Abstract
Isolated spinal artery aneurysms (ISAAs) are a rare cause of intracranial and spinal hemorrhages with unclear pathophysiology and natural history and non-standardized management strategies. We hereby present two cases of ruptured ISAAs of posterior spinal arteries treated with open surgery and embolization, respectively. Case presentations are followed by a comprehensive literature review on ISAA pathophysiology, natural history, and management strategies.
Introduction
Subarachnoid hemorrhage (SAH) from a ruptured isolated spinal artery aneurysm (ISAA) is a very rare entity (1). While ISAAs are believed to be dissecting in nature (1, 2), their exact pathophysiology and natural history have yet to be clarified, as the condition has mostly been described in case reports or small series. High heterogeneity in clinical presentation and management strategies, as well as scarcity of pertinent data from the existing literature results in the absence of standardized diagnostic and treatment guidelines. We report two cases of ruptured ISAAs of posterior spinal arteries (PSAs) and present a literature review with particular attention to imaging features and treatment options for this rare condition.
Methods
We included two cases of ruptured ISAAs from our institutions and performed a literature review on the topic. A PubMed literature search was performed using the terms “spinal”, “artery”, “aneurysm”, “subarachnoid”, “h(a)emorrhage”, and/or combinations thereof. Titles and abstracts were screened for relevance by the first author. Case reports, case series, and review articles that specifically discussed cases of ruptured ISAAs were included. To ensure that reports were not overlooked, we reviewed the references of each relevant article to identify secondary sources for inclusion. Cases of intra- or perinidal spinal arterial aneurysms associated with vascular malformations were excluded. For reports published in 2010 and earlier, information was extracted from the literature review by Kim HJ and Choi IS in 2011, which summarized 43 cases from 38 reports. Two additional case reports published before 2010 found in our literature search were also added to the case analysis, summarized in Table 1 and referenced accordingly. For reports published after 2010, all cases are summarized in Table 1, and individual cases are summarized in Table 2.
Table 1
| Year of publications/no. of studies and cases | Age/sex | Artery involved | Spinal level | Treatment | Outcome |
|---|---|---|---|---|---|
| 2010 and earlier (40 studies, 45 cases) (2, 3, 15, 21, 23–25, 27, 28, 33, 35–62) |
Mean age 55 29 F 16 M |
26 ASA 9 PSA 4 LSA 6 unknown |
24 C 17 T 4 TL |
26 Surgery 2 Embolization 14 Conservative 3 Unknown |
33 clinical improvement 9 deaths (5 conservative, 1 surgery, 1 embolization, 2 unknown) 3 unknown |
| 2011-2024 (45 studies, 70 cases) | Mean age 56 40F 30M |
38 ASA 25 PSA 4 LSA 3 unspecified |
22 C 32 Th 5 TL 11 Unknown levels |
24 Surgery 13 Embolization 26 Conservative 7 Unknown Tx |
60 clinical improvement 2 deaths (both conservatives) 8 unknown |
Summary of case studies and case series from 1930 to 2010 (40 articles) and 2011 to 2024 (45 articles).
anterior spinal artery (ASA), posterior spinal artery (PSA), lateral spinal artery (LSA), cervical (C), thoracic (T), lumbar (L), male (M), and female (F).
Table 2
| Author, year | Age, y/sex | Clinical presentation | Imaging findings | Treatment | Outcome |
|---|---|---|---|---|---|
| Iihoshi et al. 2011 (63) | 60/F | Headache and back pain | Posterior fossa and spinal SAH T12 radiculomedullary artery aneurysm | Conservative—Spontaneous occlusion with preserved ASA | Clinical improvement |
| Kim and Choi 2012 (1) | 52/M | Abdominal and back pain | Spinal SAH T8 PSA aneurysm | Embolization (coils and NBCA) | Cure Clinical improvement |
| Sato et al. 2012 (34) | 67/F | Back pain, paresthesia, sphincter disturbance | Spinal SAH and cord infarction T11 radiculomedullary and T8 radiculopial aneurysms | Conservative—Spontaneous occlusion | Clinical improvement |
| Morigaki et al. 2012 (12) | 78/M | Tetraparesis and LOC | SAH LSA aneurysm at the C2 level | Embolization (coils) | Partial PICA infarct Clinical improvement |
| Van Es et al. 2013 (58) (Case 1) | 62/F | Headache Back pain | Spinal SAH Left L1 radiculopial aneurysm | Surgery (aneurysm resection) | NA |
| Van Es et al. 2013 (58) (Case 2) | 68/M | Headache and back pain | Spinal SAH T4 radiculopial aneurysm | NA | NA |
| Son et al. 2013 (64) | 45/F | Headache and back pain | Intracranial and spinal SAH Art. of AdamKwz ASA T12 aneurysm | Conservative—Spontaneous occlusion | Clinical improvement |
| Yang 2013 (26) | 47/M | LOC | SAH ASA (from Rt Vert) dissecting aneurysm | Conservative—no change to aneurysm, no rebleed | Died @103 days post-SAH (cancer-related) |
| Marovic et al. 2013 (65) | 58/M | Back pain | Spinal SAH T3 radicular spinal artery aneurysm | Surgery (aneurysm resection) | Cure Unknown clinical outcome |
| Romero et al. 2014 (11) | 37/F | Chest pain, back pain, headache | Post fossa SAH T3 radiculomedullary aneurysm | Conservative—Spontaneous occlusion | Clinical improvement |
| Romero et al. 2014 (11) | 72/F | Neck and back pain | Intracranial and spinal SAH T11 radiculopial aneurysm | Conservative—nil imaging f/up | Clinical improvement |
| Pahl et al. 2014 (66) | 43/F | Headache, vomiting, LOC | SAH and IVH ASA (from Lt Vert) aneurysm | Conservative—Spontaneous occlusion | Clinical improvement |
| Horio et al. 2015 (4) | 84/M | Altered mental status | SAH T12 Radiculopial artery aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Ronchetti et al. 2015 (32) (Case 1) | 51/F | Headache, neck pain, lower limb numbness | Posterior fossa SAH, spinal SAH and SDH 2 thoracic PSA aneurysms in one patient | Surgical resection (aneurysm resection cx wound infection) | Cure Clinical improvement |
| Ronchetti et al. 2015 (32) (Case 2) | 68/M | Headache, back pain | Posterior fossa and spinal SAH Thoracic PSA aneurysm | Embolization (with particles) | Cure Clinical improvement |
| Ashour et al. 2015 (18) | 72/M | Headache | Cranial SAH C2 ASA aneurysm (bilateral VA occlusions) | Surgery (aneurysm clipping and wrapping) | Cure Clinical improvement |
| Nakhla et al. 2016 (8) | 88/F | Headache and neck pain | Cranial and upper spinal SAH Cervical ASA aneurysm (related to a herniated disk) | Conservative | Clinical improvement |
| Ikeda et al. 2016 (29) | 54/M | Back pain and vomiting | Spinal SAH and SDH T10 radiculopial artery aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Doberstein et al. 2016 (67) | 59/M | Back pain and lower limb weakness | SAH T11 Art. Of AdamKwz (ASA) aneurysm | Conservative | Clinical improvement |
| Takata et al. 2016 (68) | 72/M | Back pain | Spinal SAH T9 radiculopial artery | Surgery (aneurysm resection) | Cure Clinical improvement |
| Agarwal et al. 2016 (69) | 47/F | Back pain | Rt T10 intercostal artery aneurysm, close to the origin of Art. Of AdamKwz | Conservative—Spontaneous occlusion | Clinical improvement |
| Aguilar-Salinas et al. 2017 (70) | 54/F | Backpain and headache | Spinal SAH Left T10 Art. Of AdamKwz (ASA) aneurysm | Conservative—Spontaneous occlusion | Clinical improvement |
| Ren et al. 2017) (71) (Case 1) | 57/F | Headache | SAH C1 ASA aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Ren et al. 2017 (71) (Case 2) | 27/F | Lower limb pain and numbness | L2 RMA aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Singh et al. 2017 (13) (Case 1) | 18/M | Severe neck pain and UL weakness | SAH C7 ASA aneurysm (coarctation of the aorta) | Conservative | Unknown outcome |
| Singh et al. 2017 (13) (Case 2) | 25/F | Severe back pain with paraplegia | SAH T5 ASA aneurysm (Takayasu arteritis) | Conservative | Unknown outcome |
| Morozumi et al. 2017 (14) | 9/M | Back pain and gain disturbance | SAH C7-T1 intramedullary aneurysm artery (histology: few inflammatory cells) | Surgery (aneurysm resection) | Cure Clinical improvement |
| Dabus et al. 2018 (72) (4 pts) | Mean age 63/2F, 2M | 2 Back pain and sensory deficits 1 Back pain only 1 Head and neck pain |
Spinal hemorrhage (2 SAHs, 1 small intramedullary haematoma, 1 SDH) 2 ASA (AMA) aneurysms 2 PSA aneurysms 2 cervical, 2 thoracic | Conservative—Spontaneous occlusion | Clinical improvement |
| Renieri et al. 2018 (10) (Case series of 11 patients) | Mean age 60 7F, 4M | 9 Back pain 7 LL weakness |
Mostly SAH, 2 with SDH 3 ASA aneurysms (radiculomedullary) 8 PSA aneurysms (radiculopial) (level not specified) | 5 Embolization (2 with particles, 3 with coiling) 2 Surgery (1 surgical trapping, 1 aneurysm resection) 4 Conservative |
Mostly occluded aneurysms with clinical improvement 3 had no f/up |
| Simon-Gabriel et al. 2018 (17) | 65/M | Drowsy with neck stiffness | SAH Proximal ASA aneurysm arising from VA | Embolization (Flow-diverter) | Regressed aneurysm with clinical improvement |
| Aljuboori et al. 2018 (73) | 78/M | Back pain and lower limb weakness | T9 Artery of Adamkiewicz aneurysm | Surgery (aneurysm clipping) | Cure Clinical improvement |
| Roka 2019 (74) | 30/F | Headache and vomiting | Cervical ASA aneurysm | Conservative | Clinical improvement |
| Priola et al. 2019 (75) | 54/F | Upper back pain | Spinal SDH and SAH T3 radiculomedullary artery aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Yokosuka et al. 2019 (76) | 79/F | Headache and vomiting | SAH T10 radicular artery aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Hanakita et al. 2019 (77) | 77/NA | N/A | Posterior fossa SAH ASA originating from the right VA | Surgery (extirpation of aneurysm with bipolar coagulation) | Cure Clinical resolution |
| Nguyen et al. 2020 (78) | 45/M | Abdominal pain, Severe headache, seizure, paraparesis | Spinal SAH, SDH and cord compression T9 radiculomedullary artery | Surgery (aneurysm resection) | Cure Stable neurology |
| Cobb et al. 2020 (9) | 36/F | Acute low back pain and paraplegia | T3-L5 SDH and spinal cord infarct of the lower TL cord T11 ASA (radiculomedullary) aneurysm | Embolization (Onyx) | Cure Stable neurology |
| Takebayashi et al. 2020 (79) | 67/F | Back pain | Intracranial SAH T10 radiculopial | Surgery (aneurysm resection) | Cure Clinical improvement |
| Abdalkader et al. 2021 (22) | 2M (50s, 70s) 2F (40s, 60s) | 3 headaches 1 confusion |
2 ASA 2 radiculomedullary 3 Cervical 1 Thoracic | 1 Surgery (clipping) 3 Conservative |
3 improved 1 (conservative) died of vasospasm |
| Limaye et al. 2021 (80) | 43/F | Low back pain and paraesthesia | Spinal SAH and SDH T12 (supplied by L2) ASA aneurysm | Conservative—Spontaneous occlusion | Clinical improvement |
| Crobeddu et al. 2021 (81) | 62/M | Acute pain in the crural -thigh region | Spinal SAH L1 radiculopial aneurysm | Not found on surgery; repeat DSA-occluded | Clinical improvement |
| Bergeron 2021 (7) (4 isolated cases) | Mean age 52/F | Headache and back pain | Intracranial and spinal SAH 1 radiculomedullary 3 radiculopial All 4 thoracic | 1 Surgery (aneurysm resection) 3 Conservative | Cure Clinical improvement |
| Shima et al. 2021 (19) | 77/F | Headache and drowsiness | Cranial SAH C4 ASA aneurysm (bilateral VA occlusions) | Endovascular (aneurysm coiling and VA stenting) | Cure Clinical improvement |
| Gomez et al. 2023 (82) | Adult/F | Low back pain | Spinal epidural hematoma and SAH (L1-L4 levels) L2 radiculomedullary | Conservative—Spontaneous occlusion | Clinical improvement |
| Liu et al. 2023 (83) | 64/M | Headache and vomiting | Post fossa and upper cervical SAH C4 radiculomedullary artery aneurysm | Conservative—Spontaneous occlusion | Clinical resolution |
| Jeon et al. 2024 (31) | 51/F | Severe headache, neck pain | Posterior fossa SAH LSA aneurysm from PICA | Embolization | Cure Clinical improvement |
| Ha et al. 2024 (30) | 52/M | Acute lower back pain and bil. Leg pain | T12-L1 radiculomedullary (ventral lateral aspect of spine) | Surgery (resection of thrombosed aneurysm) | Cure Clinical improvement |
| Papadimitriou et al. 2024 (84) | 74/F | Headache | Post fossa SAH LSA aneurysm | Surgery (aneurysm resection) | Cure Clinical improvement |
| Song et al. 2024 (85) (3 patients) | Mean age 64/2M, 1F | Headache | Post fossa SAH LSA aneurysms | 2 Surgery (aneurysm clipping) 1 lost to f/up | Cure Clinical improvement |
| Zhao and Yu 2024 (16) | 62/M | Headache | Post fossa SAH Aneurysm along collateral circulation from chronically occluded distal V4 VA supplying ASA | Embolization (flow-diverter) | Occluded flow-diverter with partial PICA infarcts regressed aneurysm |
Summary of all published reports on isolated spinal artery aneurysms from 2011 to 2024.
Subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH), loss of consciousness (LOC), anterior spinal artery (ASA), posterior spinal artery (PSA), lateral spinal artery (LSA) male (M), female (F), posterior inferior cerebellar artery (PICA), and vertebral artery (VA).
Case reports
Case 1
A 60-year-old woman with a history of hypertension, dyslipidemia, and diabetes was admitted for workup of acute-onset chest pain after an episode of abdominal pain with vomiting and diarrhea. The next day, she developed acute lower limb weakness and numbness, along with urinary retention. On examination, she was alert, with a Glasgow Coma Scale score of 15. Power of bilateral upper extremities was 5/5 at the C5 level, 3/5 at C6–C7 levels, and 1/5 at the C8-T1 levels, with flaccid paresis (power 0/5) of bilateral lower extremities. She had absent sensation at the sensory level of T2 and below, along with saddle anesthesia.
Magnetic resonance imaging (MRI) of the spine (Figure 1) demonstrated a subdural hematoma with spinal cord edema along the lower cervical and upper thoracic spine, with SAH tracking down to T9 level. On postcontrast T1-weighted sequences, there was focal nodular enhancement within the hematoma at the level of T2. The patient underwent a spinal angiogram, which demonstrated a fusiform dilatation of the right PSAs originating at the T2 level consistent with an ISAA.
Figure 1

A 60-year-old woman presented with acute back pain and flaccid quadriparesis. A, B: Sagittal T2-weighted MRI images of the spine [(A) cervical spine and (B) thoracic spine] show a subdural hematoma extending along the lower cervical and upper thoracic spine, and spinal subarachnoid hemorrhage extending down to the level of T9. Note associated cord edema. (D, E) Axial T2-weighted (D) and gradient echo (E) images demonstrate the SDH centered predominantly along the right side of the spinal canal (arrowheads), displacing the cord to the left. (F) Postcontrast T1-weighted image shows focal nodular enhancement within the subdural hematoma (arrow), corresponding to a fusiform “tent-like” dissecting aneurysm arising from the right T2 radiculopial artery on spinal angiogram (C, arrow). This was surgically resected and characterized by histology as an organizing hematoma.
Given significant cord compression, the patient underwent a C7-T2 laminectomy with evacuation of the spinal hematoma; upon surgical exploration, there was no evidence of a spinal abscess. At the time of decompression, PSAs originating from the right T2 intersegmental artery was clipped, and the aneurysm was excised. Pathology demonstrated a disrupted vessel wall with surrounding organized hematoma in keeping with a dissecting aneurysm of the PSAs. No evidence of neutrophilic infiltration on pathology. The cause was thought to be a traumatic dissection, potentially resulting from significant abdominal hypertension from vomiting and diarrhea.
Over the following week, the patient developed septic shock requiring resuscitation and intubation. Abdominal imaging showed evidence of acute descending colitis, pyelonephritis, and a renal abscess. Blood cultures were positive for Bacteroides uniformis, likely from the gastrointestinal tract, and Morganella morganii. After successful treatment, the patient was discharged to rehab, where she recovered almost complete upper limb but remained paraplegic, with neurogenic bladder and bowel. Follow-up imaging of the spine was not performed.
Case 2
A 66-year-old woman with a history of mechanical aortic valve replacement and atrial fibrillation on warfarin, hypertension, and dyslipidemia, presented with acute left lower extremity weakness. A few days prior to admission, she had started noticing back pain radiating to the left lower extremity after picking up a crate at work. Over the following days, the pain gradually worsened, with an acute exacerbation the night prior to admission, which was followed by unsteadiness and significant left lower extremity weakness. On examination, the patient had significant weakness in left hip flexion and numbness in the left lower extremity.
MRI of the spine revealed multicompartmental spinal hemorrhage, in both the subarachnoid and subdural compartments, extending from T7 down to the sacrum, and significant swelling of the lower thoracic cord and the conus medullaris (Figure 2). There was also a trace of intracranial SAH. Contrast-enhanced sequences demonstrated a small, enhanced lesion located posteriorly in the left spinal canal at the T12 level (Figure 2). This was confirmed on spinal angiography to be an ISAA of the left PSAs originating from the left T12 intersegmental artery. The patient was treated by the endovascular coil embolization of bilateral T12 intersegmental arteries to reduce direct and indirect flow to the lesion and to promote thrombosis while sparing the affected PSAs. Postprocedure angiographic acquisitions of adjacent intersegmental arteries did not demonstrate collateral supply to the aneurysm (Figure 3). A follow-up MRI performed 48 h postoperatively no longer showed the focus of enhancement at the T12 level.
Figure 2
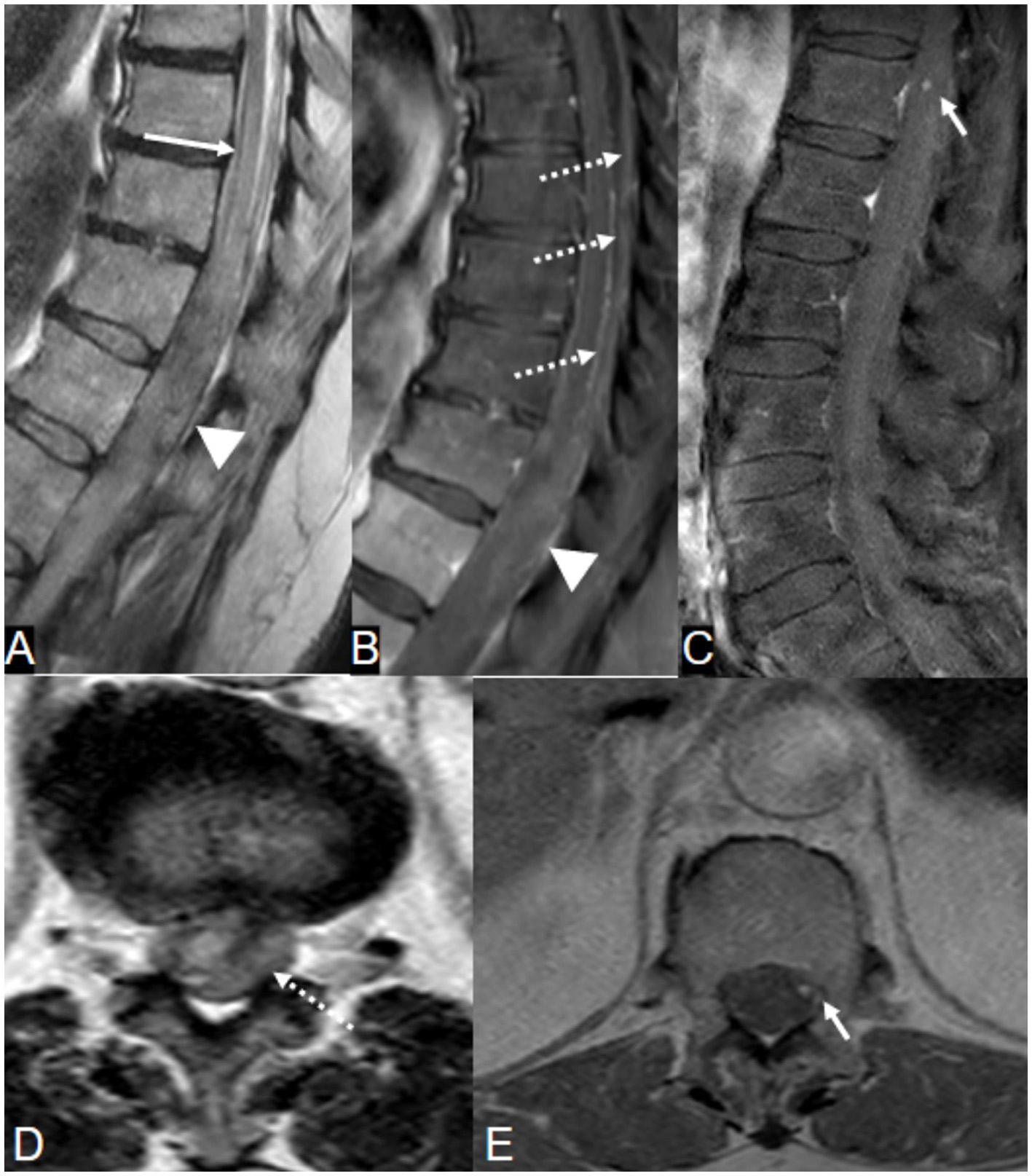
A 66-year-old woman on warfarin presented with acute severe low back pain. (A, B, D) T2-weighted MRI images demonstrate conus edema (long arrow) with spinal subdural hematoma, predominantly on the left, displacing the conus to the right (arrowheads). Note the lateral compression of the spinal cord and cerebrospinal fluid by the hematoma (long arrowhead). (C, E) Contrast-enhanced MRI shows focal nodular enhancement at the left peripheral aspect of the cord at the T12 level (short arrow), confirmed later on angiogram as a ruptured left T12 posterior spinal artery dissecting aneurysm (Figure 3A).
Figure 3
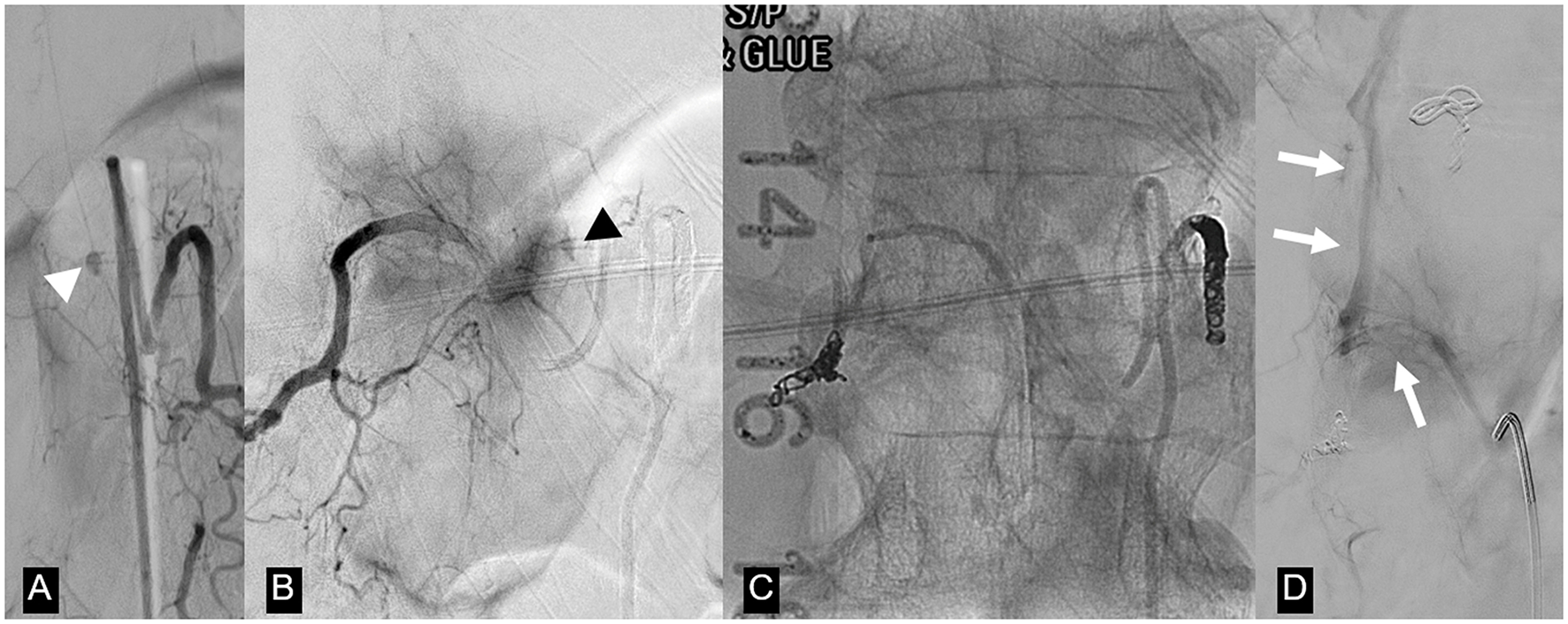
Same patient as in Figure 2. (A) Left T12 intersegmental artery (ISA) angiogram demonstrates an oval-shaped dissecting pseudoaneurysm arising from a posterior spinal artery (white arrowhead). (B) Right T12 ISA angiogram postcoil embolization of the left T12 ISA shows retrograde filling of the pseudoaneurysm through retrocorporeal collaterals (black arrowhead). (C) Unsubtracted image of the T12 vertebral body demonstrates coil and n-butyl cyanoacrylate occlusion of bilateral T12 ISAs. (D) Repeat T12 ISA angiogram obtained after the deterioration of the patient 2 weeks after the first embolization shows no filling of the treated aneurysm and a new arteriovenous shunting between the right T12 ISA and the Azygos vein (arrow). This was considered to be incidental and unrelated to the patient's clinical deterioration, but nevertheless embolized via n-butyl cyanoacrylate injection.
Fourteen days postcoiling, after a gradual improvement in functionality, the patient developed a sudden onset of severe low back pain and acute worsening of her left lower extremity weakness. MRI of the spine was negative for new hemorrhages or new areas of contrast enhancement and demonstrated decreased cord edema. There was no evidence of acute cord infarction. A repeat spinal angiogram did not demonstrate filling of the pseudoaneurysm or new intrathecal lesions. However, there was a newly developed arteriovenous shunt between the right T12 intersegmental artery and the azygous vein. This was thought to be an incidental finding unrelated to the new symptoms and probably secondary to the prior embolization, but was nevertheless occluded by coils and glue.
The patient improved over a week and was discharged to rehab with mild residual left lower extremity paresis and paresthesias.
Discussion
In addition to the two cases we described, we were able to find 115 cases of ruptured ISAAs in the literature, exclusively in the form of case reports and small case series (Tables 1, 2).
The mean age at the time of presentation is in the fifth decade, with female predominance (F:M = 2:1). Ruptured ISAAs are typically present with spinal and/or intracranial SAH, associated with subdural hematomas in some cases and rarely epidural hematomas. The compartment of hematomas could be related to the involved arterial segment as it traverses the various spinal compartments.
Although the precise pathophysiological mechanism of ISAAs remains unclear, case analyses with available histopathology indicate that they are the result of a dissecting process, as evidenced by the disruption of the internal elastic lamina and the absence of a three-layered architecture (1–4). This notion fits with the observed ISAAs being fusiform at a non-branching site and with the spontaneous angiographic resolution of lesions over time in some reported cases treated conservatively. The exact causes for spinal arterial dissecting aneurysms are not known and are difficult to elucidate, as histological confirmation is rarely available. Different from extracranial dissections that appear to be mainly related to trauma, connective tissue diseases, or pro-inflammatory conditions (5, 6), the etiology for intracranial or spinal arterial dissections is less well known and often remains “idiopathic”. Several suspected inciting events or predisposing factors have been proposed, such as: vascular trauma secondary to repeated vomiting episodes or sustained efforts like in the cases we presented (7), intervertebral disk herniation (8), collagenopathies (9, 10), hypertension and smoking (10–12), and inflammatory-infective causes (13, 14). While hemodynamic stresses may increase the chance for vascular remodeling and shear-stress related aneurysmal formation, their causal relationship to forming a histological dissection of the vessel wall remains elusive. It is of note, though, that spinal aneurysms have been described in patients with increased flow imposed by collateral pathways through the spinal vasculature in steno-occlusive disease (15–19) or in patients with concomitant intradural vascular malformations (20). Thus, increased hemodynamic stress may be another factor to consider when discussing the etiology of spinal arterial dissecting aneurysms.
Anatomically, lesions were evenly distributed between radiculomedullary feeders to the anterior spinal artery and radiculopial PSAs. The cervicothoracic spine was the most common site of involvement.
In all reported cases, the aneurysm was present along the ascending limb of either the radiculomedullary or a radiculopial artery and it may be hypothesized that, similar to cervical dissections being present where the mobile segment of an artery transitions into an immobile segment, in spinal aneurysms the dissection occurs at the segment where the artery is exposed to highest torsional shear stress forces.
Clinical presentations varied, depending on the extent and location of spinal hemorrhage and associated myelopathy (21). Patients with ISAAs at the upper cervical level often present with predominantly intracranial SAH and tetraparesis/plegia associated with severe headache, nausea, vomiting, and in some cases coma. Patients with ISAAs at the thoraco-lumbar levels presented with severe chest or back pain, and in some cases lower extremity paresis or plegia with paresthesia and urinary or bowel sphincter dysfunction.
ISAAs can be identified on MRI as foci of nodular enhancement along the surface of the spinal cord. Various types of spinal hemorrhages have been reported, with SAH being the most common, in some cases extending to the intracranial compartment and resulting in hydrocephalus and rarely vasospasm (22). Spinal angiography is the gold standard and necessary modality to establish a final diagnosis. ISAAs have often been described as having a “fusiform” or “tent-like” appearance on the ascending limbs of anterior or posterior radiculomedullary branches.
Of the 115 cases of ruptured ISAAs reported in the literature, 40 (35%) were managed conservatively. The majority of these (30/40, 75%) improved clinically, with spontaneous occlusion observed in those cases that underwent follow-up imaging. Seven out of 40 (18%) patients in the conservative group died; 2 (5%) from rebleeding (23, 24), 1 (3%) from severe vasospasm (24), 2 (5%) from causes not directly related to spinal hemorrhage (25, 26), and 2 (5%) from unclear causes (27, 28). Three out of 40 (7%) patients had no reported outcome.
Out of 115 patients, 65 (57%) underwent operative treatment, either by open surgery (50/65, 77%) or by endovascular intervention (15/65, 23%). Surgical resection, clipping, or wrapping of the aneurysms has been reported (1), with resection of the aneurysm being the most commonly used strategy (32/50, 64%). Complication rates for surgery were low (2/50, 4%), with 1 case of subdural hematoma (15) and another 1 case of wound infection (28). Postoperative neurological complications may be reduced by using intraoperative indocyanine green and neurophysiological monitoring (29, 30). Ischemic events are likely to be lower in patients with robust spinal arterial anastomotic networks. Embolizations of the aneurysm-bearing or -supplying artery(ies) were also performed, mostly with coiling (10, 12, 31), and in a small number of cases with particles (>150 μm) (10, 32), Onyx (7), n-butyl-2-cyanoacrylates (1), and flow-diverter (16, 17). Complication rates of endovascular treatment, in general, were higher (3/15, 20%) than those of open surgery and were mostly represented by ischemic events in the territories of the embolized vessels. All of these three cases were infarcts in the posterior inferior cerebellar artery (PICA) territory, from the treatment of ISAAs, in the presence of pre-existing vertebral artery steno-occlusion. Two from coiling (12, 15) and one from flow-diverter deployment in the vertebral artery giving rise to the aneurysm-bearing spinal artery (16). Two cases of reported deaths (2/65, 3%), one in each group, were not directly related to the spinal hemorrhage or the intervention (15, 33). The remainder of the treated patients for whom outcomes were reported (57/65, 88%) did not experience rebleeding and achieved varying degrees of neurological improvement. Management for 10 patients (10/115, 9%) was not specified in the individual case reports.
Conservative management was mostly reserved for patients with ISAAs located along the anterior radiculomedullary artery. This was due to the high risk for intervention-related ischemic cord injury and to the notion that these lesions have a higher chance of spontaneous thrombosis as compared to intracranial ones (34). Although most deaths occurred in the conservative group, rebleeding was the cause in only 5% of patients. Long-term rebleeding rates for conservatively managed ISAAs are therefore significantly lower when compared to those of their intracranial counterparts, presumably related to the smaller vessel caliber and thus diminished flow through the artery (23, 24, 34).
The choice between surgical resection and endovascular embolization depends on several factors such as the clinical status of the patient, the ISAA location, and institutional expertise. Patients were treated surgically due to neurological deterioration requiring decompression, inadequate vascular access to the aneurysm (for example, stenosis at the origin or small caliber artery), or absence of an acceptable safety margin for endovascular embolization (for example, in close proximity to the origin of the anterior spinal artery). Adequate safety margins are particularly critical when considering embolization with liquid embolic agents. Occlusion of the artery of Adamkiewicz or the entire anterior spinal artery is likely to lead to severe cord infarction, while occlusion of the PSAs is known to be less risky, given the robust collaterals. Occlusion of the intersegmental artery(ies) supplying the affected spinal arterial branch may be sufficient to decrease flow to the ISAAs and promote thrombosis in most cases. In cases of aneurysms originating from spinal radiculomedullary feeders arising directly from the vertebral arteries, flow diversion can be considered. In these cases, risks related to the procedure and the required dual antiplatelet regimen need to be leveraged against the benefits of a potential aneurysm occlusion (86).
In our experience, we believe that surgical management is the treatment of choice if additional decompression is clinically mandated, whereas an endovascular approach with parent vessel sacrifice, preferably at the origin of the radiculopial or radiculomedullary branch, is preferred in the remainder of the cases. We believe that the excellent collaterals present on the surface of the cord through the vasacorona or the rope ladder anastomoses will prevent cord ischemia, in particular in dissections of radiculopial arteries. Nevertheless, given the relatively low reported rebleeding risk, the management approach for each case needs to be tailored to the particular patient's clinical and anatomical characteristics.
Conclusion
ISAAs are rare vascular lesions, likely dissecting in nature. Their natural history remains poorly understood; however, available data suggest that the risk of rebleeding is relatively low compared to that of their intracranial counterparts. This difference is presumably due to the lower hemodynamic stress exerted on these lesions by the reduced flow within smaller-caliber spinal vessels. Nevertheless, the potential for substantial morbidity and mortality associated with rebleeding, as highlighted in the reviewed literature, supports a proactive management approach. Whenever feasible and associated with an acceptable risk profile, surgical or endovascular parent vessel sacrifice should be considered. In cases of radiculomedullary artery dissections, the risk of treatment-related anterior spinal cord ischemia must be carefully weighed against the relatively low risk of rebleeding.
Statements
Author contributions
TPK: Investigation, Methodology, Data curation, Formal analysis, Visualization, Writing – original draft. TK: Investigation, Methodology, Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. JP: Data curation, Investigation, Writing – review & editing. SS: Data curation, Investigation, Writing – review & editing. EO: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kim HJ Choi IS . Dissecting aneurysm of the posterior spinal artery: Case report and review of the literature. Neurosurgery. (2012) 71:E749–56. 10.1227/NEU.0b013e31825ea539
2.
Geibprasert S Krings T Apitzsch J Reinges MH Nolte KW Hans FJ . Subarachnoid hemorrhage following posterior spinal artery aneurysm. A case report and review of the literature. Interv Neuroradiol. (2010) 16:183–90. 10.1177/159101991001600211
3.
Massand MG Wallace RC Gonzalez LF Zabramski JM Spetzler RF . Subarachnoid hemorrhage due to isolated spinal artery aneurysm in four patients. AJNR Am J Neuroradiol. (2005) 26:2415–9.
4.
Horio Y Katsuta T Samura K Wakuta N Fukuda K Higashi T et al . Successfully treated isolated posterior spinal artery aneurysm causing intracranial subarachnoid hemorrhage: Case report. Neurol Med Chir. (2015) 55:915–9. 10.2176/nmc.cr.2015-0210
5.
Debette S Compter A Labeyrie MA Uyttenboogaart M Metso TM Majersik JJ et al . Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. (2015) 14:640–5410.1016/S1474-4422(15)00009-5
6.
Pfefferkorn T Saam T Rominger A Habs M Gerdes LA Schmidt C et al . Vessel wall inflammation in spontaneous cervical artery dissection: a prospective, observational positron emission tomography, computed tomography, and magnetic resonance imaging study. Stroke. (2011) 42:1563–8. 10.1161/STROKEAHA.110.599548
7.
Bergeron D Nehme A Berthelet F Farzin B Obaid S Westwick H et al . Repeated retching and vomiting in the pathophysiology of isolated spinal aneurysms. World Neurosurg. (2021) 149:e512–20. 10.1016/j.wneu.2021.01.143
8.
Nakhla J Nasser R Yassari R Pasquale D Altschul D . Ruptured anterior spinal artery aneurysm from a herniated cervical disc. A case report and review of the literature. Surg Neurol Int. (2016) 7:10. 10.4103/2152-7806.175072
9.
Cobb M Griffin A Karikari I Gonzalez LF . Endovascular treatment of ruptured enlarging dissecting anterior spinal artery aneurysm. World Neurosurg. (2020) 139:e658–62. 10.1016/j.wneu.2020.04.100
10.
Renieri L Raz E Lanzino G Krings T Shapiro M Shirani P et al . Spinal artery aneurysms: clinical presentation, radiological findings and outcome. J Neurointerv Surg. (2018) 10:646–50. 10.1136/neurintsurg-2017-013687
11.
Romero DG Batista AL Gentric JC Raymond J Roy D Weill A . Ruptured isolated spinal artery aneurysms: Report of two cases and review of the literature. Interv Neuroradiol. (2014) 20:774–80. 10.15274/INR-2014-10074
12.
Morigaki R Satomi J Shikata E Nagahiro S . Aneurysm of the lateral spinal artery: a case report. Clin Neurol Neurosurg. (2012) 114:713–6. 10.1016/j.clineuro.2011.12.003
13.
Singh V Naik S Bhoi SK Phadke RV . Anterior spinal artery aneurysm in aortic stenosis of different etiology: Report of three cases. Neuroradiol J. (2017) 30:180–5. 10.1177/1971400917690008
14.
Morozumi M Imagama S Ando K Kobayashi K Hida T Ito K et al . Surgical intervention for a pediatric isolated intramedullary spinal aneurysm. Eur Spine J. (2018) 27:342–6. 10.1007/s00586-017-5256-7
15.
Chen CC Bellon RJ Ogilvy CS Putman CM . Aneurysms of the lateral spinal artery: report of two cases. Neurosurgery. (2001) 48:949–53. 10.1227/00006123-200104000-00057
16.
Zhao Y Yu J . Treatment of medullary aneurysm in the collateral circulation with a flow diverter: a case report. Int J Surg Case Rep. (2024) 121:110038. 10.1016/j.ijscr.2024.110038
17.
Simon-Gabriel CP Urbach H Meckel S . Ruptured fusiform aneurysm of the anterior spinal artery. Clin Neuroradiol. (2018) 28:613–6. 10.1007/s00062-018-0684-2
18.
Ashour R Filippidis A Patel N . Ruptured anterior spinal artery aneurysm associated with bilateral vertebral artery occlusion treated by surgical clipping. World Neurosurg. (2015) 84:1178.e11-3. 10.1016/j.wneu.2015.06.010
19.
Shima S Sato S Motizuki T Niimi Y . Endovascular treatment for ruptured cervical anterior spinal artery aneurysm caused by occlusive disease of bilateral vertebral arteries: a case report and literature review. Clin Neurol Neurosurg. (2021) 208:106862. 10.1016/j.clineuro.2021.106862
20.
Donauer E Aguilar Pérez M Jangid N Tomandl B Ganslandt O Henkes H . Spontaneous cervical intramedullary and subarachnoid hemorrhage due to a sulco-commissural artery aneurysm. Clin Neuroradiol. (2019) 29:777–81. 10.1007/s00062-019-00772-6
21.
Karakama J Nakagawa K Maehara T Ohno K . Subarachnoid hemorrhage caused by a ruptured anterior spinal artery aneurysm. Neurol Med Chir. (2010) 50:1015–9. 10.2176/nmc.50.1015
22.
Abdalkader M Samuelsen BT Moore JM Cervantes-Arslanian A Ong CJ Setty BN et al . Ruptured spinal aneurysms: diagnosis and management paradigms. World Neurosurg. (2021) 146:e368–77. 10.1016/j.wneu.2020.10.098
23.
Yonas H Patre S White RJ . Anterior spinal artery aneurysm. Case report J Neurosurg. (1980) 53:570–3. 10.3171/jns.1980.53.4.0570
24.
Koçak A Ateş Ö Çayli SR Saraç K . Isolated posterior spinal artery aneurysm. Br J Neurosurg. (2006) 20:241–4. 10.1080/02688690600852704
25.
Yoong MF Blumbergs PC Brian North J . Primary (granulomatous) angiitis of the central nervous system with multiple aneurysms of spinal arteries. J Neurosurg. (1993) 79:603–7. 10.3171/jns.1993.79.4.0603
26.
Yang TK A . Ruptured aneurysm in the branch of the anterior spinal artery. J Cerebrovasc Endovasc Neurosurg. (2013) 15:26. 10.7461/jcen.2013.15.1.26
27.
Garcia CA Dulcey S Dulcey J . Ruptured aneurysm of the spinal artery of Adamkiewicz during pregnancy. Neurology. (1979) 29:394–394. 10.1212/WNL.29.3.394
28.
Henson RA Croft PB . Spontaneous spinal subarachnoid haemorrhage. Q JMed. (1956) 25:53–66.
29.
Ikeda S Takai K Kikkawa Y Takeda R Ikeda T Kohyama S et al . Ruptured posterior spinal artery aneurysm: Intraoperative and histologic findings with appreciable thrombosis. Spine J. (2016) 16:e215–7. 10.1016/j.spinee.2015.11.015
30.
Ha JW Lee Y Kim KH Moon BJ Park JY Chin DK et al . Surgical treatment of a ruptured isolated spinal artery aneurysm with negative angiography findings: a case report. Nerve. (2024) 10:51–6. 10.21129/nerve.2024.00514
31.
Jeon YS Park JJ Chun YI Roh HG . Lateral spinal artery aneurysm causing subarachnoid hemorrhage: literature review and case report. J Clin Med. (2024) 13:4910. 10.3390/jcm13164910
32.
Ronchetti G Morales-Valero SF Lanzino G Wald JT . A cause of atypical intracranial subarachnoid hemorrhage: posterior spinal artery aneurysms. Neurocrit Care. (2015) 22:299–305. 10.1007/s12028-014-0009-5
33.
Toyota S Wakayama A Fujimoto Y Sugiura S Yoshimine T . Dissecting aneurysm of the radiculomedullary artery originating from extracranial vertebral artery dissection in a patient with rheumatoid cervical spine disease: an unusual cause of subarachnoid haemorrhage—case report. J Neurosurg Spine. (2007) 7:660–3. 10.3171/SPI-07/12/660
34.
Sato K Roccatagliata L Depuydt S Rodesch G . Multiple aneurysms of thoracic spinal cord arteries presenting with spinal infarction and subarachnoid hemorrhage: case report and literature review. Neurosurgery. (2012) 71:E1053–8. 10.1227/NEU.0b013e3182647be4
35.
Kinal ME Sejanovich C . Spinal cord compression by an intramedullary aneurysm; case report and review of the literature. J Neurosurg. (1957) 14:561–5. 10.3171/jns.1957.14.5.0561
36.
Woollam HM Millen JW Blackwood W et al . Greenfield's Neuropathology: Vascula Disease of the Central Nervous System. London, United Kingdom: Edward Arnold Inc. (1971). p. 71–137.
37.
Hopkins CA Wilkie FL Voris DC . Extramedullary aneurysm of the spinal cord. Case report J Neurosurg. (1966) 24:1021–3. 10.3171/jns.1966.24.6.1021
38.
Leech PJ Stokes BA ApSimon T Harper C . Unruptured aneurysm of the anterior spinal artery presenting as paraparesis. Case report J Neurosurg. (1976) 45:331–3. 10.3171/jns.1976.45.3.0331
39.
Thomson RL . Aneurysm in the cervical spinal canal. Med J Aust. (1980) 1:220–222. 10.5694/j.1326-5377.1980.tb134773.x
40.
Vincent FM . Anterior spinal artery aneurysm presenting as a subarachnoid hemorrhage. Stroke. (1981) 12:230–2. 10.1161/01.STR.12.2.230
41.
Moore DW Hunt WE Zimmerman JE . Ruptured anterior spinal artery aneurysm: repair via a posterior approach. Neurosurgery. (1982) 10:626–30. 10.1227/00006123-198205000-00015
42.
Kito K Kobayashi N Mori N Kohno H . Ruptured aneurysm of the anterior spinal artery associated with pseudoxanthoma elasticum. Case report J Neurosurg. (1983) 58:126–8. 10.3171/jns.1983.58.1.0126
43.
Smith BS Penka CF Erickson LS Matsuo F . Subarachnoid hemorrhage due to anterior spinal artery aneurysm. Neurosurgery. (1986) 18:217–9. 10.1227/00006123-198602000-00020
44.
Saunders FW Birchard D Willmer J . Spinal artery aneurysm. Surg Neurol. (1987) 27:269–72. 10.1016/0090-3019(87)90041-3
45.
Goto Y Kamijyo Y Yonekawa Y Kikuchi H . Ruptured aneurysm of the posterior spinal artery of the upper cervical spinal cord: case report. Neurosurgery. (1988) 22:558–60. 10.1227/00006123-198803000-00019
46.
elMahdiMA Rudwan MA Khaffaji SM Jadallah FA . A giant spinal aneurysm with cord and root compression. J Neurol Neurosurg Psychiatry. (1989) 52:532–5. 10.1136/jnnp.52.4.532
47.
Handa T Suzuki Y Saito K Sugita K Patel SJ . Isolated intramedullary spinal artery aneurysm presenting with quadriplegia. Case report. J Neurosurg. (1992) 77:148–50. 10.3171/jns.1992.77.1.0148
48.
Bahar S Coban O Gürvit IH Akman-Demir G Gökyigit A . Spontaneous dissection of the extracranial vertebral artery with spinal subarachnoid haemorrhage in a patient with Behçet's disease. Neuroradiology. (1993) 35:352–4. 10.1007/BF00588368
49.
Rengachary SS Duke DA Tsai FY Kragel PJ . Spinal arterial aneurysm: case report. Neurosurgery. (1993) 33:125–9. 10.1227/00006123-199307000-00020
50.
Mohsenipour I Ortler M Twerdy K Schmutzhard E Attlmayr G Aichner F . Isolated aneurysm of a spinal radicular artery presenting as spinal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. (1994) 57:767–8. 10.1136/jnnp.57.6.767
51.
Vishteh AG Brown AP Spetzler RF . Aneurysm of the intradural artery of Adamkiewicz treated with muslin wrapping: technical case report. Neurosurgery. (1997) 40:207–9. 10.1227/00006123-199701000-00047
52.
Kawamura S Yoshida T Nonoyama Y Yamada M Suzuki A Yasui N . Ruptured anterior spinal artery aneurysm: a case report. Surg Neurol. (1999) 51:608–12. 10.1016/S0090-3019(98)00114-1
53.
Taniura S Watanebe T . A ruptured dissecting aneurysm of the anterior radiculomedullary artery caused by vertebral angiography. Neuroradiology. (2000) 42:539–42. 10.1007/s002340000322
54.
Berlis A Scheufler KM Schmahl C Rauer S Götz F Schumacher M et al . Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: potential etiology and treatment strategy. AJNR Am J Neuroradiol. (2005) 26:405–10.
55.
Nemecek AN Sviri G Hevner R Ghodke B Britz GW . Dissecting aneurysm of the thoracic posterior spinal artery. Case illustration. J Neurosurg Spine. (2006) 5:555. 10.3171/spi.2006.5.6.555
56.
Longatti P Sgubin D Di Paola F . Bleeding spinal artery aneurysms. J Neurosurg Spine. (2008) 8:574–8. 10.3171/SPI/2008/8/6/574
57.
Klingler JH Gläsker S Shah MJ Van Velthoven V . Rupture of a spinal artery aneurysm attributable to exacerbated Sjögren syndrome: case report. Neurosurgery. (2009) 64:E1010–1. 10.1227/01.NEU.0000344002.21699.A3
58.
Pollock JM Powers AK Stevens EA Sanghvi AN Wilson JA Morris PP . Ruptured anterior spinal artery aneurysm: a case report. J Neuroimaging. (2009) 19:277–9. 10.1111/j.1552-6569.2008.00287.x
59.
Cavuşoğlu H Ozdilmaç A Sahin Y Aydin Y . Isolated posterior spinal artery aneurysm causing intracranial acute subarachnoidal hemorrhage. Acta Neurochir. (2010) 152:721–4. 10.1007/s00701-009-0491-2
60.
Peltier J Bougeois P Baroncini M Thines L Leclerc X Lejeune JP et al . Ultra-early rebleeding of an anterior spinal artery aneurysm. Br J Neurosurg. (2010) 24:468–70. 10.3109/02688691003710502
61.
Kurita M Endo M Kitahara T Fujii K . Subarachnoid haemorrhage due to a lateral spinal artery aneurysm misdiagnosed as a posterior inferior cerebellar artery aneurysm: Case report and literature review. Acta Neurochir. (2009) 151:165–9. 10.1007/s00701-009-0183-y
62.
Kubota H Suehiro E Yoneda H Nomura S Kajiwara K Fujii M et al . Lateral spinal artery aneurysm associated with a posterior inferior cerebellar artery main trunk occlusion. J Neurosurg Spine. (2006) 4:347. 10.3171/spi.2006.4.4.347
63.
Iihoshi S Miyata K Murakami T Kaneko T Koyanagi I . Dissection aneurysm of the radiculomedullary branch of the artery of Adamkiewicz with subarachnoid hemorrhage. Neurol Med Chir. (2011) 51:649–52. 10.2176/nmc.51.649
64.
Son S Lee SG Park CW . Solitary ruptured aneurysm of the spinal artery of adamkiewicz with subarachnoid hemorrhage. J Korean Neurosurg Soc. (2013) 54:50. 10.3340/jkns.2013.54.1.50
65.
Marovic P Thani N Lu S Bala A . Spinal subarachnoid hemorrhage secondary to rupture of an isolated radicular artery aneurysm. J Neurol Surg A Cent Eur Neurosurg. (2013) 74:410–4. 10.1055/s-0032-1320025
66.
Pahl FH De Oliveira MF Rotta MAC Dias GMS Rezende AL Rotta JM . Spontaneous resolution of an isolated cervical anterior spinal artery aneurysm after subarachnoid hemorrhage. Surg Neurol Int. (2014) 5:139. 10.4103/2152-7806.141776
67.
Doberstein CA Bouley A Silver B Morrison JF Jayaraman M V . Ruptured aneurysms of the intradural artery of adamkiewicz: angiographic features and treatment options. Clin Neurol Neurosurg. (2016) 146:152–5. 10.1016/j.clineuro.2016.05.013
68.
Takata M Takayama M Yokoyama Y Hayashi H Kishida N . An isolated posterior spinal aneurysm resection in which intraoperative electrophysiological monitoring was successfully used to locate the lesion and to detect the possibility of ischemic complications. Spine. (2016) 41:E46–9. 10.1097/BRS.0000000000001081
69.
Agarwal A Weerakkody Y Marshall M Singh T . Intercostal artery pseudoaneurysm with spontaneous resolution in the setting of an artery of Adamkiewicz. BMJ Case Rep. (2016) 2016:bcr2016012802. 10.1136/bcr-2016-012802
70.
Aguilar-Salinas P Lima J Brasiliense LBC Hanel RA Sauvageau E . Ruptured aneurysm of the artery of Adamkiewicz: is conservative management the standard of treatment in the current era?BMJ Case Rep. (2017) 2017:bcr2017013194. 10.1136/bcr-2017-013194
71.
Ren Y He M You C Li J . Successful surgical resection of spinal artery aneurysms: report of 3 cases. World Neurosurg. (2018) 109:171–8. 10.1016/j.wneu.2017.09.174
72.
Dabus G Tosello RT Pereira BJA Linfante I Piske RL . Dissecting spinal aneurysms: conservative management as a therapeutic option. J Neurointerv Surg. (2018) 10:452–5. 10.1136/neurintsurg-2017-013566
73.
Aljuboori Z Sharma M Simpson J Altstadt T . Surgical management of ruptured isolated aneurysm of artery of adamkiewicz: interesting report and overview of literature. World Neurosurg. (2018) 111:36–40. 10.1016/j.wneu.2017.11.179
74.
Roka YB . Isolated cervical anterior spinal artery aneurysm: case report. Br J Neurosurg. (2019) 33:568–9. 10.1080/02688697.2017.1384790
75.
Priola SM Heyn C da Costa L . Minimally invasive approach for the removal of a ruptured radiculomedullary artery aneurysm: case report and literature review. World Neurosurg. (2019) 126:605–10. 10.1016/j.wneu.2019.03.225
76.
Yokosuka J Fukaya S Yamomoto S Ueki K Kim P . Intracranial subarachnoid hemorrhage caused by an aneurysm at the thoracic spinal region: case report and literature review. Br J Neurosurg. (2020) 34:672–6. 10.1080/02688697.2019.1690130
77.
Hanakita S Oya S Tsuchiya T Shojima M Matsui T . Extirpation of a ruptured anterior spinal artery aneurysm accompanied by dural arteriovenous fistula at the craniovertebral junction via a posterolateral approach: the management of extradural venous congestion. J Neurol Surg B Skull Base. (2019) 80:S344–5. 10.1055/s-0039-1697981
78.
Nguyen NH Le VC Nguyen TQ Nguyen TH . Subarachnoid hemorrhage due to ruptured spinal artery aneurysm: a diagnostic challenge. Case Rep Neurol. (2020) 12:169–75. 10.1159/000507953
79.
Takebayashi K Ishikawa T Murakami M Funatsu T Ishikawa T Taira T et al . Isolated posterior spinal artery aneurysm presenting with spontaneous thrombosis after subarachnoid hemorrhage. World Neurosurg. (2020) 134:544–7. 10.1016/j.wneu.2019.11.118
80.
Limaye K Kandemirli S Dlouhy K . Spinal subarachnoid hemorrhage secondary to ruptured artery of Adamkiewicz aneurysm: is conservative management the first best step?Clin Neurol Neurosurg. (2021) 205:106647. 10.1016/j.clineuro.2021.106647
81.
Crobeddu E Pilloni G Zenga F Cossandi C Garbossa D Lanotte M . Dissecting aneurysm of the L1 radiculomedullary artery associated with subarachnoid hemorrhage: a case report. J Neurol Surg A Cent Eur Neurosurg. (2022) 83:099–103. 10.1055/s-0040-1720997
82.
Gomez D Rairan LG Ramirez-Arquez E Mejia JA . Dissecting a radiculomedullary artery as an infrequent cause of low back pain: illustrative case. J Neurosurg. Case Lessons. (2023) 5:CASE22405. 10.3171/CASE22405
83.
Liu S Chan N Mahboobani N Poon T Poon W . Ruptured cervical radiculomedullary artery mycotic aneurysm presenting with intracranial and spinal subarachnoid haemorrhage: a case report. Hong Kong J Radiol. (2023) 26:266–70. 10.12809/hkjr2217540
84.
Papadimitriou K Quach ET Golub D Patsalides A Dehdashti AR . Far lateral approach with C1 hemilaminotomy for excision of a ruptured fusiform lateral spinal artery aneurysm: 2-dimensional operative video. Oper Neurosurg. (2024) 27:375. 10.1227/ons.0000000000001113
85.
Song Y Lee K Park H Hwang SH Baek HJ Park IS . Surgical treatment of ruptured aneurysms of lateral spinal artery presenting as intracranial subarachnoid hemorrhage: case series and literature review. J Korean Neurosurg Soc. (2024) 67:568–92. 10.3340/jkns.2024.0040
86.
Van Es AC Brouwer PA Willems PW . Management considerations in ruptured isolated radiculopial artery aneurysms. A report of two cases and literature review. Interv Neuroradiol. (2013) 19:60–6. 10.1177/159101991301900109
Summary
Keywords
spinal aneurysm, spinal dissection, arterial dissection, spinal hemorrhage, spinal vascular malformation
Citation
Kee TP, Krings T, Pace J, Swaminathan SK and Orru' E (2025) Ruptured isolated spinal artery aneurysms: a rare manifestation of an arterial dissecting disease. Front. Neurol. 16:1567536. doi: 10.3389/fneur.2025.1567536
Received
27 January 2025
Accepted
08 May 2025
Published
29 May 2025
Volume
16 - 2025
Edited by
Stephan Meckel, University of Freiburg Medical Center, Germany
Reviewed by
Theo Demerath, University of Freiburg Medical Center, Germany
Christoph Johannes Maurer, Augsburg University Hospital, Germany
Emanuela Crobeddu, Azienda Ospedaliero Universitaria Maggiore della Carità, Italy
Zhaolong Zhang, The Affiliated Hospital of Qingdao University, China
Updates
Copyright
© 2025 Kee, Krings, Pace, Swaminathan and Orru'.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. Krings Timo.krings@lahey.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.