- 1Department of Physiology and Pharmacology, Faculty of Pharmacy and Medicine, Sapienza University of Rome, Rome, Italy
- 2Department of Human Physiology, University of Perugia, Perugia, Italy
- 3National Institute for Insurance Against Accidents at Work (INAIL), Rome, Italy
- 4Department of Human, Social, and Health Sciences, University of Cassino, Cassino, Italy
- 5Department of Neuroscience, Università Cattolica del Sacro Cuore, Rome, Italy
Introduction: This review analyses the benefits of focal muscle vibration (FV) in the treatment of spasticity enhancing current understanding and promoting sustained improvements in motor function. Findings could support the selection of optimal FV protocols, guide future research, and provide insights into the mechanisms by which FV may improve motor function in individuals with spasticity.
Methods: A systematic search was conducted using the online databases PubMed, Web of Science, and The Cochrane Library. Including criteria: (a) participants presented with chronic spasticity; (b) the intervention involved the application of localized mechanical vibration; and (c) outcomes included neuromuscular functional parameters. Data extraction was performed independently by four reviewers, using a modified version of the 16-item Downs and Black checklist.
Results: A total of 20 studies were selected, most of which investigated on spasticity following stroke, as well as in conditions such as cerebral palsy, multiple sclerosis, and Minamata syndrome. FV effects were assessed using several methodologies: functional scales, digital analysis and electrophysiological evaluations. After-effects were positive and significant in 19 studies, while one study found non-significant results. In three studies, follow-up durations ranged between 1 and 30 days, and exceeded 1 month in seven. When adequate tests were performed, improvements extended to untreated muscles and involved complex motor behaviors.
Discussion: The after-effects of FV appear to be most relevant and long-lasting when a high-frequency (75–120 Hz), small-amplitude sinusoidal vibrations are repeatedly applied. The observed enduring improvements in complex motor behaviors suggest the involvement of sensory-motor mechanisms. These findings are discussed in the context of previous studies on FV.
Introduction
In recent years, several journals have reported the positive effect of focally administered mechanical vibration on individual muscles (focal vibration, FV) in improving various motor fitness parameters, including strength, readiness, power, and efficiency (1–6) both healthy and sick. Notably, the after-effects demonstrated prolonged, lasting up to several months. Most of the published articles on FV (2, 7–14) attribute the effects to selective, intense, and prolonged activation of the proprioceptive system, particularly neuromuscular spindle receptors, which induces central changes in the motor system (1–6).
As shown in the recent literature review, persistent positive after-effects seem to be preferably elicited by a vibratory stimulation characterized by high-frequency (70–300 Hz), small-amplitude sinusoidal muscle stretch-shortening, which must be repeated for days (4). Regards the functional effects, FV proprioceptive hyperactivation (4, 13–16) likely acts either directly on the muscle control mechanism, inducing a rearrangement of motor control, or by enhancing proprioceptive discriminative ability and refining the establishment of the spatial reference frame (13, 14, 17–19). Thus, intense and prolonged proprioceptive activation could induce persistent motor improvements, even for complex movements, in the absence of specific motor training (4, 20).
Considering these mechanisms, it is conceivable that FV may enhance motor performance in healthy individuals and support the recovery of mobility in orthopedic or neurological conditions, characterized by motor weakness or paresis or flaccidity. Interestingly, there is also consistent evidence supporting the positive effects of FV in patients experiencing undesired muscle hyperactivity linked to heightened proprioceptive reflex activity, as observed in muscle spasticity (21–40). Spastic hypertonia is a common complication following central nervous system injury, affecting 30–40% of individuals with impaired limb function after stroke (41–44). Certainly, the most prevalent cause of such motor unit hyperactivity is the spasticity following a stroke, but childhood cerebral palsy might also play a role. Spasticity severely limits movements, such as walking, and activities of daily living. Therefore, a systematic review of the literature is essential to evaluate for consistency of the positive effects of FV in the management of spasticity. Although the use of FV in spasticity is endorsed by the American Academy of Physical Medicine and Rehabilitation and the American Society of Neurorehabilitation, appropriate best-practice protocols are not yet well defined (45). Although some reviews have reported the positive effects of FV in the treatment of spasticity, they have not specifically focused on the characteristics of the FV protocols that are most effective in producing substantial and long-lasting positive improvements (46–48). Therefore, the present review aims to: (a) compile the literature in which FV interventions have been administered to patients with muscle spasticity; (b) report FV-induced changes in motor function; (c) identify the most effective intervention parameters in relation to both the extent and duration of functional performance; and (d) assess the compatibility of current findings with a recently proposed theory (4). Collectively, these objectives are intended to advance the knowledge on rehabilitation treatments in pathologies characterized by spasticity.
Materials and methods
The Preferred Reporting Items for Systematic reviews (PRISMA) guidelines were followed in this review (49).
Data sources
A systematic literature search was conducted from January 1985 to March 2024 in the online databases PubMed, Web of Science, and The Cochrane Library. Medical Subject Headings (MeSH) of the United States National Library of Medicine (NLM) and search terms were included in Boolean search syntax: (vibration) AND (stroke); (vibration) AND (spasticity); (focal vibration) AND (muscle), (segmental vibration) AND (muscle), (local vibration) AND (muscle). Searching was limited to original studies in English language, human species, and full text availability. Other studies were identified through a manual search for potential articles based on the authors’ knowledge.
Selection criteria
Two reviewers (LF and GMF) independently extracted data from each study using a structured script. The script included study design, sample characteristics (e.g., sample size and gender), experimental and control group characteristics, outcome measures, and timing of results. Inclusion criteria were decided by the consensus statements between the two reviewers. In cases of disagreement, others reviewers (AR and VEP) were consulted to resolve discrepancies. Inclusions criteria were: (a) participants showed a stable condition of muscle spasticity; (b) the intervention treatment involved localized mechanical vibration; (c) outcomes assessed neuromuscular parameters related to conditional abilities.
Study eligibility
Studies were excluded if treatment was administered to the whole body (i.e., “no focal”); did not present an original investigation (reviews or proceedings); were not published in English language.
Assessment of methodological quality
The study quality of each publication was evaluated, by LF, GMF, EM, AR, and VEP, using a 16-item checklist (50). The quality scores were classified as “low” for scores < 50\% or equal; “good” for scores between 51 and 75%; and “excellent” if the score was more than 75%.
According to the modified version of the 16-item Downs and Black checklist, the average quality score was 89.8%. All studies had quality score > 75% (excellent) ranging between 81.25 and 93.75. The inter-rater reliability analysis showed a good coherence between the observers, being 0.92 the kappa value.
Results
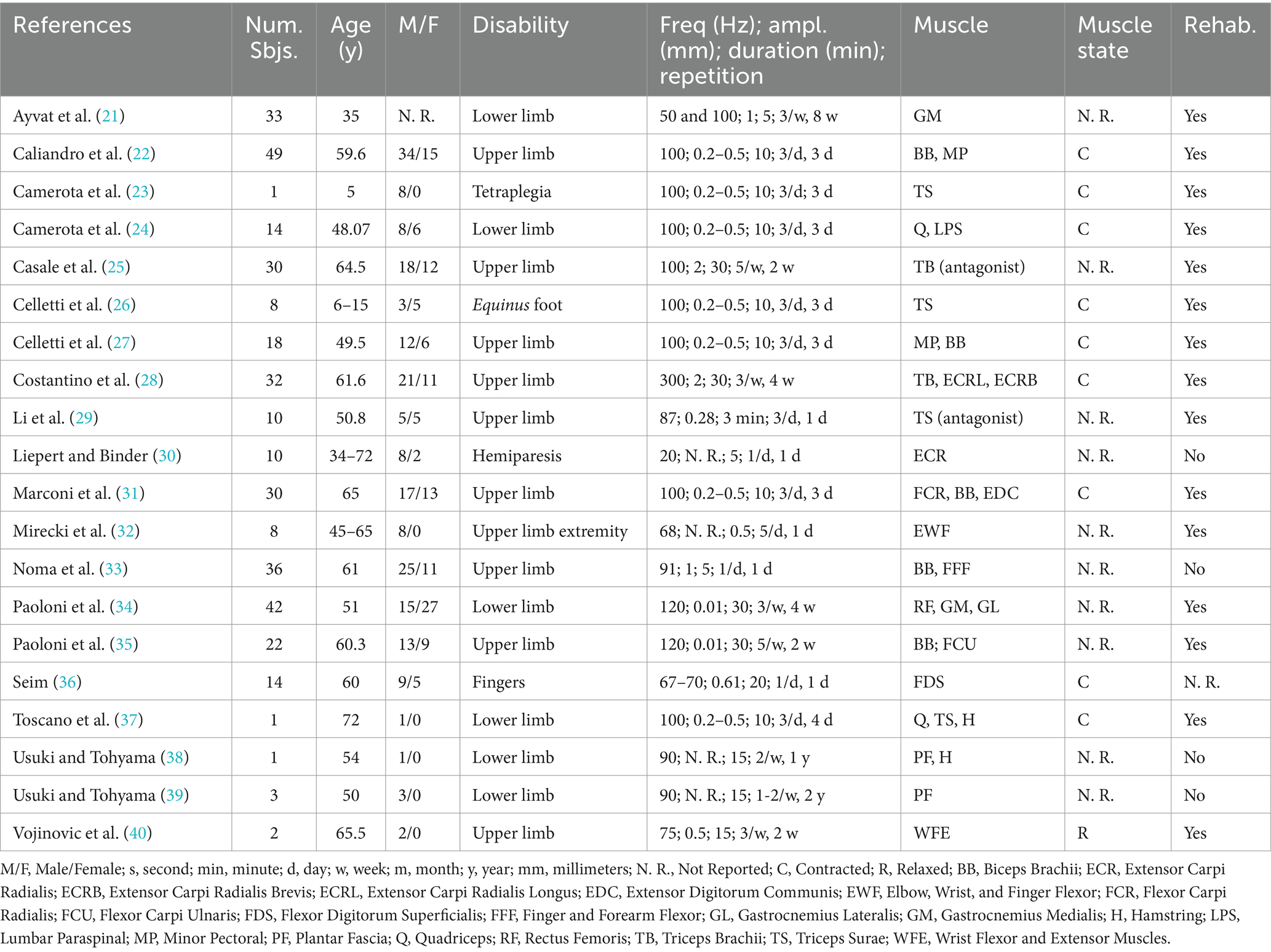
Analysis of the literature showed a positive influence of FV on function and motor abilities in neurological diseases characterized by spastic chronic hypertonia. In the present review were selected 20 studies (Figure 1) in which FV was tested on patients with several pathologies (21–40). Eleven articles (22, 25, 27–31, 33, 35–37), out of 20, analysed the effects of FV after stroke, in chronic conditions. In two other studies (23, 26), the participants were children (aged 5–15 years) with symptoms of cerebral palsy. The remaining studies involved motor disabilities associated with multiple sclerosis (21, 24, 34), spinal cord injury (32, 40) and fetal Minamata disease (38, 39). In Table 1 protocols, outcomes, and follow-up are reported.
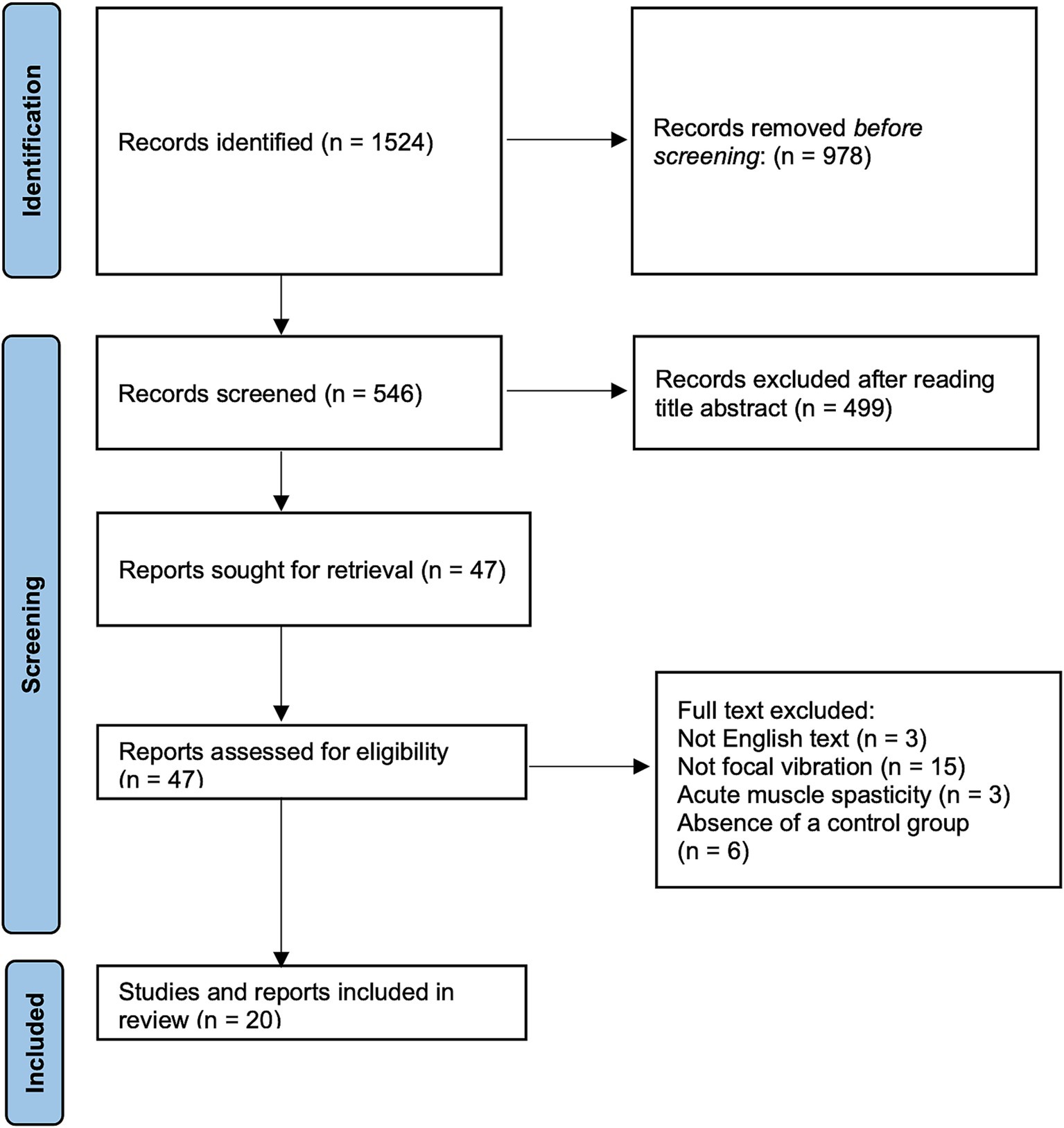
Figure 1. PRISMA workflow of included and excluded articles. PRISMA flow diagram outlining identified studies and exclusion criteria at every level of screening.
FV protocols
Two main frequencies ranges of FV can be identified. The first group applied to frequencies between 20 and 70 Hz (21, 30, 32, 36, 38), while the second group used frequencies between 75 and 120 Hz (21–29, 31, 33–35, 37, 39, 40). Three research groups applied only one vibratory session (30, 32, 33), whereas all others repeated the sessions over the course of the week(s). As pointed out in previous reviews (2–4), when vibratory sessions were repeated, two patterns emerged. The first pattern was characterized by FV single sessions distributed across 2–5 days per week (21, 25, 28, 29, 34, 35, 38–40). The second pattern followed a more intense approach, with 3 consecutive days similarly of stimulation, each consisting of 3 sessions per day. Each session lasted 10 min and was separated by 1–2 min of rest (2–4). As shown in Table 1, when amplitude was reported, FV typically ranged between 0.01 and 0.5 mm (22–24, 26, 27, 29, 31, 34–36, 39). In four studies, peak-to-peak amplitude reached up to ≥1 mm (21, 25, 28, 33), while three papers did not report this parameter (30, 38, 39).
Outcomes
Most of the papers listed in Table 1 used the Modified Ashworth Scale (MAS) (21, 22, 25–28, 31–34, 36, 38–40) and Range of Motion (ROM) as the most common tests (25, 38–40). In addition, also other functional analogue scales (21, 22, 27, 28, 30–32, 34–38, 40), digital analyses (21, 23, 24, 37) and examining electrophysiological correlates of motor effects were adopted to assess changes in complex movements and multi-joint coordination (31, 33, 37).
Regarding spasticity, FV-treated patients, although suffering from different clinical conditions (stroke, cerebral palsy, multiple sclerosis, and Minamata syndrome), achieved significant muscle relaxation, demonstrated by positive improvements in passive joint movements tested by ROM and/or MAS tests (21, 22, 25–28, 31–34, 36, 38–40).
Along with muscle relaxation, a general improvement in voluntary motor activity was reported in almost all studies not only to the treated muscle, but the effects were extended to other muscle districts. In addition, FV treatment resulted in improved movement in several motor tasks of varying degrees of complexity (21–24, 27, 28, 30–32, 36). The effects manifested very early, soon after the end of treatment (21, 23, 28–33, 35, 38, 40).
All but one of the listed authors reported statistically significant improvement in spasticity and voluntary movements (36). However, important differences emerge from the different duration of follow-up. Eight studies tested the effects of FV only immediately after the end of treatment (21, 28–30, 32, 33, 36, 40). This extremely short observation period does not rule out possible persistence of effects, but it does not document it. On the other hand, some studies have reported moderately long follow-up (≥24 h, <1 month) (22, 24, 25, 31, 37–39) and other longer observation intervals, ≥ 1 month (22–24, 26, 27, 34).
Another difference concerns the time interval between the end of FV and the first assessment test. Excluding studies in which follow-up was limited to the end of FV, two studies tested results within 24 h after the end of FV and repeated the test after 2 and 12 weeks (26, 31).
Discussion
The main findings suggested by the present review are: (a) FV stimulus can improve motor function in patients with muscle spasticity; (b) in several studies, the effects of FV are not only limited to reducing spasticity but also improving motor coordination; and (c) there are important differences among the different studies in terms of the protocols applied, observation period, and positive sequelae. It should be noted that the works listed in this review showed heterogeneity in terms of both the level of functional impairment, due to different pathologies, and the adopted tests.
Interestingly, following FV treatment, patients showed both muscle relaxation and significant improvement in motor coordination when assessed with appropriate tests. A reasonable explanation might be that the reduction of muscle spasticity in the treated muscle, by itself, eliminated important limitations, allowing the adoption of more physiological motor strategies. However, it has been observed that common rehabilitative interventions, aimed at reducing spasticity, do not result in an immediate and proportional improvement of motor gesture (51). This suggests that the early recovery of coordination in complex motor gesture achieved with FV (21–24, 27, 28, 30–32, 36) should be attributed to additional mechanisms elicited by the proprioceptive stimulation, such as those suggested for motor deficit in the presence of reduced mobility and reflexes (4).
Possible mechanism for explaining the FV effects
Repetitive FV stimulations can be considered as sensorial stimulus that can improve both proprioceptive processing (4, 5) and perception of the spatial reference frames, which can promote both refinement of already known motor behaviors and motor learning (1–4, 15). Regarding the effects of FV on motor adjustments, several authors have suggested that spindle stimulation could improve joint stabilization by acting on the control of joint stiffness (2–4, 7–14, 19). Modulation of joint impedance is a parameter that may result in changes in fatigue, speed, strength, motor task accuracy, and body balance (52, 53). Neurophysiological studies on FV, adopting transcranial magnetic stimulation, seem to propose a convincing background showing a rebalancing of agonist–antagonist activity in the primary motor cortex that can modulate joint impedance (13, 31).
Another interesting feature is the duration of FV effects as also pointed out in previous reviews (2–4). In several articles, reviewed in this study, tests were performed only and immediately at the end of FV stimulation (21, 28–30, 32, 33, 36, 40). Although persistence of the immediately detected significant improvements cannot be ruled out, however, it is not supported by the data. Consequently, this group of study articles cannot offer any insight into this important aspect. However, the remaining papers report long-lasting after-effects that persist, without showing any decay, for weeks and months, suggesting a possible and interesting application role.
Cortical and spinal plastic changes induced by FV
FV motor positive effects in case of hypotonia can be explained by considering that intense and prolonged proprioceptive activation can potentiate the sensorimotor circuitry excitability through long-term synaptic effects, such as LTP, thus restoring responsiveness of neurons and improving motor performance in the centers of movement planning and execution (3, 4, 13, 15, 20, 31). At the same time, the underlying mechanisms by which FV reduces muscle spasticity remain insufficiently understood. How can same protocol (FV) have positive effects in these opposite conditions?
Marconi and co-workers have shown a long-term increase in FV-induced intracortical inhibition, suggesting an agonist–antagonist rebalance, leading to a remodulation of joint mechanical impedance (13, 31). Such intracortical inhibition correlates with reduced unwanted contractions and improved motor performance in healthy subjects (54–56). However, spasticity is known to be related to hyperexcitability of proprioceptive reflexes. How can the further enhancement of proprioceptive signals by FV lead to both a reduction in muscle spasticity and improvement in motor performance? In the absence of direct experimental evidence, we can only speculate that in the presence of a disinhibition of proprioceptive reflexes due to an imbalance of supraspinal excitatory/inhibitory descending flow, FV could act on this disturbed balance (57). Experimental data report a transient post-FV depression of the stretch reflex, elicited at the spinal cord level by presynaptic or recurrent inhibition (28, 33, 37). These effects occur at the spinal level and, although transient, typically last on the order of minutes, with synaptic control mechanisms appearing to return to baseline within approximately 60 min. Such spinal mechanisms alone are unlikely to account for after-effects that persists for weeks or months (4). However, they could be the trigger for subsequent and persistent cortical rebalancing (31, 57), eventually by interfering with the development of a delayed spasticity (58).
Comparison between FV protocols
To foster future lines of research and application experiences, it is important to define the determinants of FV and their optimal value related to motor changes and enduring.
FV is commonly applied using sinusoidal muscle shortening-elongation sequences with a small amplitude, it is known to be selectively appropriate for activating proprioceptive muscle afferents (59–61). Likewise, as pointed out in the results, the selected studies showed differences in mechanical stimulation frequency and repetition. Regarding applied stimulation frequencies, two groups of protocols can be identified, one based on 75–120 Hz (21–29, 31, 33–35, 37, 39, 40), while the others adopted stimuli at 20–70 Hz (21, 30, 32, 36, 37). Most of the studies (17 of 20) chose to repeat the application of FV. The repetition of FV, as observed in other reviews, has two different patterns (2–4). A first scheme followed a homogeneous protocol, in which FV applications were performed for 3 consecutive days, with 3 consecutive applications each day, separated by two short rest breaks. A second scheme showed a more distributed and uneven sequence of treatments (i.e., single applications of FV during some days and over one or more weeks).
It should be noted that sustained and documented persistence of after-effects, is associated with the combination of a stimulation frequency of 75–120 Hz with repeated applications. This observation suggests that a repeated stimulation protocol based on a small-amplitude, sinusoidal frequency of 75–120 Hz may contribute to sustained improvements in motor control by inducing central plastic rearrangement (3, 4, 13–15, 20, 62). High-frequency, small-amplitude sinusoidal muscle stretch can drive afferent discharge to various muscle spindles at a correspondingly high rate (59–62). Furthermore, such a low amplitude allows avoidance of the tonic vibration reflex, which could alter the function of central proprioceptive circuits (63). The peak-to-peak shift (0.2–0.5 mm) is in the order of magnitude of the above studies. On the other hand, the effectiveness of much lower displacements, ≈0.01 mm, applied by some studies (34, 35), could be explained by considering the state of muscle spasticity. Isometric muscle contraction facilitates the transmission of mechanical energy and promotes fusimotor activation, that amplifies spindle Ia sensitivity (60, 61, 64). In spasticity, a state of overamplification of the sensitivity of Ia afferents is well known, so that even an extremely low signal could be detected by the neuromuscular spindles, eliciting a high-frequency afferent discharge. Neuronal high frequency activation is known to promote plastic changes in the central nervous system, such as long-term synaptic potentiation (14, 15, 20, 65). In addition, stimulus repetition is a well-known protocol to promote consolidation of plastic rearrangement (65). These observations are supported by a specific experimental study (14) and in tune with previous reviews in which the relationship between FV protocols and beneficial after-effects in healthy individuals and in patients with weak and inadequate muscle contraction has been described (3, 4).
Conclusion
The methods currently used for spasticity are based on continuous rehabilitative exercise, drug therapy or, sometimes, surgery as last resort (66–68). Present review indicates that FV may be an efficacious additional tool. A highly specific and repeated proprioceptive training based on a sinusoidal waveform in a bounded frequency range, appears to be specifically appropriate to an optimization of motor planning and execution, possibly improving compensatory strategies. Moreover, repeated and preferentially concentrated FV treatment emerge as a more effective protocol in relation to the quality of the effects and their persistence in the follow-up. The above-discussed mechanisms might explain the wide variety of diseases in which FV has induced positive outcomes [see also reviews (2–4)].
Appropriate FV protocols can be used in combination with traditional therapies, or, as suggested in some studies, in patients who exhibit limited or no response to pharmacological treatment (24). Several studies have combined FV with conventional rehabilitation approaches (see Table 1), reporting a booster effect from the combination. However, the current evidence is insufficient to establish a standardized best practice. Several studies have combined FV with traditional rehabilitation (see Table 1), showing a booster effect from the combination. However, the current evidence is insufficient to define a best practice. This represents an important area for further investigation, as future data may support the development of new and more effective interventions.
Limits
While the reported data provide a more detailed analysis of the effects of FV on spastic symptoms, larger and multicentre studies are warranted to confirm these findings. Moreover, a systematic analysis of these potential interaction between FV and traditional rehabilitation opportune in identifying the best approach to restoring motor function across different spasticity conditions and in different populations.
Author contributions
LF: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. VP: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. EM: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. AR: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. GF: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This paper was financed from Italian National Institute for Insurance Against Accidents at Work (INAIL) with project BRIC22 ID 12 “No Risk.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FC declared a shared affiliation with the author LF to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FV, Focal vibration; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; NLM, United States National Library of Medicine; MeSH, Medical Subject Headings; MAS, Modified Ashworth Scale; ROM, Range of Motion.
References
1. Alghadir, AH, Anwer, S, Zafar, H, and Iqbal, ZA. Effect of localised vibration on muscle strength in healthy adults: a systematic review. Physiother. (2018) 104:18–24. doi: 10.1016/j.physio.2017.06.006
2. Fattorini, L, Rodio, A, Pettorossi, VE, and Filippi, GM. Is the focal muscle vibration an effective motor conditioning intervention? A systematic review. Jd Funct Morphol Kinesiol. (2021) 6:39. doi: 10.3390/jfmk6020039
3. Fattorini, L, Rodio, A, Filippi, GM, and Pettorossi, VE. Effectiveness of focal muscle vibration in the recovery of Neuromotor Hypofunction: a systematic review. J. Funct. Morphol. Kinesiol. (2023) 8:103. doi: 10.3390/jfmk8030103
4. Filippi, GM, Rodio, A, Fattorini, L, Faralli, M, Ricci, G, and Pettorossi, VE. Plastic changes induced by muscle focal vibration: a possible mechanism for long-term motor improvements. Front Neurosci. (2023) 17:1112232. doi: 10.3389/fnins.2023.1112232
5. Murillo, N, Valls-Sole, J, Vidal, J, Opisso, E, Medina, J, and Kumru, H. Focal vibration in neurorehabilitation. Eur J Phys Rehabil Med. (2014) 50:231–42.
6. Souron, R, Besson, T, Millet, GY, and Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur J Appl Physiol. (2017) 117:1939–64. doi: 10.1007/s00421-017-3688-8
7. Brunetti, O, Filippi, GM, Lorenzini, M, Liti, A, Panichi, R, Roscini, M, et al. Improvement of posture stability by vibratory stimulation following anterior cruciate ligament reconstruction. Knee Surgery Sport Traumatol. Arthrosc. (2006) 14:1180–7. doi: 10.1007/s00167-006-0101-2
8. Brunetti, O, Botti, FM, Roscini, M, Brunetti, A, Panichi, R, Filippi, GM, et al. Focal vibration of quadriceps muscle enhances leg power and decreases knee joint laxity in female volleyball players. J. Sports Med. Phys. Fitness. (2012) 52:596–605.
9. Brunetti, O, Botti, FM, Brunetti, A, Biscarini, A, Scarponi, AM, Filippi, GM, et al. Effects of focal vibration on bone mineral density and motor performance of postmenopausal osteoporotic women. J Sports Med Phys Fitness. (2015) 55:118–27.
10. Contemori, S, Dieni, CV, Sullivan, JA, Ferraresi, A, Occhigrossi, C, Calabrese, F, et al. Sensory inflow manipulation induces learning-like phenomena in motor behavior. Eur J Appl Physiol. (2020) 120:811–28. doi: 10.1007/s00421-020-04320-w
11. Fattorini, L, Ferraresi, A, Rodio, A, Azzena, GB, and Filippi, GM. Motor performance changes induced by muscle vibration. Eur J Appl Physiol. (2006) 98:79–87. doi: 10.1007/s00421-006-0250-5
12. Filippi, GM, Brunetti, O, Botti, FM, Panichi, R, Roscini, M, Camerota, F, et al. Improvement of stance control and muscle performance induced by focal muscle vibration in young-elderly women: a randomized controlled trial. Arch Phys Med Rehabil. (2009) 90:2019–25. doi: 10.1016/j.apmr.2009.08.139
13. Marconi, B, Filippi, GM, Koch, G, Pecchioli, C, Salerno, S, Don, R, et al. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci. (2008) 275:51–9. doi: 10.1016/j.jns.2008.07.025
14. Pettorossi, VE, Panichi, R, Botti, FM, Biscarini, A, Filippi, GM, and Schieppati, M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin Neurophysiol. (2015) 126:1886–900. doi: 10.1016/j.clinph.2015.02.057
15. Beste, C, and Dinse, HR. Learning without training. Curr Biol. (2013) 23:R489–99. doi: 10.1016/j.cub.2013.04.044
16. Rosenkranz, K, and Rothwell, JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci. (2012) 32:9000–6. doi: 10.1523/JNEUROSCI.0120-12.2012
17. Karnath, HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. (1994) 117:1001–12. doi: 10.1093/brain/117.5.1001
18. Kerkhoff, G. Modulation and rehabilitation of spatial neglect by sensory stimulation. Prog Brain Res. (2003) 142:257–71. doi: 10.1016/S0079-6123(03)42018-9
19. Pettorossi, VE, and Schieppati, M. Neck proprioception shapes body orientation and perception of motion. Front Hum Neurosci. (2014) 8:895. doi: 10.3389/fnhum.2014.00895
20. Aman, JE, Elangovan, N, Yeh, IL, and Konczak, J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. (2015) 8:1075. doi: 10.3389/fnhum.2014.01075
21. Ayvat, F, Özçakar, L, Ayvat, E, Aksu Yıldırım, S, and Kılınç, M. Effects of low vs. high frequency local vibration on mild-moderate muscle spasticity: Ultrasonographical and functional evaluation in patients with multiple sclerosis. Mult Scler Relat Disord. (2021) 51:102930. doi: 10.1016/j.msard.2021.102930
22. Caliandro, P, Celletti, C, Padua, L, Minciotti, I, Russo, G, Granata, G, et al. Focal muscle vibration in the treatment of upper limb spasticity: a pilot randomized controlled trial in patients with chronic stroke. Arch Phys Med Rehabil. (2012) 93:1656–61. doi: 10.1016/j.apmr.2012.04.002
23. Camerota, F, Galli, M, Celletti, C, Vimercati, S, Cimolin, V, Tenore, N, et al. Quantitative effects of repeated muscle vibrations on gait pattern in a 5-year-old child with cerebral palsy. Case Rep Med. (2011) 2011:359126. doi: 10.1155/2011/359126
24. Camerota, F, Celletti, C, Di Sipio, E, De Fino, C, Simbolotti, C, Germanotta, M, et al. Focal muscle vibration, an effective rehabilitative approach in severe gait impairment due to multiple sclerosis. J Neurol Sci. (2017) 372:33–9. doi: 10.1016/j.jns.2016.11.025
25. Casale, R, Damiani, C, Maestri, R, Fundarò, C, Chimento, P, and Foti, C. Localized 100 Hz vibration improves function and reduces upper limb spasticity: a double-blind controlled study. Eur J Phys Rehabil Med. (2014) 50:495–504.
26. Celletti, C, and Camerota, F. Preliminary evidence of focal muscle vibration effects on spasticity due to cerebral palsy in a small sample of Italian children. Clin Ter. (2011) 162:e125–8.
27. Celletti, C, Sinibaldi, E, Pierelli, F, Monari, G, and Camerota, F. Focal muscle vibration and progressive modular rebalancing with neurokinetic facilitations in post- stroke recovery of upper limb. Clin Ter. (2017) 168:e33–6. doi: 10.7417/CT.2017.1979
28. Costantino, C, Galuppo, L, and Romiti, D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: a randomized controlled trial. Eur J Phys Rehabil Med. (2017) 53:32–40. doi: 10.23736/S1973-9087.16.04211-8
29. Li, W, Luo, F, Xu, Q, Liu, A, Mo, L, Li, C, et al. Brain oscillatory activity correlates with the relief of post-stroke spasticity following focal vibration. J Integr Neurosci. (2022) 21:96. doi: 10.31083/j.jin2103096
30. Liepert, J, and Binder, C. Vibration-induced effects in stroke patients with spastic hemiparesis - a pilot study. Restor Neurol Neurosci. (2010) 28:729–35. doi: 10.3233/RNN-2010-0541
31. Marconi, B, Filippi, GM, Koch, G, Giacobbe, V, Pecchioli, C, Versace, V, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. (2011) 25:48–60. doi: 10.1177/1545968310376757
32. Mirecki, MR, Callahan, S, Condon, KM, and Field-Fote, EC. Acceptability and impact on spasticity of a single session of upper extremity vibration in individuals with tetraplegia. Spinal Cord Ser Cases. (2022) 8:483. doi: 10.1038/s41394-022-00483-0
33. Noma, T, Matsumoto, S, Shimodozono, M, Etoh, S, and Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: a proof-of-principle study. J Rehabil Med. (2012) 44:325–30. doi: 10.2340/16501977-0946
34. Paoloni, M, Giovannelli, M, Mangone, M, Leonardi, L, Tavernese, E, di Pangrazio, E, et al. Does giving segmental muscle vibration alter the response to botulinum toxin injections in the treatment of spasticity in people with multiple sclerosis? A single-blind randomized controlled trial. Clin Rehabil. (2013) 27:803–12. doi: 10.1177/0269215513480956
35. Paoloni, M, Tavernese, E, Fini, M, and Sale, P. Segmental muscle vibration modifies muscle activation during reaching in chronic stroke: a pilot study. Neuro Rehabilitation. (2014) 35:405–14. doi: 10.3233/NRE-141131
36. Seim, C, Chen, B, Han, C, Vacek, D, Wu, LS, Lansberg, M, et al. Relief of post-stroke spasticity with acute vibrotactile stimulation: controlled crossover study of muscle and skin stimulus methods. Front Hum Neurosci. (2023) 17:1206027. doi: 10.3389/fnhum.2023.1206027
37. Toscano, M, Ricci, M, Celletti, C, Paoloni, M, Ruggiero, M, Viganò, A, et al. Motor recovery after stroke: from a Vespa scooter ride over the Roman Sampietrini to focal muscle vibration (fMV) treatment. A 99mTc-HMPAO SPECT and neurophysiological case study. Front Neurol. (2020) 11:567833. doi: 10.3389/fneur.2020.567833
38. Usuki, F, and Tohyama, S. Vibration therapy of the plantar fascia improves spasticity of the lower limbs of a patient with fetal-type Minamata disease in the chronic stage. BMJ Case Rep. Published online 2011. (2011) 2011:bcr0820114695. doi: 10.1136/bcr.08.2011.4695
39. Usuki, F, and Tohyama, S. Three case reports of successful vibration therapy of the plantar fascia for spasticity due to cerebral palsy-like syndrome, Fetal-type Minamata disease. Medicine. (2016) 95:e3385. doi: 10.1097/MD.0000000000003385
40. Vojinovic, TJ, Linley, E, Zivanovic, A, and Rui Loureiro, CV. Effects of focal vibration and robotic assistive therapy on upper limb spasticity in incomplete spinal cord injury. IEEE Int. Conf. Rehab. Robotics. (2019) 2019:542–7. doi: 10.1109/ICORR.2019.8779566
41. Dajpratham, P, Kuptniratsaikul, V, Kovindha, A, Kuptniratsaikul, PSA, and Dejnuntarat, K. Prevalence and management of poststroke spasticity in Thai stroke patients: a multicenter study. J Med Assoc Thail. (2009) 92:1354–60.
42. Watkins, CL, Leathley, MJ, Gregson, JM, Moore, AP, Smith, TL, and Sharma, AK. Prevalence of spasticity post stroke. Clin Rehabil. (2002) 16:515–22. doi: 10.1191/0269215502cr512oa
43. Mozaffarian, D, Benjamin, EJ, Go, AS, Arnett, DK, Blaha, MJ, Cushman, M, et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. (2016) 133:e38–e360. doi: 10.1161/CIR.0000000000000350
44. Ovbiagele, B, Goldstein, LB, Higashida, RT, Howard, VJ, Johnston, SC, Khavjou, OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American heart association and American stroke association. Stroke. (2013) 44:2361–75. doi: 10.1161/STR.0b013e31829734f2
45. Winstein, CJ, Stein, J, Arena, R, Bates, B, Cherney, LR, Cramer, SC, et al. Guidelines for adult stroke rehabilitation and recovery. Stroke. (2016) 47:820–1. doi: 10.1161/STR.0000000000000098
46. Alashram, AR, Padua, E, Romagnoli, C, and Annino, G. Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: a systematic review. Neuro Rehabilitation. (2019) 45:471–81. doi: 10.3233/NRE-192863
47. Avvantaggiato, C, Casale, R, Cinone, N, Facciorusso, S, Turitto, A, Stuppiello, L, et al. Localized muscle vibration in the treatment of motor impairment and spasticity in post-stroke patients: a systematic review. Eur J Phys Rehabil Med. (2021) 57:44–60. doi: 10.23736/S1973-9087.20.06390-X
48. Ritzmann, R, Stark, C, and Krause, A. Vibration therapy in patients with cerebral palsy: a systematic review. Neuropsychiatr Dis Treat. (2018) 14:1607–25. doi: 10.2147/NDT.S152543
49. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev Esp Nutr Humana y Diet. (2015) 21:i4086. doi: 10.1136/bmj.i4086
50. Low, B, Coutinho, D, Gonçalves, B, Rein, R, Memmert, D, and Sampaio, J. A systematic review of collective tactical behaviours in football using positional data. Sport Med. (2020) 50:343–85. doi: 10.1007/s40279-019-01194-7
51. Ojardias, E, Ollier, E, Lafaie, L, Celarier, T, Giraux, P, and Bertoletti, L. Time course response after single injection of botulinum toxin to treat spasticity after stroke: systematic review with pharmacodynamic model-based meta-analysis. Ann Phys Rehabil Med. (2022) 65:101579. doi: 10.1016/j.rehab.2021.101579
52. Fattorini, L, Tirabasso, A, Lunghi, A, Di Giovanni, R, Sacco, F, and Marchetti, E. Muscular forearm activation in hand-grip tasks with superimposition of mechanical vibrations. J Electromyogr Kinesiol. (2016) 26:143–8. doi: 10.1016/j.jelekin.2015.10.015
53. Fattorini, L, Tirabasso, A, Lunghi, A, Di Giovanni, R, Sacco, F, and Marchetti, E. Muscular synchronization and hand-arm fatigue. Int J Ind Ergon. (2017) 62:13–6. doi: 10.1016/j.ergon.2016.07.009
54. Dai, W, Pi, YL, Ni, Z, Tan, XY, Zhang, J, and Wu, Y. Maintenance of balance between motor cortical excitation and inhibition after long-term training. Neuroscience. (2016) 336:114–22. doi: 10.1016/j.neuroscience.2016.08.053
55. Mouthon, A, and Taube, W. Intracortical inhibition increases during postural task execution in response to balance training. Neuroscience. (2019) 401:35–42. doi: 10.1016/j.neuroscience.2019.01.007
56. Stinear, CM, and Byblow, WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol. (2003) 89:2014–20. doi: 10.1152/jn.00925.2002
57. Rocchi, L, Suppa, A, Leodori, G, Celletti, C, Camerota, F, Rothwell, J, et al. A plasticity induced in the human spinal cord by focal muscle vibration. Front Neurol. (2018) 9:935. doi: 10.3389/fneur.2018.00935
58. Toscano, M, Celletti, C, Viganò, A, Altarocca, A, Giuliani, G, Jannini, TB, et al. Short-term effects of focal muscle vibration on motor recovery after acute stroke: a pilot randomized sham-controlled study. Front Neurol. (2019) 10:115. doi: 10.3389/fneur.2019.00115
59. Bianconi, R, and van der Meulen, J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. (1963) 26:177–90. doi: 10.1152/jn.1963.26.1.177
60. Burke, D, Hagbarth, KE, Löfstedt, L, and Wallin, BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. (1976) 261:695–711. doi: 10.1113/jphysiol.1976.sp011581
61. Roll, JP, and Vedel, JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. (1982) 47:177–90. doi: 10.1007/BF00239377
62. Fallon, JB, and Macefield, VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve. (2007) 36:21–9. doi: 10.1002/mus.20796
63. Rosenkranz, K, Pesenti, A, Paulus, W, and Tergau, F. Focal reduction of intracortical inhibition in motor cortex by selective proprioceptive stimulation. Exp Brain Res. (2003) 149:9–16. doi: 10.1007/s00221-002-1330-3
64. Matthews, PBC. Mammalian muscle receptors and their central action. London: Edward Arnold (1972).
65. Smolen, P, Zhang, Y, and Byrne, JH. The right time to learn: mechanisms and optimization of spaced learning. Nat Rev Neurosci. (2016) 17:77–88. doi: 10.1038/nrn.2015.18
66. Li, S. Spasticity, motor recovery, and neural plasticity after stroke. Front Neurol. (2017) 8:120. doi: 10.3389/fneur.2017.00120
67. Tranchida, GV, and Van Heest, A. Preferred options and evidence for upper limb surgery for spasticity in cerebral palsy, stroke, and brain injury. J Hand Surg Eur. (2020) 45:34–42. doi: 10.1177/1753193419878973
Keywords: hypertonia, proprioception, rehabilitation protocol, stimulus frequency, tonic vibration reflex
Citation: Fattorini L, Pettorossi VE, Marchetti E, Rodio A and Filippi GM (2025) A review about muscle focal vibration contribution on spasticity recovery. Front. Neurol. 16:1579118. doi: 10.3389/fneur.2025.1579118
Edited by:
Claudia Celletti, Università Link Campus, ItalyReviewed by:
Simona Maria Carmignano, University of Salerno, ItalyAlessandro Viganò, Fondazione Don Carlo Gnocchi Onlus (IRCCS), Italy
Filippo Camerota, Sapienza University of Rome, Italy
Copyright © 2025 Fattorini, Pettorossi, Marchetti, Rodio and Filippi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Fattorini, bHVpZ2kuZmF0dG9yaW5pQHVuaXJvbWExLml0
 Luigi Fattorini
Luigi Fattorini Vito Enrico Pettorossi
Vito Enrico Pettorossi Enrico Marchetti
Enrico Marchetti Angelo Rodio
Angelo Rodio Guido Maria Filippi
Guido Maria Filippi