- 1Department of Rehabilitation, Binzhou Medical University Hospital, Binzhou, China
- 2School of Special Education and Rehabilitation, Department of Rehabilitation Medicine, Binzhou Medical University, Yantai, China
Objective: To investigate the impact of animated video modeling (AVM) on the brain function of children with autism spectrum disorder (ASD) using functional near-infrared spectroscopy (fNIRS).
Methods: Fifteen children with ASD and 15 matched typically developing (TD) controls were enrolled. fNIRS was used to obtain 8-min data in quiet and visually stimulated states, with the dorsolateral prefrontal cortex, medial prefrontal cortex (mPFC), and bilateral occipital lobe as regions of interest (ROIs). Based on the concentration of oxygenated hemoglobin (HbO2) over time, correlation coefficient analysis was performed to calculate functional connection strength, and the intergroup disparity was compared.
Result: The ASD group showed significantly lower functional connection strength. Comparison of the ROI–ROI functional connectivity strength revealed significant differences in connectivity patterns of the right dorsolateral prefrontal lobe (RDLPFC), left dorsolateral prefrontal lobe (LDLPFC), mPFC, right occipital lobe (ROL), and left occipital lobe (LOL) with other brain regions. Extremely significant differences were found between the RDLPFC/RPMC, ROL/RPMC, LOL/RPMC, and LOL/LIPL. The functional connectivity strength of children with ASD was significantly higher during visual stimulation than during the quiet test. Comparison between the ROI–ROI functional connectivity strengths revealed significant differences in the connectivity strength of the RDLPFC/LOL, LDLPFC/LIPL, mPFC/RPMC, mPFC/LPMC, mPFC/LIPL, ROL/RPM, ROL. RIPL, ROL/LIPL, and LOL/LIPL. Extremely significant differences were observed between the ROL/RPMC and ROL/RIPL.
Conclusion: Animated video modeling can improve visual perception and information processing in children with ASD, by strengthening the functional connectivity between the occipital and inferior parietal cortices.
Introduction
Autism spectrum disorder (ASD) encompasses a group of neurodevelopmental disorders characterized by varying degrees of impairment in social interaction and communication, restricted interests, and repetitive behaviors (1). According to data statistics from the Autism Spectrum and Developmental Disorders Surveillance (ADDM) Network, the incidence of ASD is increasing annually, with this disease emerging as a significant global issue in pediatric neurodevelopmental disorders (2). Visual perception and information processing abnormalities are common symptoms in children with ASD, significantly affecting their daily functioning and learning abilities and impeding their interaction with the environment and peers, resulting in varying degrees of social interaction and communication disorders. Yeung et al. (3) discovered that children with ASD frequently have difficulty recognizing and interpreting facial expressions, which can impede social interactions and the development of relationships. Similarly, Dakin et al. (4) reported that numerous children with ASD display abnormal patterns of visual attention, such as fixating on specific details rather than the entire scene, and that this tendency can interfere with their ability to perceive and interpret social cues. Furthermore, Posar et al. (5) noted that the integration of visual information from different sources can be problematic for children with ASD, affecting their ability to understand complex visual scenes and interactions. Therefore, understanding and addressing these visual processing deficits is crucial for developing effective interventions that can improve social interaction, communication impairments, and cognitive outcomes among children with ASD (6, 7). Owing to patients’ relatively strong dependence in responding to visual cues (8), an increasing number of educational programs are incorporating visual teaching and learning strategies (9, 10).
Animated video modeling (AVM) is a visual aid that can effectively impact the social skills of children with ASD (11). Visual dependence has been indicated to strongly influence several individuals with ASD. Children with ASD often show a keen interest in videos, acquiring significant knowledge from them (12, 13). Numerous researchers have observed a preference for visual stimuli, particularly those presented on electronic screens. This has led to speculation that this preference can be exploited to further enhance various skills (14). Some researchers have further proposed that the specific characteristics of electronic screen media make them effective tools for delivering information to individuals with ASD. The limited viewing area helps narrow the participants’ attention frame, enabling them to focus on relevant stimuli and disregard irrelevant ones (15). Many educators use this preference for electronic screen media in teaching, with video modeling being one of the most effective applications. This intervention method can help children with ASD develop various skills. This process involves recording the modeling of target behaviors and skills into videos for students to repeatedly watch and imitate, thus facilitating the acquisition of these behaviors and skills (13, 16, 17). However, the functional mechanisms underlying the action of AVM in improving social and communication skills in children with ASD remain unclear.
Functional near-infrared spectroscopy (fNIRS) is an ideal neuroimaging technique for investigating brain function in children. Owing to its advantages of affordability, portability, minimal noise interference, and high tolerance to head movements, this technique has gained popularity among many researchers and has been extensively used in the study of brain functional connectivity and activation in individuals with ASD (18–21).
The present study collected different state data of 3–7-year-old children diagnosed with ASD and matched typically developing (TD) children using fNIRS to investigate the AVM impact on brain function in children with ASD by comparing the magnitude of functional strength between the two groups.
Materials and methods
Participants
The ASD group comprised children diagnosed with ASD at the Binzhou Medical University Hospital between October 2023 and April 2024. The inclusion criteria were as follows: Patients who met the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5); aged between 3 and 7 years; right-handedness; and no discomfort experienced for nearly 1 month. The exclusion criteria were as follows: Other neurodevelopmental disorders, inability to complete fNIRS, and a history of transcranial magnetic stimulation or other neuroregulatory treatments. A total of 15 children with ASD met the inclusion criteria. Concurrently, 15 TD children enrolled in the child rehabilitation department were included in the TD group.
In addition, all of the participants’ parents were requested to report their child’s behavior using the Autism Behavior Checklist (ABC), and a parental reporting questionnaire, was used to confirm the clinical diagnosis of ASD. Fifty-seven items describing typical behaviors in different developmental areas of children with ASD were included and divided into five subscales (sensory, relating, body and object use, language, and social and self-help skills). Each item was scored on a scale from 1 to 4, depending on its importance for the diagnosis of ASD. The subscale scores were determined by summing the values of the endorsed items for each area, which were then summed to represent the total ABC score. Children with a total score of 68 points may have a high risk of ASD.
The study was conducted following approval by the Medical Ethics Committee of Binzhou Medical University Hospital (approval number 2024-KYLL-141). All methods were performed in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki and institutional ethical standards. Written informed consent was obtained from all participants and/or their legal guardians for both study participation and publication of identifying information/images in an online open-access publication. No patient names or other identifying details are included in this manuscript. All images used in this study are fully anonymized, adhering to ethical standards to protect participant privacy.
Data acquisition
NirScan-9000ET (Danyang Huichuang Medical Equipment Co., Ltd., China) was applied to continuously measure and record brain oxyhemoglobin (HbO2) and deoxyhemoglobin (Hb) concentration changes in participants in a quiet state and during visual stimulation from the AVM. The sampling frequency was 11 Hz. The device comprised 24 transmitting and 24 receiving probes with 63 effective channels; the channel coordinates were based on the standard international 10–20 electrode system. During data collection, eight resting-state data points were collected while participants either sat alone or were held by a guardian (Figure 1).

Figure 1. Schematic diagram of experimental data acquisition. (a) Photo obtained from a participant during data collection in the quiet state. (b) Photo obtained from a participant during data collection in the visual stimulation state.
In this study, the brain was divided into the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (mPFC), bilateral premotor cortex (PMC), inferior parietal lobe (IPL), and bilateral occipital lobe (OL), of which the bilateral DLPFC, mPFC, and OL were used as regions of interest (ROIs) (Figure 2). The HbO2 concentration change signal was selected as the primary data collected for the test because of its high sensitivity to cortical blood flow changes and superior signal-to-noise ratio (11).
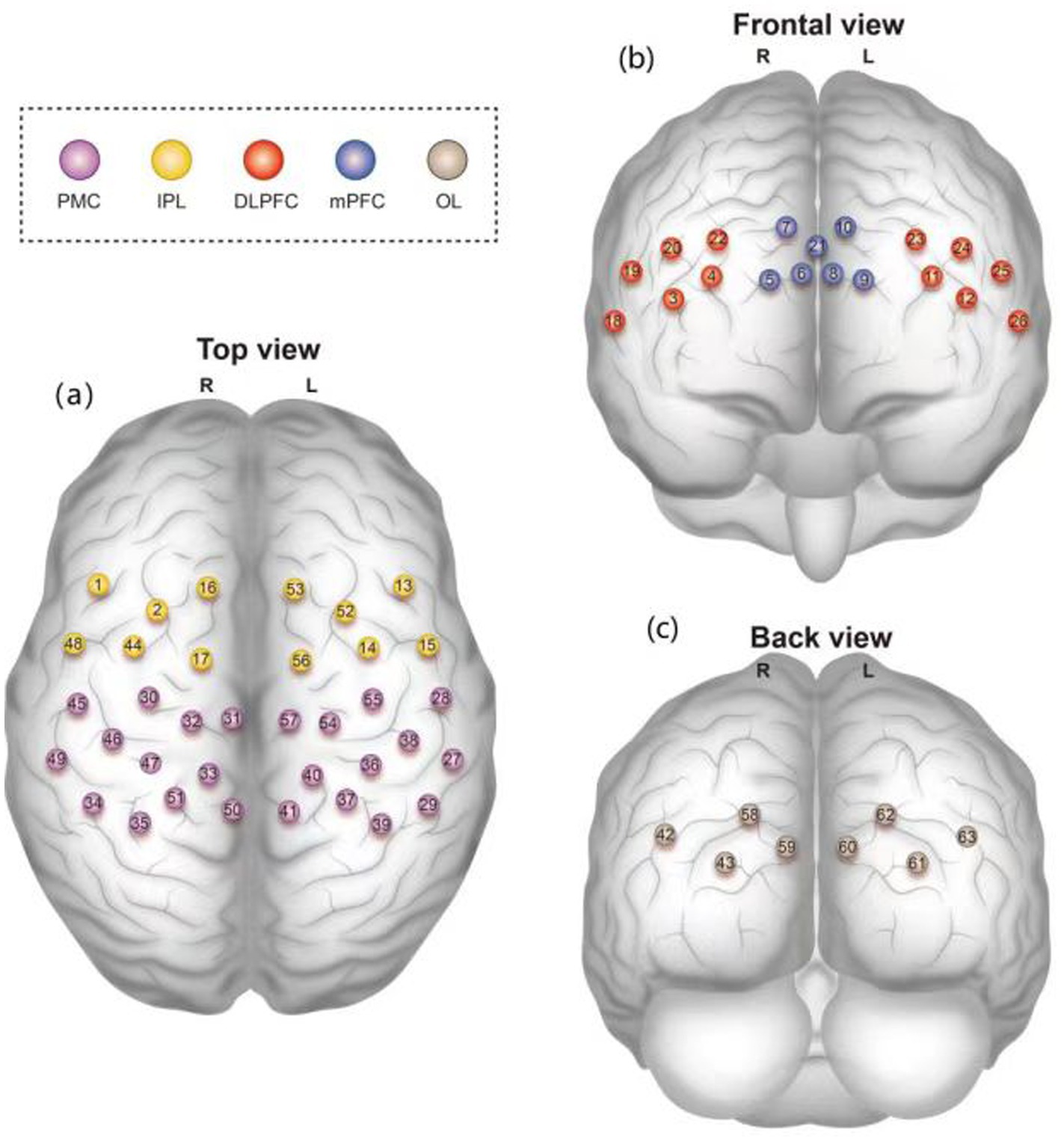
Figure 2. (a) Top view, (b) Frontal view, and (c) Back view of the brain regions covered by 63 fNIRS channels. Color-coded regions: dorsolateral prefrontal cortex (DLPFC, red), medial prefrontal cortex (mPFC, blue), premotor cortex (PMC, purple), inferior parietal lobe (IPL, yellow), and occipital lobe (OL, gray).
Animation modeling video
Videos that met the following content criteria were selected: (a) engaging a partner in an interactive play, (b) initiate and sustain conversations with a partner, and (c) start and maintain enjoyable activities with a partner. The videos showcased peers exhibiting four fundamental behaviors for each task, as follows: (1) interaction and engagement by maintaining eye contact while speaking; (2) coherence through nonverbal communication, such as direct facial expressions (e.g. smiling or frowning), to convey interest; (3) peer-to-peer conversation taking turns engaging in dialogue; and (4) politeness and etiquette by offering additional comments or asking questions related to the interlocutor’s statements.
Data analysis
NirSpark software was used for data preprocessing and analysis. During preprocessing, a quality control module was used to detect motion artifacts and filter unqualified data. The spline interpolation method was used to correct movement artifacts in the channel. Physiological noise was eliminated using a low-pass filter at approximately 0.009–0.008 Hz (22–24), while the filtered optical density data were converted into HbO2 concentration changes according to the modified Beer–Lambert law.
Functional connectivity analysis: Data from each time point were extracted for each participant, and a statistical analysis of the HbO2 concentration in each brain region over the time series was conducted. The Pearson correlation method was applied to calculate the correlation between each channel and brain region, with the correlation coefficient “r” of HbO2 valued by Fisher-Z conversion as a measure of the functional connectivity strength for each corresponding channel. Moreover, comparisons were made regarding the functional connectivity strength between ROI-ROIs.
Statistical methods
SPSS (version 25.0) software was used for statistical analysis. Data with normal distribution, such as age and functional connectivity strength, are described as the x- ± s. Independent sample t-tests were used to compare two groups, whereas paired sample t-tests were used to compare different states in the same group. The test level was set at α = 0.05 for bilateral tests.
Results
Demographic and behavioral questionnaires
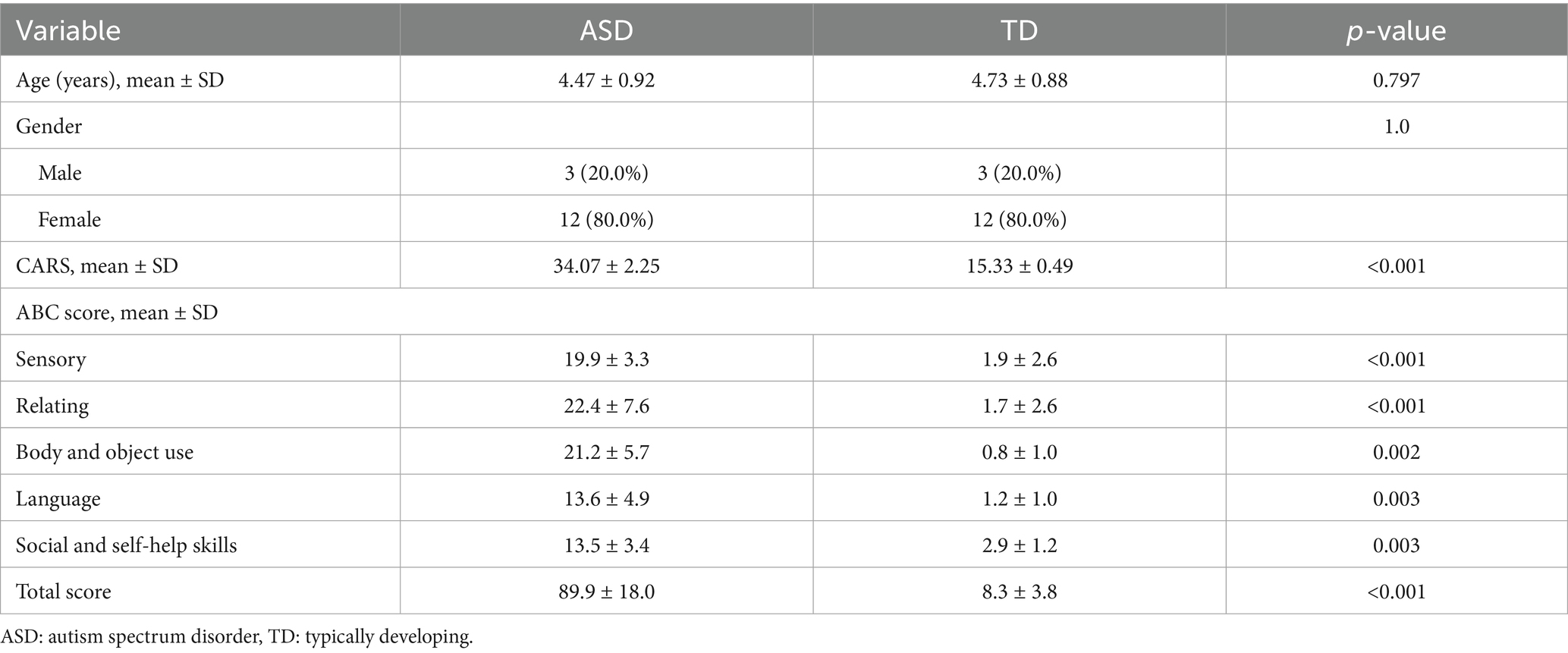
No significant differences in age and gender were observed between the ASD and TD groups, (p > 0.05), while the childhood autism rating scale (CARS) was significantly lower in the TD groups, compared with ASD group (Table 1). The subscales and total ABC scores of all children with ASD included in the data analysis were significantly higher than those of TD children (p < 0.05) (Table 1).
Functional connectivity in children with ASD and TD children
A comparison between the functional connectivity maps of the ASD and TD groups revealed that the average functional connectivity strength was lower in the ASD group (0.27 ± 0.09) than in the TD group (0.45 ± 0.13) (Figures 3a,b).
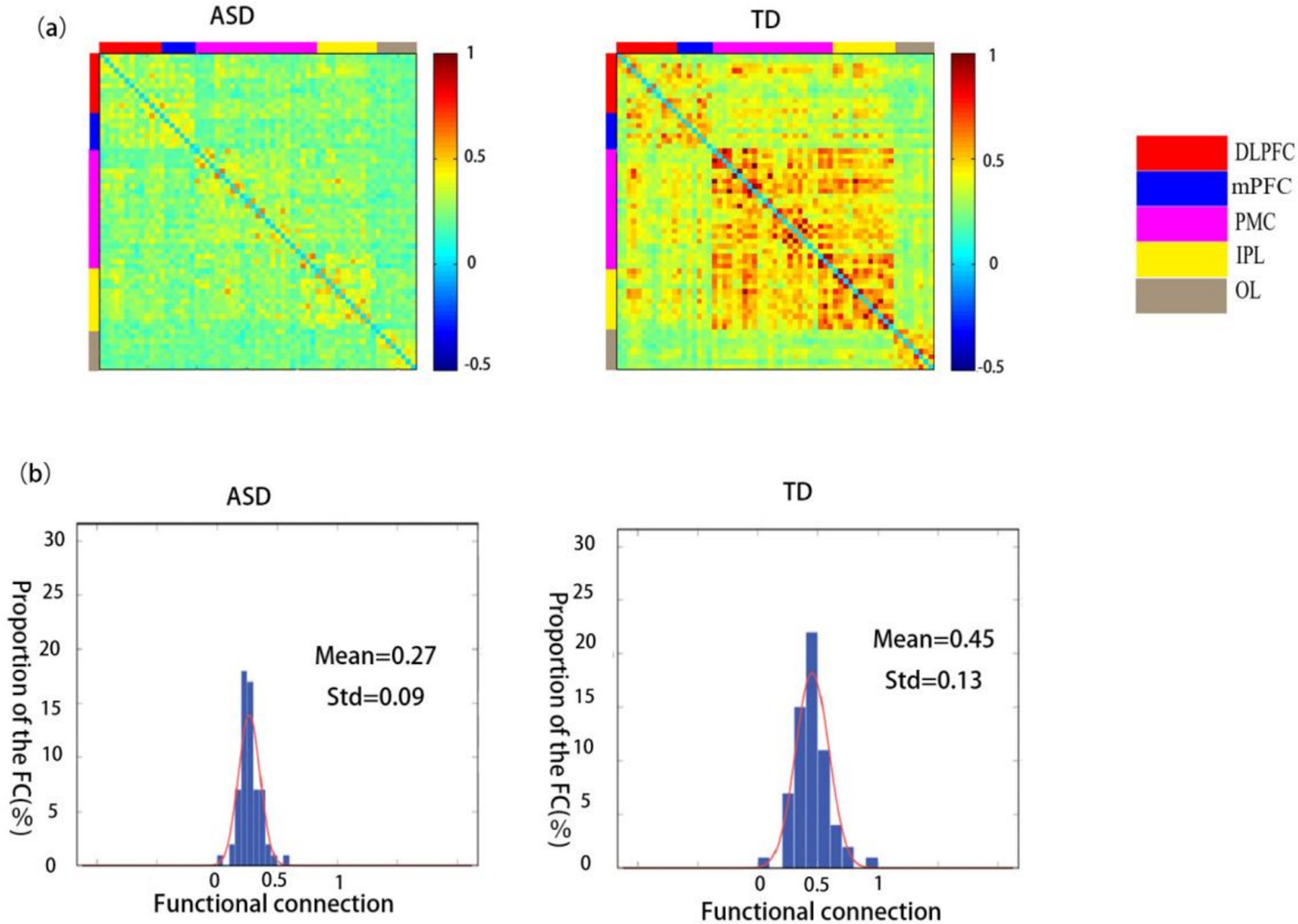
Figure 3. Spatial patterns of functional connectivity in the ASD and TD groups. (a) Functional connectivity maps for the ASD and TD groups. (b) Histograms of the functional connectivity distribution in the ASD and TD groups. TD: typically developing, ASD: autism spectrum disorder.
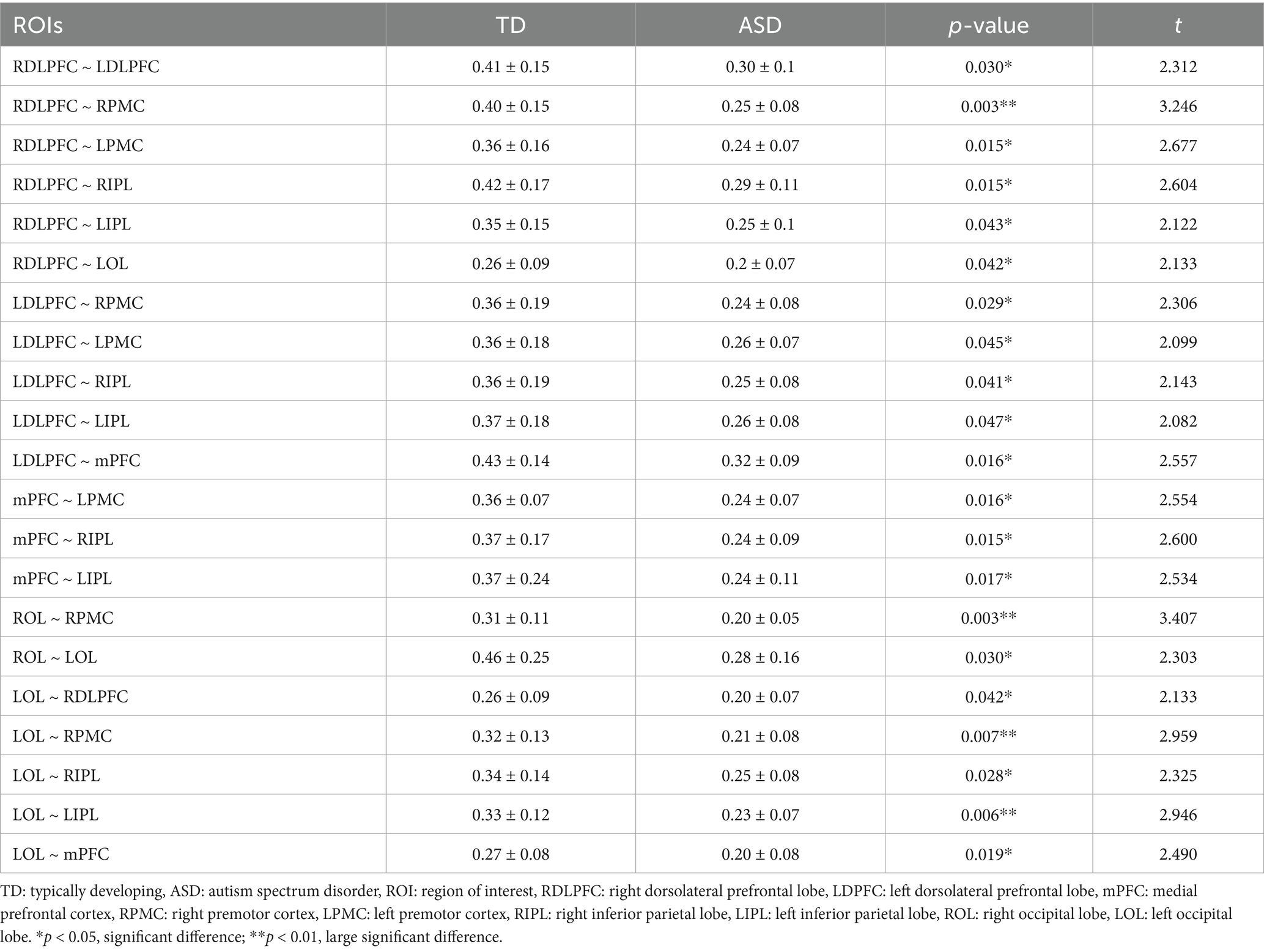
Comparison of the ROI–ROI functional connectivity strength between children in the ASD and TD groups revealed statistically significant differences in the connectivity patterns of the right dorsolateral prefrontal lobe (RDLPFC), left dorsolateral prefrontal lobe (LDLPFC), medial prefrontal cortex (mPFC), right occipital lobe (ROL), and left occipital lobe (LOL) with other brain regions (p < 0.05). Extremely significant differences were found between the RDLPFC and RPMC, ROL and RPMC, LOL and RPMC, as well as between the LOL and LIPL (p < 0.01) (Table 2).
Functional connectivity strength in children with ASD during quiet and visual stimulation
The functional connectivity strength of children in the ASD group was significantly higher during the visual stimulation state (0.32 ± 0.10) than during the quiet state (0.27 ± 0.09) (Figures 4a,b).
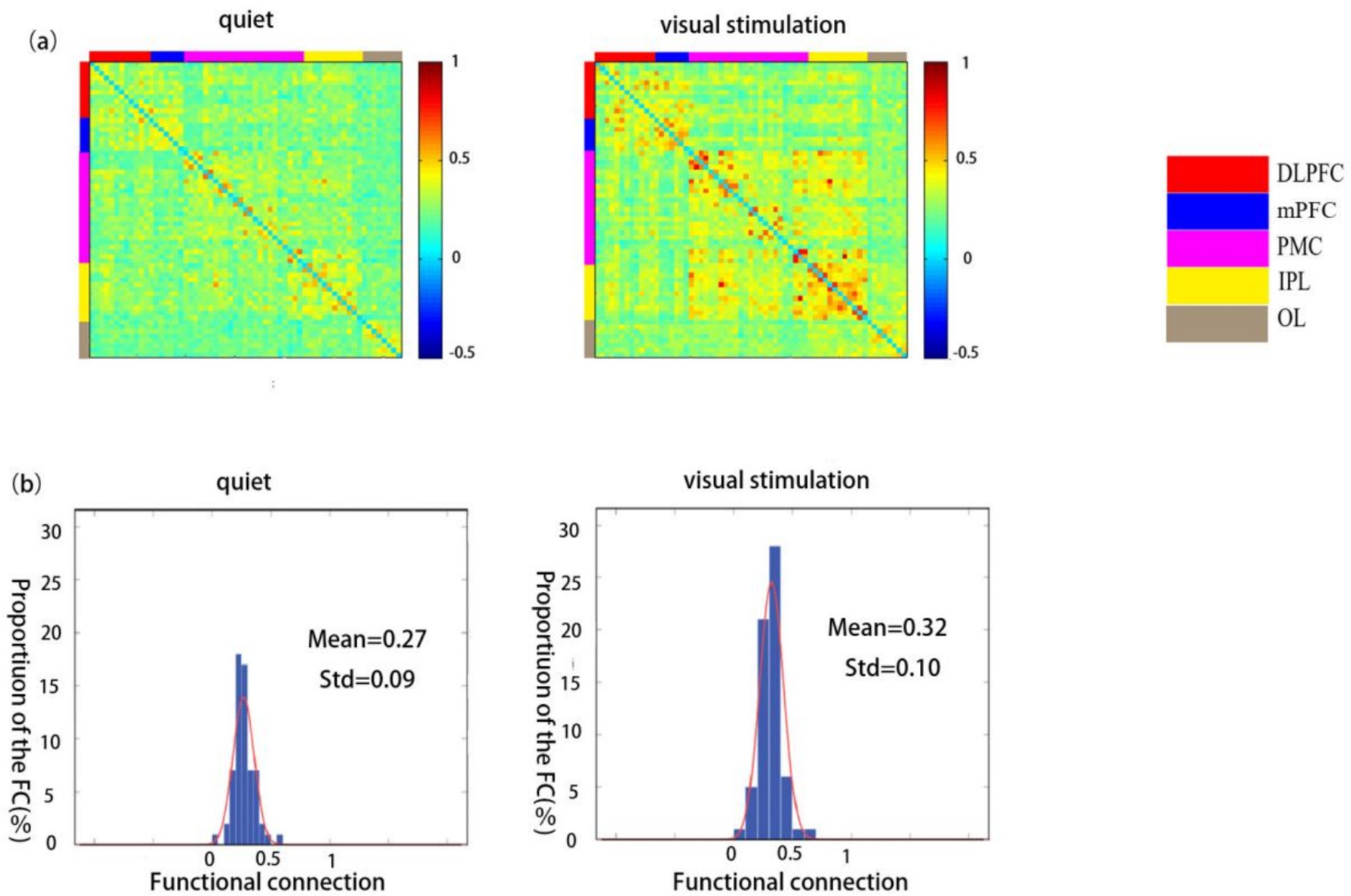
Figure 4. Spatial patterns of functional connectivity in the ASD group in the quiet and visual stimulation states. (a) Functional connectivity maps for the ASD group. (b) Histograms of the functional connectivity distribution. TD: typically developing, ASD: autism spectrum disorder.
A comparison between the ROI-ROI functional connectivity strength revealed statistically significant differences in the connectivity strength of the RDLPFC and LOL, LDLPFC and LIPL, mPFC and RPMC, mPFC and LPMC, mPFC and LIPL, ROL and RPM, ROL and RIPL, ROL and LIPL, as well as between the LOL and LIPL (p < 0.05). Further, extremely significant differences were observed between ROL and RPMC and ROL and RIPL (p < 0.01) (Table 3).
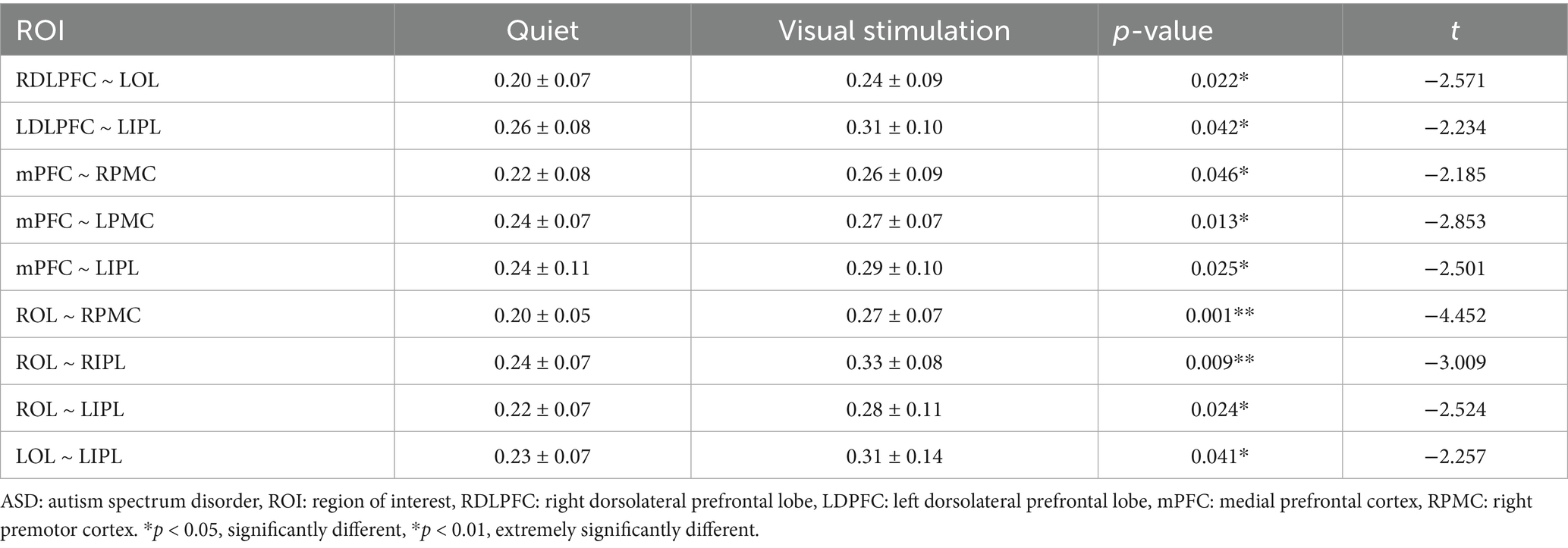
Table 3. Comparison of ROI–ROI functional connectivity strength between the quiet and visual stimulation states in children with ASD.
Functional connectivity strength in TD children during quiet and visual stimulation states
The functional connectivity strength of children in the TD group was significantly lower during visual stimulation (0.39 ± 0.14) than during the quiet state (0.45 ± 0.13) (Figures 5a,b).
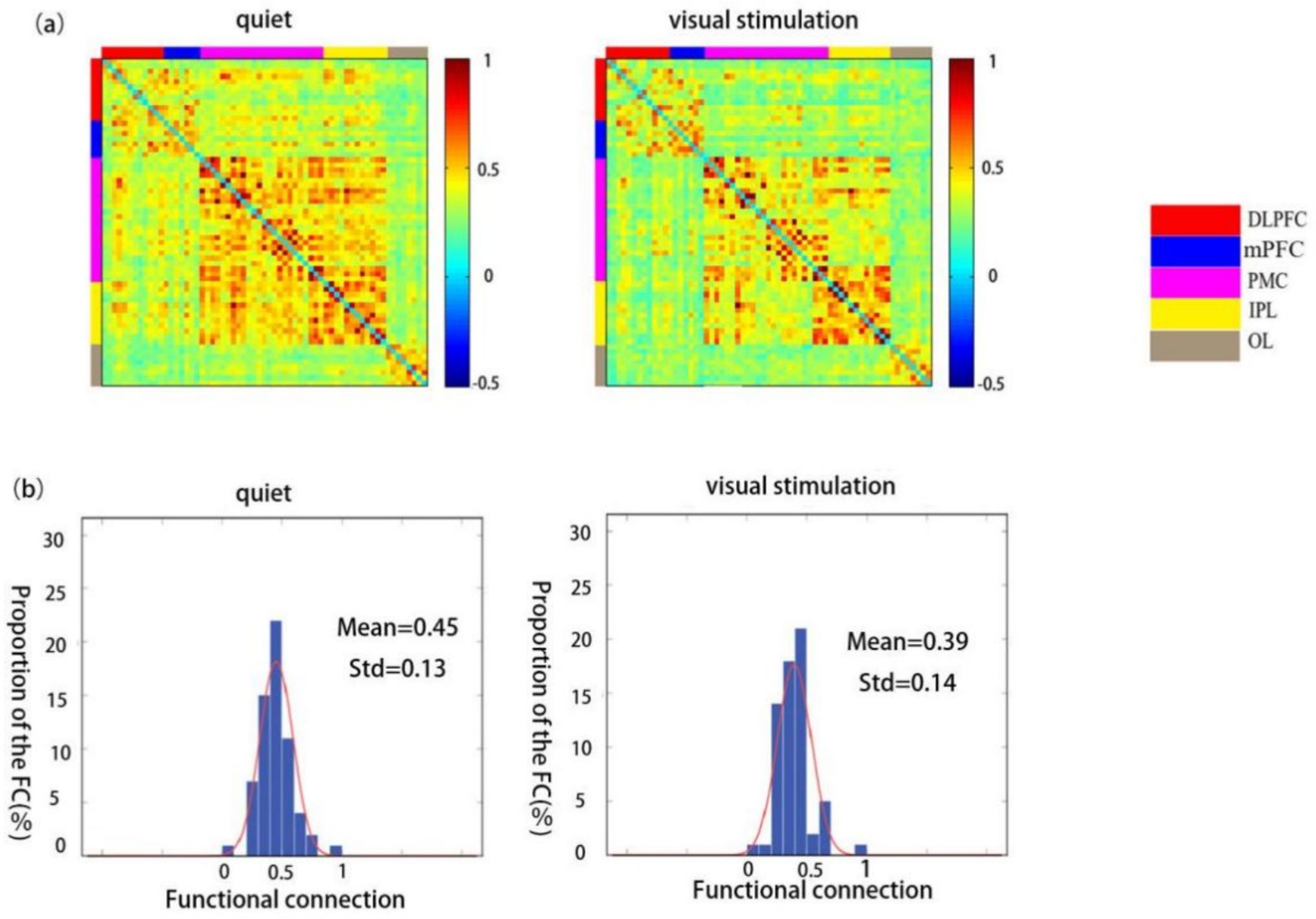
Figure 5. Spatial patterns of functional connectivity in the ASD group during quiet and visual stimulation state. (a) Functional connectivity maps of the ASD group. (b) Histograms of the functional connectivity distribution. TD: typically developing, ASD: autism spectrum disorder.
The comparison between the ROI–ROI functional connectivity strength revealed statistically significant differences in the connectivity strength of the RDLPFC and RPMC, RDLPFC and RIPL, LDLPFC and ROL, LDLPFC and LIPL, mPFC and RPMC, mPFC and RIPL, and mPFC and LIPL regions (p < 0.05). Extremely significant differences were observed between the RDLPFC and RIPL (p < 0.01) (Table 4).
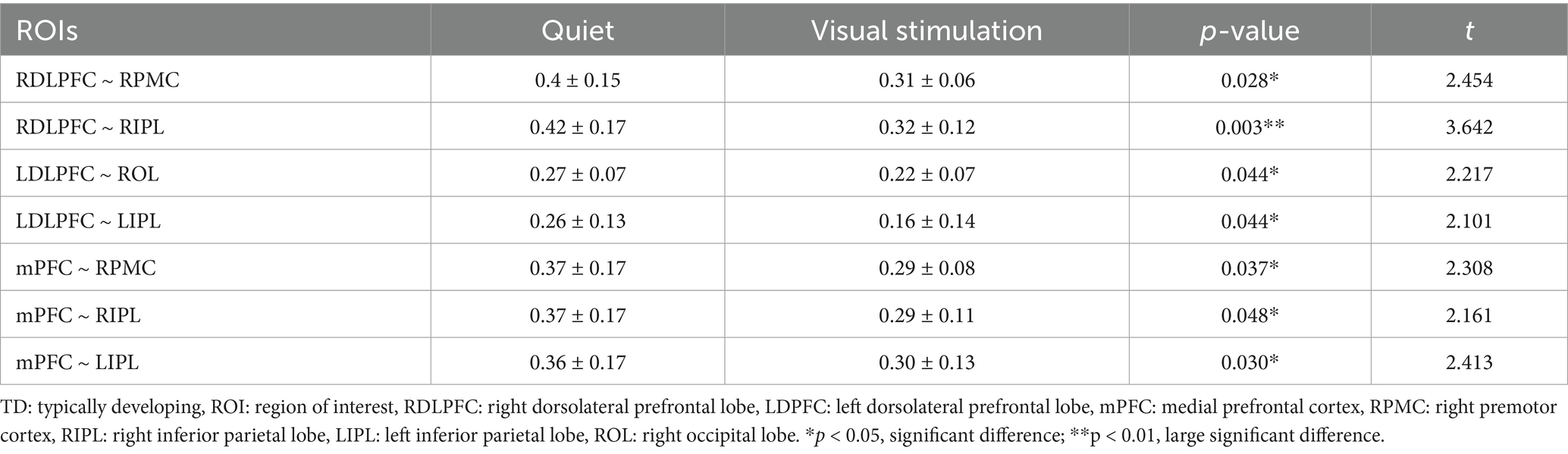
Table 4. Comparison of functional connectivity strength of ROI–ROI between the quiet and visual stimulation states in TD children.
Discussion
In recent years, an increasing number of studies have shown that ASD is associated with various brain neural network dysfunctions. Thus, the analysis of brain function connectivity in a resting state based on ROIs has emerged as a prominent topic in ASD research. This study used fNIRS to investigate brain functional connectivity strength in children with ASD and TD aged 3–7 years during the quiet test and while undergoing visual stimulation using AVM. These findings revealed that brain functional connectivity strength was lower in children with ASD than in TD children, indicating its potential as an effective neurobiological marker for the early detection of ASD.
Our ROI functional connectivity analysis revealed that connectivity between the DLPFC and other brain regions, as well as between the mPFC and other brain regions, was diminished in the ASD group. These findings align with previous research outcomes. For example, Rudie et al. (25) used resting-state fMRI to study children and adolescents with ASD and healthy controls and found that the functional connectivity between the prefrontal lobe and other brain regions in children with ASD was reduced. Zhu et al. (26) used fNIRS to show that the functional connection between the prefrontal lobe and other brain areas in children with ASD was weaker than that in healthy children in a common attention state. Mamashli et al. (27) also observed that the functional connections between the bilateral inferior frontal gyrus and temporal lobe were weaker in children with ASD than in healthy controls. Although the age of the participants and the research methods in this study were not completely consistent with those in the previously mentioned studies, we nevertheless found that children with ASD had reduced functional connectivity between ROIs. The decreases in functional connection strength observed in this study were all associated with the DLPFC and mPFC. The PFC plays a key role in higher cognition and working memory. This brain region has extensive neural fiber cross-projection connections and functional connections with other cerebral cortices and subcortical structures, making it dominant in the generation of related behaviors (28). Thus, we speculated that a decrease in the strength of functional connections between the PFC and other brain regions is the main cause of the decreased efficiency of the functional connection network in individuals with ASD.
Recently, AVM has been confirmed to be an effective teaching method for improving behavior in children with ASD. Video demonstration is commonly used to train children with ASD in various skills, mainly social behavior, functional life skills, cognitive behavior training, and problem behavior correction (29–31). Gaffrey and Samson also noted that individuals with ASD exhibit stronger visual participation than do those without when performing a series of perceptual or cognitive tasks (32, 33).
During cognition and verbal communication, individuals with ASD rely on their visual ability to acquire information through visual stimuli. The AVM method involves the application of visual support strategies and visual media as training. Out of the target behaviors, many international studies have confirmed that the video model law is consistent in children with ASD. In the present study, we found that the use of a tablet computer to play videos can better stimulate and maintain patients’ viewing motivation and observation ability and enable students to acquire and maintain the target behavior further by watching visual cues.
While fNIRS has emerged as a promising tool in neurocinematics, demonstrating significant inter-subject correlations in brain responses during multimedia experiences (34), other neuroimaging techniques such as functional Magnetic Resonance Imaging (fMRI) and Electroencephalography (EEG) have been more commonly applied in different contexts, including cognitive load assessment in multimedia learning (35) and driving behavior research (36). Despite the advantages of fNIRS, such as portability and reduced sensitivity to movement artifacts (37), the overall application of diverse neuroimaging methods in AVM remains underexplored. This gap highlights the need for further research to leverage various neuroimaging techniques to enhance understanding of cognitive and emotional responses elicited by animated video content.
Furthermore, children with ASD reportedly exhibit atypical visual perception abilities and a decrease in functional connectivity between the OL and IPL (38–40). Additionally, compared with that in a quiet state, functional connectivity between the OL and IPL was enhanced in children with ASD when they watched demonstration videos. IPL is linked to the ventral premotor cortex and plays a crucial role in processing visual and somatosensory information (41); this could be attributed to the rapid attention-grabbing nature of the demonstration videos on electronic screens for children with ASD, serving to stimulate their visual cortical responses. Conversely, in TD children, functional connectivity between the DLPFC and other brain regions, as well as between the mPFC and other brain regions, was lower when they watched demonstration videos than when they were in a quiet state. This may be due to differences in the behavior and cognitive mechanisms of TD children when watching videos, which may be influenced by their knowledge and experience. It has also been hypothesized that audiovisual modulation enhances the visual perception and information processing abilities of children with ASD by increasing the functional connectivity strength between the OL and IPL, thus serving as a potential treatment approach for ASD.
The use of virtual reality (VR) to enhance the social and cognitive skills of children with ASD has garnered widespread attention and interest across various sectors (42–44). The key feature of VR technology is its ability to provide a controlled visual and auditory experience (45, 46), allowing for the real-time assessment of patient adaptation and acceptance levels within the virtual training environment, thereby enabling appropriate adjustments. Integrating VR technology into social interaction demonstration videos offers novel methods and concepts for developing personalized rehabilitation programs for individuals with ASD.
The literature on visual tasks applied to the ASD population reveals a significant gap in recent studies, particularly regarding the integration of visual perception skills and their implications for interventions. A systematic review identified only 16 relevant studies on visual perception in ASD from 2014 to 2024, highlighting diverse findings such as enhanced visual processing and perceptual biases, yet underscoring the need for tailored interventions to address visual perception deficits that impact daily functioning (47). Additionally, a review of computer vision applications from 2009 to 2019 indicated that while computer vision techniques have potential in quantifying behavioral markers in ASD, the overall impact on visual task research remains underexplored (48). Furthermore, the application of virtual reality for vocational skills training in ASD has shown promise, but calls for more research to ensure skill maintenance and generalization (49). Overall, the current state of literature suggests a pressing need for updated studies focusing on visual tasks in ASD to better understand and leverage these unique perceptual characteristics (50, 51).
However, fNIRS presents several limitations, particularly regarding its penetration depth and spatial resolution compared to other neuroimaging techniques like functional magnetic resonance imaging (fMRI). While fNIRS is advantageous for its portability and ability to tolerate motion, it primarily measures hemodynamic responses in superficial cortical regions, which restricts its effectiveness in studying deeper brain structures (52). The technique’s reliance on scalp-located sensors and the diffusive nature of light in biological tissues complicate accurate hemoglobin evaluation, leading to potential errors in data interpretation due to hemoglobin cross talk (53). Additionally, variations in cap placement and systemic noise can further compromise signal quality, making it challenging to achieve reliable and repeatable measurements (54). Consequently, while fNIRS is a valuable tool for certain applications, it requires careful consideration, especially in studies requiring comprehensive functional connectivity assessments.
Study limitations
This study had limitations. First, due to constraints of the detecting equipment, the brain functional connectivity data included only the frontal, parietal, and occipital lobes, excluding the temporal lobe. Second, investigation into screen time in children with ASD using demonstration videos was lacking; hence, future research should focus on screen viewing time and personalized video content customization. Third, owing to the limited number of participants, larger cohort studies are required to better understand the functional connectivity characteristics of the brains of children with ASD, as well as to provide new insights for evaluating and treating sensory perception in patients with ASD.
Although our study is premature to conclude definitively that AVM constitutes an “efficient” or standalone treatment for ASD, these results serve as an initial indication of AVM’s potential to support visual processing in ASD, justifying the need for further investigation with larger cohorts, more robust experimental controls, and long-term follow-up assessments.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Binzhou Medical University Hospital (Approval number: 2024-KYLL-141). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. HL: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft. LZ: Methodology, Writing – original draft. YL: Project administration, Writing – original draft. YZ: Project administration, Writing – original draft. BH: Methodology, Project administration, Writing – original draft. WL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Shandong Natural Science Foundation (grant number ZR2022MH063).
Acknowledgments
We would like to thank all participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1590185/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington,VA, USA: American Psychiatric Association (2013).
2. Maenner, MJ, Shaw, KA, Bakian, AV, Bilder, DA, Durkin, MS, Esler, A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2012(2018). MMWR Surveill Summ. (2021) 70:1–16. doi: 10.15585/mmwr.ss6513a1
3. Yeung, MK. A systematic review and meta-analysis of facial emotion recognition in autism spectrum disorder: the specificity of deficits and the role of task characteristics. Neurosci Biobehav Rev. (2022) 133:104518. doi: 10.1016/j.neubiorev.2021.104518
4. Dakin, S, and Frith, U. Vagaries of visual perception in autism. Neuron. (2005) 48:497–507. doi: 10.1016/j.neuron.2005.10.018
5. Posar, A, and Visconti, P. Sensory abnormalities in children with autism spectrum disorder. J Pediatr. (2018) 94:342–50. doi: 10.1016/j.jped.2017.08.008
6. Bettoni, R, Cantiani, C, Riboldi, EM, Molteni, M, Bulf, H, and Riva, V. Visual statistical learning in preverbal infants at a higher likelihood of autism and its association with later social communication skills. PLoS One. (2024) 19:e0300274. doi: 10.1371/journal.pone.0300274
7. Riddiford, JA, Enticott, PG, Lavale, A, and Gurvich, C. Gaze and social functioning associations in autism spectrum disorder: a systematic review and meta-analysis. Autism Res. (2022) 15:1380–446. doi: 10.1002/aur.2729
8. Shane, HC, and Albert, PD. Electronic screen media for persons with autism spectrum disorders: results of a survey. J Autism Dev Disord. (2008) 38:1499–508. doi: 10.1007/s10803-007-0527-5
9. Ganz, JB, and Flores, MM. Effects of the use of visual strategies in play groups for children with autism spectrum disorders and their peers. J Autism Dev Disord. (2008) 38:926–40. doi: 10.1007/s10803-007-0463-4
10. Kojovic, N, Cekic, S, Castañón, SH, Franchini, M, Sperdin, HF, Sandini, C, et al. Unraveling the developmental dynamic of visual exploration of social interactions in autism. eLife. (2024) 13:e85623. doi: 10.7554/eLife.85623
11. Broder-Fingert, S, Feinberg, E, and Silverstein, M. Music therapy for children with autism spectrum disorder. JAMA. (2017) 318:523–4. doi: 10.1001/jama.2017.9477
12. Mason, RA, Rispoli, M, Ganz, JB, Boles, MB, and Orr, K. Effects of video modeling on communicative social skills of college students with Asperger syndrome. Dev Neurorehabil. (2012) 15:425–34. doi: 10.3109/17518423.2012.704530
13. Radley, KC, Ford, WB, McHugh, MB, Dadakhodjaeva, K, O'Handley, RD, Battaglia, AA, et al. Brief report: use of superheroes social skills to promote accurate social skill use in children with autism spectrum disorder. J Autism Dev Disord. (2015) 45:3048–54. doi: 10.1007/s10803-015-2442-5
14. Mineo, BA, Ziegler, W, Gill, S, and Salkin, D. Engagement with electronic screen media among students with autism spectrum disorders. J Autism Dev Disord. (2009) 39:172–87. doi: 10.1007/s10803-008-0616-0
15. LeBlanc, LA, Coates, AM, Daneshvar, S, Charlop-Christy, MH, Morris, C, and Lancaster, BM. Using video modeling and reinforcement to teach perspective-taking skills to children with autism. J Appl Behav Anal. (2003) 36:253–7. doi: 10.1901/jaba.2003.36-253
16. Frolli, A, Ricci, MC, Bosco, A, Lombardi, A, Cavallaro, A, Operto, FF, et al. Video modeling and social skills learning in ASD-HF. Children. (2020) 7:279. doi: 10.3390/children7120279
17. Syriopoulou-Delli, CK, and Sarri, K. Video-based instruction in enhancing functional living skills of adolescents and young adults with autism spectrum disorder and their transition to independent living: a review. Int J Dev Disabil. (2022) 68:788–99. doi: 10.1080/20473869.2021.1900504
18. Chen, L, Du, B, Li, K, Li, K, Hou, T, Jia, F, et al. The effect of tDCS on inhibitory control and its transfer effect on sustained attention in children with autism spectrum disorder: an fNIRS study. Brain Stimul. (2024) 17:594–606. doi: 10.1016/j.brs.2024.04.019
19. Conti, E, Scaffei, E, Bosetti, C, Marchi, V, Costanzo, V, Dell'Oste, V, et al. Looking for "fNIRS signature" in autism spectrum: a systematic review starting from preschoolers. Front Neurosci. (2022) 16:785993. doi: 10.3389/fnins.2022.785993
20. Piatti, A, Van der Paelt, S, Warreyn, P, and Roeyers, H. Neural correlates of response to joint attention in 2-to-5-year-olds in relation to ASD and social-communicative abilities: an fNIRS and behavioral study. Autism Res. (2024) 17:1106–25. doi: 10.1002/aur.3149
21. Zhang, X, Noah, JA, Singh, R, McPartland, JC, and Hirsch, J. Support vector machine prediction of individual autism diagnostic observation schedule (ADOS) scores based on neural responses during live eye-to-eye contact. Sci Rep. (2024) 14:3232. doi: 10.1038/s41598-024-53942-z
22. Cui, X, Bray, S, Bryant, DM, Glover, GH, and Reiss, AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. (2011) 54:2808–21. doi: 10.1016/j.neuroimage.2010.10.069
23. Sasai, S, Homae, F, Watanabe, H, and Taga, G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. NeuroImage. (2011) 56:252–7. doi: 10.1016/j.neuroimage.2010.12.075
24. Zhu, H, Fan, Y, Guo, H, Huang, D, and He, S. Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomed Opt Express. (2014) 5:1262–74. doi: 10.1364/BOE.5.001262
25. Rudie, JD, Brown, JA, Beck-Pancer, D, Hernandez, LM, Dennis, EL, Thompson, PM, et al. Altered functional and structural brain network organization in autism. Neuroimage Clin. (2012) 2:79–94. doi: 10.1016/j.nicl.2012.11.006
26. Zhu, H, Li, J, Fan, Y, Li, X, Huang, D, and He, S. Atypical prefrontal cortical responses to joint/non-joint attention in children with autism spectrum disorder (ASD): a functional near-infrared spectroscopy study. Biomed Opt Express. (2015) 6:690–701. doi: 10.1364/BOE.6.000690
27. Mamashli, F, Khan, S, Bharadwaj, H, Losh, A, Pawlyszyn, SM, Hämäläinen, MS, et al. Maturational trajectories of local and long-range functional connectivity in autism during face processing. Hum Brain Mapp. (2018) 39:4094–104. doi: 10.1002/hbm.24234
28. Ameis, SH, Daskalakis, ZJ, Blumberger, DM, Desarkar, P, Drmic, I, Mabbott, DJ, et al. Repetitive transcranial magnetic stimulation for the treatment of executive function deficits in autism spectrum disorder: clinical trial approach. J Child Adolesc Psychopharmacol. (2017) 27:413–21. doi: 10.1089/cap.2016.0146
29. Macpherson, K, Charlop, MH, and Miltenberger, CA. Using portable video modeling technology to increase the compliment behaviors of children with autism during athletic group play. J Autism Dev Disord. (2015) 45:3836–45. doi: 10.1007/s10803-014-2072-3
30. McConnell, K, Keenan, C, Storey, C, and Thurston, A. Video-based interventions promoting social behavioural skills for autistic children and young people: an evidence and gap map. Campbell Syst Rev. (2024) 20:e1405. doi: 10.1002/cl2.1405
31. Petursdottir, AL, and Gudmundsdottir, T. Supporting social play skill acquisition and generalization of children with autism through video modeling. J Autism Dev Disord. (2023) 53:1391–402. doi: 10.1007/s10803-021-05204-4
32. Gaffrey, MS, Kleinhans, NM, Haist, F, Akshoomoff, N, Campbell, A, Courchesne, E, et al. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. (2007) 45:1672–84. doi: 10.1016/j.neuropsychologia.2007.01.008
33. Samson, F, Mottron, L, Soulières, I, and Zeffiro, TA. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. (2012) 33:1553–81. doi: 10.1002/hbm.21307
34. Maior, HA, Ramchurn, R, Martindale, S, Cai, M, Wilson, ML, and Benford, S. fNIRS and neurocinematics In: Extended abstracts of the 2019 CHI conference on human factors in computing systems (paper LBW2522). New York, NY, USA: Association for Computing Machinery (2019). 1–6.
35. Ozel, P, Bayraktar, DM, Altan, T, Coskun, V, and Olamat, A. A review for neuroimaging techniques in multimedia learning: an experimental framework In: Y Koumpouros, A Georgoulas, and G Kremmyda, editors. Transcending humanitarian engineering strategies for sustainable futures. Hershey, PA, USA: IGI Global Scientific Publishing (2023). 106–19.
36. Haghani, M, Bliemer, MCJ, Farooq, B, Kim, I, Li, Z, Oh, C, et al. Applications of brain imaging methods in driving behaviour research. Accid Anal Prev. (2021) 154:106093. doi: 10.1016/j.aap.2021.106093
37. Kohl, SH, Mehler, DMA, Lührs, M, Thibault, RT, Konrad, K, and Sorger, B. The potential of functional near-infrared spectroscopy-based neurofeedback-a systematic review and recommendations for best practice. Front Neurosci. (2020) 14:594. doi: 10.3389/fnins.2020.00594
38. Jin, X, Zhang, K, Lu, B, Li, X, Yan, CG, Du, Y, et al. Shared atypical spontaneous brain activity pattern in early onset schizophrenia and autism spectrum disorders: evidence from cortical surface-based analysis. Eur Child Adolesc Psychiatry. (2024) 33:2387–96. doi: 10.1007/s00787-023-02333-2
39. Knight, EJ, Freedman, EG, Myers, EJ, Berruti, AS, Oakes, LA, Cao, CZ, et al. Severely attenuated visual feedback processing in children on the autism spectrum. J Neurosci. (2023) 43:2424–38. doi: 10.1523/JNEUROSCI.1192-22.2023
40. Lepping, RJ, McKinney, WS, Magnon, GC, Keedy, SK, Wang, Z, Coombes, SA, et al. Visuomotor brain network activation and functional connectivity among individuals with autism spectrum disorder. Hum Brain Mapp. (2022) 43:844–59. doi: 10.1002/hbm.25692
41. Unruh, KE, Martin, LE, Magnon, G, Vaillancourt, DE, Sweeney, JA, and Mosconi, MW. Cortical and subcortical alterations associated with precision visuomotor behavior in individuals with autism spectrum disorder. J Neurophysiol. (2019) 122:1330–41. doi: 10.1152/jn.00286.2019
42. De Luca, R, Naro, A, Colucci, PV, Pranio, F, Tardiolo, G, Billeri, L, et al. Improvement of brain functional connectivity in autism spectrum disorder: an exploratory study on the potential use of virtual reality. J Neural Transm. (2021) 128:371–80. doi: 10.1007/s00702-021-02321-3
43. Herrera, G, Alcantud, F, Jordan, R, Blanquer, A, Labajo, G, and De Pablo, C. Development of symbolic play through the use of virtual reality tools in children with autistic spectrum disorders: two case studies. Autism. (2008) 12:143–57. doi: 10.1177/1362361307086657
44. Lahiri, U, Warren, Z, and Sarkar, N. Design of a gaze-sensitive virtual social interactive system for children with autism. IEEE Trans Neural Syst Rehabil Eng. (2011) 19:443–52. doi: 10.1109/TNSRE.2011.2153874
45. Bernal-Berdun, E, Vallejo, M, Sun, Q, Serrano, A, and Gutierrez, D. Modeling the impact of head-body rotations on audiovisual spatial perception for virtual reality applications. IEEE Trans Vis Comput Graph. (2024) 30:2624–32. doi: 10.1109/TVCG.2024.3372112
46. Massiceti, D, Hicks, SL, and van Rheede, JJ. Stereosonic vision: exploring visual-to-auditory sensory substitution mappings in an immersive virtual reality navigation paradigm. PLoS One. (2018) 13:e0199389. doi: 10.1371/journal.pone.0199389
47. Redkar, SS, Ganapathy, SU, and Deepak, VR. Understanding visual perception skills in autism spectrum disorder: a systematic review. J Associated Med Sci. (2025) 58:245–59. doi: 10.12982/jams.2025.027
48. de Belen, RAJ, Bednarz, T, Sowmya, A, and Del Favero, D. Computer vision in autism spectrum disorder research: a systematic review of published studies from 2009 to 2019. Transl Psychiatry. (2020) 10:333. doi: 10.1038/s41398-020-01015-w
49. Sarri, K, Syriopoulou-Delli, C, Gkiolnta, E, and Zygopoulou, M. The application of virtual reality for vocational skills of individuals with autism spectrum disorder: a systematic review. Eximia. (2022) 5:581–95. doi: 10.47577/eximia.v5i1.179
50. Chung, S, and Son, JW. Visual perception in autism spectrum disorder: a review of neuroimaging studies. J Child Adolesc Psychiatry. (2020) 31:105–20. doi: 10.5765/jkacap.200018
51. Tribull, M. (2014). Visual search and task-irrelevant shape information in autism spectrum disorder. (master’s thesis, University College London). UCL Discovery. Available online at: https://discovery.ucl.ac.uk/id/eprint/1470117/ (Accessed 15 December, 2024).
52. Scarapicchia, V, Brown, C, Mayo, C, and Gawryluk, JR. Functional magnetic resonance imaging and functional near-infrared spectroscopy: insights from combined recording studies. Front Hum Neurosci. (2017) 11:419. doi: 10.3389/fnhum.2017.00419
53. Chiarelli, AM, Perpetuini, D, Filippini, C, Cardone, D, and Merla, A. Differential pathlength factor in continuous wave functional near-infrared spectroscopy: reducing hemoglobin’s cross talk in high-density recordings. Neurophotonics. (2019) 6:035005. doi: 10.1117/1.NPH.6.3.035005
Keywords: autism spectrum disorder, animated video modeling, brain functional connectivity, visual stimulation, functional near-infrared spectroscopy
Citation: Zhu C, Li H, Zhang L, Liu Y, Zhang Y, Huang B and Li W (2025) Functional near-infrared spectroscopy characteristics in children with autism spectrum disorder under animated video modeling therapy. Front. Neurol. 16:1590185. doi: 10.3389/fneur.2025.1590185
Edited by:
Lingshan Gou, Xuzhou Maternity and Child Health Care Hospital Affiliated to Xuzhou Medical University, ChinaReviewed by:
Elena Scaffei, Stella Maris Foundation (IRCCS), ItalyFerdinando Suvini, Conservatorio di Musica Girolamo Frescobaldi, Italy
Copyright © 2025 Zhu, Li, Zhang, Liu, Zhang, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, eWlzaGVuZ2xpd2VpQDE2My5jb20=
†These authors have contributed equally to this work
 Chuanhua Zhu
Chuanhua Zhu Hongwei Li
Hongwei Li Lina Zhang1
Lina Zhang1 Binbin Huang
Binbin Huang Wei Li
Wei Li