Abstract
Introduction:
Tourette syndrome (TS), a neurodevelopmental disorder, requires attention to the physical and psychological impacts of tics and associated comorbidities. Researchers are making efforts to clarify the pathophysiology of TS and develop effective treatments amidst its rising global prevalence. This study aimed to retrieve publications discussing TS in the context of the dopaminergic system from 1994 to 2023, summarize previous research, and analyze the general information and hotspots to provide references for future research and clinical applications.
Methods:
Literature was filtered from the Web of Science Core Collection. Excel, CiteSpace, VOSviewer, and Scimago Graphica were used to analyze and visualize the results.
Results:
A total of 482 related publications were included in the study. The United States has consistently led in research output, and Yale University demonstrates excellence in workload, impact, and collaboration. Harvey S. Singer has the highest number of publications. The hotspots include comorbidities, dopaminergic Components, candidate genes, and deep brain stimulation.
Discussion:
The analysis reveals that the understanding of TS is gradually evolving towards neuronal and genetic mechanisms. Concurrently, deep brain stimulation is being investigated as a treatment for refractory TS. These findings suggest a need for more in-depth research to produce higher-level evidence.
1 Introduction
In an article published in 1885, the French neurologist Georges Gilles de la Tourette described nine patients exhibiting involuntary movements and vocalizations, a condition that would later be named Tourette syndrome (TS) in his honor. Current estimates indicate that TS affects approximately 0.3–0.9% of children and 0.002–0.08% of adults worldwide (1). These prevalence rates highlight TS as a condition of growing public health concern. TS is a chronic neurodevelopmental disorder whose core symptoms include motor and phonic tics (1). Motor tics involve sudden, involuntary contractions of muscles in the face, neck, shoulders, or limbs, while phonic tics result from contractions of muscles in the oral, nasal, or throat regions that produce sounds. Tics are typically sudden, rapid, repetitive, non-rhythmic, and can be suppressed only temporarily. These symptoms significantly impact patients’ quality of life and social functioning. Moreover, TS is frequently associated with comorbid psychiatric conditions such as obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), anxiety, and depression (2).
Dopamine (DA) is a central neurotransmitter regulating a variety of critical brain functions. Dysregulation of dopaminergic signaling in the central nervous system (CNS) has been implicated in several neuropsychiatric disorders. In particular, the dopaminergic system remains the most extensively studied and clinically validated target in TS to date, an emphasis supported by both pathophysiology and treatment evidence. The established central role of dopamine in TS has spawned multifaceted, and often fragmented, pathophysiological hypotheses, encompassing diverse brain circuits, dopaminergic components (transporters, receptors, enzymes), and interacting neurotransmitter systems. To integrate this scattered landscape of cross-level complexity, a bibliometric analysis was conducted to quantitatively map global research trends. By synthesizing three decades of evidence, our study aims to delineate the evolving knowledge architecture, identify latent collaborative opportunities, thereby providing a data-driven framework to guide future research.
Bibliometrics emerged as a scientific discipline in 1969 and has since been applied extensively across various research fields (3). It employs quantitative methods to analyze scholarly publications, enabling the identification of knowledge structures, evolutionary patterns, and emerging trends within a discipline. However, to date, no bibliometric study has systematically explored the dopaminergic mechanisms underlying TS. To address this gap, this study collects and analyzes relevant literature from 1994 onward and uses computational tools to visually represent the research landscape. The aim is to identify current research hotspots, uncover underexplored issues, and provide guidance for future investigations, thereby contributing to advancements in the field of TS.
2 Methods
2.1 Data collection
The Web of Science (WOS) database is widely recognized as an authoritative and rigorously curated scientific platform, offering comprehensive and regularly updated content that is highly suitable for bibliometric analysis (4). To ensure data authority and breadth of coverage, this study utilized the Web of Science Core Collection (WOSCC) as the primary source (5). The literature search was meticulously initiated on October 18, 2024. The search strategy was input in the advanced search bar as (TS = (“Tourette syndrome” OR “Tic Disorder”) AND TS = (Dopamine)) AND DT = (Article OR Review) AND LA = (English), with the time range limited from January 1, 1994, to December 31, 2023. The literature screening and data extraction processes were conducted independently by two authors. A third author adjudicated discrepancies to ensure consensus and methodological rigor. Ultimately, 482 non-duplicate valid documents were acquired. The full record and references of the 482 documents were saved in plain text file format. Figure 1 provides a detailed description of the search process.
Figure 1

Flowchart of the search.
2.2 Data analysis
Microsoft Excel 2019, CiteSpace 6.1.6 (6), VOSviewer 1.6.20.0 (7), and Scimago Graphica (8) were utilized for data analysis and visualization, as these tools complement each other’s strengths in handling different aspects of bibliometric analysis. Excel was employed for statistical calculations and the creation of data tables. CiteSpace, a Java-based application developed by Dr. Chaomei Chen at Drexel University (9), was instrumental in conducting time-sliced network analyses. In this study, CiteSpace was configured with time slices spanning from 1994 to 2023 (one-year intervals), using appropriate linking, selection criteria, and pruning methods. It was used to examine the distribution relationships among countries, institutions, authors, and journals, as well as the co-occurrence of keywords. VOSviewer, which employs probabilistic normalization techniques, was applied to generate and visualize bibliometric networks in various formats, including network, overlay, and density maps (7).
2.3 Quality control
To ensure the highest standards of methodological rigor and reporting transparency in our bibliometric analysis, this study was conducted and reported in accordance with the Reporting and Measurement of Items for Bibliometric Studies (RAMIBS) guidelines (10). This checklist provides a comprehensive framework for bibliometric research, and we adhered to its recommendations regarding data sourcing, processing, analysis, and interpretation.
3 Results
The 482 papers in this study, from 2010 authors affiliated with 668 organizations in 40 countries, appeared in 220 journals and cited 21,671 references from 3,355 journals.
3.1 Annual publication trends
From 1994 to 2023, 482 documents have been published, comprising 356 articles and 126 reviews. In Figure 2, it can be observed that before 1997, research was in its infancy, then there was a slight fluctuation but an overall upward trend. Notable increases compared to the previous year were observed in 1997, 2003, 2013, peaking in 2016 (n = 32). To further clarify the publication growth trend, we established a polynomial fit curve, yielding the equation Y = 0.2982X2 + 7.3837X + 5.264 (R2 = 0.9984), indicating a strong correlation.
Figure 2

Trend of publications in the field.
3.2 Contribution of countries and institutions
This study assessed publications from 668 organizations across 40 countries to identify leading countries and institutions in the field. As shown in Figure 3A, the countries involved in the research are widely distributed and are divided into three groups. The United States, leading in the field, collaborates with numerous countries across the Americas, Europe, Asia, and Australia. The other two collaborative clusters are primarily centered in Asia and Europe.
Figure 3

Analysis of the countries. (A) Geographical distribution and cooperation of global by Scimago Graphica. (B) Links and cooperation between countries by CiteSpace. (C) The yearly output of the top five publishing nations.
In Figure 3B, certain purple-outlined countries are more prominent than others, including USA, UK, Germany, Netherlands, Canada, and China. The magnitude of a node’s centrality, which signifies its importance, is represented by the thickness of the purple ring. A centrality value above 0.1 denotes a node’s prominent position. Table 1 lists the top 10 countries that play a crucial role.
Table 1
| Rank | Country | Centrality | Country | Count |
|---|---|---|---|---|
| 1 | USA | 0.47 | USA | 221 |
| 2 | UK | 0.43 | China | 59 |
| 3 | Germany | 0.15 | Germany | 55 |
| 4 | Netherlands | 0.15 | UK | 47 |
| 5 | Canada | 0.11 | Canada | 39 |
| 6 | China | 0.1 | Italy | 29 |
| 7 | Italy | 0.04 | Netherlands | 21 |
| 8 | Denmark | 0.03 | France | 19 |
| 9 | France | 0.03 | Japan | 13 |
| 10 | South Korea | 0.02 | Israel | 12 |
The top 10 countries with the highest centrality and number of publications.
Figure 3C presents that while the United States has consistently led, China’s output has increased over the past decade, occasionally exceeding that of the USA. Germany and the UK have also shown potential in recent years.
Table 2 lists the top five institutions by centrality and publication volume, which are highlighted in Figures 4A,B displays the collaborative relationships. The top five institutions are all from the USA and Germany. Yale University, which produced the highest number of publications and achieved the greatest centrality, was ranked first and maintains collaborative relationships with numerous institutions.
Table 2
| Rank | Institutions | Centrality | Country | Institutions | Count | Country |
|---|---|---|---|---|---|---|
| 1 | Yale University | 0.39 | USA | Yale University | 28 | USA |
| 2 | Hannover Medical School | 0.21 | Germany | Johns Hopkins University | 19 | USA |
| 3 | Charité – Universitätsmedizin Berlin | 0.15 | Germany | Hannover Medical School | 15 | Germany |
| 4 | New York University | 0.12 | USA | Baylor College of Medicine | 13 | USA |
| 5 | Baylor College of Medicine | 0.11 | USA | Technical University of Dresden | 11 | Germany |
The top five institutions with the highest centrality and number of publications.
Figure 4

Analysis of the institutions. (A) Links and cooperation between institutions by CiteSpace. (B) Contribution and cooperation of different institutions by chord chart.
3.3 Outstanding authors and cited authors
Two thousand and ten authors participated in the publications. In Table 3, Harvey S. Singer published the most, totaling 15. Following him are David E. Comings and Donald L. Gilbert, who published 14 and 10, respectively. However, it is noteworthy that, as shown in Figure 5A, apart from clusters centered around Singer, Gilbert, Leckman, and Martino, there are few connections between other groups.
Table 3
| Rank | Author | Publications | Institutions |
|---|---|---|---|
| 1 | Harvey S. Singer | 15 | Johns Hopkins University |
| 2 | David E. Comings | 14 | City of Hope National Medical Center |
| 3 | Donald L. Gilbert | 10 | University of Cincinnati |
| 4 | Muenchau, Alexander | 9 | University Hospital Medical Center |
| 5 | Worbe, Yulia | 9 | Sorbonne University |
| 6 | James F. Leckman | 9 | Yale University |
| 7 | Pittenger, Christopher | 9 | Yale University |
| 8 | Sandor, Paul | 9 | University of Toronto |
| 9 | Bortolato, Marco | 8 | University of Utah |
| 10 | Jankovic, Joseph | 8 | Baylor College of Medicine |
The top 10 most publication authors.
Figure 5
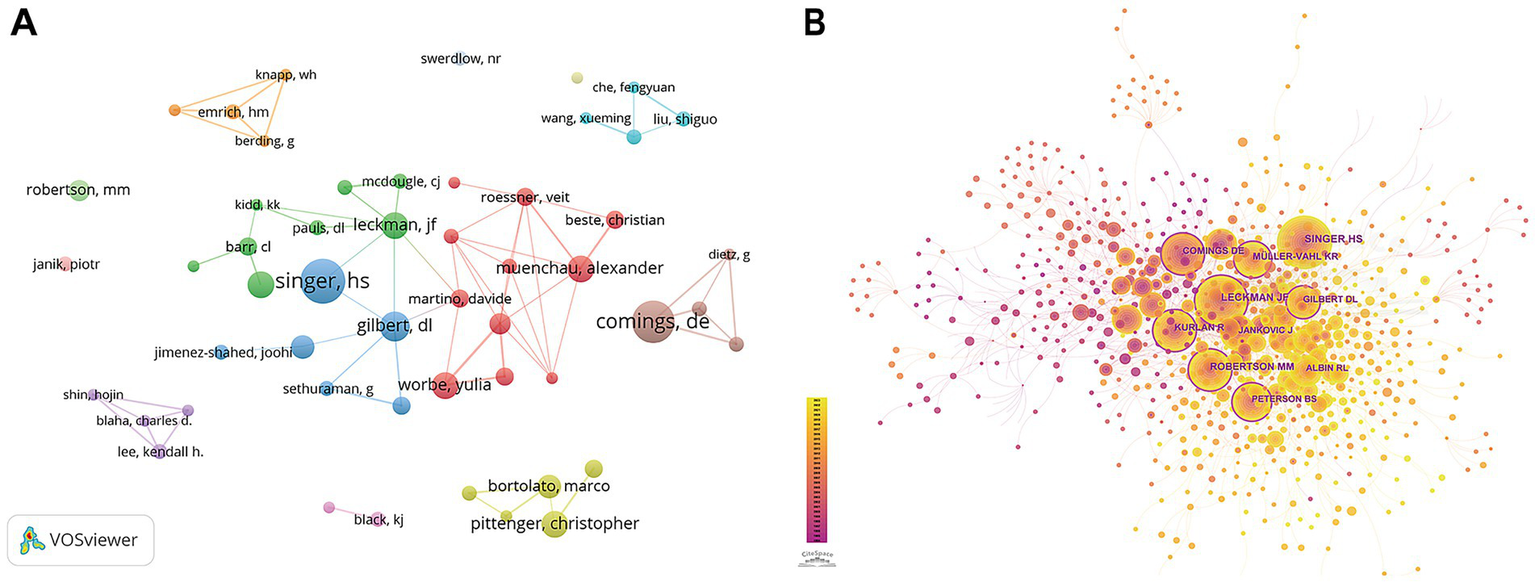
Analysis of the authors. (A) Visualization of authors. (B) Links and cooperation between cited authors by CiteSpace.
Co-citation analysis refers to the relationship between two authors when they are cited together by a third author or paper (11). Table 4 displays the top 10 cited authors by Centrality, with those having a centrality of 0.1 or higher being highlighted in Figure 5B. David E. Comings, Donald L. Gilbert, and Bradley S. Peterson are the top three authors and James F. Leckman has the most citations.
Table 4
| Rank | Cited-author | Centrality | Frequency | Institution |
|---|---|---|---|---|
| 1 | David E. Comings | 0.13 | 98 | City of Hope National Medical Center |
| 2 | Donald L. Gilbert | 0.13 | 79 | University of Cincinnati |
| 3 | Bradley S. Peterson | 0.13 | 77 | University of Southern California |
| 4 | James F. Leckman | 0.12 | 188 | Yale University |
| 5 | Mary M. Robertson | 0.12 | 133 | University College London |
| 6 | Kurlan, Roger | 0.12 | 94 | Cognitive and Research Center of New Jersey |
| 7 | Kirsten R. Mueller-Vahl | 0.11 | 91 | Hannover Medical School |
| 8 | Harvey S. Singer | 0.09 | 183 | Johns Hopkins University |
| 9 | Francisco X. Castellanos | 0.09 | 45 | New York University |
| 10 | Roger L. Albin | 0.08 | 89 | University of Michigan |
The top 10 cited authors with the highest centrality.
3.4 Distribution of journals and cited journals
In Table 5, we observe that 60% of top 10 journals are in the Q1 JCR region, 50% have an Impact factor (IF) greater than 5, and 40% have published more than 10 related articles. Biological Psychiatry ranks first, which makes it prominent in Figure 6A. Among these journals, Brain boasts the highest IF. The map of cited journals, as shown in Figure 6B, is divided into three clusters.
Table 5
| Rank | Journal | Publications | IF | JCR |
|---|---|---|---|---|
| 1 | Biological Psychiatry | 15 | 9.6 | Q1 |
| 2 | Movement Disorders | 13 | 7.4 | Q1 |
| 3 | Molecular Psychiatry | 10 | 9.6 | Q1 |
| 4 | Neuroscience and Biobehavioral Reviews | 10 | 7.6 | Q1 |
| 5 | American Journal Of Medical Genetics | 9 | 1.7 | Q3 |
| 6 | Advances in the Neurochemistry and Neuropharmacology of Tourette Syndrome | 8 | 4.28 | Q2 |
| 7 | American Journal of Medical Genetics Part B-Neuropsychiatric Genetics | 8 | 1.6 | Q3 |
| 8 | Behavioral Brain Research | 8 | 2.6 | Q2 |
| 9 | Brain | 7 | 11.9 | Q1 |
| 10 | Journal of the Neurological Sciences | 7 | 3.7 | Q1 |
Top 10 journals by number of publications.
Figure 6

Analysis of the journals. (A) The weight of different publications in the density map. (B) Visualization of cited journals. (C) Dual-map overlay of journals.
The dual-map overlay technique in journal mapping reflects the disciplinary mobility at the journal level (12). Figure 6C shows that fields of molecular/biology/genetics and psychology/education/social serve as the knowledge base for the frontier research areas of molecular/biology/immunology, psychology/education/health, and neurology/sports/ophthalmology.
3.5 Occurrence of keywords
In this study, a total of 639 keywords were identified, as shown in Figure 7A. Table 6 presents the top 10 keywords with the highest frequency and centrality. These include “Tourette syndrome,” “OCD,” “ADHD,” “Parkinson disease,” and “Schizophrenia” which represent neuropsychiatric disorders. Keywords such as “Basal ganglia,” “dopamine,” “linkage disequilibrium,” “brain,” “nucleus accumbens,” and “dopamine transporter” are associated with mechanistic aspects of TS. Additionally, “children,” “adolescent,” “double blind,” “behavior,” and “deep brain stimulation” relate to clinical trials and treatment strategies.
Figure 7

Analysis of the keywords by CiteSpace. (A) Links and cooperation between keywords. (B) Top 18 representative burst keywords.
Table 6
| Rank | Keyword | Occurrences | Keyword | Centrality |
|---|---|---|---|---|
| 1 | Tourette syndrome | 327 | basal ganglia | 0.23 |
| 2 | OCD | 117 | disorder | 0.19 |
| 3 | ADHD | 111 | brain | 0.19 |
| 4 | basal ganglia | 89 | ADHD | 0.18 |
| 5 | children | 85 | children | 0.17 |
| 6 | dopamine | 70 | dopamine | 0.16 |
| 7 | disorder | 52 | OCD | 0.12 |
| 8 | tic disorder | 50 | double blind | 0.12 |
| 9 | double blind | 44 | Parkinson disease | 0.12 |
| 10 | deep brain stimulation | 39 | association | 0.11 |
The top 10 keywords with the highest frequency and centrality of occurrence.
A burst keyword refers to the phenomenon where any keyword appears frequently within a specific time period. This information reveals the evolution and trends of hotspots over time. In Figure 7B, the red lines indicate the times when the usage of these keywords suddenly increases. “ADHD” has the strongest citation burst, and “linkage disequilibrium” has the longest burst duration. Notably, since 2020, “prevalence” has emerged as a new burst term and has continued to the present.
3.6 Co-cited representative literature
The cited references are displayed in Figure 8A and Table 7 provides details of the top 10 articles. The article published by Vijay A. Mittal in 2011 ranks first in citation count. In Figure 8B, the work “Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET” by Dean F. Wong exhibits the highest citation strength. Additionally, “Dopaminergic disturbances in Tourette syndrome: an integrative account” by Tiago V. Maia, which has shown a notable citation burst since 2020, provides an in-depth discussion on hypotheses of TS and is regarded as a foundational reference in the new era.
Figure 8

Analysis of the references. (A) Links and cooperation between references. (B) The top 15 references with the highest citation burst values.
Table 7
| Rank | References | Author | Frequency |
|---|---|---|---|
| 1 | DOI: 10.1016/j.psychres.2011.06.006 | Mittal VA | 24 |
| 2 | DOI: 10.1038/sj.npp.1301528 | Wong DF | 23 |
| 3 | DOI: 10.1016/j.neubiorev.2012.10.004 | Buse J | 23 |
| 4 | DOI: 10.1016/j.neuron.2013.10.052 | Baldan LC | 19 |
| 5 | DOI: 10.1093/brain/aws104 | Lerner A | 17 |
| 6 | DOI: 10.1016/j.tins.2006.01.001 | Albin RL | 16 |
| 7 | DOI: 10.1038/ng0196-81 | Benjamin J | 15 |
| 8 | DOI: 10.1097/WNF.0000000000000017 | Gilbert DL | 14 |
| 9 | PMID: 9118321 | LaHoste GJ | 14 |
| 10 | DOI: 10.1002/cne.22206 | Kataoka Y | 14 |
The top 10 references by the frequency of occurrence.
4 Discussion
As far as we know, this represents the first comprehensive bibliometric analysis mapping the research landscape of dopamine’s role in Tourette syndrome. The field has evolved from initial observations on the efficacy of dopamine antagonists in alleviating TS symptoms to the accumulation of neurobiological and molecular evidence in the 1980s and 1990s, which significantly advanced understanding of dopaminergic dysfunction in TS and guided the development of pharmacological treatment strategies. However, the rising prevalence of TS, along with challenges such as treatment-resistant cases and frequent comorbidities, has prompted a shift towards deeper genetic investigations. Concurrently, emerging technologies have created new possibilities for non-pharmacological interventions.
By synthesizing global research trends, our study uncovers the dynamic trajectory of TS research, charting its progression from fundamental mechanistic discoveries to clinical applications, while identifying key knowledge gaps and future opportunities for therapeutic development.
4.1 Current general information
An in-depth analysis presents a comprehensive overview of the field spanning 1994 to 2023. Keyword analysis identified research hotspots and trends.
Over the past 30 years, alongside the advancement of medical science, research on TS has been progressing, but there remains significant room for further research. In terms of countries and institutions, the United States has always been a leader, and countries like China, Germany, and the UK have also been actively developing their efforts. Yale University, demonstrating superior performance in workload, influence, and collaboration, far surpasses other organizations and holds an unshakable position in field.
In terms of authors, Harvey S. Singer, who has published the most papers, primarily researches neurosciences and psychiatry. Dr. Singer has been researching TS since the 1970s, focusing on the basal ganglia (13), dopaminergic neurotransmission (14), behavior rating scales (15), and Behavior Therapy (16). James F. Leckman, the most cited researcher, is a pioneer in TS. He is highly skilled in the diagnosis, participating in the revision of DSM-5 (17) and Yale Global Tic Severity Scale (18). He began focusing on the DA-TS link in 1986 (19), with his research mainly on animal models (20), genetic (21), and treatment (22).
Analysis of reference sources revealed that the majority of cited publications originated from high-impact scientific journals. Among these, JAMA Psychiatry, which has the highest impact factor in its category, emerged as one of the most influential journals in the field of psychiatry. These high-quality references ensure the scientific rigor and reliability of the literature survey and form a solid foundation for the research outcomes presented in this study.
4.2 Hotspots and frontier
Keyword analysis can reveal research hotspots and trends (12). Our analysis revealed that many high-frequency keywords correspond to comorbidities of TS. The burst keywords related to DA include “dopamine receptor,” and “dopamine transporter” reflecting ongoing investigations into how disruptions in the dopaminergic system contribute to the pathogenesis of TS. The sustained prominence of “linkage disequilibrium” as a keyword indicates a growing research focus on genetic mechanisms (23) particularly genome-wide association studies (24). Additionally “deep brain stimulation” has emerged as a prominent area of interest reflecting its evolving role as a therapeutic strategy for refractory cases.
4.2.1 Comorbidities
“Isolated Tourette syndrome is the exception, not the rule” (1). 85.7% of individuals with TS had at least one psychiatric comorbidity, and 57.7% had two or more disorders (25), including ADHD, OCD, depressive disorder, anxiety disorder, bipolar disorder, schizophrenia, etc. (26). Approximately 72% of individuals with TS have ADHD or OCD as the most common comorbidities, with females more likely to have OCD and males more prone to ADHD (26). TS, ADHD, and OCD are frequently described as a “clinical triad” (27) due to their high rate of co-occurrence. These extensive comorbidities are likely attributable to overlapping neurobiological mechanisms and shared genetic vulnerabilities.
Comorbidities significantly increase the complexity of TS and complicate both diagnosis and therapeutic management. These associated conditions adversely affect learning, social functioning, and psychological development. Furthermore, individuals with comorbidities face a higher natural and unnatural mortality risk than those with isolated TS (28). Therefore, early assessment and regular reevaluation of comorbidities, accompanied by increased clinical attention and timely tailored interventions, are crucial.
4.2.2 From dopaminergic components to pharmacological treatment
DA is an important catecholamine neurotransmitter in the CNS (29), playing a significant role in regulating various complex functions. Tyrosine hydroxylase (TH) and dopa decarboxylase (DDC) play key roles in the synthesis of DA. After synthesis, DA is actively transported into synaptic vesicles via the vesicular monoamine transporter 2 (VMAT2) (30), then released into the synaptic cleft through exocytosis.
DA has two modes of release, namely the tonic-phasic release (31): phasic firing, which occurs after a neuron is activated, leads to a transient release of DA, which is then rapidly removed via reuptake, forming the basis for most of DA’s functions. Tonic firing, which occurs without presynaptic input, refers to the sustained low-level release and is regarded as background activity that helps maintain stability. At this level, DA is insufficient to activate intrasynaptic dopamine receptors (DRs); instead activating extrasynaptic DRs, which inhibit phasic DA release. In synaptic cleft, DA can bind to postsynaptic receptors or dopamine terminal autoreceptors (32). Dopamine receptors (DRs) are classified into two groups based on their ability to activate or inhibit adenylate cyclase (AC): D1-like receptors and D2-like receptors. Different DRs are distributed across various brain regions, transmitting distinct signals and performing functions. After the successful transmission, the dopamine transporter (DAT) detaches DA from DRs and reuptakes it back into the presynaptic neuron (33); therefore, DAT plays a crucial role in the spatiotemporal distribution of DA in the cleft. A portion of the recycled DA is repackaged into synaptic vesicles for the next release, while another part is metabolized by monoamine oxidase (MAO), catechol-O-methyltransferase (COMT), and aldehyde dehydrogenase (ALDH) into homovanillic acid (HVA) (34), which is then excreted from the body.
Current first-line pharmacotherapies for TS, such as aripiprazole, haloperidol, risperidone, and tiapride, primarily exert their therapeutic effects through antagonism or partial agonism of the dopamine D2 receptor (35). While effective in reducing tics, this D2-centric mechanism is frequently associated with significant side effects, including extrapyramidal symptoms, sedation, and headache (35), which has motivated the pursuit of novel agents with alternative mechanisms of action. In recent years, research efforts have yielded several promising compounds that act on distinct components of the dopaminergic system. This therapeutic diversification is exemplified by the development of agents such as the D1 receptor antagonist ecopipam (36), the D3 receptor-targeting compound BL-1020 (37), and valbenazine, a selective VMAT2 inhibitor (38).
These emerging directions reflect a conceptual shift from historically predominant D2-based hypotheses toward targeting specific receptor subtypes and presynaptic regulatory proteins. This represents a pivotal advance in the neuropharmacology of TS with the goal of achieving superior efficacy alongside a more favorable tolerability profile.
4.2.3 Candidate dopaminergic genes
Family, twin studies provide evidence for the strong heritability of TS (39). Candidate genes on the dopaminergic pathway primarily include DRs (DRD1, DRD2, DRD4, and DRD5), monoamine oxidase-A (MAO-A), the dopamine transporter gene (DAT1) (40).
Beyond the study of candidate genes, genome-wide association studies (GWAS) have offered novel insights into TS genetics. For instance, a large-scale GWAS of TS (41) identified several significant risk loci. The SNP rs7868992 is located within an intron of COL27A1 and may be involved in neurodevelopmental processes. Another key variant, rs6539267, is situated on chromosome 12q23 within an intron of POLR3B; recessive mutations in this gene are associated with severe neurological impairment. Collectively, these findings indicate that the genetic basis of TS extends beyond a few candidate genes to encompass broader neurodevelopmental mechanisms.
4.2.4 Deep brain stimulation
Deep brain stimulation (DBS) involves precise implanting electrodes in specific brain areas for continuous stimulation (42). Back in the late 1990s (43), DBS was attempted for the treatment of highly severe, drug-resistant tics (44). However, the use of this method for TS is still experimental, as there is currently uncertainty regarding best target choice. The common brain targets for TS are the globus pallidus internus (GPi) and the centromedian-parafascicular complex (CM-Pf) of the thalamus (44). The GPi, a key basal ganglia structure, regulates motor functions, and the CM-Pf complex is crucial for motor control and sensory processing. Evidence shows that CM-Pf DBS, via striatal cholinergic interneurons, triggers synaptic dopamine release and increases tonic DA levels (45).
The sustained scholarly focus on DBS underscores its value as a therapeutic option for treatment-refractory cases. However, larger multicentre studies are urgently needed to develop specific predictors of treatment response, which will be crucial to optimise patient-tailored brain target selection. Furthermore, the long-term efficacy and safety of DBS remain to be fully established to confirm its sustained therapeutic benefits and comprehensive risk profile. Simultaneously, given the ethical considerations of a brain surgery, it’s important to implement more thorough informed consent procedures. Especially for pediatric patients with developing brains, the long-term cognitive impacts of DBS are largely unknown, necessitating a careful assessment of the treatment benefits against the risks of invasive surgery and side effects. This requires a collaborative effort among ethicists, psychiatrists, and neurosurgeons.
4.3 Limitation
In this study, there are certain limitations. First, while TS is a multifactorial disorder involving multiple potential pathophysiological mechanisms, our analysis focused primarily on the dopaminergic system, which, although predominant, does not encompass all relevant pathways. Second, the literature was sourced exclusively from the WOSCC and limited to English-language articles published between 1994 and 2023, which may not fully represent the entire origin and the latest developments. Although these limitations do not invalidate the overall findings or the robustness of the bibliometric analysis, achieving large-scale, cross-platform integration and analysis of diverse literature sources remains an important objective for future research.
5 Conclusion
There are few studies that have employed bibliometric analysis to examine the temporal evolution, spatial distribution, and trend hotspots in the field of TS. This study combines bibliometric and visual analytic techniques to map the knowledge landscape of dopamine-related research in TS over the past three decades. Although the absolute number of publications remains modest, the steady increase in annual publications reflects sustained research interest and anticipates future expansion. Future efforts should focus on enhancing regional and international collaboration to overcome the limitations inherent in single-institution studies and promote the implementation of large-scale, multicenter clinical trials. At the same time, rapidly evolving scientific and technological tools should be fully leveraged to facilitate the generation of more robust and clinically impactful research.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
QL: Data curation, Investigation, Visualization, Writing – original draft. ZZ: Validation, Writing – original draft. FL: Methodology, Software, Writing – original draft. YD: Writing – review & editing. YW: Supervision, Writing – review & editing. CZ: Conceptualization, Funding acquisition, Writing – review & editing. BY: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the General Project of Scientific Research Project of Wuxi Municipal Health Commission (M202231) and Jiangsu Association of Chinese Medicine Research Project (ZXFZ2024042).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Johnson KA Worbe Y Foote KD Butson CR Gunduz A Okun MS . Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. (2023) 22:147–58. doi: 10.1016/S1474-4422(22)00303-9
2.
Sambrani T Jakubovski E Müller-Vahl KR . New insights into clinical characteristics of Gilles de la Tourette syndrome: findings in 1032 patients from a single German center. Front Neurosci. (2016) 10:415. doi: 10.3389/fnins.2016.00415
3.
Pritchard A . Statistical bibliography or bibliometrics. J Doc. (1969) 25:348–9.
4.
Ding X Yang Z . Knowledge mapping of platform research: a visual analysis using VOSviewer and cite space. Electron Commer Res. (2022) 22:787–809. doi: 10.1007/s10660-020-09410-7
5.
Wang C. Dai J. Xu L. . (2022). Big data and data mining in education: a bibliometrics study from 2010 to 2022. 2022 7th International Conference on Cloud Computing and Big Data Analytics (ICCCBDA). 507–512.
6.
Chen C . Cite space II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
7.
van Eck NJ Waltman L . Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
8.
Hassan-Montero Y De-Moya-Anegon F Guerrero-Bote VP . Scimago Graphica: a new tool for exploring and visually communicating data. Prof Inferm. (2022) 31:e310502. doi: 10.3145/epi.2022.sep.02
9.
Yang C Zhang J Zhao Q Zhang J Zhou J Wang L . Trends of Tourette syndrome in children from 2011 to 2021: a bibliometric analysis. Front Behav Neurosci. (2022) 16:991805. doi: 10.3389/fnbeh.2022.991805
10.
Mayta-Tovalino F Diaz-Barrera ME Runzer-Colmenares FM Pacheco-Mendoza J . RAMIBS: reporting and measurement of items for bibliometric or scientometric studies in health sciences. J Int Oral Health. (2024) 16:253–6. doi: 10.4103/jioh.jioh_23_24
11.
Cui Z Luo F Wang J Diao J Pan Y . Bibliometric and visual analysis of Kawasaki disease in children from 2012 to 2022. Front Pediatr. (2023) 11:1142065. doi: 10.3389/fped.2023.1142065
12.
Zhu Z Wu J Chen W Luo F Zhao X . Bibliometric analysis of cough variant asthma from 1993 to 2022. J Asthma Allergy. (2024) 17:517–37. doi: 10.2147/JAA.S452097
13.
Singer HS Reiss AL Brown JE Aylward EH Shih B Chee E et al . Volumetric MRI changes in basal ganglia of children with Tourette’s syndrome. Neurology. (1993) 43:950–6. doi: 10.1212/WNL.43.5.950
14.
Wong DF Brasić JR Singer HS Schretlen DJ Kuwabara H Zhou Y et al . Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. (2008) 33:1239–51. doi: 10.1038/sj.npp.1301528
15.
Mahone EM Cirino PT Cutting LE Cerrone PM Hagelthorn KM Hiemenz JR et al . Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch Clin Neuropsychol. (2002) 17:643–62. doi: 10.1093/arclin/17.7.643
16.
Essoe JKY Ramsey KA Singer HS Grados M McGuire JF . Mechanisms underlying behavior therapy for Tourette’s disorder. Curr Dev Disord Rep. (2021) 8:161–74. doi: 10.1007/s40474-021-00225-1
17.
Walkup JT Ferrão Y Leckman JF Stein DJ Singer H . Tic disorders: some key issues for DSM-V. Depress Anxiety. (2010) 27:600–10. doi: 10.1002/da.20711
18.
McGuire JF Piacentini J Storch EA Murphy TK Ricketts EJ Woods DW et al . A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology. (2018) 90:e1711–9. doi: 10.1212/WNL.0000000000005474
19.
Riddle M Leckman J Cohen D Anderson M Ort S Caruso K et al . Assessment of central dopaminergic function using plasma-free homovanillic-acid after debrisoquin administration. J Neural Transm. (1986) 67:31–43. doi: 10.1007/BF01243357
20.
Taylor JR Morshed SA Parveen S Mercadante MT Scahill L Peterson BS et al . An animal model of Tourette’s syndrome. Am J Psychiatry. (2002) 159:657–60. doi: 10.1176/appi.ajp.159.4.657
21.
Willsey AJ Fernandez TV Yu D King RA Dietrich A Xing J et al . De novo coding variants are strongly associated with Tourette disorder. Neuron. (2017) 94:486–499.e9. doi: 10.1016/j.neuron.2017.04.024
22.
Martinez-Ramirez D Jimenez-Shahed J Leckman JF Porta M Servello D Meng FG et al . Efficacy and safety of deep brain stimulation in Tourette syndrome: the international Tourette syndrome deep brain stimulation public database and registry. JAMA Neurol. (2018) 75:353–9. doi: 10.1001/jamaneurol.2017.4317
23.
Grice DE Leckman JF Pauls DL Kurlan R Kidd KK Pakstis AJ et al . Linkage disequilibrium between an allele at the dopamine D4 receptor locus and Tourette syndrome, by the transmission-disequilibrium test. Am J Hum Genet. (1996) 59:644–52.
24.
Yu D Mathews CA Scharf JM Neale BM Davis LK Gamazon ER et al . Cross-disorder genome-wide analyses suggest a complex genetic relationship between Tourette’s syndrome and OCD. Am J Psychiatry. (2015) 172:82–93. doi: 10.1176/appi.ajp.2014.13101306
25.
Singer HS . Tics and Tourette syndrome. Continuum. (2019) 25:936–58. doi: 10.1212/CON.0000000000000752
26.
Hirschtritt ME Lee PC Pauls DL Dion Y Grados MA Illmann C et al . Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. (2015) 72:325–33. doi: 10.1001/jamapsychiatry.2014.2650
27.
Shprecher DR Rubenstein LA Gannon K Frank SA Kurlan R . Temporal course of the Tourette syndrome clinical triad. Tremor Other Hyperkinet Mov. (2014) 4:243. doi: 10.7916/D8HD7SV6
28.
Meier SM Dalsgaard S Mortensen PB Leckman JF Plessen KJ . Mortality risk in a nationwide cohort of individuals with tic disorders and with Tourette syndrome. Mov Disord. (2017) 32:605–9. doi: 10.1002/mds.26939
29.
Missale C Nash SR Robinson SW Jaber M Caron MG . Dopamine receptors: from structure to function. Physiol Rev. (1998) 78:189–225. doi: 10.1152/physrev.1998.78.1.189
30.
Wang Y Zhang P Chao Y Zhu Z Yang C Zhou Z et al . Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines. Cell Res. (2024) 34:47–57. doi: 10.1038/s41422-023-00906-z
31.
Bressan RA Crippa JA . The role of dopamine in reward and pleasure behaviour—review of data from preclinical research. Acta Psychiatr Scand Suppl. (2005) 427:14–21. doi: 10.1111/j.1600-0447.2005.00540.x
32.
Ford CP . The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. (2014) 282:13–22. doi: 10.1016/j.neuroscience.2014.01.025
33.
Yang L Wang X Liu X Chen X . Striatal syntaxin 1A is associated with development of Tourette syndrome in an Iminodipropionitrile-induced animal model. Dis Markers. (2022) 2022:1–6. doi: 10.1155/2022/1148191
34.
Napolitano A Cesura AM Da Prada M . The role of monoamine oxidase and catechol O-methyltransferase in dopaminergic neurotransmission. J Neural Transm Suppl. (1995) 45:35–45.
35.
Roessner V Eichele H Stern JS Skov L Rizzo R Debes NM et al . European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part III: pharmacological treatment. Eur Child Adolesc Psychiatry. (2022) 31:425–41. doi: 10.1007/s00787-021-01899-z
36.
Gilbert DL Murphy TK Jankovic J Budman CL Black KJ Kurlan RM et al . Ecopipam, a D1 receptor antagonist, for treatment of Tourette syndrome in children: a randomized, placebo-controlled crossover study. Mov Disord. (2018) 33:1272–80. doi: 10.1002/mds.27457
37.
Geffen Y Keefe R Rabinowitz J Anand R Davidson M . Bl-1020, a new γ-aminobutyric acid-enhanced antipsychotic: results of 6-week, randomized, double-blind, controlled, efficacy and safety study. J Clin Psychiatry. (2012) 73:e1168–74. doi: 10.4088/JCP.12m07642
38.
Farber RH Angelov A Kim K Carmack T Thai-Cuarto D Roberts E . Clinical development of valbenazine for tics associated with Tourette syndrome. Expert Rev Neurother. (2021) 21:393–404. doi: 10.1080/14737175.2021.1898948
39.
O’Rourke JA Scharf JM Yu D Pauls DL . The genetics of Tourette syndrome: a review. J Psychosom Res. (2009) 67:533–45. doi: 10.1016/j.jpsychores.2009.06.006
40.
Paschou P . The genetic basis of Gilles de la Tourette syndrome. Neurosci Biobehav Rev. (2013) 37:1026–39. doi: 10.1016/j.neubiorev.2013.01.016
41.
Scharf JM Yu D Mathews CA Neale BM Stewart SE Fagerness JA et al . Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. (2013) 18:721–8. doi: 10.1038/mp.2012.69
42.
Camacho-Conde JA Gonzalez-Bermudez MDR Carretero-Rey M Khan ZU . Brain stimulation: a therapeutic approach for the treatment of neurological disorders. CNS Neurosci Ther. (2022) 28:5–18. doi: 10.1111/cns.13769
43.
Lozano AM Lipsman N Bergman H Brown P Chabardes S Chang JW et al . Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. (2019) 15:148–60. doi: 10.1038/s41582-018-0128-2
44.
Aydin S Darko K Jenkins A Detchou D Barrie U . Deep brain stimulation for Tourette’s syndrome. Neurosurg Rev. (2024) 47:734. doi: 10.1007/s10143-024-02958-0
45.
Rusheen AE Rojas-Cabrera J Goyal A Shin H Yuen J Jang DP et al . Deep brain stimulation alleviates tics in Tourette syndrome via striatal dopamine transmission. Brain. (2023) 146:4174–90. doi: 10.1093/brain/awad142
Summary
Keywords
Tourette syndrome, dopamine, bibliometric, visual analysis, hotspots
Citation
Liu Q, Zhu Z, Luo F, Ding Y, Wang Y, Zhao C and Yuan B (2025) Dopamine in Tourette syndrome: a 30-year bibliometric analysis of hotspot evolution. Front. Neurol. 16:1589842. doi: 10.3389/fneur.2025.1589842
Received
08 March 2025
Accepted
31 August 2025
Published
17 September 2025
Volume
16 - 2025
Edited by
Félix Javier Jiménez-Jiménez, Hospital Universitario del Sureste, Spain
Reviewed by
Natalia Szejko, Medical University of Warsaw, Poland
Carlos Quispe-Vicuña, National University of San Marcos, Peru
Updates
Copyright
© 2025 Liu, Zhu, Luo, Ding, Wang, Zhao and Yuan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changjiang Zhao, 78189982@qq.com; Bin Yuan, yuanbin68358@163.com
†These authors have contributed equally to this work
†ORCID: Changjiang Zhao, orcid.org/0009-0006-6450-965X
Bin Yuan, orcid.org/0000-0002-6071-8925
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.