- 1Department of Psychology, University of Virginia, Charlottesville, VA, United States
- 2UVA School of Data Science, Charlottesville, VA, United States
Introduction: Current concussion assessments used by the NCAA are generally applied to both male and female athletes to evaluate the effects of sports-related head impacts. However, increasing evidence indicates that female athletes show different physiological and psychosocial responses to concussions compared to their male counterparts, raising concerns about the suitability of gender blind concussion assessments.
Methods: This study analyzes data from N = 1,021 NCAA athletes (379 females, 642 males) who completed the SCAT3 Symptom Severity Checklist after a concussion. A systematic use of multivariate statistical methods, including Exploratory Graph Analysis (EGA), Principal Component Analysis (PCA), Exploratory Factor Analysis (EFA), Linear Discriminant Analysis (LDA), and Rasch Partial Credit Modeling (PCM), was applied to this 22-item instrument to explore the underlying factor structure and identify assessment items sensitive to gender differences. Differential Item Functioning (DIF) analysis examined gender disparities in symptom reporting.
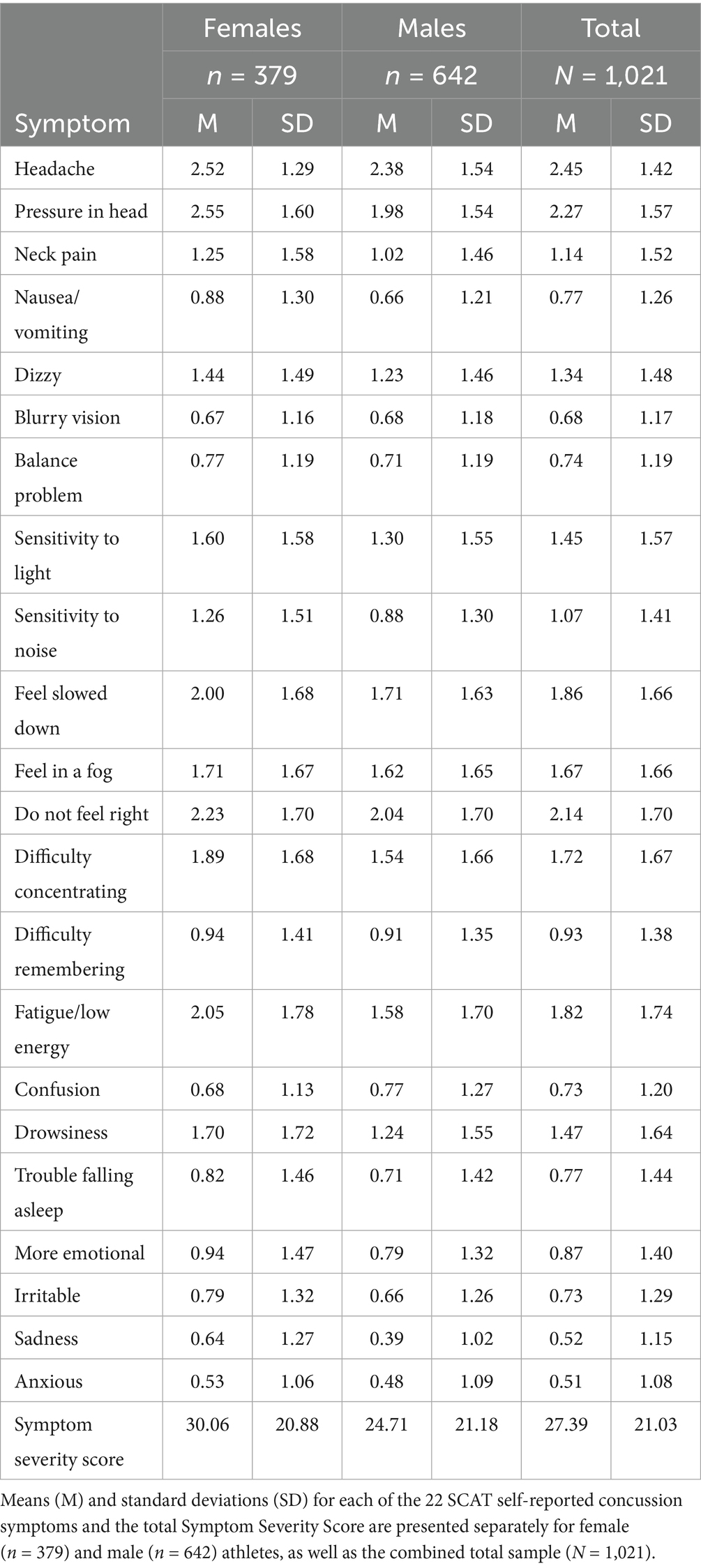
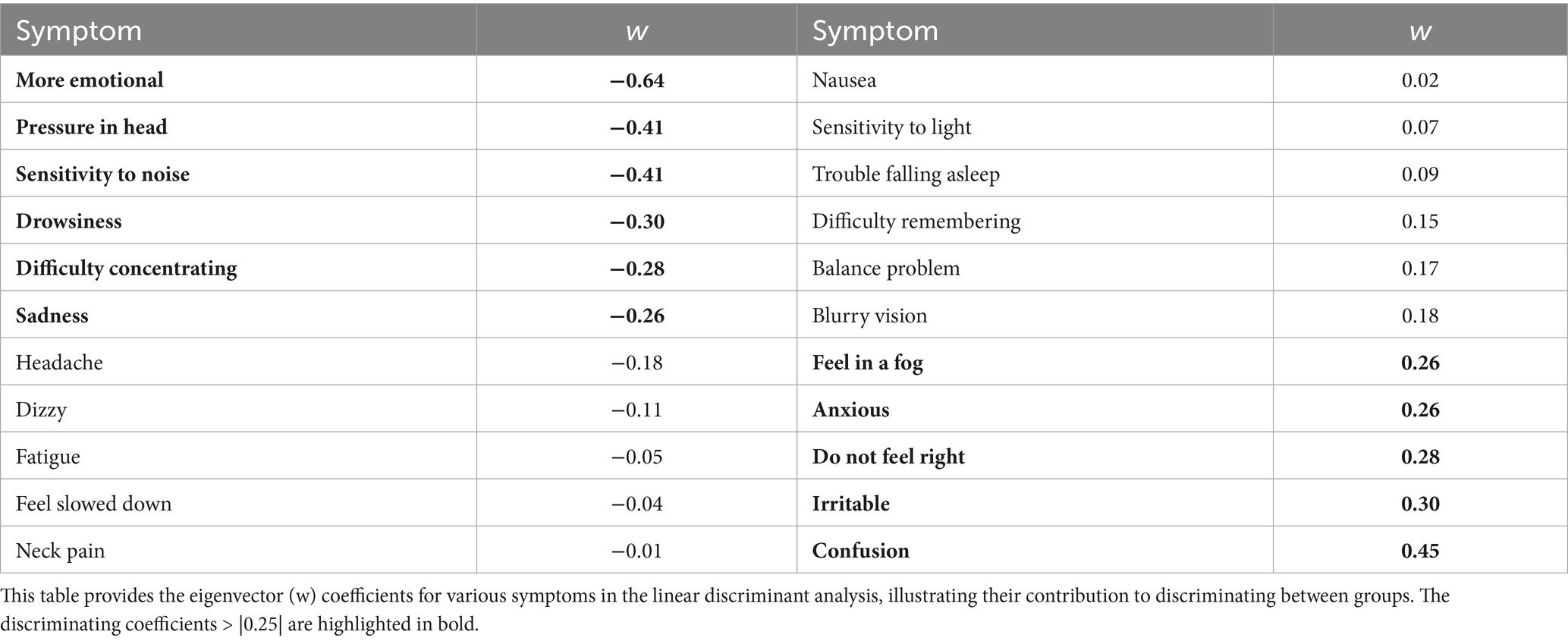
Results: Based on EGA and PCA, the SCAT3 showed a four-factor substructure, with EFA accounting for 62.44% of the variance. LDA comparing males and females revealed a significant difference in their multivariate score distributions (χ2 (22) = 130.56, p < 0.001), with emotional and physical symptom items loading negatively, and cognitive and sensory items loading positively. This suggests emotional and physical symptoms contribute oppositely to cognitive and sensory symptoms, implying these domains may represent opposite ends of a single symptom dimension. Rasch analysis of each assessment item identified three items with no difference between genders. Conversely, nine symptoms showed males were more likely to report higher severity. Nonetheless, females generally reported divergent overall symptom severity scores (Mean = 30.06, SD = 20.88) than males (Mean = 24.71, SD = 21.18), t(765.06) = 3.85, p < 0.001.
Discussion: Differences in symptom presentation post-concussion may suggest that: (1) males tend to be more conservative in reporting and only endorse symptoms when they are more intense, leading to higher scores on fewer symptoms, whereas (2) females may more readily emphasize emotional and physical symptoms. The findings imply that considering gender differences in concussion symptom reporting is important when making clinical recommendations.
Introduction
Sports-related concussions (SRC) represent a significant health concern for student-athletes regardless of age, sport, athletic division/conference/etc., or gender. The National Collegiate Athletic Association (NCAA) employs a broad, multifaceted approach to determining SRC, but one which relies heavily upon symptom self-reporting as a primary method of assessment (1–6). However, increasing evidence suggests that male and female athletes exhibit distinct physiological and psychosocial responses to concussions, raising concerns about the adequacy of the current gender-neutral diagnostic framework (7–14). Despite this growing body of research, NCAA concussion assessments, such as the widely used Sport Concussion Assessment Tool (SCAT), do not specifically account for gender differences in symptom reporting or recovery trajectories (15).
Concussion rates among NCAA student-athletes underscore the importance of refining diagnostic tools. Over 460,000 student-athletes participate in NCAA competitions each year, nearly half (43.7%) of whom are female (16, 58). For instance, between the 2009–2010 and 2013–2014 academic years, approximately 4.47 concussions occurred per 10,000 athlete exposures, resulting in around 10,560 concussions annually (17, 18). Women’s soccer ranked second among sports with the highest concussion rates (19). In spite of differences in concussion exposure, NCAA concussion assessment protocols have tended to remain gender-agnostic, applying the same self-report diagnostic tools to male and female athletes alike (4, 6, 20).
Research consistently shows that male and female athletes differ in both the frequency and severity of reported concussion symptoms. Studies have indicated that female athletes tend to report a higher frequency of concussions, along with more severe symptoms, compared to their male counterparts (7–9, 21, 59, 60). These gender differences suggest that the current gender-neutral assessment methods may overlook key factors influencing symptom expression and recovery outcomes, potentially compromising the accuracy of concussion management (22–24). Furthermore, a systematic review of gender differences in SRC revealed that female athletes often experience longer recovery times, though the exact reasons for these differences remain uncertain (25). Such discrepancies may stem from biases in the diagnostic tools, which have historically been developed using predominantly male populations (26, 27).
The Sports Concussion Assessment Tool (SCAT)
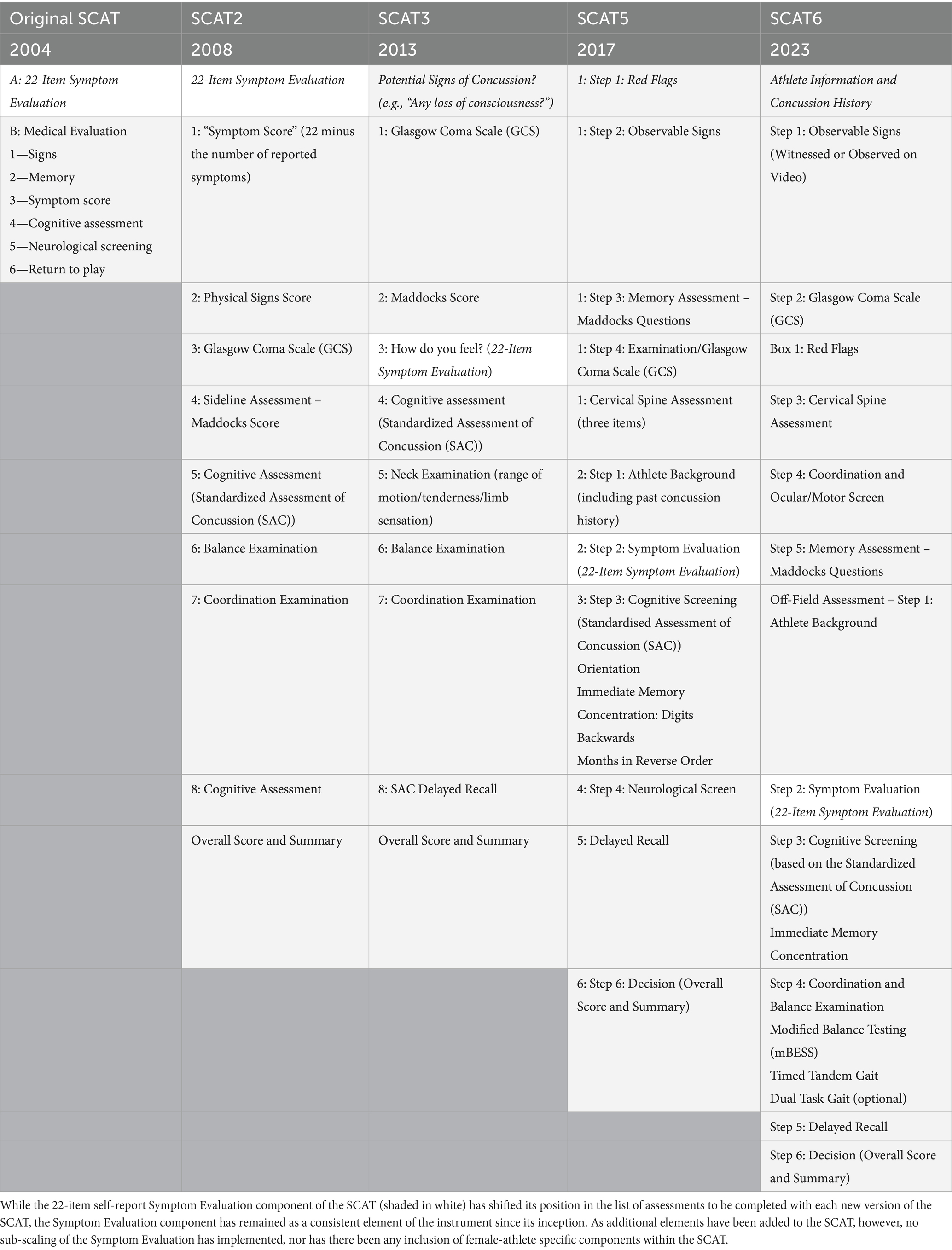
The Sports Concussion Assessment Tool (SCAT) is a widely used assessment measure developed to systematically evaluate symptoms and cognitive functioning in athletes who may have experienced a concussion (61, 62). Initially introduced in 2004, following the 2nd International Symposium on Concussion in Sport, organized held in Prague, Czech Republic, and subsequently updated (SCAT2 in 2008, SCAT3 in 2013, SCAT5 in 2017, and SCAT6 in 2023; The version number SCAT version 5 was chosen to align the version number with the 5th International Consensus Conference on Concussion in Sport, held in Berlin, Germany, in 2016, meeting number and, as such, there is no SCAT4), the SCAT reflects advancements in concussion research and clinical feedback aimed at standardizing concussion assessment and improving its sensitivity. A depiction of the historical development of the SCAT components is presented in Table 1.
The SCAT incorporates both subjective symptom self-reporting and objective cognitive and physical assessments. The symptom evaluation component, which lists common concussion symptoms such as headaches, dizziness, nausea, and mental fog, requires athletes to self-rate the severity of each symptom. This self-reporting is meant to align with the recognition that subjective symptomology is an essential indicator of concussion severity and recovery. Alongside symptom evaluation, SCAT includes a cognitive assessment that tests immediate and delayed memory, concentration, and orientation, as well as balance testing through the modified Balance Error Scoring System (BESS). SCAT has been widely adopted in sports, particularly at professional and amateur levels, and is endorsed by major organizations such as the International Olympic Committee (IOC) and World Rugby.
The SCAT5 introduced several refinements over the SCAT3 to enhance usability and clinical accuracy. One notable change was the expansion of the symptom checklist to accommodate a broader range of neurological symptoms and increased sensitivity to symptom severity. This update was performed in response to research on the diverse ways concussions present, aiming to capture subtle changes that could be missed in earlier iterations. SCAT5 also offers updated guidance on interpreting symptom severity scores, including clearer thresholds to guide clinical decisions about when athletes should return to play (28–30, 63). Additionally, it emphasizes cognitive and neurological examination components, enhancing sections on memory, concentration, and balance testing. For instance, the SCAT5 includes more detailed instructions for assessing balance using the modified Balance Error Scoring System (BESS), an essential metric for detecting vestibular and motor impairments post-concussion. Moreover, the SCAT5 provided updated guidelines on its utility across age groups, particularly recommending modifications for athletes under 13 with the Child SCAT5. Overall, changes in SCAT5 sought to provide a more robust framework, allowing clinicians to identify and manage concussions with greater confidence and precision compared to the SCAT3. The current SCAT6 version (released in 2023), extends the set of neurological assessment domains still further (see below as well as in Table 1).
However, the self-report symptom rating portion of the SCAT has remained a consistent core feature across all iterations, from its initial version in 2004 to the SCAT6, despite numerous enhancements to other sections of the tool (15). This feature in the assessment reflects the central influence of subjective symptom reporting in concussion diagnosis and management, as athletes’ descriptions of their symptoms provide critical insights that objective or more clinical observation measures may not fully capture.
In the self-report portion of the SCAT, athletes rate the severity of various concussion symptoms—such as headache, nausea, dizziness, and cognitive fog—on a scale from 0 (none) to 6 (severe), creating a total symptom score. This scoring approach has not changed, even as additional assessment components, like more detailed cognitive testing, neurological assessment, and balance evaluations, have been added to enrich the SCAT’s comprehensiveness. Notably, throughout the history of the SCAT, there has never been any particular differentiation between the experience of female versus male athletes in response to the perceived symptoms of concussion and the assessment provides no sub-scaling nor differentiation between male and female symptom reporting (see Table 2).
The NCAA-DoD concussion assessment, research, and education (CARE) consortium
The NCAA-DoD Concussion Assessment, Research, and Education (CARE) Consortium represents a large-scale, multi-institutional research initiative focused on understanding SRC, primarily in college athletes and military cadets. Launched in 2014 as a collaboration between the NCAA and the U.S. Department of Defense (DoD), CARE aims to advance knowledge on concussion diagnosis, management, and recovery, as well as long-term health outcomes (31). This consortium integrates data from baseline assessments, injury evaluations, and post-injury follow-ups to study concussion trajectories comprehensively. Involving over 30,000 athletes across 30 + institutions, CARE represents one of the largest databases of concussion data globally. Its assessments encompass a wide range of modalities, including self-reported symptoms, neurocognitive testing, neuroimaging, and genetic analyses, allowing for a multidimensional understanding of concussion’s impact on brain health. The CARE Consortium collects pre-injury baseline data from athletes, enabling comparisons between pre-and post-concussion states and for tracking recovery progress in detail (31). This data-design has allowed for insights into gender, age, and sport-related differences in concussion risk and recovery, as well as the development of sophisticated predictive models for post-concussive outcomes. Through its broad dataset, CARE supports numerous research initiatives to develop evidence-based clinical guidelines, improve safety protocols, and identify biomarkers that could lead to more accurate diagnosis and personalized treatment for concussions, with impacts extending beyond sports medicine to military and civilian healthcare.
All data from the CARE Consortium have been made openly available on the Federal Inter-Agency Traumatic Brain Injury Resource (FITBIR) data archive (fitbir.nih.gov). This includes clinical assessments, neuroimaging, balance test metrics, etc. The version of the SCAT used by the CARE Consortium for which data is available in the FITBIR archive was obtained using the SCAT3 version of the assessment, so while not reflective of the current state-of-the-art concerning the SCAT, it reflects the same core set of 22 self-report items as the more recent, SCAT6, assessment.
Scoring the SCAT self-report assessment
The SCAT6 incorporates several clinical and cognitive test components designed to provide a comprehensive assessment of concussion effects on an athlete. The clinical component begins with an “Immediate or On-Field Assessment” to identify any severe injury markers, termed “Red Flags,” such as neck pain or altered consciousness, which necessitate urgent medical attention. This is followed by an orientation and memory section, in which athletes answer basic questions regarding time, place, and recent events, aiding in the identification of disorientation or memory loss. The cognitive portion includes immediate memory recall, where athletes repeat a list of words presented to them, testing short-term memory, and a concentration test involving number sequencing and reverse recitation, which assesses focus and mental processing. Additionally, the delayed recall component tests retention by asking athletes to recall the initial list of words after a brief delay, providing insight into memory consistency over time. The SCAT6 also integrates a modified Balance Error Scoring System (BESS), which assesses postural stability by having the athlete balance in various stances while clinicians score any errors in posture or movement. Together, these tests evaluate an athlete’s cognitive functioning, memory, and balance—key areas frequently affected by concussion—helping clinicians make informed decisions about diagnosis, treatment, and readiness for return to play. Specifically, athletes complete a cognitive screening that includes orientation questions, immediate memory recall, concentration tasks (like “serial 7 s” or months-in-reverse-order), and delayed recall; each scored separately. Physical testing, such as the modified Balance Error Scoring System (BESS), evaluates the athlete’s postural stability by measuring errors made during various stances.
Total scores on the SCAT do not yield a simple “pass/fail” outcome; instead, high scores indicate a generally more significant symptom burden and/or level of impairment. While no universal threshold score dictates whether an athlete is concussed, clinicians compare SCAT scores against baseline scores, if available, to detect changes and monitor recovery. However, the underlying basis of all versions of the SCAT checklist is the assumption that symptom severity can be captured by the Total Symptom Score, regardless of gender (32, 33). This overall number is then frequently employed to make clinical determinations on an athlete’s concussion severity and, ultimately, any clinical response. However, research by the CARE Consortium has suggested that the pooling of responses from across the range of SCAT items may not reflect the more subtle elements of head injuries in both male and female athletes (31, 34).
Considering the definition of gender
The interchangeability of the terms “gender” and “sex” in SRC research further complicates the issue of considering the differential effects concerning a spectrum of gender identities. “Sex” refers to biological differences between males and females, while “gender” encompasses the social roles, behaviors, and identities associated with each (64). Sports are commonly classified as “men’s” or “women’s” versions, based upon the biological interpretation. This distinction is nuanced but essential for understanding how social and cultural factors may influence symptom reporting and recovery. The SCAT also makes no attempt to capture differences which may be relevant to transgendered athletes. Beyond these issues, most modern concussion assessment tools, including the SCAT checklist, would ideally need to adequately consider how factors related to the gender of the athlete could skew symptom evaluation and recovery outcomes. In a broader sense, the consideration of SRC across the spectrum of perceived gender identities is beyond the scope, per se, of the present investigation, and the consideration of gender is limited to male and female labels, as reported by the CARE Consortium in the FITBIR archive.
Examining the structure of the SCAT and the potential for gender differences
Under the SCAT assessment, female athletes are more likely to report a broader range of symptoms with greater severity than males (35, 65). Thus, these findings highlight the need to reexamine the psychometric properties of the SCAT checklist to ensure it accurately reflects the symptomatology of both genders (27).
To address these gaps, the present investigation seeks to deconstruct the multidimensional nature of SRC symptom reporting, focusing on how gender may influence the perception and reporting of symptoms. By drawing on a robust dataset from the NCAA and DoD CARE Consortium, the study applies a suite of advanced multivariate statistical methods to assess the underlying dimensionality of concussion symptoms and to unravel the complexity of symptom reporting across genders. Exploratory Graph Analysis (EGA), Principal Component Analysis (PCA), Exploratory Factor Analysis (EFA), and Linear Discriminant Analysis (LDA) are employed to explore and clarify the dimensional structures that underlie the symptom clusters reported on the SCAT checklist, providing insights into how different symptoms co-vary and whether specific patterns emerge across male or female athletes.
As a complement to these methods, Differential Item Functioning (DIF) analysis and Rasch Modeling are used to rigorously investigate whether the SCAT checklist might disproportionately represent symptom severity scores in both male and female athletes, even when adjusting for differing individual trait level differences between genders. This layered approach aims to go beyond simple univariate symptom reporting or intensity comparisons and seeks to identify whether any gender-specificity exists in the underlying assessments themselves. Importantly, this study seeks to identify distinct clusters of concussion symptoms that more accurately reflect gender differences, helping to present a more nuanced, multidimensional framework for concussion assessment.
Given the SCAT’s historical consistency and comprehensive coverage of subjective concussion symptoms, a multivariate analysis of its self-report items is both timely and highly relevant, especially in light of the tool’s lack of adjustments or thresholds that account for the athlete’s gender. Since the SCAT self-report section has remained largely unchanged across iterations, this stability offers a unique opportunity for researchers to analyze symptom reporting trends over time and across diverse populations. Despite research showing that gender differences may influence concussion symptomatology and recovery trajectories, the SCAT does not differentiate between scores or assessment criteria based on gender, potentially overlooking nuanced variations in symptom reporting between male and female athletes. Multivariate analysis could reveal patterns and dimensions within self-reported symptoms that vary by gender, identifying clusters or specific symptom profiles that might be more predictive of prolonged recovery in one group compared to the other. By examining the dimensionality of symptom reporting with statistical rigor, this approach could provide valuable insights that might improve individualized concussion management. Such an analysis could support the development of more tailored concussion guidelines, refining both diagnostic and recovery protocols to account for gender-related differences, ultimately enhancing the clinical utility of the SCAT for both male and female athletes.
Methods
Demographics
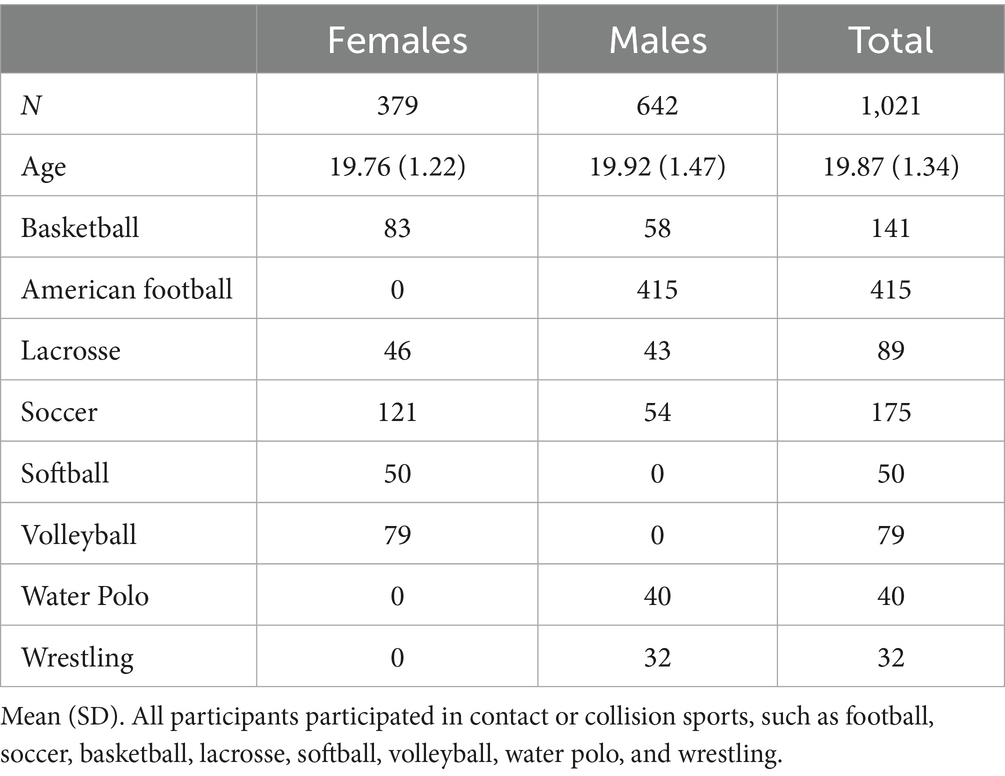
N = 1,021 NCAA student-athletes (379 females and 642 males) completed the SCAT Version 3.0 (SCAT3) Symptom Severity Checklist within 48 h post-concussion, which was obtained from the Federal Interagency of Traumatic Brain Injury Research (FITBIR) in collaboration with the NCAA and DoD CARE Consortium. As noted above, the checklist includes 22 symptoms, each assessed using a 7-point Likert scale ranging from 0-to-6. These symptoms are summed for a Total Symptom Severity Score, in which values may range from 0-to-132.
Statistical approaches
A systematic approach was utilized to evaluate the SCAT concussion assessment instrument’s underlying dimensionality. First, an exploratory graph analysis (EGA) was performed to illustrate the SCAT’s potential underlying multivariate structure (36). This was followed by a Principal Component Analysis (PCA) (37), which formed the basis for a subsequent Exploratory Factor Analysis (EFA) (38), which was used to determine which assessment items load the most onto latent symptom constructs (39). A linear discriminant analysis (LDA) was also performed to determine the most discriminating SCAT items between males and females (66). Lastly, the Masters (40) Partial Credit Model (PCM) and (67) Differential Item Functioning (DIF) analysis were conducted to confirm LDA results and provide greater specificity to gender-related symptoms on the SCAT symptom checklist most sensitive to differences between male and female athletes. PCA, EFA, and LDA analysis were also conducted through R version 4.2.2 (68). In what follows, we describe the details involved in each of these steps:
Exploratory graph analysis (EGA)
Utilizing R version 4.2.2 in conducting EGA, running the EGAnet package version 1.2.3 (36). These network-based models use nodes to represent random variables connected by edges, indicating the level of unique interaction between them rather than individuals in networks, aiding in determining the number of dimensions through cluster detection.
Principle component analysis (PCA)
PCA was utilized for dimensionality reduction while preserving as much of the variability in the data as possible, deconstructing the item-wise correlation matrix, and transforming the original variables into a new set of linear combinations of the original variables. These new variables, called principal components (PCs), are orthogonal (independent of one another) and ordered, so the first few retain most of the variation in all the original variables. The eigenvalues associated with each PC were examined, and those greater than or exceeding unity (e.g., Kaiser’s Criterion) were taken as indicative of the SCAT assessment’s multivariate sub-space.
Exploratory factor analysis (EFA)
Building from the PCA and assessing the number of putative factors, an EFA was done to determine the final number of factors and the subsequent standardized loadings for each assessment item, loading onto each factor. Three and four-factor models with varimax rotation were compared by analyzing fit indices, computing the 𝜒2 fit statistic, RMSEA, the Comparative Fit Index (CFI), and the Tucker-Lewis Index (TLI) (41).
To determine clusters of items and their corresponding factor, a cut-off score for the standardized factor loadings of 0.21 comes from the approach where the smallest acceptable absolute factor loading is determined as one over the square root of the number of items (69). This is consistent with (70) concerning psychometric validation for ensuring robust factor interpretation and dimensionality.
Linear discriminant analysis (LDA)
LDA finds the best linear combination of assessment items, which maximally separates two or more distributions relative to within-distribution variability (39). Moreover, LDA is solving an eigenvector (w) problem to maximize group separation. To determine this separation, the coefficients and corresponding eigenvalues (λ) were used to determine directions along the axes, computing the identification of symptoms best separated by gender. The eigenvector coefficient equation comes from solving the eigenvalue equation where A is a square matrix of the between-class and within-class scatter matrices (42). The eigenvector coefficients are the elements of the eigenvector, and these coefficients can be found by solving the linear system derived from the matrix A (Trendafilov and Gallo, 2021). Furthermore, these coefficients define how the features combine to form the maximal gender separation between symptoms.
Finally, the statistical significance of this discriminant function was evaluated using Wilk’s Lambda (⋀) statistic and its approximate F-ratio test statistic. A low ⋀ value approaching 0 and a significant p-value indicates that the discriminant function explains a substantial portion of the variance between the groups (43).
Rasch analysis
Rasch partial credit model (PCM)
The PCM, a model within the family of Rasch measurement theories, was employed to analyze the response data (44). This model is particularly suited for handling ordinal response categories typical of symptom severity scales, offering robust estimations of item difficulty parameters without assuming equal distances between each category. The PCM equation can be interpreted as follows: r is the current step, x is the current step, and m is the full set of categories. The numerator sums up to the current category, while the denominator is the sum of all the categories:
Delta parameters δij are specified per item; δij is the step difficulty or location where 2 categories intersect (category intersections). Item locations (𝛽i’s) are typically obtained by taking an average of all the deltas (δij), the points along the latent trait continuum at which the likelihood of endorsing successive response categories increased (45, 46). The estimated thresholds for each symptom were used to identify the levels at which respondents were likely to move between response categories. Symptoms with disordered thresholds were marked for further review.
Before applying the PCM, the assumptions of unidimensionality, local independence, and monotonicity were tested. Unidimensionality was assessed through Exploratory Factor Analysis (EFA), ensuring that all symptom items measured a single latent trait (46). PCM assumes that the item parameters (e.g., symptom difficulties and thresholds) are invariant across different genders. This assumption implies that the model should work equally well across genders. Lastly, evaluating the assumption of monotonicity ensures that as the underlying trait increases, the probability of endorsing higher response categories also increases (40, 47).
Differential item functioning (DIF)
DIF occurs when individuals from different groups (e.g., genders) respond differently to a symptom despite having similar underlying latent trait levels. In the context of concussion symptom reporting, DIF quantifies the extent to which male and female athletes may interpret or report symptoms differently. In such cases, one gender may be more likely to endorse a symptom at a higher severity level than the other, even though their actual level of concussion-related impairment may be the same (40, 48). While measurement invariance across groups and time is desirable, cases in which symptoms are endorsed more severely for one gender versus another indicate lack of support for it. Ensuring measurement invariance is essential to ensure that the assessment accurately reflects an equivalent probability of endorsement for items for all individuals, regardless of gender (46). The following equation further computes the overall DIF measure or the difficulty for each gender and corresponding item
The DIF equation for the PCM involves several key variables (40). To compute the DIF measure for the corresponding gender and item βig, the θn takes the ability level of person n, while δig is the difficulty of the item within each gender, g. The τimg are step thresholds that define the boundaries between response categories.
DIF contrast quantifies the difference in item difficulty between groups (e.g., genders) for a specific symptom. The size and direction of the contrast indicate whether and to what extent males and females tend to report a higher severity (49). The equation below shows how the DIF contrast was computed, which takes the difference between the item difficulty for the prechosen reference group (females) and the focal group (males). This contrast value helps identify DIF direction and assess whether different genders respond differently to the same item after controlling for trait level (48). This is found using the following Masters (40) equation
Where i = item, f = female, and m = male. A negative contrast value of βif − βim suggests that females tend to more frequently report the symptom as relatively more severe than males at the same level of concussion severity (67). In other words, for the same overall concussion effects, females are more likely to endorse higher ratings for that symptom than males. Contrasts in the opposite direction, or a positive contrast, suggest that males tend to more frequently report the symptoms as relatively more severe than females.
To further determine significant symptoms that display DIF, Mantel–Haenszel probability statistics were used to determine whether an item exhibits uniform DIF between two observed groups, that is, whether an item is more frequently endorsed by one gender relative to the other, considering the latent trait. To avoid alpha inflation and Type I errors stemming from multiple comparisons, Benjamini & Hochberg (B-H) post-hoc tests (50) were conducted, as testing item DIF for many items poses an increased risk of Type I errors due to multiple tests with α < 0.05. Therefore, B-H correction was appropriate to control the false discovery rate (FDR) associated with the multiple comparisons (51).
Results
Demographics
As seen in Table 3, this analysis revealed a statistically significant difference in the mean Total Symptom Severity Scores between females (M = 30.06, SD = 20.88) and males (M = 24.71, SD = 21.18), t(765.06) = 3.85, p < 0.001. All participants were enrolled based upon participation in contact or collision sports, including football, soccer, basketball, lacrosse, softball, volleyball, water polo and wrestling, to ensure a consistent level of concussion exposure risk across the sample. The structure and breakdown of each sport can be described elsewhere (31).
Dimensionality of the SCAT self-report items
Exploratory graph analysis
The EGA network visualization and corresponding network loadings represent different symptoms or states grouped into clusters based on their underlying correlations (Figure 1). The analysis identified five clusters. However, one factor contained only three items, and two had relatively low loadings, minimizing the variance. A total of five clusters were identified, which served as justification for further analysis using PCA and EFA to achieve a more parsimonious factor structure, reflecting symptom reporting subspaces in the SCAT. In summary, while five clusters were identified via EGA, the instability and low variance contribution of some clusters justified the use of PCA and EFA to refine the dimensional structure and improve the construct validity of the SCAT symptom domains. This step supports downstream modeling by anchoring the latent constructs in a more stable and theoretically interpretable structure.
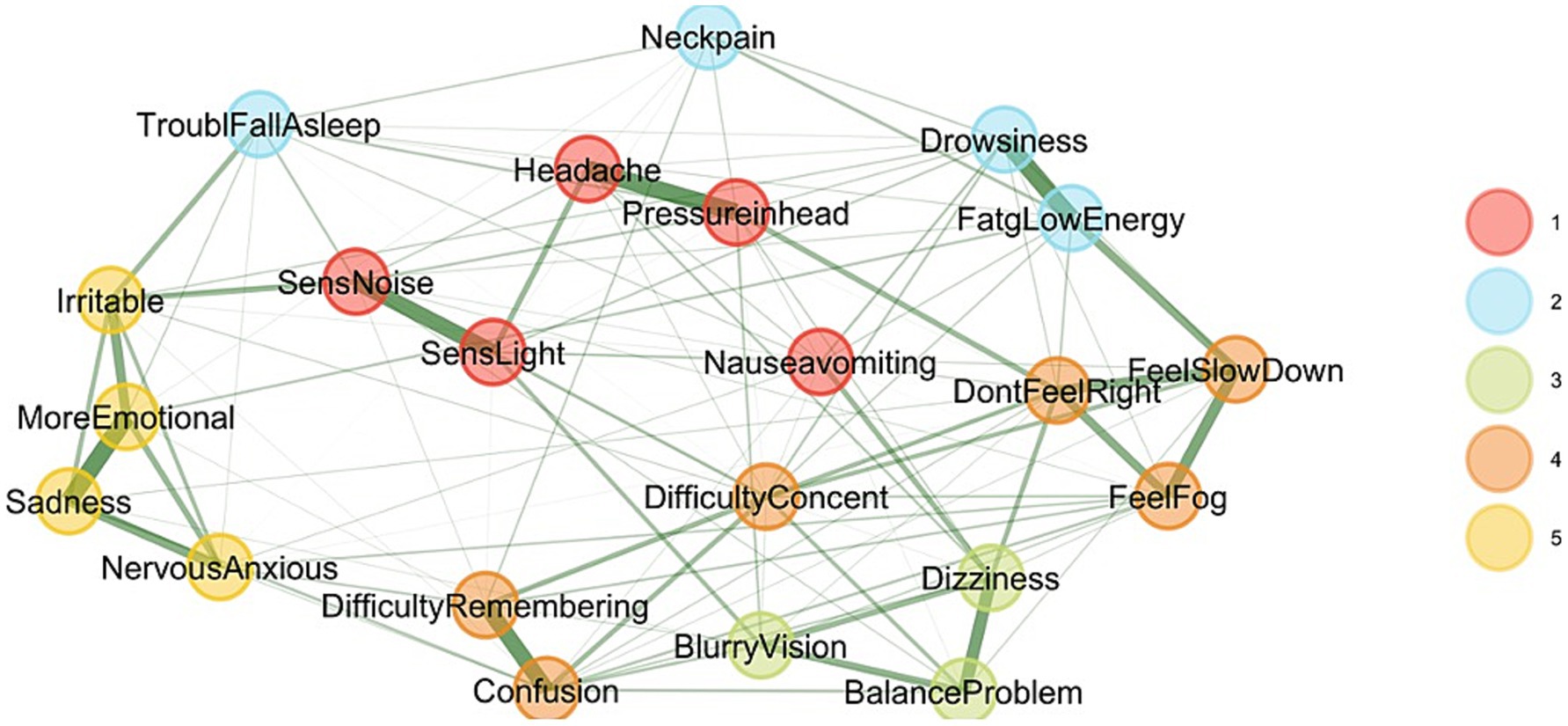
Figure 1. Exploratory graph analysis. This graph illustrates the SCAT3 Symptom Checklist, revealing five correlated factors. Each node represents a symptom, with lines indicating relationships between symptoms based on the stages the instrument intended to measure.
To further explore the structure of inter-item associations, a clustered heatmap was generated using the EGA-derived association matrix. Items were reordered using hierarchical clustering based on correlation distance (1–r), a method that prioritizes pattern similarity in item responses rather than raw magnitude (Figure 2). The resulting heatmap revealed distinct diagonal blocks of high association strength, indicating strong within-cluster coherence. In contrast, off-diagonal regions displayed weaker associations, reflecting reduced connectivity between symptom groups. The application of correlation distance was particularly appropriate in this context, as it preserved the psychological meaning of item interrelationships and supported the interpretability of the clustering solution.
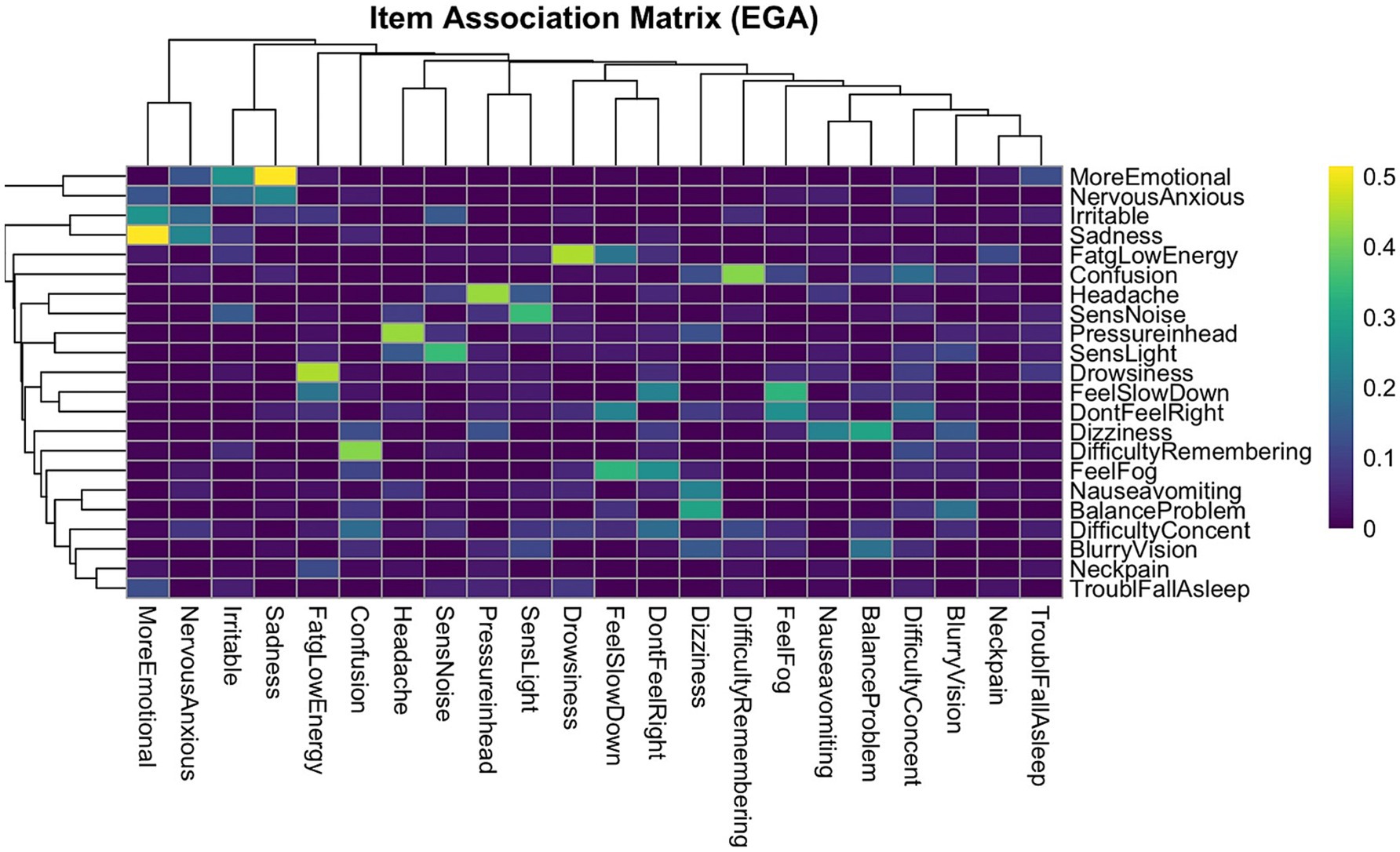
Figure 2. EGA item association heatmap. This dendrogram heatmap displays the strength of pairwise associations between symptoms. Items are ordered based on hierarchical clustering to visually group symptoms with similar association patterns. Color intensity reflects the magnitude of the association between symptoms, with the brighter colors indicating stronger correlations. Distinct diagonal blocks of higher association strength reflect strong within-cluster coherence, supporting the factorial structure identified by EGA. The dendrogram further reveals the nested structure of symptom groupings, offering complementary insight into the dimensional organization of post-concussion symptoms. Four distinct symptom clusters were observed; these clusters align with the four-factor structure identified through EGA and support the presence of coherent, multidimensional symptom domains in concussion presentations.
Principal components analysis
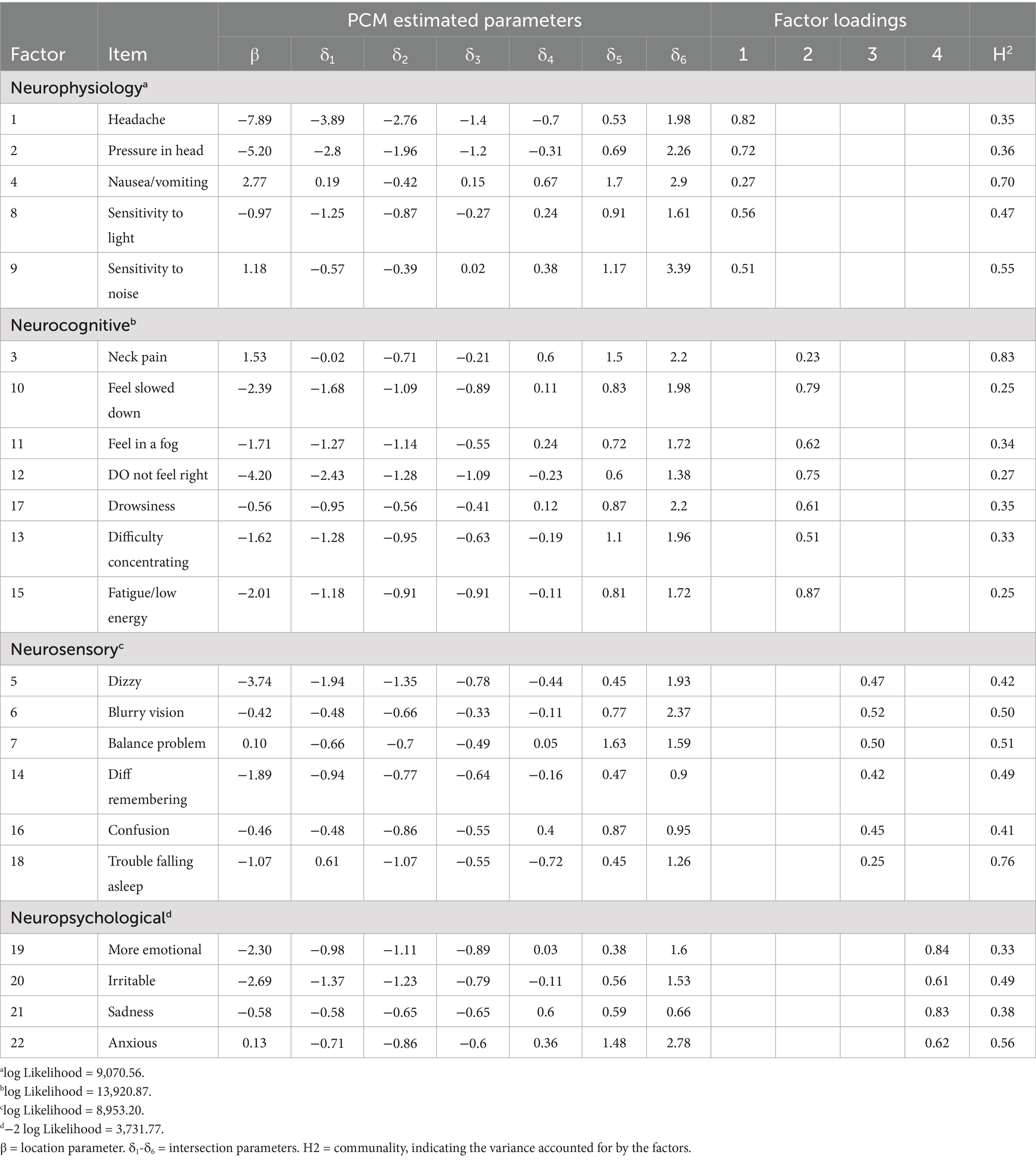
PCA identified four significant components, as rendered through a scree plot (Figure 3), which were 9.82, 1.63, 1.20, and 0.998, respectively; the fourth component’s eigenvalue nearly meets the Kaiser criterion, suggesting potential additional information. The fourth dimension explains 4.53% of the variance, leading to a higher cumulative explanation of 62.44% in the four-factor model. Including the additional fourth factor may result in a more comprehensive representation of the dataset, ensuring that subtler yet important patterns are accounted for.
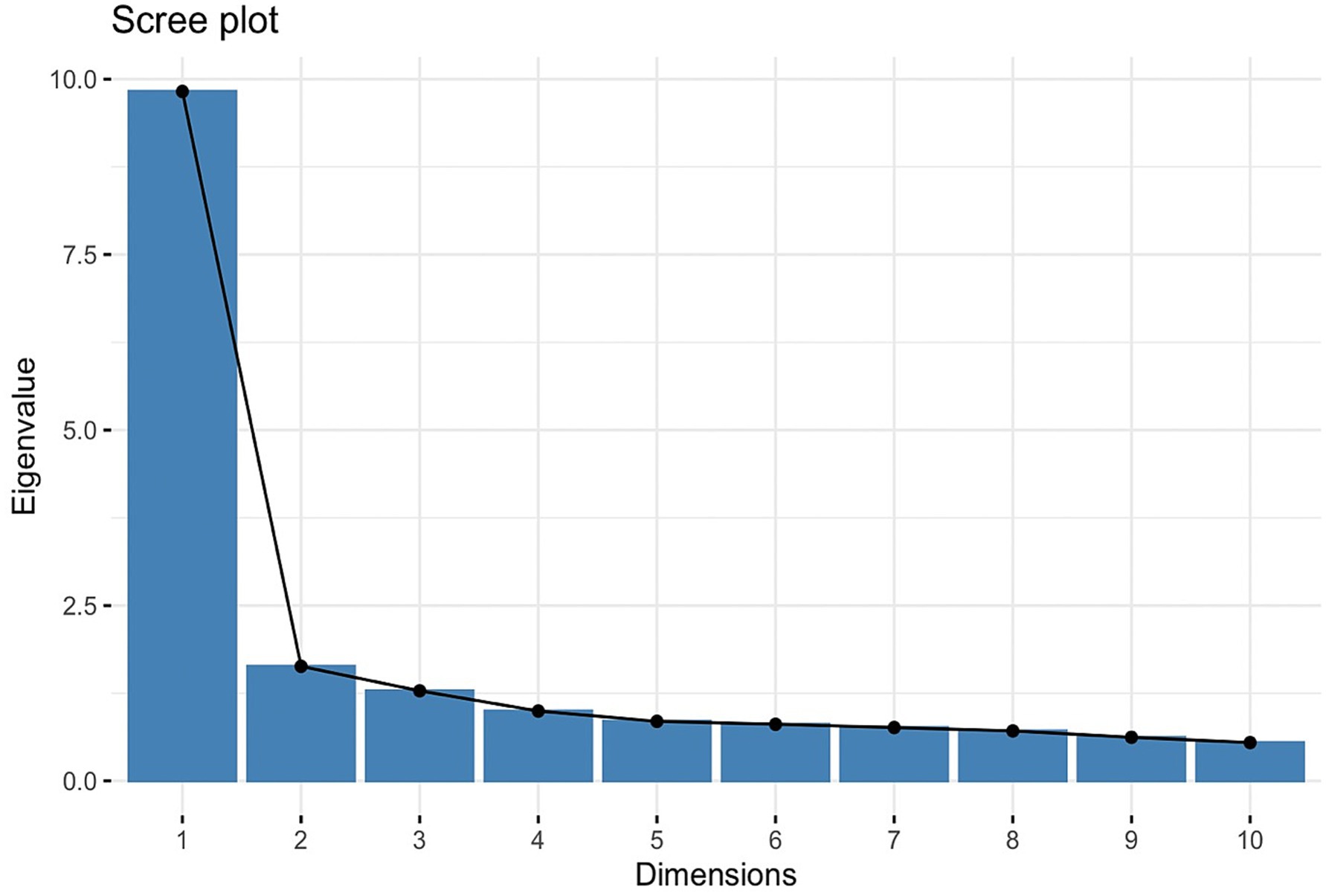
Figure 3. PCA scree plot. The scree plot displays eigenvalues (9.82, 1.63, 1.20, 0.998) against the dimensions, helping to identify the four factors to retain in exploratory factor analysis.
Exploratory factor analysis (EFA)
Two models, a three-factor and a four-factor model, were compared to identify the most suitable number of factors. The four-factor model demonstrated a significantly better fit, as the three-factor model (x2(168) = 1,527.04, CFI = 0.89, TLI = 0.85, RMSEA = 0.09) demonstrated lower fit indices compared to the four-factor model to (x2(149) = 957.23, CFI = 0.94, TLI = 0.90, RMSEA = 0.07). The statistical improvements in fit support the inclusion of a fourth factor, as it better captures the multivariate subspace of the data. In turn, if the inclusion of an additional factor aligns well with known constructs, it is often best to include it. In concert with these fit values, the PCA results indicating a fourth eigenvalue close to unity suggest a four-factor model will result in a more parsimonious model.
Latent factor names
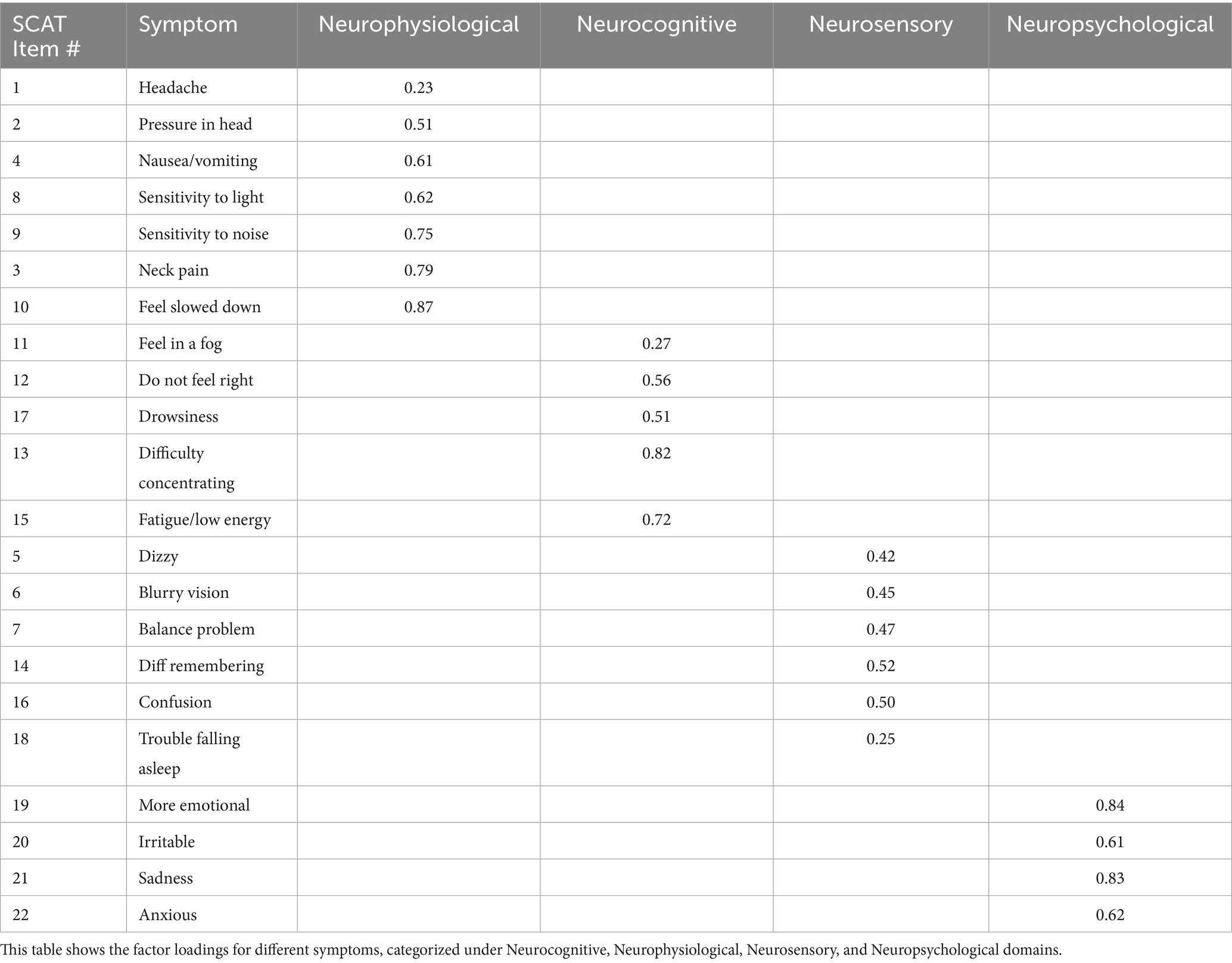
Based on the factor analysis shown in Table 4, the latent factors labeled as Neurocognitive, Neurophysiological, Neurosensory, and Neuropsychiatric were selected to encompass various dimensions of post-concussion symptoms. Each factor aggregates specific symptoms based on their underlying relationships and shared characteristics, providing a comprehensive understanding of the multifaceted impacts of concussions. The prefix “Neuro-” in each factor name effectively underscores the neurological basis of the symptoms associated with post-concussion syndrome:
1. Neurocognitive: Cognitive impairments, such as difficulty concentrating, memory problems, and mental fog, typically manifest from neurological disruption following a concussion. Labeling this factor as “neurocognitive” highlights the brain-based origin of these dysfunctions.
2. Neurophysiological: This factor includes symptoms that, while physical (e.g., headaches, sensitivity to light and noise), are a direct result of neurological damage resulting from concussion. This emphasizes that these symptoms are linked to neurological processes related to SRC, not just physical ailments involving other parts of the body.
3. Neurosensory: Symptoms grouped under this factor (e.g., blurry vision, balance problems, dizziness) are sensory-related and tied to the sensory pathways in the brain affected by the concussion. “Neurosensory” underscores the neurological origin of these sensory disturbances.
4. Neuropsychiatric: Emotional and behavioral symptoms (e.g., irritability, sadness, anxiety) often have neurological underpinnings. Labeling them as “neuropsychiatric” acknowledges that these symptoms are psychiatric potentially resulting from their SRC.
Thus, each factor aggregates specific symptoms based on their underlying relationships and shared characteristics with respect to self-reported ratings. Importantly, males and females tend to load onto these factors differently which prompts further examination into those items which may be driving these differences.
Linear discriminant analysis (LDA)
LDA was conducted to identify items which, in a weighted linear combination, maximized differences in how groups responded and to identify potential item biases in advance of conducting a more item-specific DIF analysis (see below). The LDA revealed significant differences in symptom reporting between males and females (Wilk’s Λ = 0.82, χ2 (22) = 130.56, p < 0.001), with an accuracy of 91% in distinguishing between the two groups. Thus, the differences between the groups are statistically significant and that the discriminant function is capturing meaningful distinctions between the genders, even though the effect size might be modest. Therefore, symptoms such as emotional distress, pressure in the head, sensitivity to noise, drowsiness, and sadness contributed most to these differences, with females reporting having these symptoms more frequently (Table 5).
This density plot in Figure 4 of the LDA-derived distributions illustrates the separation of optimized symptom scores between male and female NCAA athletes. The overlap indicates some shared symptom presentation across genders. Still, the shift in the peak density for males compared to females suggests that females report specific symptoms at a slightly higher discriminant score, reflecting potential differences in symptom severity or reporting behavior between the two groups. Thus, the discriminant function holds practical importance in differentiating males from females in terms of symptom reporting.
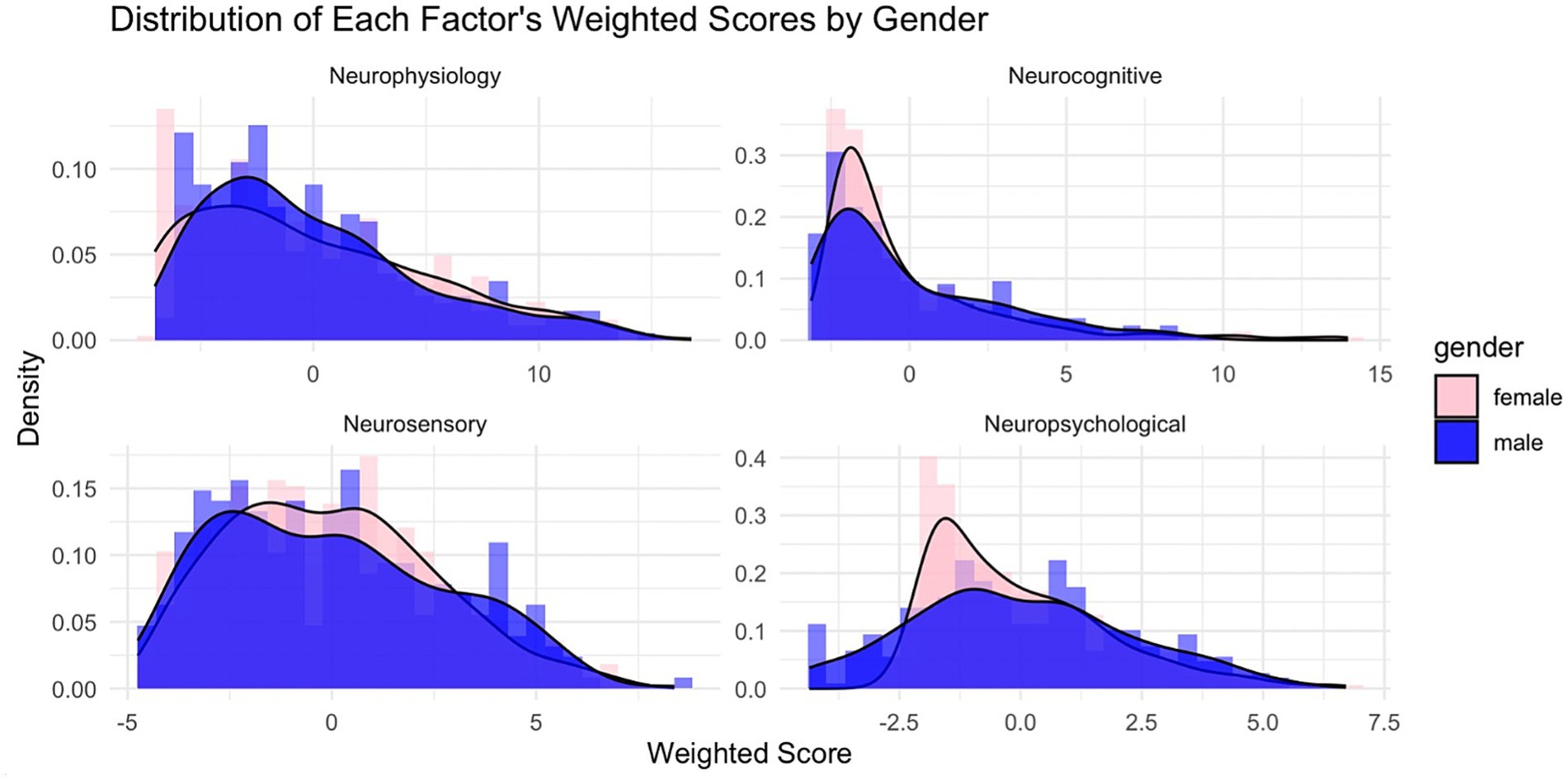
Figure 4. Factor score distributions by factor by gender. Factor scores were computed on each of the, so-named, Neurophysiology, Neurocognitive, Neurosensory, and Neuropsychological factors using the extracted weightings as presented in Table 3. Males (blue) and females (light red) are distinctly shown.
Rasch partial credit model (PCM)
This Rasch model analysis utilizing the Partial Credit Model (PCM) was conducted through WINSTEPS Version 5.8.5 (52) to examine the item thresholds for various symptoms related to a specified condition. The analysis delineated at which points along the latent trait continuum individuals were more likely to endorse successive response categories for each symptom, thus providing insight into the differential sensitivity of items as the underlying condition intensifies (45, 46) (see Figure 5).
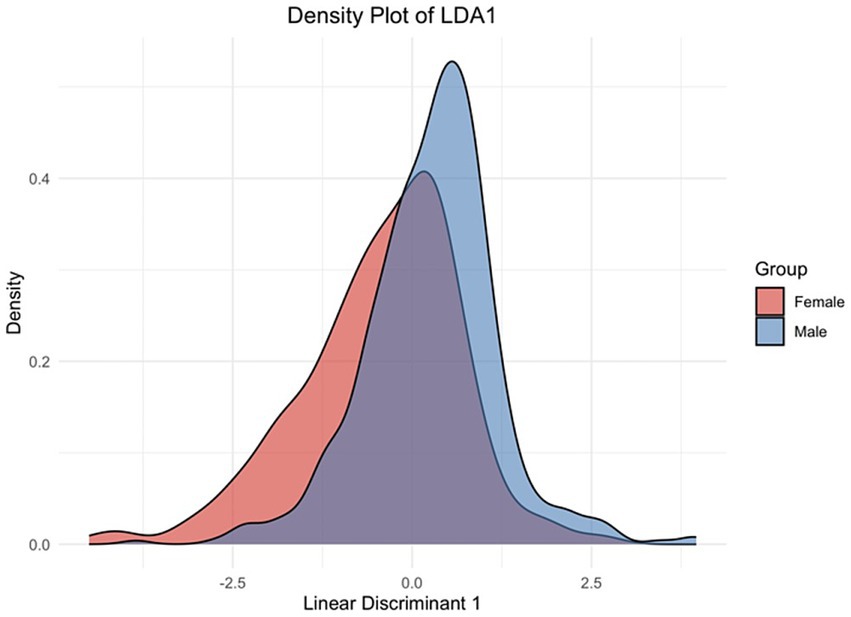
Figure 5. Linear discriminant density plot. The graph shows how the linear discriminant function distinguishes between the male and female groups based on the underlying symptoms or features included in the model. The peaks of the curves indicate the most common LDA1 scores for each gender, while the spread of the curves reflects the variability within each group.
The results in Table 6 indicated that the “Headache” has step difficulties ranging from −3.89 to 1.98, suggesting a wide range where this symptom progressively becomes more likely to be reported as the latent trait level increases from very low to high. For “Pressure in Head,” step difficulties spanned from −2.8 to 2.26, demonstrating that this symptom is relevant across a broad spectrum of the latent trait severity, with both genders starting to report this symptom at moderately low levels of the underlying trait. However, a few items displayed non-monotonic step difficulties patterns, where athletes report specific symptoms more efficiently at low severity levels but underreport them at moderate or high severity levels. Symptoms like “Nausea/Vomiting,” “Irritability,” “Sadness,” “Neck Pain,” and “Balance Problems,” respectively, suggest varied levels of the trait.
In examining monotonicity, most items indicated that as severity levels increase, the frequency of reporting symptoms as severe decreases. However, several symptoms display varying step difficulties, meaning reporting symptoms as a 1–2 or 5–6 symptoms may be more easily endorsed; however, reporting symptoms as moderate may be more difficult. For example, the symptom “Nausea/Vomiting” shows distorted thresholds as reporting symptoms as a 1 (δ1 = 0.19) or a 3 (δ1 = 0.15) and was more difficult to endorse than symptoms as 2 (δ1 = −0.42). As a result, certain symptoms may exhibit nonlinear characteristics. This means the relationship between these symptoms and their underlying causes does not follow a straightforward, predictable pattern.
Differential item functioning (DIF)
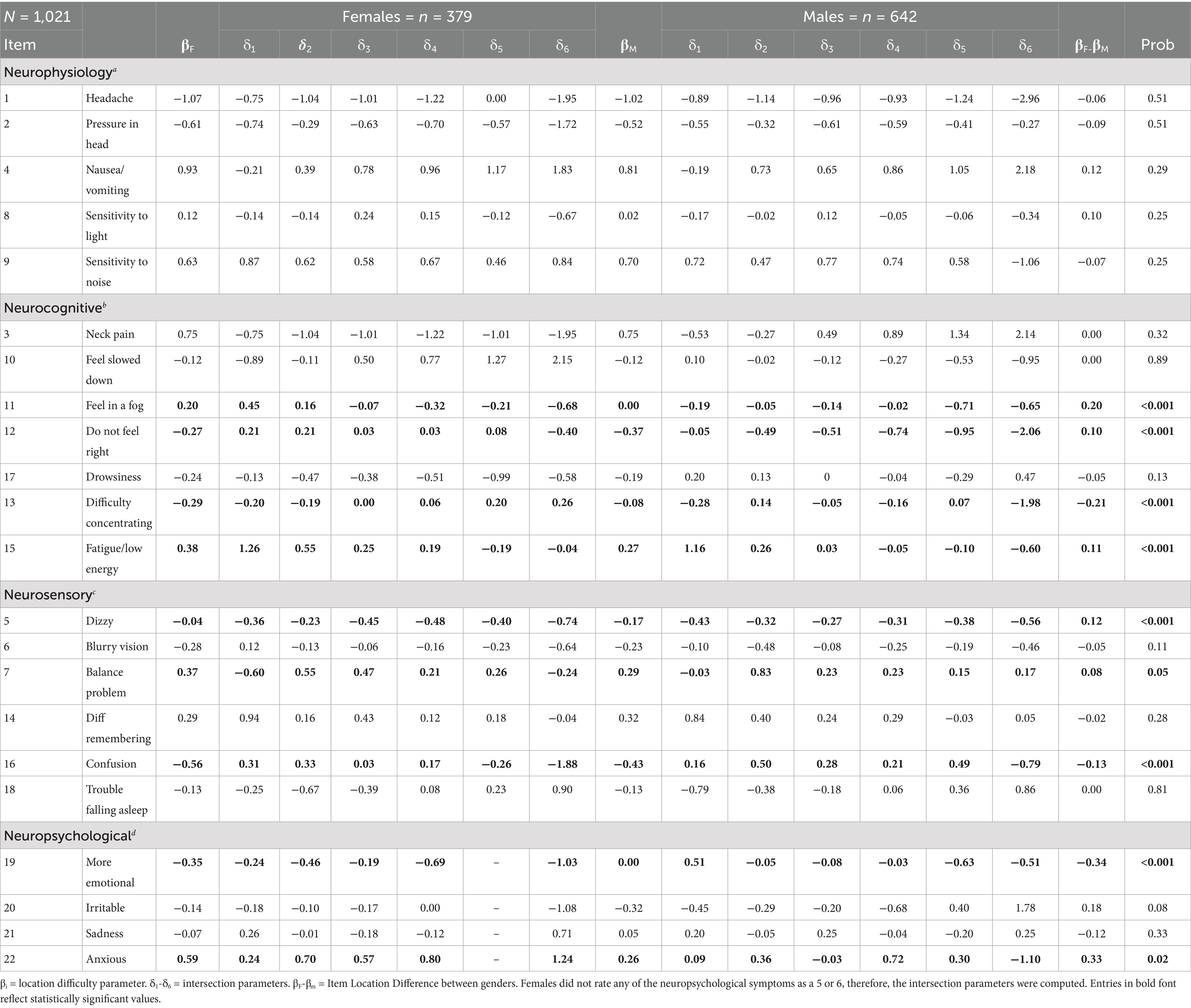
The results highlight significant gender differences in the reporting of concussion symptoms, with notable findings in several key symptom dimensions. DIF analysis reveals that specific symptom dimensions—such as neuropsychological, neurosensory, and neurocognitive—exhibit the most substantial gender-based differences in symptom reporting.
Table 7 shows that nine symptoms in three dimensions exhibited significant DIF. Symptoms such as “Feel in a Fog,” “Do not Feel Right,” “Fatigue,” “Dizzy,” “Balance Problem,” and “Anxious” exhibit positive levels of DIF, with female athletes consistently having reported lower symptom severity These findings support the PCM findings, as symptoms such as “Feeling in a Fog,” “Fatigue,” and “Balance Problem” indicating that males report higher severity more frequently, potentially due to underreporting at lower levels. In contrast, other symptoms such as “Difficulty Concentrating,” “More Emotional,” and “Confusion” demonstrate DIF in the opposite direction, indicating that male athletes are significantly less likely to endorse these symptoms at higher severity levels compared to females.
These results, in addition to those of the LDA, provide robust evidence for gender differences in concussion symptom reporting, particularly in how male and female athletes respond to varying levels of symptom severity. Moreover, males’ symptom profile post-concussion aligns within cognitive and sensory domains, which might not affect their overall perception of symptom burden as much as the emotional and physical symptoms reported by females. Furthermore, the inflation in total symptom scores for females could be indicative of a reporting pattern where more diffuse symptoms contribute to a perception of greater severity, potentially leading to an overestimation of symptom burden.
Discussion
The results of this study have several specific implications for the diagnosis, management, and treatment of female athletes with SRC. Firstly, the findings from this examination underscore the need for gender-sensitive approaches to concussion assessment. The SCAT self-report items, while widely used, may not be sufficient to capture the full spectrum of symptoms experienced by female athletes when used in its unidimensional form. Clinicians may need to account for the higher likelihood of emotional and sensory symptoms in females, which could contribute to a higher total symptom score but may not necessarily reflect more severe neurological impairment. Future revisions of the SCAT self-report questions and other concussion assessment tools should consider including gender-specific norms or symptom weightings to improve diagnostic accuracy and provide a more comprehensive assessment with respect to concussion symptoms experienced by women (53).
Secondly, the results suggest that female athletes may require more individualized post-concussion considerations. The presence of emotional symptoms, such as anxiety and sadness, reported more frequently by female athletes, emphasizes the importance of providing comprehensive mental health support as part of concussion recovery. Additional psychological counseling, monitoring for depression and anxiety, and ensuring that emotional symptoms likely need particular attention and best not overlooked during clinical evaluations in female athletes (13).
Third, one potential limitation of the current analysis is the unequal distribution of male and female participants, which introduces statistical and interpretive challenges when examining gender differences. Uneven sample sizes can affect the precision of parameter estimates, reduce statistical power in the smaller group, and increase the likelihood of Type II error (50). In multigroup modeling, disparities in group sizes can also inflate fit indices in favor of the larger group, potentially obscuring meaningful effects in the underrepresented group. Moreover, imbalanced samples may raise concerns about generalizability and representation, especially in studies aiming to identify sex-based disparities in concussion outcomes. To mitigate these concerns, measurement invariance testing was conducted, and sensitivity analyses were run on sport-matched subsamples (e.g., soccer, lacrosse) to reduce sport-specific variability. These additional steps helped ensure that the observed gender differences were not solely attributable to sample size disparities or contextual differences in sport exposure.
However, the analyses performed here on the SCAT’s self-reporting portion reveal that this portion of the SCAT – the most consistent component over its history—encompasses a more nuanced set of symptom sub-scales within its broader collection of symptom ratings, highlighting complexities not captured by its single aggregated score. Certain symptom clusters—such as those related to mood, migraine-like pain, and cognitive fogginess—tend to emerge as distinct factors within the overall self-reported symptomatology, suggesting that a multivariate understanding could better capture the multidimensional experience of concussion. Additionally, male and female athletes often report differing symptom severities on specific items, with female athletes generally rating symptoms like headache, nausea, and emotional sensitivity more intensely than their male counterparts. These differences point to a need for gender-specific symptom profiling within the SCAT’s self-report section to capture the variations in how concussions manifest and are experienced across athletes. Recognizing and addressing these sub-scales and gender-related response differences could make the SCAT a more finely tuned tool for assessing and monitoring concussion, leading to more individualized and effective clinical management.
Multivariate analyses revealed the presence of four distinct symptom clusters, reinforcing the argument for a multidimensional approach to concussion symptom assessment. Prior studies support that an effective concussion care protocol must acknowledge the neurocognitive, neurophysiological, neurosensory, and neuropsychiatric dimensions of symptoms (4, 34). The unidimensional model employed by SCAT3, as analyzed here, is likely insufficient to capture the intricate interplay among these dimensions, potentially leading to misdiagnoses or mismanagement in athletic settings.
Gender differences in SRC self-reporting
The present investigation highlights significant gender disparities in post-concussion symptom reporting among a large sample of NCAA student-athletes obtained, underscoring the limitations of the SCAT3 Symptom Severity Checklist’s traditionally scored unidimensional structure. Female athletes demonstrated a higher overall symptom severity, particularly within the emotional and sensory domains, suggesting an inherent bias in symptom assessment that warrants further clinical attention. These findings are crucial to understanding the implications of personalized concussion management strategies, especially given the historical tendency to underestimate the severity of symptoms in male athletes.
Additionally, it has been suggested that gender differences in symptom reporting may stem from cultural influences, with male athletes often underreporting symptoms due to societal pressures to exhibit “toughness” in competitive environments (71). Such behavioral discrepancies can compromise the accuracy of symptom assessment and contribute to inflated severity scores for female athletes within the critical period following injury. This calls for a reevaluation of current assessment tools, as failing to account for these gender-based differences may result in male athletes being inaccurately perceived as less symptomatic, thereby jeopardizing their health and recovery trajectory.
The evidence emphasizes the need to modify existing concussion assessment methods, particularly the SCAT3. Clinicians must adopt a nuanced understanding of symptom reporting that integrates gender-specific considerations to enhance the accuracy of diagnoses, inform appropriate management protocols, and ultimately improve outcomes for all athletes.
Limitations of the present investigation
This investigation was conducted on NCAA athletes, and it is important to note that these results cannot be generalized to youth, high school, or professional sports. Most of the participants in the CARE Consortium sample attended academic institutions with well-funded athletic programs, which may reflect a greater attention to concussion symptoms, better quality of treatment, and more formalized programs for concussion management. Therefore, it is unclear if such results would be the same or similar in participants drawn from smaller athletic programs, historically Black colleges and universities (HBCUs), or other ethnically unique programs. Consequently, it would be advantageous to conduct further studies on more comprehensive, national samples including different age groups, contact vs. non-contact, youth, collegiate, and professional sports, as well as socioeconomic backgrounds to better understand and generalize the properties of SCAT as they pertain to both male and female athletes.
Indeed, this examination could not consider any psychological or social factors impeding symptom reporting. It will be important for future research to include variables that reflect how psychological and social factors influence gender biases at different stages of recovery (20, 31, 54, 55, 72). Researchers may want to expand upon the current Rasch Partial Credit Model to account for additional parameters to understand better how much external factors influence accurate symptom reporting. Rasch modeling will be a useful tool for researchers in the concussion field to evaluate the relationship between sociological pressures, such as reporting intentions, and diagnostic measures, such as symptom presentation, on the variability of recovery length.
Future SCAT assessment recommendations
A female-athlete-specific section in future versions of the SCAT is essential due to accumulating evidence that female athletes experience and report concussion symptoms differently from their male counterparts. Female athletes are more likely to report symptoms such as migraines, mood disturbances, and neck pain after a concussion, which may be linked to anatomical, hormonal, and physiological differences. These differences not only affect symptom severity but can also influence recovery duration, as female athletes often report prolonged symptom durations compared to male athletes.
The current SCAT assessment, however, remains largely agnostic to gender differences, potentially leading to under-recognition or misinterpretation of symptoms in female athletes. By incorporating a new section in future versions of the SCAT dedicated to symptoms and issues more commonly reported by female athletes, such as hormonal influences on mood and menstrual cycle irregularities, the SCAT could provide a more accurate and comprehensive picture of concussion specifically as it pertains to women. This tailored approach would support clinicians in identifying concussion effects more precisely and creating individualized care plans that consider the unique recovery patterns of female athletes. A female-athlete-specific section in the SCAT would, thus, represent a critical step forward in equitable, evidence-based concussion care for athletes across all sports and competition levels.
Our analysis revealed that gender differences in the self-reporting of concussion symptoms, as measured by the SCAT assessment, are best conceptualized through a multidimensional structure comprising four distinct symptom subscales: neurocognitive, neurophysiological, neurosensory, and neuropsychological. This novel factor solution advances the field by offering a more nuanced framework for interpreting symptom patterns following sport-related concussion, particularly in the context of sex-specific variation. By identifying these four latent domains through combined exploratory graph analysis, principal component analysis, and linear discriminant analysis, the current study contributes to a growing body of research that emphasizes the value of domain-specific symptom modeling over traditional total score comparisons. Importantly, this work builds on—and helps reconcile—prior studies that have reported inconsistent SCAT factor structures across different populations and statistical approaches (28, 29, 34). Clinically, these findings support the development of more individualized and gender-informed approaches to post-injury symptom tracking, which may ultimately improve diagnostic precision, monitoring of recovery, and return-to-play decision-making in both male and female athletes (28, 29, 34).
More specifically, to enhance the SCAT’s sensitivity to the unique experiences of female athletes with SRC, based upon the discriminant analysis performed here, five additional assessment items concerning the following might be considered:
Menstrual Cycle Changes and Symptoms: Concussions can impact the menstrual cycle resulting in irregularities or heightened premenstrual symptoms, which, themselves, may complicate recovery. An item asking about recent changes in menstrual patterns or cycle-related symptom severity would allow clinicians to monitor potential hormonal impacts that may influence both symptoms and healing time in female athletes.
Mood Changes and Emotional Sensitivity: Research indicates that female athletes are more likely to report mood swings, irritability, and emotional sensitivity post-concussion. Adding an item specifically assessing mood disturbances (“Have you experienced increased mood swings, irritability, or emotional sensitivity?”) could help clinicians monitor this common symptom and inform recovery strategies.
Sleep Disturbances Related to Hormonal Fluctuations: Likewise, hormonal fluctuations can affect sleep quality in female athletes, which may exacerbate concussion recovery. A targeted item assessing sleep issues with attention to any recent menstrual or hormonal changes (e.g., “Have you experienced disrupted sleep, especially during your menstrual cycle?”) could give a fuller picture of factors influencing recovery.
Migraine-Like Symptoms: While headache is included in the SCAT, female athletes often report migraine-like symptoms, such as throbbing pain and heightened sensitivity to light or sound, more frequently than male athletes following concussion. A more specific item assessing the nature and intensity of headache symptoms (e.g., “Is the headache migraine-like, with throbbing or sensitivity to light/sound?”) could capture this experience more accurately.
Neck Pain and Whiplash Sensitivity: Female athletes suffer higher instances of neck pain and whiplash-like symptoms post-injury, likely due to anatomical and muscular differences (56). A new item assessing neck-related symptoms or pain more carefully would allow athletic trainers and clinicians to differentiate these from primary concussion symptoms, thereby refining diagnosis and treatment options.
Incorporating a new section including items on these topics could make future versions of the SCAT more responsive to the unique symptom experiences of female athletes, potentially leading to more personalized and effective concussion management strategies.
Conclusion
In conclusion, this quantitatively-focused examination highlights the multidimensional nature of concussion self-reporting of symptoms as measured by the SCAT Symptom Severity Checklist and underscores the importance of (1) the multi-factorial nature of the SCAT symptom self-reporting, as well as (2) more carefully considering gender differences in concussion assessment. The findings suggest that an athlete-gender-agnostic approach to concussion symptom severity may not be appropriate and that gender-specific considerations should be integrated into clinical assessments and treatment plans. By adopting a more nuanced, multidimensional approach, athletic training staff and healthcare providers can ensure more precise diagnosis and tailored interventions, ultimately improving outcomes for all athletes. Neuropsychological testing is recommended to remain a key component in evaluating complex concussions, although it is not currently considered essential for assessing simple concussions. While the SCAT assessment and the analyses examined here significantly enhances both the understanding of concussion effects and the potential for management of individual athletes, they should not serve as the sole basis for decisions regarding time away from play or return-to-play readiness. Nevertheless, the clinical management of concussions specific to the symptoms female athletes tend to report is not often part of current SRC assessments (57) as currently practiced, as exemplified by the SCAT. If included in future versions of the SCAT, the additions of self-report items, as recommended here, strategically added to the sub-scale structure or as its own sub-scale, altogether, would undoubtedly provide important context to individualized SRC treatment approaches.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://fitbir.nih.gov.
Ethics statement
The studies involving humans were approved by International Review Board University of Virginia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing. JH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to thank contributions to an earlier version of this manuscript from Ms. Sydney Cushing of the Department of Psychology at the University of Virginia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Broglio, SP, Cantu, RC, Gioia, GA, Guskiewicz, KM, Kutcher, J, Palm, M, et al. National athletic trainers' association position statement: management of sport concussion. J Athl Train. (2014) 49:245–65. doi: 10.4085/1062-6050-49.1.07
2. Dick, RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. (2009) 43:i46–50. doi: 10.1136/bjsm.2009.058172
3. Harmon, KG, Drezner, JA, Gammons, M, Guskiewicz, KM, Halstead, M, Herring, SA, et al. American medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. (2013) 47:15–26. doi: 10.1136/bjsports-2012-091941
4. Kroshus, E, Lowry, SJ, Garrett, K, Hays, R, Hunt, T, and Chrisman, SPD. Development of a scale to measure expected concussion reporting behavior. Inj Epidemiol. (2021) 8:70. doi: 10.1186/s40621-021-00364-4
5. McCrory, P, Meeuwisse, W, Aubry, M, Cantu, B, Dvořák, J, Echemendia, R, et al. Consensus statement on concussion in sport – the 4th international conference on concussion in sport held in Zurich, November 2012. Phys Ther Sport. (2013) 14:e1–e13. doi: 10.1016/j.ptsp.2013.03.002
6. Meehan, WP, Mannix, RC, O’Brien, MJ, and Collins, MW. The prevalence of undiagnosed concussions in athletes. Clin J Sport Med. (2013) 23:339–42. doi: 10.1097/JSM.0b013e318291d3b3
7. Gessel, LM, Fields, SK, Collins, CL, Dick, RW, and Comstock, RD. Concussions among United States high school and collegiate athletes. J Athl Train. (2007) 42:495–503.
8. Granito, VJ. Psychological response to athletic injury: gender differences. J Sport Behav. (2002) 25:243.
9. Kieffer, EE, Brolinson, PG, Maerlender, AE, Smith, EP, and Rowson, S. In-season concussion symptom reporting in male and female collegiate Rugby athletes. Neurotrauma Reports. (2021) 2:503–11. doi: 10.1089/neur.2021.0050
10. Mihalik, JP, Ondrak, KS, Guskiewicz, KM, and McMurray, RG. The effects of menstrual cycle phase on clinical measures of concussion in healthy college-aged females. J Sci Med Sport. (2009) 12:383–7. doi: 10.1016/j.jsams.2008.05.003
11. O’Connor, KL, Baker, MM, Dalton, SL, Dompier, TP, Broglio, SP, and Kerr, ZY. Epidemiology of sport-related concussions in high school athletes: National Athletic Treatment, injury and outcomes network (NATION), 2011–2012 through 2013–2014. J Athl Train. (2017) 52:175–85. doi: 10.4085/1062-6050-52.1.15
12. Schatz, P, Moser, RS, Covassin, T, and Karpf, R. Early indicators of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery. (2011) 68:1562–7. doi: 10.1227/NEU.0b013e31820e382e
13. Wallace, J, Covassin, T, and Beidler, E. Gender differences in high school athletes’ knowledge of sport-related concussion symptoms and reporting behaviors. J Athl Train. (2017) 52:682–8. doi: 10.4085/1062-6050-52.3.06
14. Zuckerman, SL, Apple, RP, Odom, MJ, Lee, YM, Solomon, GS, and Sills, AK. Effect of gender on symptoms and return to baseline in sport-related concussion: clinical article. J Neurosurg Pediatr. (2014) 13:72–81. doi: 10.3171/2013.9.PEDS13257
15. Echemendia, RJ, Meeuwisse, W, McCrory, P, Davis, GA, Putukian, M, Leddy, J, et al. The sport concussion Assessment tool 5th edition (SCAT5). Br J Sports Med. (2017). doi: 10.1136/bjsports-2017-097506
16. Garcia, G-GP, Yang, J, Lavieri, MS, McAllister, TW, McCrea, MA, Broglio, SP, et al. Optimizing components of the sport concussion assessment tool for acute concussion assessment. Neurosurgery. (2020) 87:971–81. doi: 10.1093/neuros/nyaa150
17. Chandran, A, Boltz, AJ, Morris, SN, Robison, HJ, Nedimyer, AK, Collins, CL, et al. Epidemiology of concussions in National Collegiate Athletic Association (NCAA) sports: 2014/15-2018/19. Am J Sports Med. (2022) 50:526–36. doi: 10.1177/03635465211060340
18. Edelstein, R, and Van Horn, JD. Modulating factors affecting sports-related concussion exposures: a systematic review and analysis. medRxiv. (2023). doi: 10.1101/2023.03.08.23286974
19. Zuckerman, SL, Kerr, ZY, Yengo-Kahn, A, Wasserman, E, Covassin, T, and Solomon, GS. Epidemiology of sports-related concussion in NCAA athletes from 2009-2010 to 2013-2014: incidence, recurrence, and mechanisms. Am J Sports Med. (2015) 43:2654–62. doi: 10.1177/0363546515599634
20. Beran, KM, and Scafide, KN. Factors related to concussion knowledge, attitudes, and reporting behaviors in US high school athletes: a systematic review. J Sch Health. (2022) 92:406–17. doi: 10.1111/josh.13140
21. Broshek, DK, Kaushik, T, Freeman, JR, Erlanger, D, Webbe, F, and Barth, JT. Gender differences in outcome following sports-related concussion. J Neurosurg. (2005) 102:856–63. doi: 10.3171/jns.2005.102.5.0856
22. Brown, DA, Elsass, JA, Miller, AJ, Reed, LE, and Reneker, JC. Differences in symptom reporting between males and females at baseline and after a sports-related concussion: a systematic review and meta-analysis. Sports Med. (2015) 45:1027–40. doi: 10.1007/s40279-015-0335-6
23. Cantu, RC, and Register-Mihalik, JK. Considerations for return-to-play and retirement decisions after concussion. PM&R. (2011) 3:S440–4. doi: 10.1016/j.pmrj.2011.07.013
24. Covassin, T, and Elbin, RJ. The female athlete: the role of gender in the assessment and management of sport-related concussion. Clin Sports Med. (2011) 30:125–31. doi: 10.1016/j.csm.2010.08.001
25. Edelstein, R, Gutterman, S, Newman, B, and Van Horn, JD. Assessment of sports concussion in female athletes: a role for neuroinformatics? Neuroinformatics. (2024) 22:607–18. doi: 10.1007/s12021-024-09680-8
26. D’Lauro, C, Jones, ER, Swope, LM, Anderson, MN, Broglio, S, and Schmidt, JD. Under-representation of female athletes in research informing influential concussion consensus and position statements: an evidence review and synthesis. Br J Sports Med. (2022) 56:981–7. doi: 10.1136/bjsports-2021-105045
27. Randolph, C, Millis, S, Barr, WB, McCrea, M, Guskiewicz, KM, Hammeke, TA, et al. Concussion symptom inventory: an empirically derived scale for monitoring resolution of symptoms following sport-related concussion. Arch Clin Neuropsychol. (2009) 24:219–29. doi: 10.1093/arclin/acp025
28. Alsalaheen, B, Li, Y, Almeida, A, Eckner, J, Freeman, J, Popovich, M, et al. Factor structure for the sport concussion Assessment tool symptom scale in adolescents after concussion. Clin J Sport Med. (2022) 32:400–7. doi: 10.1097/JSM.0000000000000959
29. Anderson, M, Petit, KM, Bretzin, AC, Elbin, RJ, Stephenson, KL, and Covassin, T. Sport concussion Assessment tool symptom inventory: healthy and acute postconcussion symptom factor structures. J Athl Train. (2020) 55:1046–53. doi: 10.4085/1062-6050-393-19
30. D'Alonzo, BA, Barnett, IJ, Master, CL, Hamilton, RH, Wiebe, DJ, Schneider, ALC, et al. Factor structure and measurement invariance across sex of the sport concussion assessment tool symptom inventory. Clin J Sport Med. (2024) 35:e61–8. doi: 10.1097/JSM.0000000000001301
31. CARE Consortium InvestigatorsBroglio, SP, Katz, BP, Zhao, S, McCrea, M, and McAllister, T. Test-retest reliability and interpretation of common concussion assessment tools: findings from the NCAA-DoD CARE consortium. Sports Med. (2018) 48:1255–68. doi: 10.1007/s40279-017-0813-0
32. Chin, EY, Nelson, LD, Barr, WB, McCrory, P, and McCrea, MA. Reliability and validity of the sport concussion assessment tool–3 (SCAT3) in high school and collegiate athletes. Am J Sports Med. (2016) 44:2276–85. doi: 10.1177/0363546516648141
33. Wilmoth, K, Magnus, BE, McCrea, MA, and Nelson, LD. Preliminary validation of an abbreviated acute concussion symptom checklist using item response theory. Am J Sports Med. (2020) 48:3087–93. doi: 10.1177/0363546520953440
34. Nelson, LD, Kramer, MD, Patrick, CJ, and McCrea, MA. Modeling the structure of acute sport-related concussion symptoms: a Bifactor approach. J Int Neuropsychol Soc. (2018) 24:793–804. doi: 10.1017/S1355617718000462
35. Guskiewicz, KM, Register-Mihalik, J, McCrory, P, McCrea, M, Johnston, K, Makdissi, M, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3: table 1. Br J Sports Med. (2013) 47:289–93. doi: 10.1136/bjsports-2013-092225
36. Golino, HF, and Epskamp, S. Exploratory graph analysis: a new approach for estimating the number of dimensions in psychological research. PLoS One. (2017) 12:e0174035. doi: 10.1371/journal.pone.0174035
37. Pearson, K. On lines and planes of closest fit to systems of points in space. Philos Mag. (1901) 2:559–72.
38. Spearman, C. “General intelligence,” objectively determined and measured. Am J Psychol. (1904) 15:201–92. doi: 10.2307/1412107
39. Rencher, AC. Interpretation of canonical discriminant functions, canonical variates, and principal components. Am Stat. (1992) 46:217–25. doi: 10.1080/00031305.1992.10475889
40. Masters, GN. A Rasch model for partial credit scoring. Psychometrika. (1982) 47:149–74. doi: 10.1007/BF02296272
41. Bakker, LA, Schröder, CD, Van Es, MA, Westers, P, Visser-Meily, JMA, and Van Den Berg, LH. Assessment of the factorial validity and reliability of the ALSFRS-R: a revision of its measurement model. J Neurol. (2017) 264:1413–20. doi: 10.1007/s00415-017-8538-4
42. Xanthopoulos, P, Pardalos, PM, and Trafalis, TB. Linear discriminant analysis In: P Xanthopoulos, PM Pardalos, and TB Trafalis, editors. Robust data mining. New York: Springer (2013). 27–33.
44. Masters, GN, and Wright, BD. The partial credit model In: WJ Van Der Linden and RK Hambleton, editors. Handbook of modern item response theory. New York: Springer (1997). 101–21.
45. de Ayala, RJ. Theory and practice of item response theory. New York: Guilford Publications (2013).
46. Embretson, SE, and Reise, SP. Item response theory for psychologists. Mahwah, NJ: Erlbaum (2000).
47. Tennant, A, and Conaghan, PG. The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper? Arthritis Care Res. (2007) 57:1358–62. doi: 10.1002/art.23108
48. Bollmann, S, Berger, M, and Tutz, G. Item-focused trees for the detection of differential item functioning in partial credit models. Educ Psychol Meas. (2018) 78:781–804. doi: 10.1177/0013164417722179
49. Dorans, NJ, and Holland, PW. Dif detection and description: Mantel-Haenszel and standardization. ETS Res. Rep. Series. (1992) 1992:40. doi: 10.1002/j.2333-8504.1992.tb01440.x
50. Benjamini, Y, and Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
51. Buchardt, A-S, Christensen, KB, and Jensen, SN. Visualizing Rasch item fit using conditional item characteristic curves in R. Psychol Test Assess Model. (2023) 2:206–219. Available online at: https://www.psychologie-aktuell.com/fileadmin/Redaktion/Journale/ptam_2023-2/PTAM__2-2023_2_kor.pdf
52. Linacre, J. WINSTEPS®; (2023). Available online at: https://www.winsteps.com/index.htm
53. McCrory, P, Meeuwisse, W, Johnston, K, Dvorak, J, Aubry, M, Molloy, M, et al. Consensus statement on concussion in sport 3rd international conference on concussion in sport held in Zurich, November 2008. Clin J Sport Med. (2009) 19:185–200. doi: 10.1097/JSM.0b013e3181a501db
54. Lempke, LB, Oldham, JR, Passalugo, S, Willwerth, SB, Berkstresser, B, Wang, F, et al. Influential factors and preliminary reference data for a clinically feasible, functional reaction time assessment: the standardized assessment of reaction time. J Athl Train. (2023) 58:112–9. doi: 10.4085/1062-6050-0073.22
55. Sinnott, AM, Eagle, SR, Kochick, V, Bricker, IR, Collins, MW, Sparto, PJ, et al. Test-retest, interrater reliability, and minimal detectable change of the dynamic exertion test (EXiT) for concussion. Sports Health. (2023) 15:410–21. doi: 10.1177/19417381221093556
56. Lin, CY, Casey, E, Herman, DC, Katz, N, and Tenforde, AS. Gender differences in common sports injuries. PM&R. (2018) 10:1073–82. doi: 10.1016/j.pmrj.2018.03.008
57. Malcolm, D. Some problems of research exploring gender differences in sport-related concussions: a narrative review. Res Sports Med. (2023):1–10. doi: 10.1080/15438627.2023.2271604
58. Musko, PA, and Demetriades, AK. Are Sex Differences in Collegiate and High School Sports-Related Concussion Reflected in the Guidelines? A Scoping Review. Brain Sciences. (2023) 13:1310. doi: 10.3390/brainsci13091310
59. Iverson, GL, Karr, JE, Maxwell, B, Zafonte, R, Berkner, PD, and Cook, NE. Examining Criteria for Defining Persistent Post-concussion Symptoms in Children and Adolescents. Frontiers in Neurology. (2021) 12:614648. doi: 10.3389/fneur.2021.614648
60. Resch, JE, Rach, A, Walton, S, and Broshek, DK. Sport Concussion and the Female Athlete. Clinics in Sports Medicine. (2017) 36:717–39. doi: 10.1016/j.csm.2017.05.002
61. Alla, S, Sullivan, SJ, Hale, L, and McCrory, P. Self-report scales/checklists for the measurement of concussion symptoms: A systematic review. British Journal of Sports Medicine. (2009) 43:i3–i12. doi: 10.1136/bjsm.2009.058339
62. Bretzin, AC, Esopenko, C, D’Alonzo, BA, and Wiebe, DJ. Clinical Recovery Timelines After Sport-Related Concussion in Men’s and Women’s Collegiate Sports. Journal of Athletic Training. (2022) 57:678–87. doi: 10.4085/601-20
63. Joyce, AS, Labella, CR, Carl, RL, Lai, J-S, and Zelko, FA. The Post-Concussion Symptom Scale: Utility of a Three-Factor Structure. Med Sci Sports Exercise. (2015) 47:1119–23. doi: 10.1249/MSS.0000000000000534
64. Heidari, S, Babor, TF, De Castro, P, Tort, S, and Curno, M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Research Integrity and Peer Review. (2016) 1:2. doi: 10.1186/s41073-016-0007-6
65. McCrea, M, Guskiewicz, KM, Marshall, SW, Barr, W, Randolph, C, Cantu, RC, et al. Acute Effects and Recovery Time Following Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA. (2003) 290:2556. doi: 10.1001/jama.290.19.2556
66. Trendafilov, N, and Gallo, M. Multivariate Data Analysis on Matrix Manifolds: (With Manopt). Springer International Publishing. (2021). doi: 10.1007/978-3-030-76974-1
67. Angoff, WH, and Schrader, WB. A Study of Alternative Methods for Equating Rights Scores to Formula Scores. Princeton, NJ. (1981) 167. doi: 10.1002/j.2333-8504.1981.tb01241.x
68. R Core Team. (2022). R: A language and environment for statistical computing. Available online at: https://web.mit.edu/r_v3.3.1/fullrefman.pdf
69. Comrey, AL, and Lee, HB. A First Course in Factor Analysis. Psychology Press. (2013). doi: 10.4324/9781315827506
71. Master, A, Meltzoff, AN, and Cheryan, S. Gender stereotypes about interests start early and cause gender disparities in computer science and engineering. Proceedings of the National Academy of Sciences. (2021) 118:e2100030118. doi: 10.1073/pnas.2100030118
Keywords: SCAT3, concussion, gender differences, symptom reporting, NCAA athletes, psychometrics
Citation: Edelstein R, Schmidt KM and Van Horn JD (2025) Revealing gender differences in concussion reporting: a detailed analysis of SCAT assessment self-report symptom ratings. Front. Neurol. 16:1584875. doi: 10.3389/fneur.2025.1584875
Edited by:
Ansgar J. Furst, War Related Illness and Injury Study Center, United StatesReviewed by:
Michael F. La Fountaine, Seton Hall University, United StatesKryshawna Beard, Traumatic Brain Injury Center of Excellence, United States
Copyright © 2025 Edelstein, Schmidt and Van Horn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Edelstein, cWJqNm12QHZpcmdpbmlhLmVkdQ==
†ORCID: Rachel Edelstein, orcid.org/0000-0002-9904-186X
John Darrell Van Horn, orcid.org/0000-0003-1537-0816
 Rachel Edelstein
Rachel Edelstein Karen M. Schmidt
Karen M. Schmidt John Darrell Van Horn
John Darrell Van Horn