- 1Institute for Health, Health Care Policy and Aging Research, Rutgers University, New Brunswick, NJ, United States
- 2Department of Neurology, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, United States
- 3Center for Stroke Rehabilitation Research, Kessler Foundation, East Hanover, West Orange, NJ, United States
- 4Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers University, Newark, NJ, United States
- 5Center for Neuropsychology and Neuroscience, Kessler Foundation, East Hanover, NJ, United States
- 6Department of Occupational Therapy, Steinhardt School of Culture, Education, and Human Development, New York University, NY, United States
Background: Visual problems are common among persons with multiple sclerosis (MS) and may interfere with the assessment of cognitive functioning using visually mediated neuropsychological tests. The current study explored visual discomfort symptoms among persons with MS compared to healthy controls (HCs), using the Visual Discomfort Scale (VDS), which measures somatic and perceptual visual discomfort symptoms that interfere with reading.
Methods: Eighty-nine persons with MS and 30 HCs completed the VDS and the Symbol Digit Modalities Test (SDMT), a visually mediated test of information processing speed and gold standard for screening for MS-related cognitive dysfunction.
Results: Persons with MS endorsed higher frequencies of visual discomfort symptoms, including seeing the text or background moving or fading, headache/eye soreness, blurriness/diplopia, having to re-read, and slow reading, compared to HCs. More frequent visual discomfort symptoms were associated with worse performance on the SDMT. For participants with MS reporting moderate/high levels of visual discomfort symptoms, having a longer disease duration or progressive disease courses were correlated with worse performance on the SDMT.
Conclusion: It is important for clinicians to ask about specific visual discomfort problems that the patient experiences when interpreting a visually-mediated neuropsychological test such as the SDMT, especially for MS patients with longer disease duration or a progressive disease course.
Introduction
Multiple sclerosis (MS) is a demyelinating, inflammatory, and neurodegenerative condition, affecting 2.2 million individuals globally (1). Demyelination and inflammation of the optic nerve (optic neuritis) is one of the most common presenting symptoms of MS (2). Although optic neuritis is transient, many persons with MS report persistent visual problems even after resolution of optic neuritis (3). Using structural imaging techniques, such as optical coherence tomography (OCT) and magnetic resonance imaging (MRI), we now know that there is neurodegeneration in the visual pathway over time among persons with MS in addition to the acute neuro-ophthalmic episodes (4).
Visual impairment significantly disrupts health-related quality of life among persons with MS (5, 6). Most commonly reported problems include blurry or double vision, trouble seeing at night or under bright sunlight, difficulty with reading or looking at a computer, and driving or parking a car (5, 6). The 25-Item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) is the most widely used vision-related patient reported outcome measure in MS. The NEI-VFQ-25 composite and subscale scores have been validated in the MS population, including discriminating between MS and healthy control (HC) participants as well as correlating with performance on visual function tests, such as visual acuity and low contrast sensitivity (5–8). A 10-item Neuro-Ophthalmic Supplement to the NEI-VFQ-25 was developed to increase the measure’s sensitivity in the detection of neuro-ophthalmologic symptoms, including blurry vision, trouble following moving objects, and double vision (9).
While the NEI-VFQ-25 is a well validated measure that effectively represents the real-life impact of visual impairment, its emphasis is on gauging the degree to which visual symptoms disrupt daily activities rather than the manner in which they are disrupted. Visual Discomfort Scale (VDS) (10, 11)explores in greater detail the somatic and perceptual symptoms experienced by those with visual discomfort that interfere with reading and similar tasks. The VDS is a 23-item measure that probes physical symptoms such as strained eyes and headache; perceptual symptoms such as moving, floating, or flickering text; and compensatory strategies employed such as squinting, repetitive blinking, or using one’s finger to guide the eyes across the text. The VDS can therefore provide more clinically relevant information that may direct intervention to reduce somatic and perceptual symptoms of visual stress during reading that the NEI-VFQ-25 cannot.
Cognitive impairment is also prevalent and debilitating at onset and throughout the course of MS (12). Although variability in cognitive impairment has been reported (13, 14), it occurs across all MS types with prevalence in the range of 25–75% of the overall MS population (13, 15). It most often affects processing speed, executive functioning, visual and verbal memory, and visuospatial processing (13). Furthermore, cognitive impairment may predate other symptoms of MS (13, 14). Cognitive dysfunction has been shown to negatively impact quality of life, including employment and social functioning (15–18) [cf. Chow et al. (19), Glanz et al. (20), and Baumstarck-Barrau et al. (21)].
Tests used to assess the most commonly affected cognitive domains in MS, such as information processing speed, visual memory, and executive functions, are often exclusively visually-mediated. It is thus necessary to examine how visual problems may confound performance on these neuropsychological tests. Accurate detection of cognitive deficits is vital in monitoring disease progression and treatment planning.
We have previously shown that a history of neuro-ophthalmic syndromes and oculomotor speed deficits are associated with poorer performance on the Symbol Digit Modalities Test (SDMT) (22, 23), a visually-mediated measure of information processing speed and a gold standard screening measure for MS-related cognitive dysfunction (24). Other studies have also found that those who performed worse on visual function tests also performed poorly on visually-mediated neuropsychological tests (25–27). The current study will extend these findings by examining if self-reported visual discomfort problems would also be associated with SDMT performance.
The overarching objective of the current investigation is to explore which visual discomfort problems are commonly reported among persons with MS and whether they are discrepant from HCs. We hypothesize that persons with MS would report more frequent visual discomfort symptoms compared to HCs. Moreover, the current study will examine whether self-reported visual discomfort symptoms are associated with performance on the SDMT. We hypothesize that more frequent visual discomfort symptoms would be associated with poorer performance on the SDMT. Finally, we will evaluate the role of MS disease characteristics in visual discomfort symptoms and their association with SDMT performance. We hypothesize that longer disease duration and progressive MS disease course would be linked to more frequent visual discomfort problems.
Methods
Participants
Data from four studies were included in the current investigation. Inclusion and exclusion criteria were similar across studies. Inclusion criteria included: (1) diagnosis of MS (or no neurological conditions for the HC group), (2) able to speak English fluently, and (3) age between 18 and 70 years. Exclusion criteria included: (1) history of stroke or neurologic disease other than MS, (2) history of significant psychiatric disorders (e.g., major depressive disorder, bipolar disorder, schizophrenia), (3) history of alcohol or substance abuse, (4) MS relapses within the past month, and (5) use of medications that may influence cognition, such as steroids, benzodiazepines, neuroleptics, and opiates, within the past month. Clinical variables such as, but not limited to, time since diagnosis, relapsed history, use of disease modifying therapies, were retrieved from medical records, when available, or by participant self-report. All studies were approved by the Kessler Foundation Institutional Review Board, and all participants provided written informed consent before enrollment.
Measures
The SDMT assesses information processing speed and is a gold standard for evaluating cognition in MS research (28, 29). The oral version of the SDMT was used for this study, as recommended in MS consensus neuropsychological batteries (30, 31), due to motor difficulties among MS participants that may confound performance on the written version. Participants were presented with an 8.5 × 11 sheet of paper with a key of nine symbols and digit pairs along the top. Below the key were several lines of symbols without the corresponding number. The participant was asked to verbally call out numbers for different symbols as quickly as they could within 90 s, after completing several practice trials. The total number of correct responses was used as an outcome for this study.
The VDS is 23-item questionnaire assessing the frequency of visual discomfort symptoms (10, 11). Responses were recorded in a four-point Likert scale, from “never occurs” to “almost always.” Higher scores indicated more frequent visual discomfort symptoms. Besides a total score, we also calculated scores for each domain, including seeing the text or background moving or fading, headache/eye soreness, blurriness/diplopia, having to re-read, experience of glare, and slow reading, based on factor analyses performed in prior literature (11). The total score was further divided into three clinical ranges based on previous literature (low = 0–24; moderate = 25–48; high = 49–69) (10, 11).
Statistical analyses
R version 4.0.4 was used for all analyses. Group differences (MS vs. HC) in demographic characteristics were determined using Welch’s Two Samples t tests for continuous variables and Pearson’s chi-squared tests for categorical variables. Group differences in VDS scores were calculated using generalized linear models with Poisson distributions, adjusted for age. For VDS clinical ranges, because only one person was in the high visual discomfort group, they were combined with the moderate group for subsequent analyses; group differences in clinical ranges were determined using a generalized linear model with binomial distribution (logistic). Relationships between MS disease variables (duration and disease courses) and VDS scores were examined using Poisson generalized linear models (binomial models for clinical ranges), adjusted for age. In this analysis, MS disease course was dichotomized into relapsing–remitting or progressive types. Associations between VDS scores and the SDMT score were calculated using Poisson generalized linear models, adjusted for both age and education, within the overall sample (MS and HC). Potential moderating effects of MS disease variables on the relationship between VDS and SDMT scores were investigated with additional MS disease × VDS interaction terms. Predictors were centered on reducing the correlation between the interaction terms and their component predictors.
Results
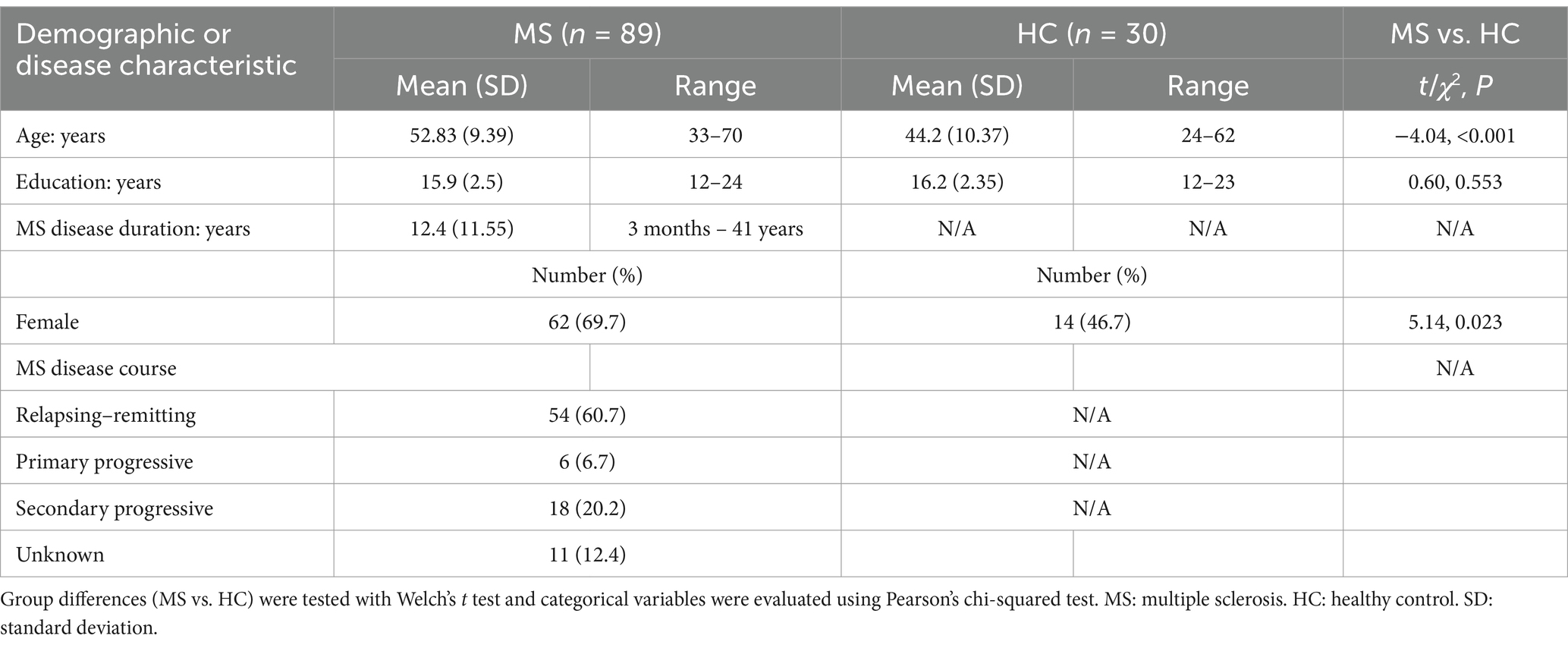
Demographic and clinical characteristics
The study sample included 89 participants with MS and 30 healthy controls (HCs). See Table 1 for summary of demographic and disease characteristics. The MS group was significantly older [t (46.09) = −4.03, p < 0.001] and had more females than the HC group [χ2(1) = 5.14, p = 0.023].
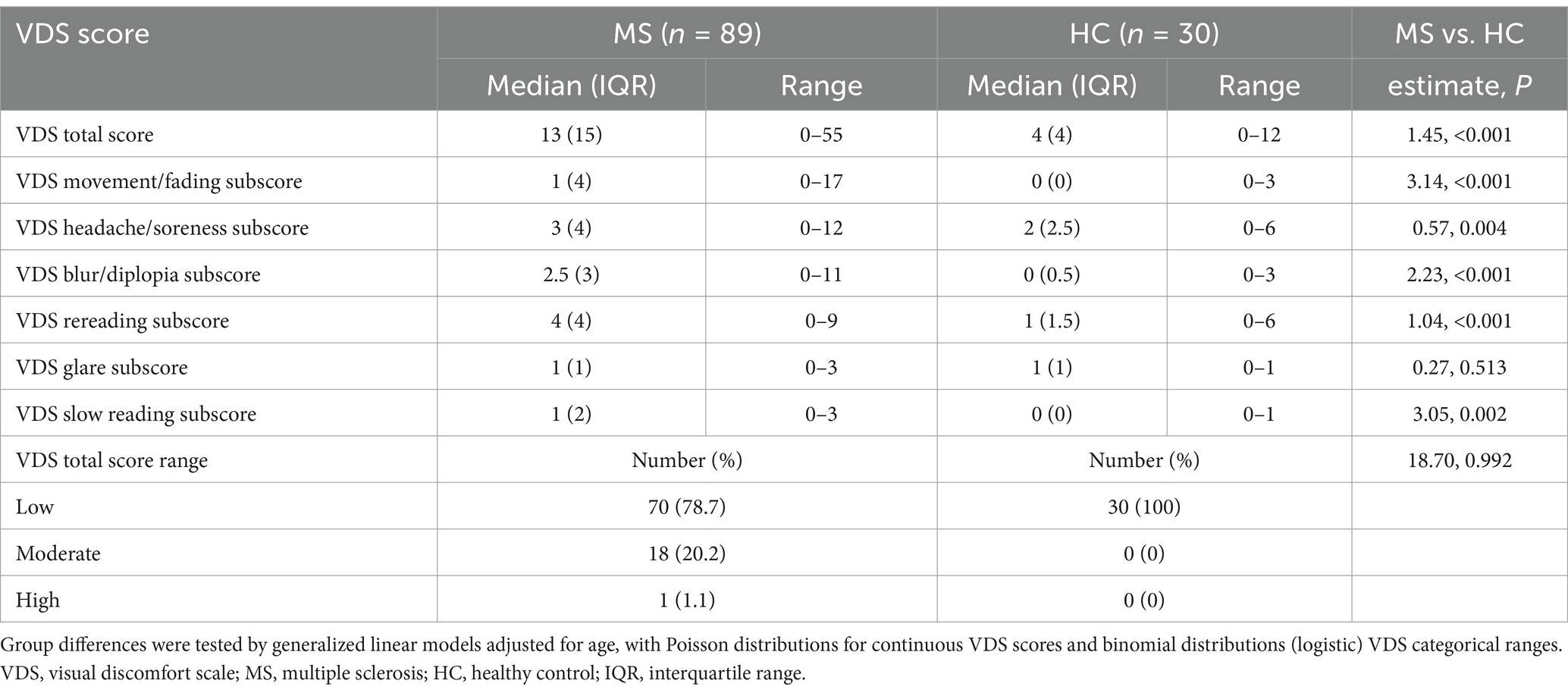
Group differences on the VDS
The MS group endorsed more frequent visual discomfort symptoms on the VDS on all domains except for experience of glare (Table 2). Using Poisson generalized linear models adjusted for age, the group effects were: estimate = 1.45, p < 0.001 for total score; estimate = 3.14, p < 0.001 for movement/fading; estimate = 0.57, p = 0.004 for headache/soreness; estimate = 2.23, p < 0.001 for blur/diplopia; estimate = 1.04, p < 0.001 for rereading; estimate = 0.27, p = 0.513 for glare; estimate = 3.05, p = 0.003 for slow reading. All HCs and the majority of persons with MS were in the low visual discomfort group, but 20% of persons with MS fell in the moderate visual discomfort group and one person with MS fell in the high visual discomfort group. However, formal testing of group differences was not significant (p > 0.05).
Associations between visual discomfort symptoms and SDMT performance
In Poisson generalized linear models adjusted for both age and education within the overall sample, higher frequency in most visual discomfort symptoms was associated with poorer performance on the SDMT. Specifically, all but headache/soreness and glare symptom scores were associated with performance on the SDMT (total score: estimate = −5.94 × 10−3, p < 0.001; movement/fading: estimate = −0.02, p < 0.001; headache/soreness: estimate = −5.21 × 10−3, p = 0.330; blur/diplopia: estimate = −0.01, p = 0.012; rereading: estimate = −0.03, p < 0.001; glare: estimate = −0.03, p = 0.160; slow reading: estimate = −0.07, p < 0.001; Figure 1). Participants in the low visual discomfort group had higher SDMT scores than participants in the moderate or high visual discomfort group (estimate = −0.14, p < 0.001).
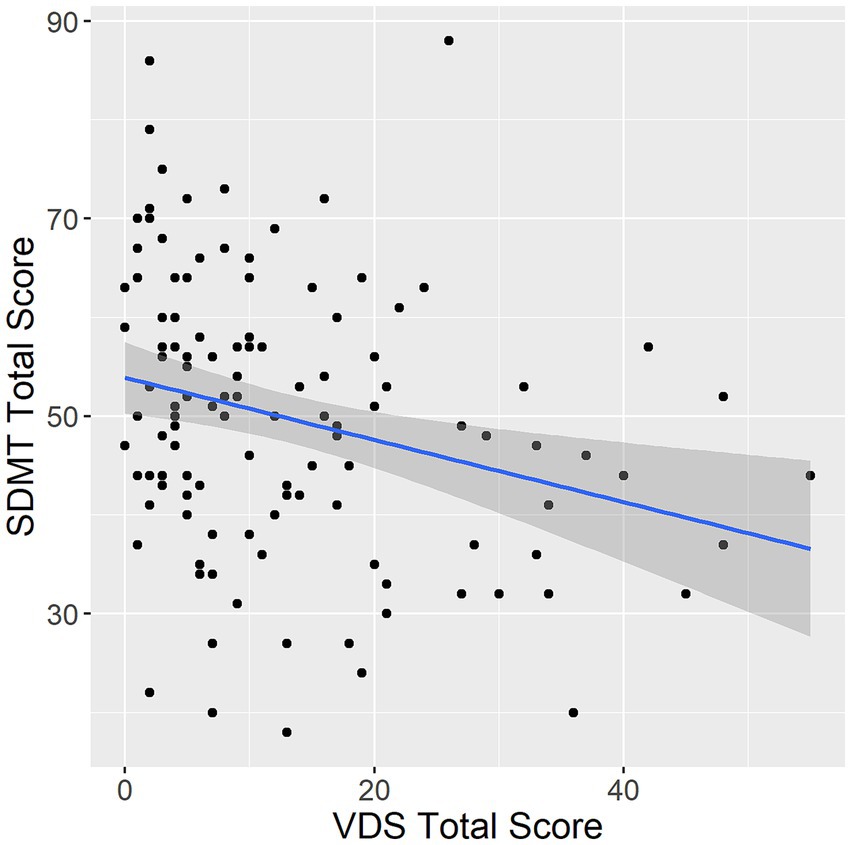
Figure 1. Association between VDS and SDMT. VDS, visual discomfort scale. SDMT, symbol digit modalities test. Error band represents 95% confidence intervals.
Role of MS disease characteristics
MS disease duration was associated with only the headache/soreness symptom (estimate = 0.001, p = 0.042) but not VDS total score or any other visual discomfort symptoms, after adjusting for age. Interestingly, after adjusting for age, MS participants with the relapsing–remitting disease course endorsed more frequent visual discomfort symptoms compared to those with the progressive disease course in the VDS total score (estimate = 0.17, p = 0.004). However, this association was not maintained with individual symptom scores (p > 0.05). Neither MS disease duration nor disease course was associated with VDS clinical ranges (p > 0.05).
With regard to the role of MS disease variables in moderating the relationships between VDS and SDMT scores, MS participants with longer disease duration exhibited a stronger negative correlation between VDS total score and SDMT score (interaction estimate = −2.88 × 10−5, p = 0.019), adjusting for age and education. The same moderating effect was observed in the blur/diplopia (interaction estimate = −1.66 × 10−4, p = 0.001), glare (interaction estimate = −3.05 × 10−4, p = 0.042), and slow reading (interaction estimate = −2.95 × 10−4, p = 0.015) symptom scores. Having a progressive course yielded a stronger negative correlation between visual discomfort symptoms and SDMT performance only for the glare symptom (interaction estimate = 0.09, p = 0.029), adjusting for age and education.
Notably, the negative correlation between MS disease duration and SDMT score was stronger among participants in the moderate/high visual discomfort group relative to the low visual discomfort group (interaction estimate = 7.03 × 10−4, p = 0.04; Figure 2A), adjusting for age and education. MS participants with a progressive course in the moderate/high visual discomfort group had lower SDMT score than those with a progressive course in the low visual discomfort group or MS participants with relapsing–remitting course in either low or high visual discomfort group (interaction estimate = 0.34, p < 0.001; Figure 2B).
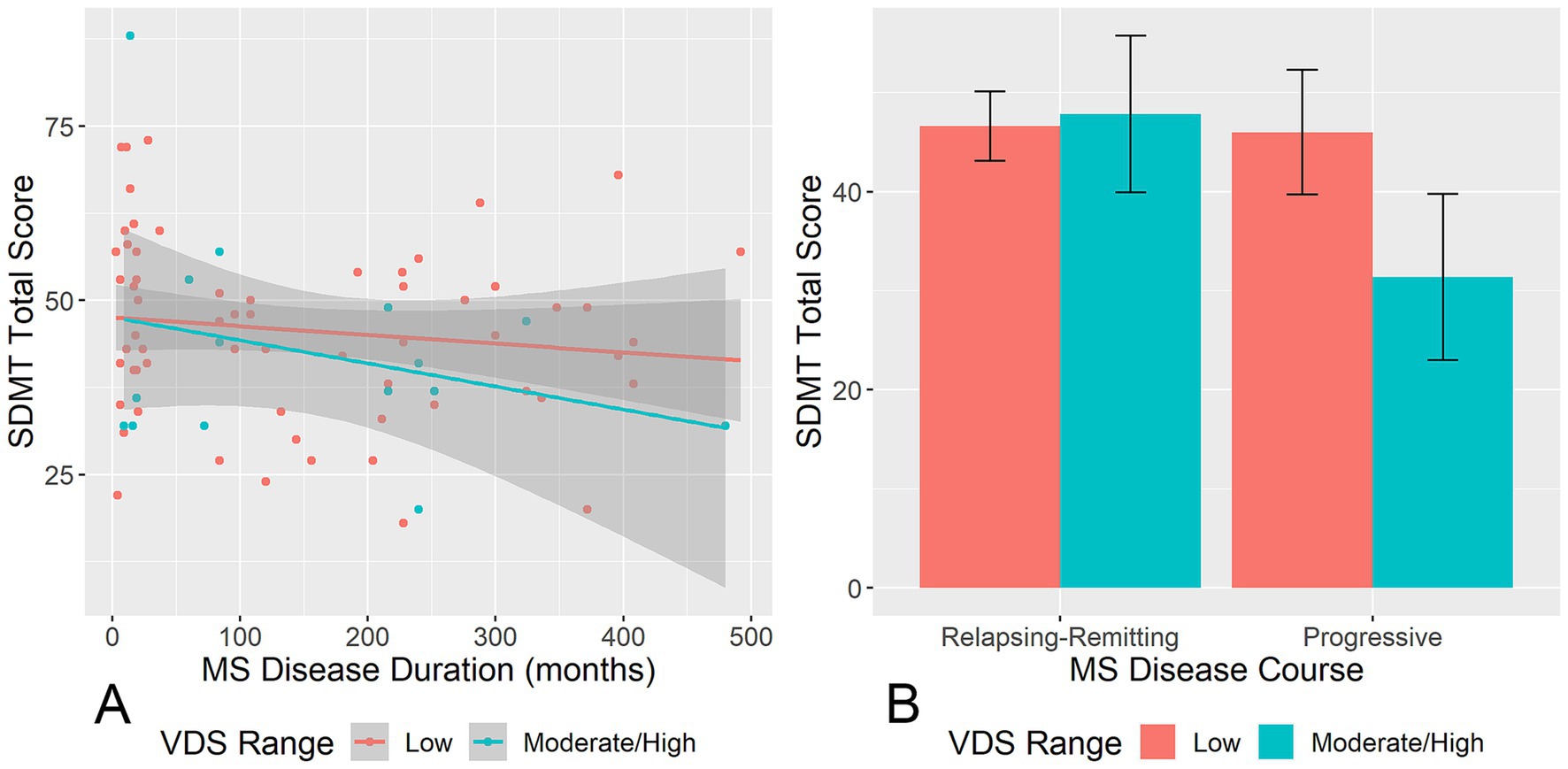
Figure 2. Moderating effects of MS disease characteristics on the relationships between VDS and SDMT. (A) Shows the moderating effect of VDS range on the relationship between MS disease duration and SDMT score. (B) Shows the moderating effect of VDS range on the relationship between MS disease course and SDMT score. VDS, visual discomfort scale; SDMT, symbol digit modalities test. Error bands/bars represent 95% confidence intervals.
Discussion
The current study was the first to examine somatic and perceptual visual discomfort problems using the VDS in persons with MS. Across a variety of domains, persons with MS endorsed higher frequencies of visual discomfort symptoms relative to HCs. More frequent visual discomfort symptoms were associated with worse performance on the SDMT. For participants with MS reporting moderate/high levels of visual discomfort symptoms, having a longer disease duration or progressive disease courses (primary or secondary) were linked with worse performance on the SDMT.
The current study extends previous vision research in MS. While studies that use neuroimaging or visual/oculomotor assessment as outcome measures elucidate the neurodegenerative processes that affect vision and ocular motility, they do not capture the subjective experience of visual discomfort and its impact on daily living. Clinicians rely on data derived from both objective and subjective measurement to implement targeted, evidence-based interventions customized to the unique needs of each patient. The most widely used patient-reported outcome measure in MS research, the NEI-VFQ-25 (32), measures the degree of interference of visual problems on various activities of daily living but is not specific to the type of visual problem that is occurring. The emphasis of the VDS, on the other hand, is on the individual experience of somatic and perceptual symptoms that typically accompany visual stress. Therefore, the VDS may better inform treatment than the NEI-VFQ-25.
Our finding that more frequent visual discomfort symptoms were associated with worse performance on the SDMT is consistent with our prior investigation, which found that visual and oculomotor deficits were related to worse SDMT performance (22). Similarly, other studies have found that poor performance on visual function tests (e.g., visual acuity, and low-contrast sensitivity) was linked to worse performance on visually-mediated neuropsychological tests among persons with MS (16–18, 25–27). Importantly, specific symptoms that were related to SDMT performance included seeing the text or background moving or fading, blurriness/diplopia, having to re-read, and reading slowly. In contrast, headache/eye soreness and experience of glare did not impact SDMT performance. Additional evidence of interaction between cognitive and visual impairments has been provided by studies using Optical Coherence Tomography (OCT). OCT metrics, such as, retinal nerve fiber layer thickness, have been proven to be a sensitive biomarker of neurodegeneration in MS (33–35) and is highly correlated with performance on cognitive tests, such as the SDMT (36, 37). Thus, it is important for clinicians to objectively and subjectively assess visual discomfort problems that the patient experiences when interpreting a visually-mediated neuropsychological test such as the SDMT, especially among those with a longer disease duration or progressive disease course. The VDS is quick to administer in a clinical setting, with 23 items using a four-point Likert scale.
Additionally, we found that having a longer disease duration and progressive disease course were associated with worse performance on the SDMT only among those with a clinically moderate or high level of visual discomfort (relative to a low level). This suggests that poor performance observed on the SDMT among patients with longer disease duration and progressive disease course may be partially due to their visual problems, rather than purely cognitive dysfunction. It is well known that age and lifestyle factors, such as diet, can influence both cognitive and visual outcomes (38, 39); future research should extend the current research by exploring the multifaceted processes that influence both cognitive and visual outcomes.
There were a few limitations to this study that warrant discussion. First, the cross-sectional nature of the current investigation precludes us from making statements regarding causation. While it is tempting to conclude that visual discomfort symptoms were a confounder of SDMT performance, it is also possible that MS-related neurodegeneration manifests in both visual discomfort and cognitive deficits. More longitudinal research examining vision and cognition, with neuroimaging measures, are needed to disentangle these cause-and-effect relationships. Second, we used groups of unequal size (30 healthy controls vs. 89 MS patients). Finally, the VDS has not been formally validated in the MS population, and the current study lacked some important clinical measures, such as objective visual function tests and neurologic disability measures (e.g., Kurtzke Expanded Disability Status Scale). Future research should include these measures which will add to our understanding of visual discomfort symptoms among persons with MS.
In conclusion, MS patients with greater self-report of symptoms of visual discomfort showed worse performance on the SDMT. This could reflect that MS-related neurodegeneration manifests in both visual discomfort and cognitive impairment, or that performance on visually-mediated neuropsychological tests such as the SDMT is mediated by symptoms of visual discomfort. Thus, clinicians should identify specific visual discomfort problems that the patient experiences and take them into account when interpreting a visually-mediated neuropsychological test such as the SDMT, especially for MS patients with longer disease duration or a progressive disease course.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Kessler Foundation Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MC: Conceptualization, Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing, Formal analysis, Visualization. TR: Writing – original draft, Writing – review & editing. NC: Writing – original draft, Writing – review & editing. YG: Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The preparation of this manuscript was supported in part by the Multiple Sclerosis Collaborative Network of NJ grant 1069-A7 (PI: Silvana Costa), National Multiple Sclerosis Society (NMSS) grant PP2280 (PI: John DeLuca), NMSS grant 4997A5 (PI: Nancy Chiaravalloti), NMSS grant CA 1069-A-7 (PI: Yael Goverover), NMSS grant MB-0024 (PI: Yael Goverover), NMSS grant MB-1606-08779 (PI: John DeLuca), and Kessler Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wallin, MT, Culpepper, WJ, Nichols, E, Bhutta, ZA, Gebrehiwot, TT, Hay, SI, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:269–85. doi: 10.1016/S1474-4422(18)30443-5
2. Costello, F. The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol. (2013) 2013:1–17. doi: 10.1155/2013/134858
3. Jasse, L, Vukusic, S, Durand-Dubief, F, Vartin, C, Piras, C, Bernard, M, et al. Persistent visual impairment in multiple sclerosis: prevalence, mechanisms and resulting disability. Mult Scler. (2013) 19:1618–26. doi: 10.1177/1352458513479840
4. Balcer, LJ, Miller, DH, Reingold, SC, and Cohen, JA. Vision and vision-related outcome measures in multiple sclerosis. Brain. (2015) 138:11–27. doi: 10.1093/brain/awu335
5. Ma, S-L, Shea, JA, Galetta, SL, Jacobs, DA, Markowitz, CE, Maguire, MG, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. (2002) 133:686–92. doi: 10.1016/S0002-9394(02)01337-5
6. Mowry, EM, Loguidice, MJ, Daniels, AB, Jacobs, DA, Markowitz, CE, Galetta, SL, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. (2009) 80:767–72. doi: 10.1136/jnnp.2008.165449
7. Noble, J, Forooghian, F, Sproule, M, Westall, C, and O’Connor, P. Utility of the National eye Institute VFQ-25 questionnaire in a heterogeneous Group of Multiple Sclerosis Patients. Am J Ophthalmol. (2006) 142:464–8. doi: 10.1016/j.ajo.2006.04.051
8. Balcer, LJ, Baier, ML, Kunkle, AM, Rudick, RA, Weinstock-Guttman, B, Simonian, N, et al. Self-reported visual dysfunction in multiple sclerosis: results from the 25-item National eye Institute visual function questionnaire (VFQ-25). Mult Scler. (2000) 6:382–5. doi: 10.1177/135245850000600604
9. Raphael, BA, Galetta, KM, Jacobs, DA, Markowitz, CE, Liu, GT, Nano-Schiavi, ML, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. (2006) 142:1026–1035.e2. doi: 10.1016/j.ajo.2006.06.060
10. Conlon, EG, Lovegrove, WJ, Chekaluk, E, and Pattison, PE. Measuring visual discomfort. Vis Cogn. (1999) 6:637–63. doi: 10.1080/135062899394885
11. Borsting, E, Chase, CH, and Ridder, WH. Measuring visual discomfort in college students. Optom Vis Sci. (2007) 84:745–51. doi: 10.1097/OPX.0b013e31812f5f51
12. Chiaravalloti, ND, and DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. (2008) 7:1139–51. doi: 10.1016/S1474-4422(08)70259-X
13. Benedict, RHB, Amato, MP, DeLuca, J, and Geurts, JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. (2020) 19:860–71. doi: 10.1016/S1474-4422(20)30277-5
14. Rao, SM, Leo, GJ, Bernardin, L, and Unverzagt, F. Cognitive dysfunction in multiple sclerosis.: I. Frequency, patterns, and prediction. Neurology. (1991) 41:685–91. doi: 10.1212/WNL.41.5.685
15. Patti, F, Leone, C, and D’Amico, E. Treatment options of cognitive impairment in multiple sclerosis. Neurol Sci. (2010) 31:265–9. doi: 10.1007/s10072-010-0438-7
16. Cutajar, R, Ferriani, E, Scandellari, C, Sabattini, L, Trocino, C, Marchello, LP, et al. Cognitive function and quality of life in multiple sclerosis patients. Journal of Neurovirology. (2000) 6:S186–S190.
17. Nabizadeh, F, Balabandian, M, Rostami, MR, Owji, M, Sahraian, MA, Bidadian, M, et al. Association of cognitive impairment and quality of life in patients with multiple sclerosis: a cross-sectional study. CJN. (2022) 21:144–50. doi: 10.18502/cjn.v21i3.11106
18. Ruet, A, Deloire, M, Hamel, D, Ouallet, J-C, Petry, K, and Brochet, B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. (2013) 260:776–84. doi: 10.1007/s00415-012-6705-1
19. Chow, HH, Højsgaard Chow, H, Schreiber, K, Magyari, M, Ammitzbøll, C, Börnsen, L, et al. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav. (2018) 8:e00875. doi: 10.1002/brb3.875
20. Glanz, BI, Healy, BC, Rintell, DJ, Jaffin, SK, Bakshi, R, and Weiner, HL. The association between cognitive impairment and quality of life in patients with early multiple sclerosis. J Neurol Sci. (2010) 290:75–9. doi: 10.1016/j.jns.2009.11.004
21. Baumstarck-Barrau, K, Simeoni, MC, Reuter, F, Klemina, I, Aghababian, V, Pelletier, J, et al. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurol. (2011) 11:17. doi: 10.1186/1471-2377-11-17
22. Chen, MH, Chiaravalloti, ND, Genova, HM, and Costa, SL. Visual and motor confounds on the symbol digit modalities test. Mult Scler Relat Disord. (2020) 45:102436. doi: 10.1016/j.msard.2020.102436
23. Costa, SL, Gonçalves, ÓF, Chiaravalloti, ND, DeLuca, J, and Almeida, J. Neuro-ophthalmic syndromes and processing speed in multiple sclerosis. J Neuroophthalmol. (2016) 36:23–8. doi: 10.1097/WNO.0000000000000272
24. Kalb, R, Beier, M, Benedict, RHB, Charvet, L, Costello, K, Feinstein, A, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. (2018) 24:1665–80. doi: 10.1177/1352458518803785
25. Bruce, JM, Bruce, AS, and Arnett, PA. Mild visual acuity disturbances are associated with performance on tests of complex visual attention in MS. J Inter Neuropsych Soc. (2007) 13:544–8. doi: 10.1017/S1355617707070658
26. Feaster, HT, and Bruce, JM. Visual acuity is associated with performance on visual and non-visual neuropsychological tests in multiple sclerosis. Clin Neuropsychol. (2011) 25:640–51. doi: 10.1080/13854046.2011.565075
27. Wieder, L, Gäde, G, Pech, LM, Zimmermann, H, Wernecke, KD, Dörr, JM, et al. Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: a cross-sectional pilot study. BMC Neurol. (2013) 13:167. doi: 10.1186/1471-2377-13-167
28. Benedict, RH, DeLuca, J, Phillips, G, LaRocca, N, Hudson, LD, Rudick, R, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. (2017) 23:721–33. doi: 10.1177/1352458517690821
29. Strober, L, DeLuca, J, Benedict, RHB, Jacobs, A, Cohen, JA, Chiaravalloti, N, et al. Symbol digit modalities test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler. (2019) 25:1781–90. doi: 10.1177/1352458518808204
30. Benedict, RHB, Benedict, RH, Fischer, JS, Archibald, CJ, Arnett, PA, Beatty, WW, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. (2002) 16:381–97. doi: 10.1076/clin.16.3.381.13859
31. Langdon, D, Amato, MP, Boringa, J, Brochet, B, Foley, F, Fredrikson, S, et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler. (2012) 18:891–8. doi: 10.1177/1352458511431076
32. Mangione, CM, Lee, PP, Gutierrez, PR, Spritzer, K, Berry, S, and Hays, RD. Development of the 25-item National eye Institute visual function questionnaire. Arch Ophthalmol. (2001) 119:1050–8. doi: 10.1001/archopht.119.7.1050
33. Zanghì, A, Greco, A, Giancipoli, E, Tumani, H, Avolio, C, and D’Amico, E. Unraveling the inflammation–degeneration tangle in early MS: preliminary insights from ferritin, neurogranin, TREM2, and retinal ganglion cell layer. J Neurol. (2025) 272:109. doi: 10.1007/s00415-024-12797-0
34. Alonso, R, Gonzalez-Moron, D, and Garcea, O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult Scler Relat Disord. (2018) 22:77–82. doi: 10.1016/j.msard.2018.03.007
35. Christensen, R, Jolly, A, Yam, C, Yiannakas, MC, Toosy, AT, Pitteri, M, et al. Investigating the complementary value of OCT to MRI in cognitive impairment in relapsing-remitting multiple sclerosis. Mult Scler. (2025) 31:218–30. doi: 10.1177/13524585241304356
36. Dreyer-Alster, S, Gal, A, and Achiron, A. Optical coherence tomography is associated with cognitive impairment in multiple sclerosis. J Neuroophthalmol. (2022) 42:e14:–e21. doi: 10.1097/WNO.0000000000001326
37. Mirmosayyeb, O, Zivadinov, R, Weinstock-Guttman, B, Benedict, RHB, and Jakimovski, D. Optical coherence tomography (OCT) measurements and cognitive performance in multiple sclerosis: a systematic review and meta-analysis. J Neurol. (2023) 270:1266–85. doi: 10.1007/s00415-022-11449-5
38. Godos, J, Caraci, F, Micek, A, Castellano, S, D’Amico, E, Paladino, N, et al. Dietary phenolic acids and their major food sources are associated with cognitive status in older Italian adults. Antioxidants. (2021) 10:700. doi: 10.3390/antiox10050700
Keywords: visual impairment, visual discomfort scale, symbol digit modalities test, information processing speed, multiple sclerosis
Citation: Chen MH, Rich TJ, Chiaravalloti N, Goverover Y and Costa SL (2025) Impact of visual discomfort symptoms on SDMT performance among persons with MS. Front. Neurol. 16:1569451. doi: 10.3389/fneur.2025.1569451
Edited by:
Emanuele D’amico, University of Foggia, ItalyReviewed by:
Samar Farouk Ahmed, Minia University, EgyptIoannis Nikolaidis, Hippokration General Hospital, Greece
Copyright © 2025 Chen, Rich, Chiaravalloti, Goverover and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvana L. Costa, U2Nvc3RhQGtlc3NsZXJmb3VuZGF0aW9uLm9yZw==; Michelle H. Chen, bWljaGVsbGUuY2hlbjJAcnV0Z2Vycy5lZHU=
 Michelle H. Chen
Michelle H. Chen Timothy J. Rich
Timothy J. Rich Nancy Chiaravalloti
Nancy Chiaravalloti Yael Goverover
Yael Goverover Silvana L. Costa
Silvana L. Costa