Abstract
Background:
Sciatic nerve gives off branches that supply the back of the thigh, leg, and foot. Classically, this nerve emerges from the greater sciatic foramen below the piriformis muscle and subsequently divides into the common fibular and tibial nerves in the distal third of the posterior thigh. However, the course of the sciatic nerve varies among individuals, potentially resulting in nerve compressions. Understanding these variations helps prevent injuries during diagnostic and therapeutic procedures. This study examined the branching patterns of this nerve in the Scottish cadavers using the Beaton and Anson classification, which categorizes them into Type A–G.
Methods:
Twelve gluteal regions (4 males, 8 females) with a mean age of 87.3 years, were obtained from a Scottish University regulated by the Human Tissue (Scotland) 2006. The sciatic nerve and its branches were carefully dissected, and the relationship between the nerve and the piriformis muscle was observed and documented.
Results:
One left gluteal region of an 89-year-old female (n = 1, 8.3%) showed sciatic nerve variation. This variant exhibited early bifurcation, with the common fibular nerve piercing through the piriformis and the tibial nerve passing beneath it (referred to as Type B). The remaining 91.7% of cases, the sciatic nerve exhibited classical presentation (referred to as Type A).
Conclusion:
The Type B variation of the sciatic nerve is found in 8.3% of the elderly Scottish cadavers. While it is rare, it is crucial to acknowledge nerve variants to prevent injuries during posterior approach total hip arthroplasty or inadequate sciatic nerve blockade.
Introduction
The sciatic nerve (SN) is the body’s largest nerve, originating from spinal nerve roots L4 to S3. It innervates the posterior thigh muscles and provides sensory and motor innervation to the skin and muscles of the leg and foot. The nerve exits the pelvis through the greater sciatic foramen, beneath the piriformis muscle (PM), travels through the gluteal region, and enters the posterior thigh. There, it splits into two primary branches: the common fibular nerve (CFN) and the tibial nerve (TN) (1). However, it is important to note that the course and branches of the sciatic nerve can differ among individuals, which can potentially result in nerve compressions. In individuals with an anatomical variation, the sciatic nerve, or parts of it, may exit through or above the piriformis muscle (2). Nerve compressions are more likely to occur at the site where the CFN and TN originate from the pelvis (3).
Beaton and Anson first classified the relationships between the PM and the SN into six categories and then updated by Tomaszewski which then, became seven categories (4, 5). This classification system is the preferred system for sciatic nerve variations due to its comprehensive detail, clinical relevance, clarity, and simplicity (4, 6). It is widely used in surgical settings, particularly in hip surgery, to help anticipate and mitigate the risks of nerve injury. Anomalous relationships are categorized from type “B” to type “G” (non-Type A), with type “A” representing the normal relationship between the PM and the SN (4, 7) (Table 1; Figure 1). This classification provides a framework for understanding the clinical implications of sciatic nerve variations, which are especially important in conditions such as piriformis syndrome. In 16.9% of patients with piriformis syndrome, there is a variation in the bifurcation of the sciatic nerve (8). Of these, 80.9% are classified as type “B,” 7.6% as type “C,” 3.1% as type “D,” and 0.5% each as types “E” and “F” (8).
Table 1
| Types | Description |
|---|---|
| A | The SN exits medially under the piriformis muscle PM. |
| B | The SN is pre-bifurcated, with the CFN piercing the muscle belly of the PM. |
| C | The SN is pre-bifurcated, with the CFN exiting above the PM, and the TN exits medially underneath the PM. |
| D | The entire SN pierces the PM as a single trunk. |
| E | The SN is pre-bifurcated, with the CFN exiting above the PM, and the TN piercing the PM. |
| F | The entire SN exits above the PM as a single trunk. |
| G | The SN is pre-bifurcated, with both the CFN and TN exiting beneath the PM |
Beaton and Anson’s classification system for the sciatic nerve distinguishes between Type A (normal) and various anomalous variations (B, C, D, E, F, or G) (4, 5, 7).
SN = sciatic nerve; PM = piriformis muscle; CFN = common fibular nerve; TN = tibial nerve.
Figure 1

Beaton and Anson classification system of the sciatic nerve as depicted by Type A or Non-Type A (B, C, D, E, F or G variations) (4, 5, 7). This illustration is made by the author using Biorender.com.
The variability in the course of the sciatic nerve can be attributed to its embryonic development, which involves the autonomous formation of the common fibular and tibial divisions (9). The lumbar and sacral plexuses develop at the base of the lower limb bud, forming the primary nerve supply of the lower limb. Nerves from the plexuses differentiate into dorsal and ventral components as they extend into the limb to innervate the respective musculature (10, 11). The common fibular nerve originates from the large dorsal component, whereas the tibial nerve forms from the ventral portion (12). As the subdivisions of the sciatic nerve arise from distinct components, these divisions may separate at varying levels and follow different pathways as they descend to their targets in the lower limb (10, 11).
Interestingly, anatomical variations of the sciatic nerve have been shown to exhibit ethnic and geographical differences (3, 13–19). Both Types A and B variations of the sciatic nerve are observed across all human populations (20). The prevalence of the Type B variation has been reported to range from 1.3 to 33.9% across different studies (3, 14–19, 21).
In relationship to this, non-type A variations in the SN-PM relationship are associated with an increased risk of injury, depending on the clinical approach used (22–25). When anatomical variations of the sciatic nerve are not adequately recognised, they can pose challenges during clinical interventions such as total hip arthroplasty via the posterior approach and sciatic nerve blockade (5, 26, 27). The inability to identify these variations is a common technical error that may result in iatrogenic injury, emphasising the need for a thorough understanding of variant anatomy (28).
Sciatic nerve variants in close relation to the piriformis muscle are at greater risk of intraoperative injury, including stretching, compression and lacerations during total hip arthroplasty (16, 29). Specifically, the location of the common fibular nerve traversing through the piriformis in the Type B variant is highly susceptible to injury from traction, not only during surgery, but also in cases of traumatic hip dislocation (29). Failure to recognise high bifurcation of the sciatic nerve can lead to incomplete sciatic nerve block during popliteal block anaesthesia, as only one subdivision of the nerve may be anaesthetized (17, 30). This variant was also suggested to be more commonly associated with piriformis syndrome due to the entrapment of the common fibular nerve between the piriformis muscle (15). These anomalies are particularly relevant for clinicians performing procedures like imaging-guided injections of the PM, total hip arthroplasty, and piriformis tenotomy for piriformis syndrome. Therefore, the understanding of the prevalence of piriformis and sciatic nerve anomalies is crucial in various clinical contexts. Hence, the aim of this study was to examine the patterns of sciatic nerve branching in the Scottish cadavers.
Methods
Specimens in this study were obtained from a Scottish Univeristy, regulated by the Human Tissue (Scotland) Act 2006. Ethical approval number ANATED_0036 was given for the use of cadaveric images.
A total of 12 gluteal regions from 6 cadaveric bodies, consisting of two males and four females, with a mean age of 87.3 years. Cadavers with previous trauma or surgery to the gluteal region were excluded. The gluteus maximus muscles of bilateral gluteal regions in each cadaver are dissected and cleaned. The gluteus maximus muscle is then incised along its origin and reflected laterally to expose the underlying gluteus medius, piriformis muscle, gemellus superior, obturator internus, and gemellus inferior muscles along with their neurovascular supply. The fascia covering PM, the sheath covering SN and its branches were carefully dissected. Subsequently, the relationship between the nerve and the piriformis muscle was observed and documented.
Since samples in this study were recruited from a university-based body donation volunteers, this method may introduce sampling bias as the participants are predominantly elderly and from a specific geographic region. These characteristics may limit the generalizability of the results to represent the Scottish population.
Results
The anatomical relationships between the sciatic nerve, its branches and the piriformis muscle were documented in twelve dissected gluteal regions. According to Beaton and Anson’s classification (1938b), a Type B variation of the sciatic nerve was identified in 8.3% (n = 1) of the specimens, observed unilaterally in the left gluteal region of an 89-year-old female cadaver. In this case, the left sciatic nerve emerged from the greater sciatic foramen and showed an early bifurcation, with the common fibular nerve piercing through the piriformis muscle, while the tibial nerve emerged below the muscle (Figure 2). Distal to their emergence from the piriformis, the common fibular and tibial nerves followed their typical courses separately to innervate the posterior compartment of the lower limb. In the remaining 91.7% (n = 11) of gluteal regions, the sciatic nerve demonstrated the classical Type A pattern, passing undivided inferior to the piriformis muscle. No other variation types were observed in this sample.
Figure 2
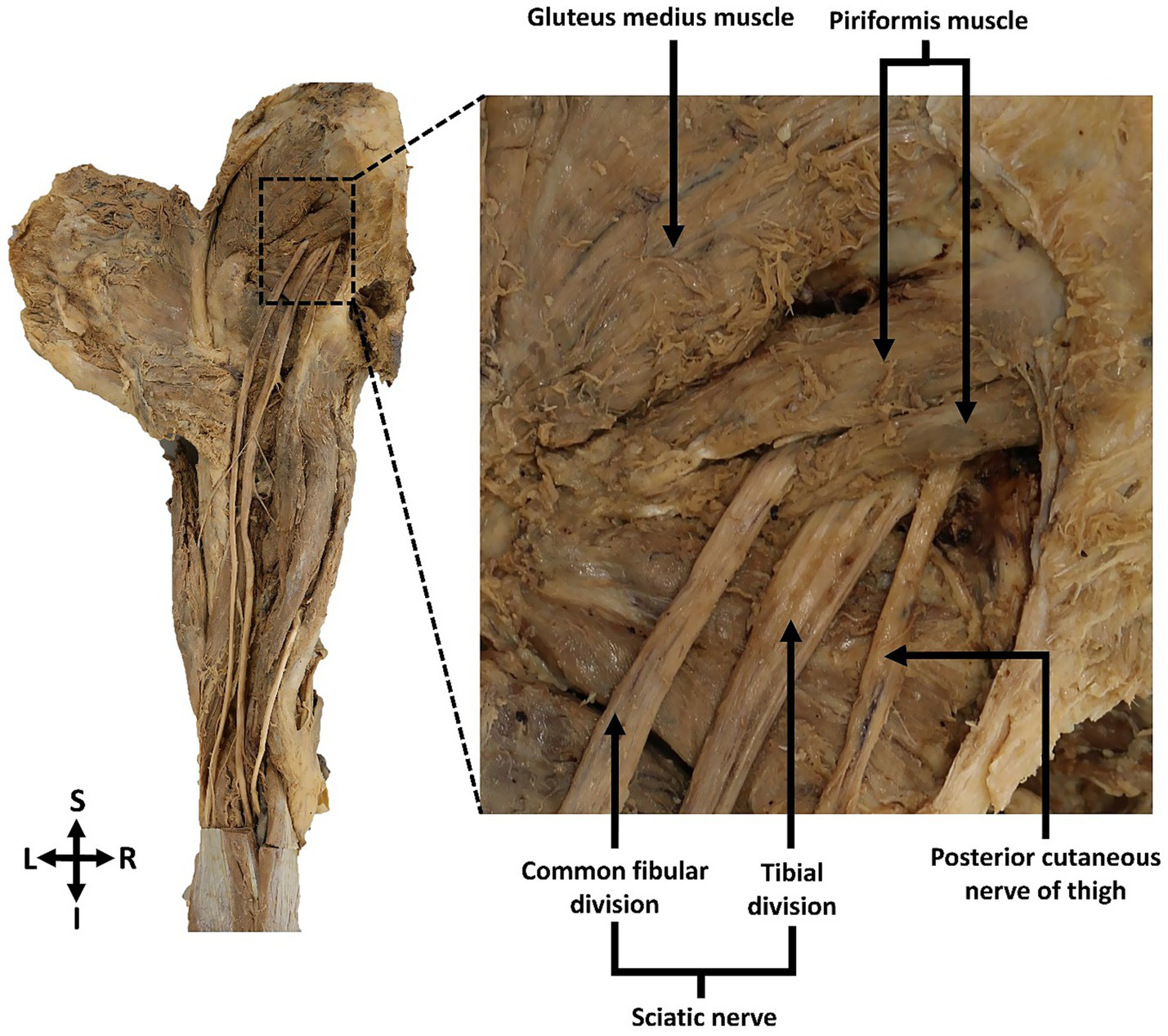
Posterior view of the left gluteal region showing the Type B sciatic nerve variation. The common fibular division of the left sciatic nerve pierced through and exited the piriformis muscle, while the tibial nerve passed inferior to the muscle. The common fibular and tibial nerve divisions remained separate as they proceeded distally to their target muscles in the lower limb.
Discussion
This exploratory study documented the variation in the sciatic nerve and its prevalence within the cadaveric population at the University of Edinburgh, highlighting the significance of such anatomical variations and their implications for clinical practice. In comparison with prevalence data from a meta-analysis, Type A sciatic nerve presentation is the most common, with a reported pooled prevalence of 90% (8), closely aligning with the 91.7% observed in this study. The Type B variant, which is characterised by the sciatic nerve subdivisions passing between and below the piriformis muscle, exhibited an 8.3% prevalence in this sample, consistent with the previous pooled prevalence of 8% (8). The course and bifurcation pattern of sciatic nerve showed great variability as categorised by Beaton and Anson (4). The nerve subdivisions emerged above and below the piriformis muscle in Type C whereas the undivided nerve pierced through the muscle in Type D. In contrast to previously reported cases (8), Types C, D, E, F and G were absent in the current study.
The findings of this study were also in line with the previous reports on sciatic nerve variation across different demographical and geographical backgrounds. In this study, the prevalence of the Type B variation is present in 10% of the Scottish cadavers, which lies within the prevalence range of 1.3 to 33.9% across different studies (3, 14–19, 21). The wide range of reported prevalence values (1.3–33.9%) suggests that ethnic differences may influence sciatic nerve variations. Notably, the prevalence is significantly higher in East Asia (24%) compared to Europe (9%) (8), where the Scottish cadavers from this study can be classified. Contrary to previous studies indicating no significant differences in the laterality of sciatic nerve variations (8), the Type B variant in this study was observed exclusively in the left gluteal region of a female cadaver. Similar to observations in this study, sciatic nerve variations were more frequently identified in females compared to males (29, 31, 32). A previous meta-analysis found that the Type B variant was twice as common in females as in males, though this difference was not statistically significant (8). This gender-based prevalence disparity remains unclear, but it has been suggested that the development of the sciatic nerve close to the female reproductive organs may play a role (8).
Differences in the embryological development timing of the sciatic nerve and piriformis muscle may contribute to variations in their anatomical relationship (8). Formation of the sciatic nerve begins approximately the sixth week of embryonic development, while the piriformis muscle develops by the eighth week (33). This discrepancy in developmental timing and speed may contribute to variations in the anatomical relationship between the two structures before the definitive insertion of piriformis forms at around 15 weeks (33). The formation of piriformis muscle from two separate myotome structures may also allow the common fibular nerve to traverse the muscle before these myotomes fuse into a single structure (34, 35), leading to the Type B variation. While direct genetic studies specifically linking genetic factors to sciatic nerve variations are currently limited, Kasapuram et al. (34) proposed that nerve pathway morphology may be influenced by the expression levels of molecules which regulate nerve growth and direction, such as netrins, slits, semaphorins and ephrins (36). Genetic predisposition resulting in these molecular changes could contribute to population-based differences in sciatic nerve anatomy. Further genetic studies are needed to establish a direct association.
Awareness of sciatic nerve variations not only improves treatment outcomes, but also aids in the diagnosis of related pathologies. While sciatic nerve variations do not always correlate to piriformis syndrome, they are potential causes of this condition, leading to pain in the gluteal region (2, 37). Although sciatica commonly results from spinal degenerative disc disorders, nerve entrapment in piriformis syndrome can mimic sciatica symptoms and has been reported to account for 6–8% of sciatica cases (38, 39). Therefore, recognising sciatic nerve variants is crucial for accurately diagnosing associated pathologies to ensure appropriate treatment is administered (40, 41). The Type B variant was previously suggested to be more commonly associated with piriformis syndrome due to the entrapment of the common fibular nerve between the piriformis muscle (15). Although sciatica commonly results from spinal degenerative disc disorders, nerve entrapment in piriformis syndrome can mimic sciatica symptoms and has been reported to account for 6–8% of sciatica cases (38, 39).
This study presented several limitations, including small sample size, which restricted the examination of other sciatic nerve variations, specific variant laterality, age, gender prevalence, and also sampling bias. Furthermore, the absence of the donor’s medical histories did not allow investigation into potential correlations between anatomical variations of sciatic nerve and clinical presentations. Despite these limitations, the sciatic nerve variant observed in the current study highlighted the importance of an in-depth understanding of anatomical variability and the need for future research into how such variations impact clinical practice and outcomes. Future studies should also aim to include a more diverse sample by recruiting from multiple locations and using random sampling methods to enhance external validity.
In conclusion, variation in the sciatic nerve anatomy is present in 8.3% of the Scottish elderly cadaveric population. In this case, the left sciatic nerve exhibited early bifurcation, with the common fibular nerve piercing through piriformis and the tibial nerve passing beneath it (referred to as Type B). In the remaining 91.7% of cases, the sciatic nerve exhibits the classical presentation (referred to as Type A). The Type B variation of the sciatic nerve is found in 8.3% of the elderly Scottish cadavers. Although anatomical variations of the sciatic nerve are relatively uncommon, it is essential for medical practitioners to understand such variants and consider preoperative imaging for high-risk patients to prevent injuries during medical procedures. Future studies using imaging techniques in living patients, or genetic research would be most beneficial and should be conducted on a larger scale to comprehensively investigate the prevalence of sciatic nerve variations among the Scottish population.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Anatomy, Old Medical School: Deanery of Biomedical Sciences, Edinburgh University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RR: Writing – original draft, Writing – review & editing, Formal analysis, Data curation, Visualization, Methodology, Project administration. JT: Data curation, Formal analysis, Writing – review & editing, Writing – original draft. SC: Writing – review & editing, Formal analysis, Data curation, Project administration, Validation. RA: Writing – review & editing, Formal analysis, Data curation, Validation. ASA: Validation, Writing – review & editing, Formal analysis, Data curation. AA: Validation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors convey their utmost gratitude to all body donors of The University of Edinburgh who have dedicated their bodies to the development of anatomical knowledge and advancement of science. None of the authors have a conflict of interest to disclose.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Drake RL Vogl AW Mitchell AWM . Gray's anatomy for students. Philadelphia: Elsevier (2024).
2.
Smoll NR . Variations of the piriformis and sciatic nerve with clinical consequence: a review. Clinical anatomy (New York, NY). (2010) 23:8–17. doi: 10.1002/ca.20893
3.
Güvençer M Iyem C Akyer P Tetik S Naderi S . Variations in the high division of the sciatic nerve and relationship between the sciatic nerve and the piriformis. Turk Neurosurg. (2009) 19:139–44.
4.
Beaton LE Anson BJ . The sciatic nerve and the piriformis muscle: their interrelation a possible cause of coccygodynia. JBJS. (1938) 20:686–688.
5.
Tomaszewski KA Graves MJ Henry BM Popieluszko P Roy J Pękala PA et al . Surgical anatomy of the sciatic nerve: a meta-analysis. J Orthop Res. (2016) 34:1820–7. doi: 10.1002/jor.23186
6.
Asmall T. Gunston G. Venter R. Henry B. Keet K. (2020). Surgical anatomy of the sciatic nerve and its relationship to the piriformis muscle with a description of a rare variant. SA Orthop. J. doi: 10.17159/2309-8309/2020/v19n1a5
7.
Charles RM Aaron LG Benjamin JP David D Bradley AC Jennifer FD . Beaton and Anson type a classification of the sciatic nerve and piriformis complex: clinical considerations for sex and laterality. Anatomia. (2024) 3:182–91. doi: 10.3390/anatomia3030014
8.
Poutoglidou F Piagkou M Totlis T Tzika M Natsis K . Sciatic nerve variants and the piriformis muscle: a systematic review and meta-analysis. CureusPalo Alto, Ca. (2020) 12:e11531. doi: 10.7759/cureus.11531
9.
Keibel FM Franklin P . Manual of human embryology ii, Philadelphia. Philadelphia & London: J. B Lippincott Company (1912).
10.
Singh D Sharma R . Relationship between the sciatic nerve and piriformis muscle. Neurosci Res Letter. (2011)
11.
Umarji BN Karambelkar RR Shewale AD . Study of variations in the divisions, course and termination of the sciatic nerve. J Krishna Institute Med Sci Univ. (2013) 2:62–8.
12.
Demiryurek D Bayramoglu A Erbil K Aldur M Mustafa ES . Bilateral divided piriformis muscle together with the high division of the sciatic nerve. Gazi Med J. (2002) 13:41–4.
13.
Berihu BA Debeb YG . Anatomical variation in bifurcation and trifurcations of sciatic nerve and its clinical implications: in selected university in Ethiopia. BMC Res Notes. (2015) 8:633–3. doi: 10.1186/s13104-015-1626-6
14.
Chiba S . Multiple positional relationships of nerves arising from the sacral plexus to the piriformis muscle in humans. Kaibogaku Zasshi. (1992) 67:691–724.
15.
Pećina M . Contribution to the etiological explanation of the piriformis syndrome. Acta Anat (Basel). (1979) 105:181–7. doi: 10.1159/000145121
16.
Pokorny D Jahoda D Veigl D Pinskerova V Sosna A . Topographic variations of the relationship of the sciatic nerve and the piriformis muscle and its relevance to palsy after total hip arthroplasty. Surg Radio Anatomy. (2006) 28:88–91. doi: 10.1007/s00276-005-0056-x
17.
Shailesh P Mitesh S Rakesh V Ankur Z Rathod SP . A variation in the high division of the sciatic nerve and its relation with piriformis muscle. Natl J Med Res. (2011) 1:27–30.
18.
Sulak O Sakalli B Ozguner G Kastamoni Y . Anatomical relation between sciatic nerve and piriformis muscle and its bifurcation level during fetal period in human. Surg Radio Anatomy (English ed.). (2014) 36:265–72. doi: 10.1007/s00276-013-1179-0
19.
Ugrenovic S Jovanovic I Krstic V Stojanovic V Vasovic L Antic S et al . The level of the sciatic nerve division and its relations to the piriform muscle. Vojnosanit Pregl. (2005) 62:45–9. doi: 10.2298/vsp0501045u
20.
Dogood A. Oyinbo C. Francis D. Tabowei U. (2022) Anatomic variation of the sciatic nerve: a study on the prevalence, and bifurcation loci in relation to the piriformis and popliteal Fossa. Acta Medica Academica. doi: 10.5644/ama2006-124.370
21.
Vicente EJD Viotto MJS Barbosa CAA Vicente PC . Estudo das relações anatômicas e suas variações entre o nervo ciático e o músculo piriforme. Rev Bras Fisioter. (2007) 11:227–32. doi: 10.1590/S1413-35552007000300009
22.
Gänsslen A Grechenig S Nerlich M Müller M . Standard approaches to the acetabulum part 1: Kocher-Langenbeck approach. Acta Chir Orthop Traumatol Cechoslov. (2016) 83:141–6. doi: 10.55095/achot2016/021
23.
Jung Kim H Hyun Park S . Sciatic nerve injection injury. J Int Med Res. (2014) 42:887–97. doi: 10.1177/0300060514531924
24.
Kline DG Kim D Midha R Harsh C Tiel R . Management and results of sciatic nerve injuries: a 24-year experience. J Neurosurg. (1998) 89:13–23. doi: 10.3171/jns.1998.89.1.0013
25.
Moretti VM Post ZD . Surgical approaches for Total hip arthroplasty. Indian J Orthop. (2017) 51:368–76. doi: 10.4103/ortho.IJOrtho_317_16
26.
Farina D Lombardi D Bertuletti M Palumbo G Zorza I Ravanelli M . An additional challenge for head and neck radiologists: anatomic variants posing a surgical risk – a pictorial review. Insights Imaging. (2019) 10:112–06. doi: 10.1186/s13244-019-0794-7
27.
Prakash BAK Devi MN Sridevi NS Rao PK Singh G . Sciatic nerve division: a cadaver study in the Indian population and review of the literature. Singapore Med J. (2010) 51:721–3. PMID:
28.
Alraddadi A . Literature review of anatomical variations: clinical significance, identification approach, and teaching strategies. Curēus. (2021) 13:e14451–1. doi: 10.7759/cureus.14451
29.
Gomes BA Ramos MRF Fiorelli RKA Almeida CRD Fiorelli SKA . Topographic anatomical study of the sciatic nerve relationship to the posterior portal in hip arthroscopy. Rev Col Bras Cir. (2014) 41:440–4. doi: 10.1590/0100-69912014006010
30.
Kirschner JS Foye PM Cole JL . Piriformis syndrome, diagnosis and treatment. Muscle Nerve. (2009) 40:10–8. doi: 10.1002/mus.21318
31.
Nizankowski C Slociak J Szybejko J . Variations in the anatomy of the sciatic nerve in man. Folia Morphol (Warsz). (1972) 31:507–13.
32.
Uluutku MH Kurtoğlu Z . Variations of nerves located in deep gluteal region. Okajimas Folia Anat Jpn. (1999) 76:273–6. doi: 10.2535/ofaj1936.76.5_273
33.
Reynoso JP De Jesus Encarnacion M Nurmukhametov R Melchenko D Efe IE Goncharov E et al . Anatomical variations of the sciatic nerve exit from the pelvis and its relationship with the piriformis muscle: a cadaveric study. Neurol Int. (2022) 14:894–902. doi: 10.3390/neurolint14040072
34.
Kasapuram D Ganapathy A Harisha K Bhukya S Rani N Singh S . Neuromuscular variations in the gluteal region - embryological basis and clinical significance. Clin Ter. (2021) 172:91–3. doi: 10.7417/CT.2021.2290
35.
Yadav Y . Superior gluteal nerve entrapment between two bellies of piriformis muscle. Ijav. (2010) 3:203–4.
36.
Bashaw GJ Klein R . Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. (2010) 2:a001941. doi: 10.1101/cshperspect.a001941
37.
Papadopoulos EC Khan SN . Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am. (2004) 35:65–71. doi: 10.1016/S0030-5898(03)00105-6
38.
Jankovic D Peng P Van Zundert A . Brief review: piriformis syndrome: etiology, diagnosis, and management. Can J Anesth. (2013) 60:1003–12. doi: 10.1007/s12630-013-0009-5
39.
Yeoman W . The relation of arthritis of the Sacro-iliac joint to sciatica, with an analysis of 100 cases. Lancet (British edition). (1928) 212:1119–23.
40.
Benzon HT Katz JA Benzon HA Iqbal MS . Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. (2003) 98:1442–8. doi: 10.1097/00000542-200306000-00022
41.
Fishman LM Dombi GW Michaelsen C Ringel S Rozbruch J Rosner B et al . Piriformis syndrome: diagnosis, treatment, and outcome—a 10-year study. Arch Phys Med Rehabil. (2002) 83:295–301. doi: 10.1053/apmr.2002.30622
Summary
Keywords
anatomy, early bifurcation, piriformis, sciatic nerve, variant
Citation
Rehir R, Teoh JM, Chan S, Almansour RA, Alshaya AS and Alashkham A (2025) An exploratory study of Type B variation of the sciatic nerve. Front. Neurol. 16:1592879. doi: 10.3389/fneur.2025.1592879
Received
14 March 2025
Accepted
10 July 2025
Published
31 July 2025
Volume
16 - 2025
Edited by
Ngan Pan Bennett Au, University of Surrey, United Kingdom
Reviewed by
Vanina Usach, Universidad de Buenos Aires, Argentina
Natalie Winter, Hertie Institute for Clinical Brain Research (HIH), Germany
Updates
Copyright
© 2025 Rehir, Teoh, Chan, Almansour, Alshaya and Alashkham.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rasyidah Rehir, rasyidahrehir@ukm.edu.my
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.