- 1Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, United States
- 2Department of Rehabilitation Medicine, University of Washington, Seattle, WA, United States
- 3Moss Rehabilitation Research Institute, Elkins Park, PA, United States
- 4Department of Physical Medicine and Rehabilitation, University of Texas Southwestern, Dallas, TX, United States
- 5Department of Brain Injury Medicine, Womack Army Medical Center, Fort Bragg, NC, United States
- 6Herbert Wertheim School of Public Health and Human Longevity Science, University of California, San Diego, La Jolla, CA, United States
- 7Alzheimer’s Therapeutic Research Institute, University of Southern California, Los Angeles, CA, United States
- 8Department of Neurological Surgery, University of Washington, Seattle, WA, United States
- 9Department of Health Management and Policy, University of Michigan, Ann Arbor, MI, United States
- 10Department of Psychiatry, University of California, San Diego, La Jolla, CA, United States
- 11VA San Diego Healthcare System, San Diego, CA, United States
- 12Department of Biostatistics, University of Washington, Seattle, WA, United States
Objective: Many active duty service members with mild traumatic brain injury (mTBI) report comorbidities such as depression, anxiety, PTSD, insomnia, and pain. We analyzed data from a prior randomized controlled trial (RCT) to examine the effects of evidence-based treatment modules, delivered by telephone, on the number and symptom burden of five common comorbidities.
Setting and participants: 356 service members from two military medical centers who had sustained deployment-related mTBI in the preceding 2 years.
Design: Secondary analysis of RCT comparing 6 months of telephone-delivered problem-solving treatment (PST) with comorbidity-specific modules to education only (EO).
Main measures: Comorbidity burden measured by Patient Health Questionnaire-9, Brief Symptom Inventory-Anxiety, PTSD Checklist, Pittsburgh Sleep Quality Inventory, Rivermead Postconcussion Symptoms Questionnaire (headache item) assessed at baseline and 6 and 12 months.
Results: 47% of service members endorsed ≥ 3 comorbidities at baseline. At 6 months, the PST group had significantly fewer comorbidities, greater improvement in depression, anxiety, PTSD, and sleep, but not headache, and higher response/remission rates for depression and sleep, compared to EO. There were no significant group differences at 12 months.
Conclusion: Telephone-delivered PST with comorbidity-specific modules reduces burden of comorbidities after deployment-related mTBI. Research is needed on how to maintain improvements over time.
Introduction
Over more than 20 years of U.S. military engagement in Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND), many service members sustained at least one mild traumatic brain injury (mTBI), often leading to persisting neuropsychiatric difficulties. However, many questions remain about the best approaches to treating these challenges in Veterans, particularly those with deployment-related mTBI. Veterans with mTBI have a higher prevalence of comorbid conditions such as post-traumatic stress disorder (PTSD), depression, anxiety, and substance use disorders compared to those without TBI (1). Other common comorbidities include sleep disturbance (2) and pain (3), especially headache (4, 5). These problems often co-occur and may persist for months or years. In a population-based study of OEF/OIF/OND Veterans with any TBI, almost one-third had one psychiatric diagnosis and half had two or more diagnoses (6). In both military and civilian samples, TBI accompanied by these comorbid mental health problems is associated with impaired coping, functional impairment, and decreased quality of life (5, 7–9).
The treatment of mTBI and its mental and physical comorbidities was noted by the Institute of Medicine as a critical area of focus among service members and Veterans (10). However, a systematic review of treatment approaches for deployment-related psychiatric conditions that are comorbid with mTBI revealed insufficient evidence to support treatment guidelines, despite the fact that such guidelines exist for PTSD, depression, and other conditions in the absence of TBI (11). It was noted that some psychotherapies designed for the general population do result in symptom reduction in those with mTBI; however, many military personnel fail to follow through with treatment recommendations due to perceived stigma, lack of access to care, and other barriers (12–14). Thus, there remains a need for flexible, accessible treatments to address the comorbidities that accompany mTBI in this population (15).
In the randomized controlled CONTACT (Concussion Treatment after Combat Trauma) study, we compared the effects of a multifaceted problem-solving treatment (PST) delivered by telephone versus education only (EO) in a group of service members with deployment-related mTBI (16). The PST group showed significantly more improvement on a measure of psychological distress after 6 months of treatment, but reduction in postconcussive symptoms did not differ by group (17). Although the PST treatment arm included evidence-based treatment modules targeting several commonly occurring comorbidities, the effects on comorbidities was not directly examined.
This secondary analysis seeks to investigate whether the person-centered, modular components of the treatment intervention reduce comorbidity burden in this population. While the primary study focused on group differences in overall psychological distress and postconcussive symptoms, the current analysis focuses on the presence, severity, and response rates of five common individual comorbidities (depression, anxiety, PTSD, insomnia, and headache), thus providing a finer-grained view of the efficacy of a novel, phone-based intervention at the individual level. Specifically, we examined the effects of the person-centered intervention package that included a system of detection, education, and therapeutic support tailored to address identified symptoms among the five comorbid conditions. We hypothesized that, although participation in the CONTACT study did not require the presence of particular symptoms, (1) participants in both treatment arms would endorse a high burden of comorbid conditions at baseline; and (2) those enrolled in the PST intervention, with modules addressing specific comorbidities, would report significantly lower comorbid symptom burden and decreased symptom severity compared to those in the EO condition, both immediately after 6 months of treatment and at 12-month follow-up.
Materials and methods
The study was approved by the participating academic and military institutional review boards and all participants gave informed consent. Comprehensive study procedures, including inclusion and exclusion criteria, descriptions of measures, and specifics regarding the intervention are detailed elsewhere (16). The clinical trial was registered at: clinicaltrials.gov, identifier NCT01387490.
Participants
In brief, participants were 356 service members from the TBI Clinics of two military medical centers (Madigan Army Medical Center and Womack Army Medical Center) who had sustained deployment-related mTBI during OEF/OIF/OND within the previous 2 years. Participants were excluded for moderate to severe TBI requiring hospitalization, psychosis, active suicidal ideation, or participation in a formal TBI treatment program on base.
Measures
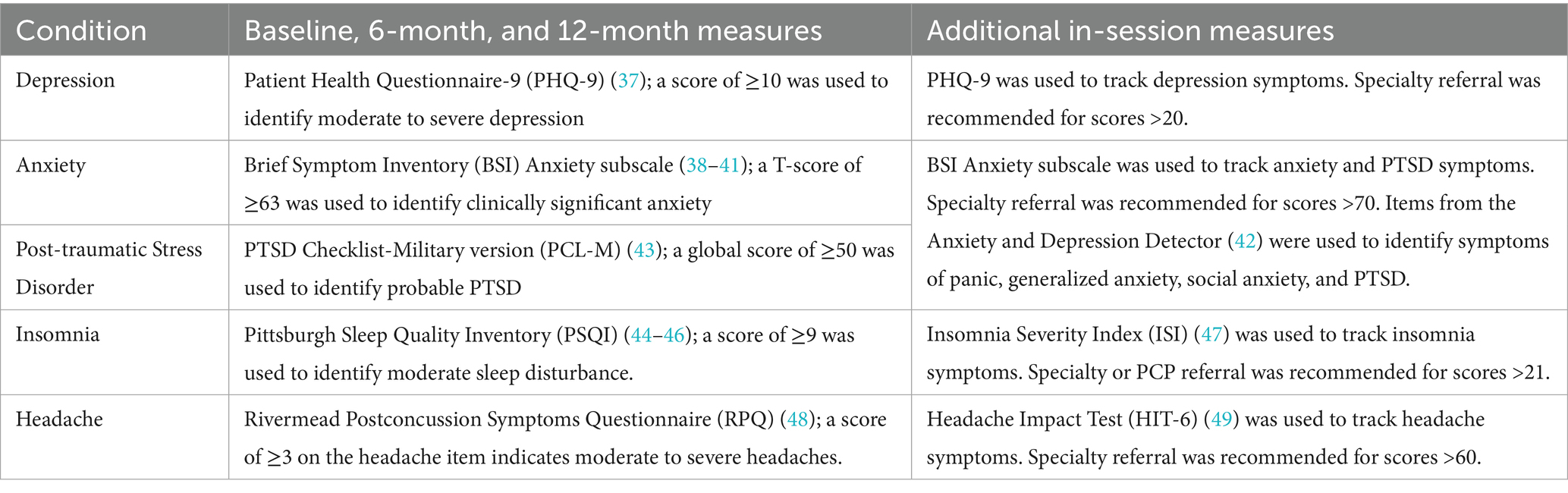
The five comorbid conditions (depression, anxiety, PTSD, insomnia, and headache) were assessed at baseline, after 6 months of treatment, and at 12-month follow-up. Depression was assessed using the Patient Health Questionnaire-9 (PHQ-9); anxiety with the Brief Symptom Inventory (BSI) Anxiety subscale; PTSD with the PTSD Checklist–Military version (PCL-M); insomnia with the Pittsburgh Sleep Quality Index (PSQI); and headache with the headache item from the Rivermead Postconcussion Symptoms Questionnaire (RPQ). Table 1 also shows the measures (if different) that were used to track symptoms during intervention, the cutoff scores used to determine the presence of each comorbidity, and the cutoff scores when a specialty referral was recommended. Basic demographic information, TBI-related information, and additional clinical measures (for use as secondary outcome measures not pertinent to the current analysis) were also collected at baseline (16).
Interventions
Both treatment arms included 178 randomly assigned participants. All participants received a study binder that included educational brochures on problems commonly experienced by Service Members returning from deployment (e.g., cognitive deficits, finances, sleep disturbance). Participants in the EO condition received a second copy of the brochures by mail, one every two weeks.
The PST intervention consisted of 12 scheduled biweekly calls placed by Masters-trained counselors, called Concussion Support Specialists (CSS), who were trained and supervised by a psychiatrist, licensed psychologist and physicians throughout the study. During these calls, service members learned and practiced a manualized 6-step strategy for selecting, characterizing, and solving problems affecting their daily lives (16). In addition, clinical status was monitored during each call for elevated symptoms of each of the five comorbidities. If participants reported clinically significant depression, anxiety, PTSD, sleep disturbance, or headache, the CSS suggested augmenting the PST intervention with brief, comorbidity-specific interventions, or modules, each designed to span 2–4 sessions. These modules, which were also manualized, included additional assessment, education, and evidence-based therapeutic strategies for each comorbidity. We used principles of behavioral activation (BA) (18–22) for the depression and anxiety/PTSD modules, with content adapted for the specific disorder; psychoeducation (including stimulus control and sleep hygiene) and components of cognitive behavioral therapy for insomnia (CBT-I) (23–25); and psychoeducation and mindfulness-based strategies (26–29) for the headache module. Problem-specific educational packets and worksheets were provided and symptoms were monitored using the validated instruments shown in Table 1. After completing a module, the CSS continued to monitor and support the problem during the remainder of the intervention and, when indicated, recommended referral for further treatment.
Data analysis
We examined the effect of the interventions on the severity of comorbidities at 6 and 12 months using generalized estimating equations to estimate the parameters of a generalized linear model (30). We analyzed the effect of the intervention on the prevalence of dichotomous comorbidity outcomes by examining the number and percent of those in the PST and EO groups with each of the comorbidities at baseline, 6 months, and 12 months. Values of missing items on individual instruments were imputed by prorating if at least half of the items were completed for that instrument. We also identified the proportion of those who were responders, defined as those who screened positive for a comorbidity at baseline but did not meet case criteria for that comorbidity at the 6- or 12-month outcome assessment. Differences in prevalence between the PST and EO groups were assessed using mixed-effects logistic regression, which safeguards against any potential bias due to unobserved outcomes under the assumption that they are missing at random (MAR). Differences in response were assessed using exact logistic regression. Site, military status (active duty vs. National Guard/ reserve) and the baseline Brief Symptom Inventory (31). Global Severity Index T-score were used as covariates in the analyses. We considered the analysis of each comorbidity to provide crucial information independent of the other analyses. Because the risk of Type II error was as important as the risk of Type I error in this instance, we set alpha = 0.05 for each statistical comparison.
Using the dichotomous comorbidity outcomes, we created a variable indicating the number of comorbidities that each participant had at baseline, 6 months, and 12 months. This variable was classified as missing for a given time point if the participant did not complete all 5 measures. We used SPSS (version 19) and SAS (version 9.3) for all analyses.
Results
Participant characteristics
Characteristics of the sample recruited into the parent trial and the flow of participants from screening to follow-up are reported elsewhere (17). In brief, trial participants were 29 years old on average, 93% male, 77% white, and had a mean education level of 13.4 years. The average participant had undergone 2–3 deployments, and the majority (73%) had sustained 3 or more mTBIs, mostly involving blast injury. As previously reported, there were no significant differences between the groups in their baseline demographic, injury, or clinical characteristics (17). At the 6 month assessment, 78% of the PST participants and 93% of the EO participants provided outcomes data; at 12 months, the follow-up rates were 80 and 88%, respectively. The mean number of treatment sessions completed by the PST participants was 6.6 (SD 4.6, range 0–12, median 7); 119 (67%) completed 4 or more sessions.
Comorbid conditions
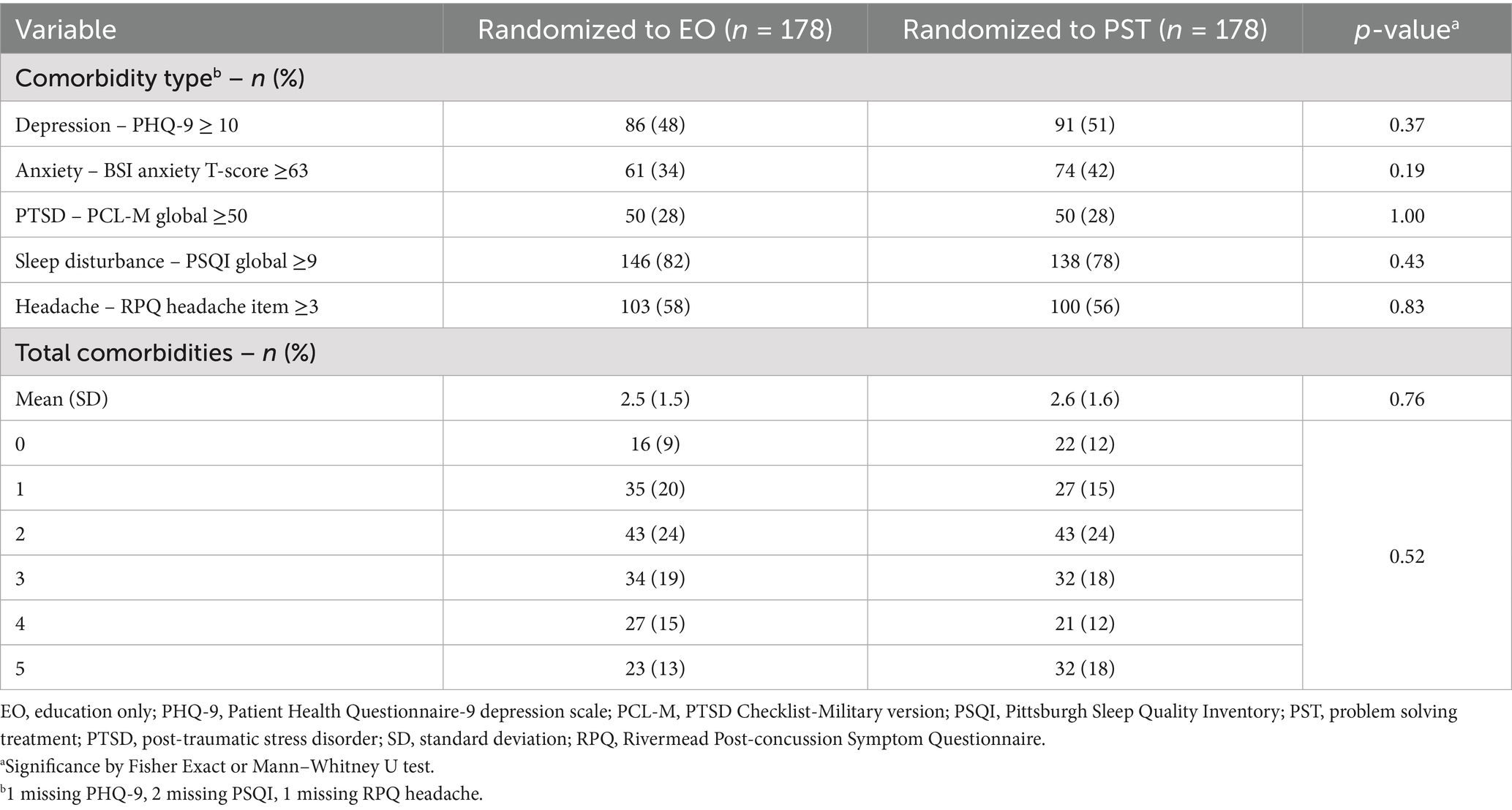
Table 2 shows the numbers and proportions of participants who screened positive for each comorbid condition at baseline; the groups were equivalent on these measures. Nearly half of participants endorsed three or more comorbidities. Sleep disturbance was the most common (80%), followed by headache (57%) and depression (50%). Among the 62 participants with one comorbidity, sleep disturbance was the most common (n = 42); among the 86 participants with two comorbidities, sleep disturbance and headache was the most common combination (n = 55); among the 66 participants with three comorbidities, the combination of sleep disturbance, headache, and depression was the most common (n = 33); among the 48 participants with four comorbidities, the combination of sleep disturbance, depression, anxiety, and PTSD was the most common (n = 24).
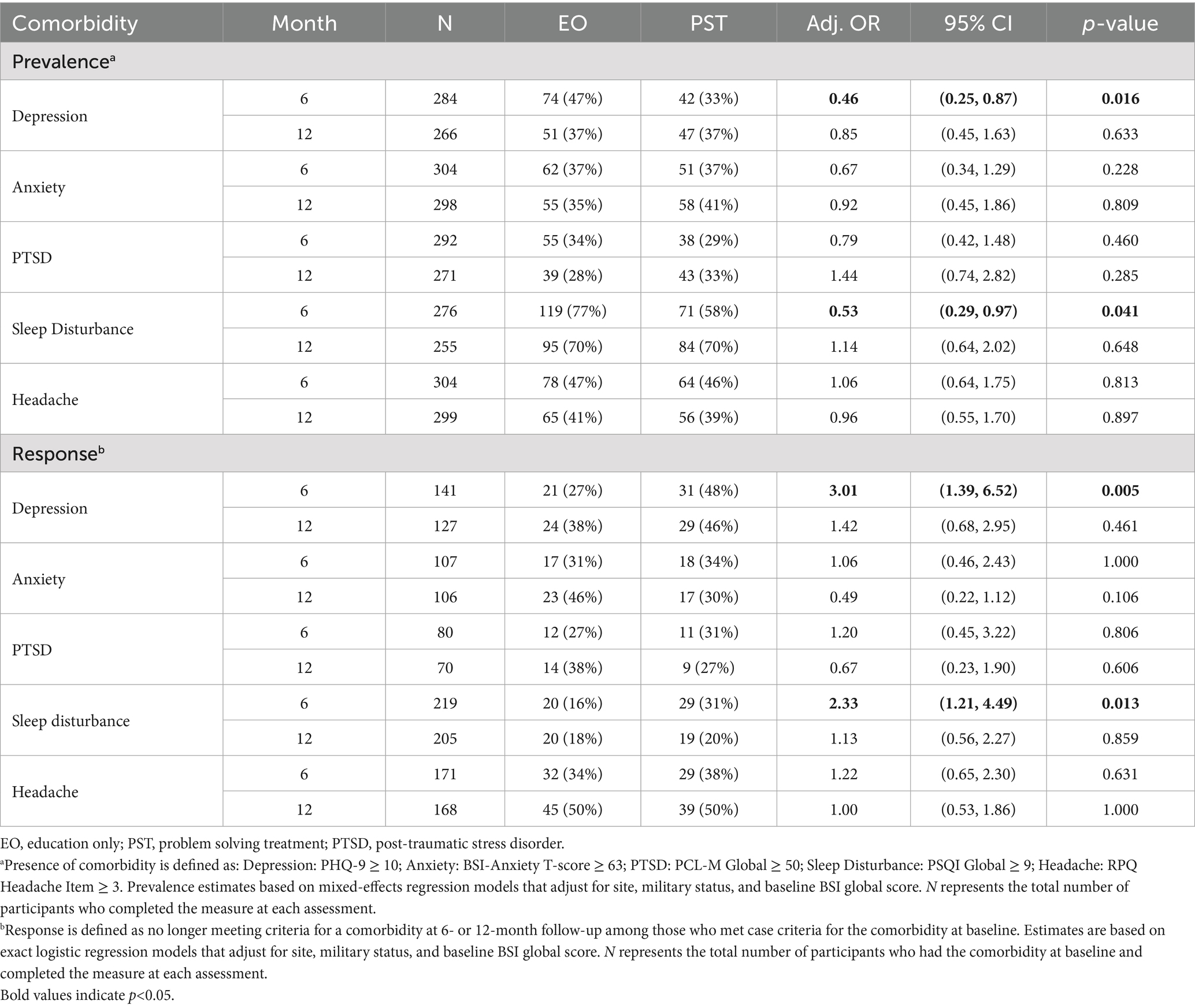
Results of the analysis of comorbidity outcome measures at 6 and 12 months are summarized in Table 3. Depression, anxiety, PTSD and sleep were significantly improved in the PST group at 6 months, compared to the EO group, while headache was not. The mean number of comorbidities was also significantly lower in the PST group compared to the EO group at 6 months. Among the PCL-M subscales, re-experiencing symptoms were significantly improved in the PST group compared to the EO group (p < 0.05). There were no statistically significant differences between the PST and EO groups for any of the comorbidity outcomes or the total number of comorbidities at 12 months.
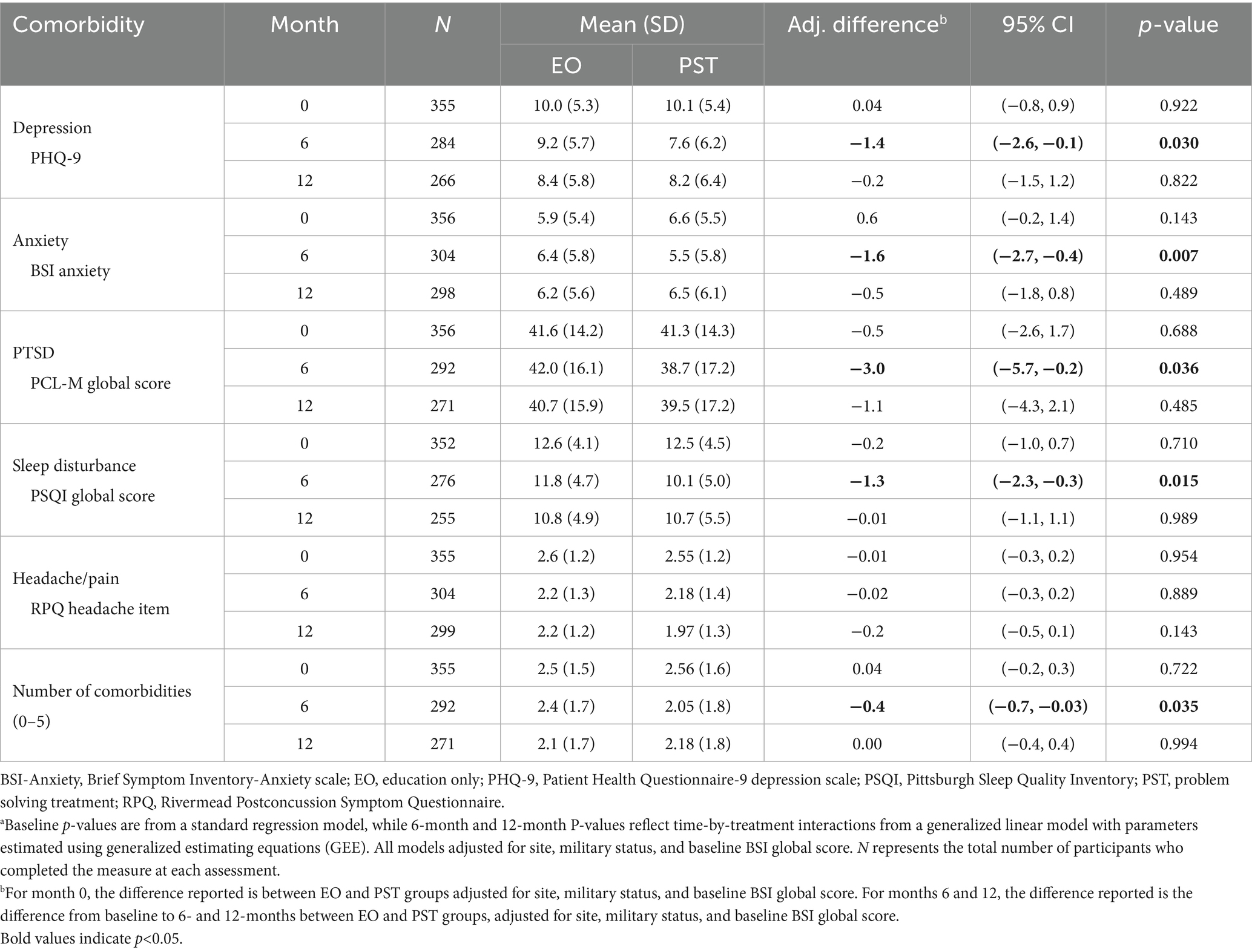
Table 3. Comorbidity outcomes by treatment groups at 6 and 12 monthsa.
Table 4 shows the prevalence and response rates for each comorbidity at 6 and 12 months. Depression and sleep disturbance were both significantly less prevalent at 6 months in the PST group than the EO group. Similarly, among those with the respective comorbidity at baseline, more PST participants than EO participants were responders (i.e., no longer met case criteria) for depression (48% vs. 27%, p = 0.005) and sleep disturbance (31% vs. 16%, p = 0.013) at 6 months. At 6 months, the PST group also demonstrated significantly higher remission rates than the EO group for depression (PHQ-9 < 5; 22% vs. 8%, p = 0.026) and sleep disturbance (PSQI < 6; 15% vs. 5%, p = 0.017). There were no significant differences in prevalence, response, or remission rates at 12 months.
Examining the sleep disturbance and headache findings in greater detail, 43 participants chose to participate in the “insomnia module” for sleep disturbance (mean 3.6 ± 1.6 sessions) and only 3 participated in the “headache module” (mean 4.3 ± 0.6 sessions). Among participants who had significant sleep disturbance on the PSQI at baseline, those who received the insomnia module had higher mean baseline scores than those who did not (13.9 ± 3.9 vs. 12.0 ± 4.6, p < 0.001), with scores of 11.0 ± 5.2 at 6 months and 12.1 ± 6.4 at 12 months. The in-session ISI score significantly improved among participants in the insomnia module, with the mean dropping from 17.0 ± 5.0 to 11.3 ± 6.5, (p < 0.001). There was no significant improvement in mean HIT-6 scores among the 3 headache module participants (64 ± 10 to 63 ± 17, p = 0.84). Similar data are not available for the depression and anxiety/PTSD modules because components of these modules were used by the counselors and integrated throughout the intervention in response to evidence of depression or anxiety/PTSD on routine in-session screening for overall distress, depression and anxiety.
Discussion
In a previous report on the efficacy of a telehealth intervention for service members with mTBI, we focused on group means to show that a problem-solving treatment (PST) was superior to education alone (EO) for reducing psychological distress, but not post-concussive symptoms (17). This secondary analysis, focused on the comorbidities that often accompany mTBI, provides further information on the impact of using a person-centered modular approach to supplement the general problem-solving approach in the active treatment arm. We found that this approach significantly improved both the number and the symptom burden of multiple common comorbidities and overall symptom burden. Specifically, symptoms of depression, anxiety, PTSD, and sleep disturbance all improved after the end of the 6-month treatment period, and the proportion of service members with clinically significant symptoms of depression and sleep disturbance also significantly decreased from baseline to 6 months in the group receiving this modular treatment. Unfortunately, superiority of the PST group was not maintained to the 12-month assessment as comorbidities and symptoms were equivalent to the EO group at this timepoint. These results are similar to the follow-up findings in the parent trial (17). Additionally, headache pain improved equally in both groups.
Our study sample exhibited a high baseline level of symptomatology in the five comorbidities of interest, despite not requiring any symptoms for enrollment. The improvement in both the number of comorbidities and the proportion of service members with clinically significant symptoms may translate into improved readiness to return to duty and reduced burden on healthcare systems. The fact that multiple, potentially debilitating, inter-related symptoms showed improvement suggests that this brief, flexible treatment model could be adapted to the complex array of problems experienced by Veterans of overseas conflicts, with the possible exception of headache. The reasons why headache did not show a greater treatment response in the PST group need to be further explored; meanwhile, inclusion of a more traditional medical management approach for headache may be appropriate.
Similar to prior findings (17), these results support the use of the telephone to extend the clinical reach of behavioral interventions to service members; two-thirds of our participants completed at least four sessions, which has been cited as a “minimally effective dose” of psychotherapy in some studies (32, 33). Even for comorbidities such as these, which are quite disruptive to daily functioning and characterized as difficult to treat (34), telehealth interventions hold promise for reducing barriers to evidence-based care by overcoming stigma, lack of access, transportation difficulties, and avoidance behaviors. A qualitative study conducted at the close of the parent trial confirmed that very few participants would have preferred face-to-face treatment (35).
For the comorbidities that showed improvement with modular treatment at 6 months (depression, anxiety, PTSD, and sleep disturbance), the reason for the lack of group differences at 12 months appears to be two-fold. The PST group showed some relapse in symptom severity between 6 and 12 months, while the EO group showed symptom improvement during the same interval. More research is needed to elucidate the reasons for the symptom relapse after completion of the intervention and to explore strategies for maintaining the initial gains, such as booster sessions, or “apps” for reminding participants of personally effective strategies.
Several limitations of this study should be noted when interpreting results. First, most participants were male and served exclusively in the Army, with few National Guard and reserve members represented. Gender differences in treatment response would be valuable to explore in future research. Second, our loss to follow-up rate was higher in the PST group. Several reasons for this may be possible, including dropping out due to fatigue from the number of calls or feeling that their outcomes were already being assessed as part of the PST intervention. Third, we lack detailed data on referrals made as a result of participation in the modules, which could have affected treatment response; however, an analysis examining the impact of the PST intervention on healthcare utilization did show increased use of hospital services (36). Finally, we enrolled participants on the basis of their having sustained mTBI during deployment, rather than on the basis of symptom severity. Results may have differed had we attempted to recruit participants with a minimum level of symptomatology at baseline.
In conclusion, telephone-based PST, supplemented with brief, condition-specific modules to reduce the cumulative burden of comorbidities such as depression, anxiety, PTSD and sleep disturbance is a promising approach for mTBI in military populations. Future studies should extend this approach to more diverse Veteran and civilian populations with mTBI. It would also be helpful to develop and test brief modules for other common comorbidities, such as cognitive problems and anger/ irritability.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving humans was approved by the institutional review boards at University of Washington, Madigan Army Medical Center, T2 Headquarters, Womack Army Medical Center, University of California San Diego, and the Army Human Rights Protection Office. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JF: Supervision, Methodology, Writing – review & editing, Investigation, Conceptualization, Writing – original draft, Validation, Funding acquisition. TH: Writing – original draft, Writing – review & editing. KB: Writing – review & editing, Supervision, Investigation, Conceptualization, Validation, Methodology, Funding acquisition, Resources, Project administration, Formal analysis, Data curation, Writing – original draft. WC: Investigation, Writing – review & editing, Resources, Data curation, Writing – original draft, Project administration. SJ: Writing – review & editing, Writing – original draft. RR: Writing – review & editing, Writing – original draft. JB: Formal analysis, Validation, Writing – review & editing, Writing – original draft, Data curation. SD: Writing – review & editing, Funding acquisition, Writing – original draft. JR: Validation, Writing – review & editing, Writing – original draft, Formal analysis. MS: Funding acquisition, Writing – review & editing, Writing – original draft. NT: Conceptualization, Writing – review & editing, Funding acquisition, Formal analysis, Validation, Writing – original draft, Data curation, Methodology.
Group member of the CONTACT investigators
The CONTACT study team members: Jef St. De Lore, Sara E. Fey-Hinckley, Jocelyn L. Savage, and Michael Warren, University of Washington, Seattle, WA; Gregory Gahm, Elissa Thomas, Karina Boykin, and Derek Smolensky, National Center for Telehealth and Technology, T2, Tacoma, WA; Leila Forbes and Danielle Benton, Defense and Veterans Brain Injury Center, now the TBI Center of Excellence; and the Geneva Foundation, Tacoma, WA, in particular Kasey Zink.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grant number W81XWH-08-2-0159 from the U.S. Army Medical Research and Materiel Command (USAMRMC).
Acknowledgments
The authors are thankful to all study participants and their families; the CONTACT study team members: Jef St. De Lore MPH, Sara E. Fey-Hinckley, MA, LMFT, CBIS, Jocelyn L. Savage, LICSW, Michael Warren, MA (University of Washington, Seattle, WA), Gregory Gahm, PhD, Elissa Thomas, Karina Boykin, Derek Smolensky, PhD, MPH (National Center for Telehealth and Technology, T2, Tacoma, WA); Leila Forbes and Danielle Benton (Defense and Veterans Brain Injury Center, now the TBI Center of Excellence); and the Geneva Foundation (Tacoma, WA), in particular Kasey Zink.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed herein are those of the author(s) and do not reflect the official policy of the Department of the Army, Department of Defense, or the U.S. Government.
References
1. Greer, N, Ackland, P, Sayer, N, Spoont, M, Taylor, B, MacDonald, R, et al. VA evidence-based synthesis program reports. Relationship of deployment-related mild traumatic brain injury to posttraumatic stress disorder, depressive disorders, substance use disorders, suicidal ideation, and anxiety disorders: A systematic review. Washington (DC): Department of Veterans Affairs (US) (2019).
2. Vuletic, S, Bell, KR, Jain, S, Bush, N, Temkin, N, Fann, JR, et al. Telephone problem-solving treatment improves sleep quality in service members with combat-related mild traumatic brain injury: results from a randomized clinical trial. J Head Trauma Rehabil. (2016) 31:147–57. doi: 10.1097/HTR.0000000000000221
3. Mac Donald, CL, Johnson, AM, Wierzechowski, L, Kassner, E, Stewart, T, Nelson, EC, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol. (2014) 71:994–1002. doi: 10.1001/jamaneurol.2014.1114
4. Patil, VK, St Andre, JR, Crisan, E, Smith, BM, Evans, CT, Steiner, ML, et al. Prevalence and treatment of headaches in veterans with mild traumatic brain injury. Headache. (2011) 51:1112–21. doi: 10.1111/j.1526-4610.2011.01946.x
5. Theeler, BJ, Flynn, FG, and Erickson, JC. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache. (2010) 50:1262–72. doi: 10.1111/j.1526-4610.2010.01700.x
6. Iverson, KM, Hendricks, AM, Kimerling, R, Krengel, M, Meterko, M, Stolzmann, KL, et al. Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: a gender comparison. Womens Health Issues. (2011) 21:S210–7. doi: 10.1016/j.whi.2011.04.019
7. Lippa, SM, Fonda, JR, Fortier, CB, Amick, MA, Kenna, A, Milberg, WP, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J Trauma Stress. (2015) 28:25–33. doi: 10.1002/jts.21979
8. Bombardier, CH, Fann, JR, Temkin, NR, Esselman, PC, Barber, J, and Dikmen, SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA J Am Med Assoc. (2010) 303:1938–45. doi: 10.1001/jama.2010.599
9. King, NS. PTSD and traumatic brain injury: folklore and fact? Brain Inj. (2008) 22:1–5. doi: 10.1080/02699050701829696
10. Institute of Medicine. Returning home from Iraq and Afghanistan: Preliminaary assessment of readjustment needs of veterans, service members, and their families. Washington, D.C.: National Academies Press (2010).
11. Ackland, PE, Greer, N, Sayer, NA, Spoont, MR, Taylor, BC, MacDonald, R, et al. Effectiveness and harms of mental health treatments in service members and veterans with deployment-related mild traumatic brain injury. J Affect Disord. (2019) 252:493–501. doi: 10.1016/j.jad.2019.04.066
12. Hoge, CW, Auchterlonie, JL, and Milliken, CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. (2006) 295:1023–32. doi: 10.1001/jama.295.9.1023
13. Kim, PY, Thomas, JL, Wilk, JE, Castro, CA, and Hoge, CW. Stigma, barriers to care, and use of mental health services among active duty and national guard soldiers after combat. Psychiatr Serv. (2010) 61:572–88. doi: 10.1176/ps.2010.61.6.582
14. Chase, RP, and Nevin, RL. Population estimates of undocumented incident traumatic brain injuries among combat-deployed US military personnel. J Head Trauma Rehabil. (2015) 30:E57–64. doi: 10.1097/HTR.0000000000000061
15. French, LM. Military traumatic brain injury: an examination of important differences. Ann N Y Acad Sci. (2010) 1208:38–45. doi: 10.1111/j.1749-6632.2010.05696.x
16. Bell, KR, Brockway, JA, Fann, JR, Cole, WR, St De Lore, J, Bush, N, et al. Concussion treatment after combat trauma: development of a telephone based, problem solving intervention for service members. Contemp Clin Trials. (2015) 40:54–62. doi: 10.1016/j.cct.2014.11.001
17. Bell, KR, Fann, JR, Brockway, JA, Cole, WR, Bush, NE, Dikmen, S, et al. Telephone problem solving for service members with mild traumatic brain injury: a randomized, clinical trial. J Neurotrauma. (2017) 34:313–21. doi: 10.1089/neu.2016.4444
18. Dimidjian, S, Barrera, M Jr, Martell, C, Munoz, RF, and Lewinsohn, PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. (2011) 7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535
19. Dimidjian, S, Hollon, SD, Dobson, KS, Schmaling, KB, Kohlenberg, RJ, Addis, ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. (2006) 74:658–70. doi: 10.1037/0022-006X.74.4.658
20. Hopko, D, Robertson, S, and Lejuez, C. Behavioral activation for anxiety disorders. Behav Anal Today. (2006) 7:212–24.
21. Wagner, A, Zatzick, D, Ghesquiere, A, and Jurkovich, J. Behavioral activation as an early intervention for PTSD and depression among physically injured trauma survivors. Cogn Behav Pract. (2007) 14:341–9. doi: 10.1016/j.cbpra.2006.05.002
22. Jakupcak, M, Wagner, A, Paulson, A, Varra, A, and McFall, M. Behavioral activation as a primary care-based treatment for PTSD and depression among returning veterans. J Trauma Stress. (2010) 23:491–5. doi: 10.1002/jts.20543
23. Ouellet, MC, and Morin, CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil. (2007) 88:1581–92. doi: 10.1016/j.apmr.2007.09.006
24. Ruff, RL, Riechers, RG 2nd, Wang, XF, Piero, T, and Ruff, SS. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. (2012) 49:1305–20. doi: 10.1682/jrrd.2011.12.0251
25. Buysse, DJ, Germain, A, Moul, DE, Franzen, PL, Brar, LK, Fletcher, ME, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. (2011) 171:887–95. doi: 10.1001/archinternmed.2010.535
27. Loder, E, and Biondi, D. Disease modification in migraine: a concept that has come of age? Headache. (2003) 43:135–43. doi: 10.1046/j.1526-4610.2003.03033.x
28. Day, MA, Halpin, J, and Thorn, BE. An empirical examination of the role of common factors of therapy during a mindfulness-based cognitive therapy intervention for headache pain. Clin J Pain. (2016) 32:420–7. doi: 10.1097/AJP.0000000000000277
29. Lorig, K, Sobel, D, Ritter, P, Laurent, D, and Hobbs, M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. (2001) 4:256–62.
30. Liang, KY, and Zeger, SL. Longitudinal data analysis using generalized linear models. Biometrics. (1986) 73:13–22. doi: 10.1093/biomet/73.1.13
31. Derogatis, L. Brief symptom inventory (BSI): Administration, scoring and procedures manual. 4th ed. Minneapolis, MN: NCS Pearson, Inc (1993).
32. Baldwin, SA, Berkeljon, A, Atkins, DC, Olsen, JA, and Nielsen, SL. Rates of change in naturalistic psychotherapy: contrasting dose-effect and good-enough level models of change. J Consult Clin Psychol. (2009) 77:203–11. doi: 10.1037/a0015235
33. Horvitz-Lennon, M, Normand, SL, Frank, RG, and Goldman, HH. Usual care for major depression in the 1990s: characteristics and expert-estimated outcomes. Am J Psychiatry. (2003) 160:720–6. doi: 10.1176/appi.ajp.160.4.720
34. Mohr, DC, Carmody, T, Erickson, L, Jin, L, and Leader, J. Telephone-administered cognitive behavioral therapy for veterans served by community-based outpatient clinics. J Consult Clin Psychol. (2011) 79:261–5. doi: 10.1037/a0022395
35. Brockway, JA, St De Lore, J, Fann, JR, Hart, T, Hurst, S, Fey-Hinckley, S, et al. Telephone-delivered problem-solving training after mild traumatic brain injury: qualitative analysis of service members' perceptions. Rehabil Psychol. (2016) 61:221–30. doi: 10.1037/rep0000077
36. Richardson, JS, Fann, JR, Bell, KR, and Temkin, N. Impact of telephone-based problem-solving treatment on the use of medical and psychological services in the military. J Head Trauma Rehabil. (2018) 33:E1–E6. doi: 10.1097/HTR.0000000000000299
37. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
38. Meachen, S, Hanks, R, Millis, S, and Rapport, L. The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch Phys Med Rehabil. (2008) 89:958–65. doi: 10.1016/j.apmr.2007.12.028
39. Granger, CV, Divan, N, and Fiedler, RC. Functional assessment scales: a study of persons after traumatic brain injury. Am J Phys Med Rehabil. (1995) 74:107–13.
40. Dikmen, S, Machamer, J, Powell, J, and Temkin, N. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. (2003) 84:1449–57. doi: 10.1016/s0003-9993(03)00287-9
41. Slaughter, J, Johnstone, G, Petroski, G, and Flax, J. The usefulness of the brief symptom inventory in the neuropsychological evaluation of traumatic brain injury. Brain Inj. (1999) 13:125–30. doi: 10.1080/026990599121782
42. Means-Christensen, AJ, Sherbourne, CD, Roy-Byrne, PP, Craske, MG, and Stein, MB. Using five questions to screen for five common mental disorders in primary care: diagnostic accuracy of the anxiety and depression detector. Gen Hosp Psychiatry. (2006) 28:108–18. doi: 10.1016/j.genhosppsych.2005.08.010
43. Weathers, F, Huska, J, and Keane, T. The PTSD checklist military version (PCL-M). Boston: National Center for PTSD (1991).
44. Buysse, D, Reynolds, C, Monk, T, Berman, S, and Kupfer, D. The Pittsburgh sleep quality index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. (1989) 28:193–213.
45. Mollayeva, T, Thurairajah, P, Burton, K, Mollayeva, S, Shapiro, CM, and Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
46. Fichtenberg, NL, Zafonte, RD, Putnam, S, Mann, NR, and Millard, AE. Insomnia in a post-acute brain injury sample. Brain Inj. (2002) 16:197–206. doi: 10.1080/02699050110103940
47. Morin, CM, Belleville, G, Belanger, L, and Ivers, H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. (2011) 34:601–8. doi: 10.1093/sleep/34.5.601
48. King, N, Crawford, S, Wenden, F, Moss, N, and Wade, D. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. (1995) 242:587–92. doi: 10.1007/BF00868811
Keywords: traumatic brain injury, concussion, comorbidity, telehealth, clinical trial
Citation: Fann JR, Hart T, Bell KR, Cole W, Jain S, Raman R, Barber J, Dikmen S, Richardson J, Stein MB and Temkin N (2025) Effects of a modular telehealth intervention on comorbid conditions in service members with mild traumatic brain injury. Front. Neurol. 16:1594748. doi: 10.3389/fneur.2025.1594748
Edited by:
Bradley Dengler, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Michael J. Roy, Uniformed Services University, United StatesSharon Mburu, University of Florida, United States
Copyright © 2025 Fann, Hart, Bell, Cole, Jain, Raman, Barber, Dikmen, Richardson, Stein and Temkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesse R. Fann, ZmFubkB1dy5lZHU=
†Present addresses: Wesley Cole, Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
John Richardson, Stop Soldier Suicide, Durham, NC, United States
 Jesse R. Fann
Jesse R. Fann Tessa Hart
Tessa Hart Kathleen R. Bell4
Kathleen R. Bell4 Nancy Temkin
Nancy Temkin