- 1Department of Neurology, China-Japan Union Hospital of Jilin University, Changchun, China
- 2Department of Traditional Chinese Medicine, China-Japan Union Hospital of Jilin University, Changchun, China
- 3Department of Nursing, China-Japan Union Hospital of Jilin University, Changchun, China
Objectives: To present the latest systematic review and meta-analysis of high-quality randomized controlled trials (RCTs) comparing high-intensity exercise with routine rehabilitation in stroke patients.
Methods: PubMed, Web of Science, and Cochrane were used to searching literature up to October 2024. RCTs with sample size of ≥50 individuals were included. Primary outcomes assessed were the Six-Minute Walking Test (6MWT), Ten-Meter Walk Test (10MWT), VO2peak, Berg Balance Scale (BBS), Timed Up and Go test (TUG), and Montreal Cognitive Assessment (MoCA). Standardized mean differences (SMD) with 95% confidence intervals (CI) were used for pooling data. Stability was evaluated by sensitivity analysis.
Results: Seven RCTs with 724 participants were included. Meta-analysis revealed significant improvements in the 6MWT (SMD: 0.28; 95% CI: 0.11, 0.45) and BBS (SMD: 0.35; 95% CI: 0.03, 0.67) in the high-intensity exercise group. However, high-intensity exercise had no significant effect on VO2peak, TUG, or 10MWT. Sensitivity analysis showed that all outcomes were stable except for the 10MWT. No significant publication bias was detected for any indicator.
Conclusion: High-intensity exercise significantly improves 6MWT and BBS in stroke patients, but does not significantly affect TUG, VO2peak, 10MWT, or MoCA. Clinicians should encourage stroke patients with walking function to engage in structured high-intensity exercise to improve cardiopulmonary function.
Systematic review registration: CRD42024623036 Publicly accessible URL: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024623036.
1 Introduction
Stroke has become a major global public health issue. The Global Burden of Disease report indicates that the stroke’ incidence in China is higher than in other developed countries and continues to rise, with the burden of stroke-related diseases growing increasingly severe (1). Motor dysfunction is a leading cause of disability among stroke survivors (2). Statistics show that over 80% of stroke survivors experience varying degrees of motor function loss, and 50% still have motor dysfunction 3 months after the stroke (3, 4). Studies have shown that, compared to age-and gender-matched healthy individuals, stroke patients have about 50% reduced cardiopulmonary fitness in the early stages (5). In some patients, this cardiopulmonary fitness continues to deteriorate, significantly increasing the risk of cardiovascular disease. Improved cardiopulmonary reserve can optimize peripheral muscle oxygen supply and promote limb function recovery by improving metabolic efficiency, thereby improving quality of life and reducing the risk of recurrence (6, 7). Therefore, improving the cardiopulmonary reserve in stroke patients is crucial for enhancing limb function, improving quality of life, and preventing disease recurrence. Exercise interventions, as a key rehabilitation method, play a vital role in promoting the recovery of daily living abilities and reintegration into family and society for stroke survivors (8).
Currently, two common exercise intervention approaches are used to improve mobility and aerobic capacity: moderate-intensity training and high-intensity training. Moderate-intensity training is typically based on moderate-intensity continuous training (MICT), which involves continuous exercise at 64 to 75% of the maximum heart rate (HRmax) or 40 to 59% of the heart rate reserve (HRR). On the other hand, the most common form of high-intensity training is high-intensity interval training (HIIT), which alternates between short bursts of intense activity and longer recovery periods (9). Studies have shown that HIIT is more effective than low-and moderate-intensity exercise in improving aerobic capacity and other functions in healthy adults and patients with cardiovascular diseases (10–13). In addition, studies have shown that HIIT also has significant advantages in improving executive function and metabolic regulation (14, 15). Due to safety considerations, The American Heart Association now recommends moderate exercise for stroke patients to enhance their exercise volume and aerobic fitness (16, 17). However, numerous studies have demonstrated that high-intensity exercise offers greater benefits than low-and moderate-intensity exercise for stroke rehabilitation (18–20).
The meta-analysis by Baricich et al. (21), which included randomized controlled trials (RCTs) published before May 2023, found that high-intensity exercise significantly improved the Six-Minute Walking Test (6MWT) and VO2peak in stroke patients. However, no significant differences were observed in the Berg Balance Scale (BBS), Ten-Meter Walk Test (10MWT), or Timed Up and Go test (TUG). This meta-analysis, however, had several limitations, including a high proportion of small-sample studies, which introduced significant heterogeneity and potential small sample size effects that reduced the quality of evidence. Moreover, several recent well-designed, large-sample RCTs have reported the rehabilitation effects of high-intensity exercise on stroke patients, potentially altering the current understanding of its role in post-stroke rehabilitation (22–26). Therefore, this article aims to conduct an updated meta-analysis of high-quality RCTs to more precisely evaluate the effects of high-intensity exercise on post-stroke rehabilitation.
2 Methods
2.1 Literature search
According to the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), meta-analysis was conducted (27) and registered with PROSPERO (CRD42024623036). Literature searching was performed via PubMed, Embase, Web of Science, and Cochrane up to October 2024 for randomized controlled trials (RCTs) comparing high-intensity exercise with routine rehabilitation for stroke patients. The search terms included “exercise,” “stroke,” and “high-intensity.” Additionally, we manually screened the reference lists of all included RCTs. Two authors independently retrieved and assessed eligible articles. Disagreements in the literature selection were resolved through discussion. The full literature search strategy is provided in Supplementary Table S1.
2.2 Inclusion and exclusion criteria
2.2.1 Eligibility criteria
P (Population): The study subjects were patients diagnosed with stroke by imaging.
I (Intervention): High-intensity exercise is the intervention method that this study focuses on, usually used alone or in combination with other conventional rehabilitation.
C (Comparison): Control measures included routine rehabilitation, including moderate or low-intensity exercise, or no exercise intervention.
(Outcomes): The main outcome measures including 6MWT, 10MWT, VO2peak, TUG, BBS, MoCA, etc.
S (Study design): To minimize the effect of small sample size, only randomized controlled trials (RCTs) with a total sample size ≥50 participants were included.
2.2.2 Exclusion criteria
Study protocols; unpublished studies; non-original studies (including meeting abstracts, corrections, and replies); non-RCT studies; studies without sufficient data; reviews; RCTs with sample size of less than 50 participants.
2.3 Data abstraction
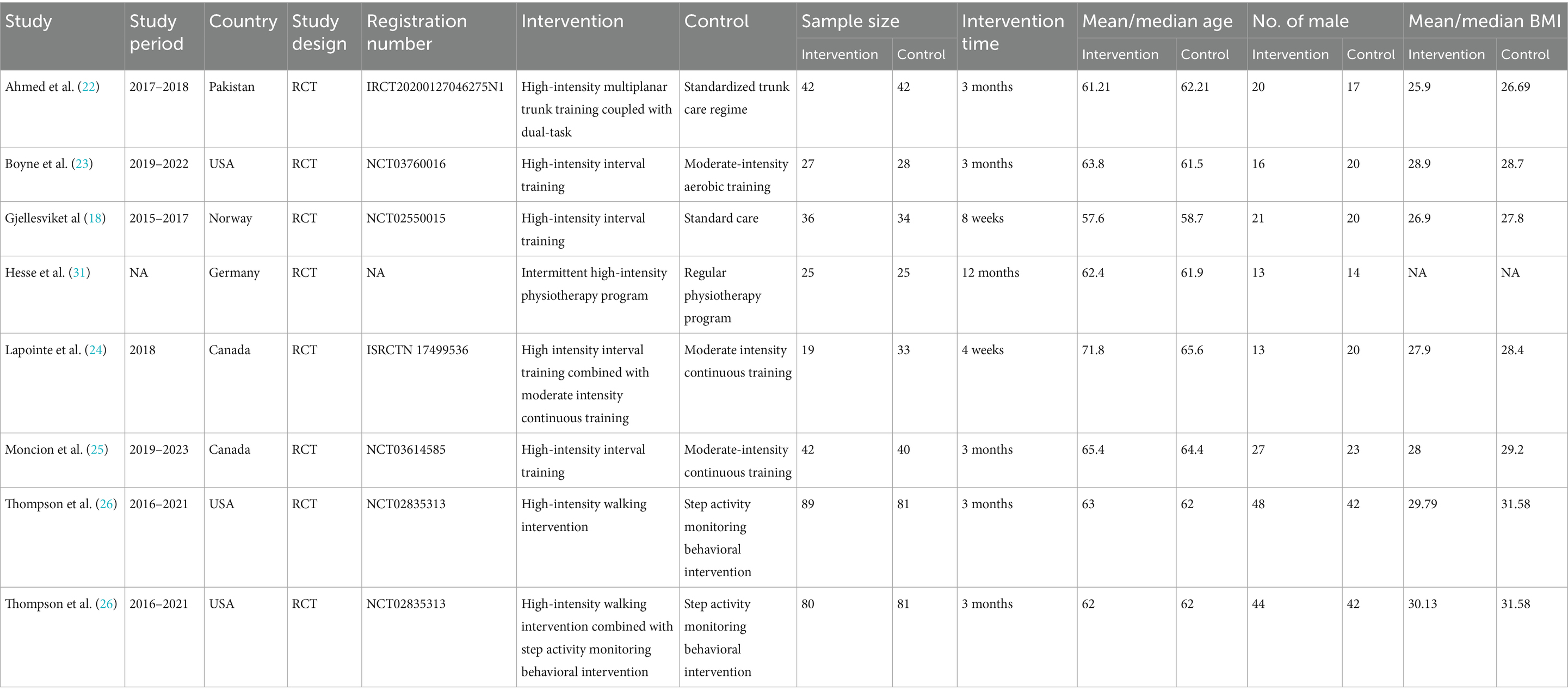
Two researchers conducted data abstraction independently, with any differences resolved by a third author. The following information was abstracted from eligible RCTs: first author name, publication year, research period, study region, study design, registration number, intervention, control, sample size, age, gender, intervention time, 6MWT, 10MWT, VO2peak, TUG, BBS, and MoCA (because cognitive impairment is a common sequela of stroke, MoCA is included to evaluate the impact of intervention on cognitive function). If research data were insufficient, corresponding authors were contacted for complete data when available.
2.4 Quality evaluation
Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 was used to assess the RCTs’ quality, focusing on seven domains: sequence generation, allocation concealment, blinding of participants and personnel, outcome assessment blinding, incomplete outcome data, selective outcome reporting, and other potential biases (28). Each domain was rated as low, high, or unclear risk. Studies with more low-risk evaluations were considered of higher quality. Two authors independently assessed the quality, resolving disagreements through discussion.
2.5 Statistical analysis
Review Manager 5.4.1 was used for performing data synthesis. For continuous outcomes, standardized mean differences (SMD) with 95% confidence intervals (CI) were calculated. Chi-squared (χ2) test (Cochran’s Q) and the inconsistency index was conducted to assess heterogeneity (29). Substantial heterogeneity was defined as a χ2 p-value <0.1 or I2 > 50%. The overall SMD was computed using the random-effects model. For outcomes with more than two studies, sensitivity analysis with one-by-one exclusion was conducted to assess the impact of each individual RCT on the overall result. Funnel plots and Egger’s regression test was conducted for evaluating publication bias (30) in Stata 15.1 (Stata Corp, College Station, Texas, United States). A p-value <0.05 was considered indicative of significant publication bias.
3 Results
3.1 Literature retrieval, study characteristics, and baseline
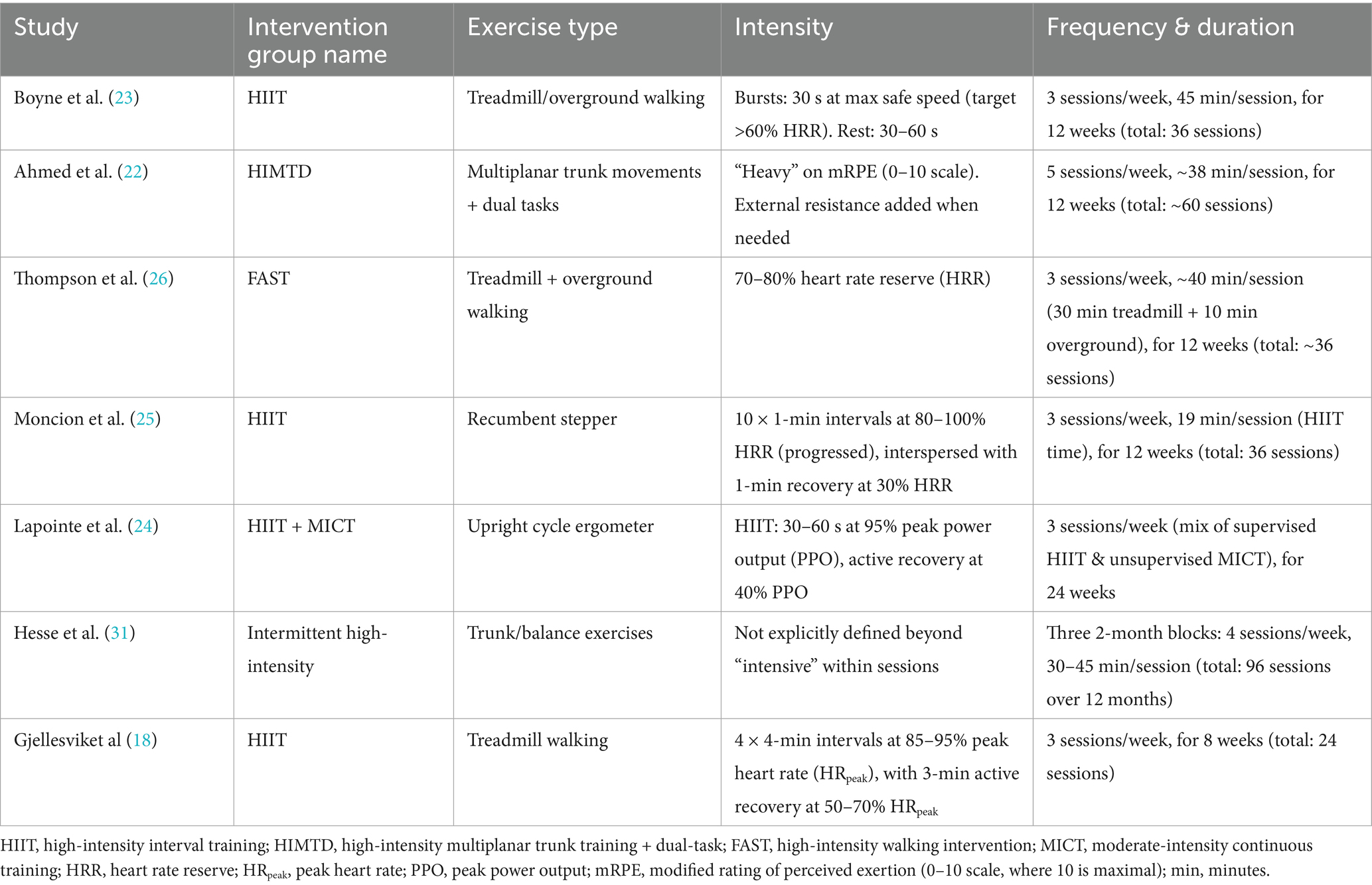
Figure 1 illustrates the literature retrieval and selection process. Eight hundred and eighty-seven studies were identified in PubMed (n = 271), Embase (n = 201), Web of Science (n = 203), and Cochrane (n = 212). After removing duplicates, 408 titles and abstracts were screened, and seven RCTs (18, 22–26, 31) involving 724 patients were included in the meta-analysis. Table 1 outlines the characteristics of each eligible RCT, and Figure 2 presents the quality evaluation details for all included studies. Table 2 presents the detailed high-intensity exercise protocols in each study.
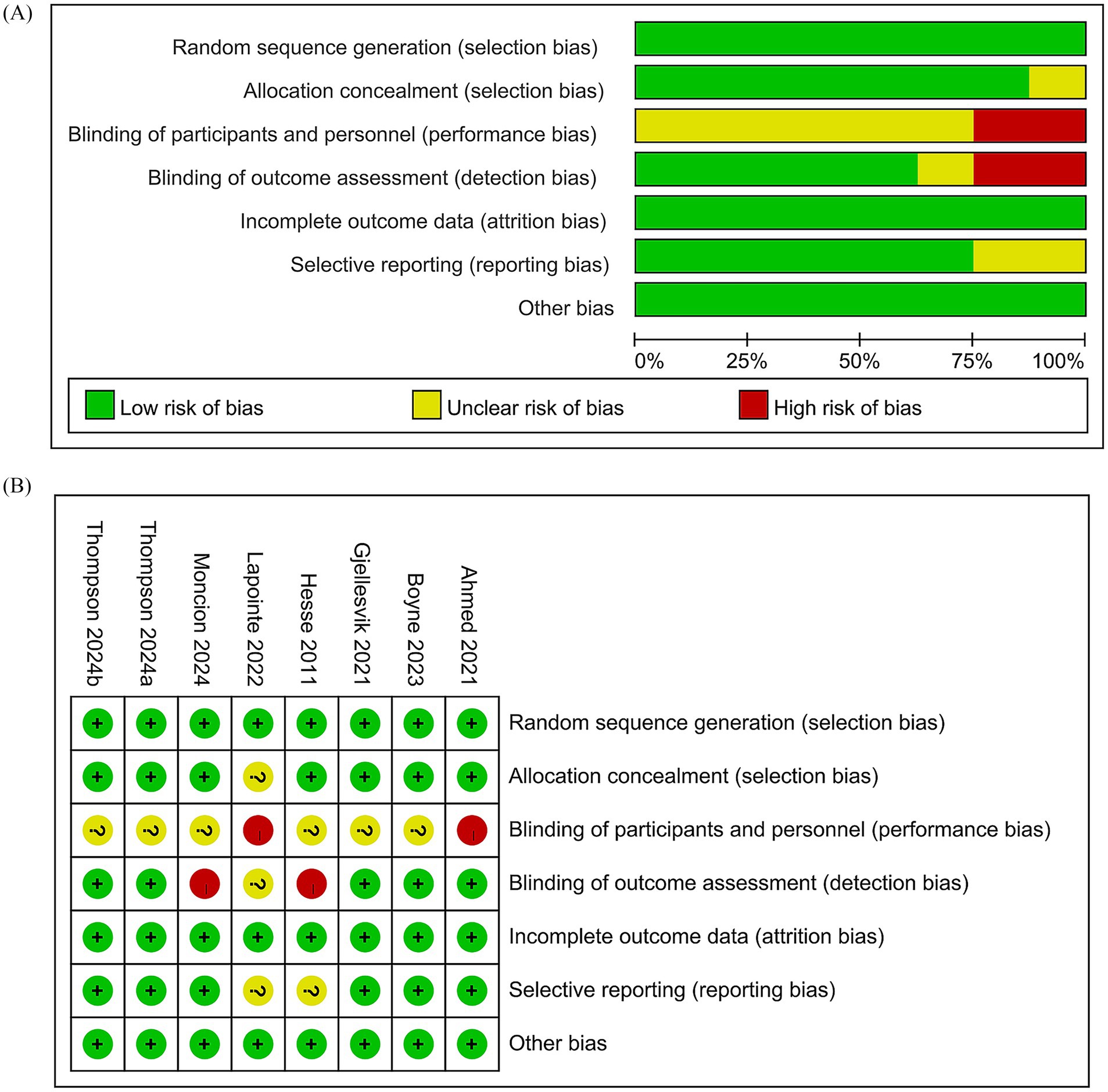
Figure 2. Details of the quality evaluation for included RCTs. (A) Risk bias of summary. (B) Risk bias of graph.
3.2 6MWT
Results from five RCTs with 538 individuals were pooled for the 6MWT. Meta-analysis showed a significant improvement in the 6MWT for the high-intensity exercise group (SMD: 0.27; 95% CI: 0.08, 0.46; p = 0.006), with no significant heterogeneity (I2 = 16%, p = 0.31) (Figure 3A).
3.3 10MWT
Results from six RCTs with 622 patients were pooled for the 10MWT. Meta-analysis showed a similar change in the 10MWT between the two groups (SMD: 0.27; 95% CI: −0.02, 0.57; p = 0.07), with significant heterogeneity (I2 = 68%, p = 0.007) (Figure 3B).
3.4 VO2peak
Results from five RCTs with 520 patients were pooled for VO2peak. Meta-analysis showed a similar change in VO2peak (SMD: 0.14; 95% CI: −0.03, 0.32; p = 0.10), with no significant heterogeneity (I2 = 0%, p = 0.58) (Figure 3C).
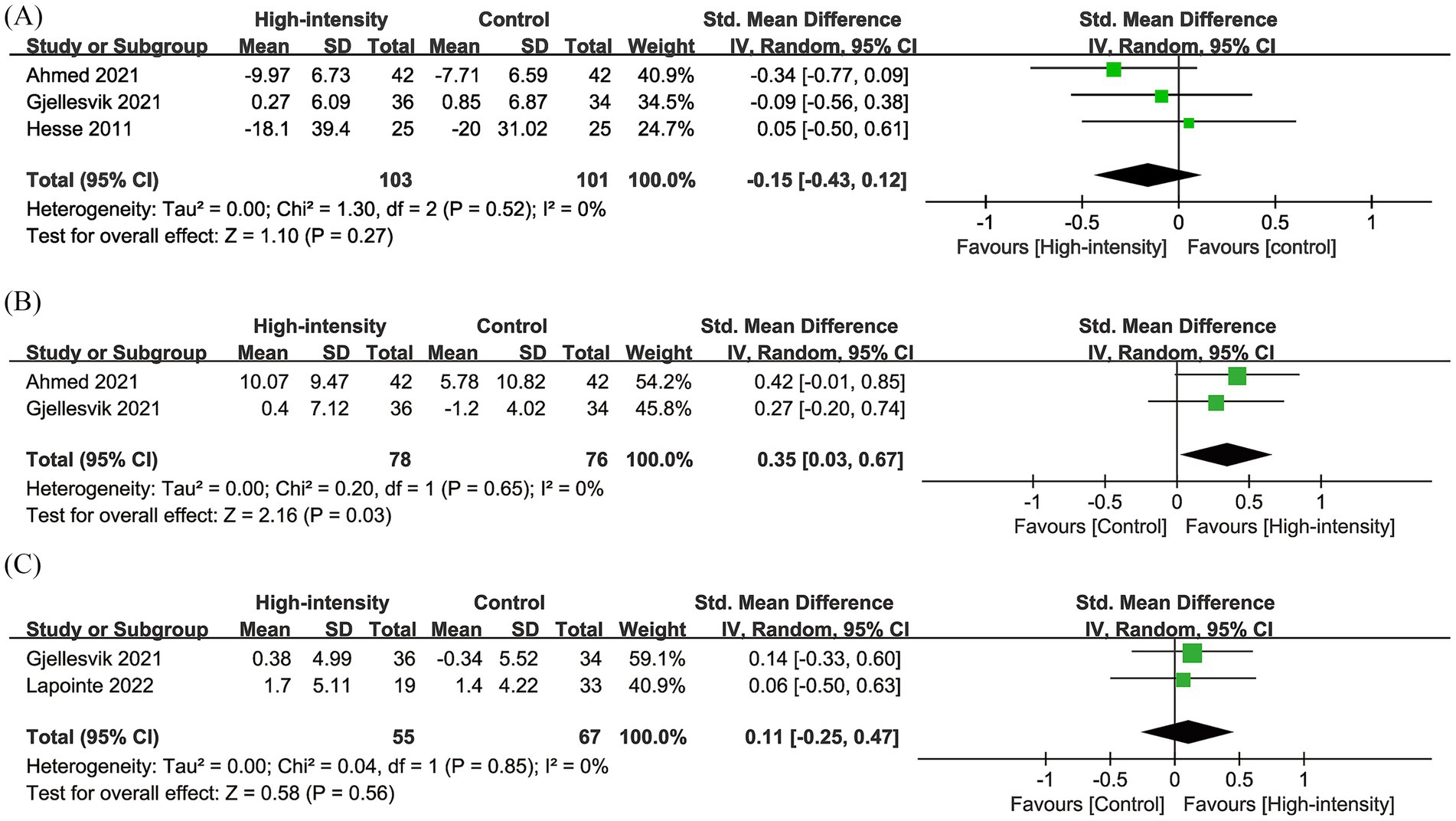
3.5 TUG
Results from three RCTs with 204 patients were pooled for TUG. Meta-analysis showed a similar change in TUG (SMD: −0.15; 95% CI: −0.43, 0.12; p = 0.27), with no significant heterogeneity (I2 = 0%, p = 0.52) (Figure 4A).
3.6 BBS
Results from two RCTs with 154 patients were pooled for BBS. Meta-analysis showed a significantly greater increase in BBS in the high-intensity exercise group (SMD: 0.35; 95% CI: 0.03, 0.67; p = 0.03), with no significant heterogeneity (I2 = 0%, p = 0.65) (Figure 4B).
3.7 MoCA
Results from two RCTs with 122 patients were pooled for MoCA. Meta-analysis showed no significant change in MoCA between the two groups (SMD: 0.11; 95% CI: −0.25, 0.47; p = 0.56), with no significant heterogeneity (I2 = 0%, p = 0.85) (Figure 4C).
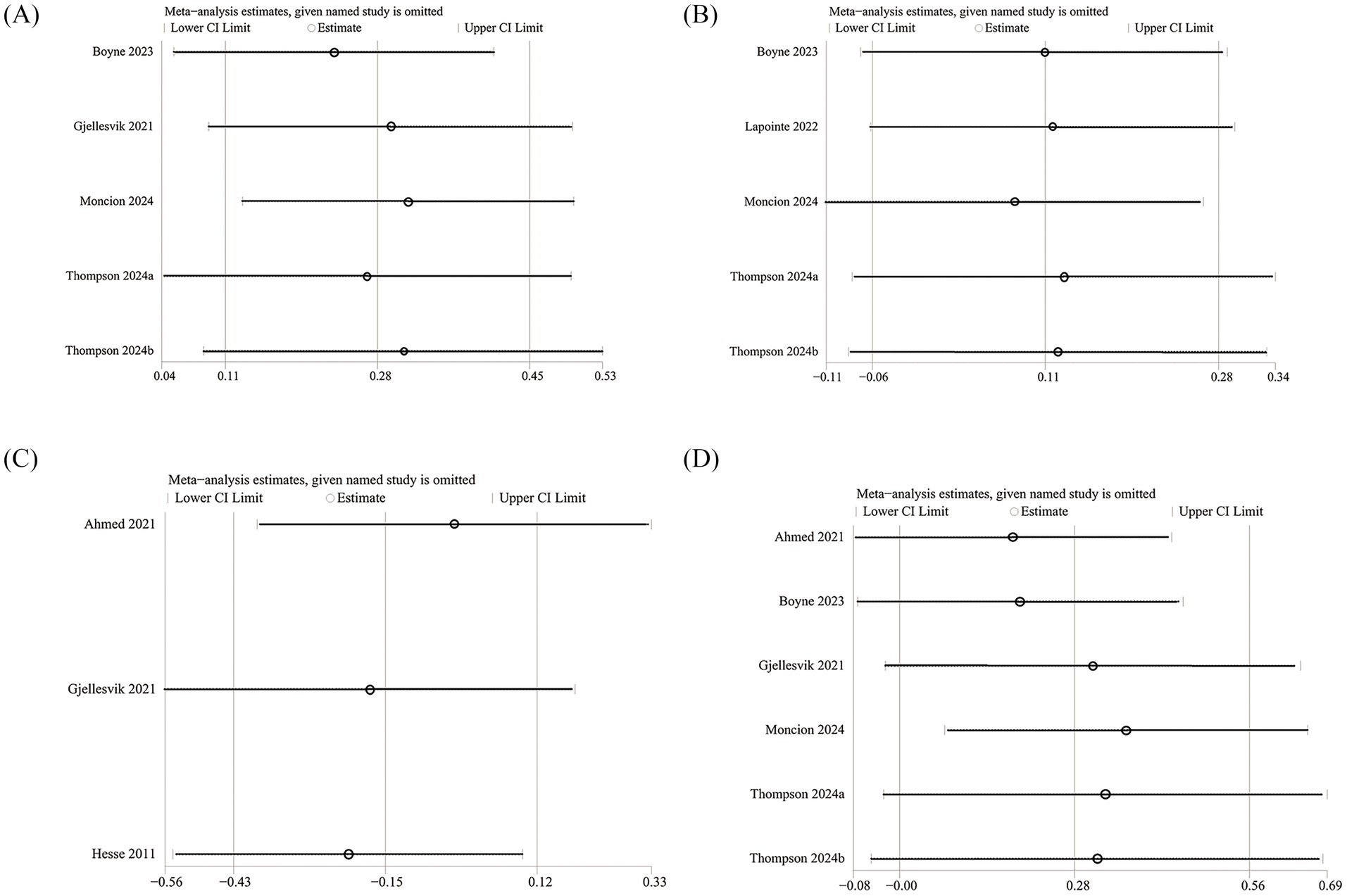
3.8 Sensitivity analysis and publication bias
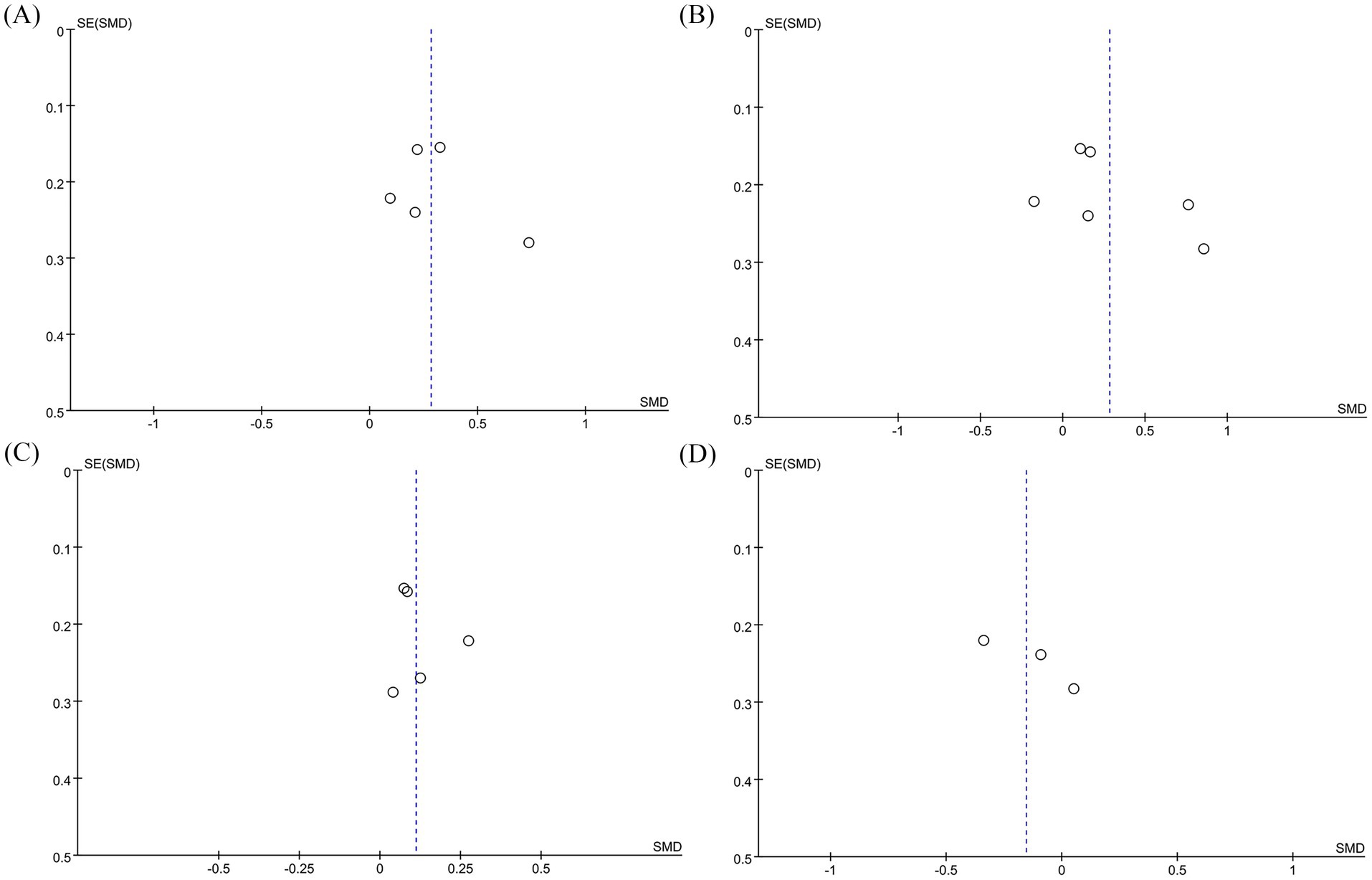
We conducted sensitivity analyses on the 6MWT, 10MWT, VO2peak, and TUG results by sequentially excluding each RCT to assess its impact on the overall SMD. The sensitivity analysis showed that the total SMD remained stable after excluding individual RCTs for 6MWT (Figure 5A), VO2peak (Figure 5B), and TUG (Figure 5C). However, excluding Moncion’s et al. (25) data caused the 10MWT result to shift from nonsignificant to significant (SMD: 0.36; 95% CI: 0.07, 0.66), indicating that the results are not stable enough and need to be interpreted with caution (Figure 5D). No significant asymmetry was observed in the funnel plots for 6MWT (Figure 6A), 10MWT (Figure 6B), VO2peak (Figure 6C), and TUG (Figure 6D). Additionally, Egger’s tests for 6MWT (p = 0.543), 10MWT (p = 0.305), VO2peak (p = 0.654), and TUG (p = 0.247) did not indicate publication bias.
4 Discussion
This study found that high-intensity exercise significantly improved patients’ 6MWT compared to conventional rehabilitation, with low heterogeneity. Sensitivity analysis showed no significant instability, and no potential publication bias was detected, confirming the evidence’s high quality and credibility. However, the included RCTs employed diverse high-intensity exercise protocols, as detailed in Table 2, which may contribute to the heterogeneity observed in some outcomes (notably the 10MWT) and influence the interpretation of overall effects. In addition, it should be emphasized that the conclusions of this article all indicate that there is no statistical difference compared with conventional rehabilitation. These findings suggest that adding high-intensity exercise to conventional rehabilitation can enhance stroke patients’ walking speed. Consistent with this, a meta-analysis on the effects of high-intensity interval training on post-stroke function and quality of life found that high-intensity interval training significantly improved gait speed in stroke patients (32). Despite significant heterogeneity, the meta-analysis by Baricich et al. (21) also found that high-intensity training significantly improved the 6MWT in stroke patients. Additionally, the current study suggests that high-intensity exercise may improve BBS, but due to the limited number of studies and the low quality of evidence, further research is needed to confirm this.
This study found no significant improvement in VO2peak in stroke patients following high-intensity exercise, likely due to negative results reported in all included studies. This contrasts with Baricich et al. (21), who reported that high-intensity exercise significantly improved VO2peak in stroke patients. However, Baricich’s et al. results showed substantial heterogeneity and may have been influenced by the small sample size effect. In contrast, no significant heterogeneity or publication bias was detected in this study, and sensitivity analysis showed no instability. The evidence quality of this study suggests that high-intensity exercise has a limited effect on VO2peak in stroke patients. This negative result may be due to the fact that autonomic dysfunction in stroke patients limits the heart rate response (33). However, due to the limited number of studies, further research is needed to confirm whether high-intensity exercise can improve VO2peak in stroke patients.
This study found that high-intensity exercise had no significant effect on TUG and 10MWT in stroke patients, consistent with Baricich et al. (21). Due to limitations such as small sample size and instability, more multicenter, large-sample RCTs are needed to confirm the efficacy of high-intensity exercise on TUG and 10MWT. Additionally, this study included MoCA, an unreported indicator, but found no significant effect of high-intensity exercise on MoCA in stroke patients. Although cognitive function is a key outcome for stroke survivors, the results suggest that high-intensity exercise does not improve cognitive function, which aligns with previous research.
The primary modes of exercise varied considerably across studies. Treadmill walking, either alone (18, 23, 26) or combined with overground walking (23, 26), was the most common modality. This focus on gait-specific training likely underpins the significant improvement in the walking endurance measure (6MWT). In contrast, Moncion et al. (25) utilized a recumbent stepper, Lapointe et al. (24) used an upright cycle ergometer, and Ahmed et al. (22) focused on high-intensity multiplanar trunk training with dual tasks. Hesse et al. (31) employed intensive trunk and balance exercises. These non-ambulatory or non-gait-specific modalities may partially explain the lack of significant effect on gait speed (10MWT) and the sensitivity of this outcome to the removal of the Moncion et al. study (which used a stepper).
The definition and control of “high-intensity” also differed. Most studies used percentage of heart rate reserve (HRR) as the intensity target (18, 23–26), with target intensities ranging from >60% HRR bursts (23) to 80–100% HRR intervals (18, 25). Ahmed et al. (22) relied on a modified rating of perceived exertion (mRPE) scale (targeting “heavy”), while Hesse et al. (31) described sessions as “intensive” without a specific physiological metric. Lapointe et al. (24) used peak power output (PPO) on a cycle ergometer. These variations in intensity quantification and the physiological systems primarily stressed (aerobic vs. neuromuscular) represent a source of potential heterogeneity in outcomes like VO2peak and BBS.
Furthermore, the structure of the high-intensity stimulus varied. While most implemented HIIT protocols with defined work:rest ratios (e.g., Boyne: 30 s bursts; Moncion: 1-min intervals; Gjellesvik: 4-min intervals), Thompson et al. (26) and Ahmed et al. (22) prescribed continuous high-intensity walking or trunk training, respectively. Hesse et al. (31) delivered intensive intermittent sessions over a very long period. The duration of the intervention programs also spanned a wide range, from 8 weeks (18) to 24 weeks (24) and even 12 months (31), with corresponding variations in the total number of sessions (24 to 96).
This heterogeneity in exercise modality, intensity definition and target, session structure, and program duration highlights a significant challenge in synthesizing evidence for “high-intensity exercise” in stroke rehabilitation. While our analysis suggests a beneficial effect on walking endurance and balance, the inconsistent effects on other outcomes, particularly gait speed and peak oxygen consumption, may partly stem from these fundamental differences in how the intervention was applied. Future research would benefit from greater standardization in defining and reporting high-intensity parameters and matching the exercise modality more closely to the functional outcomes being targeted.
Preliminary evidence suggests that stroke patients can physiologically adapt to exercise training. However, the clinical application of exercise as a therapeutic intervention remains insufficient in the general stroke population. The decline in cardiopulmonary function after stroke may result from inadequate physical activity, leading to reduced muscle activation, impaired oxygen transport, and decreased cellular metabolism. More research is needed to fully understand the consequences and remedies of cardiopulmonary dysfunction after stroke. Systematic reviews by Luo et al. (34) and Weiner et al. (35) reported that high-intensity exercise benefits the cardiopulmonary health of stroke survivors and may be a safe and effective intervention for rehabilitation. This study confirmed that exercise training improves cardiopulmonary function in stroke patients. The mechanism may involve enhanced microvascular density, increased cardiac output, and improved mitochondrial respiration and citrate synthase activity (36). Additionally, exercise training, when added to conventional rehabilitation, can inhibit macrophage-mediated inflammatory responses in paralyzed skeletal muscles after ischemic stroke, prevent muscle atrophy, and improve physical function (37).
A meta-analysis by Machado et al. (38) on the sustained effects of high-intensity training found that stroke patients can maintain cardiopulmonary health in the short, medium, and long term after discontinuing rehabilitation. However, few studies have explored the long-term maintenance of cardiopulmonary health post-exercise, and the optimal intervention parameters for sustaining function remain unclear. Due to limited data, this study did not conduct a subgroup analysis based on training duration. Future studies should focus on longer-term follow-up to determine whether HIIT is more effective than other programs in maintaining cardiopulmonary function and whether a plateau occurs in its improvement. Clinicians should encourage stroke patients with walking function to engage in planned high-intensity training to enhance cardiopulmonary fitness. Further research is needed to assess whether stroke patients without walking function can also benefit from HIIT and to define the most suitable training model.
This meta-analysis has several limitations. First, due to the inherent limitations of exercise intervention studies, none of the seven included RCTs adequately blinded participants and personnel, which may affect the reliability of the results. Second, the RCTs varied in intervention methods (high-intensity exercise types, duration, and frequency), introducing potential heterogeneity. However, most indicators in this study showed no significant heterogeneity, minimizing its impact on the results. Third, due to the limited number of studies, we could not analyze the effects of high-intensity exercise on outcomes such as quality of life and psychological state, which requires further investigation. Additionally, data limitations prevented subgroup analysis based on intervention methods, intensity, frequency, age, race, etc., so it remains unclear whether these factors influenced the findings. Fourth, most studies were from Europe and North America (mainly the United States, Canada, and Germany), with limited data from regions such as Asia and Africa. Therefore, the global generalizability of these findings remains uncertain. Despite these limitations, this updated meta-analysis overcomes previous issues such as significant heterogeneity and small sample sizes and further supports the positive effects of high-intensity exercise on cardiopulmonary rehabilitation in stroke patients.
5 Conclusion
This study found that compared with usual care, high-intensity exercise significantly improves 6MWT and BBS in stroke patients, but has no significant effect on TUG, VO2peak, 10MWT, or MoCA. Clinicians should encourage stroke patients with walking function to engage in planned high-intensity training to enhance cardiopulmonary function. Given the limitations of this study, such as the small sample size, regional selection bias, and potential instability, future large-scale, multi-center RCTs are needed to further confirm the rehabilitation effects of high-intensity exercise on stroke patients and identify potential influencing factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. YD: Resources, Software, Supervision, Validation, Writing – original draft. HL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was financially supported by the Jilin Provincial Scientific and Technological Development Program (YDZJ202201ZYTS532).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1565118/full#supplementary-material
References
1. He, Q, Wang, W, Zhang, Y, Xiong, Y, Tao, C, Ma, L, et al. Global, regional, and national burden of stroke, 1990–2021: a systematic analysis for Global Burden of Disease 2021. Stroke. (2024) 55:2815–24. doi: 10.1161/STROKEAHA.124.048033
2. Benjamin, EJ, Muntner, P, Alonso, A, Bittencourt, MS, Callaway, CW, Carson, AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
3. Einstad, MS, Saltvedt, I, Lydersen, S, Ursin, MH, Munthe-Kaas, R, Ihle-Hansen, H, et al. Associations between post-stroke motor and cognitive function: a cross-sectional study. BMC Geriatr. (2021) 21:103. doi: 10.1186/s12877-021-02055-7
4. Selves, C, Stoquart, G, and Lejeune, T. Gait rehabilitation after stroke: review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol Belg. (2020) 120:783–90. doi: 10.1007/s13760-020-01320-7
5. de Sousa, JCS, Torriani-Pasin, C, Tosi, AB, Fecchio, RY, Costa, LAR, and de Moraes Forjaz, CL. Aerobic stimulus induced by virtual reality games in stroke survivors. Arch Phys Med Rehabil. (2018) 99:927–33. doi: 10.1016/j.apmr.2018.01.014
6. Bernhardt, J, Hayward, KS, Kwakkel, G, Ward, NS, Wolf, SL, Borschmann, K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
7. Boissoneault, C, Rose, DK, Grimes, T, Waters, MF, Khanna, A, Datta, S, et al. Trajectories of stroke recovery of impairment, function, and quality of life in response to 12-month mobility and fitness intervention. NeuroRehabilitation. (2021) 49:573–84. doi: 10.3233/NRE-210147
8. Saunders, DH, Sanderson, M, Hayes, S, Kilrane, M, Greig, CA, Brazzelli, M, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. (2016) 3:CD003316. doi: 10.1002/14651858.CD003316.pub6
9. Boyne, P, Dunning, K, Carl, D, Gerson, M, Khoury, J, Rockwell, B, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther. (2016) 96:1533–44. doi: 10.2522/ptj.20150277
10. Chen, X, Zhang, T, Hu, X, Wen, Z, Lu, W, and Jiang, W. High-intensity interval training programs versus moderate-intensity continuous training for individuals with heart failure: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2024) 106:98–112. doi: 10.1016/j.apmr.2024.05.028
11. Costache, AD, Maștaleru, A, Leon, MM, Roca, M, Gavril, RS, Cosău, DE, et al. High-intensity interval training vs. medium-intensity continuous training in cardiac rehabilitation programs: a narrative review. Medicina. (2024) 60:1875. doi: 10.3390/medicina60111875
12. Oliveira, A, Fidalgo, A, Farinatti, P, and Monteiro, W. Effects of high-intensity interval and continuous moderate aerobic training on fitness and health markers of older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2024) 124:105451. doi: 10.1016/j.archger.2024.105451
13. Wang, Z, and Wang, J. The effects of high-intensity interval training versus moderate-intensity continuous training on athletes’ aerobic endurance performance parameters. Eur J Appl Physiol. (2024) 124:2235–49. doi: 10.1007/s00421-024-05532-0
14. Guo, L, Chen, J, and Yuan, W. The effect of HIIT on body composition, cardiovascular fitness, psychological well-being, and executive function of overweight/obese female young adults. Front Psychol. (2023) 13:1095328. doi: 10.3389/fpsyg.2022.1095328
15. Reyes-Amigo, T, Bezerra, A, Gomez-Mazorra, M, Boppre, G, Martins, C, Carrasco-Beltran, H, et al. Effects of high-intensity interval training on executive functions in children and adolescents: a systematic review and meta-analysis. Phys Act Rev. (2022) 10:77–87. doi: 10.16926/par.2022.10.23
16. Billinger, SA, Arena, R, Bernhardt, J, Eng, JJ, Franklin, BA, Johnson, CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2532–53. doi: 10.1161/STR.0000000000000022
17. Lloyd, M, Skelton, DA, Mead, GE, Williams, B, and van Wijck, F. Physical fitness interventions for nonambulatory stroke survivors: a mixed-methods systematic review and meta-analysis. Brain Behav. (2018) 8:e01000. doi: 10.1002/brb3.1000
18. Gjellesvik, TI, Becker, F, Tjønna, AE, Indredavik, B, Lundgaard, E, Solbakken, H, et al. Effects of high-intensity interval training after stroke (the HIIT stroke study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. (2021) 102:1683–91. doi: 10.1016/j.apmr.2021.05.008
19. Gjellesvik, TI, Becker, F, Tjønna, AE, Indredavik, B, Nilsen, H, Brurok, B, et al. Effects of high-intensity interval training after stroke (the HIIT-stroke study): a multicenter randomized controlled trial. Arch Phys Med Rehabil. (2020) 101:939–47. doi: 10.1016/j.apmr.2020.02.006
20. Miller, A, Reisman, DS, Billinger, SA, Dunning, K, Doren, S, Ward, J, et al. Moderate-intensity exercise versus high-intensity interval training to recover walking post-stroke: protocol for a randomized controlled trial. Trials. (2021) 22:457. doi: 10.1186/s13063-021-05419-x
21. Baricich, A, Borg, MB, Battaglia, M, Facciorusso, S, Spina, S, Invernizzi, M, et al. High-intensity exercise training impact on cardiorespiratory fitness, gait ability, and balance in stroke survivors: a systematic review and meta-analysis. J Clin Med. (2024) 13:5498. doi: 10.3390/jcm13185498
22. Ahmed, U, Karimi, H, Amir, S, and Ahmed, A. Effects of intensive multiplanar trunk training coupled with dual-task exercises on balance, mobility, and fall risk in patients with stroke: a randomized controlled trial. J Int Med Res. (2021) 49:3000605211059413. doi: 10.1177/03000605211059413
23. Boyne, P, Billinger, SA, Reisman, DS, Awosika, OO, Buckley, S, Burson, J, et al. Optimal intensity and duration of walking rehabilitation in patients with chronic stroke: a randomized clinical trial. JAMA Neurol. (2023) 80:342–51. doi: 10.1001/jamaneurol.2023.0033
24. Lapointe, T, Houle, J, Sia, YT, Payette, M, and Trudeau, F. Addition of high-intensity interval training to a moderate intensity continuous training cardiovascular rehabilitation program after ischemic cerebrovascular disease: a randomized controlled trial. Front Neurol. (2022) 13:963950. doi: 10.3389/fneur.2022.963950
25. Moncion, K, Rodrigues, L, De Las Heras, B, Noguchi, KS, Wiley, E, Eng, JJ, et al. Cardiorespiratory fitness benefits of high-intensity interval training after stroke: a randomized controlled trial. Stroke. (2024) 55:2202–11. doi: 10.1161/STROKEAHA.124.046564
26. Thompson, ED, Pohlig, RT, McCartney, KM, Hornby, TG, Kasner, SE, Raser-Schramm, J, et al. Increasing activity after stroke: a randomized controlled trial of high-intensity walking and step activity intervention. Stroke. (2024) 55:5–13. doi: 10.1161/STROKEAHA.123.044596
27. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
29. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
30. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
31. Hesse, S, Welz, A, Werner, C, Quentin, B, and Wissel, J. Comparison of an intermittent high-intensity vs continuous low-intensity physiotherapy service over 12 months in community-dwelling people with stroke: a randomized trial. Clin Rehabil. (2011) 25:146–56. doi: 10.1177/0269215510382148
32. Maskeliunas, R, Damasevicius, R, Paulauskas, A, Ceravolo, MG, Charalambous, M, Kambanaros, M, et al. Deep reinforcement learning-based iTrain serious game for caregivers dealing with post-stroke patients. Information. (2022) 13:564. doi: 10.3390/info13120564
33. Jimenez-Ruiz, A, Racosta, JM, Kimpinski, K, Hilz, MJ, and Sposato, LA. Cardiovascular autonomic dysfunction after stroke. Neurol Sci. (2021) 42:1751–8. doi: 10.1007/s10072-021-05128-y
34. Luo, L, Meng, H, Wang, Z, Zhu, S, Yuan, S, Wang, Y, et al. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2020) 63:59–68. doi: 10.1016/j.rehab.2019.07.006
35. Wiener, J, McIntyre, A, Janssen, S, Chow, JTY, Batey, C, and Teasell, R. Effectiveness of high-intensity interval training for fitness and mobility post stroke: a systematic review. PM R. (2019) 11:868–78. doi: 10.1002/pmrj.12154
36. Rosenblat, MA, Granata, C, and Thomas, SG. Effect of interval training on the factors influencing maximal oxygen consumption: a systematic review and meta-analysis. Sports Med. (2022) 52:1329–52. doi: 10.1007/s40279-021-01624-5
37. Liu, MX, Luo, L, Fu, JH, He, JY, Chen, MY, He, ZJ, et al. Exercise-induced neuroprotection against cerebral ischemia/reperfusion injury is mediated via alleviating inflammasome-induced pyroptosis. Exp Neurol. (2022) 349:113952. doi: 10.1016/j.expneurol.2021.113952
Keywords: high-intensity, exercise, stroke, randomized controlled trials, meta-analysis
Citation: Li S, Dou Y and Li H (2025) Effects of high-intensity exercise on rehabilitation of patients after stroke: a systematic review and meta-analysis of randomized controlled trials with high quality. Front. Neurol. 16:1565118. doi: 10.3389/fneur.2025.1565118
Edited by:
Shihao He, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Timothy F. Boerger, Medical College of Wisconsin, United StatesYookyung Lee, Chung-Ang University Gwangmyeong Hospital, Republic of Korea
Copyright © 2025 Li, Dou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li, SG9uZ2xpMjAxOEBqbHUuZWR1LmNu
 Shuang Li
Shuang Li Yuchang Dou
Yuchang Dou Hong Li
Hong Li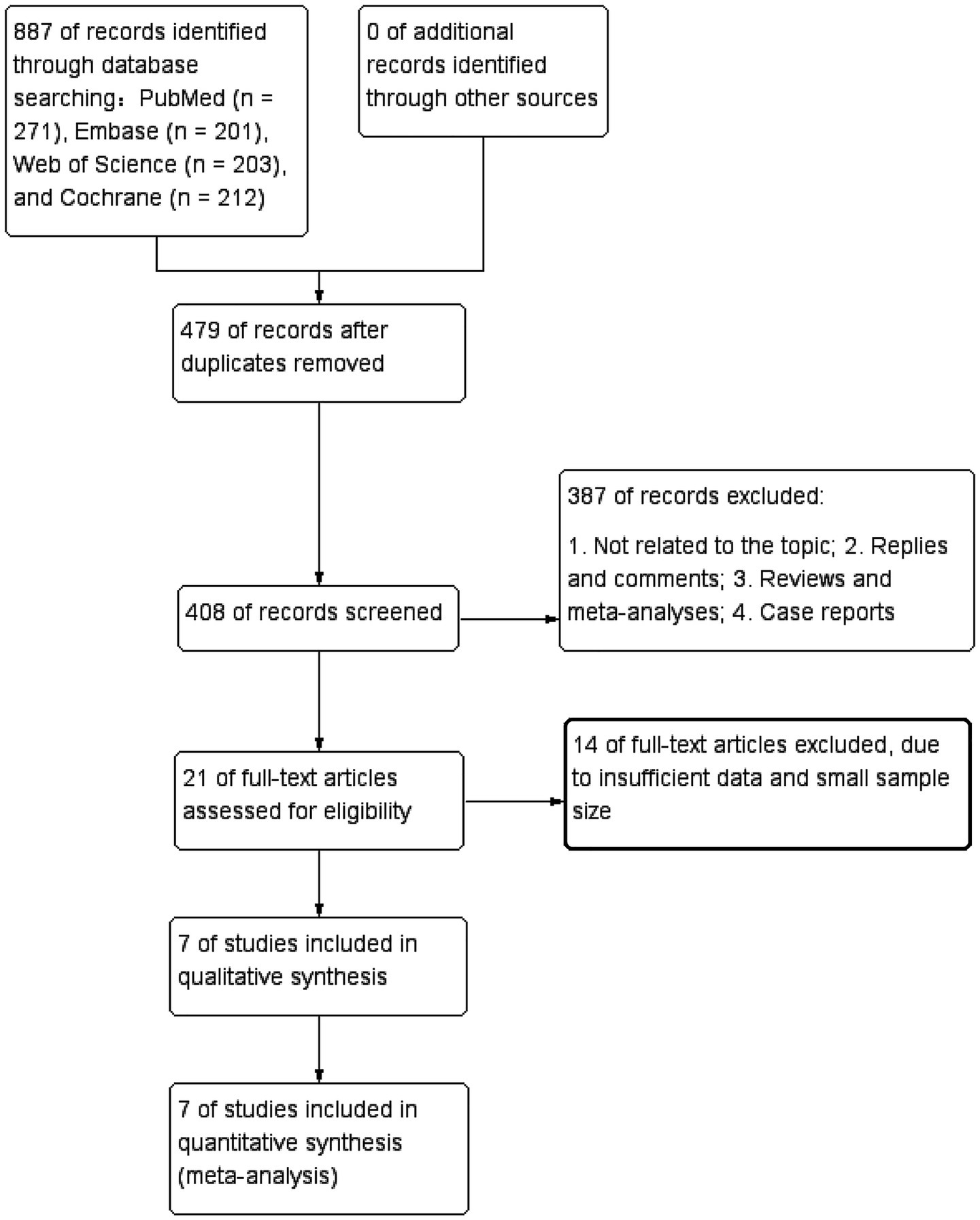
![Forest plots A, B, and C compare high-intensity vs. control groups across multiple studies. Panel A shows a standard mean difference favoring high-intensity (0.27 [0.08, 0.46]) with moderate heterogeneity (I² = 16%). Panel B also favors high-intensity (0.27 [-0.02, 0.57]) with higher heterogeneity (I² = 68%). Panel C shows a slight favor towards high-intensity (0.14 [-0.03, 0.32]) with no heterogeneity (I² = 0%). Each plot illustrates study-specific and overall confidence intervals, using a random-effects model.](https://www.frontiersin.org/files/Articles/1565118/fneur-16-1565118-HTML/image_m/fneur-16-1565118-g003.jpg)