- 1Department of Neurology, Xuanwu Hospital of Capital Medical University, Beijing, China
- 2Department of Neurology, Suzhou Municipal Hospital, Suzhou, Anhui, China
- 3Department of Neuro-intervention, Zhangzhou Municipal Hospital, Zhangzhou, Fujian, China
- 4Department of Neuro-intervention, Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou, Fujian, China
- 5Department of Neurology, Nanyang Central Hospital, Nanyang, Henan, China
- 6Department of Neurology, Maanshan People's Hospital, Maanshan, Anhui, China
- 7Department of Neurology, Luoyang Central Hospital Affiliated To Zhengzhou University, Luoyang, Henan, China
- 8Department of Neurology, Liaocheng Third People's Hospital, Liaocheng, Shandong, China
- 9Department of Neurology, WanBei Coal-Electricity Group General Hospital, Suzhou, Anhui, China
- 10Department of Neurology, Si County People's Hospital, Anhui, China
- 11Department of Neurology, Sui Xi County Hospital, Huaibei, Anhui, China
- 12Department of Neurology, The First People’s Hospital of Zhengzhou, Zhengzhou, Henan, China
- 13Department of Neurology, Xihua County People's Hospital, Zhoukou, Henan, China
- 14Department of Cerebrovascular Disease, Fujian Medical University Union Hospital, Fujian, China
- 15China-America Institute of Neuroscience and Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China
- 16Center for Evidence Based Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
- 17Beijing Institute for Brain Disorders, Capital Medical University, Beijing, China
Background: Albumin is a multifunctional plasma protein that is mainly synthesized in the liver and may play a neuroprotective role in treating acute ischemic stroke (AIS). The efficacy of albumin in patients with AIS receiving reperfusion therapy remains unknown.
Methods: ARISE is a multicenter, randomized, double-blind, placebo-controlled, phase 2 study. We will recruit 134 patients aged 18–80 years with AIS due to large-vessel occlusion in the anterior circulation, within 24 h of symptom onset, with an Alberta Stroke Program Early CT Score of 3–10 points and an infarct core volume of ≤100 mL at baseline. Eligible patients will be randomly assigned, on a 1:1 ratio, to undergo endovascular therapy (EVT) and receive albumin therapy (0.5 g/kg; intravenous injection) once daily for 4 days or to undergo EVT and receive placebo therapy once daily for 4 days. The primary efficacy outcome is the change in infarct volume from baseline to day 5.
Conclusion: The ARISE trial will provide valuable evidence on the efficacy and safety of albumin in patients with AIS receiving EVT.
Clinical trial registration: www.clinicaltrials.gov, NCT06538844.
Background
Stroke is the most common cause of death and disability worldwide, with acute ischemic stroke (AIS) accounting for 80% of all cases (1, 2). Effective reperfusion therapies, such as intravenous thrombolysis and endovascular therapy (EVT), have been widely used in the treatment of AIS patients (3–5). While recanalization of occluded arteries is central to the treatment of AIS, patient prognosis may be further improved with additional treatment options that preserve and enhance brain function through neuroprotection (6, 7). Neuroprotective drugs can freeze the ischemic penumbra and target the ischemic cascade following cerebral infarction, including mechanisms such as oxidative stress and inflammation. This may reduce the inflammatory response, limit hemorrhagic transformation, and improve neurological prognosis after cerebral infarction (8, 9). However, neuroprotection in AIS has a long history of clinical trial failures over the past several decades (9, 10). One possible reason is that many of these drugs were not combined with reperfusion therapy (11, 12). Therefore, it is recommended that previously unsuccessful neuroprotective drugs, especially those supported by strong preclinical research evidence and confirmed to be safe in clinical studies, be reconsidered in the context of reperfusion therapy (9).
Albumin, the predominant protein in plasma, is mainly synthesized in the liver (13). Although the exact molecular mechanisms underlying albumin’s effects remain unclear, preclinical studies have shown its neuroprotective effects in several animal models of cerebral infarction. These effects include reducing cerebral edema through dehydration and increasing cerebral blood flow in ischemic areas (14–16). However, the Albumin in Acute Ischemic Stroke (ALIAS) trial, conducted from 2009 to 2012, did not confirm that high-dose albumin improves neurological prognosis in AIS patients (17). In this study, only 21% of participants received EVT. EVT devices and therapeutic techniques, along with the expansion of indications for EVT and the reduction of barriers to EVT selection through imaging, were not fully developed at the time of the study (17). The fact that neuroprotective treatment with albumin was not combined with efficient reperfusion therapy may have contributed to the failure of the study. Therefore, it is important to reevaluate the neuroprotective role of albumin in the context of reperfusion therapy for AIS.
This protocol describes the rationale and design of the ARISE (Albumin for Patients with Acute Large-Vessel Occlusive Stroke Undergoing Endovascular Therapy) trial, which aims to investigate albumin’s safety and efficacy for AIS patients undergoing EVT.
Methods
Ethical approval and informed consent
The clinical trial complies with the ethical guidelines outlined in the Declaration of Helsinki, which governs medical research involving human subjects. The ethics committees and institutional review boards of all participating clinical sites approved the study protocol. Prior to participation, written informed consent will be obtained from all participants or their legal guardians.
Study design
ARISE is a multicenter, randomized, double-blind, placebo-controlled trial with two parallel (1:1) groups (albumin or placebo), designed to evaluate the safety and efficacy of albumin for patients with AIS receiving EVT. All participants will be followed for 3 months. The study has been registered in www.clinicaltrials.gov (registration number NCT06538844).
Patient population
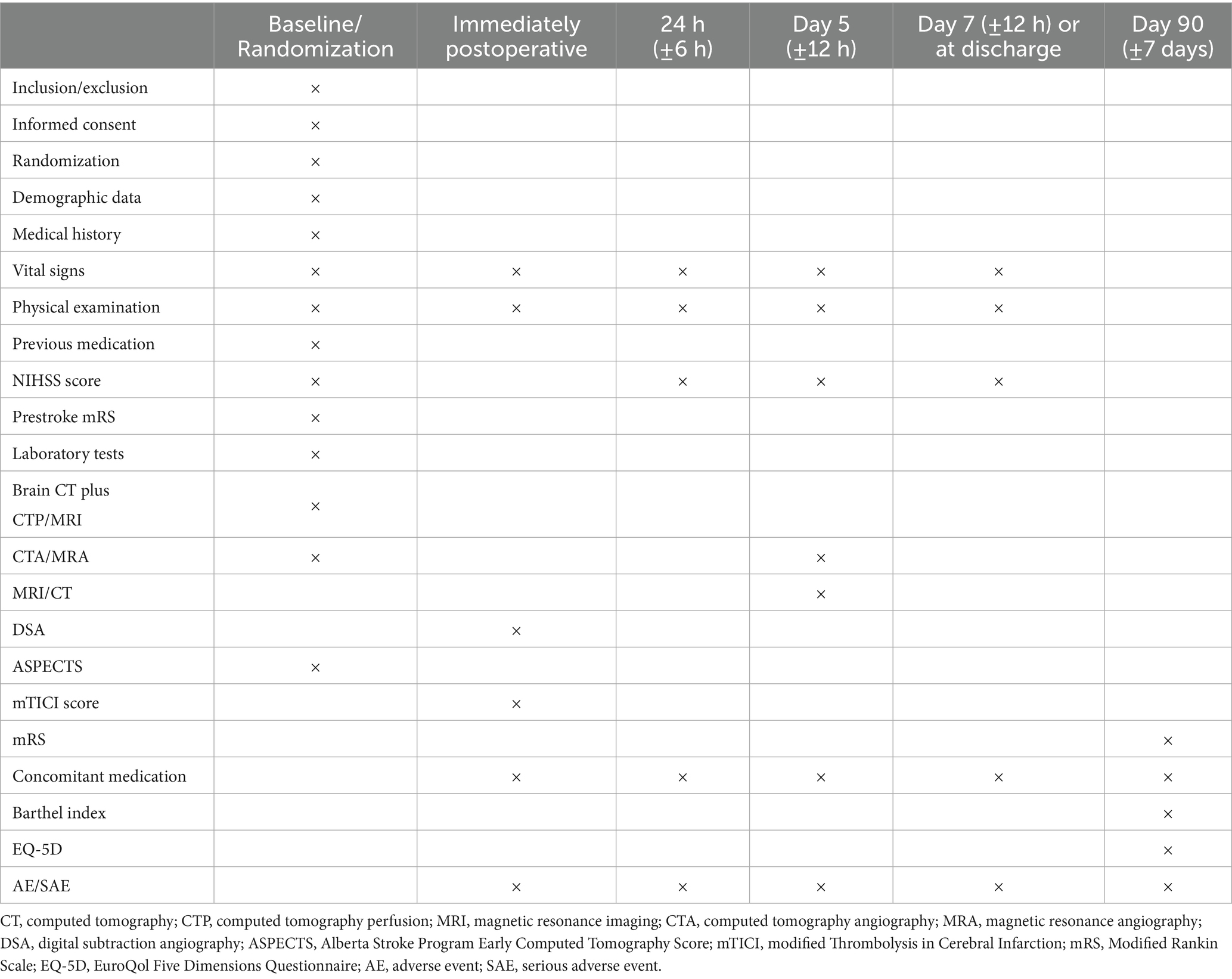
Patients will be eligible for enrollment if they have an acute ischemic stroke due to a large-vessel occlusion in the intracranial segment of the internal carotid artery or the M1 or M2 segment of the middle cerebral artery. Eligible patients must be aged 18–80 years, have a modified Rankin Scale (mRS) score of ≤1 the onset of the disease, have a National Institute of Health Stroke Scale (NIHSS) score of ≥6, have an Alberta Stroke Program Early CT Score (ASPECTS) of ≥3, and have ischemic core volume of ≤100 mL. Patients must be enrolled up to 24 h after the onset of stroke symptoms. A complete list of inclusion and exclusion criteria is provided in Table 1.
Randomization
Eligible patients will be randomly assigned (1:1) to receive albumin or placebo via a web-based interactive response system. A minimization process will be used to balance the stratification factors of age (≤ 60 or > 60 years) and baseline ASPECTS (3 to 6 or ≥6) at each medical site.
Treatments
After qualifying imaging, patients will undergo EVT using currently available devices as quickly as possible and will receive intravenous thrombolysis if indicated (before or during EVT, at a primary hospital prior to transfer, or at the comprehensive stroke center). The experimental group will receive a 25% albumin solution (0.5 g/kg; maximum dose 150 mL) administered via intravenous infusion once daily for 4 days. The control group will receive an equivalent volume of placebo injection intravenously once daily for the first 4 days (Figure 1). The trial drugs will be manufactured and provided by Shanghai RAAS Blood Products Co., Ltd., Shanghai, China. All researchers and patients will be blinded to treatment allocation. The slightly differently colored study drugs will be concealed. The first infusion will be administered within 1 h after randomization. The steering committee will make recommendations for concurrent treatments. Equal care following the American Heart Association/American Stroke Association guidelines for AIS during admission will be provided for all patients.
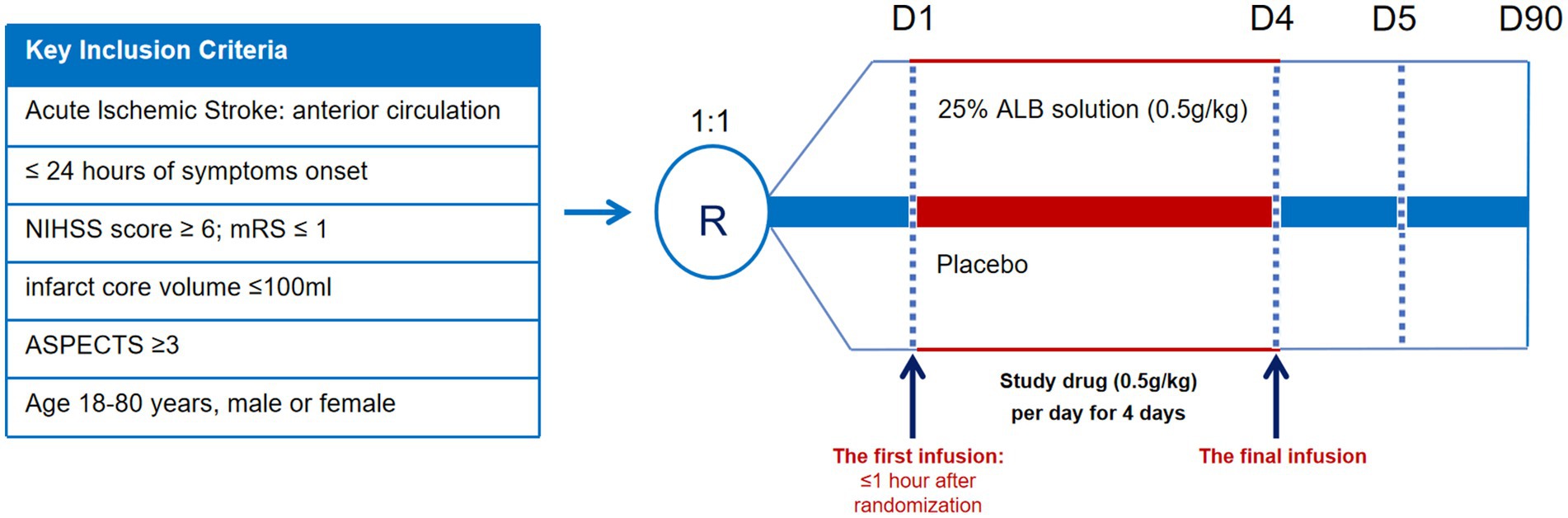
Figure 1. Graphical study design. NIHSS, National Institute of Health Stroke Scale; mRS, Modified Rankin Scale; ASPECTS, Alberta Stroke Program Early CT Score.
Study outcomes
The primary efficacy endpoint is the change in infarct volume from baseline (as measured by computed tomography perfusion (CTP)) to day 5 (as measured by fluid-attenuated inversion recovery (FLAIR)). Infarct volume assessed by CTP will be calculated using NeuBrainCARE® software (version 1.0, Neusoft, Beijing, China). All investigators responsible for enrollment will receive training on the imaging protocol and on the use of NeuBrainCARE® software. For the baseline assessment, the infarction core volume will be considered to be those areas with a relative cerebral blood flow (rCBF) of less than 30%, as indicated by CTP. FLAIR images will be used to calculate the infarct volume at follow-up. If magnetic resonance imaging (MRI) at follow-up time is unavailable, computed tomography (CT) can be used.
The secondary efficacy outcomes include (1) early neurological improvement within 24 h: a decrease in NIHSS score of ≥8 points or NIHSS score of 0–2 within 24 h (2); neurological deterioration within 24 h: an increase in NIHSS score by ≥4 points within 24 h (3); NIHSS score at 24 h (4); proportion of successful reperfusion (mTICI 2b/3) (5); NIHSS score at 5 days (6); proportion of patients with functional independence (mRS 0–1) (7); mRS of 0–2 at 90 days (8); European quality of Life-5 dimensions (EQ-5D) at 90 days; and (9) proportion of patients with Barthel Index (BI) of 95–100 at 90 days. The safety endpoints will mainly include (1) intracerebral hemorrhage within 24 h (2), symptomatic intracerebral hemorrhage (ICH) within 24 h (3), new-onset atrial fibrillation within 5 days (4), pulmonary edema or congestive heart failure within 5 days (5), intracerebral hemorrhage within 5 days (6), decompressive craniectomy within 7 days (7), death within 90 days (8), adverse events (AEs), and (9) serious adverse events (SAEs).
Sample size calculation
In historical controls, the mean growth in the post-EVT infarct core volume from baseline is 18.7 mL, with a variance of 9.7. Based on our pilot study (unpublished data), we assume that there will be a 30% reduction in infarct volume in the albumin group compared to the sham group. A sample size of 128 participants would provide the trial with 90% power at a two-sided alpha level of 0.05. Considering a 5% loss to follow-up, the total sample size estimate was 134 participants (67 per group).
Statistical analysis
The main analysis will be performed in the intention-to-treat population. The primary efficacy outcome will be analyzed by a linear regression model or median regression model, depending on the distribution, to compare the change in infarct volume from baseline to day 5 between the intervention group and the control group, after adjusting for baseline ASPECTS, age, and baseline infarct core volume. The effect of the intervention is presented as a mean difference (or median difference) and 95% confidence intervals (CIs). For missing infarct volume data due to stroke-related death, the worst infarct growth will be imputed. Other missing data will be handled using multiple imputation.
For other outcomes, continuous variables will be analyzed using the same method as the primary efficacy outcome, and a mean difference (or median difference) with 95% CIs will be calculated. Binary variables will be estimated using a modified Poisson regression model, and the result will be presented as relative risk and corresponding 95% CIs. All analyses will be conducted at a two-sided level with a significance level of 0.05. Statistical tests will be performed using the R programming language (version 4.3.3).
Visits and data collection
Study visit will be conducted at baseline, 24 h, day 5, day 7, or before discharge (the follow-up point will be determined by the earliest or latest event occurring between the two specified instances), and day 90 (Table 2). At baseline, computed tomography (CT), CT angiography (CTA), and CT perfusion (CTP) should be performed. On day 5 after randomization, MRI and MRA should be conducted. If MRI is unavailable, a CT scan may be performed. At baseline and during follow-up visits, clinical information will be collected using an electronic data capture system.
Data management and quality control
The Data Safety Monitoring Board (DSMB) monitors the schedule and progress of the trial to ensure patient safety. The DSMB consists of a neurologist, a neuroradiologist, and a methodologist. These experts are not allowed to participate in the trial. The responsibilities of the DSMB include reviewing data completeness and safety, revising the trial protocol, and terminating the trial. The DSMB reviews all SAEs in a blinded manner, adjudicates their unexpectedness and relatedness to the study drug, and assesses adverse outcome events.
Discussion
Our trial aimed to evaluate the safety and efficacy of albumin intravenous infusion for AIS patients who are receiving EVT. The study was designed with lessons learned from previous studies that failed to prove the effectiveness of the treatment (17). First, our trial ensures that all enrolled patients will undergo EVT and assess recanalization rates. Second, in the ALIAS trial, patients treated with albumin experienced a higher incidence of pulmonary edema and congestive heart failure compared to those treated with saline. According to the results of our pilot study, administering an albumin dose of 2 g/kg (as in the ALIAS trial) as four separate injections of 0.5 g/kg, given continuously over 4 days, results in a greater reduction in infarct volume and fewer adverse reactions. We anticipate that this modified dosing strategy will reduce the likelihood of pulmonary edema and congestive heart failure.
The ARISE trial will enroll patients with an infarct core volume of less than 100 mL, encompassing both small and large core infarcts. The inclusion of patients with a small infarct core volume is consistent with numerous studies, such as the ACTION II trial (Natalizumab in acute ischemic stroke) and the ApTOLL study, in patients with AIS (18, 19). Patients with large core infarcts are more prone to developing cerebral edema, which can lead to further ischemic damage (20). Albumin increases plasma colloid osmolality and has been widely supported in animal studies to reduce cerebral edema and improve neurological prognosis (14). Including patients with large core infarct volumes will provide an answer to the question of whether albumin is effective for patients with large core infarct volumes (20). The benefits of expanding the time window for patients to use neuroprotective medications are worth waiting for. Therefore, the ARISE study sets the treatment window at 24 h. Subsequent subgroup analyses may focus on age, infarct volume, last known good status to randomization time, NIHSS score, and stroke type.
The primary endpoint of the study is the change in infarct volume from baseline to day 5 in the intervention versus control groups. An increase in infarct volume (instead of clinical assessment at 3 months) was chosen because this biomarker is recommended by the Academic Industry Roundtable on Stroke Care and the Stroke Imaging Study (21, 22). It is an objective outcome and may be closely associated with clinical outcomes.
Conclusion
To sum up, if the study outcomes are positive, they will provide significant evidence to support a phase 3 trial assessing the efficacy of albumin for AIS patients.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xuanwu Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YuL: Investigation, Methodology, Writing – original draft, Writing – review & editing. XD: Writing – original draft. XC: Writing – original draft. ZM: Writing – original draft. TY: Writing – original draft. CWe: Writing – original draft. YiL: Writing – original draft. JS: Writing – original draft. JX: Writing – original draft. WL: Writing – original draft. LY: Writing – original draft. BW: Writing – original draft. LS: Writing – original draft. JL: Writing – original draft. XZ: Writing – original draft. ChaL: Writing – original draft. WC: Writing – original draft. ChuL: Writing – original draft. DW: Writing – original draft. CH: Writing – original draft. CZ: Writing – original draft. ML: Writing – original draft. YX: Writing – original draft. CWu: Writing – original draft. XJ: Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. National Natural Science Foundation of China (82271507); Beijing Natural Science Foundation (JQ24041); Noncommunicable Chronic Diseases - National Science and Technology Major Project (2023ZD0505403); Beijing Physician Scientist Training Project (BJPSTP-2024-04).
Acknowledgments
We appreciate the staff and participants of ARISE studies for their outstanding contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eren, F, and Yilmaz, SE. Neuroprotective approach in acute ischemic stroke: a systematic review of clinical and experimental studies. Brain Circ. (2022) 8:172–9. doi: 10.4103/bc.bc_52_22
2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
3. Jolugbo, P, and Ariëns, RAS. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. (2021) 52:1131–42. doi: 10.1161/strokeaha.120.032810
4. Elmadhoun, A, Wang, H, and Ding, Y. Impacts of futile reperfusion and reperfusion injury in acute ischemic stroke. Brain Circ. (2024) 10:1–4. doi: 10.4103/bc.bc_9_24
5. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344-e418. doi: 10.1161/str.0000000000000211
6. Stoll, G, and Pham, M. Beyond recanalization - a call for action in acute stroke. Nat Rev Neurol. (2020) 16:591–2. doi: 10.1038/s41582-020-00417-0
7. Tymianski, M. Combining neuroprotection with endovascular treatment of acute stroke: is there hope? Stroke. (2017) 48:1700–5. doi: 10.1161/strokeaha.117.017040
8. Savitz, SI, Baron, JC, Yenari, MA, Sanossian, N, and Fisher, M. Reconsidering neuroprotection in the reperfusion era. Stroke. (2017) 48:3413–9. doi: 10.1161/strokeaha.117.017283
9. Fisher, M, and Savitz, SI. Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nat Rev Neurol. (2022) 18:193–202. doi: 10.1038/s41582-021-00605-6
10. Lyden, PD. Cerebroprotection for acute ischemic stroke: looking ahead. Stroke. (2021) 52:3033–44. doi: 10.1161/strokeaha.121.032241
11. Schneider, AM, Regenhardt, RW, Dmytriw, AA, Patel, AB, Hirsch, JA, and Buchan, AM. Cerebroprotection in the endovascular era: an update. J Neurol Neurosurg Psychiatry. (2023) 94:267–71. doi: 10.1136/jnnp-2022-330379
12. Dmytriw, AA, Zhang, Y, and Mendes Pereira, V. Mechanical thrombectomy and the future of acute stroke treatment. Eur J Radiol. (2019) 112:214–21. doi: 10.1016/j.ejrad.2019.01.029
13. Prajapati, KD, Sharma, SS, and Roy, N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. (2011) 22:355–63. doi: 10.1515/rns.2011.028
14. Belayev, L, Zhao, W, Pattany, PM, Weaver, RG, Huh, PW, Lin, B, et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. (1998) 29:2587–99. doi: 10.1161/01.str.29.12.2587
15. Defazio, RA, Zhao, W, Deng, X, Obenaus, A, and Ginsberg, MD. Albumin therapy enhances collateral perfusion after laser-induced middle cerebral artery branch occlusion: a laser speckle contrast flow study. J Cereb Blood Flow Metab. (2012) 32:2012–22. doi: 10.1038/jcbfm.2012.102
16. Huh, PW, Belayev, L, Zhao, W, Busto, R, Saul, I, and Ginsberg, MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. (1998) 804:105–13. doi: 10.1016/s0006-8993(98)00674-x
17. Ginsberg, MD, Palesch, YY, Hill, MD, Martin, RH, Moy, CS, Barsan, WG, et al. High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. (2013) 12:1049–58. doi: 10.1016/s1474-4422(13)70223-0
18. Elkind, MSV, Veltkamp, R, Montaner, J, Johnston, SC, Singhal, AB, Becker, K, et al. Natalizumab in acute ischemic stroke (ACTION II): a randomized, placebo-controlled trial. Neurology. (2020) 95:e1091–104. doi: 10.1212/wnl.0000000000010038
19. Hernández-Jiménez, M, Abad-Santos, F, Cotgreave, I, Gallego, J, Jilma, B, Flores, A, et al. Safety and efficacy of ApTOLL in patients with ischemic stroke undergoing endovascular treatment: a phase 1/2 randomized clinical trial. JAMA Neurol. (2023) 80:779–88. doi: 10.1001/jamaneurol.2023.1660
20. Winstein, CJ, Stein, J, Arena, R, Bates, B, Cherney, LR, Cramer, SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98-e169. doi: 10.1161/str.0000000000000098
21. Warach, SJ, Luby, M, Albers, GW, Bammer, R, Bivard, A, Campbell, BC, et al. Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke. (2016) 47:1389–98. doi: 10.1161/strokeaha.115.012364
Keywords: albumin, neuroprotection, endovascular therapy, acute ischemic stroke
Citation: Liu Y, Dong X, Chu X, Ma Z, Yi T, Wen C, Liu Y, Sun J, Xu J, Li W, Yang L, Wang B, Shi L, Li J, Zhang X, Li C, Chen W, Li C, Wu D, Hou C, Zhou C, Li M, Xu Y, Wu C and Ji X (2025) Albumin for patients with acute large-vessel occlusive stroke undergoing endovascular therapy (ARISE): the protocol of a randomized double-blind trial. Front. Neurol. 16:1570184. doi: 10.3389/fneur.2025.1570184
Edited by:
Patricia Martínez Sánchez, Torrecárdenas University Hospital, SpainReviewed by:
Shenqiang Yan, Zhejiang University, ChinaSharon Yeatts, Medical University of South Carolina, United States
Yu Xie, Wuhan University, China
Copyright © 2025 Liu, Dong, Chu, Ma, Yi, Wen, Liu, Sun, Xu, Li, Yang, Wang, Shi, Li, Zhang, Li, Chen, Li, Wu, Hou, Zhou, Li, Xu, Wu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanjie Wu, d3VjaHVhbmppZUBjY211LmVkdS5jbg==; Xunming Ji, aml4bUBjY211LmVkdS5jbg==
†These authors have contributed equally to this work
 Yuanyuan Liu1†
Yuanyuan Liu1† Tingyu Yi
Tingyu Yi Changming Wen
Changming Wen Jing Xu
Jing Xu Wenhuo Chen
Wenhuo Chen Di Wu
Di Wu Chengbei Hou
Chengbei Hou Chen Zhou
Chen Zhou Xunming Ji
Xunming Ji