- 1Ningxia Hui Autonomous Region Hospital of Traditional Chinese Medicine, Ningxia Hui Autonomous Region Academy of Traditional Chinese Medicine, Yinchuan, Ningxia, China
- 2General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
Purpose: Urinary incontinence (UI) is a prevalent clinical manifestation in spinal cord injury (SCI) patients, occurring in approximately 70% of these individuals. This systematic review aims to comprehensively evaluate the research evidence on electroacupuncture (EA) for UI after SCI, assess its clinical efficacy and safety, and provide a reference for clinical practice.
Method: Eight databases were searched for randomized controlled trials (RCTs) published from inception to May 20th, 2025. RCTs comparing EA (with or without conventional rehabilitation, CR) to CR alone for managing UI after SCI were included. Data were analyzed using R version 3.6.3. In accordance with PRISMA-2020 guidelines, two reviewers independently extracted data and assessed the risk of bias using the Cochrane risk of bias tool (ROB 2.0). The certainty of the evidence was graded using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) according to GRADE handbook.
Results: A total of 15 studies were included, comprising 1,394 patients with UI after SCI. The meta-analysis indicated that, compared to the CR group, the EA group showed a significant improvement in 24 h incontinence frequency (MD = −1.42, 95% CI [−1.88, −0.96], p < 0.01), maximum urine output in 24 h (MD = 18.98, 95% CI [9.27, 28.69], p < 0.01), and single urination volume in 24 h (MD = 30.76, 95% CI [21.45, 40.08], p < 0.01). Regarding the Urodynamic outcome indices, the EA group displayed significant improvement in residual urine volume (MD = −20.06, 95% CI [−28.73, −11.38], p < 0.01), bladder volume (MD = 38.86, 95% CI [19.98, 57.75], p < 0.01), maximum urine flow rate (Qmax) (MD = 2.68, 95% CI [1.66, 3.70], p < 0.01), detrusor pressure (PdetQmax) (MD = −6.77, 95% CI [−9.54, −4.00], p < 0.01), and bladder compliance (BC) (MD = 1.41, 95% CI [0.88, 1.93], p < 0.01). Trial Sequential Analysis (TSA) confirmed the superior treatment outcomes of EA compared to CR. The reported adverse events related to acupuncture were minimal and less severe.
Conclusion: EA exhibits considerable potential to enhance self-control of bladder function in patients with UI following SCI. However, this study has certain limitations, and higher quality randomized controlled trials are necessary to confirm these findings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024594516.
1 Introduction
The spinal cord functions as both the neural pathway and the central hub for urinary reflexes. After spinal cord injury (SCI), a significant majority of patients experience urinary dysfunction, characterized by reduced self-control over urination and impaired regulation of urinary movements. Urinary incontinence (UI) is a prevalent clinical manifestation in SCI patients, affecting approximately 70% individuals (1). Bladder detrusor hyperactivity and abnormal urethral sphincter contractions following SCI are major contributing factors to UI (2).
UI not only imposes substantial inconvenience on patients’ lives but also poses a risk for various complications (3, 4), including physical, social, and emotional impairments, and an increased risk of febrile urinary tract infections. Effective bladder management is crucial to prevent these infections (5, 6). Traditional approaches for the treatment of urinary incontinence caused by neurological disorders include pharmacological therapies, surgical interventions, and behavioral training (7). Alternative therapies encompass physical neuromodulation techniques and integrative approaches combining traditional Chinese and Western medicine (such as electroacupuncture of the pudendal nerve). The latter has been shown to significantly improve bladder dysfunction associated with conditions like multiple sclerosis by enhancing pelvic floor muscle contraction (8).
Acupuncture, as a complementary alternative therapy, offers certain advantages in alleviating incontinence symptoms (9, 10). In this context, Electroacupuncture (EA) refers to the technique in which pulse current is applied after elicitation of “Deqi” via filiform needle insertion, modality that integrates traditional acupuncture with modern electrical stimulation techniques, holds promise for the treatment of UI following SCI patients (11). EA stimulation may induce electrophysiologic changes and modulate neurotransmitter activity in the bladder, exhibiting both excitatory and inhibitory effects (12, 13). This dual modulation can enhance voiding function by improving the contraction of a weakened detrusor muscle and inhibiting hyperreflexia, thus enhancing the bladder’s storage function (14). Additionally, EA can regulate the coordination between the bladder detrusor and urethral sphincter.
Recent research has increasingly focused on the effects of EA on UI following SCI patients (15–17). And this systematic review aims to comprehensively evaluate on the efficacy and safety of EA for UI following SCI and provide guidelines for clinical practice. We will employ trial sequential analysis (TSA) to assess whether the included trials have reached the optimal information size and if the cumulative data are adequately powered to evaluate outcomes.
2 Materials and methods
2.1 Literature search strategy
We registered the protocol on the PROSPERO (ID: CRD42024594516) and conducted our study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 (PRISMA-2020) (18) guidelines in Supplementary material. We searched the following eight databases for Chinese and English articles from inception to May 20th, 2025: PubMed, Embase (Ovid), Web of Science, Cochrane Library, CBM, CNKI, Wanfang, and VIP. The search strategy details are provided in Supplementary Tabl S1.
2.2 Inclusion and exclusion criteria
All enrolled UI patients met the diagnostic criteria for SCI established by the American Spinal Injury Association, and had completed the spinal shock stage, neurogenic bladder was diagnosed as incontinence following spinal cord injury. The experimental group was treated with EA (involves piercing the skin and eliciting a deqi sensation), which can be supplemented with conventional rehabilitation (CR) measures. The CR including Western medicine, instrumental assistance, physical therapy. The control group was treated with CR measures or combined with sham acupuncture. The outcome measures included: urination diary indices (24 h incontinence frequency, 24 h maximum urine output, 24 h single urination volume) and urodynamic outcome indices [residual urine volume, bladder volume, maximum urine flow rate (Qmax), detrusor pressure (PdetQmax), bladder compliance (BC)].
Only RCTs that utilized EA for treating UI after SCI were included. We excluded articles that were not available in full text and other publication types such as letters, comments, and conference abstracts. Studies for which complete data could not be obtained or that used the same patient data as other included articles were also excluded. The eligible trials met the following PICOS (participants, interventions, comparisons, outcomes, and study design) criteria.
2.3 Data extraction
Two researchers independently selected the studies, collected the data, and imported the determined studies into EndNote 20. Any disagreements were resolved by a third researcher. Initially, articles with duplicate data were excluded. Subsequently, unrelated research was excluded based on the title and abstract. Then, the remaining studies were reviewed in detail to determine the final selection. Data for each included study were entered into Microsoft Excel (2016), including the study ID, age, sex, sample size, disease duration, intervention time, study design, acupuncture points and the outcomes.
2.4 Quality assessment
Two reviewers independently assessed the risk of bias for each included study using the Cochrane Risk of Bias (ROB) tool 2.0 (19). The certainty of the evidence was graded using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) according to (GRADE handbook). Disagreements between reviewers were resolved by a third researcher.
2.5 Strategy for data synthesis
Meta-analysis was conducted whenever outcomes were comparable across studies. Data analysis was performed using R 3.6.3. Continuous data were presented as mean difference (MDs) with 95% CI, and dichotomous data were presented as relative risk (RR) with 95% CI. Standardized mean differences (SMDs) with 95% CIs were calculated for studies using different outcome scales, and MDs with 95% CIs were calculated for studies using the same outcome scale (20). Heterogeneity was categorized as low (I2 < 50%), moderate (I2 = 50–74%), or high (I2 ≥ 75%) (21). Due to conceptual heterogeneity in acupuncture RCTs, a random effect model was used. Publication bias was assessed using Egger’s test when more than 10 studies were included in the analysis (22). If the heterogeneity was considerable, we would conduct subgroup analysis. The sensitivity analyses were conducted to assess robustness of the synthesized results. Trial sequential analysis (TSA) was conducted to determine if the optimal information size was reached and if the cumulative data were sufficiently powered to evaluate the outcomes. TSA software 0.9.5.10 beta (Copenhagen Trial Unit, Denmark) was used (23–25). An optimal information size was defined with a two -sided 5% risk of a type I error or a 20% risk of a type II error (80%power).
3 Results
3.1 Description of included trials
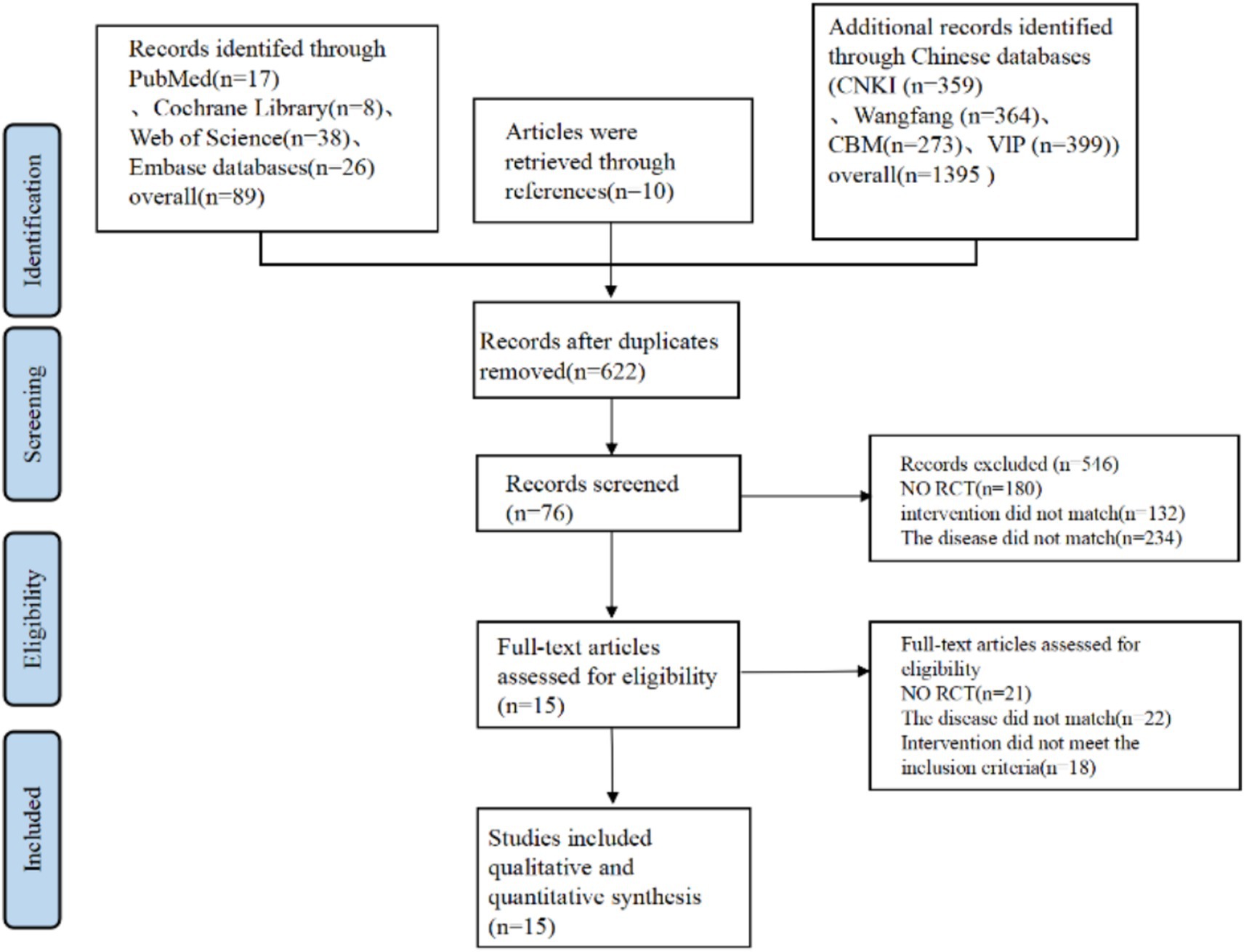
At first, a total of 1,484 articles were identified through database searches, with 89 from Pubmed, Cochrane, Embase, and Web of Science. 1,395 from CNKI, CBM, VIP, and Wan fang Data. Additional 10 records were identified through reference lists. After removing 862 duplicate articles, 622 articles underwent title and abstract screening. Of these, 546 articles were excluded due to lack of relevance, leaving 76 articles that met the inclusion criteria. Subsequently, 61 articles were excluded for the following reasons: not RCT (21 articles), disease mismatch (12 articles), patients mismatch (10 articles), and interventions not meeting inclusion criteria (18 articles). Ultimately, 15 RCTs (15–17, 26–37) were included (Figure 1).
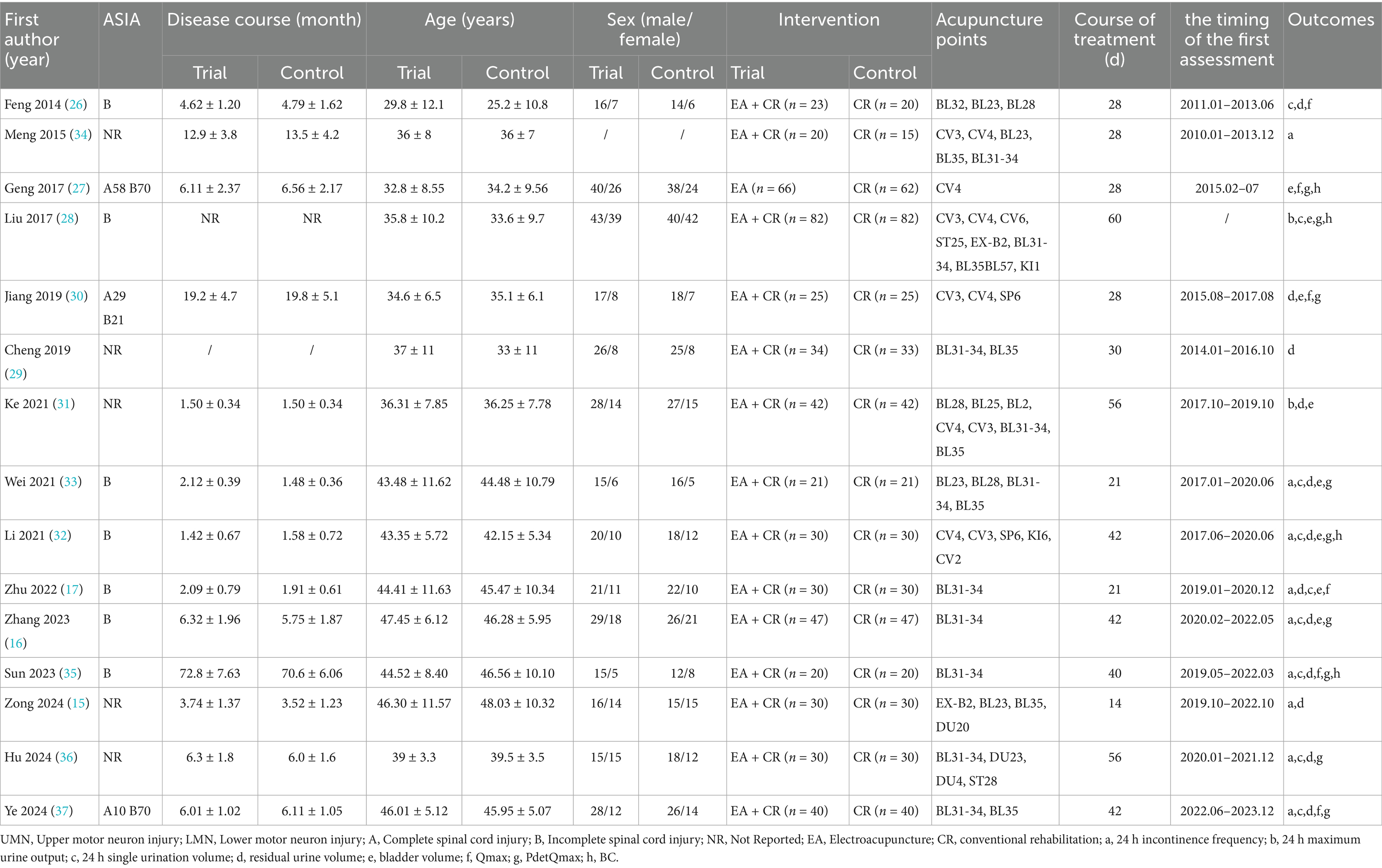
All included studies were published and conducted in Chinese (Table 1). 14 studies employed a two-armed, one study employed a three-armed (35), parallel design, with sample sizes ranging from 35 to 164 participants. The included studies comprised 1,394 patients with a mean age ranging from 20 to 68 years. All diagnosed with UI following SCI. And all the studies included were uppermotor neuron injuries. The experimental interventions included EA, EA combined with conventional rehabilitation, while the control groups received conventional rehabilitation across all studies. Outcome measures included urination diary indices (24-h incontinence frequency, 24-h maximum urine output, 24-h single urination volume) and urodynamic indices [residual urine volume, bladder volume, maximum urine flow rate (Qmax), detrusor pressure (PdetQmax), bladder compliance (BC)].
3.2 Risk of bias
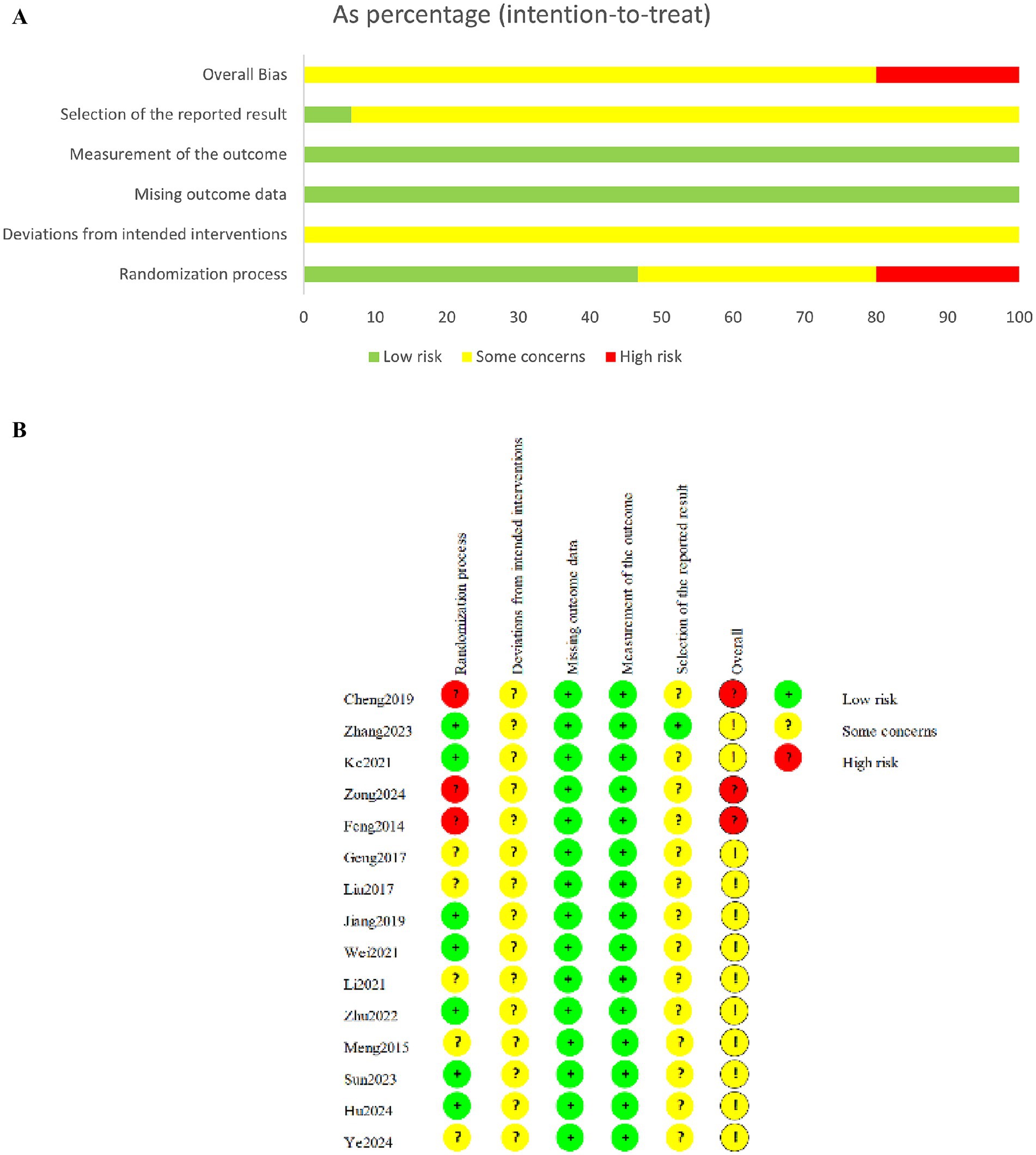
Three studies (15, 26, 29) showed a high risk of bias, while the remaining studies exhibited some concerns. The randomization process was mentioned in all studies, nine articles used randomized table method and were rated as low risk, three articles sorted participants according to consultation order and admission time, and were rated as high risk, and three articles used unspecified grouping methods, leading to some concerns regarding potential selection bias. Due to the specific nature of acupuncture therapy, all studies were not blinded. However, the included outcome measures were less susceptible to bias from lack of blinding, thus deviations from intended interventions were assessed as low risk for all included studies. Additionally, none studies had registered protocols, leading to some concerns regarding biases in outcome confirmation and reporting (Figures 2A,B).
3.3 The forest of outcome index of urination diary
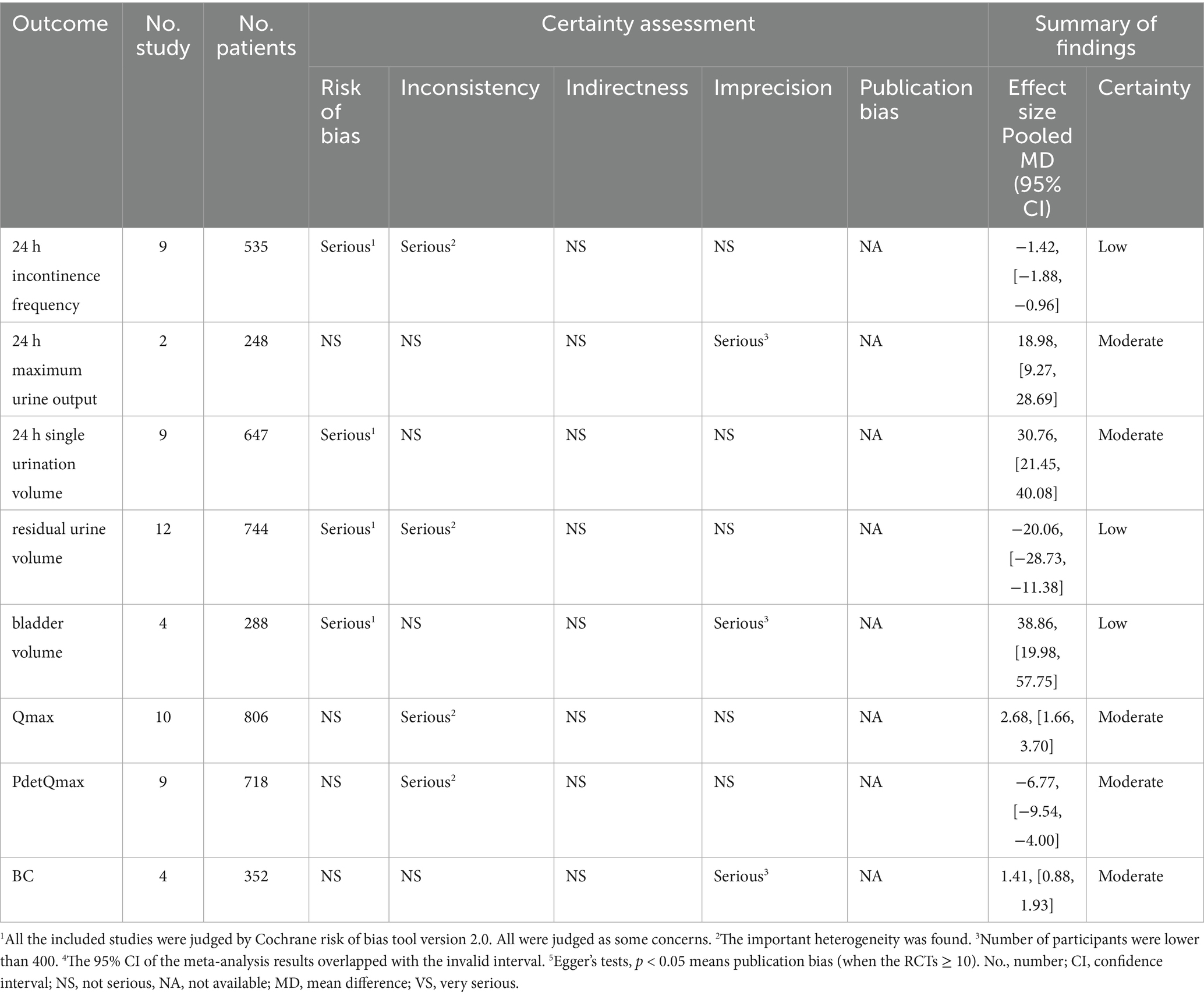
3.3.1 24 h incontinence frequency
In 9 studies (15–17, 32–37), the effect of EA (or combined with CR) on 24 h incontinence frequency was compared with that of CR. The meta-analysis showed that EA had superior effects compared to CR (n = 535, MD = −1.42, 95% CI (−1.88, −0.96), p < 0.01), with high heterogeneity (I2 = 83%) (Figure 3A).
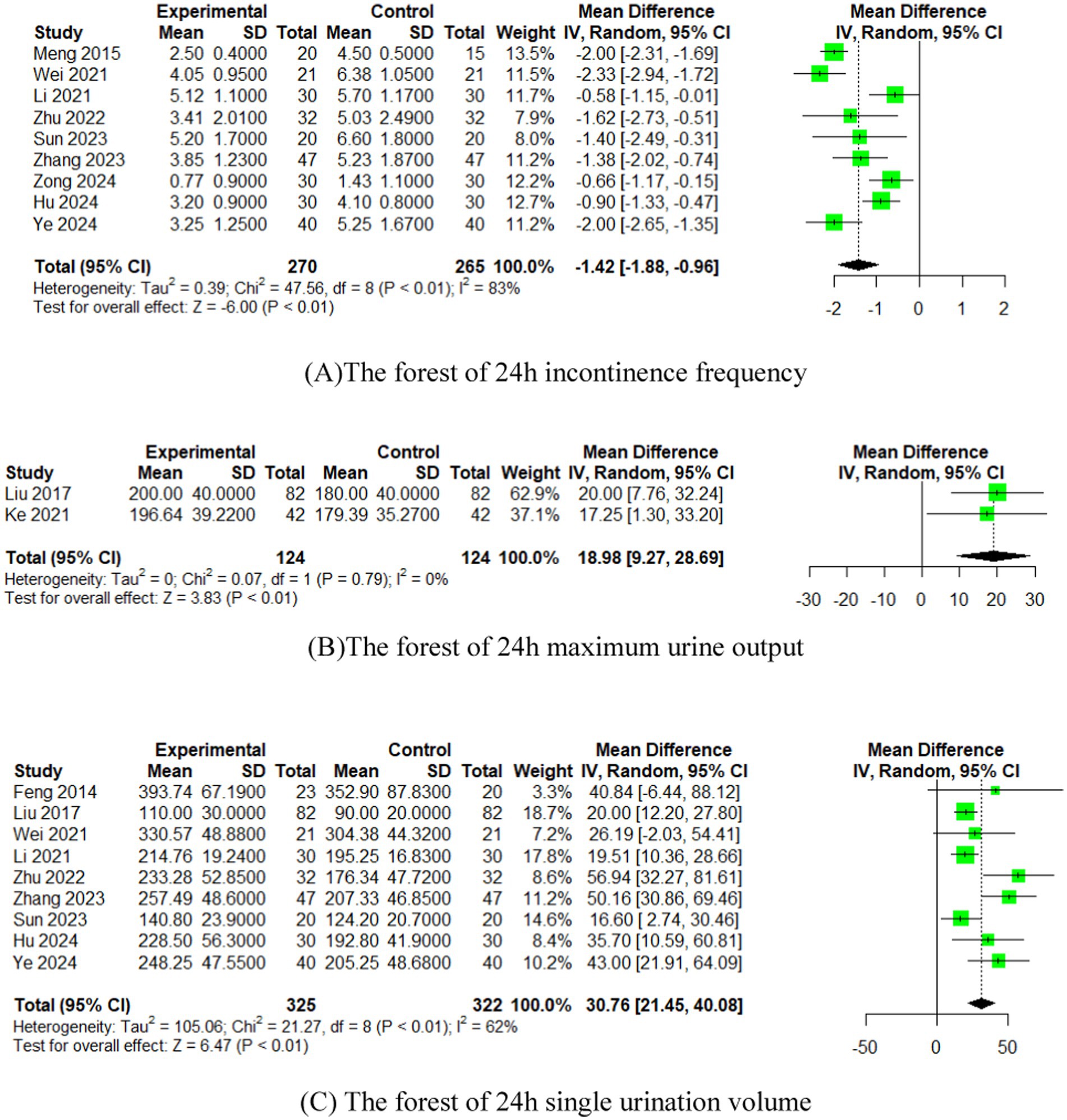
Figure 3. The forest of outcome index of urination diary. (A) The forest of 24 h incontinence frequency. (B) The forest of 24 h maximum urine output. (C) The forest of 24 h single urination volume.
3.3.2 24 h maximum urine output
In 2 studies (28, 31), the effect of EA (or combined with CR) on 24 h maximum urine output was compared with that of CR. The meta-analysis indicated superior effects of EA over CR (n = 248, MD = 18.98, 95% CI (9.27, 28.69), p < 0.01), with low heterogeneity (I2 = 0%) (Figure 3B).
3.3.3 24 h single urination volume
In 9 studies (16, 17, 26, 28, 32, 33, 35–37), the effect of EA (or combined with CR) on 24 h single urination volume was compared with that of CR. The meta-analysis demonstrated superior effects of EA compared to CR (n = 647, MD = 30.76, 95% CI (21.45, 40.08), p < 0.01), with moderate heterogeneity (I2 = 62%) (Figure 3C).
3.4 The forest of urodynamic outcome index
3.4.1 Residual urine volume
In 12 studies (15–17, 26, 29–33, 35–37), the effect of EA (or combined with CR) on residual urine volume was compared with that of CR. The meta-analysis showed that EA had superior effects compared to CR (n = 744, MD = −20.06, 95% CI (−28.73, −11.38), p < 0.01), with high heterogeneity (I2 = 87%) (Figure 4A).
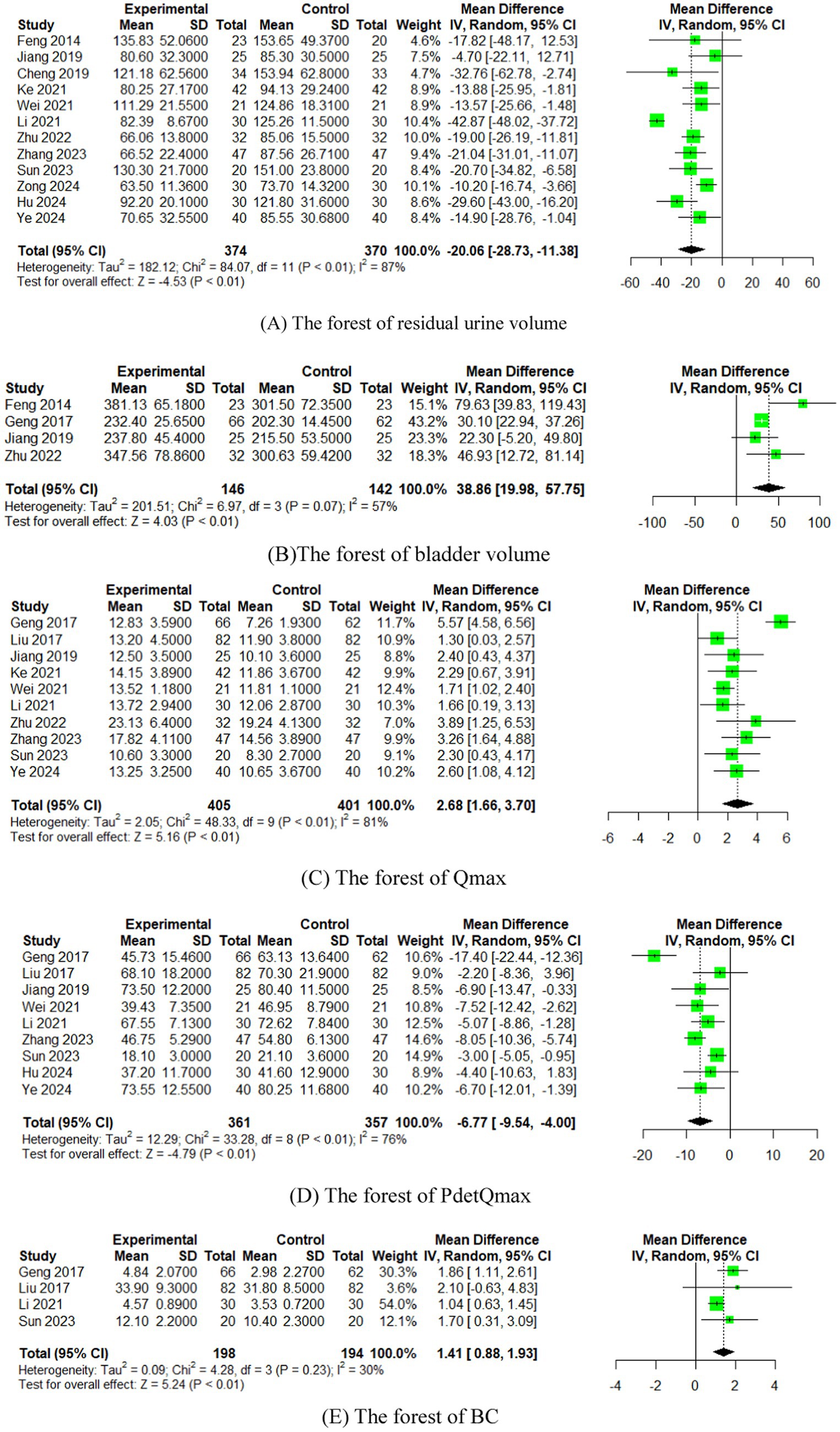
Figure 4. The forest of urodynamic outcome index. (A) The forest of residual urine volume. (B) The forest of bladder volume. (C) The forest of Qmax. (D) The forest of PdetQmax. (E) The forest of PdetQmax.
3.4.2 Bladder volume
In four studies (17, 26, 27, 30), the effect of EA (or combined with CR) on bladder volume was compared with that of CR. The meta-analysis indicated superior effects of EA over CR CR (n = 288, MD = 38.86, 95% CI (19.98, 57.75), p < 0.01), with moderate heterogeneity (I2 = 57%) (Figure 4B).
3.4.3 Qmax
In 10 studies (16, 17, 27, 28, 30–33, 35, 37), the effect of EA (or combined with CR) on Qmax was compared with that of CR. The meta-analysis showed that EA had superior effects compared to CR (n = 806, MD = 2.68, 95% CI (1.66, 3.70), p < 0.01), with high heterogeneity (I2 = 81%) (Figure 4C).
3.4.4 PdetQmax
In nine studies (16, 27, 28, 30, 32, 33, 35–37), the effect of EA (or combined with CR) on PdetQmax was compared with that of CR. The meta-analysis demonstrated superior effects of EA compared to CR (n = 718, MD = −6.77, 95% CI (−9.54, −4.00), p < 0.01), with high heterogeneity (I2 = 76%) (Figure 4D).
3.4.5 BC
In four studies (27, 28, 32, 35), the effect of EA (or combined with CR) on BC was compared with that of CR. The meta-analysis indicated superior effects of EA over CR (n = 392, MD = 1.41, 95% CI (0.88, 1.93), p < 0.01), with low heterogeneity (I2 = 30%) (Figure 4E).
3.5 The sensitivity analysis of outcome index of urination diary
We performed a leave-one-out sensitivity analysis by iteratively removing one study at a time. The point estimates remained within the 95% confidence interval (CI) of the complete analysis for the outcomes, namely 24 h incontinence frequency and 24 h single urination volume (Figure 5). This suggests that the results were stable.
Figure 5. The sensitivity analysis of outcome index of urination diary [(A) 24 h incontinence frequency, (B) 24 h single urination volume].
3.6 The sensitivity analysis of urodynamic outcome index
We performed a leave-one-out sensitivity analysis by iteratively removing one study at a time. The point estimates remained within the 95%CI of the complete analysis for the outcomes, which include residual urine volume, bladder volume, Qmax, PdetQmax, BC (Figure 6). This indicates that the results were stable.
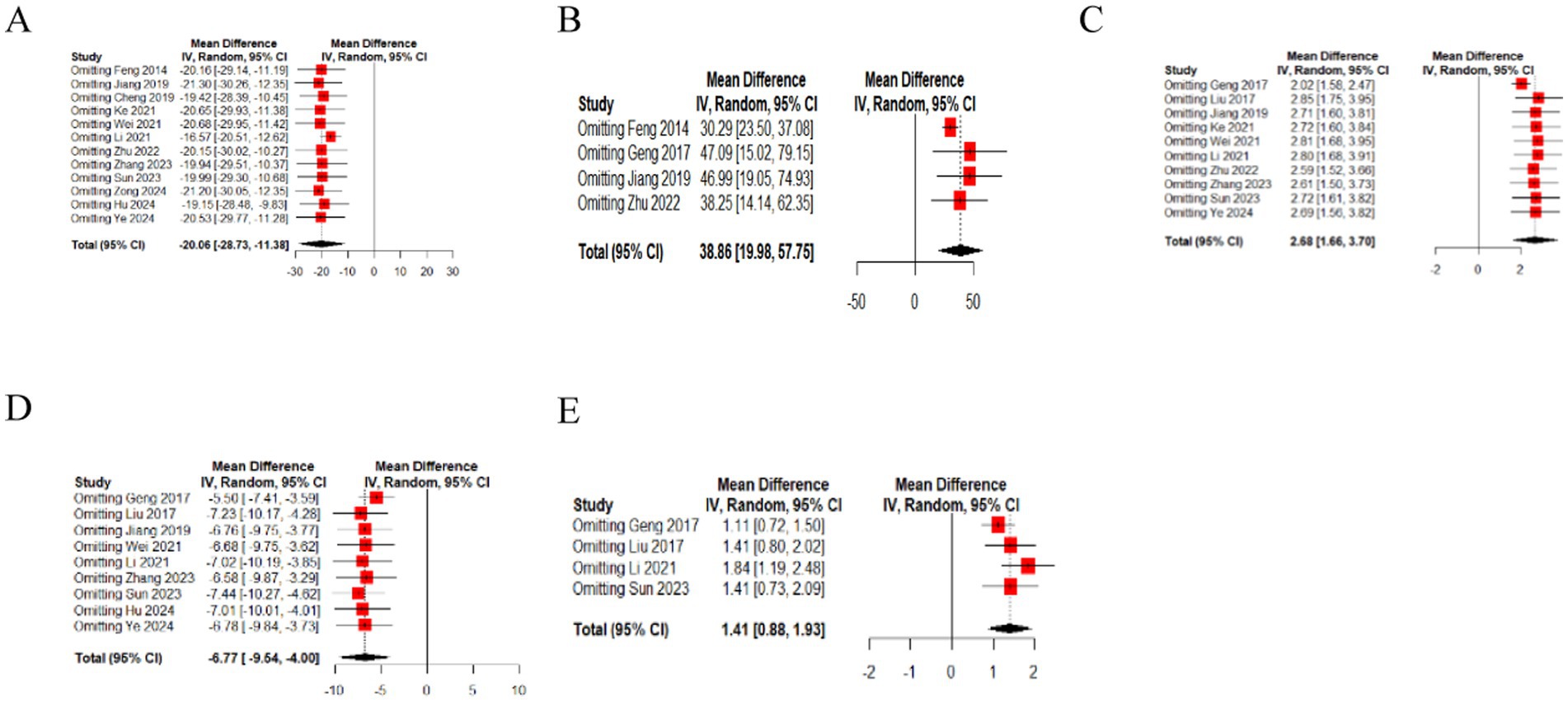
Figure 6. The sensitivity analysis of urodynamic outcome index [(A) residual urine volume, (B) bladder volume, (C) Qmax, (D) PdetQmax, (E) BC].
3.7 Publication bias
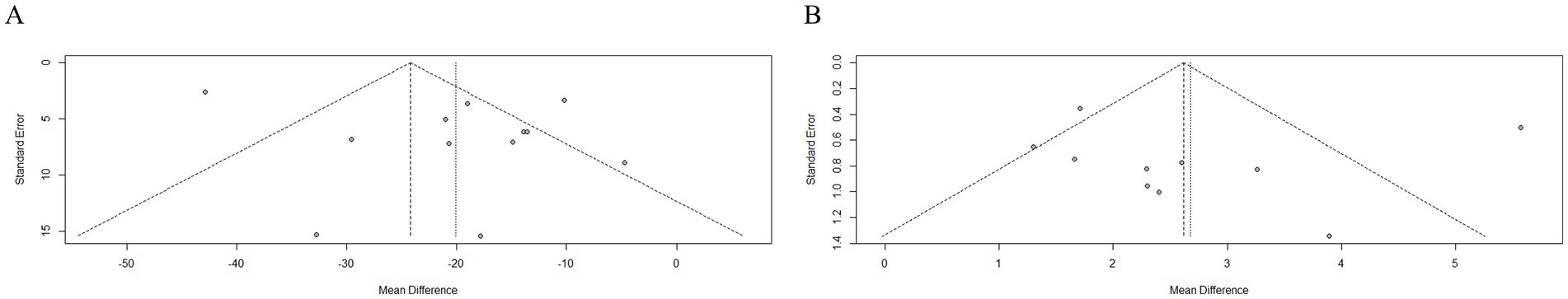
Eggers tests were performed to detect publication bias when more than 10 studies with the same outcome were included in the analysis. Since Residual urine volume and Qmax indicators exceeded this threshold, a funnel plot and Egger’s tests could be made to assess publication bias (Figure 7). After Egger’s tests, the p-values of the two were 0.2096 and 0.8085 respectively, so there was no risk of bias in either of them.
3.8 GRADE evidence profile for the studies in the meta-analysis
We extracted all relevant outcomes reported in the 15 included RCTs, specifically 24 h incontinence frequency, 24 h maximum urine output, 24 h single urination volume residual urine volume, bladder volume, Qmax, PdetQmax, BC. The GRADE analysis results indicated that the overall quality of evidence for various outcome indicators ranged from low to moderate, which was not conducive to our recommendation of the results. The reasons for downgrading were clarified with superscripts for each outcome (Table 2).
3.9 The TSA analysis of outcome index of urination diary
The TSA was conducted for the 24 h incontinence frequency and single urination volume. Due to the relatively high heterogeneity and potential bias in the trials, a random-effect model (BT) was employed (37). The TSA plots for EA (alone or with CR) versus CR showed that Z-curve crossed both the trial sequential monitoring boundary and conventional monitoring boundary, and surpassed the required information size (RIS) axis. This indicates conclusive evidence for the efficacy of EA in improving incontinence in patients with UI (Figure 8).
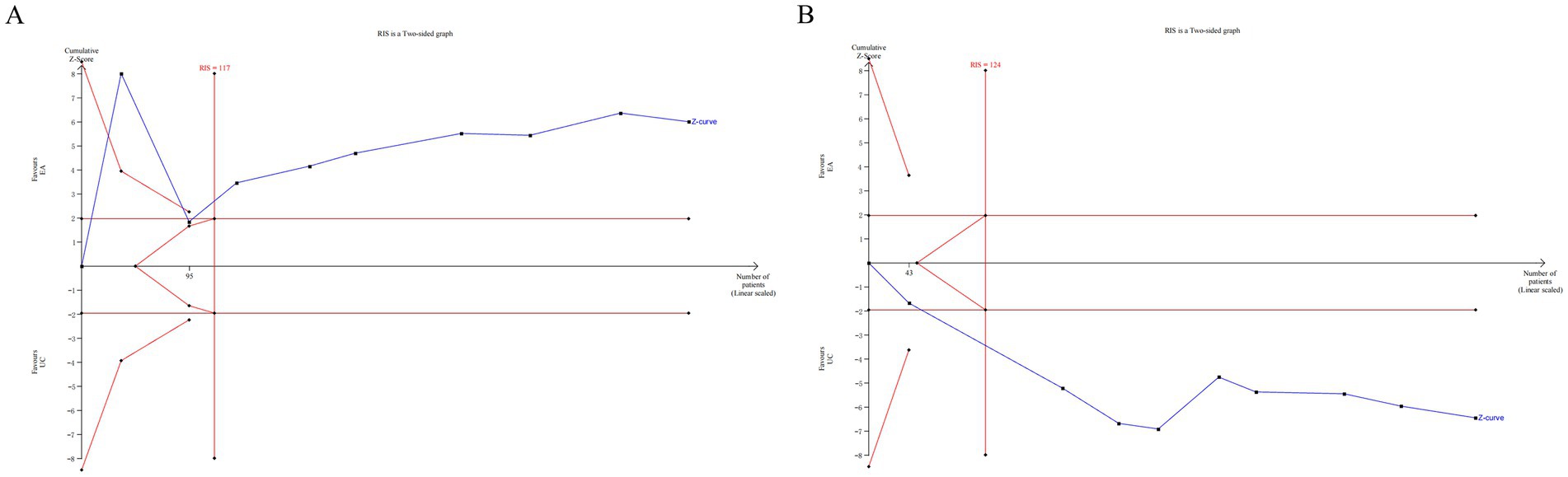
Figure 8. The TSA analysis of outcome index of urination diary [(A) 24 h incontinence frequency, (B) single urination volume].
3.10 The TSA analysis of urodynamic outcome index
The TSA was performed for the outcomes of residual urine volume, bladder volume, Qmax, PdetQmax, BC. The TSA plots for EA (alone or with CR) versus CR showed that Z-curve crossed both the trial sequential monitoring boundary and conventional monitoring boundary, and surpassed the RIS axis. This confirms conclusive evidence for the efficacy of EA in improving urodynamic outcomes in patients with UI (Figure 9).
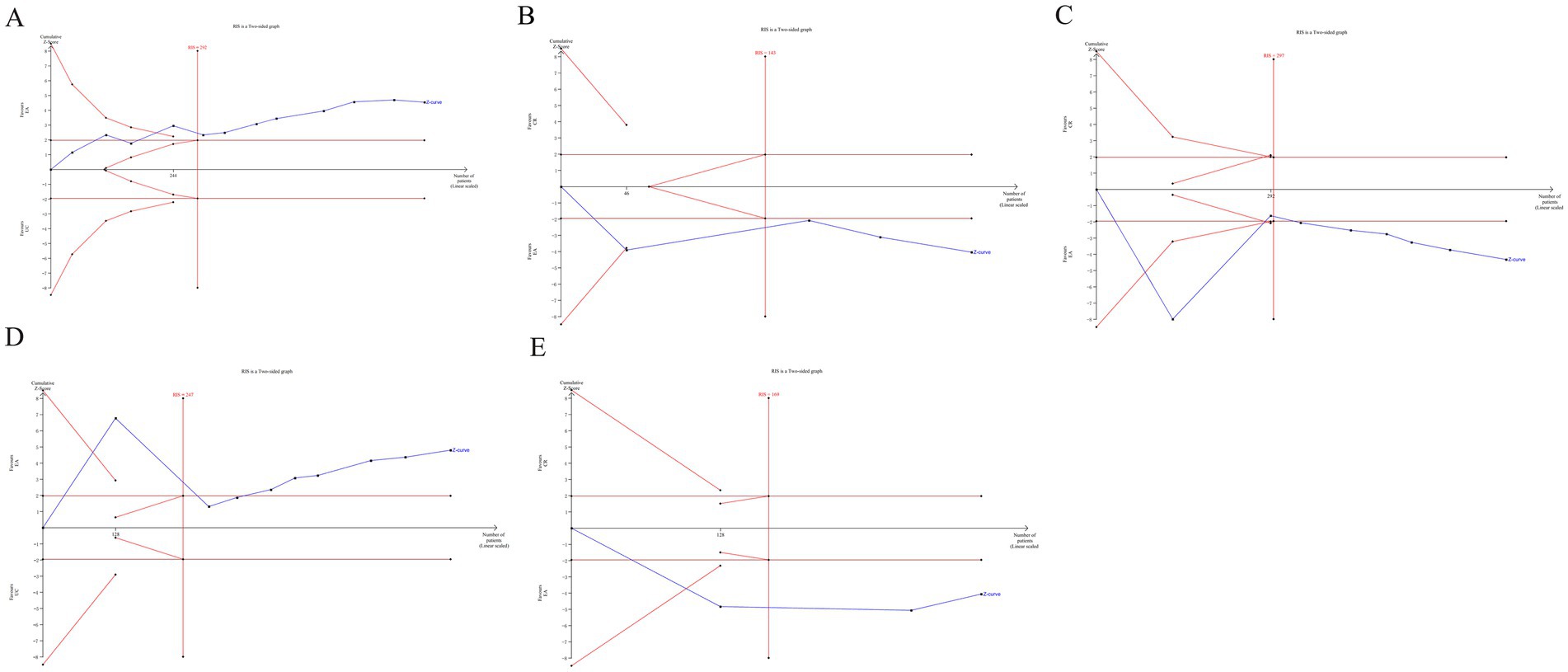
Figure 9. The TSA analysis of urodynamic outcome index [(A) residual urine volume, (B) bladder volume, (C) Qmax, (D) PdetQmax, (E) BC].
4 Discussion
4.1 Main findings
To the best of our knowledge, the effectiveness of EA for UI following SCI remains controversial. This meta-analysis is the first to evaluate the clinical efficacy of EA for UI. We included 15 studies in which all showed that EA (alone or with conventional rehabilitation, CR) was more effective for UI recovery compared to CR alone. This review indicates that EA has significant potential to enhance the recovery of UI in patients with SCI. Specifically, EA significantly reduces the frequency of 24 h urinary incontinence, increase the maximum 24 h urinary output and improves single urinary output. It effectively reduces the residual urine volume in the bladder, increases bladder capacity, and enhances maximum urinary flow rate. Moreover, EA decreases detrusor muscle pressure, improves bladder compliance, and overall bladder function.
A reduction of 1.42 episodes of urinary incontinence within 24 h has achieved the minimal clinically important difference (MCID). The Clinical Practice Guidelines for Comprehensive Management of Neurogenic Bladder1 suggest that a reduction of ≥1 episode of 24-h urinary incontinence can lower the risk of urinary tract infections and improve quality of life. According to the consensus on the management of overactive bladder (OAB) (38), an increase in single voided volume by ≥30 mL may result in a ≥25% reduction in daily voiding frequency (e.g., from 10 times to 7–8 times). Improved voiding efficiency can reduce post-void residual volume and decrease the risk of urinary tract infections. The European Association of Urology (EAU) recommends an increase in Qmax by ≥2 mL/s as a clinically significant indicator of relief from urethral obstruction. This suggests reduced bladder outlet resistance and enhanced urinary flow rate. An increase in Qmax reflects improved urethral sphincter coordination and enhanced detrusor contractility. PdetQmax ≥5 cm H₂O reduction indicates improved synchronized detrusor contractions and optimized urethral resistance during voiding. If PdetQmax decreases while Qmax increases, it suggests a significant improvement in detrusor-urethral coordination. The combined improvement of these parameters can predict effective upper urinary tract protection (39). Adverse reactions to EA were generally mild, primarily including bleeding at needle sites, numbness or soreness. Despite the very low to moderate certainty of evidence due to poor methodological quality and significant heterogeneity among studies, this review synthesizes the existing RCT evidence regarding the effect of EA on UI after SCI. EA may be a valuable addition to treatment protocols for UI following SCI and warrants integration into clinical guidelines.
SCI can disrupt the neural pathways between the bladder and the brain, resulting in a loss of voluntary control over the urination process; SCI may lead to either hyperreflexia or hyporeflexia of the bladder, commonly manifesting as detrusor overactivity (hyperactive detrusor muscle), which results in urgency and incontinence (40). Furthermore, SCI can affect the balance between the sympathetic and parasympathetic nervous systems, leading to dysfunction of the bladder and urethra. It may also cause changes in the bladder wall, including reduced capacity and compliance, ultimately resulting in incontinence (41).
Electroacupuncture (EA) can influence the activity of the sympathetic and parasympathetic nervous systems by stimulating relevant acupoints (42). The parasympathetic nervous system, in particular, plays a crucial role in promoting bladder emptying. Through EA treatment, the activity of the parasympathetic nerves can be regulated to control the relaxation and contraction of the bladder’s smooth muscles, thereby alleviating symptoms of incontinence. EA therapy can also modulate the release of various neurotransmitters, such as serotonin (5-HT), norepinephrine, and opioid substances (43), which in turn regulate neural activity and bladder function. These neurotransmitters are essential for the transmission of neural signals and the coordinated control of bladder muscles. Additionally, EA may reduce the release of inflammatory factors and modulate immune functions, thereby decreasing inflammatory responses and aiding in the management of incontinence (44). Studies have shown that EA can promote the regeneration and repair of nerve cells (45, 46). By stimulating the spinal cord and related neural regions, EA has the potential to foster the regeneration of nerve cells and the reconnection of synapses, thus partially restoring urinary control functions. This systematic review aims to comprehensively evaluate the research evidence on EA for UI following SCI. It assesses the clinical efficacy and safety of EA and provides a reference for clinical practice.
4.2 Quality summaries
Three studies exhibited a high risk of bias due to incorrect randomization method. Variations in acupuncture treatment protocols, practitioner techniques, and the type of SCI (with unclear grouping by injury degree and segment) could contribute to the observed heterogeneity in therapeutic outcomes. None of the included studies implemented blinding due to the nature of electroacupuncture, potentially causing implementation bias. The studies did not specify whether outcome measurements were conducted by an independent third party, raising the possibility of measurement bias if performed by the same physician administering EA. Additionally, none of the studies mentioned pre-registration in the clinical trial registry, making it difficult to ascertain if all intended outcomes were reported, thus introducing reporting bias. The GRADE analysis results indicate that the overall quality of evidence across outcome indicators ranges from low to moderate, which was not conducive to our recommendation of the results.
4.3 Outlook and recommendations
Future RCTs should adhere to the STRICTA and CONSORT guidelines, clearly describe random number generation and allocation concealment, and pre-registration trial protocol. Moreover, outcome assessors, participants, and physicians should be blinded, and any adverse effects should be clearly documented. Improved methods for assessing the effectiveness of blinding in acupuncture RCTs are needed. Bang et al. (47) developed a high-quality blinding assessment tool for clinical trials, which should be widely adopted in the future.
4.4 Strengths and limitations
This paper presents the first meta-analysis on the efficacy of EA for treating UI following SCI, thereby filling a significant research gap. TSA analysis was employed to validate the robustness of the research on multiple outcome indicators, minimizing false positives. However, several limitations should be considered. Firstly, the overall methodology and reporting quality of the included studies were poor, affecting the credibility of the results. Secondly, significant heterogeneity among the studies impacted the meta-analysis findings. Moreover, the limited published literature precluded a comprehensive analysis of the long-term efficacy of EA. Moreover, the studies included were predominantly domestic, with few international reports, reducing the applicability of the findings. Future high-quality RCTs are necessary to provide a reliable basis for using EA to treat UI after SCI. Lastly, the search strategy primarily focused on incontinence. All the qualifying studies involved patients with upper motor neuron injury, which is commonly associated with overactive bladder. Future literature reviews should expand the search terminology to include “overactive bladder” and/or “upper motor neuron bladder” to achieve a more comprehensive evaluation of incontinence and assess the efficacy of EA in populations with SCI.
5 Conclusion
EA can significantly reduce the frequency of 24 h urinary incontinence, increase both maximum 24 h urinary output and single urinary output. It effectively reduces the residual urine volume in the bladder, increases bladder capacity, improves maximum urinary flow rate, decreases detrusor muscle pressure, and enhances bladder compliance. EA shows great potential for improving bladder function control in patients with UI following SCI. However, this study has some limitations and additional high-quality RCTs are required to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
P-YZ: Methodology, Writing – original draft. C-LX: Data curation, Formal analysis, Writing – review & editing. G-XY: Methodology, Software, Writing – review & editing. LM: Methodology, Software, Writing – review & editing. FW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1573090/full#supplementary-material
Footnotes
References
1. Anderson, KD . Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. (2004) 21:1371–83. doi: 10.1089/neu.2004.21.1371
2. Tapia, CI, Khalaf, K, Berenson, K, Globe, D, Chancellor, M, and Carr, LK. Health-related quality of life and economic impact of urinary incontinence due to detrusor overactivity associated with a neurologic condition: a systematic review. Health Qual Life Outcomes. (2013) 11:13. doi: 10.1186/1477-7525-11-13
3. Perez, NE, Godbole, NP, Amin, K, Syan, R, and Gater, DR. Neurogenic bladder physiology, pathogenesis, and management after spinal cord injury. J Pers Med. (2022) 12:968. doi: 10.3390/jpm12060968
4. Nseyo, U, and Santiago-Lastra, Y. Long-term complications of the neurogenic bladder. Urol Clin North Am. (2017) 44:355–66. doi: 10.1016/j.ucl.2017.04.003
5. Albayrak, H, Atli, E, Aydin, S, and Ozyemisci-Taskiran, O. Successful outcome following a multimodal pelvic rehabilitation program in a woman with neurogenic bladder and bowel dysfunction: A case report. Physiother Theory Pract. (2024) 40:1083–90. doi: 10.1080/09593985.2022.2144561
6. McKibben, MJ, Seed, P, Ross, SS, and Borawski, KM. Urinary tract infection and neurogenic bladder. Urol Clin North Am. (2015) 42:527–36. doi: 10.1016/j.ucl.2015.05.006
7. Musco, S, Ecclestone, H, t Hoen, L, Blok, B, Padilla-Fernández, B, Del Popolo, G, et al. Efficacy and safety of surgical treatments for neurogenic stress urinary incontinence in adults: a systematic review. Eur Urol Focus. (2022) 8:1090–02. doi: 10.1016/j.euf.2021.08.007
8. Vecchio, M, Chiaramonte, R, and DI Benedetto, P. Management of bladder dysfunction in multiple sclerosis: a systematic review and meta-analysis of studies regarding bladder rehabilitation. Eur J Phys Rehabil Med. (2022) 58:387–96. doi: 10.23736/S1973-9087.22.07217-3
9. Miao, C, Li, X, and Zhang, Y. Effect of acupuncture on BDNF signaling pathways in several nervous system diseases. Front Neurol. (2023) 14:1248348. doi: 10.3389/fneur.2023.1248348
10. Su, H, Zhou, B, Ji, X, Li, B, Yang, X, and Li, Z. Meta-analysis on the recovery effect of acupuncture combined with rehabilitation therapy in spinal cord injury patients. Altern Ther Health Med. (2024) 5:AT10337.
11. Chen, T, Zhang, WW, Chu, YX, and Wang, YQ. Acupuncture for pain management: molecular mechanisms of action. Am J Chin Med. (2020) 48:793–811. doi: 10.1142/S0192415X20500408
12. Mei, JL, Yang, ZX, and Li, XN. Effect of electroacupuncture of "Jiaji" (EX-B2) on NOD-like receptor protein 3 mediated pyroptosis in spinal cord tissue of rats with acute spinal cord injury. Zhen Ci Yan Jiu. (2024) 49:110–8. doi: 10.13702/j.1000-0607.20230701
13. Lu, J, Cheng, B, and Lin, L. Urodynamic analysis of the effect of electroacupuncture at different acupoints on the bladder after spinal cord injury. Neurourol Urodyn. (2024) 43:2065–75. doi: 10.1002/nau.25534
14. Kumar, V, Templeman, L, Chapple, CR, and Chess-Williams, R. Recent developments in the management of detrusor overactivity. Curr Opin Urol. (2003) 13:285–91. doi: 10.1097/00042307-200307000-00004
15. Kai, ZONG, Qinian, PING, Jin, SHI, and Gang, L. Jiaji (EX-B2) electric field therapy in the treatment of neurogenic bladder urinary incontinence after Suprasacral spinal cord injury. CJGMCM. (2024) 39:3729–32.
16. Zhang, Q, Wang, X, Shen, R, Ma, C, Yang, J, and W, L. Influences of electroacupuncture at Baliao point combined with pelvic floor muscle electrical stimulation on urination, urodynamics and quality of life in patients with urinary incontinence after spinal cord injury. Anhui Med Pharm J. (2023) 12:2421–4.
17. Jia-Min, ZHU, Zhong-Ren, SUN, Yang, CUI, and Hong-Na, Y. Clinical observation on deep Electroacupuncture at Baliao points in the treatment of neurogenic bladder after spinal cord injury. J Guangzhou Univ Tradition Chin Med. (2022) 39:328–33.
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:I4898. doi: 10.1136/bmj.l4898
20. Jin, W, and Youping, L. Selection of effect scale indexes in meta analysis. Chin J Evid Based Med. (2007) 8:606–13.
21. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
23. Trial Sequential Analysis . Copenhagen Trial Unit: Centre for Clinical Intervention Research, (2021). https://www.ctu.dk/tsa/ (Accessed June 22, 2021).
24. Wetterslev, J, Jakobsen, JC, and Gluud, C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. (2017) 17:39. doi: 10.1186/s12874-017-0315-7
25. Thorlund, JK, Engstrøm, J, Wetterslev, J, Brok, G, and Imberger, G. User manual for trial sequential analysis (TSA). Copenhagen: Centre for Clinical Intervention Research (2011).
26. Feng, x, Wei, X, and Wu, CH. Electroacupuncture treatment of neurogenic bladder with incomplete spinal cord injury in 23 cases. J Anhui Univ Chin Med. (2014) 33:43–6.
27. Geng, D, and Dan, Z. Analysis on the curative effect of electroacupuncture guanyuan acupoint in the treatment of spinal cord injury patients with detrusor reflex hyperfunction. Chin Commun Doctors. (2017) 33:57–9.
28. Liu, C, Wong, J, yongbo, J, Liu, J, Li, C, and S, h. Clinical efficacy and mechanism of electroacupuncture combined with bladder function training in the treatment of neurogenic bladder dysfunction caused by detrusor hyperreflexia. Modern J Integr Tradition Chin West Med. (2017) 26:3804–6.
29. Cheng, X-k, and Ying-chun, S. Clinical study on electroacupuncture treatment for urinary incontinence in spinal cord injury-induced neurogenic bladder. Shanghai J Acu-mox. (2019) 38:646–9.
30. Jiang, H, Ai, K, and Z, Y. Effects of electroacupuncture combined with bladder synthesis training on bladder function of patients with treating neurogenic bladder (detrusor hyperreflex type) after supraorbital spinal cord injury. J Hunan Univ Chin Med. (2019) 39:627–30.
31. Hua-jing, KE, Yu, ZHANG, and Yu-xiang, W. Effect of electroacupuncture combined with modern rehabilitation technology on rehabilitation of patients with bladder urinary incontinence caused by spinal cord injury. China Mod Med. (2021) 28:50–2.
32. Li, D, Qin, J, He, X, Hou, X, and Weirong, W. Effects of electroacupuncture combined with pelvic floor electromyography biofeedback on micturition diary and urodynamic indexes in patients with neurogenic bladder after spinal cord injury. Clin Res Pract. (2021) 6:136–8.
33. Wei, X, Zhou, y, Wu, J, Xu, j, Feng, x, and Zhang, R. Clinical observation on the treatment of neurogenic bladder after spinal cord injury by electroacupuncture at bladder meridian points combined with bladder therapeutic instrument. Chin J Rehabil. (2021) 36:396–400.
34. Meng, Z, Wang, T, Yin, Z, and Wang, J. Clinical study on electroacupuncture combined with perineal BTX-A injection for the treatment of neurogenic bladder after spinal cord injury. Chin Acupunct Moxibustion. (2015) 35:17–20.
35. Sun, Y, Zhong, L, Tao, L, Fu, J, Wu, C, Shen, Y, et al. Effects of hyperbaric oxygen combined with electroacupuncture at Baliao acupoints on urodynamics in patients with neurogenic bladder due to spinal cord injury. Chin Rehabil. (2023) 38:422–5.
36. Hu, J, Liang, H, Qin, M, Zhu, F, Zhang, Z, and Sun, N. Effects of biofeedback combined with electroacupuncture at shu-mu acupoints on urodynamics and urinary function in patients with SCI NB. Zhejiang Clin Med. (2024) 26:194–6.
37. Jiangeng, Y, Xing, W, Ying, Y, Xiaoping, L, Bin, W, and Huibin, H. Effects of electroacupuncture combined with pelvic floor muscle electrical stimulation on urinary function and urodynamic parameters in patients with urinary incontinence after spinal cord injury. Capital Food Med. (2024) 31:160–2.
38. Cameron, AP, Chung, DE, Dielubanza, EJ, Enemchukwu, E, Ginsberg, DA, Helfand, BT, et al. The AUA/SUFU guideline on the diagnosis and treatment of idiopathic overactive bladder. J Urol. (2024) 212:11–20. doi: 10.1097/JU.0000000000003985
39. Deininger, S, Herrmann, T, Schönburg, S, Törzsök, P, Kunit, T, and Lusuardi, L. Die chirurgische Therapie der benignen Prostataobstruktion (BPO) beim antikoagulierten Patienten: eine Übersichtsarbeit über die Blutungsrisiken etablierter Techniken [Surgical treatment of benign prostatic obstruction (BPO) in patients under anticoagulation: a review of the bleeding risks of established techniques]. Urologe A. (2020) 59:1187–94. doi: 10.1007/s00120-020-01319-1
40. Ahuja, CS, Wilson, JR, Nori, S, Kotter, MRN, Druschel, C, Curt, A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. (2017) 3:17018. doi: 10.1038/nrdp.2017.18
41. Kozomara, M, Birkhäuser, V, Anderson, CE, Bywater, M, Gross, O, Kiss, S, et al. Neurogenic lower urinary tract dysfunction in the first year after spinal cord injury: A descriptive study of urodynamic findings. J Urol. (2023) 209:225–32. doi: 10.1097/JU.0000000000003021
42. Gao, L, Zhang, JF, Williams, JP, Yan, YN, Xiao, XL, Shi, WR, et al. Neuropathic pain creates systemic ultrastructural changes in the nervous system corrected by Electroacupuncture but not by Pregabalin. J Pain Res. (2021) 14:2893–905. doi: 10.2147/JPR.S322964
43. Li, J, Yao, D, Zhang, T, Tong, T, Shen, J, Yan, S, et al. GABAB modulate NF-κB/NLRP3 pathways in electroacupuncture prevention of depression in CUMS rats. Brain Res Bull. (2024) 218:111108. doi: 10.1016/j.brainresbull.2024.111108
44. Zhang, J, Xu, J, Li, S, Chen, W, and Wu, Y. Electroacupuncture relieves HuR/KLF9-mediated inflammation to enhance neurological repair after spinal cord injury. eNeuro. (2023) 10:ENEURO.0190-23.2023. doi: 10.1523/ENEURO.0190-23.2023
45. Deng, Q, Ma, L, Yang, Y, Chen, T, Zhan, L, He, Q, et al. Effect of Electroacupuncture stimulation on proliferation and differentiation of endogenous neural stem cells in rats with spinal cord injury. Mol Neurobiol. (2024) 61:635–45. doi: 10.1007/s12035-023-03577-4
46. Xu, H, Yang, Y, Deng, QW, Zhang, BB, Ruan, JW, Jin, H, et al. Governor vessel electro-acupuncture promotes the intrinsic growth ability of spinal neurons through activating calcitonin gene-related peptide/α-calcium/Calmodulin-dependent protein kinase/Neurotrophin-3 pathway after spinal cord injury. J Neurotrauma. (2021) 38:734–45. doi: 10.1089/neu.2020.7155
Keywords: electroacupuncture, spinal cord injury, urinary incontinence, meta-analysis, trial sequential analysis
Citation: Zhang P-Y, Xu C-L, Ye G-X, Meng L and Wang F (2025) Clinical efficacy of electroacupuncture for urinary incontinence following spinal cord injury: a meta-analysis and trial sequential analysis. Front. Neurol. 16:1573090. doi: 10.3389/fneur.2025.1573090
Edited by:
Fernando Zanela Arêas, Baylor Scott & White Research Institute (BSWRI), United StatesReviewed by:
Argyrios Stampas, University of Texas Health Science Center at Houston, United StatesRita Chiaramonte, University of Catania, Italy
Copyright © 2025 Zhang, Xu, Ye, Meng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, dGdiejEyMzQ1NkAxMjYuY29t
 Ping-Yan Zhang1
Ping-Yan Zhang1 Feng Wang
Feng Wang