- 1Department of Neurology, Mass General Brigham, Boston, MA, United States
- 2Department of Neurology, University of Pennsylvania, Philadelphia, PA, United States
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the brain resulting from infection of oligodendrocytes by JC virus (JCV) typically occurring in association with defects of cell-mediated immunity. The clinical presentation of PML depends on its area of effect in the central nervous system and can include a broad spectrum of deficits such as focal weakness, speech difficulties, visual changes, cognitive disruptions, or ataxia. While the disease was first described in patients with B cell malignancies (Hodgkins’s lymphoma and chronic lymphocytic leukemia), a large array of immunosuppressive conditions, most notably human immunodeficiency virus, may predispose to the disorder. From 2005 on, PML was observed in patients with multiple sclerosis (MS) and Crohn’s disease being treated with natalizumab, a monoclonal antibody inhibiting α4β1 and α4β7 integrins. Risk factors for PML development were identified, and an effective risk mitigation strategy chiefly predicated on the JCV antibody index was established (an antibody index less than 0.4 is considered negative, 0.4 to 0.9 low risk, 0.9 to 1.5 medium risk, and greater than 1.5 high risk). Here we review risk stratification, diagnosis, and treatment of PML in patients receiving natalizumab.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a devastating disease of the central nervous system (CNS) that occurs most commonly in immunosuppressed patients in whom JC virus (JCV), a human polyomavirus, transforms from a latent to a more clinically active form. PML develops as a stochastic event that requires viral replication, infection of oligodendrocytes following viral genetic modification, and impaired immunosurveillance permitting viral persistence and replication within the CNS (1). Productive infection of oligodendrocytes, the myelin-producing cells of the central nervous system (CNS), results in demyelination within the brain leading to PML. More rarely, granule cells of the cerebellum are infected, leading to cerebellar degeneration in a condition known as granule cell neuronopathy. On magnetic resonance imaging (MRI), PML is often characterized by multifocal, asymmetric, T2-weighted hyperintensities with patchy diffusion restriction that can be contrast enhancing, particularly in cases of natalizumab-associated PML or after immune reconstitution has begun (2). The diagnosis can be confirmed by testing cerebrospinal fluid for JCV DNA via polymerase chain reaction when coupled with clinical and radiographic features consistent with PML or through tissue examination demonstrating the classic histopathological triad of PML (demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei) (3).
Seroepidemiological studies reveal that approximately 50–90% of adults have been infected by JCV but remain asymptomatic (4). PML most commonly develops in the context of immune suppression in patients with human immunodeficiency virus (HIV) or hematologic malignancies, and in those receiving various immunosuppressive medications that are used in rheumatologic or autoimmune conditions (5). One such medication is natalizumab, a monoclonal antibody that binds to alpha-4 integrin. The Food and Drug Administration (FDA) approved natalizumab for the treatment of relapsing–remitting multiple sclerosis (RRMS) in 2004, and the first cases of PML associated with natalizumab were recognized in 2005 (6–8). As of February 2024, the overall global incidence of natalizumab-associated PML (per Biogen) was 3.43 per 1,000 patients.
Despite the substantial risk of PML, natalizumab continues to have a role in the treatment of RRMS, as it is a highly effective medication with low rates of relapse and high rates of no evidence of disease activity (NEDA-3) status (39.0% NEDA-3 after 2 years in natalizumab compared to 22.0% in fingolimod) that are similar to the more recently introduced anti-CD20 monoclonal antibodies (9–11). Prior studies have shown similar beneficial effects on rates of relapse, MRI activity, and disability progression when comparing natalizumab and the CD-20 inhibitor ocrelizumab (12, 13). However, natalizumab is associated with risks of PML that are orders of magnitude higher than anti-CD20 monoclonal antibodies like ocrelizumab. The stratification of PML risk in patients on natalizumab is crucial for ensuring patients receive effective treatment while minimizing possible harm.
Methods
Articles for this mini-review were primarily identified through a Pubmed search of “natalizumab and PML.” Additional resources were further found from more targeted searches for specific sections (“PML imaging” and “PML” combined with various treatments were also search terms utilized to obtain additional articles). Information was also obtained from Biogen’s website.
Natalizumab and progressive multifocal leukoencephalopathy
PML has become increasingly associated with a variety of immunosuppressive medications, including mycophenolate mofetil, dimethyl fumarate, fingolimod, cyclosporin, cyclophosphamide, methotrexate, infliximab, and rituximab (14–17). By blocking α4β1 integrin, natalizumab prevents the entry of inflammatory cells into the CNS in a mechanism that is unique compared to other therapies used for RRMS. In patients receiving this medication, levels of CD4 + and CD8 + T cells as well as CD19 + and CD138 + positive B cells are significantly reduced in cerebrospinal fluid (CSF), and CD4/CD8 ratios in the CSF are similar to those who are infected with HIV (18). While this immunomodulatory effect can be beneficial in reducing autoimmune damage in patients with RRMS, it may also predispose to certain infections, particularly PML, though the precise mechanisms underlying this risk remain uncertain. Currently, it is believed that those most important are its ability to enhance the likelihood of transformation of the archetype virus to the prototype (neurotropic) virus, to increase expression of JCV within CD34 lymphocytes (19), and to limit the ability of JCV-specific T lymphocytes to clear the infection from the brain (20).
Studies have since been conducted to help better evaluate the risk of PML in patients on natalizumab, specifically looking at duration of therapy and JCV antibody status prior to and while on treatment. One such study of 3,180 patients examined JCV status and seroconversion in patients with RRMS who were receiving natalizumab. Prior to treatment, 56.3% of patients were JCV positive. Of the patients who were initially JCV negative, 10.9% became JCV positive during the course of their treatment (21). JCV serostatus can be quantified into a JCV index, which calculates levels of anti-JCV antibodies in the serum to classify patients into the following categories: Negative (less than 0.4), low risk (0.4 to 0.9), medium risk (0.9 to 1.5), and high risk (greater than 1.5). One study evaluated how the JCV index changed over time for 525 patients being treated with natalizumab. They found that patients’ JCV indices tended to increase with duration of therapy: 20 patients went from negative to positive, the medium risk group grew from 24 to 31, and the high risk group went from 117 to 131 (21).
JCV serostatus is crucial in assessing a patient’s risk of PML. A negative JCV serostatus suggests that the individual has not been exposed to the virus and is at no risk of PML, although a false negative rate of 2.5% has been reported (22). However, the frequency with which individuals convert from negative to positive status when serially determined suggests that there may simply be a low level of antibody production in a previously infected person and that viral replication allowed by immunosuppression has resulted in an anamnestic response that increases antibody production sufficiently to allow detection. There is a clear association between JCV index and PML; a prior study found a difference in JCV index levels in those treated with natalizumab who did not develop PML (average JCV index of 1.4) compared to those who did develop PML (average JCV index of 2.4) (23). Other studies estimate that, for patients on natalizumab, a negative JCV antibody has an associated PML risk of 1 in 10,000, a JCV index that is positive but less than 1.5 has a risk of 1 in 5,882, and an index of greater than 1.5 has a risk of 1 in 855. The risk continues to increase the longer a patient is receiving the medication; after 24–48 months of being on the medication, an index of greater than 1.5 is associated with a 1/113 risk of developing PML (24).
A risk-stratification table of risk for PML in patients receiving natalizumab has been provided by Biogen (64) based on exposure time to the medication, antibody index value, and prior immunosuppressant use. For this reason, it has been recommended to check an anti-JCV antibody approximately every 6 months while on natalizumab (24) and discontinue the drug when levels exceed 1.5. Additionally, prior studies have shown that the use of extended interval dosing at 5–6 week intervals was associated with a reduced risk of PML (25). Alternatively, the risk of PML can be virtually eliminated by confining natalizumab treatment to patients who are JCV seronegative and remain so, regardless of the level. In 2023, the FDA approved the first biosimilar to natalizumab (known as natalizumab-sztn) for use in RRMS. To go along with the new biosimilar, a new JCV index test was developed (as the standard Stratify platform that is offered by Biogen is only available to those on natalizumab and not the biosimilar). This is known as the Immunowell platform, and it is also an enzyme-linked immunosorbent assays (ELISA) test. A recent study evaluated JCV indices in a cohort of 39 patients with MS as they transitioned from natalizumab to natalizumab-sztn (26). They found that JCV antibody levels increased from 13% (via the Stratify platform) to 52% (via the Immunowell platform), which may be related to differences in the tests rather than an increase in JCV antibody (JCV indices were only tested with the Immunowell platform after the patients had switched to the biosimilar in this cohort). However, another recent study compared Stratify and Immunowell JCV indices on patients who were receiving natalizumab and found an 85.5% agreement between the two tests, with the Immunowell platform tending to categorize patients as higher risk (27).
If a clinician elects to treat a patient who is JCV seropositive with natalizumab, more frequent MRI monitoring (every 3–4 months) is recommended to determine whether PML has developed (28). Brain MRI may detect PML in a presymptomatic phase (24), which can lead to earlier intervention (cessation of immunosuppression) and improved outcomes. One challenge with presymptomatic PML detection is distinguishing it from RRMS disease progression. Some studies have found certain radiologic patterns may help differentiate these disease entities. For instance, the punctate pattern or “Milky Way sign” refers to the appearance of multiple punctate areas of T2-weighted hyperintensities surrounding the main component of a new lesion (29). One study that compared 20 patients with PML (14 of which were associated with natalizumab and 9 of which were presymptomatic) to 80 patients without PML but with multiple sclerosis found that the punctate pattern was found in 18 of the patients with PML (including all 14 associated with Natalizumab) and in none of the patients without PML (30). Thus, the appearance of the punctate pattern may be more indicative of PML than RRMS activity. Other factors on MRI that may be suggestive of PML rather than RRMS activity include a more irregular shape with ill-defined border toward the white matter and being less hypointense on T1-weighted images compared to what is expected for acute lesions due to multiple sclerosis (24). Solitary lesions that are periventricular are more associated with multiple sclerosis activity (29).
Management of natalizumab-associated progressive multifocal leukoencephalopathy
While there are ways to help predict risk of PML in patients receiving natalizumab, cases of natalizumab-associated PML continue to occur. Prior studies have found survival rates as high as, or higher than, 70%. One study that followed 15 patients for an average of 21 months had no fatalities, although patients can have significant and lasting disability (31–34). Younger age at diagnosis, better functional status prior to diagnosis, lower JC viral load, and more localized brain involvement at time of diagnosis have all been associated with improved survival (24). Patients who are asymptomatic at time of diagnosis also have improved functional outcomes and survival (35).
Natalizumab should be discontinued in a patient who develops PML while receiving the medication. However, because the medication’s immunosuppressive effects can persist even up to 12 weeks after the last administration, plasma exchange or immunoadsorption have been used in an attempt to more quickly wash out the monoclonal antibody to expedite immune reconstitution (36). While it has previously been demonstrated that plasma exchange accelerate natalizumab clearance (37), whether this translates to clinical benefit remains uncertain. A study that followed 15 patients with natalizumab-associated PML treated with plasma exchange or immunoadsorption reported no mortalities after 21 months, although as all patients received interventions and survived, no inter-group comparisons for treatment or survival could be made (33). A larger study evaluated 219 patients with natalizumab-associated PML where 84% underwent plasma exchange, and, after a mean follow-up time of 11.5 months, there was no difference in survival or clinical outcomes compared to those who did not receive plasma exchange (38). Another, smaller study of 42 patients with natalizumab-associated PML found similar results of plasma exchange not providing a significant benefit after a 12-month follow-up period (39). While undergoing plasma exchange to expedite natalizumab clearance mechanistically makes sense, more evidence is needed to better determine its clinical utility for natalizumab-associated PML. The implementation of either plasma exchange or immunoadsorption is probably best reserved for individuals who have received their natalizumab within 3 weeks of diagnosis as the half-life of natalizumab is about 11 days in adults.
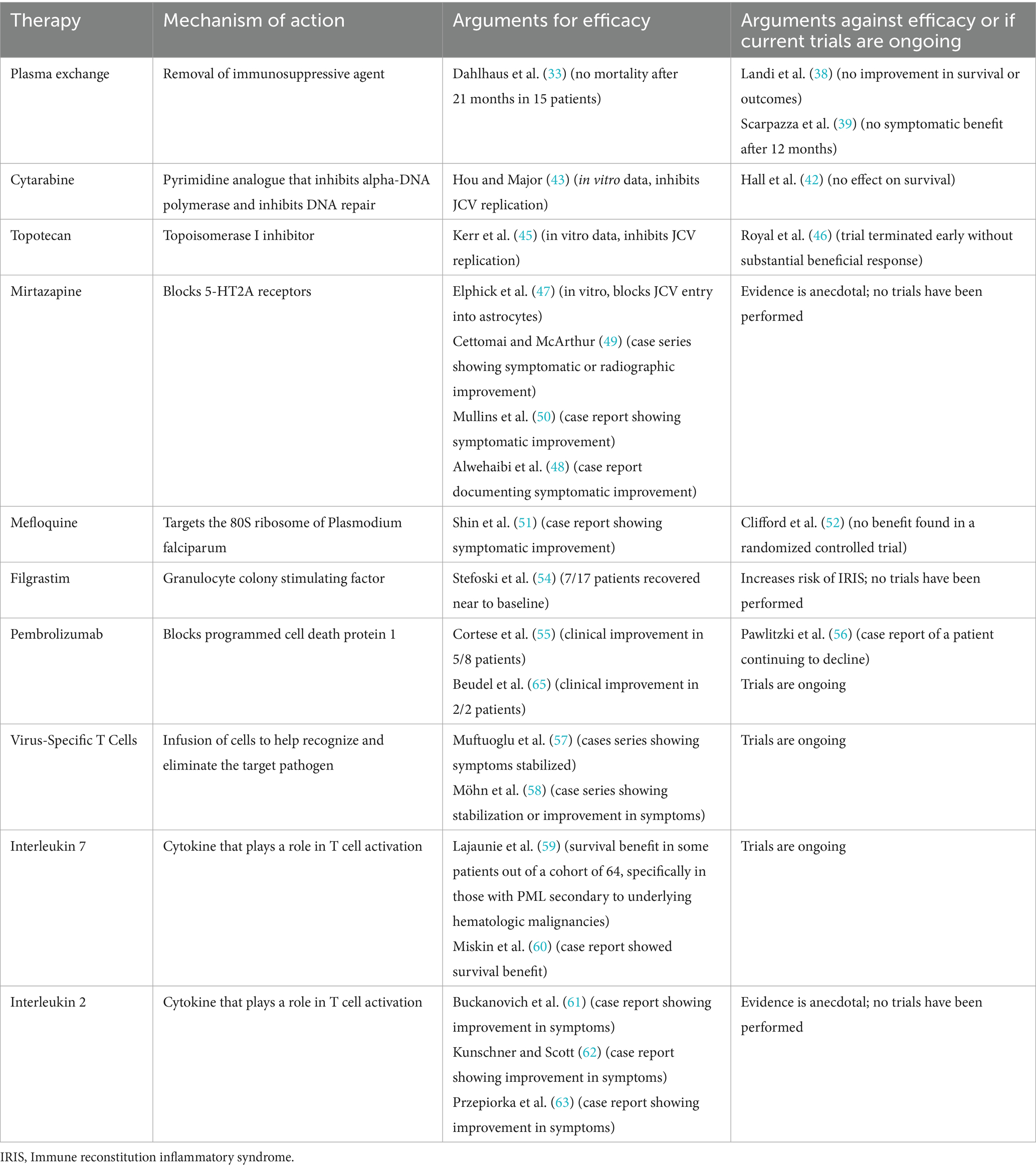
The single best treatment of PML is the restoration of the immune system. Fortunately, in natalizumab-associated PML, removing the offending agent can return the immune system to its baseline state. To date, no adjunctive therapy has been demonstrated to be effective in treating PML in a randomized, controlled trial (although additional trials are underway). Among the adjunctive therapies that have been explored are medications such as cytarabine, topotecan, mirtazapine, and mefloquine that mainly target JCV replication or entry into host cells (see Table 1 for a summary of therapies that have been evaluated for efficacy in the treatment of PML) (40, 41). In a phase II trial, the largest clinical trial of PML treatment to date, cytarabine, though effective in preventing viral replication in vitro, had no effect on PML survival in HIV-associated PML (42–44). Another trial investigated topotecan in a small series of patients with HIV-associated PML as topoisomerase inhibitors had previously been shown to have in vitro efficacy against JCV replication (45). This trial was terminated early without a substantial beneficial response (46). Mirtazapine, by blocking 5-HT2A receptors, appears to block entry of JCV into astrocytes in vitro (47) resulting in its use and occasional case reports documenting symptomatic improvement after its initiation (48–50). However, no large-scale studies have shown substantial benefit. Mefloquine, an anti-malarial that is thought to target the 80S ribosome of Plasmodium falciparum, has been shown in some case reports to also result in symptomatic improvement in patients with PML (51). However, other studies have found no benefit from mefloquine administration (52), and some differences in response may be mediated by genetic polymorphisms in the p-glycoprotein transporter, which can affect the drug’s absorption and elimination (53). Granulocyte-colony stimulating factor G-CSF (known as filgrastim), which has been used to help restore immune system function after chemotherapy, has also previously been investigated to see if confers a benefit to patients with natalizumab-associated PML, as it may accelerate the development of IRIS to more effectively clear JCV (54). Patients who received filgrastim did develop IRIS more rapidly than has generally been described in the literature, suggesting that filgrastim did help promote immune reconstitution. However, IRIS carries its own dangers such as increased risk of seizure and potentially fatal cerebral edema.
As restoration of the immune system appears fundamental to PML outcome, attempts have also been made to increase neuroimmunosurveillance. One strategy employs the use of checkpoint inhibitors. A small trial of pembrolizumab (which blocks programmed cell death protein 1) in 8 patients with PML secondary to a variety of causes suggested improvement in some patients (55). However, mixed results from pembrolizumab have also been reported in case reports (56). There are also ongoing trials evaluating the efficacy of cell-based therapies for PML. For instance, the use of allogenic BK virus-specific T cell infusions for the treatment of PML has previously been shown in case reports to have a beneficial effect for patients with PML. Given the genetic similarities between the polyomaviruses BK and JC viruses, T cells that had been developed against the BK virus were hypothesized to also be effective against JCV. Three patients with PML were infused with these T cells. Two improved symptomatically and radiographically, and cleared JCV from their CSF, while the other patient had a stabilization of symptoms and reduction in JCV viral load (57). Additionally, the use of virus-specific T-cells from partially human leukocyte antigen (HLA) matched donors has been shown to result in clinical stabilization or improvement of symptoms with reduction of viral load in 22 patients (from a cohort of 28 who received the virus-specific T-cells) (58).
Trials are also underway for the use of recombinant interleukins (IL) in PML. Studies of IL-7, which promotes T cell function, found that IL-7 may confer a survival benefit but in PML secondary to hematologic malignancies and post-transplant immunosuppression patients but not underlying HIV (59, 60). IL-2 has been demonstrated in case reports to have benefit in patients with PML; however, data with larger cohorts or in trials are lacking (61–63).
Clinical summary
The use of natalizumab in the treatment of RRMS leads to an increased risk of developing PML. Duration of treatment and JCV antibody seropositivity (and specifically antibody index) can be used to risk-stratify patients on natalizumab and allow for more individualized conversations regarding benefits and potential hazards of therapy. Tracking the JCV antibody index while on treatment and monitoring for the possible development of PML with serial MRI scans can help further personalize a patient’s possible risk of PML, as well as potentially capture PML in the presymptomatic stage, thus leading to improved outcomes. While RRMS disease activity can appear similar to PML lesions on MRI, certain features have been identified (such as the punctate pattern or “Milky Way” sign) that are more closely associated with PML.
If a patient develops PML while on natalizumab, immunosuppressive therapy should be discontinued. The benefit of plasma exchange is unclear. Other therapies such as mirtazapine, mefloquine, or pembrolizumab have been evaluated for possible benefit in patients with PML with mixed results or slight symptomatic improvement, and trials in interleukin- or cell-based therapies are ongoing. The most effective current management paradigm for natalizumab-associated PML is to screen patients for JCV antibody index to avoid development of the disease and to discontinue the therapy if any signs of PML development are detected.
Author contributions
TG: Writing – original draft. JB: Writing – review & editing. CM: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
In the last 2 years, JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Celgene/BMS. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Cycle Pharma. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Dice Therapeutics. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Genentech/Roche. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Gilead. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Janssen/J&J. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Merck. Berger has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Morphic. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Novartis. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Sandoz. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Seagen. JB has received personal compensation in the range of $500–$4,999 for serving as a Consultant for Takeda. JB has received personal compensation in the range of $5,000–$10,000 for serving as a Consultant for TG Therapeutics. JB has received personal compensation in the range of $500–$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for MAPI. JB has received personal compensation in the range of $500–$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for ExcisionBio. In the last 2 years, the institution of JRB has received research support from Genentech/Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rocchi, A, Sariyer, IK, and Berger, JR. Revisiting JC virus and progressive multifocal leukoencephalopathy. J Neurovirol. (2023) 29:524–37. doi: 10.1007/s13365-023-01164-w
2. Baldassari, LE, Wattjes, MP, Cortese, ICM, Gass, A, Metz, I, Yousry, T, et al. The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective. Brain. (2021) 145:426–40. doi: 10.1093/brain/awab419
3. Berger, JR, Aksamit, AJ, Clifford, DB, Davis, L, Koralnik, IJ, Sejvar, JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious disease section. Neurology. (2013) 80:1430–8. doi: 10.1212/WNL.0b013e31828c2fa1
4. Bellizzi, A, Anzivino, E, Rodio, DM, Palamara, AT, Nencioni, L, and Pietropaolo, V. New insights on human polyomavirus JC and pathogenesis of progressive multifocal leukoencephalopathy. Clin Dev Immunol. (2013) 2013:839719:1–17. doi: 10.1155/2013/839719
5. Anand, P, Hotan, GC, Vogel, A, Venna, N, and Mateen, FJ. Progressive multifocal leukoencephalopathy. Neurol Neuroimmunol Neuroinflam. (2019) 6:e618. doi: 10.1212/NXI.0000000000000618
6. Kleinschmidt-DeMasters, BK, and Tyler, KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. (2005) 353:369–74. doi: 10.1056/NEJMoa051782
7. Langer-Gould, A, Atlas, SW, Green, AJ, Bollen, AW, and Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with Natalizumab. N Engl J Med. (2005) 353:375–81. doi: 10.1056/NEJMoa051847
8. Van Assche, G, Van Ranst, M, Sciot, R, Dubois, B, Vermeire, S, Noman, M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. (2005) 353:362–8. doi: 10.1056/NEJMoa051586
9. Curti, E, Tsantes, E, Baldi, E, Caniatti, LM, Ferraro, D, Sola, P, et al. The real-world effectiveness of natalizumab and fingolimod in relapsing-remitting multiple sclerosis. An Italian multicentre study. Mult Scler Relat Disord. (2019) 33:146–52. doi: 10.1016/j.msard.2019.05.026
10. Guerra, T, Caputo, F, Orlando, B, Paolicelli, D, Trojano, M, and Iaffaldano, P. Long-term comparative analysis of no evidence of disease activity (NEDA-3) status between multiple sclerosis patients treated with natalizumab and fingolimod for up to 4 years. Neurol Sci. (2021) 42:4647–55. doi: 10.1007/s10072-021-05127-z
11. Prosperini, L, Saccà, F, Cordioli, C, Cortese, A, Buttari, F, Pontecorvo, S, et al. Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naïve patients with multiple sclerosis. J Neurol. (2017) 264:284–94. doi: 10.1007/s00415-016-8343-5
12. Barbuti, E, Castiello, A, Pozzilli, V, Carotenuto, A, Tomasso, I, Moccia, M, et al. Comparative effectiveness, safety and persistence of ocrelizumab versus natalizumab in multiple sclerosis: a real-world, multi-center, propensity score-matched study. Neurotherapeutics. (2025) 22:e00537. doi: 10.1016/j.neurot.2025.e00537
13. Iaffaldano, P, Lucisano, G, Guerra, T, Paolicelli, D, Portaccio, E, Inglese, M, et al. A comparison of natalizumab and ocrelizumab on disease progression in multiple sclerosis. Ann Clin Transl Neurol. (2024) 11:2008–15. doi: 10.1002/acn3.52118
14. Berger, JR, and Houff, S. Opportunistic infections and other risks with newer multiple sclerosis therapies. Ann Neurol. (2009) 65:367–77. doi: 10.1002/ana.21630
15. Calabrese, LH, and Molloy, ES. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann Rheum Dis. (2008) 67:iii64-65. doi: 10.1136/ard.2008.097972
16. Clifford, DB, Ances, B, Costello, C, Rosen-Schmidt, S, Andersson, M, Parks, D, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. (2011) 68:1156–64. doi: 10.1001/archneurol.2011.103
17. Kumar, D, Bouldin, TW, and Berger, RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. (2010) 62:3191–5. doi: 10.1002/art.27687
18. Stüve, O, Marra, CM, Jerome, KR, Cook, L, Cravens, PD, Cepok, S, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. (2006) 59:743–7. doi: 10.1002/ana.20858
19. Frohman, EM, Monaco, MC, Remington, G, Ryschkewitsch, C, Jensen, PN, Johnson, K, et al. JC virus in CD34+ and CD19+ cells in patients with multiple sclerosis treated with natalizumab. JAMA Neurol. (2014) 71:596–602. doi: 10.1001/jamaneurol.2014.63
20. Carruthers, RL, and Berger, J. Progressive multifocal leukoencephalopathy and JC virus-related disease in modern neurology practice. Mult Scler Relat Disord. (2014) 3:419–30. doi: 10.1016/j.msard.2014.01.005
21. Schwab, N, Schneider-Hohendorf, T, Pignolet, B, Breuer, J, Gross, CC, Göbel, K, et al. Therapy with natalizumab is associated with high JCV seroconversion and rising JCV index values. Neurol Neuroimmunol Neuroinflam. (2016) 3:e195. doi: 10.1212/NXI.0000000000000195
22. Gorelik, L, Lerner, M, Bixler, S, Crossman, M, Schlain, B, Simon, K, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. (2010) 68:295–303. doi: 10.1002/ana.22128
23. Plavina, T, Subramanyam, M, Bloomgren, G, Richman, S, Pace, A, Lee, S, et al. Anti–JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. (2014) 76:802–12. doi: 10.1002/ana.24286
24. McGuigan, C, Craner, M, Guadagno, J, Kapoor, R, Mazibrada, G, Molyneux, P, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. (2016) 87:117–25. doi: 10.1136/jnnp-2015-311100
25. Ryerson, LZ, Foley, J, Chang, I, Kister, I, Cutter, G, Metzger, RR, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. (2019) 93:e1452–62. doi: 10.1212/WNL.0000000000008243
26. Høgestøl, EA, Brustad, ÅW, Celius, EG, Meling, M, Berg-Hansen, P, Kro, GB, et al. Real-world experience with switching from originator to biosimilar natalizumab (2025). doi: 10.1101/2025.02.05.25320428 [Pre print].
27. Zanghì, A, Marinelli, F, Filippo, PSD, Avolio, C, and D’Amico, E. Comparing STRATIFY JCV™ DxSelect™ and IMMUNOWELL™ JCV IgG tests in RRMS to assess PML risk. Curr Neuropharmacol. (2025). doi: 10.2174/011570159X372688250226110925
28. Scarpazza, C, Signori, A, Cosottini, M, Sormani, MP, Gerevini, S, and Capra, R. Should frequent MRI monitoring be performed in natalizumab-treated MS patients? A contribution to a recent debate. Mult Scler. (2020) 26:1227–36. doi: 10.1177/1352458519854162
29. Wijburg, MT, Witte, BI, Vennegoor, A, Roosendaal, SD, Sanchez, E, Liu, Y, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. (2016) 87:1138–45. doi: 10.1136/jnnp-2016-313772
30. Hodel, J, Darchis, C, Outteryck, O, Verclytte, S, Deramecourt, V, Lacour, A, et al. Punctate pattern. Neurology. (2016) 86:1516–23. doi: 10.1212/WNL.0000000000002586
31. Blankenbach, K, Schwab, N, Hofner, B, Adams, O, Keller-Stanislawski, B, and Warnke, C. Natalizumab-associated progressive multifocal leukoencephalopathy in Germany. Neurology. (2019) 92:e2232–9. doi: 10.1212/WNL.0000000000007451
32. Clifford, DB, DeLuca, A, Simpson, DM, Arendt, G, Giovannoni, G, and Nath, A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. (2010) 9:438–46. doi: 10.1016/S1474-4422(10)70028-4
33. Dahlhaus, S, Hoepner, R, Chan, A, Kleiter, I, Adams, O, Lukas, C, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. (2013) 84:1068–74. doi: 10.1136/jnnp-2013-304897
34. Dong-Si, T, Gheuens, S, Gangadharan, A, Wenten, M, Philip, J, McIninch, J, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. (2015) 21:637–44. doi: 10.1007/s13365-015-0316-4
35. Dong-Si, T, Richman, S, Wattjes, MP, Wenten, M, Gheuens, S, Philip, J, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. (2014) 1:755–64. doi: 10.1002/acn3.114
36. Yaldizli, Ö, and Putzki, N. Natalizumab in the treatment of multiple sclerosis. Ther Adv Neurol Disord. (2009) 2:115–28. doi: 10.1177/1756285608101861
37. Khatri, BO, Man, S, Giovannoni, G, Koo, AP, Lee, J-C, Tucky, B, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. (2009) 72:402–9. doi: 10.1212/01.wnl.0000341766.59028.9d
38. Landi, D, De Rossi, N, Zagaglia, S, Scarpazza, C, Prosperini, L, Albanese, M, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology. (2017) 88:1144–52. doi: 10.1212/WNL.0000000000003740
39. Scarpazza, C, Prosperini, L, De Rossi, N, Moiola, L, Sormani, MP, Gerevini, S, et al. To do or not to do? Plasma exchange and timing of steroid administration in progressive multifocal leukoencephalopathy. Ann Neurol. (2017) 82:697–705. doi: 10.1002/ana.25070
40. Cortese, I, Reich, DS, and Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. (2021) 17:37–51. doi: 10.1038/s41582-020-00427-y
41. Schweitzer, F, Laurent, S, Cortese, I, Fink, GR, Silling, S, Skripuletz, T, et al. Progressive multifocal leukoencephalopathy. Neurology. (2023) 101:700–13. doi: 10.1212/WNL.0000000000207622
42. Hall, CD, Dafni, U, Simpson, D, Clifford, D, Wetherill, PE, Cohen, B, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 team. N Engl J Med. (1998) 338:1345–51. doi: 10.1056/NEJM199805073381903
43. Hou, J, and Major, EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol. (1998) 4:451–6. doi: 10.3109/13550289809114545
44. Pavlovic, D, Patera, AC, Nyberg, F, Gerber, M, and Liu, MProgressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. (2015) 8:255–73. doi: 10.1177/1756285615602832
45. Kerr, DA, Chang, CF, Gordon, J, Bjornsti, MA, and Khalili, K. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology. (1993) 196:612–8. doi: 10.1006/viro.1993.1517
46. Royal, W, Dupont, B, McGuire, D, Chang, L, Goodkin, K, Ernst, T, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. (2003) 9:411–9. doi: 10.1080/13550280390201740
47. Elphick, GF, Querbes, W, Jordan, JA, Gee, GV, Eash, S, Manley, K, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. (2004) 306:1380–3. doi: 10.1126/science.1103492
48. Alwehaibi, AI, AlJaber, MI, Nahrir, S, Alwehaibi, AI, AlJaber, MI, and Nahrir, S. Favorable response to mirtazapine in John Cunningham virus-related gray matter lesion in a patient with human immunodeficiency virus. Cureus. (2019) 11. doi: 10.7759/cureus.4255
49. Cettomai, D, and McArthur, JC. Mirtazapine use in human immunodeficiency virus–infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. (2009) 66:255–8. doi: 10.1001/archneurol.2008.557
50. Mullins, C, Miranda, J, Sandoval, H, Ramos-Duran, L, and Tonarelli, SB. The benefit of mirtazapine in the treatment of progressive multifocal leukoencephalopathy in a young HIV-positive patient. Innov Clin Neurosci. (2018) 15:33–5.
51. Shin, J-W, Jung, K-H, Lee, S-T, Moon, J, Lim, J-A, Byun, J-I, et al. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with immunoglobulin a nephropathy. J Clin Neurosci. (2014) 21:1661–4. doi: 10.1016/j.jocn.2013.12.031
52. Clifford, DB, Nath, A, Cinque, P, Brew, BJ, Zivadinov, R, Gorelik, L, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. (2013) 19:351–8. doi: 10.1007/s13365-013-0173-y
53. Aarnoudse, ALHJ, van Schaik, RHN, Dieleman, J, Molokhia, M, van Riemsdijk, MM, Ligthelm, RJ, et al. MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin Pharmacol Ther. (2006) 80:367–74. doi: 10.1016/j.clpt.2006.07.003
54. Stefoski, D, Balabanov, R, Waheed, R, Ko, M, Koralnik, IJ, and Sierra Morales, F. Treatment of natalizumab-associated PML with filgrastim. Ann Clin Transl Neurol. (2019) 6:923–31. doi: 10.1002/acn3.776
55. Cortese, I, Muranski, P, Enose-Akahata, Y, Ha, S-K, Smith, B, Monaco, M, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. (2019) 380:1597–605. doi: 10.1056/NEJMoa1815039
56. Pawlitzki, M, Schneider-Hohendorf, T, Rolfes, L, Meuth, SG, Wiendl, H, Schwab, N, et al. Ineffective treatment of PML with pembrolizumab. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e627. doi: 10.1212/NXI.0000000000000627
57. Muftuoglu, M, Olson, A, Marin, D, Ahmed, S, Mulanovich, V, Tummala, S, et al. Allogeneic BK virus–specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. (2018) 379:1443–51. doi: 10.1056/NEJMoa1801540
58. Möhn, N, Grote-Levi, L, Wattjes, MP, Bonifacius, A, Holzwart, D, Hopfner, F, et al. Directly isolated allogeneic virus–specific T cells in progressive multifocal leukoencephalopathy. JAMA Neurol. (2024) 81:1187–98. doi: 10.1001/jamaneurol.2024.3324
59. Lajaunie, R, Mainardi, I, Gasnault, J, Rousseau, V, Tarantino, AG, Sommet, A, et al. Outcome of progressive multifocal leukoencephalopathy treated by interleukin-7. Ann Neurol. (2022) 91:496–505. doi: 10.1002/ana.26307
60. Miskin, DP, Chalkias, SG, Dang, X, Bord, E, Batson, S, and Koralnik, IJ. Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e213. doi: 10.1212/NXI.0000000000000213
61. Buckanovich, RJ, Liu, G, Stricker, C, Luger, SM, Stadtmauer, EA, Schuster, SJ, et al. Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin’s lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann Hematol. (2002) 81:410–3. doi: 10.1007/s00277-002-0481-4
62. Kunschner, L, and Scott, TF. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology. (2005) 65:1510. doi: 10.1212/01.wnl.0000183064.10227.b5
63. Przepiorka, D, Jaeckle, KA, Birdwell, RR, Fuller, GN, Kumar, AJ, Huh, YO, et al. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. (1997) 20:983–7. doi: 10.1038/sj.bmt.1701010
64. PML tools and StratifyJCV, (2025) Available online at:https://biogenlinc.co.uk/en/products/ms-portfolio/tysabri/pml/ (accessed 3.19.25).
Keywords: progressive multifocal leukoencephalopathy, natalizumab, JC virus, relapsing remitting multiple sclerosis, demyelimating diseases
Citation: Glenn T, Berger JR and McEntire CRS (2025) Natalizumab-associated progressive multifocal leukoencephalopathy. Front. Neurol. 16:1575653. doi: 10.3389/fneur.2025.1575653
Edited by:
Mahsa Ghajarzadeh, Johns Hopkins University, United StatesReviewed by:
Mohammadali Nahayati, Mashhad University of Medical Sciences, IranCopyright © 2025 Glenn, Berger and McEntire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trevor Glenn, dGdsZW5uQG1nYi5vcmc=
 Trevor Glenn
Trevor Glenn Joseph R. Berger2
Joseph R. Berger2 Caleb R. S. McEntire
Caleb R. S. McEntire