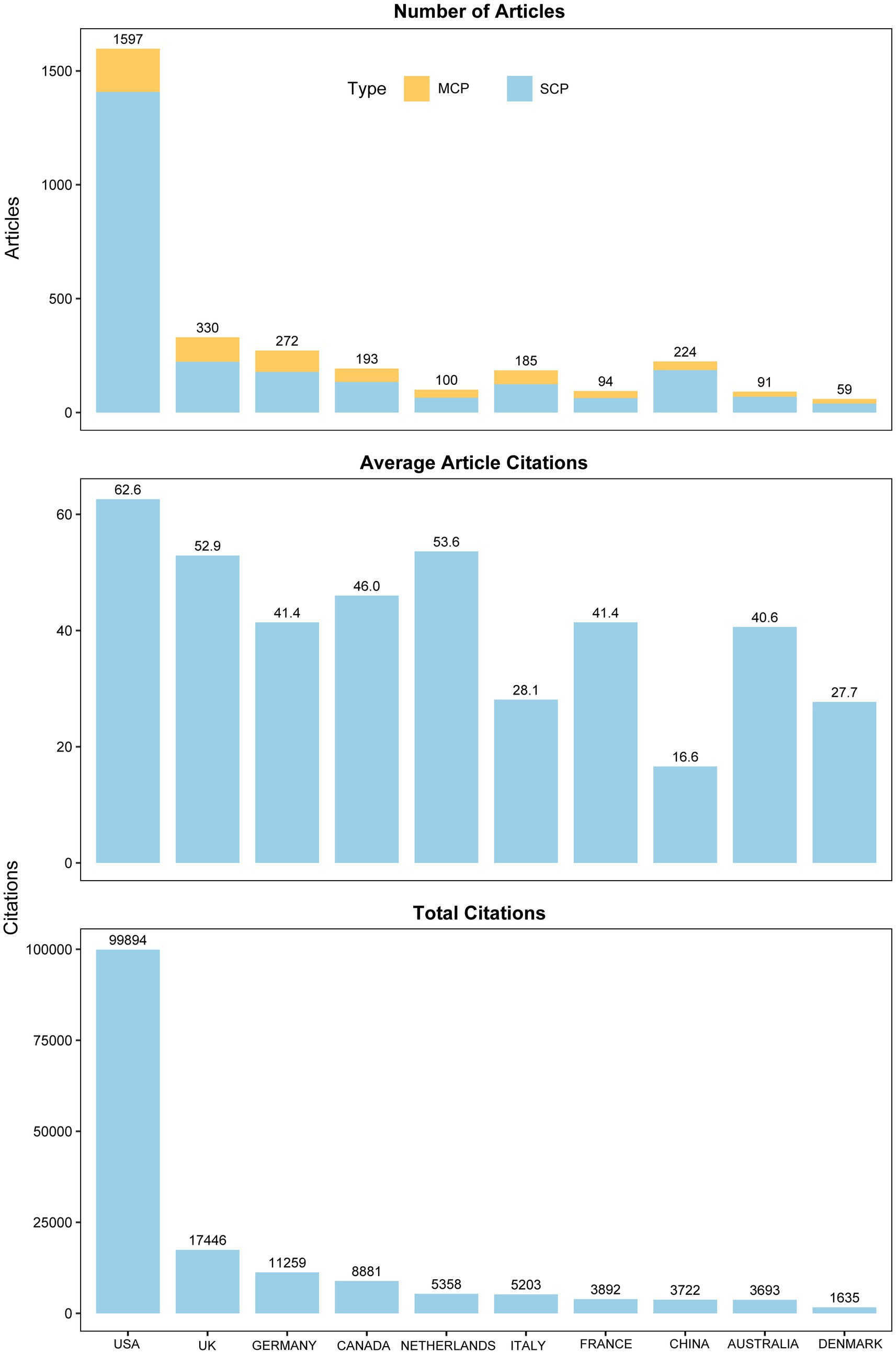
Figure 5. Research performance of top 10 countries: collaboration types and citations. SCP, Single Country Publication; MCP, Multinational Cooperative Publication.
3.5 Leading source journals
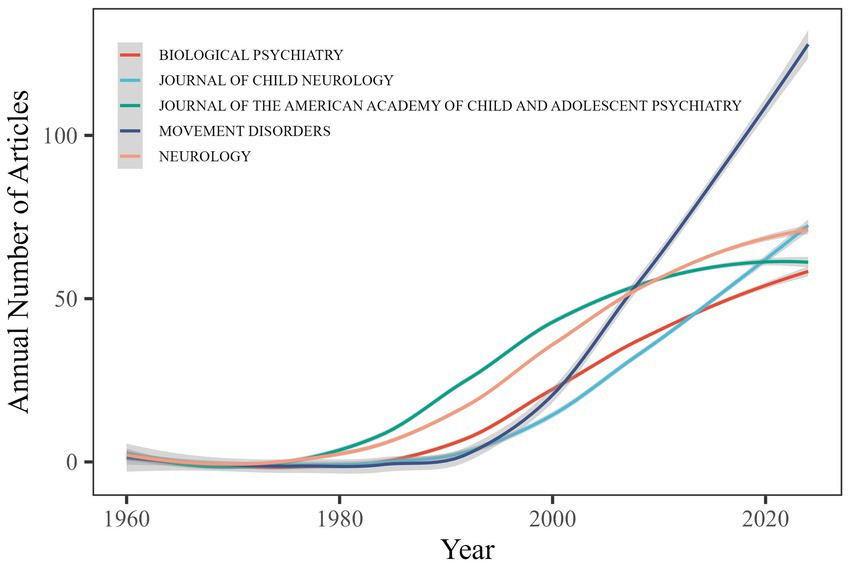
TS research findings are disseminated across 991 journals, yet their publication patterns closely follow Bradford’s Law of Scattering, displaying a marked core–periphery structure. Specifically, the top five outlets—Movement Disorders (114 papers), Neurology (22), Journal of Child Neurology (23), Journal of the American Academy of Child and Adolescent Psychiatry (24) and Biological Psychiatry (25)—have published a total of 369 papers (Figure 7). In contrast, 540 journals (54.4%) each published only one TS article, and 894 journals (90.2%) published 10 or fewer. This long-tail distribution is characteristic of a mature yet dispersed research field.
Regarding local citation impact, JAMA Psychiatry demonstrates the highest citation frequency among TS publications (Table 3). The top 10 journals identified through local citation analysis include multiple journals recognized as core sources for TS research, such as Neurology, Movement Disorders and Biological Psychiatry. These journals have significantly contributed to advancing TS research through their influential scholarly output.
3.6 Most influential authors and organizations
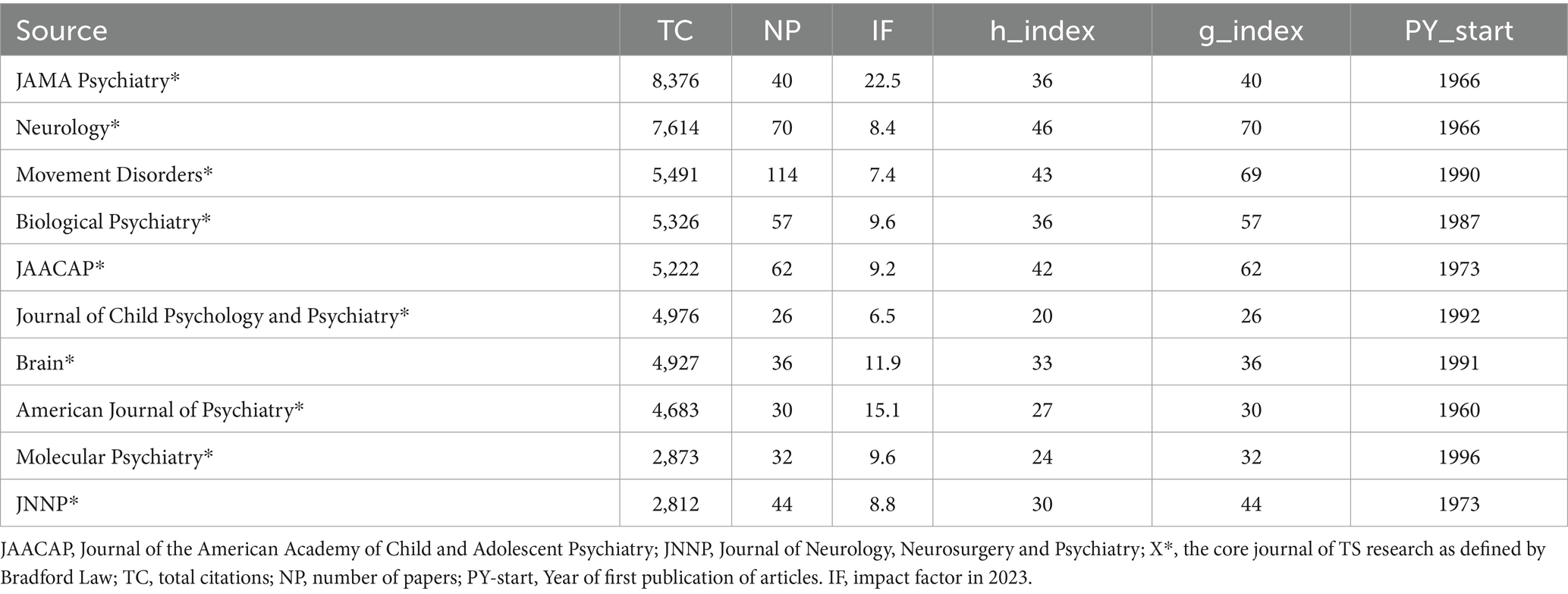
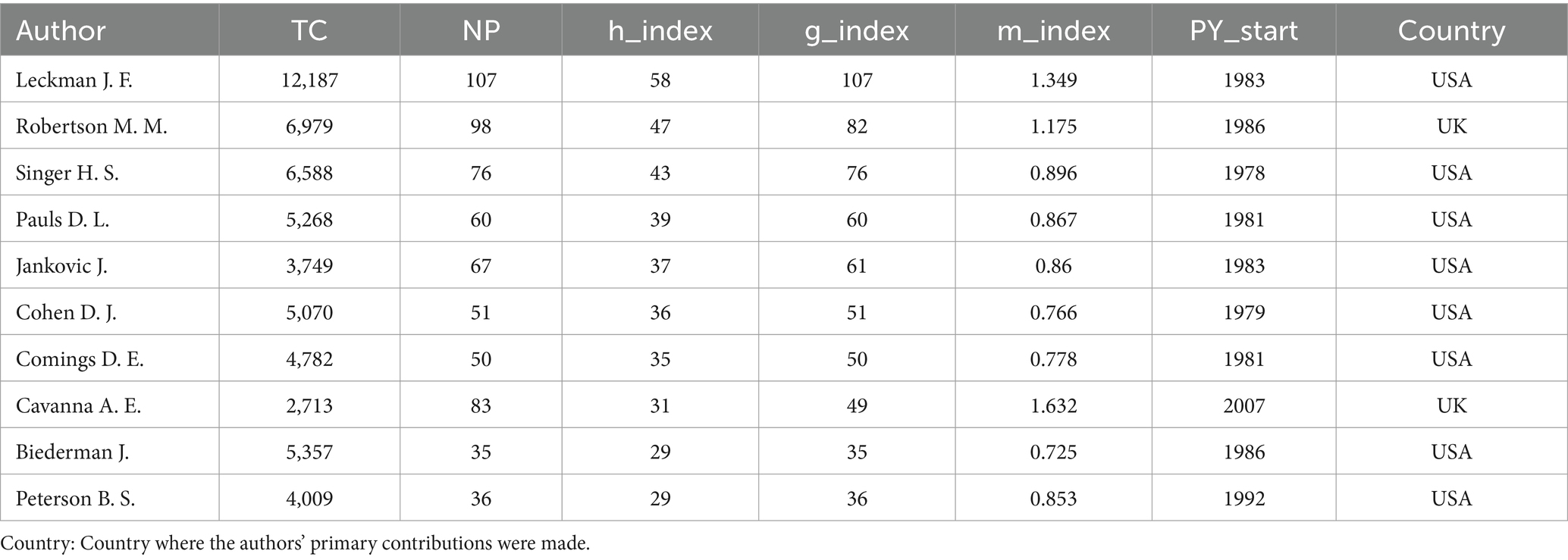
The H-index serves as a prominent metric to gage a researcher’s academic impact, taking into account the citation frequencies of their published works (26). In this study, we determined the H-index for every author involved in the 4,011 TS research articles, irrespective of their ranking in the author sequence. The authors with the highest H-indices include Leckman J. F. with 107 publications, Robertson M. M. with 98, Singer H. S. with 76, as detailed in Table 4. Leckman J. F., boasting an M-index of 1.349, stands out as the leading author in terms of both publication volume and citation metrics. Dr. James F. Leckman, a distinguished professor at Yale University School of Medicine, is renowned for his pioneering work in the neurobiology of TS and related developmental disorders. His research has significantly advanced our understanding of the genetic and environmental factors influencing TS, and he has been instrumental in developing comprehensive assessment tools for tic disorders (27–29). Among the top 10 influential researchers, eight are affiliated with institutions in the United States, one in the United Kingdom, and one in Canada. Overall, our analysis encompassed 4,011 papers, which involved a total of 12,860 authors, among whom 332 produced single-authored works. With an average of 5.43 co-authors per paper, this suggests a strong inclination toward collaborative efforts in TS research.
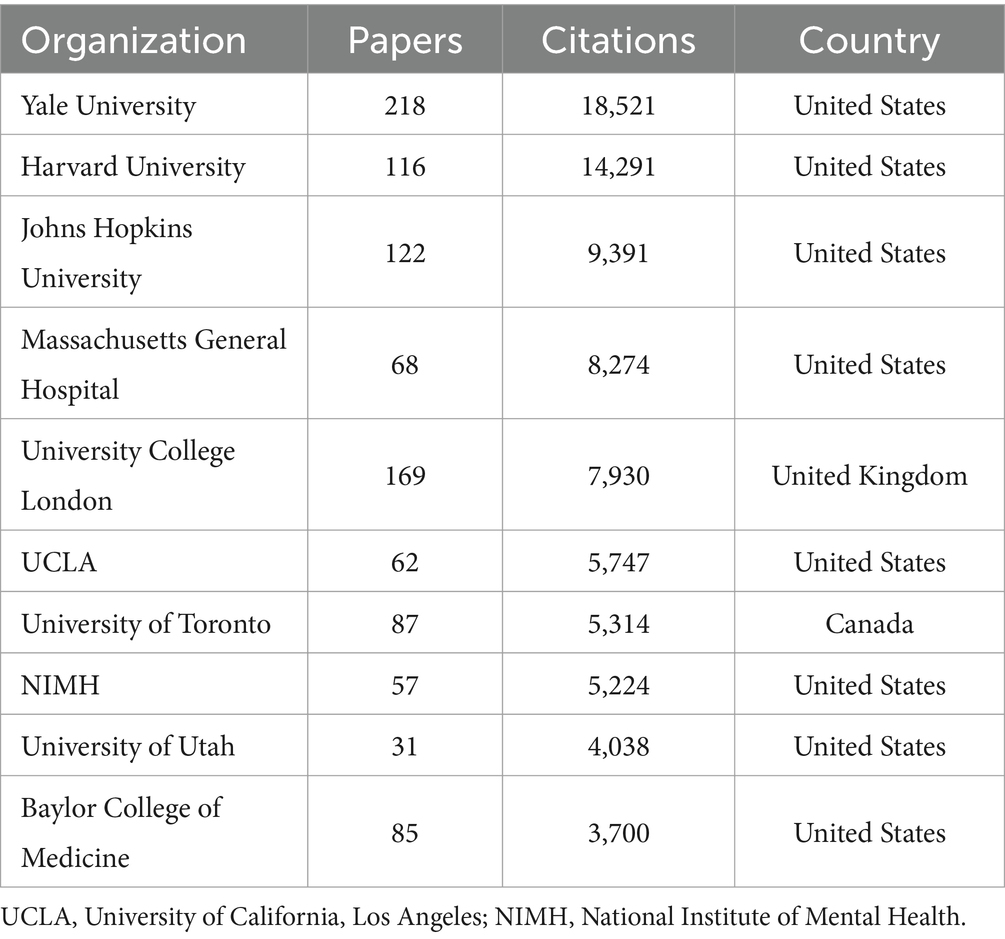
Table 5 presents the top 10 institutions ranked by academic productivity and citation impact. Yale University leads with 218 papers and 18,521 citations, reflecting both high productivity and broad influence. Harvard University, despite fewer publications (116 papers), demonstrates exceptional scholarly impact through 14,291 citations. Johns Hopkins University ranks third with 122 papers and 9,391 citations, indicating sustained research engagement. Collectively, these institutions exemplify global leadership in research and academic excellence, with the United States prominently featuring as a hub for scientific inquiry.
3.7 Most influential papers
This study incorporated 121,908 references, with citation counts defined by local citations (LC) (30)—that is, the number of times each work was cited within our dataset—and Table 6 presents the 10 most influential papers ranked by LC.
The most cited paper, by Pauls et al. (31), employed semi-structured interviews of TS probands and their first-degree relatives to demonstrate that obsessive-compulsive disorder (OCD) aggregates with TS in families, providing the first clear evidence of shared heritable susceptibility and laying the methodological groundwork for subsequent genome-wide linkage and SLITRK1 candidate-gene studies (32) as well as revisions to DSM comorbidity criteria; Freeman et al. (33) analyzed a multicenter cohort of 3,500 TS patients from 22 countries to characterize sex ratio, age at onset, comorbidity (88% ADHD), and regional variation, supplying robust epidemiological data that have informed international practice guidelines and the design of multicenter intervention trials; Comings et al. (34) compared 246 TS patients with 47 controls using DSM-III–based questionnaires to quantify familial co-occurrence of ADHD, learning disorders, and school difficulties, establishing a phenotypic screening paradigm subsequently adopted in candidate-gene investigations; Leckman et al. (27) conducted longitudinal follow-up of TS children, identifying a peak in tic severity during adolescence followed by marked remission in early adulthood, thereby refining prognostic counseling and optimizing intervention timing; Peterson et al. (35) used fMRI in 22 adult TS patients to compare neural activity during voluntary tic suppression versus free expression, revealing enhanced activation in basal ganglia–thalamocortical regions inversely related to symptom severity and guiding TMS/DBS target selection (36, 37); in a subsequent structural MRI study (38), quantitative analyses demonstrated reduced caudate nucleus volumes in TS subjects relative to controls, with volumetric reductions correlating with comorbid OCD and symptom persistence, thereby validating a neuroanatomical biomarker and motivating machine-learning–based prognostic models; Albin & Mink (39) synthesized evidence for dysregulation of the cortico–striato–thalamo–cortical circuitry and interactions among dopamine, GABA, and glutamate systems to propose a unified pathophysiological model that has underpinned VMAT2 inhibitor development and neuromodulation approaches (40, 41); Bohlhalter et al. (42) demonstrated via task-based fMRI that TS patients exhibit weakened fronto–parietal–basal ganglia connectivity during motor inhibition tasks, highlighting network-level deficits that have informed network-guided TMS protocols and subtype stratification; Kalanithi et al. (43) provided the first postmortem evidence of reduced parvalbumin-positive GABAergic interneurons in the basal ganglia of TS cases, corroborating an inhibitory signaling deficit hypothesis and directly supporting GABA-targeted therapeutic development (44, 45); and Singer (46) assessed adult TS patients’ neuropsychological and motor functions, showing that cognitive and attentional deficits often persist despite tic remission, thereby emphasizing the need for lifelong neuropsychological monitoring and individualized rehabilitation plans.
Together, these studies span family-based epidemiology, natural history, functional and structural neuroimaging, and pathophysiological synthesis, mapping a comprehensive research trajectory from genetic susceptibility to clinical intervention in TS.
3.8 Analysis of prominent research trends
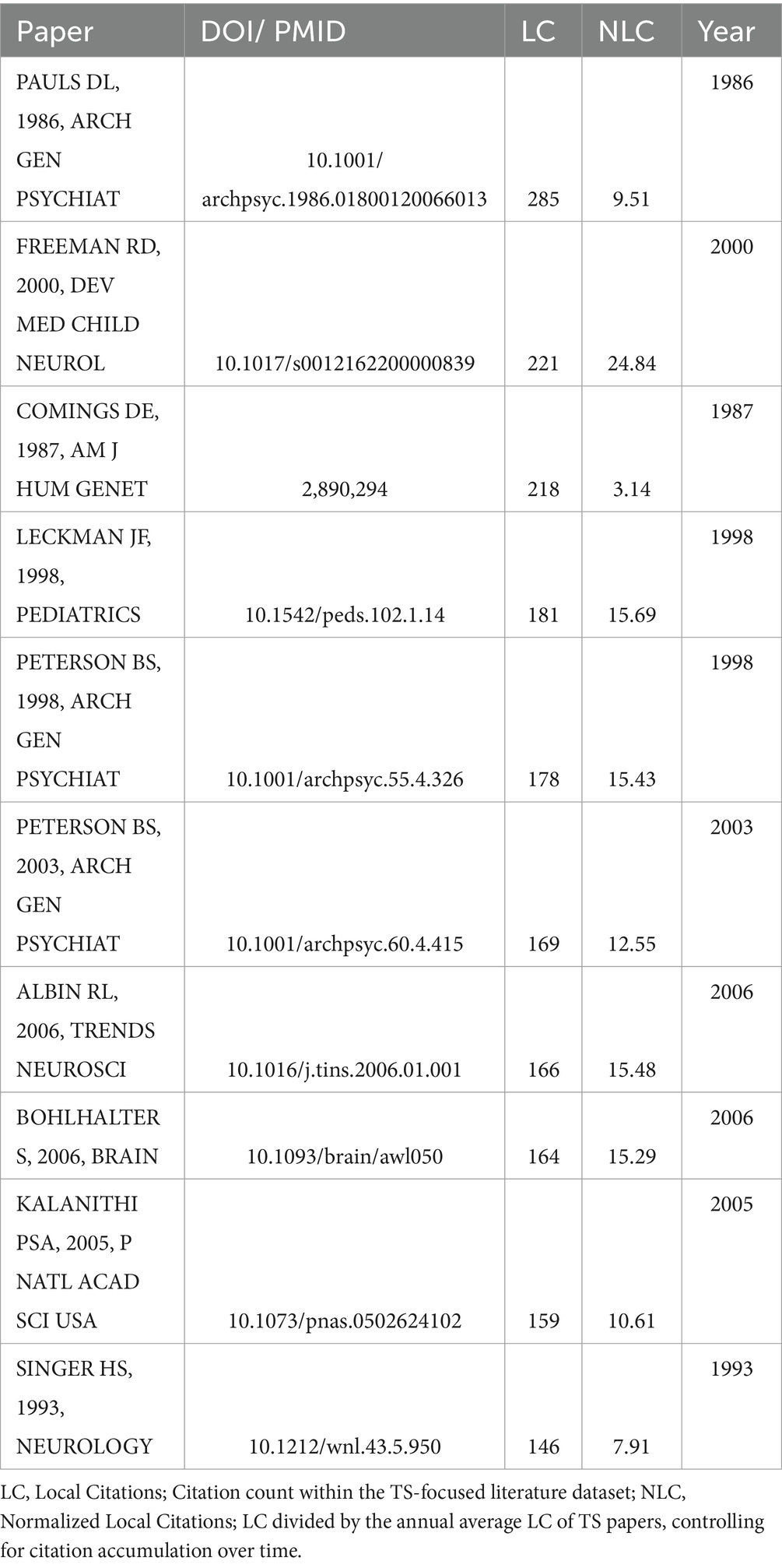
This study employed bibliometric methods to analyze research trends in TS. Figure 8 illustrates the temporal trends in author keywords, with the x-axis representing publication years and the y-axis showing keywords. Each keyword’s publication years are represented by three quartiles: green dots indicate the first quartile, blue dots the median publication year, and red dots the third quartile. The size of the blue dots corresponds to keyword frequency, with larger dots indicating higher frequencies.
The analysis of keywords reveals the top 10 most frequently occurring terms in TS research: Tourette syndrome, Children, obsessive-compulsive disorder, basal ganglia, disorder, double-blind, scale, Parkinsons-disease, disorders and adolescents. Among these, ‘Tourette syndrome’ stands out as a core topic, reflecting its central role in the TS research domain.
The bibliometric analysis delineates five temporal phases in TS research, reflecting evolving methodological and conceptual priorities. Initial investigations (mid-1980s–1991) focused on establishing diagnostic criteria (DSM-III) (47, 48) and epidemiological foundations, with Tourette syndrome, family aggregation (31, 49), and prevalence (50, 51) as key terms. Subsequent clinical phenotyping efforts (1992–1996) prioritized symptom characterization, emphasizing multiple tics and comorbidities like ADHD (46, 52, 53), alongside early neurochemical studies of dopaminergic pathways (25, 54, 55).
The molecular genetics era (1997–2003) saw advancements in candidate gene studies (32, 56, 57) (e.g., SLITRK1 and HDC variants linked to synaptic pruning and histaminergic dysfunction) and structural neuroimaging (35, 38) (magnetic-resonance imaging), alongside immunological hypotheses involving antineuronal antibodies (58, 59). From 2004 to 2014, circuit-level models dominated, driven by deep brain stimulation (60–62) targeting the subthalamic nucleus and dorsal striatum (63, 64), supported by genome-wide association studies (24, 65, 66) integrating genetic risks with cortico-striato-thalamo-cortical circuit abnormalities. Recent research (2015–2024) prioritizes patient-centered outcomes (67), marked by quality-of-life (23, 68, 69) metrics, behavior-therapy for premonitory urges (70), and trials of delta (9)-tetrahydrocannabinol (Δ9-THC) (22, 71). Emerging challenges include differentiating organic tics from pseudo-tics and addressing autism spectrum disorder comorbidity (72, 73). Methodologically, machine learning applications and clinical management frameworks now aim to unify genetic, environmental, and neural circuit insights into actionable care models. This progression illustrates TS research’s shift from descriptive nosology to mechanism-driven, functionally oriented paradigms.
4 Discussion
4.1 From descriptive epidemiology to precision science
The trajectory of TS research publications reveals a field that has steadily evolved from descriptive epidemiology to sophisticated, patient-centered science. Prior to 1990, annual outputs were modest, reflecting TS’s absence from mainstream nosology; the advent of DSM-III (74) and the first family-aggregation (31, 75) studies in the mid-1980s marked the first sustained uptick as investigators began to quantify heritable risk. The 1990s saw another acceleration, driven by the adoption of MRI and fMRI and landmark longitudinal cohort analyses that illuminated basal-ganglia circuitry and tic-suppression mechanisms. A more pronounced surge in the early 2000s coincided with the rollout of genome-wide linkage and association studies, and the emergence of CSTC circuit models (76), which underpinned the first clinical trials of deep brain stimulation and transcranial magnetic stimulation. Publication rates peaked around the release of DSM-5 (77, 78) in 2013—when expanded diagnostic categories and comorbidity specifiers broadened the scope of inquiry—and then plateaued at a high level as the community embraced standardized quality-of-life measures, digital biomarkers, and new pharmacotherapies such as VMAT2 inhibitors (41). The brief dip in 2020–2021 likely reflects the pandemic’s interruption of in-person research, but the rapid rebound demonstrates the field’s agility in deploying telehealth and remote assessment tools. Together, these inflection points underscore how evolving diagnostic frameworks, methodological innovations, and clinical imperatives have shaped TS research into the multidisciplinary endeavor it is today.
4.2 Collaborative capacity, challenges, and future synergies in TS research
The concentration of TS research within a handful of countries and institutions highlights both remarkable capacity and untapped opportunities for collaboration. The United States’ leading position in publication volume and citation impact stems from its abundant funding and well-established clinical–research infrastructure; however, its evaluation system—which places greater weight on high-impact domestic papers than on multinational projects—may inadvertently discourage cross-border partnerships despite ample resources (30, 31). In contrast, European nations such as the United Kingdom, Germany and Denmark benefit from EU initiatives like Horizon Europe, which explicitly reward multinational consortia and have driven their higher proportions of internationally coauthored work and strong citation metrics. This contrast suggests that, while U. S. teams have propelled foundational advances in TS genetics and circuitry, a more concerted engagement with global networks could further enrich study populations, foster methodological innovation and accelerate the translation of mechanistic insights into diverse clinical settings. Facilitating joint grant schemes, shared data platforms and co-mentorship between North American centers and emerging hubs would help distribute expertise more evenly and build a truly global TS research community.
China’s rapid ascent into the top five producers of TS literature reflects successful capacity building, yet its comparatively modest citation rate signals room to enhance study design, target leading journals and forge strategic international alliances. Likewise, burgeoning research pockets in Canada and Australia stand to gain from closer collaborations with established laboratories.
At the individual level, the enduring influence of investigators at Yale, Harvard and Johns Hopkins—particularly figures such as Leckman, Robertson and Singer—has set high benchmarks for cohort size, longitudinal follow-up and translational scope; nevertheless, nurturing a new generation of scholars across diverse geographic and career stages will be vital to sustain this momentum.
Looking forward, deliberate expansion of collaborative frameworks is essential. Multinational genomics consortia, standardized neuroimaging repositories and shared clinical-trial networks can unify efforts, while partnerships between high-output centers and emerging research communities can drive cultural sensitivity and inclusivity in study design (17). By reinforcing these global alliances, the field can ensure that advances in TS research benefit patients everywhere.
4.3 Core journal concentration: benefits, drawbacks, and future directions
The concentration of TS research in a small set of core journals—following Bradford’s Law—has clear benefits and drawbacks. On the upside, specialist outlets such as Movement Disorders and Neurology uphold rigorous peer review and ensure that major advances [for example, Yale’s contributions to genetic research and key imaging studies on TS have been pivotal (31, 32, 43)] reach the right audience quickly. On the downside, more than half of all journals publish only one TS paper, which means that useful innovations—like early smartphone apps for automated tic tracking or small telehealth pilot programs—can slip under the radar when they appear outside the core literature.
Looking ahead, balancing depth and breadth will be essential. Leading journals can help by dedicating space to both specialized studies and practical innovations—like testing AI tools that spot tic patterns in home videos, or refining apps that help track symptoms between clinic visits. We also need smarter ways to connect findings from different fields. Envisioning a shared online platform where engineers developing motion sensors collaborate with therapists implementing school-based interventions may facilitate a deeper understanding of why certain treatments demonstrate greater effectiveness in real-world settings. At the same time, annual review collections or simple online consortia could bring together findings from peripheral outlets, whether they describe wearable sensors tested in engineering journals or community-based support models reported in rehabilitation publications. Finally, to close geographic gaps, the TS community should back regional registries (for example, in Latin America or Southeast Asia) and fund translation and validation of key rating scales in multiple languages. These steps will help ensure that TS research remains both rigorous and relevant to patients and clinicians worldwide.
4.4 Gene–environment interactions and translational research in Tourette syndrome: from mechanistic insights to precision prevention
The evolving focus of TS research—from mapping cortico-striatal circuits to decoding genetic risks and ultimately improving patients’ daily lives—mirrors both scientific breakthroughs and societal demands for holistic care. Seminal discoveries include SLITRK1—whose rare variants disrupt synaptogenesis and neural circuit connectivity—and HDC, the rate-limiting enzyme in central histamine synthesis, together anchoring TS pathophysiology in both neurodevelopmental and neurochemical pathways (79–81).
Increasing emphasis on quality-of-life outcomes in neurological disorders, including TS, underscores the need to translate laboratory insights into clinical and public health contexts. A central challenge is understanding how genetic predispositions interact with environmental exposures in TS. Epidemiological observations—for instance, rising TS incidence in urban populations and in post-pandemic cohorts—suggest that modifiable environmental factors (e.g., noise, air pollution, chronic stress) may influence disease onset or severity, yet robust study designs to disentangle these interactions are lacking.
To systematically explore this research gap, a three-part translational framework is designed. First, a prospective birth cohort would enroll children carrying known TS-associated risk genotypes (such as SLITRK1 or HDC variants) and follow them from the prenatal period through adolescence. Detailed prenatal and early-life exposure data (including fine particulate matter [PM2.5] levels and serial maternal cytokine profiles) would be collected and correlated with epigenetic modifications (for example, SLITRK1 promoter methylation) and neurodevelopmental outcomes. In vivo neuroimaging markers—such as striatal GABA concentration measured by magnetic resonance spectroscopy—would provide objective neural correlates. This design builds on evidence linking air pollution to neurotransmitter dysregulation relevant to TS, but will require careful sample stratification to ensure adequate numbers of variant carriers, long-term follow-up, and rigorous ethical oversight for pediatric genetic research. Second, geospatial analysis combined with machine learning could identify high-risk environments and gene–environment signatures. By integrating neighborhood-level pollution maps, individual gut microbiome profiles (via shotgun metagenomics), and psychosocial stress indicators (e.g., exposure to bullying), AI models may reveal patterns such as HDC variant carriers in heavily polluted areas developing early pharmacoresistance—a phenomenon suggested by studies in other inflammatory disorders. This approach demands large, multi-modal datasets and must address confounding factors and data privacy concerns. Finally, hypothesis-driven intervention trials would test causality and therapeutic strategies. For example, SLITRK1 variant carriers with gut dysbiosis could be enrolled in a randomized trial of the probiotic Bifidobacterium longum to assess effects on tic severity and microbial metabolites (such as butyrate). Similarly, HDC-variant subjects in high-pollution regions might receive combined interventions (HEPA air purifiers and histamine H3 receptor antagonists) to mitigate neuroinflammatory pathways implicated in TS. These trials would include rigorous controls, safety monitoring, and ethical safeguards appropriate for pediatric populations, with outcomes assessed at both clinical and molecular levels. Together, this integrative framework links genetic, environmental, and mechanistic perspectives on TS and provides a feasible roadmap for translational research from bench to bedside in neurology.
In conclusion, although genetic research has significantly advanced our understanding of TS, its practical impact depends on transcending disciplinary boundaries. Insights from ADHD research (82), where integration of genetic data with educational outcomes enabled personalized interventions, illustrate the potential of such interdisciplinary approaches. For TS, bridging genomic insights with real-world measures may clarify why the same genetic variant manifests as tics in some individuals and obsessive thoughts in others. Ultimately, this integration can transform molecular discoveries into meaningful improvements in patient care.
4.5 Treatment modalities in TS
A 2024 meta-analysis (83) demonstrated that repetitive transcranial magnetic stimulation (TMS) targeting the supplementary motor area or prefrontal cortex yields modest yet sustained tic reduction in TS, with mild, transient headache reported as the most common adverse effect; the authors recommend the adoption of standardized TMS protocols and extended follow-up to assess long-term efficacy. For refractory cases, a recent Cochrane review (84) confirmed that deep brain stimulation (DBS)—primarily directed at the centromedian–parafascicular thalamic complex—produces significant and durable symptom improvement, although patient selection criteria and optimal stereotactic targets warrant further refinement. Complementing these findings, a 2024 clinical series (85) reported that combining DBS with anterior capsulotomy provides additional benefit for patients with severe psychiatric comorbidities, albeit necessitating careful monitoring of postoperative cognitive and emotional outcomes. Meanwhile, a recent review of gut microbiome interventions in children (86) suggests that probiotic supplementation and diet-based strategies aimed at restoring microbial balance may help alleviate tic severity and comorbid behaviors, though larger randomized controlled trials are required to validate these preliminary observations and identify precise microbial targets. In the pharmacological domain, an updated review (87) reaffirms the use of α₂-adrenergic agonists (e.g., clonidine, guanfacine) as first-line agents and antipsychotics (e.g., aripiprazole, risperidone) as second-line options, while emerging therapies—including VMAT2 inhibitors and cannabinoid-based compounds—show promise in early-phase studies for reducing tics with potentially fewer metabolic side effects.
4.6 Advantages over prior bibliometric analyses and clinical implications
Compared with earlier, narrower-scope studies of TS literature (15), our work extends the temporal window from 1960 to 2024—capturing foundational milestones such as the introduction of DSM-III, the rise of genome-wide linkage studies, and pandemic-related publication shifts—rather than focusing solely on a single decade. Methodologically, we combine R/Biblioshiny, Tidyverse visualizations, VOSviewer network mapping, and Bradford’s Law to provide a multifaceted portrait of research trends, core journals, and author impact, whereas prior analyses relied primarily on CiteSpace. Rigorous data refinement—including iterative search optimization documented in a detailed flowchart and systematic imputation of missing references, affiliations, DOIs, and impact factors—ensures a reproducible dataset of 4,011 records. Beyond descriptive mapping, we introduce a tripartite translational framework that links genetic discoveries (SLITRK1, HDC) with environmental exposures and targeted intervention trials, offering a roadmap for precision prevention.
Clinically, these enhancements guide the formation of multinational TS consortia with more inclusive recruitment strategies, highlight validated assessment tools and novel therapies (e.g., VMAT2 inhibitors, digital biomarkers), and lay the groundwork for prospective G × E cohort studies. By integrating bibliometric insights with actionable trial designs, our study not only charts the evolution of TS research but also directly informs the development of personalized diagnostics and early-intervention strategies that can ultimately improve patient outcomes.
4.7 Limitations
This study has several limitations that may affect the comprehensiveness and representativeness of the findings. First, the analysis was restricted to the Web of Science (WoS) Core Collection, which, despite its broad coverage, may exclude relevant studies indexed in other databases, potentially overlooking regional or discipline-specific contributions. Second, the search was limited to publications from 1960 to 2024, which may have excluded earlier studies and the most recent preprints. Third, only English-language literature was included, introducing a potential linguistic and geographic bias by underrepresenting non-English research outputs. Additionally, the inclusion criteria were limited to articles and review articles, which may have excluded other meaningful publication types such as conference proceedings, editorials, or clinical guidelines. The use of a specific search strategy focused on Tourette syndrome may also have led to the exclusion of related research on comorbid conditions, such as ADHD or OCD, that did not explicitly mention TS in the title or abstract. These constraints may have influenced the observed patterns in journal distribution, author affiliations, and thematic evolution. Future research should expand the database scope (such as Scopus, PubMed, CNKI, and SciELO), incorporate multilingual and multi-format inclusion strategies, and refine search approaches to better capture the interdisciplinary and comorbid dimensions of TS research.
5 Conclusion
This analysis outlines the evolution of TS research from symptom-focused studies (1960s) to today’s integration of genetics, environmental triggers, and patient-centered care. The United States has led global efforts, with JAMA Psychiatry and pioneers like Yale’s Dr. Leckman shaping key insights. Future efforts must prioritize multidisciplinary collaboration integrating neuroimaging, environmental data, and AI, while advancing genetic insights to refine personalized therapies. Building inclusive global cohorts will ensure discoveries translate into equitable solutions, bridging research with clinical and societal needs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
ZS: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. TD: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. TX: Funding acquisition, Project administration, Supervision, Writing – review & editing. XG: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Yunnan Innovation Team for Acupuncture and Tuina in Prevention and Treatment of Brain Diseases (grant no. 202405AS350007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hartmann, A, Ansquer, S, Brefel-Courbon, C, Burbaud, P, Castrioto, A, Czernecki, V, et al. French guidelines for the diagnosis and management of tourette syndrome. Rev Neurol (Paris). (2024) 180:818–27. doi: 10.1016/j.neurol.2024.04.005
2. Marazziti, D, Palermo, S, Arone, A, Massa, L, Parra, E, Simoncini, M, et al. Obsessive-compulsive disorder, pandas, and tourette syndrome: immuno-inflammatory disorders In: Y Kim, editor. Advances in experimental medicine and biology. 1411. Singapore: Springer Nature Singapore (2023). 275–300.
3. Martino, D, Ganos, C, and Pringsheim, TM. Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. (2017) 134:1461–90. doi: 10.1016/bs.irn.2017.05.006
4. Robertson, MM, Eapen, V, and Cavanna, AE. The international prevalence, epidemiology, and clinical phenomenology of tourette syndrome: a cross-cultural perspective. J Psychosom Res. (2009) 67:475–83. doi: 10.1016/j.jpsychores.2009.07.010
5. Robertson, MM. The prevalence and epidemiology of Gilles de la Tourette syndrome: part 1: the epidemiological and prevalence studies. J Psychosom Res. (2008) 65:461–72. doi: 10.1016/j.jpsychores.2008.03.006
6. Hirschtritt, ME, Lee, PC, Pauls, DL, Dion, Y, Grados, MA, Illmann, C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in tourette syndrome. JAMA Psychiatry. (2015) 72:325–33. doi: 10.1001/jamapsychiatry.2014.2650
7. Robertson, MM. The prevalence and epidemiology of Gilles de la Tourette syndrome: part 2: tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res. (2008) 65:473–86. doi: 10.1016/j.jpsychores.2008.03.007
8. Horesh, N, Shmuel-Baruch, S, Farbstein, D, Ruhrman, D, Milshtein, N, Fennig, S, et al. Major and minor life events, personality and psychopathology in children with Tourette syndrome. Psychiatry Res. (2018) 260:1–9. doi: 10.1016/j.psychres.2017.11.016
9. Steinberg, T, Shmuel-Baruch, S, Horesh, N, and Apter, A. Life events and tourette syndrome. Compr Psychiatry. (2013) 54:467–73. doi: 10.1016/j.comppsych.2012.10.015
11. van Eck, NJ, and Waltman, L. Software survey: vosviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
12. Aria, M, and Cuccurullo, C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Inf Secur. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
13. Wu, X, Deng, H, Jian, S, Chen, H, Li, Q, Gong, R, et al. Global trends and hotspots in the digital therapeutics of autism spectrum disorders: a bibliometric analysis from 2002 to 2022. Front Psychol. (2023) 14:1126404. doi: 10.3389/fpsyt.2023.1126404
14. Deng, H, Huang, Z, Li, Z, Cao, L, He, Y, Sun, N, et al. Systematic bibliometric and visualized analysis of research hotspots and trends in attention-deficit hyperactivity disorder neuroimaging. Front Neurosci. (2023) 17:1098526. doi: 10.3389/fnins.2023.1098526
15. Yang, C, Zhang, J, Zhao, Q, Zhang, J, Zhou, J, and Wang, L. Trends of tourette syndrome in children from 2011 to 2021: a bibliometric analysis. Front Behav Neurosci. (2022) 16:991805. doi: 10.3389/fnbeh.2022.991805
16. Strom, NI, Halvorsen, MW, Grove, J, Ásbjörnsdóttir, B, Luðvígsson, P, Thorarensen, Ó, et al. Genome-wide association study meta-analysis of 9619 cases with tic disorders. Biol Psychiatry. 97:743–52. (2024). doi: 10.1016/j.biopsych.2024.07.025
17. Johnson, KA, Worbe, Y, Foote, KD, Butson, CR, Gunduz, A, and Okun, MS. Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. (2023) 22:147–58. doi: 10.1016/S1474-4422(22)00303-9
18. Senthil, R, Anand, T, Somala, CS, and Saravanan, KM. Bibliometric analysis of artificial intelligence in healthcare research: trends and future directions. Future Healthcare J. (2024) 11:100182. doi: 10.1016/j.fhj.2024.100182
19. Nguyen, H, and Voznak, M. A bibliometric analysis of technology in digital health: exploring health metaverse and visualizing emerging healthcare management trends. IEEE Access. (2024) 12:23887–913. doi: 10.1109/ACCESS.2024.3363165
20. AlRyalat, S, Malkawi, LW, and Momani, SM. Comparing bibliometric analysis using PubMed, Scopus, and web of science databases. J Vis Exp. (2019) 152. doi: 10.3791/58494
21. Brookes, BC. Sources of information on specific subjects by s. c. Bradford. J Inf Sci. (1985) 10:173–5.
22. Serag, I, Elsakka, MM, Moawad, M, Ali, HT, Sarhan, K, Shayeb, S, et al. Efficacy of cannabis-based medicine in the treatment of Tourette syndrome: a systematic review and meta-analysis. Eur J Clin Pharmacol. (2024) 80:1483–93. doi: 10.1007/s00228-024-03710-9
23. Cavanna, AE. Quality of life in tourette syndrome. Psychiatr Clin North Am. (2025) 48:123–50. doi: 10.1016/j.psc.2024.08.010
24. State, MW. The genetics of child psychiatric disorders: focus on autism and tourette syndrome. Neuron. (2010) 68:254–69. doi: 10.1016/j.neuron.2010.10.004
25. Comings, DE, Comings, BG, Muhleman, D, Dietz, G, Shahbahrami, B, Tast, D, et al. The dopamine d sub 2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. (1991) 266:1793.
26. Hirsch, JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. (2005) 102:16569–72. doi: 10.1073/pnas.0507655102
27. Leckman, JF, Zhang, H, Vitale, A, Lahnin, F, Lynch, K, Bondi, C, et al. Course of tic severity in tourette syndrome: the first two decades. Pediatrics. (1998) 102:14–9.
28. Leckman, JF, Riddle, MA, Hardin, MT, Ort, SI, Swartz, KL, Stevenson, J, et al. The Yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. (1989) 28:566–73.
29. Leckman, JF, and Peterson, BS. The pathogenesis of tourette’s syndrome: epigenetic factors active in early cns development. Biol Psychiatry. (1993) 34:425–7.
30. Garfield, E. (1977). Introducing citation classics-human side of scientific reports : Inst Sci Inform Inc., Philadelphia, PA, USA 5–07
31. Pauls, DL, Towbin, KE, Leckman, JF, Zahner, GE, and Cohen, DJ. Gilles de la tourette’s syndrome and obsessive-compulsive disorder. Evidence supporting a genetic relationship. Arch Gen Psychiatry. (1986) 43:1180–2. doi: 10.1001/archpsyc.1986.01800120066013
32. Abelson, JF, Kwan, KY, O’Roak, BJ, Baek, DY, Stillman, AA, Morgan, TM, et al. Sequence variants in Slitrk1 are associated with Tourette’s syndrome. Science. (2005) 310:317–20. doi: 10.1126/science.1116502
33. Freeman, RD, Fast, DK, Burd, L, Kerbeshian, J, Robertson, MM, and Sandor, P. An international perspective on Tourette syndrome: selected findings from 3, 500 individuals in 22 countries. Dev Med Child Neurol. (2000) 42:436–47.
34. Comings, DE, and Comings, BG. A controlled study of tourette syndrome. I. Attention-deficit disorder, learning disorders, and school problems. Am J Hum Genet. (1987) 41:701–41.
35. Peterson, BS, Skudlarski, P, Anderson, AW, Zhang, H, Gatenby, JC, Lacadie, CM, et al. A functional magnetic resonance imaging study of tic suppression in tourette syndrome. Arch Gen Psychiatry. (1998) 55:326–33.
36. Loh, A, Gwun, D, Chow, CT, Boutet, A, Tasserie, J, Germann, J, et al. Probing responses to deep brain stimulation with functional magnetic resonance imaging. Brain Stimul. (2022) 15:683–94. doi: 10.1016/j.brs.2022.03.009
37. Haense, C, Müller-Vahl, KR, Wilke, F, Schrader, C, Capelle, HH, Geworski, L, et al. Effect of deep brain stimulation on regional cerebral blood flow in patients with medically refractory tourette syndrome. Front Psychol. (2016) 7:118. doi: 10.3389/fpsyt.2016.00118
38. Peterson, BS, Thomas, P, Kane, MJ, Scahill, L, Zhang, H, Bronen, R, et al. Basal ganglia volumes in patients with gilles de la tourette syndrome. Arch Gen Psychiatry. (2003) 60:415–24. doi: 10.1001/archpsyc.60.4.415
39. Albin, RL, and Mink, JW. Recent advances in Tourette syndrome research. Trends Neurosci. (2006) 29:175–82. doi: 10.1016/j.tins.2006.01.001
40. Neudorfer, C, and Maarouf, M. Neuroanatomical background and functional considerations for stereotactic interventions in the h fields of forel. Brain Struct Funct. (2018) 223:17–30. doi: 10.1007/s00429-017-1570-4
41. Jankovic, J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. (2016) 17:2461–70. doi: 10.1080/14656566.2016.1258063
42. Bohlhalter, S, Goldfine, A, Matteson, S, Garraux, G, Hanakawa, T, Kansaku, K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. (2006) 129:2029–37. doi: 10.1093/brain/awl050
43. Kalanithi, PS, Zheng, W, Kataoka, Y, DiFiglia, M, Grantz, H, Saper, CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with tourette syndrome. Proc Natl Acad Sci USA. (2005) 102:13307–12. doi: 10.1073/pnas.0502624102
44. Draper, A, Stephenson, MC, Jackson, GM, Pépés, S, Morgan, PS, Morris, PG, et al. Increased gaba contributes to enhanced control over motor excitability in tourette syndrome. Curr Biol. (2014) 24:2343–7. doi: 10.1016/j.cub.2014.08.038
45. Jackson, GM, Draper, A, Dyke, K, Pépés, SE, and Jackson, SR. Inhibition, disinhibition, and the control of action in tourette syndrome. Trends Cogn Sci. (2015) 19:655–65. doi: 10.1016/j.tics.2015.08.006
46. Singer, HS, Reiss, AL, Brown, JE, Aylward, EH, Shih, B, Chee, E, et al. Volumetric mri changes in basal ganglia of children with tourette’s syndrome. Neurology. (1993) 43:950–6.
47. Pope, HJ, and Hudson, JI. A supplemental interview for forms of “affective spectrum disorder”. Int J Psychiatry Med. (1991) 21:205–32.
48. Kano, Y, Ohta, M, Nagai, Y, Yokota, K, and Shimizu, Y. Tourette’s disorder coupled with infantile autism: a prospective study of two boys. Jpn J Psychiatry Neurol. (1988) 42:49–57.
49. Kurlan, R, Behr, J, Medved, L, Shoulson, I, Pauls, D, Kidd, JR, et al. Familial tourette’s syndrome: report of a large pedigree and potential for linkage analysis. Neurology. (1986) 36:772–6.
50. Caine, ED, McBride, MC, Chiverton, P, Bamford, KA, Rediess, S, and Shiao, J. Tourette’s syndrome in Monroe county school children. Neurology. (1988) 38:472–5.
51. Burd, L, Kerbeshian, J, Wikenheiser, M, and Fisher, W. A prevalence study of gilles de la tourette syndrome in North Dakota school-age children. J Am Acad Child Psychiatry. (1986) 25:552–3.
52. Jensen, PS, Martin, D, and Cantwell, DP. Comorbidity in adhd: implications for research, practice, and dsm-v. J Am Acad Child Adolesc Psychiatry. (1997) 36:1065–79.
53. Landgren, M, Pettersson, R, Kjellman, B, and Gillberg, C. Adhd, damp and other neurodevelopmental/psychiatric disorders in 6-year-old children: epidemiology and co-morbidity. Dev Med Child Neurol. (1996) 38:891–906.
54. Blum, K, Sheridan, PJ, Wood, RC, Braverman, ER, Chen, TJ, and Comings, DE. Dopamine d2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. (1995) 5:121–41.
55. Comings, DE, Wu, S, Chiu, C, Ring, RH, Gade, R, Ahn, C, et al. Polygenic inheritance of tourette syndrome, stuttering, attention deficit hyperactivity, conduct, and oppositional defiant disorder: the additive and subtractive effect of the three dopaminergic genes--drd 2, d beta h, and dat1. Am J Med Genet. (1996) 67:264–88.
56. Ercan-Sencicek, AG, Stillman, AA, Ghosh, AK, Bilguvar, K, O’Roak, BJ, Mason, CE, et al. L-histidine decarboxylase and tourette’s syndrome. N Engl J Med. (2010) 362:1901–8. doi: 10.1056/NEJMoa0907006
57. Stillman, AA, Krsnik, Z, Sun, J, Rasin, MR, State, MW, Sestan, N, et al. Developmentally regulated and evolutionarily conserved expression of slitrk1 in brain circuits implicated in tourette syndrome. J Comp Neurol. (2009) 513:21–37. doi: 10.1002/cne.21919
58. Singer, HS, Giuliano, JD, Hansen, BH, Hallett, JJ, Laurino, JP, Benson, M, et al. Antibodies against human putamen in children with tourette syndrome. Neurology. (1998) 50:1618–24.
59. Hallett, JJ, Harling-Berg, CJ, Knopf, PM, Stopa, EG, and Kiessling, LS. Anti-striatal antibodies in tourette syndrome cause neuronal dysfunction. J Neuroimmunol. (2000) 111:195–202.
60. Krack, P, Hariz, MI, Baunez, C, Guridi, J, and Obeso, JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. (2010) 33:474–84. doi: 10.1016/j.tins.2010.07.002
61. Servello, D, Porta, M, Sassi, M, Brambilla, A, and Robertson, MM. Deep brain stimulation in 18 patients with severe gilles de la tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. (2008) 79:136–42. doi: 10.1136/jnnp.2006.104067
62. Johnson, MD, Miocinovic, S, McIntyre, CC, and Vitek, JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics. (2008) 5:294–308.
63. Aliane, V, Pérez, S, Deniau, JM, and Kemel, ML. Raclopride or high-frequency stimulation of the subthalamic nucleus stops cocaine-induced motor stereotypy and restores related alterations in prefrontal basal ganglia circuits. Eur J Neurosci. (2012) 36:3235–45. doi: 10.1111/j.1460-9568.2012.08245.x
64. Luigjes, J, van den Brink, W, Feenstra, M, van den Munckhof, P, Schuurman, PR, Schippers, R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. (2012) 17:572–83. doi: 10.1038/mp.2011.114
65. Sundaram, SK, Huq, AM, Wilson, BJ, and Chugani, HT. Tourette syndrome is associated with recurrent exonic copy number variants. Neurology. (2010) 74:1583–90. doi: 10.1212/WNL.0b013e3181e0f147
66. Tylee, DS, Sun, J, Hess, JL, Tahir, MA, Sharma, E, Malik, R, et al. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am J Med Genet B Neuropsychiatr Genet. (2018) 177:641–57. doi: 10.1002/ajmg.b.32652
67. Gao, Y, Wang, S, Wang, A, Fan, S, Ge, Y, Wang, H, et al. Comparison of children and adults in deep brain stimulation for tourette syndrome: a large-scale multicenter study of 102 cases with long-term follow-up. BMC Med. (2024) 22:218. doi: 10.1186/s12916-024-03432-w
68. Muniz, JA, McGuire, JF, and Ramsey, KA. Evidence-based assessment of Tourette syndrome. Psychiatr Clin North Am. (2025) 48:61–75. doi: 10.1016/j.psc.2024.08.006
69. Atkinson-Clement, C, Duflot, M, Lastennet, E, Patsalides, L, Wasserman, E, Sartoris, TM, et al. How does tourette syndrome impact adolescents’ daily living? A text mining study. Eur Child Adolesc Psychiatry. (2023) 32:2623–35. doi: 10.1007/s00787-022-02116-1
70. Matsuda, N, Nonaka, M, Kono, T, Fujio, M, Nobuyoshi, M, and Kano, Y. Premonitory awareness facilitates tic suppression: subscales of the premonitory urge for tics scale and a new self-report questionnaire for tic-associated sensations. Front Psychol. (2020) 11:592. doi: 10.3389/fpsyt.2020.00592
71. Anis, S, Zalomek, C, Korczyn, AD, Rosenberg, A, Giladi, N, and Gurevich, T. Medical cannabis for gilles de la tourette syndrome: an open-label prospective study. Behav Neurol. (2022) 2022:5141773. doi: 10.1155/2022/5141773
72. Szejko, N, Fletcher, J, Martino, D, and Pringsheim, T. Premonitory urge in patients with tics and functional tic-like behaviors. Mov Disord Clin Pract. (2024) 11:276–81. doi: 10.1002/mdc3.13951
73. Müller-Vahl, KR, Pisarenko, A, Fremer, C, Haas, M, Jakubovski, E, and Szejko, N. Functional tic-like behaviors: a common comorbidity in patients with tourette syndrome. Mov Disord Clin Pract. (2024) 11:227–37. doi: 10.1002/mdc3.13932
74. Bornstein, RA. Neuropsychological performance in children with tourette’s syndrome. Psychiatry Res. (1990) 33:73–81.
75. Pauls, DL, and Leckman, JF. The inheritance of Gilles de la Tourette’s syndrome and associated behaviors. Evidence for autosomal dominant transmission. N Engl J Med. (1986) 315:993–7.
76. Burton, FH. Back to the future: circuit-testing ts & ocd. J Neurosci Methods. (2017) 292:2–11. doi: 10.1016/j.jneumeth.2017.07.025
77. Messent, P. Dsm-5. Clin Child Psychol Psychiatry. (2013) 18:479–82. doi: 10.1177/1359104513502138
78. Pomeroy, EC, and Anderson, K. The dsm-5 has arrived. Soc Work. (2013) 58:197–200. doi: 10.1093/sw/swt028
79. Lin, WD, Tsai, FJ, and Chou, IC. Current understanding of the genetics of tourette syndrome. Biom J. (2022) 45:271–9. doi: 10.1016/j.bj.2022.01.008
80. Jindachomthong, K, Yang, C, Huang, Y, Coman, D, Rapanelli, M, Hyder, F, et al. White matter abnormalities in the hdc knockout mouse, a model of tic and ocd pathophysiology. Front Mol Neurosci. (2022) 15:1037481. doi: 10.3389/fnmol.2022.1037481
81. Hatayama, M, and Aruga, J. Developmental control of noradrenergic system by slitrk1 and its implications in the pathophysiology of neuropsychiatric disorders. Front Mol Neurosci. (2022) 15:1080739. doi: 10.3389/fnmol.2022.1080739
82. Gao, T, Yang, L, Zhou, J, Zhang, Y, Wang, L, Wang, Y, et al. Development and validation of a nomogram prediction model for adhd in children based on individual, family, and social factors. J Affect Disord. (2024) 356:483–91. doi: 10.1016/j.jad.2024.04.069
83. Steuber, ER, and McGuire, JF. A meta-analysis of transcranial magnetic stimulation in Tourette syndrome. J Psychiatr Res. (2024) 173:34–40. doi: 10.1016/j.jpsychires.2024.02.057
84. Wang, S, Zhang, Y, Wang, M, Meng, F, Liu, Y, and Zhang, J. Deep brain stimulation for tourette’s syndrome. Cochrane Database Syst Rev. (2024) 8:CD015924. doi: 10.1002/14651858.CD015924
85. Wang, S, Fan, S, Gan, Y, Zhang, Y, Gao, Y, Xue, T, et al. Efficacy and safety of combined deep brain stimulation with capsulotomy for comorbid motor and psychiatric symptoms in tourette’s syndrome: experience and evidence. Asian J Psychiatr. (2024) 94:103960. doi: 10.1016/j.ajp.2024.103960
86. Borrego-Ruiz, A, and Borrego, JJ. Neurodevelopmental disorders associated with gut microbiome dysbiosis in children. Children (Basel). (2024) 11:796. doi: 10.3390/children11070796
Keywords: Tourette syndrome, bibliometric analysis, global research trends, visualization, collaborative networks, genetic-environmental interaction
Citation: Siying Z, Dong T, Xiantao T and Guangyi X (2025) Global bibliometric analysis of Tourette syndrome research (1960–2024): trends, collaborations and emerging themes. Front. Neurol. 16:1564511. doi: 10.3389/fneur.2025.1564511
Edited by:
Diego Iacono, Atlantic Health System, United StatesReviewed by:
Shu Wang, Capital Medical University, ChinaCarlos Quispe-Vicuña, National University of San Marcos, Peru
Copyright © 2025 Siying, Dong, Xiantao and Guangyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiong Guangyi, MzA3OTI0MDAyQHFxLmNvbQ==
 Zhu Siying
Zhu Siying Tian Dong1
Tian Dong1 Tai Xiantao
Tai Xiantao