Abstract
Basilar atherosclerosis is a major cause of posterior circulation ischemic stroke and it can affect neurological function negatively. Little is known about subclinical atherosclerotic changes before plaque formation. However, imaging changes in atherosclerotic plaque rupture and thrombosis have been extensively studied, which are major factors leading to the development of stroke. This study included 51 patients with posterior circulation ischemic stroke, 55 patients with high-risk factors for atherosclerosis, and 36 patients without high-risk factors for atherosclerosis. Based on high-resolution vascular wall imaging (HR-VWI) data, we quantitatively analyzed the differences in morphological abnormalities of the basilar artery wall between the three groups, including wall thickness, wall area, vessel area, and lumen area. This study aims to investigate the impact of abnormal basilar artery wall morphology, particularly on arterial wall thickness, on posterior circulation stroke development and its potential utility as a predictive biomarker. The results revealed that the stroke group exhibited significantly higher rates of cardiovascular risk factors (diabetes, hypertension, and elevated LDL-C) and increased basilar artery wall thickness compared to the high-risk group (p < 0.05). Logistic regression identified previous stroke, diabetes, and increased wall thickness as independent stroke risk factors. ROC analysis revealed strong associations between posterior circulation ischemic stroke and both vessel wall thickness alone (AUC = 0.806) and its combination with clinical features (AUC = 0.841), indicating potential diagnostic utility. These findings highlight the association between basilar artery wall morphology and stroke, supporting its potential role in risk stratification.
1 Introduction
Intracranial atherosclerosis is a leading cause of ischemic stroke, contributing to 30–50% of cases in Asian populations (1). It precipitates stroke through multiple mechanisms, including plaque formation, arterial stenosis, plaque instability, and hemodynamic alterations (2). These pathological changes can result in cerebral hypoperfusion, thromboembolism, or complete vessel occlusion, ultimately leading to ischemic brain injury (3). While high-resolution vessel wall imaging (HR-VWI) has advanced our understanding of plaque rupture in acute stroke (4–6), less is known about earlier, subclinical atherosclerotic changes preceding plaque formation.
Subclinical atherosclerosis is the pathophysiological stage characterized by pathological intimal thickening - the accumulation of lipids, inflammatory cells, and extracellular matrix in the arterial wall before visible plaque develops (7, 8). Studies have found that if the patient is treated with a small carotid intima-media thickness, plaque growth can be slowed or stopped (9, 10). Therefore, evaluating of subclinical atherosclerotic intima thickness can suggest early intervention, slow down or even reverse the atherosclerotic process, and reduce the risk of future disease and poor prognosis. Atherosclerosis progression differs substantially between intracranial and extracranial arteries. While extracranial disease progresses linearly, intracranial atherosclerosis develops later and shows distinct patterns (11), necessitating direct evaluation of intracranial vessels.
However, most existing studies have focused on changes in the intima thickness of the large blood vessels in the neck and the factors influencing these changes, emphasizing the correlation between the increase in extracranial blood vessel wall thickness and the formation of atherosclerotic plaque (12). Quantitative analysis of intracranial artery wall thickness based on HR-VWI has only been mentioned in a few assessments of non-atherosclerotic diseases (13, 14), such as vasculitis, and the long-term blood transfusion population. Posterior circulation strokes, though representing only 20–30% of ischemic strokes (1, 15), carry higher morbidity and mortality than anterior circulation events. Basilar artery (BA) occlusion accounts for approximately 1% of all ischemic strokes and about 10% of strokes due to an intracranial large vessel occlusion (16, 17). Although this condition may be relatively uncommon, it leads to potentially devastating consequences, including death or lock-in syndrome (18). Therefore, we selected BA as the object of analysis, aiming to study whether there is a difference in BA wall thickness between healthy people and patients with high-risk factors for atherosclerosis. Using our findings, we hope to guide early intervention in this group of people, ultimately preventing the occurrence and development of atherosclerotic post-circulation ischemic stroke.
2 Materials and methods
2.1 Image acquisition and processing
Instruments and parameters were set by the Vero 3.0 T MRI scanner (Siemens, Munich, Germany) with a 32-channel head and neck coil. The patient was positioned supine, and whole-brain HR-VWI was performed. The imaging protocol included conventional T1WI, T2WI, and coronal T2-FLAIR sequences, and diffusion-weighted imaging (DWI). Additionally, HR-VWI sequences, utilizing the T1-weighted sampling perfection with application-optimized contrasts using different flip angle evolution (T1-SPACE) and T2-weighted SPACE (T2-SPACE) sequences, were obtained by three-dimensional time-leap scanning, followed by axial scanning. HR-VWI scanning parameters included the following: TR, 800 ms; TE, 20 ms; slice thickness, 0.70 mm; voxel size, 0.70 × 0.70 × 0.70 (for T1-SAPCE) and TR, 1000 ms; TE, 81 ms; slice thickness, 0.80 mm; and voxel size, 0.80 × 0.80 × 0.80 mm (for T2-SPACE).
All image data were mapped using a commercial high-resolution magnetic resonance vessel wall analysis software (Vessel Explorer 2; TSImaging Healthcare, Beijing, China), and the mapping results were calculated automatically. The results included the area of the vascular lumen, the area of the lumen wall, the area of blood vessels, and the thickness of the blood vessel wall.
Two radiologists independently reconstructed the BA along the vascular centerline to obtain axial images perpendicular to the lumen wall. The layers of reconstructed images were both 1 mm thick, and 10 layers of BA images were continuously outlined after the initial outlining of the confluent bilateral vertebral arteries. If this layer was the basal artery branch level in the sketch, it was skipped because of the irregular lumen; the layer of atherosclerotic plaque in the plaque group was not sketched; instead, continuously sketched the relatively normal vessel walls of upper and lower layers of the plaque (Figures 1, 2). The results of demographic, angiographic, and other physician measurements were double-anonymized.
Figure 1

T1-SPACE and T2-SPACE images of a female patient aged 26 years from the control group. Parts (a–d) are T1-SPACE images; Parts (e–h) are T2-SPACE images. Images show the process of centerline extraction and vascular delineation.
Figure 2

T1-SPACE images of a male patient aged 50 years from the stroke group. Parts (a–e) are T1-SPACE images. Images show the process of centerline extraction and the interface of vessel delineation.
2.2 Study design and subjects
This retrospective study analyzed 170 consecutive patients who underwent HR-VWI at Yongchuan Hospital of Chongqing Medical University between April 2021 and September 2023.
Inclusion criteria: (1) presence of suspected acute stroke symptoms (headache, dizziness, vomiting, limb weakness/numbness, speech impairment, or visual disturbances); (2) completion of both cranial DWI and HR-VWI examinations within 1 week post-admission; (3) Availability of complete clinical data.
Exclusion criteria: (1) diagnosis of other cerebrovascular diseases (Moyamoya disease, hemorrhagic stroke, or arterial dissection); (2) poor image quality precluding accurate boundary delineation.
After excluding 47 patients for incomplete data or inadequate image quality and 17 for other cerebrovascular diseases, the final cohort comprised 106 patients, subdivided into a high-risk group (n = 55) and stroke group (n = 51). The study also included 36 healthy controls with normal clinical, imaging and laboratory findings for comparison. The high-risk factor group was defined as patients who met at least two of the following atherosclerotic risk factors based on laboratory tests and clinical evaluation: (1) hypertension (blood pressure ≥ 140/90 mmHg or use of antihypertensive medications); (2) diabetes mellitus (fasting glucose ≥ 7.0 mmol/L or hemoglobin A1c ≥ 6.5% or use of glucose-lowering medications); (3) hyperlipidemia (low-density lipoprotein cholesterol ≥ 3.4 mmol/L or use of lipid-lowering therapy); (4) current smoking status; (5) coronary artery disease history; and/or (6) previous stroke history. The stroke group was defined as patients who had posterior circulation infarction confirmed by DWI, characterized by high signal intensity on DWI. Collected clinical data encompassed smoking history, alcohol consumption, medical history (coronary heart disease, hypertension, diabetes, previous stroke), and lipid profiles. The institutional review board approved the study protocol, and all participants provided written informed consent (Figure 3).
Figure 3

Flowchart of patients selection.
2.3 Statistical analysis
SPSS 25.0 statistical software (IBM Corporation, Armonk, NY, USA) was used to analyze the data. Depending on data distribution, continuous variables are presented as the means ± SD or medians (Q1, Q3), while categorical variables are expressed as counts and percentages. Categorical variables were analyzed with the χ2 test or Fisher’s exact test. Continuous variables were analyzed with ANOVA or the Kruskal-Wallis test, and pairwise comparisons were conducted between groups. Variables identified as statistically significant (p < 0.05) in the univariate analysis were subsequently included in a binary logistic regression model to further assess their association with the outcome. In addition, the correlation between vascular wall indices and age in the control group was examined by Pearson’s correlation test. A two-tailed p < 0.05 was considered indicative of statistical significance. The discriminative performance of vascular wall indicators and their combination with clinical indicators for posterior circulation ischemic stroke was assessed using the area under the receiver operating characteristic (ROC) curve (AUC), with model calibration evaluated by the Hosmer-Lemeshow test (Figure 4).
Figure 4

Hosmer–Lemeshow test model calibration histogram.
3 Results
3.1 Difference analysis of clinical data and laboratory indicators
Based on the standardized HR-VWI acquisition and measurement protocols described in the Methods section, the study population comprised 142 participants divided into control (n = 36), high-risk (n = 55), and stroke (n = 51) groups. As shown in Table 1, demographic analysis revealed comparable age (F = 2.217, p = 0.113) and sex distribution (χ2 = 0.040, p = 0.980) across groups. The stroke group demonstrated significantly higher prevalence of stroke history (33.3% vs. 9.1%, χ2 = 9.456, p = 0.002), coronary heart disease (22.0% vs. 5.5%, χ2 = 6.205, p = 0.013), hypertension (70.6% vs. 47.3%, χ2 = 5.925, p = 0.015), and diabetes (37.3% vs. 20.0%, χ2 = 3.883, p = 0.049) compared to the high-risk group. Lipid analysis showed significantly elevated triglycerides (35.3% vs. 18.2%, χ2 = 3.987, p = 0.046) and low-density lipoprotein cholesterol (LDL-C) levels (58.8% vs. 36.4%, χ2 = 5.357, p = 0.021) in stroke patients, while total cholesterol (p = 0.881) and high-density lipoprotein cholesterol (HDL-C) (p = 0.183) showed no significant differences. Smoking (p = 0.656) and alcohol consumption (p = 0.500) histories were comparable between stroke and high-risk groups. These findings demonstrate significant associations between vascular comorbidities/dyslipidemia and stroke occurrence, underscoring the importance of monitoring these factors in high-risk populations.
Table 1
| Variable | Control group (n = 36) | High-risk factor group (n = 55) | Stroke group (n = 51) | F/X2 | p |
|---|---|---|---|---|---|
| Age (years) * | 53.81 ± 15.51 | 54.87 ± 13.68 | 56.69 ± 14.38 | 2.217 | 0.113 |
| Sex (male) | 13 (36.1) | 21 (38.2) | 19 (37.3) | 0.040 | 0.980 |
| Smoking history | — | 12 (21.8) | 13 (25.5) | 0.198 | 0.656 |
| Drinking history | — | 11 (20.0) | 13 (25.5) | 0.455 | 0.500 |
| Stroke history | — | 5 (9.1) | 17 (33.3) | 9.456 | 0.002 |
| Coronary heart disease | — | 3 (5.5) | 11 (22.0) | 6.205 | 0.013 |
| Hypertension | — | 26 (47.3) | 35 (70.6) | 5.925 | 0.015 |
| Diabetes | — | 11 (20.0) | 19 (37.3) | 3.883 | 0.049 |
| High level of total cholesterol | — | 37 (67.3) | 35 (68.6) | 0.022 | 0.881 |
| High level of triglycerides | — | 10 (18.2) | 18 (35.3) | 3.987 | 0.046 |
| High level of low-density lipoprotein | — | 20 (36.4) | 30 (58.8) | 5.357 | 0.021 |
| High level of high-density lipoprotein | — | 32 (58.2) | 36 (70.6) | 1.771 | 0.183 |
Differential analysis of clinical data.
*Data is mean ± SD.
3.2 Difference analysis of calculated indices for vascular wall parameters
Consistent with the image processing methodology, wall thickness showed progressive increases from control (0.90 ± 0.09 mm) to high-risk (1.08 ± 0.09 mm) to stroke groups (1.22 ± 0.14 mm), with all inter-group comparisons being statistically significant (p < 0.001). For wall area, both the high-risk group (median = 14.63 mm2, IQR = 12.99–17.00) and stroke group (16.74 ± 4.99 mm2) demonstrated significantly larger values than the control group (12.38 ± 3.21 mm2, p < 0.008), though no significant difference existed between the two patient groups (p > 0.05). Vessel area was significantly larger in the stroke group (26.31 ± 8.11 mm2) compared to controls (22.08 ± 6.74 mm2, p = 0.029), while the high-risk group (median = 23.57 mm2, IQR = 19.78–26.67) showed intermediate values. Lumen area remained comparable across all groups (Table 2). The observed gradual thickening of arterial walls across the risk spectrum, particularly without corresponding lumen changes, suggests that vascular remodeling occurs early in the disease process. These findings support the potential clinical utility of wall thickness measurement as a sensitive marker for vascular health assessment in at-risk populations.
Table 2
| Variable | Control group (n = 36) | High-risk factor group (n = 55) | Stroke group (n = 51) | H/X2 | p |
|---|---|---|---|---|---|
| Wall thickness (mm) | 0.90 ± 0.09* | 1.08 ± 0.09* | 1.22 ± 0.14* | 84.328 | < 0.001 abc |
| Wall area (mm2) | 12.38 ± 3.21* | 14.63 (12.99–17.00)♯ | 16.74 ± 4.99* | 20.881 | < 0.008 ab |
| Vessel area (mm2) | 22.08 ± 6.74* | 23.57 (19.78–26.67)♯ | 26.31 ± 8.11* | 6.741 | 0.029 b |
| Lumen area (mm2) | 9.18 (7.21, 11.15)♯ | 8.51 (6.84–10.95)♯ | 8.95 (7.37–11.09)♯ | 0.892 | 0.649 |
Differential analysis of vascular wall indices.
aControl group vs. High-risk factor group.
bControl group vs. Stroke group.
cHigh-risk factor group vs. Stroke group.
*means ± SD.
♯medians (Q1, Q3).
3.3 Difference analysis of age for vascular wall parameters
In the control group, we analyzed age-related correlations with vascular wall parameters. The results demonstrated significant positive correlations between age and all measured wall characteristics: wall thickness (r = 0.418, p < 0.01), wall area (r = 0.407, p < 0.01), and vessel area (r = 456, p < 0.05) (Figure 5).
Figure 5
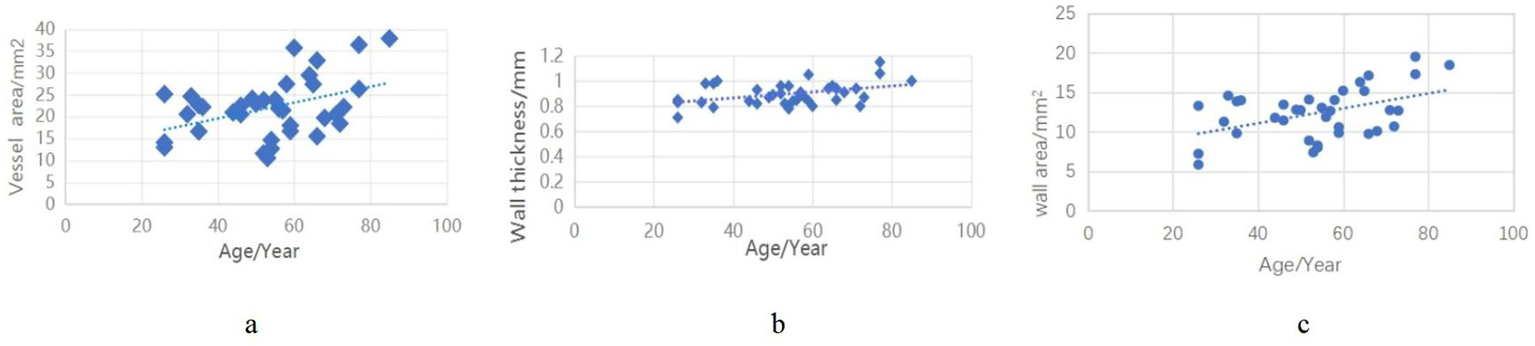
Correlation of vascular wall indices in the control group with age.
3.4 Logistic regression analysis of stroke and high-risk factors
Binary logistic regression analysis with stroke occurrence as the dependent variable revealed that previous stroke history (p = 0.036), diabetes mellitus (p = 0.048), and increased wall thickness (p < 0.001) emerged as independent risk factors for stroke in the multivariable analysis. Although wall area demonstrated significance in univariable analysis (t = 3.26, p = 0.001), it failed to maintain independent predictive value when adjusted for other variables (p = 0.093) (Table 3).
Table 3
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| t/χ2 | p value | OR value | 95% CI | p value | |
| Stroke history | 19.346 | <0.001 | 4.338 | 1.097–17.151 | 0.036 |
| Diabetes | 11.094 | 0.001 | 3.029 | 1.011–9.075 | 0.048 |
| Wall area (mm2) | 3.260 | 0.001 | 0.889 | 0.776–1.020 | 0.093 |
| Wall thickness (mm) | 8.215 | <0.001 | 780008.612 | 1302.933–466, 956,927.0 | < 0.001 |
Binary logistic regression analysis of stroke and vascular wall indexes.
3.5 Evaluation for identification of posterior circulation ischemic stroke
Following the predefined statistical analysis plan, ROC analysis was performed to assess the discriminative performance of: (1) vessel wall thickness alone, and (2) vessel wall thickness combined with clinical features (including diabetes mellitus and stroke history) for post-cardiac surgery stroke (Figure 6). The AUC for identifying posterior circulation ischemic stroke based on vessel wall thickness was 0.806 (sensitivity 74.50%, specificity 75.90%). The combined model showed improved discriminative ability with an AUC of 0.841 (sensitivity 68.60%, specificity 88.90%) (Table 4). As shown in Figure 4, the Hosmer-Lemeshow test indicated good model calibration (χ2 = 12.347, p = 0.136). These findings support the potential clinical utility of this combined approach for stroke risk stratification, though external validation is warranted to confirm generalizability.
Figure 6

Basilar atherosclerosis assessment by ROCs.
Table 4
| Variable | AUC | 95% CI | p | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|---|---|---|
| Wall thickness | 0.806 | 0.766–0.917 | < 0.001 | 74.50 | 75.90 | 0.504 |
| Collaborative forecasting | 0.841 | 0.722–0.891 | < 0.001 | 68.60 | 88.90 | 0.575 |
Association between wall thickness measurements and basilar artery atherosclerosis severity in stroke patients.
Collaborative forecasting: basilar artery wall thickness, history of stroke and history of diabetes were combined for analysis.
4 Discussion
HR-VWI, as a non-invasive magnetic resonance examination, has the advantage of high soft tissue resolution. Previous studies have found that HR-VWI has good repeatability in measuring BA wall thickness in healthy individuals, and the intra-class correlation coefficient range is 0.83–0.94, meaning it can meet the requirements for qualitative and quantitative assessment of intracranial vascular wall lesions (14, 19). The application of HR-VWI can improve the identification of basilar artery anomalies (20). Based on HR-VWI imaging, in the comparative analysis between the high-risk factor and stroke groups in this study, it was found that the lumen wall thickness in the stroke group was greater than that in the high-risk factor group. Additionally, there were statistically significant differences in the history of hypertension, diabetes, stroke, coronary heart disease, and hyperlipidemia in the stroke group (p < 0.05). This may be due to factors such as hypertension and hyperlipidemia that can induce dysregulation of lipid metabolism and inflammatory response in the body (6). According to the injury response hypothesis of the early onset of atherosclerosis, the essence of atherosclerosis emphasizes endothelial dysfunction, which is characterized by the reduced bioavailability of nitric oxide by vascular endothelium and the retention of cholesterol-carrying LDL-C under the combined action of these factors, resulting in the accumulation of extracellular matrix and endothelial activation. The above pathophysiological mechanisms further promote the development of atherosclerosis (21, 22). Cardiovascular risk factors affect specific segments of intracranial vessels in high-resolution vessel wall imaging (23). Previous studies of diabetic patients found that abnormal blood sugar levels can lead to thickening of the carotid intima (24). In experiments of mice fed a high-fructose, high-fat diet, it was observed that a high-fat diet resulted in an increase in the thickness of the BA wall of mice and that the leptin level increased with the increase in wall thickness (22). Since leptin is associated with hyperinsulinemia and glucose intolerance and has atherogenic effects (25), it is speculated that abnormal blood glucose in the intracranial arteries is also associated with lumen wall thickening. In our stroke group, the average thickness of the lumen wall was greater than that in the control and high-risk factor groups, and the proportion of diabetes patients was also higher than that in the high-risk factor group. These data indicate that the thickening of the lumen wall and the development of diabetes will increase the risk of stroke, but it cannot be confirmed that the increase in lumen wall thickness directly relates to blood glucose. The relationship between blood glucose and lumen wall thickness needs to be verified by single-factor analysis. There was no statistically significant difference in lumen area between the three groups. This result may be due to the large congenital difference in vessel diameter among different individuals. The narrow diameter and small lumen area do not necessarily suggest the existence of stenosis or atherosclerosis. This study confirms that diabetes mellitus, history of stroke, and vessel wall thickening are independent risk factors for stroke. However, the presence of vascular positive remodeling (characterized by increased wall thickness and vessel area without luminal stenosis) makes relying solely on wall thickness unreliable for risk assessment. Therefore, a multivariable approach incorporating multiple indicators is recommended to improve the accuracy of stroke risk prediction. The combination of these three factors demonstrates superior discriminative power compared to wall thickness alone, underscoring the importance of comprehensive risk stratification and the implementation of primary and secondary prevention strategies in patients with vessel wall thickening.
In addition, the correlation analysis of vascular wall indicators and age in the control group in this study found that lumen wall thickness, vessel area, and lumen wall area were positively correlated with age. This is consistent with the slight increase observed by Cogswell et al. in the scatterplot of BA thickness and age distribution in healthy screening populations. However, whether the increasing trend of intracranial artery wall thickness is an early-stage reaction of atherosclerotic changes or a physiological change during aging is still unclear and needs to be further clarified by screening and longitudinal follow-up of healthy people. The thickness and area of the BA wall in the high-risk factor and stroke groups were greater than those in the control group, and there was also a statistical difference in wall thickness between the high-risk factor and stroke groups. It was confirmed from imaging that atherosclerosis is a continuous development process. As the thickness of the artery wall increases, atherosclerosis changes from simple vessel wall thickening to plaque formation, which increases the risk of stroke. In non-atherosclerotic diseases, vascular reactivity has been demonstrated to decrease with thickening of the intracranial artery walls (14). The increase in vascular area in the stroke group compared to the control group may reflect positive remodeling of the BA wall under the combined effects of high-risk atherosclerotic factors, leading to an overall expansion of the vessel wall area. This expansion correlates with arterial wall thickness.
Several important limitations of this study must be acknowledged. First, as a single-center retrospective study, certain clinically relevant atherosclerosis risk factors, including body mass index and dietary patterns, were not systematically collected or analyzed. Furthermore, the absence of longitudinal follow-up data limits our ability to assess the predictive value of vascular wall thickening over time—a critical consideration for future research. Second, the control group comprised only 36 participants, raising concerns about selection bias and potentially limiting the generalizability of our findings to healthy populations. Third, we did not account for disease duration or severity stratification among cases, which precludes analysis of how chronicity or metabolic control of conditions such as hypertension and diabetes may influence vascular remodeling. Fourth, the logistic regression analysis did not adjust for potential confounders including age, sex, hypertension, and cholesterol levels, which may affect the interpretation of identified risk factors. Fifth, while we focused on early subclinical atherosclerosis through wall thickness measurements, the lack of systematic plaque characterization (presence/absence, morphology, and composition) prevents direct assessment of the relationship between pre-plaque changes and established atherosclerotic lesions. Sixth, our study did not distinguish degrees of wall thickening, instead relying on binary classification, which may overlook clinically meaningful variations in vascular pathology. Finally, technical constraints must be considered: accurate wall thickness measurement requires the vessel wall to be at least twice the voxel size (26). Given the small diameter of the basilar artery, measurement precision may be compromised, potentially obscuring subtle pathological changes. Advances in high-resolution MRI technology are needed to improve spatial resolution in future studies. We further recognize that our imaging-based approach cannot determine the underlying etiology of wall thickening—whether inflammatory, lipid-driven, or hypertrophic—without histopathological correlation. This represents a key limitation in interpreting the biological significance of our findings.
In conclusion, the BA wall thickness is greater in people at high risk of atherosclerosis than in normal healthy people. BA wall thickening combined with atherosclerosis risk factors is more likely to lead to atherosclerotic-related stroke. Therefore, at the level of subclinical atherosclerosis, the graded management of lumen wall thickness in high-risk populations before plaque formation can prevent intracranial plaque formation to a certain extent to achieve the purpose of health maintenance.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Yongchuan Hospital of Chongqing Medical University; Yongchuan Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZZ: Conceptualization, Data curation, Investigation, Writing – original draft, Methodology, Writing – review & editing. LY: Conceptualization, Data curation, Investigation, Writing – original draft, Methodology, Writing – review & editing. JZ: Data curation, Writing – original draft, Writing – review & editing. XH: Methodology, Writing – review & editing. MY: Data curation, Writing – original draft. BX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Chongqing Yongchuan District Natural Science Fund, grant number 2022yc-jckx20019. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Acknowledgments
The authors thank all participating patients for their willingness to contribute to medical research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kang HG Lee CH Shin BS Chung GH Kwak HS . Characteristics of symptomatic basilar artery stenosis using high-resolution magnetic resonance imaging in ischemic stroke patients. J Atheroscler Thromb. (2021) 28:1063–70. doi: 10.5551/jat.58214
2.
Zhou S Xue J Shan J Hong Y Zhu W Nie Z et al . Gut-Flora-dependent metabolite trimethylamine-N-oxide promotes atherosclerosis-associated inflammation responses by indirect ROS stimulation and signaling involving AMPK and SIRT1. Nutrients. (2022) 14:3338. doi: 10.3390/nu14163338
3.
Lee HN Ryu CW Yun SJ . Vessel-Wall magnetic resonance imaging of intracranial atherosclerotic plaque and ischemic stroke: a systematic review and Meta-analysis. Front Neurol. (2018) 9:1032. doi: 10.3389/fneur.2018.01032
4.
Mushenkova NV Summerhill VI Zhang D Romanenko EB Grechko AV Orekhov AN . Current advances in the diagnostic imaging of atherosclerosis: insights into the pathophysiology of vulnerable plaque. Int J Mol Sci. (2020) 21:2992. doi: 10.3390/ijms21082992
5.
Ahmadi A Argulian E Leipsic J Newby DE Narula J . From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:1608–17. doi: 10.1016/j.jacc.2019.08.012
6.
Koutsaliaris IK Moschonas IC Pechlivani LM Tsouka AN Tselepis AD . Inflammation, oxidative stress, vascular aging and atherosclerotic ischemic stroke. Curr Med Chem. (2022) 29:5496–509. doi: 10.2174/0929867328666210921161711
7.
Burtenshaw D Kitching M Redmond EM Megson IL Cahill PA . Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front Cardiovasc Med. (2019) 6:89. doi: 10.3389/fcvm.2019.00089
8.
Sakamoto A Torii S Jinnouchi H Finn AV Virmani R Kolodgie FD . Pathologic intimal thickening: are we any closer to understand early transitional plaques that lead to symptomatic disease?Atherosclerosis. (2018) 274:227–9. doi: 10.1016/j.atherosclerosis.2018.04.033
9.
Formanowicz D Krawczyk JB . Controlling the thickness of the atherosclerotic plaque by statin medication. PLoS One. (2020) 15:e0239953. doi: 10.1371/journal.pone.0239953
10.
Goldberg IJ Sharma G Fisher EA . Atherosclerosis: making a U turn. Annu Rev Med. (2020) 71:191–201. doi: 10.1146/annurev-med-042418-011108
11.
Wang Y Meng R Liu G Cao C Chen F Jin K et al . Intracranial atherosclerotic disease. Neurobiol Dis. (2019) 124:118–32. doi: 10.1016/j.nbd.2018.11.008
12.
Tschiderer L Klingenschmid G Seekircher L Willeit P . Carotid intima-media thickness predicts carotid plaque development: Meta-analysis of seven studies involving 9341 participants. Eur J Clin Investig. (2020) 50:e13217. doi: 10.1111/eci.13217
13.
Edjlali M Qiao Y Boulouis G Menjot N Saba L Wasserman BA et al . Vessel wall MR imaging for the detection of intracranial inflammatory vasculopathies. Cardiovasc Diagn Ther. (2020) 10:1108–19. doi: 10.21037/cdt-20-324
14.
Yuan S Jordan LC Davis LT Cogswell PM Lee CA Patel NJ et al . A cross-sectional, case-control study of intracranial arterial wall thickness and complete blood count measures in sickle cell disease. Br J Haematol. (2021) 192:769–77. doi: 10.1111/bjh.17262
15.
Ng AC . Posterior circulation Ischaemic stroke. Am J Med Sci. (2022) 363:388–98. doi: 10.1016/j.amjms.2021.10.027
16.
Lindsberg PJ Pekkola J Strbian D Sairanen T Mattle HP Schroth G . Time window for recanalization in basilar artery occlusion: speculative synthesis. Neurology. (2015) 85:1806–15. doi: 10.1212/WNL.0000000000002129
17.
Archer CR Horenstein S . Basilar artery occlusion: clinical and radiological correlation. Stroke. (1977) 8:383–90. doi: 10.1161/01.str.8.3.383
18.
Siow I Tan BYQ Lee KS Ong N Toh E Gopinathan A et al . Bridging thrombolysis versus direct mechanical Thrombectomy in stroke due to basilar artery occlusion. J Stroke. (2022) 24:128–37. doi: 10.5853/jos.2021.02082
19.
Cogswell PM Lants SK Davis LT Donahue MJ . Vessel wall and lumen characteristics with age in healthy participants using 3T intracranial vessel wall magnetic resonance imaging. J Magn Reson Imaging. (2019) 50:1452–60. doi: 10.1002/jmri.26750
20.
Lu T Zou Y Jiang T Yang Y Wu A Chen H et al . Intracranial artery injury in HIV-negative tuberculous meningitis: a high-resolution Vessel Wall imaging study. Clin Neuroradiol. (2020) 30:381–8. doi: 10.1007/s00062-019-00766-4
21.
Kitayama J Kitazono T Ooboshi H Ago T Ohgami T Fujishima M et al . Chronic administration of a tyrosine kinase inhibitor restores functional and morphological changes of the basilar artery during chronic hypertension. J Hypertens. (2002) 20:2205–11. doi: 10.1097/00004872-200211000-00020
22.
Toklu HZ Muller-Delp J Sakaraya Y Oktay S Kirichenko N Matheny M et al . High dietary fructose does not exacerbate the detrimental consequences of high fat diet on basilar artery function. J Physiol Pharmacol. (2016) 67:205–16.
23.
Montes D Vranic J Lim JC Song JW Silverman SB González RG et al . Cardiovascular risk factors affect specific segments of the intracranial vasculature in high-resolution (HR) Vessel Wall imaging (VWI). J Stroke Cerebrovasc Dis. (2021) 30:106026. doi: 10.1016/j.jstrokecerebrovasdis.2021.106026
24.
Guo HJ Li CC Bian XY Hao Q . Correlation study on the relationship between dyslipidemia and carotid intima-media thickness in patients with diabetes mellitus. Pak J Med Sci. (2023) 39:875–9. doi: 10.12669/pjms.39.3.6866
25.
Zusi C Csermely A Rinaldi E Bertoldo K Bonetti S Boselli ML et al . Crosstalk between genetic variability of adiponectin and leptin, glucose-insulin system and subclinical atherosclerosis in patients with newly diagnosed type 2 diabetes. The Verona newly diagnosed type 2 diabetes study 14. Diabetes Obes Metab. (2023) 25:2650–8. doi: 10.1111/dom.15152
26.
van Hespen KM Zwanenburg JJM Harteveld AA Luijten PR Hendrikse J Kuijf HJ . Intracranial Vessel Wall magnetic resonance imaging does not allow for accurate and Precise Wall thickness measurements: an ex vivo study. Stroke. (2019) 50:e283–4. doi: 10.1161/STROKEAHA.119.026497
Summary
Keywords
high-resolution magnetic resonance vascular wall imaging, basilar artery, vessel wall thickness, atherosclerosis, subclinical atherosclerosis
Citation
Zhang Z, Yi L, Zhou J, He X, Yuan M and Xiang B (2025) The association between basilar artery wall thickness and posterior circulation ischemic stroke: insights from high-resolution vascular wall imaging in subclinical atherosclerosis. Front. Neurol. 16:1575681. doi: 10.3389/fneur.2025.1575681
Received
10 March 2025
Accepted
09 May 2025
Published
30 May 2025
Volume
16 - 2025
Edited by
Gador Canton, University of Washington, United States
Reviewed by
Beibei Sun, Shanghai Jiao Tong University, China
Javid Azadbakht, Private Clinic of Tabesh Radiology and Sonography, Iran
Dan Cheng, University of Washington, United States
Updates
Copyright
© 2025 Zhang, Yi, Zhou, He, Yuan and Xiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Xiang, 25286772@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.