- Department of Neurosurgery, Haikou Hospital Affiliated with Xiangya Medical College, Central South University, Haikou, China
As an emerging therapeutic strategy, stem cell transplantation has demonstrated promising potential in the management of refractory epilepsy. Epilepsy, a prevalent neurological disorder characterized by recurrent seizures, affects approximately one-third of patients worldwide who exhibit resistance to existing antiepileptic drugs (AEDs). Consequently, exploring novel treatment modalities is imperative. Recent studies have indicated that stem cell transplantation improves neurological function in epilepsy through multiple mechanisms. Research has revealed that transplanted stem cells mitigate seizure-induced neuronal damage by replacing dead or dysfunctional neurons, secreting beneficial neurotrophic factors (e.g., brain-derived neurotrophic factor, BDNF), and releasing anti-inflammatory cytokines. Preclinical studies and early-phase clinical trials have shown that stem cell transplantation significantly reduces seizure frequency and enhances patients’ quality of life. However, current research is limited by small sample sizes and short-term follow-ups, necessitating further validation of long-term efficacy. Despite its therapeutic promise, stem cell transplantation faces critical challenges. First, technical details such as the cell source, processing, method of transplantation, and timing have yet to be standardized, leading to potential variability in efficacy and safety across different stem cell types. Second, complications like immune rejection and tumorigenesis pose significant safety risks. Future research should focus on optimizing stem cell selection and processing, designing robust clinical trials to evaluate long-term safety and efficacy, exploring combinatorial approaches with existing therapies, and developing advanced biomaterials to enhance transplantation success. Additionally, monitoring post-transplant cell survival and functionality, along with identifying epilepsy-specific biomarkers, will be crucial to refining the precision and safety of stem cell-based therapies.
1 Introduction
Epilepsy represents a prevalent neurological disorder characterized by recurrent seizures arising from abnormal hypersynchronous neuronal electrical activity in the brain (1). Affecting over 65 million individuals worldwide, epilepsy constitutes a significant global public health challenge (2). The clinical manifestations of epileptic seizures encompass transient paroxysmal signs and symptoms resulting from excessive neuronal discharges, with classification into focal onset seizures (originating within discrete neural networks) and generalized seizures (involving bilateral distributed networks), the latter frequently presenting with impaired consciousness and bilateral motor manifestations (2). The etiological spectrum of epilepsy is remarkably diverse, encompassing genetic predisposition, structural brain injuries (e.g., traumatic brain injury from accidents or falls), central nervous system infections (including meningitis and encephalitis), neoplastic lesions, metabolic disturbances (such as hypoglycemia or hypocalcemia), and neurodegenerative conditions (3, 4). Notably, a substantial proportion of cases (approximately 30–40%) remain classified as idiopathic epilepsy with undetermined etiology despite extensive diagnostic evaluation (5). Status epilepticus (SE), a severe and emergent condition within epilepsy, is operationally defined as a seizure persisting beyond 5 min or two or more discrete seizures without full recovery of consciousness between them. According to the International League Against Epilepsy (ILAE) 2015 definition, SE is a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms that lead to abnormally prolonged seizures. For generalized convulsive status epilepticus (GCSE), the operational time points are defined as t1 = 5 min (the time at which a seizure is considered prolonged and unlikely to stop spontaneously, thus warranting treatment) and t2 = 30 min (the time at which there is a significant risk of long-term complications) (6). Status epilepticus (SE) manifests in various forms. Common types include: (1) Generalized Convulsive Status Epilepticus (GCSE): Characterized by paroxysmal or sustained rhythmic tonic, clonic, or tonic–clonic muscle activity, impaired consciousness during seizures, and lack of complete consciousness recovery between seizures. It is frequently accompanied by severe autonomic symptoms including hyperpyrexia, tachycardia, respiratory abnormalities, blood pressure fluctuations, pupillary changes, and pathological reflexes. (2) Non-Convulsive Status Epilepticus (NCSE): Absence Status Epilepticus (ASE): Predominantly affects children, presenting with abrupt, relatively mild impairment of consciousness manifesting as lethargy, confusion, reduced voluntary movement, and slowed speech. Complex Partial Status Epilepticus (CPSE): Exhibits diverse levels of impaired consciousness and EEG abnormalities that are periodic and prolonged. Patients may remain in a twilight state for extended periods, accompanied by unresponsiveness and automatisms. (3) Simple Partial Status Epilepticus (SPSE): Consciousness is largely preserved. Patients experience continuous focal sensory or motor seizures. Transient post-ictal hemiparesis (Todd’s paralysis) may occur in the affected region following seizure cessation (6, 7). Failure to effectively control status epilepticus (SE) may lead to progression into refractory status epilepticus (RSE), defined as persistence of seizures despite adequate treatment with first-line (benzodiazepines) and second-line (e.g., levetiracetam, phenytoin, valproate sodium, or phenobarbital) antiseizure medications. This may further progress to super-refractory status epilepticus (SRSE), characterized by ongoing or recurrent seizures 24 h or more after the initiation of anesthetic therapy, or during its weaning or withdrawal phase. Furthermore, SE is closely associated with the development of chronic epilepsy (8). Repeated or prolonged SE episodes can accelerate the process of epileptogenesis, altering brain neural circuitry and function. This leads to recurrent seizures that are often more difficult to control.
The underlying mechanisms involve multiple aspects, including sustained activation of neuroinflammation, neuronal injury and death, disruption of neurotransmitter systems, and aberrant changes in brain plasticity. For instance, the massive release of pro-inflammatory cytokines during SE not only exacerbates acute neuronal damage but may also chronically alter gene expression and function in neural cells. This impacts the remodeling and stability of neural circuits, thereby promoting epileptogenesis. Concurrently, seizure-induced neuronal death and abnormal neurogenesis can contribute to the formation of aberrant synaptic connections, establishing a structural basis for persistent seizure activity (9, 10).
The pathophysiological mechanisms underlying epileptogenesis involve complex interactions between neuronal hyperexcitability and impaired inhibition. Emerging evidence implicates multiple interconnected biological processes, including substantial loss of specific interneuron subpopulations (particularly somatostatin- and parvalbumin-expressing GABAergic cells), astrocytic dysfunction in glutamate homeostasis, and dysregulation of synaptic plasticity (11, 12). Among these mechanisms, the loss or functional impairment of GABAergic inhibitory interneurons is recognized as one of the core mechanisms underlying hyperexcitability within epileptic networks. These neurons regulate the firing frequency of excitatory neurons through GABA release. Their deficiency disrupts the brain’s excitation-inhibition (E/I) balance, directly triggering or amplifying seizure activity. For example, in patients with temporal lobe epilepsy (TLE), hippocampal tissue exhibits neuronal loss, altered immunohistochemical profiles, and changes in electrophysiological properties. Specifically, in medial temporal lobe epilepsy (mTLE), there is a loss of neurons immunoreactive for neuropeptide Y (NPY), somatostatin (SST), and substance P (SP) within the subgranular polymorphic zone of the dentate gyrus. Furthermore, the mTLE group demonstrates significantly reduced overall hippocampal neuronal density compared to other groups, accompanied by electrophysiological evidence of diminished inhibitory function and enhanced excitability. These pathological alterations are strongly implicated in the pathogenesis of epilepsy (11, 13).
Recent studies have highlighted the critical role of oxidative stress pathways, with ferroptosis—an iron-dependent form of regulated cell death characterized by mitochondrial dysfunction and lipid peroxidation—being implicated in temporal lobe epilepsy pathogenesis (14–16). During epileptic seizures, excessive neuronal firing leads to a surge in oxygen consumption and disruption of mitochondrial respiratory chain function, resulting in massive overproduction of reactive oxygen species (ROS). Concurrently, the activity of the antioxidant defense system – including enzymes such as superoxide dismutase (SOD) and glutathione – is suppressed. This creates an imbalance between oxidative stress and antioxidant capacity. Excess ROS not only directly damage neuronal lipids, proteins, and DNA, but also compromise the integrity of the blood–brain barrier, promote the release of pro-inflammatory cytokines, and further exacerbate mitochondrial dysfunction. This cascade establishes a self-perpetuating vicious cycle of “oxidative stress → neuronal damage → epileptic seizures” (15, 16). Targeting this mechanism, stem cells, particularly mesenchymal stem cells (MSCs), can exert protective effects by mitigating cellular oxidative damage and aberrant mitochondrial function. This is achieved through the secretion of antioxidant factors (such as superoxide dismutase), miRNAs within exosomes, and modulation of the Nrf2-HO-1 antioxidant pathway, thereby promoting neuronal survival (17, 18). Neuroinflammation also plays a significant role in both the initiation and progression of epilepsy. Seizures or brain injury can activate microglia and astrocytes, leading to the release of pro-inflammatory cytokines (such as IL-1β, TNF-α, and IL-6) and chemokines. These inflammatory mediators further enhance neuronal excitability and disrupt synaptic homeostasis. For instance, IL-1β enhances neuronal calcium influx by activating NMDA receptors, while TNF-α regulates blood–brain barrier permeability and promotes aberrant neurogenesis. This chronic inflammatory microenvironment not only exacerbates acute seizures but also contributes to the chronic process of epilepsy (i.e., epileptogenesis) (19). Moreover, perturbations in ionic homeostasis (particularly involving sodium, potassium, and calcium channels) and genetic mutations affecting voltage-gated or ligand-gated ion channels have been identified as key contributors to neuronal hyperexcitability (20, 21).
Despite the availability of numerous antiepileptic drugs (AEDs) targeting various molecular pathways, approximately 30% of patients develop drug-resistant epilepsy with inadequate seizure control. Current pharmacotherapeutic limitations include significant adverse effects (e.g., cognitive impairment, hepatotoxicity, and metabolic disturbances), narrow therapeutic indices, and the emergence of pharmacoresistance necessitating complex polypharmacy regimens (22). While surgical interventions (e.g., temporal lobectomy or laser ablation) may benefit select patients with focal epileptogenic zones, inherent risks of neurological deficits and variable efficacy underscore the need for alternative therapeutic strategies (23). Recent advances in regenerative medicine have positioned stem cell therapy as a promising investigational approach for refractory epilepsy. Pluripotent and multipotent stem cells possess the remarkable capacity for self-renewal and multilineage differentiation, offering the potential for neural circuit reconstruction through multiple mechanistic pathways (24, 25). Preclinical studies have suggested that stem cell transplantation ameliorates epilepsy through multiple mechanisms: (1) Differentiation into inhibitory interneurons: Transplanted stem cells can differentiate into pallial medial ganglionic eminence (MGE)-derived GABAergic interneurons, restoring inhibitory tone and suppressing seizure activity (26–29); (2) Secretion of neurotrophic factors: Stem cells release neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), which enhance neuronal survival, maturation, and synaptic plasticity (30–32); (3) Anti-inflammatory modulation: Stem cells mitigate neuroinflammation by secreting cytoprotective, immunomodulatory, and anti-inflammatory factors (33). Stem cells also attenuate neuroinflammatory responses by improving mitochondrial autophagy (34). For example, mesenchymal stem cells (MSCs) attenuate post-epileptic brain injury by upregulating anti-inflammatory cytokines and downregulating pro-inflammatory mediators (19, 35, 36). Despite these promising preclinical findings, critical challenges remain in clinical translation. Key unresolved issues include optimal cell source selection (embryonic vs. induced pluripotent vs. adult stem cells), the method of transplantation (intraparenchymal vs. intravenous delivery), long-term graft survival/functional integration, and the risk of tumorigenicity or aberrant network formation. Moreover, standardization of protocols for cell dosing, timing of intervention relative to epileptogenesis phases, and development of reliable biomarkers for treatment monitoring require systematic investigation. Future research directions should emphasize multimodal approaches combining stem cell therapy with optogenetic modulation or biomaterial scaffolds to enhance graft-host integration. Large-scale randomized controlled trials (RCTs) using standardized seizure outcome measures (e.g., ILAE classification) and advanced neuroimaging modalities (functional MRI, PET) are imperative to establish clinical efficacy. Concurrent basic science investigations must elucidate molecular mechanisms of stem cell-mediated epileptogenesis suppression through single-cell transcriptomics, in vivo calcium imaging, and opto−/chemogenetic manipulation of grafted cells. The ultimate therapeutic paradigm will likely require personalized cell therapy regimens tailored to individual epileptogenic network pathologies and genetic profiles.
2 Classification of stem cells
Different types of stem cells perform distinct biological functions. To better understand their potential and limitations in biological research and medical applications, thereby enabling more targeted therapeutic strategies, the specific classifications of most stem cells are summarized below.
2.1 Classification by origin
2.1.1 Embryonic stem cells
Source: Derived from the inner cell mass of early-stage embryos.
Characteristics: Pluripotent, capable of differentiating into all cell types within the embryo. Although clinical use of ESCs faces scientific, ethical, and legal challenges, clinical trials utilizing human ESCs (hESCs) for therapy have commenced, with protocols established to differentiate hESCs into specialized cell types suitable for transplantation. Scientifically, precisely controlling the differentiation of ESCs into the desired neural cell types remains a complex task. The process needs to be highly regulated to ensure the production of pure populations of functional cells (37, 38). Once the limitations of ESCs are fully overcome, they may become the optimal source of stem cells.
2.1.2 Adult stem cells
Source: Isolated from developed tissues, such as bone marrow, adipose tissue, nervous tissue, and skin.
Characteristics: Exhibit multipotency within their tissue of origin, typically differentiating into lineage-specific cell types. Examples include bone marrow MSCs (BMSCs), neural stem cells (NSCs), and dental pulp stem cells (DPSCs) (39, 40).
2.1.3 Induced pluripotent stem cells
Source: Generated by reprogramming somatic cells (e.g., adult fibroblasts) via genetic or epigenetic modifications to regain pluripotency (41).
Characteristics: iPSCs closely resemble ESCs in their differentiation capacity, enabling generation of cells from all three embryonic germ layers. They also possess robust self-renewal capabilities. A key advantage lies in their autologous origin, minimizing the risk of immune rejection. Additionally, iPSCs can be derived from diverse donor populations (healthy or diseased), circumventing ethical controversies associated with ESCs and broadening their clinical applicability (42–44). iPSCs have emerged as a revolutionary resource in the quest for effective stem cell therapies for epilepsy. Generated by reprogramming somatic cells, such as adult fibroblasts, through genetic or epigenetic modifications to regain pluripotency, iPSCs closely mimic ESCs in their differentiation capacity. The robust self—renewal capabilities of iPSCs further enhance their potential in epilepsy treatment. This characteristic enables the generation of large quantities of cells for transplantation, ensuring an adequate supply for clinical applications. Moreover, a key advantage of iPSCs lies in their autologous origin (45). Since iPSCs can be derived from the patient’s own cells, so the risk of immune rejection is significantly minimized (46). This is a major breakthrough compared to traditional transplantation therapies, as immune-related complications often pose significant challenges in long-term treatment success.
2.2 Classification by differentiation potential
2.2.1 Totipotent stem cells
TSCs represent the most potent stem cell type, capable of generating all embryonic and extraembryonic cell lineages (e.g., placenta), thereby supporting the development of a complete organism (47). In the context of epilepsy, this unparalleled potency could, in theory, offer novel therapeutic strategies. However, their use raises significant ethical concerns.
2.2.2 Pluripotent stem cells
PSCs can differentiate into derivatives of all three germ layers (endoderm, mesoderm, ectoderm) but lack the ability to form extraembryonic tissues (48). These cells have been adapted to in vitro culture systems distinct from their native developmental niches, facilitating their accessibility and utility in research and therapy (49).
2.2.3 Unipotent stem cells
USCs exhibit the most restricted differentiation potential and are committed to a single cell lineage (e.g., muscle satellite cells) (50). They maintain long-term self-renewal within specific tissues and differentiate to repair localized damage. Their limited ethical controversy, high safety profile, and reduced tumorigenic risk compared to PSCs make them valuable tools in regenerative medicine.
2.3 Classification by tissue specificity
2.3.1 Hematopoietic stem cells
HSCs are a class of adult stem cells capable of self-renewal and differentiation into all blood cell lineages and immune cells. They serve as the foundation for the maintenance and regeneration of the hematopoietic and immune systems (51). Clinically, HSCs have been extensively utilized in the treatment of hematologic and immunologic disorders (52, 53). Despite challenges such as donor matching, transplantation-associated complications, and insufficient cell yield, advances in in vitro expansion, gene editing, and immune tolerance technologies are expected to broaden their clinical applicability. In the context of epilepsy, emerging research suggests potential roles for HSCs that extend beyond their traditional hematologic and immunologic applications. There is growing evidence of a complex interplay between the immune system and epilepsy (54).
2.3.2 Neural stem cells
NSCs possess the ability to differentiate into diverse cell types of the central nervous system (CNS), including neurons, astrocytes, and oligodendrocytes (55). Their differentiation potential positions NSCs as transformative agents for CNS disease therapies (56). A recent study has highlighted the therapeutic relevance of NSC-derived secretory products, which contribute to host cell survival, neuroplasticity, and neuroimmune modulation (57). In epilepsy, where there is often neuronal damage and impaired neuroplasticity, the secretory products of NSCs can play a crucial role. The paracrine mechanisms of NSCs are considered pivotal for their interaction with neural tissues, as they endogenously generate a multifaceted secretome consisting of growth factors, cytokines, chemokines, morphogens, microRNAs (miRNAs), and other bioactive molecules. For instance, miRNAs within the NSC secretome can regulate gene expression in neighboring cells, influencing processes such as cell survival, differentiation, and synaptic plasticity (58, 59). These properties underscore the immense potential of NSCs in future neurologic disease interventions. As research continues to uncover the complex interplay between NSCs and the epileptic brain, further optimization of NSC-based therapies, including the manipulation of their secretory profiles and differentiation pathways, holds great promise for effectively treating epilepsy and improving the quality of life for patients suffering from this debilitating disorder.
2.3.3 Mesenchymal stem cells
MSCs exhibit multipotent differentiation into mesoderm-derived lineages, such as osteocytes, chondrocytes, adipocytes, myocytes, and bone marrow stromal cells (60, 61). In the context of epilepsy, this multipotency could potentially be harnessed in novel ways. Although the primary goal in epilepsy treatment is often to correct neural dysfunctions, MSCs’ ability to differentiate into stromal cells might contribute to the creation of a more supportive microenvironment in the epileptic brain. For example, bone marrow stromal cell-like derivatives from MSCs could help in remodeling the extracellular matrix, which may be disrupted in epilepsy due to chronic seizures and associated inflammation. This remodeling could, in turn, enhance the survival and function of existing neural cells. Epilepsy is frequently associated with neuroinflammation, which can exacerbate seizure activity and contribute to neuronal damage. MSCs, with their immunomodulatory properties, could play a crucial role in mitigating this inflammation. MSCs are characterized by low immunogenicity, enabling them to suppress excessive immune responses and modulate the local immune microenvironment via secretion of cytokines and bioactive molecules, thereby attenuating inflammatory cascades (62). Preclinical studies have demonstrated that MSCs improve outcomes in animal models of neurologic disorders, including multiple sclerosis, stroke, Alzheimer’s disease, and traumatic brain injury (63). Bone marrow-derived and other tissue-specific MSCs have demonstrated favorable safety profiles in human applications (64, 65). Their versatility in administration routes—such as intravenous, intra-arterial, intraperitoneal, intrathecal, and intranasal delivery—enhances their therapeutic adaptability (66, 67). For instance, intranasal delivery of MSCs could potentially bypass the blood–brain barrier, allowing the cells to directly reach the brain tissue in a more efficient manner. This could be particularly advantageous as the blood–brain barrier may be compromised in epilepsy, but a direct and targeted method of delivery could still optimize the therapeutic effect of MSCs. MSCs are relatively accessible, with human adipose tissue serving as a robust source (68). Additionally, dental-derived MSCs can be isolated from various dental tissues (69), and OM-MSCs located in the nasal cavity have emerged as a viable source of neural stem cells (70). The ease of procurement and long-term viability of OE-MSCs render them particularly suitable for neurologic disease modeling and therapeutic development (71–74). As research progresses, further understanding of the optimal source, differentiation potential, and delivery methods of MSCs will be essential to fully realizing their potential in revolutionizing epilepsy treatment and improving the quality of life for patients suffering from this challenging neurological disorder.
3 Stem cell therapy for epilepsy
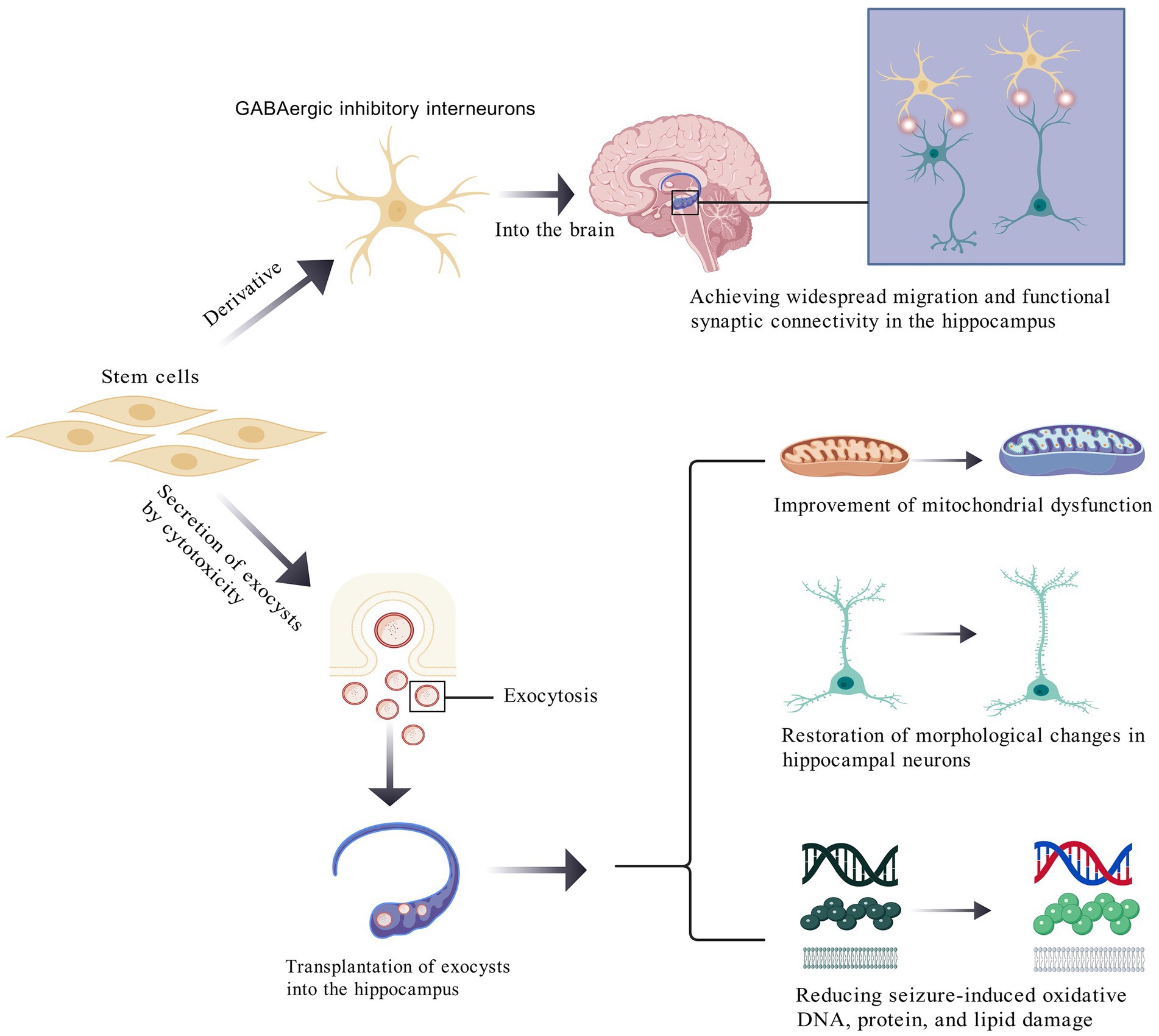
Stem cells injected into the body can alleviate epileptic seizures by differentiating into interneurons (75–79), while also secreting neurotrophic factors to promote neuroprotection and neuroregeneration, thereby improving neural network function (80, 81). Additionally, stem cells may have therapeutic effects by reducing neuroinflammation and modulating immune responses (62, 82–84). Notably, stem cell therapy has demonstrated efficacy in ameliorating spinal muscular atrophy with progressive myoclonic epilepsy (SMA-PME) caused by enzymatic deficiencies (85). Key mechanisms of stem cell therapy in epilepsy are shown in Figure 1.
A key pathophysiological mechanism of epilepsy may involve the loss of inhibitory interneurons. First, stem cell-derived GABAergic inhibitory interneurons can extensively migrate within the hippocampus, functionally integrating into synaptic networks, and releasing inhibitory neurotransmitters to alleviate seizure activity. Second, stem cells release extracellular vesicles (EVs) via exocytosis, which mitigate seizure-induced mitochondrial dysfunction and restore hippocampal neuronal morphology. Post-epilepsy, pyramidal neurons in the hippocampus exhibit shortened total dendritic length, reduced spine density, and altered dendritic morphology. EVs reverse these pathological changes and ameliorate oxidative damage to DNA, proteins, and lipids within hippocampal neurons.
3.1 Stem cell differentiation into inhibitory interneurons
Although the precise mechanisms underlying epilepsy remain unclear, the dysfunction or loss of GABAergic inhibitory interneurons is a widely proposed hypothesis. Early studies suggested a link between hippocampal interneuron loss and epilepsy (86). Recent evidence has further indicated that impaired inhibitory interneuron function within hippocampal circuits contributes to seizure generation (87). Therapeutically, Cunningham et al. demonstrated that human pluripotent stem cell (PSC)-derived mature GABAergic interneurons (mGINs) extensively migrate and functionally integrate into the brains of post-seizure mice, releasing GABA to suppress synaptic excitation via presynaptic inhibition of host hippocampal neurons and postsynaptic reception of excitatory inputs, thereby exhibiting anti-epileptic action (88).
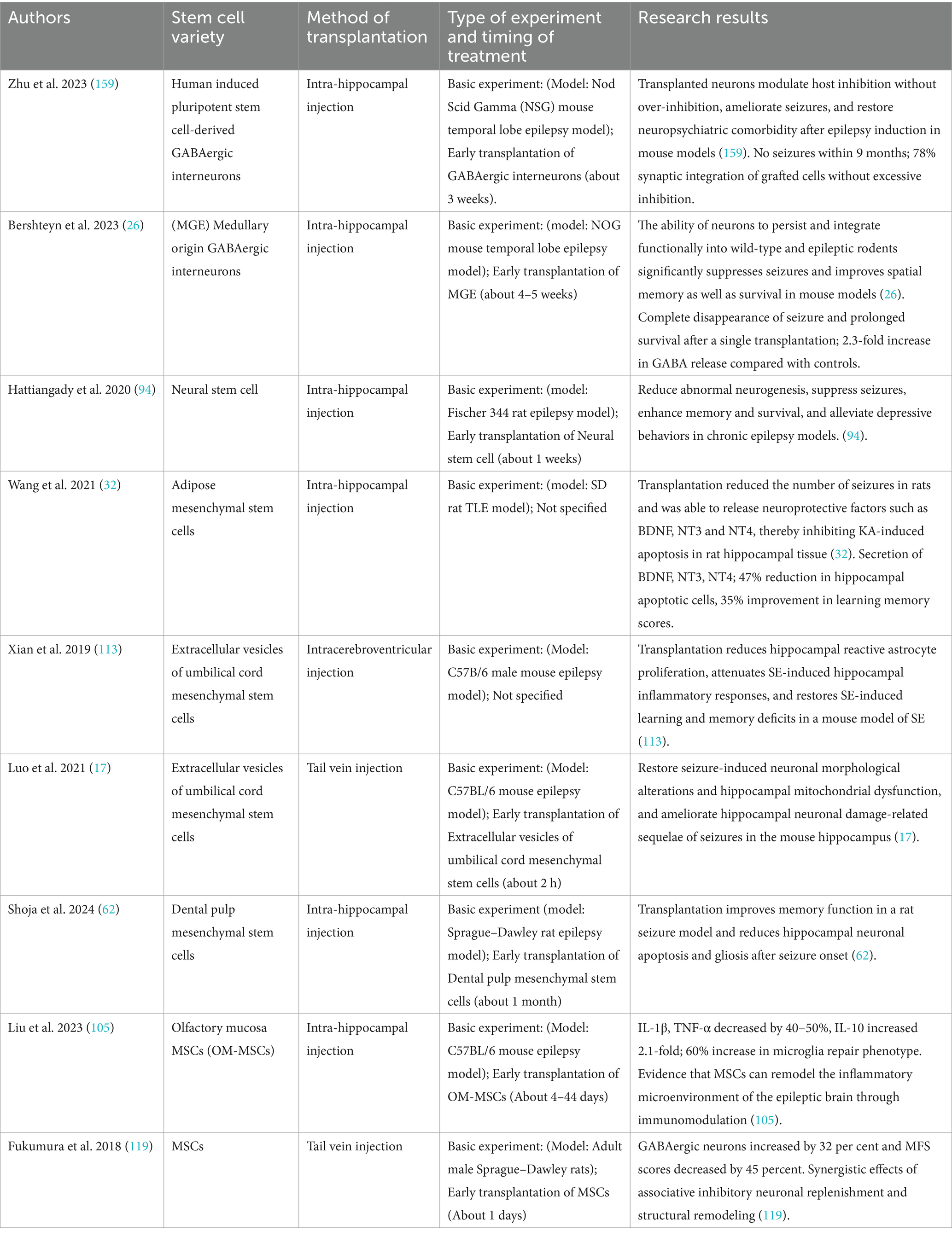
Zhu et al. explored methods of transplantation using human PSC (hPSC)-derived interneurons, which proved effective in both pilocarpine-induced temporal lobe epilepsy (TLE) and intrahippocampal kainate models.
Bershteyn et al. demonstrated that medial ganglionic eminence (MGE)-derived GABAergic interneurons from human ESCs (hESCs), delivered via single-dose intrahippocampal injection, markedly reduced seizures in medial temporal lobe epilepsy models, even achieving seizure-free outcomes and prolonged survival (26). Human iPSC (hiPSC)-derived MGE cells further attenuate progression from status epilepticus (SE) to chronic epilepsy. Notably, hiPSC-MGE transplantation during the late phase of SE reduces host interneuron loss and aberrant mossy fiber sprouting (MFS), while promoting physiological neurogenesis. Animals receiving hiPSC-MGE grafts post-SE exhibited improved cognitive and emotional function, potentially linked to the restoration of normal neuronal populations and suppression of pathological neurons (89–92).
Hattiangady et al. transplanted neural stem cells (NSCs) into animal models and observed that NSC-derived mature neurons migrated to the CA3 pyramidal cell layer or dentate granule cell layer, with approximately 17% differentiating into GABAergic interneurons. The percentage of normal hippocampal GABAergic neurons is about 10–15% (93), suggesting that transplanted cells can effectively replenish lost inhibitory circuits. Extrapolation of NeuN+ and GABA+ neuron counts per hippocampus revealed that NSC transplantation increased NeuN+ neurons by 21,280 and GABA+ interneurons by 19,040 per hippocampus. Neural stem cells differentiate into GABA+ inhibitory interneurons that replenish hippocampal neurons and significantly reduce seizure frequency (94). As NeuN is a marker for mature neurons, this quantitative evidence confirms that grafted NSCs successfully differentiated into functional neurons, directly replenishing neuronal losses induced by status epilepticus (SE). Critically, the specific restoration of inhibitory interneurons is essential, given that the loss of GABAergic interneurons constitutes a central mechanism underlying network hyperexcitability in epilepsy. The addition of 19,000 GABAergic interneurons effectively rebuilt the foundational units for inhibitory regulation within hippocampal circuitry.
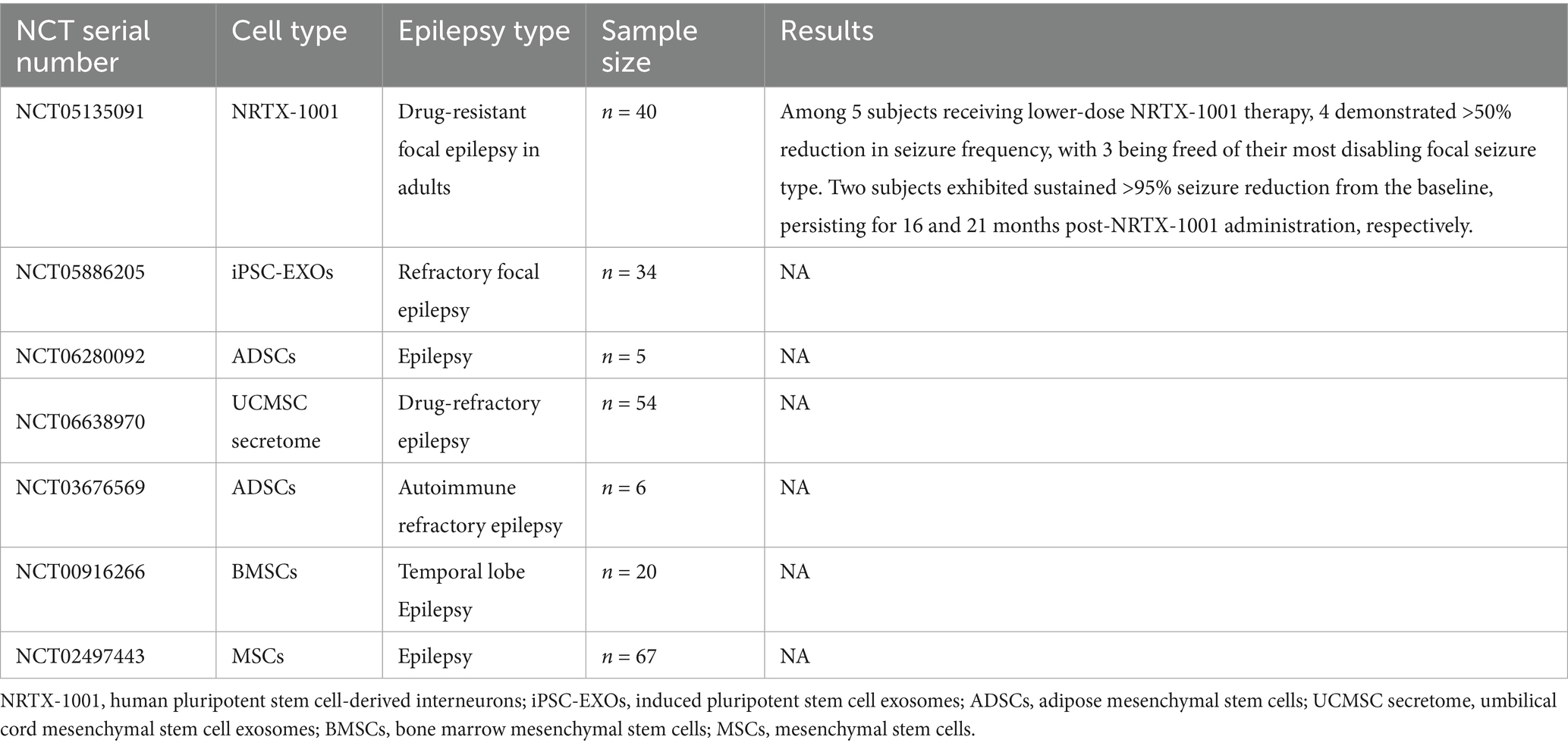
Recent clinical trials by Neurona Therapeutics, focusing on inhibitory interneuron-based therapy, highlight the promise of NRTX-1001—a regenerative cell therapy derived from human PSCs (95). NRTX-1001 delivers long-term GABAergic inhibition to hyperexcitable neural networks via a single dose. Interim results show that 4 out of 5 low-dose recipients experienced >50% seizure reduction, with 3 being freed of debilitating focal seizures. Two subjects reported >95% seizure reduction at 16 and 21 months post-treatment. To date, NRTX-1001 has been well-tolerated in all participants (96), underscoring its potential as a groundbreaking clinical intervention for epilepsy.
3.2 Stem cells secrete neurotrophic factors
MSCs secrete diverse molecules via soluble factors and extracellular vesicles (EVs), including microvesicles (MVs), exosomes (EXs), and apoptotic bodies (97–99). EXs, which are critical mediators of intercellular communication, act through paracrine and endocrine pathways, delivering bioactive components such as chemokines, cytokines, interleukins, growth factors, proteins, free nucleic acids, and lipids (100, 101).
Waldau et al. demonstrated that medial ganglionic eminence-derived neural stem cells (MGE-NSCs) not only secrete bioactive factors but also differentiate into NeuN+ neurons (13%), S−100β + astrocytes (57%), and NG2 + oligodendrocyte progenitor cells (3%) in the post-epileptic hippocampus. The newly differentiated NeuN+ neurons may integrate into hippocampal neural circuitry reconstruction, likely contributing to the amelioration of epileptic symptoms. During epileptogenesis, astrocytic function may become impaired; however, graft-derived S-100β+ astrocytes (constituting a substantial proportion of differentiated cells) facilitate the restoration and maintenance of neural microenvironmental homeostasis. This attenuates excitotoxic damage while providing essential trophic support and neuroprotection to vulnerable neuronal populations. Additionally, oligodendrocyte progenitor cells can further mature into oligodendrocytes, which are critical for myelination and efficient neuronal impulse conduction. Given that myelination deficits are prevalent in the epileptic brain, these modest yet significant populations of graft-derived oligodendrocyte precursors may participate in remyelination and the functional recovery of neural transmission. Ten percent of these derivatives expressed γ-aminobutyric acid (GABA), while 50% expressed glial cell line-derived neurotrophic factor (GDNF). Replenishing depleted GABAergic inhibitory interneurons in the post-epileptic hippocampus directly restores inhibitory circuitry, thereby counteracting neuronal hyperexcitability and attenuating seizure activity. Furthermore, transplanted neural stem cells (NSCs) elevate glial cell line-derived neurotrophic factor (GDNF) expression in host astrocytes. This dual mechanism establishes a sustained reservoir of anti-epileptic factors that promotes neuronal survival/repair and maintains neural microenvironmental homeostasis, collectively mediating robust neuroprotective effects. Transplanted NSCs also restored GDNF levels in host astrocytes, suggesting that increased GDNF+ cells and astrocytic GDNF recovery underlie the therapeutic benefits of MGE-NSC grafts (102). Similarly, NSC-derived grafts exhibited variable expression of fibroblast growth factor-2 (FGF-2, 66%), insulin-like growth factor-1 (IGF-1, 59%), brain-derived neurotrophic factor (BDNF, 25%), and GDNF (45%) (94). The expression of neurotrophic factors within neural grafts underscores their contributory role in neuroprotection, neural regeneration, and tissue repair. Critically, distinct neurotrophic factors may engage in synergistic or complementary interactions during neural repair processes, collectively orchestrating the functional restoration of epileptically damaged neural tissue.
In a rat model of kainic acid (KA)-induced epilepsy, Wang et al. observed that transplanted adipose-derived stem cells (ADSCs) released BDNF, neurotrophin-3 (NT3), and neurotrophin-4 (NT4) in hippocampal tissues (32). These neurotrophic factors have broad anti-apoptotic effects (87), with BDNF modulating hippocampal long-term potentiation (LTP) and synaptic plasticity to enhance learning and memory (103). These restorative effects may be attributed to: Repair of hippocampal-prefrontal cortical circuitry, which critically regulates emotional processing. Modulation of neurotransmitter systems (e.g., serotonin [5-HT], dopamine). Rehabilitation of damaged limbic circuitry, particularly the hippocampus which governs both cognitive and affective functions. Thus, ADSC transplantation mitigates KA-induced hippocampal apoptosis and restores cognitive function. Additionally, Hattiangady et al. reported that NSC grafts express FGF-2 and vascular endothelial growth factor (VEGF), facilitating post-epileptic repair (104).
3.3 Stem cells attenuate epilepsy and complications via antioxidant and anti-inflammatory effects
Liu et al. found that olfactory mucosa-derived MSCs (OM-MSCs) reduced post-epileptic neuroinflammation by suppressing astrogliosis and microgliosis, alleviating demyelination and neuronal loss. OM-MSC-treated groups exhibited lower pro-inflammatory cytokines (Tnf, Il1b, and Il6) and elevated anti-inflammatory IL-10, promoting neural repair. OM-MSCs also recruited regulatory T cells (Tregs) to suppress inflammation and enhance microglial repair activity, ultimately restoring cognitive function (105, 106). The therapeutic benefits of MSCs in inflammatory brain disorders may arise from the paracrine actions of EVs and soluble factors (107, 108). Notably, MSC-derived EVs exhibit potent anti-inflammatory properties post-brain injury (109–111), capitalizing on their low immunogenicity, immunomodulatory capacity, and multipotency (112).
Xian et al. demonstrated that MSC-derived EXs (MSC-Exo) attenuated lipopolysaccharide (LPS)-induced cytotoxicity, reactive astrogliosis, and inflammatory cytokine secretion (TNF-α and IL-1β) in vitro (113). Luo et al. highlighted the antioxidant activity of MSC-EVs, which mitigated oxidative stress, reversed hippocampal neuronal morphological changes, and restored mitochondrial dysfunction post-seizures, improving cognitive outcomes (17). Long et al. reported that intranasal delivery of human bone marrow MSC-derived A1-EXs post-SE suppressed pro-inflammatory cytokines (TNF-α, IL-1β, MCP-1, SCF, MIP-1α, GM-CSF, and IL-12) while elevating anti-inflammatory factors, reducing microglial activation, and attenuating the inflammatory cascade (114). Shoja et al. demonstrated that dental pulp stem cells (DPSCs) reduced astrocyte proliferation and complexity in the hippocampal CA1 region, alleviating post-epileptic inflammation and cell death (62).
3.4 Stem cell grafts protect neuronal survival and suppress aberrant neurogenesis
Dentate gyrus neurogenesis post-SE follows a biphasic pattern: excessive aberrant neurogenesis occurs during the acute/subacute phases (115, 116), while diminished neurogenesis occurs in the chronic phase (91). Hattiangady et al. showed that early NSC transplantation in SE models significantly reduced aberrant neurogenesis and protected neuronal survival. Compared to controls, SE alone reduced normal neurons by 87%, whereas SE + NSC grafts limited loss to 47%. NSC grafts also suppressed MFS, a hallmark of hippocampal hyperexcitability (94).
Huang et al. observed that human umbilical cord MSC (HUMSC) transplantation preserved hippocampal pyramidal neuron integrity and inhibitory circuits in chronic epilepsy, mitigating pilocarpine-induced neuronal loss in CA1 and CA3. Timm staining revealed excessive MFS into the inner molecular layer (IML) and CA3 in SE rats, which HUMSC grafts significantly attenuated (117, 118). Fukumura et al. confirmed that MSC infusion increased GABAergic interneurons and NeuN+ neurons in SE hippocampi, while suppressing MFS according to Timm staining (119). Stem Cell Therapy Mitigates SE-Induced Aberrant Neurogenesis by Suppressing Ectopic Migration and Modulating Overall Neurogenic Dynamics: 1. Suppression of Ectopic Migration: Neural stem cell (NSC) transplantation into SE models significantly reduces mossy fiber sprouting (MFS) area by 58%, indirectly indicating inhibition of newborn neuron ectopic migration (120, 121). This effect is mediated through: Secretion of neurotrophic factors (e.g., BDNF, FGF-2) and extracellular matrix components (e.g., integrins) that guide neuronal migration along physiological paths to the dentate granule layer, minimizing aberrant colonization of the hilus and CA3 (122). Synaptic anchoring of ectopic neurons by NSC-derived mature neurons, restricting abnormal projections via functional connectivity (123). 2. Modulation of Aberrant Neurogenesis NSC transplantation reduces pathological neurogenesis by 73% while restoring chronic-phase neurogenesis to physiological levels (124). Key mechanisms include: 1. Promotion of neuronal maturation: BDNF released by ADSCs accelerates dendritic development and synaptic integration of newborn neurons, reducing their epileptogenicity (125, 126). 2. Neurogenic cycle rebalancing: MSC-derived extracellular vesicles (MSC-EVs) restore mitochondrial function in neural stem cells, normalizing the proliferation-differentiation equilibrium to prevent chronic-phase neurogenic suppression (17, 127, 128).
Collectively, preclinical evidence supports stem cell therapy in counteracting epileptogenic neurogenesis (Table 1).
3.5 Stem cell therapy ameliorates enzyme deficiency-induced epilepsy
Rybova et al. demonstrated that hematopoietic stem cell transplantation prevented disease progression and spinal demyelination in a mouse model of spinal muscular atrophy with progressive myoclonic epilepsy (SMA-PME) caused by acid ceramidase (ACDase) deficiency, improving behavioral outcomes (85). However, the underlying mechanisms warrant further exploration.
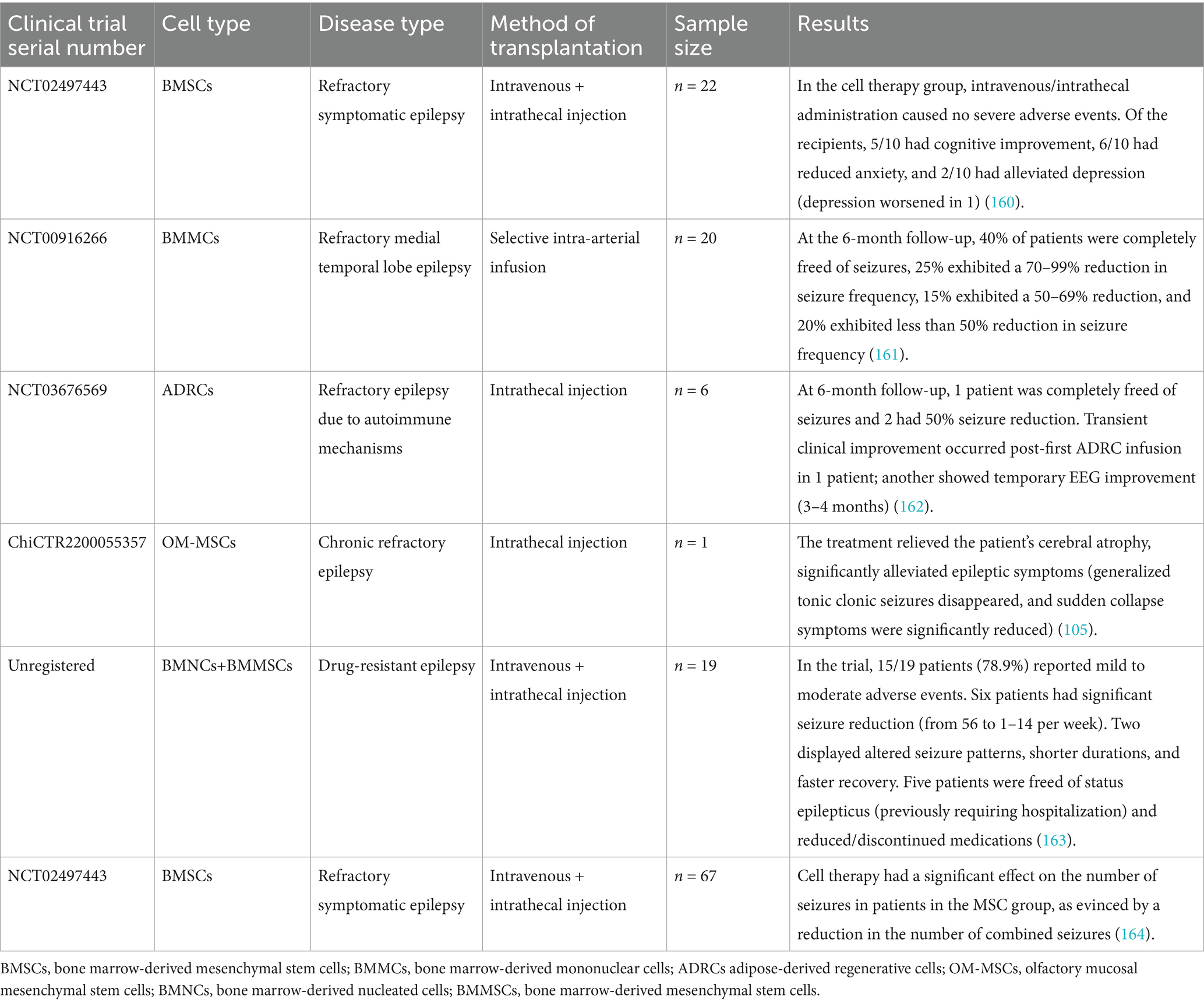
Emerging evidence from completed and ongoing clinical trials supports the therapeutic efficacy of stem cell-based interventions in epilepsy, as summarized in Tables 2, 3.
4 Discussion
Stem cell therapy holds immense potential for epilepsy treatment; however, significant challenges remain, including ensuring safety, enhancing efficacy, overcoming technical bottlenecks, and addressing ethical concerns. Future research must focus on optimizing methodologies and conducting large-scale, long-term clinical trials to advance stem cell therapy toward clinical use, ultimately delivering safe, effective, and sustainable treatments for patients.
4.1 Current challenges
4.1.1 Sources of and selection of stem cells
Stem cells are derived from diverse sources, including ESCs, adult stem cells, and iPSCs, each possessing distinct advantages and limitations that necessitate careful selection for optimal therapeutic outcomes. Currently, MSCs have gained prominence in clinical use due to their multifaceted therapeutic properties. These include superior regenerative potential, antioxidant stress mitigation, and anti-apoptotic capabilities, which collectively contribute to their critical role in disease intervention. Moreover, MSCs exhibit remarkable secretory functions through the production of bioactive cytokines and EVs, facilitating intercellular communication and tissue repair. The relative ease of isolation from accessible tissues (e.g., adipose tissue, bone marrow, and the umbilical cord) combined with minimal ethical concerns has significantly facilitated their widespread clinical utilization (112–114). While other stem cell types demonstrate unique therapeutic potentials, their full clinical implementation requires resolution of ethical controversies and optimization of accessibility. Future advances in overcoming these challenges may unlock substantial therapeutic prospects across various stem cell platforms, enabling personalized regenerative strategies tailored to specific pathological contexts (129–131). Other stem cell types, while promising, require the addressing of ethical concerns and the overcoming of accessibility barriers to unlock their full potential.
4.1.2 Post-transplantation survival and function
Ensuring stem cell survival and functional integration into host neural networks post-transplantation remains a major hurdle. Key factors requiring further investigation include transplantation targets, mechanisms of action, cell types, and therapeutic outcomes (132). Key obstacles include inflammatory mediators (e.g., IL-1β, TNF-α), oxidative stress, and synaptic mismatch, which collectively impair graft viability and neural circuit integration (133). Enhancing cell survival requires strategies such as preconditioning stem cells with antioxidants (e.g., N-acetylcysteine) or genetic modifications to overexpress anti-apoptotic proteins (e.g., BCL-2). Concurrently, promoting synaptic connectivity between donor-derived neurons and host networks necessitates precise delivery timing and microenvironmental optimization, including neurotrophic support (BDNF, GDNF) and activity-dependent plasticity modulation. Critical research priorities are as follows:
Transplantation protocol optimization: Determining ideal cell types (e.g., GABAergic progenitors vs. MSCs), doses, and delivery routes (intrahippocampal vs. intravenous). Microenvironmental modulation: Utilizing biomaterials like injectable hydrogels to sustain neuroprotective factor release and shield grafts from inflammatory damage. Mechanistic clarification: Distinguishing therapeutic effects from direct synaptic integration versus paracrine signaling using optogenetic silencing and lineage-tracing technologies. Emerging tissue engineering approaches, such as 3D-printed scaffolds with topographical cues, show promise for guiding axonal growth and improving graft-host connectivity (134). Standardized metrics for evaluating therapeutic efficacy—including a reduction in seizure frequency, cognitive recovery, and functional MRI-based graft tracking—are essential for clinical translation. Future advances will require interdisciplinary efforts to address immune compatibility, long-term safety, and scalability, ultimately positioning stem cell therapy as a viable option for drug-resistant epilepsy (135).
4.1.3 Long-term efficacy and safety
The long-term safety profile and sustained therapeutic effects of stem cell transplantation remain incompletely characterized, necessitating rigorous longitudinal investigations to address critical knowledge gaps. Key unresolved concerns include: (1) immune-mediated rejection of allogeneic grafts, particularly in non-immunoprivileged brain regions; (2) tumorigenic potential associated with residual undifferentiated pluripotent cells; and (3) infection risks from xenogenic components in culture systems. Moreover, heterogeneity in cell sourcing (autologous vs. allogeneic) and preparation protocols (enzymatic dissociation vs. mechanical isolation) may introduce variability in clinical outcomes and safety profiles (136). Current evidence from preclinical models demonstrates sustained seizure suppression (>6 months post-transplantation) through GABAergic interneuron integration and neurotrophic modulation. However, clinical translation requires systematic evaluation of the following.
Chronic neuroinflammatory responses: Monitoring microglial activation (via TSPO-PET imaging) and cytokine profiles (IL-6, TGF-β1).
Oncogenic surveillance: Implementing multimodal screening protocols combining liquid biopsy (circulating tumor DNA) and annual MRI for tumor detection.
4.1.4 Standardization of stem cell processing
The clinical translation of stem cell therapies for epilepsy remains hindered by a critical lack of standardized protocols spanning cell selection, processing, expansion, and transplantation (132). Variability in cell sources (e.g., autologous vs. allogeneic), culture conditions, and methods of differentiation compromises both reproducibility and therapeutic reliability (137). Establishing unified industry standards is imperative to ensuring consistency in cell viability (>90%), purity (<1% undifferentiated cells), and functional potency across studies. Advances in automated, closed-system bioreactors enable scalable production of clinical-grade stem cells while minimizing contamination risks. Integration of chemically defined culture matrices and AI-driven monitoring systems further enhances batch-to-batch consistency. Collaborative efforts between regulatory bodies and scientific consortia are essential to harmonizing epilepsy-specific benchmarks, including synaptic integration capacity and subtype-specific differentiation ratios, thereby facilitating multicenter trial comparability and accelerating therapeutic validation.
4.1.5 Ethical and legal considerations
Stem cell advances raise ethical, moral, and legal challenges, particularly regarding human embryo use, therapeutic cloning, and tissue sourcing. Informed consent for tissue procurement is essential to mitigating ethical disputes. Ethical debates often center on treatment risks, side effects, safety, and societal impact (138). Clinical trials must adhere to internationally recognized ethical standards, be scientifically rigorous, and adhere to principles of patient protection. These issues demand thorough consideration during research and clinical translation.
4.2 Future research directions
4.2.1 Basic research
The use of stem cells in epilepsy treatment hinges on elucidating their capacity to modulate hyperexcitable neuronal networks and repair epileptogenic circuitry (139). Focal epilepsies, and particularly temporal lobe epilepsy (TLE), are characterized by hippocampal neuron loss, gliosis, and aberrant synaptic reorganization—pathological features that may be ameliorated through stem cell-derived interventions (140). Preclinical studies have highlighted the potential of MSCs to suppress neuroinflammation via anti-apoptotic and immunomodulatory cytokine secretion (e.g., TGF-β and IL-10), while iPSC-derived GABAergic interneurons may restore inhibitory tone in seizure-prone regions such as the dentate gyrus. However, challenges persist in ensuring long-term survival, targeted migration, and functional synaptic integration of transplanted cells within sclerotic hippocampi. Recent advances in optogenetic control of grafted cells and single-cell transcriptomic profiling of host-graft interactions offer novel tools to elucidate mechanisms of circuit repair (141). Further research must prioritize optimizing methods of delivery (e.g., biomaterial scaffolds for localized grafting) and mitigating risks such as ectopic cell proliferation or unintended network dysregulation (142). Further understanding of epigenetic and metabolic crosstalk between stem cells and the epileptic microenvironment will be critical to translating these therapies into clinically viable, precision-based interventions.
4.2.2 Clinical trials
Large-scale, multicenter, RCTs need to be conducted to rigorously evaluate the efficacy and safety of stem cell therapy in epilepsy. Stem cell therapies are increasingly being tested in clinical trials for various conditions, such as diabetes (143–145) and neurodegenerative disorders (e.g., Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis) (146–148). However, challenges persist in standardizing cell types (e.g., MSCs vs. iPSC-derived inhibitory interneurons), delivery routes (intravenous vs. intracerebral), and dosing protocols across diverse cohorts. Recent advances in epilepsy research have prompted multiple international trials assessing stem cell safety and efficacy, with preliminary results showing promise. Increasing multicenter and multinational collaboration will enhance sample diversity and enable cross-regional comparisons of therapeutic outcomes. A critical gap remains in validating objective efficacy endpoints beyond seizure diaries, such as electrophysiological biomarkers or functional neuroimaging correlates of network stabilization. Concurrently, regulatory agencies must balance accelerated approval pathways for urgent unmet needs with robust post-marketing surveillance to monitor risks like graft overgrowth or autoimmune reactions. By integrating patient-reported outcomes with mechanistic biomarkers, future trials can bridge the translational gap while maintaining ethical and scientific rigor.
4.2.3 Personalized treatment
In the context of epilepsy, stem cell therapy has emerged as a promising avenue for personalized treatment. Given the highly heterogeneous nature of epilepsy, with diverse etiologies and clinical manifestations among patients, a one-size-fits-all approach is often ineffective. Precision biomarkers, genetic profiling, and advanced neuroimaging can guide patient stratification, real-time monitoring of transplanted cells, and evaluation of therapeutic responses. Tailoring treatments to individual biological, genetic, and clinical profiles remains a key challenge and opportunity for improving outcomes (149). Precision biomarkers, such as seizure-associated miRNA profiles or PET/MRI-based metabolic signatures, could further stratify patients for autologous vs. allogeneic cell therapies and track graft viability post-transplantation (150). Genetic profiling is another essential tool. Epilepsy is known to have a significant genetic component, and different genetic mutations can lead to distinct pathophysiological mechanisms. Through comprehensive genetic profiling, researchers can determine the genetic background of a patient’s epilepsy. This knowledge can then be used to engineer stem cells that are genetically modified to correct the underlying genetic defects. However, tailoring stem cell treatments to individual biological, genetic, and clinical profiles is not without challenges. The complexity of the epileptic brain, with its multiple interacting neural networks and compensatory mechanisms, hampers the precise prediction of how the transplanted stem cells will behave.
4.2.4 Technological innovations
In the pursuit of effective stem cell therapies for epilepsy, emerging technologies are playing a transformative role. CRISPR-based gene editing has emerged as a powerful tool with immense potential. Epilepsy is often associated with various genetic mutations, and CRISPR technology allows for the precise targeting and correction of these epilepsy-associated genetic defects (151). By modifying the genetic code within stem cells, researchers can potentially create cells that are resistant to abnormal neural firing patterns characteristic of epilepsy (152). If, for example, a specific gene mutation is causing an over-excitability of neurons in the epileptic brain, CRISPR can be used to edit the gene to restore normal neuronal function. This not only holds promise for treating the underlying cause of epilepsy but also for enhancing the therapeutic properties of stem cells, making them more effective in suppressing epileptic seizures. Single-cell genetic reprogramming techniques are also revolutionizing the field. These techniques enable scientists to exercise precise control over stem cell differentiation and function. In the context of epilepsy treatment, this means the ability to generate specific types of neural cells that can integrate seamlessly into the epileptic brain and restore normal neural circuitry (153). For instance, precisely reprogramming stem cells at the single-cell level enables the creation of inhibitory neurons, which are often deficient in epileptic brains. These inhibitory neurons can then be transplanted into the epileptic focus to counterbalance the excessive excitatory activity, potentially halting seizure propagation. This targeted approach, made possible by single-cell genetic reprogramming, offers a novel cell source for epilepsy treatment that was previously unattainable. Advances in cell processing and methods of transplantation are equally crucial. Biomaterial scaffolds, for example, can provide a supportive framework for transplanted stem cells. In the epileptic brain, which has a complex and often damaged neural environment, these scaffolds can help guide the migration and integration of stem cells to the appropriate regions (154). They can also mimic the natural extracellular matrix, promoting cell survival and differentiation. Combining these innovative cell processing and transplantation techniques will enable the safety and effectiveness of stem cell therapies for epilepsy to be significantly enhanced, bringing us closer to a viable treatment option for this challenging neurological disorder.
4.2.5 Combination therapies
Combining stem cell therapy with existing treatments—such as AEDs, the ketogenic diet (KD), or neuromodulation—may yield synergistic benefits. AEDs are currently the cornerstone of epilepsy treatment, aiming to control seizure frequency and severity (155). However, a significant proportion of patients remain refractory to these medications. Stem cell therapy, with its potential to modulate the epileptic brain at a cellular and molecular level, could complement AEDs. The KD, a high-fat, low-carbohydrate regimen for drug-resistant epilepsy, modulates biochemical pathways to reduce seizures (156). The KD increases the production of ketone bodies, which can act as an alternative energy source for the brain. In the context of epilepsy, this metabolic shift may also have a direct impact on neuronal excitability. Investigating its synergy with stem cell therapy could enhance efficacy. Stem cells, when transplanted, might respond to the altered metabolic environment induced by the KD. For instance, the increased availability of ketone bodies could potentially facilitate the survival and differentiation of transplanted stem cells into functional neural cells, thereby enhancing their overall anti-epileptic effect (157). Investigating its synergy with stem cell therapy could enhance efficacy. Similarly, integrating stem cell grafts with deep brain stimulation (DBS) targeting the anterior nucleus of the thalamus (ANT) may offer novel strategies for drug-refractory epilepsy (158). This multi-modal approach holds great promise for improving treatment outcomes for patients with drug-refractory epilepsy.
4.2.6 Ethical and legal frameworks
Establishing robust ethical and legal guidelines is essential to ensuring compliance and protecting patient rights. Transparent reporting of clinical trial data, public disclosure of research protocols, and rigorous oversight will foster trust and standardization. As regulations evolve, stem cell research and use of those cells in epilepsy must align with international ethical standards, balancing innovation with societal and moral considerations.
5 Conclusion
Stem cell therapy for epilepsy, as an emerging therapeutic strategy, has demonstrated significant potential and promise in preclinical studies and early-phase clinical trials. It offers multifaceted mechanisms—including differentiation into interneurons, antioxidant stress mitigation, secretion of cytokines and EVs, neuroprotection, and immunomodulation—to alleviate clinical symptoms, partially restore neural function, reduce seizure frequency, and delay disease progression. However, addressing existing challenges, such as optimizing safety and efficacy, remains critical to ensuring successful clinical translation. Future research will further our understanding of stem cell biology and accelerate their widespread use in epilepsy treatment. Innovations in this field will prioritize precision-based, personalized, and safe approaches, with interdisciplinary collaboration and technological advances serving as key drivers. As research and technology evolve, stem cell therapy may provide more effective and safer treatment options for patients with epilepsy. With progress in regenerative medicine, stem cell-based approaches hold promise as viable therapies for diverse neurological disorders, ultimately improving the quality of life of patients and their families.
Author contributions
ZW: Writing – original draft, Writing – review & editing. YM: Writing – review & editing. XH: Writing – review & editing. YX: Investigation, Resources, Funding acquisition, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the National Natural Science Foundation of China (No. 82460268) and the Hainan Province Clinical Medical Research Center (Nos. LCYX202410, LCYX202309).
Acknowledgments
Illustrations were created with BioGDP.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fisher, RS, Acevedo, C, Arzimanoglou, A, Bogacz, A, Cross, JH, Elger, CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55:475–82. doi: 10.1111/epi.12550
2. Kanner, AM, and Bicchi, MM. Antiseizure medications for adults with epilepsy: a review. JAMA. (2022) 327:1269–81. doi: 10.1001/jama.2022.3880
3. Ma, YN, Xia, Y, Karako, K, Song, P, and Hu, X. Extrachromosomal DNA: molecular perspectives in aging and neurodegenerative diseases. Intractable Rare Dis Res. (2024) 13:251–4. doi: 10.5582/irdr.2024.01058
4. Ma, YN, Xia, Y, Karako, K, Song, P, Tang, W, and Hu, X. Serum proteomics reveals early biomarkers of Alzheimer's disease: the dual role of APOE-epsilon4. Biosci Trends. (2025) 19:1–9. doi: 10.5582/bst.2024.01365
5. Devinsky, O, Vezzani, A, O'Brien, TJ, Jette, N, Scheffer, IE, de Curtis, M, et al. Epilepsy. Nat Rev Dis Primers. (2018) 4:18024. doi: 10.1038/nrdp.2018.24
6. Trinka, E, Cock, H, Hesdorffer, D, Rossetti, AO, Scheffer, IE, Shinnar, S, et al. A definition and classification of status epilepticus--report of the ILAE task force on classification of status epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
7. Aguglia, U, Sueri, C, Gasparini, S, Beghi, E, Labate, A, Gambardella, A, et al. Relevance of clinical context in the diagnostic-therapeutic approach to status epilepticus. Epilepsia. (2016) 57:1527–9. doi: 10.1111/epi.13475
8. Hu, Y, Peng, X, and Wang, X. Therapeutic approaches for super-refractory status epilepticus: an update of the literature. Expert Rev Neurother. (2025) 25:929–37. doi: 10.1080/14737175.2025.2524103
9. Shorvon, S, and Ferlisi, M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. (2011) 134:2802–18. doi: 10.1093/brain/awr215
10. Pitkanen, A, Lukasiuk, K, Dudek, FE, and Staley, KJ. Epileptogenesis. Cold Spring Harb Perspect Med. (2015) 5:5. doi: 10.1101/cshperspect.a022822
11. de Lanerolle, NC, Kim, JH, Williamson, A, Spencer, SS, Zaveri, HP, Eid, T, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. (2003) 44:677–87. doi: 10.1046/j.1528-1157.2003.32701.x
12. Purnell, BS, Alves, M, and Boison, D. Astrocyte-neuron circuits in epilepsy. Neurobiol Dis. (2023) 179:106058. doi: 10.1016/j.nbd.2023.106058
13. Shetty, AK, and Upadhya, D. GABA-ergic cell therapy for epilepsy: advances, limitations and challenges. Neurosci Biobehav Rev. (2016) 62:35–47. doi: 10.1016/j.neubiorev.2015.12.014
14. Akyuz, E, Polat, AK, Eroglu, E, Kullu, I, Angelopoulou, E, and Paudel, YN. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. (2021) 265:118826. doi: 10.1016/j.lfs.2020.118826
15. Patel, M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. (2004) 37:1951–62. doi: 10.1016/j.freeradbiomed.2004.08.021
16. Su, Y, Cao, N, Zhang, D, and Wang, M. The effect of ferroptosis-related mitochondrial dysfunction in the development of temporal lobe epilepsy. Ageing Res Rev. (2024) 96:102248. doi: 10.1016/j.arr.2024.102248
17. Luo, Q, Xian, P, Wang, T, Wu, S, Sun, T, Wang, W, et al. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics. (2021) 11:5986–6005. doi: 10.7150/thno.58632
18. Su, H, Wang, Z, Zhou, L, Liu, D, and Zhang, N. Regulation of the Nrf2/HO-1 axis by mesenchymal stem cells-derived extracellular vesicles: implications for disease treatment. Front Cell Dev Biol. (2024) 12:1397954. doi: 10.3389/fcell.2024.1397954
19. Vezzani, A, Balosso, S, and Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. (2019) 15:459–72. doi: 10.1038/s41582-019-0217-x
20. Jiao, D, Xu, L, Gu, Z, Yan, H, Shen, D, and Gu, X. Pathogenesis, diagnosis, and treatment of epilepsy: electromagnetic stimulation-mediated neuromodulation therapy and new technologies. Neural Regen Res. (2025) 20:917–35. doi: 10.4103/NRR.NRR-D-23-01444
21. Bleakley, LE, McKenzie, CE, Soh, MS, Forster, IC, Pinares-Garcia, P, Sedo, A, et al. Cation leak underlies neuronal excitability in an HCN1 developmental and epileptic encephalopathy. Brain. (2021) 144:2060–73. doi: 10.1093/brain/awab145
22. Loscher, W, Potschka, H, Sisodiya, SM, and Vezzani, A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. (2020) 72:606–38. doi: 10.1124/pr.120.019539
23. Cossu, M, Fuschillo, D, Casaceli, G, Pelliccia, V, Castana, L, Mai, R, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases. J Neurosurg. (2015) 123:1358–67. doi: 10.3171/2014.12.JNS141968
24. Alayli, A, Lockard, G, Gordon, J, Connolly, J, Monsour, M, Schimmel, S, et al. Stem cells: recent developments redefining epilepsy therapy. Cell Transplant. (2023) 32:9636897231158967. doi: 10.1177/09636897231158967
25. Lee, DH, Lee, EC, Lee, JY, Lee, MR, Shim, JW, and Oh, JS. Neuronal cell differentiation of iPSCs for the clinical treatment of neurological diseases. Biomedicine. (2024) 12:1350. doi: 10.3390/biomedicines12061350
26. Bershteyn, M, Broer, S, Parekh, M, Maury, Y, Havlicek, S, Kriks, S, et al. Human pallial MGE-type GABAergic interneuron cell therapy for chronic focal epilepsy. Cell Stem Cell. (2023) 30:1331–1350.e11. doi: 10.1016/j.stem.2023.08.013
27. Tremblay, R, Lee, S, and Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. (2016) 91:260–92. doi: 10.1016/j.neuron.2016.06.033
28. Waloschkova, E, Gonzalez-Ramos, A, Mikroulis, A, Kudlacek, J, Andersson, M, Ledri, M, et al. Human stem cell-derived GABAergic interneurons establish efferent synapses onto host neurons in rat epileptic Hippocampus and inhibit spontaneous recurrent seizures. Int J Mol Sci. (2021) 22:13243. doi: 10.3390/ijms222413243
29. Mesraoua, B, Deleu, D, Kullmann, DM, Shetty, AK, Boon, P, Perucca, E, et al. Novel therapies for epilepsy in the pipeline. Epilepsy Behav. (2019) 97:282–90. doi: 10.1016/j.yebeh.2019.04.042
30. Lee, ST, Chu, K, Jung, KH, Im, WS, Park, JE, Lim, HC, et al. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. (2009) 66:671–81. doi: 10.1002/ana.21788
31. Jeon, D, Chu, K, Lee, ST, Jung, KH, Kang, KM, Ban, JJ, et al. A cell-free extract from human adipose stem cells protects mice against epilepsy. Epilepsia. (2011) 52:1617–26. doi: 10.1111/j.1528-1167.2011.03182.x
32. Wang, L, Zhao, Y, Pan, X, Zhang, Y, Lin, L, Wu, Y, et al. Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. (2021) 1750:147121. doi: 10.1016/j.brainres.2020.147121
33. Wang, C, Zhang, J, Chen, W, Gao, L, He, J, and Xia, Y. Exosomal lncRNA RMRP-shuttled by olfactory mucosa-mesenchymal stem cells suppresses microglial Pyroptosis to improve spinal cord injury via EIF4A3/SIRT1. Mol Neurobiol. (2025) 62:8150–65. doi: 10.1007/s12035-025-04756-1
34. He, J, Peng, J, Li, Y, Jiang, J, Li, J, Lin, L, et al. SENP1 facilitates OM-MSC differentiation through activating OPTN-mediated mitophagy to mitigate the neurologic impairment following ICH. iScience. (2024) 27:109865. doi: 10.1016/j.isci.2024.109865
35. Ryu, JK, Cho, T, Wang, YT, and McLarnon, JG. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J Neuroinflammation. (2009) 6:39. doi: 10.1186/1742-2094-6-39
36. Salari, V, Mengoni, F, Del Gallo, F, Bertini, G, and Fabene, PF. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: possible treatments and future perspectives. Int J Mol Sci. (2020) 21:21. doi: 10.3390/ijms21249683
37. Thomson, JA, Itskovitz-Eldor, J, Shapiro, SS, Waknitz, MA, Swiergiel, JJ, Marshall, VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. (1998) 282:1145–7. doi: 10.1126/science.282.5391.1145
38. Golchin, A, Chatziparasidou, A, Ranjbarvan, P, Niknam, Z, and Ardeshirylajimi, A. Embryonic stem cells in clinical trials: Current overview of developments and challenges. Adv Exp Med Biol. (2021) 1312:19–37. doi: 10.1007/5584_2020_592
39. Chagastelles, PC, and Nardi, NB. Biology of stem cells: an overview. Kidney Int Suppl. (2011) 1:63–7. doi: 10.1038/kisup.2011.15
40. Ge, F, and Du, L. Study and application of multidirectional differentiation potential of dental pulp stem cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. (2019) 36:172–6. doi: 10.7507/1001-5515.201804045
41. Takahashi, K, and Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) 126:663–76. doi: 10.1016/j.cell.2006.07.024
42. Stoberl, N, Maguire, E, Salis, E, Shaw, B, and Hall-Roberts, H. Human iPSC-derived glia models for the study of neuroinflammation. J Neuroinflammation. (2023) 20:231. doi: 10.1186/s12974-023-02919-2
43. Takahashi, K, Tanabe, K, Ohnuki, M, Narita, M, Ichisaka, T, Tomoda, K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
44. Aboul-Soud, MAM, Alzahrani, AJ, and Mahmoud, A. Induced pluripotent stem cells (iPSCs)-roles in regenerative therapies, disease modelling and drug screening. Cells. (2021) 10:10. doi: 10.3390/cells10092319
45. Pan, Y, Lin, H, Chung, M, Yang, Y, Zhang, L, Pan, X, et al. Generation of phenotypically stable and functionally mature human bone marrow MSCs derived Schwann cells via the induction of human iPSCs-derived sensory neurons. Stem Cell Res Ther. (2025) 16:106. doi: 10.1186/s13287-025-04217-5
46. Abe, K, Yamashita, T, Takizawa, S, Kuroda, S, Kinouchi, H, and Kawahara, N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow Metab. (2012) 32:1317–31. doi: 10.1038/jcbfm.2011.187
47. Smith, AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. (2001) 17:435–62. doi: 10.1146/annurev.cellbio.17.1.435
48. Yamanaka, S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. (2020) 27:523–31. doi: 10.1016/j.stem.2020.09.014
49. Du, P, and Wu, J. Hallmarks of totipotent and pluripotent stem cell states. Cell Stem Cell. (2024) 31:312–33. doi: 10.1016/j.stem.2024.01.009
50. Morrissey, JB, Cheng, RY, Davoudi, S, and Gilbert, PM. Biomechanical origins of muscle stem cell signal transduction. J Mol Biol. (2016) 428:1441–54. doi: 10.1016/j.jmb.2015.05.004
51. Orkin, SH, and Zon, LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. (2008) 132:631–44. doi: 10.1016/j.cell.2008.01.025
52. Ross, JB, Myers, LM, Noh, JJ, Collins, MM, Carmody, AB, Messer, RJ, et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature. (2024) 628:162–70. doi: 10.1038/s41586-024-07238-x
53. Sakurai, M, Ishitsuka, K, Ito, R, Wilkinson, AC, Kimura, T, Mizutani, E, et al. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature. (2023) 615:127–33. doi: 10.1038/s41586-023-05739-9
54. Ghanta, MK, Merchant, N, and Bhaskar, L. A review on hematopoietic stem cell treatment for epilepsy. CNS Neurol Disord Drug Targets. (2021) 20:644–56. doi: 10.2174/1871527320666210218085816
55. Bond, AM, Ming, GL, and Song, H. Ontogeny of adult neural stem cells in the mammalian brain. Curr Top Dev Biol. (2021) 142:67–98. doi: 10.1016/bs.ctdb.2020.11.002
56. Willis, CM, Nicaise, AM, Peruzzotti-Jametti, L, and Pluchino, S. The neural stem cell secretome and its role in brain repair. Brain Res. (2020) 1729:146615. doi: 10.1016/j.brainres.2019.146615
57. Zhang, Q, Li, J, An, W, Fan, Y, and Cao, Q. Neural stem cell secretome and its role in the treatment of neurodegenerative disorders. J Integr Neurosci. (2020) 19:179–85. doi: 10.31083/j.jin.2020.01.1142
58. Shoemaker, LD, and Kornblum, HI. Neural stem cells (NSCs) and proteomics. Mol Cell Proteomics. (2016) 15:344–54. doi: 10.1074/mcp.O115.052704
59. Dause, TJ, Denninger, JK, Smith, BM, and Kirby, ED. The neural stem cell secretome across neurodevelopment. Exp Neurol. (2022) 355:114142. doi: 10.1016/j.expneurol.2022.114142
60. Pittenger, MF, Mackay, AM, Beck, SC, Jaiswal, RK, Douglas, R, Mosca, JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. (1999) 284:143–7. doi: 10.1126/science.284.5411.143
61. Rodriguez-Fuentes, DE, Fernandez-Garza, LE, Samia-Meza, JA, Barrera-Barrera, SA, Caplan, AI, and Barrera-Saldana, HA. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res. (2021) 52:93–101. doi: 10.1016/j.arcmed.2020.08.006
62. Shoja, A, Sani, M, Mirzohreh, ST, Ebrahimi, MJ, Moafi, M, Balaghirad, N, et al. Dental stem cells improve memory and reduce cell death in rat seizure model. Anat Sci Int. (2024) 100:37–53. doi: 10.1007/s12565-024-00781-7
63. Slavin, S, Kurkalli, BG, and Karussis, D. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg. (2008) 110:943–6. doi: 10.1016/j.clineuro.2008.01.014
64. Trounson, A, Thakar, RG, Lomax, G, and Gibbons, D. Clinical trials for stem cell therapies. BMC Med. (2011) 9:52. doi: 10.1186/1741-7015-9-52
65. Lalu, MM, McIntyre, L, Pugliese, C, Fergusson, D, Winston, BW, Marshall, JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. (2012) 7:e47559. doi: 10.1371/journal.pone.0047559
66. Donega, V, Nijboer, CH, van Tilborg, G, Dijkhuizen, RM, Kavelaars, A, and Heijnen, CJ. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. (2014) 261:53–64. doi: 10.1016/j.expneurol.2014.06.009
67. Oh, JY, Kim, TW, Jeong, HJ, Lee, HJ, Ryu, JS, Wee, WR, et al. Intraperitoneal infusion of mesenchymal stem/stromal cells prevents experimental autoimmune uveitis in mice. Mediat Inflamm. (2014) 2014:624640. doi: 10.1155/2014/624640
68. Lim, MH, Ong, WK, and Sugii, S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev Mol Med. (2014) 16:e8. doi: 10.1017/erm.2014.8
69. Liu, J, Yu, F, Sun, Y, Jiang, B, Zhang, W, Yang, J, et al. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. (2015) 33:627–38. doi: 10.1002/stem.1909
70. Wang, H, and Dwamena, A. Olfactory Ecto-mesenchymal stem cells in modeling and treating Alzheimer's disease. Int J Mol Sci. (2024) 25:25. doi: 10.3390/ijms25158492
71. Hernandez, R, Jimenez-Luna, C, Perales-Adan, J, Perazzoli, G, Melguizo, C, and Prados, J. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther (Seoul). (2020) 28:34–44. doi: 10.4062/biomolther.2019.065
72. Ni, WF, Wu, AM, Li, QL, Huang, ZY, Xu, HZ, and Yin, LH. Induced human bone marrow stromal cells differentiate into neural cells by bFGF and cocultured with olfactory ensheathing cells. Curr Stem Cell Res Ther. (2014) 9:291–6. doi: 10.2174/1574888x09666140115114350
73. Alizadeh, R, Kamrava, SK, Bagher, Z, Farhadi, M, Falah, M, Moradi, F, et al. Human olfactory stem cells: as a promising source of dopaminergic neuron-like cells for treatment of Parkinson's disease. Neurosci Lett. (2019) 696:52–9. doi: 10.1016/j.neulet.2018.12.011
74. Feron, F, Perry, C, Girard, SD, and Mackay-Sim, A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol. (2013) 1059:107–14. doi: 10.1007/978-1-62703-574-3_10
75. Hunt, RF, and Baraban, SC. Interneuron transplantation as a treatment for epilepsy. Cold Spring Harb Perspect Med. (2015) 5:a022376. doi: 10.1101/cshperspect.a022376
76. Baraban, SC, Southwell, DG, Estrada, RC, Jones, DL, Sebe, JY, Alfaro-Cervello, C, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci USA. (2009) 106:15472–7. doi: 10.1073/pnas.0900141106
77. Lim, L, Mi, D, Llorca, A, and Marin, O. Development and functional diversification of cortical interneurons. Neuron. (2018) 100:294–313. doi: 10.1016/j.neuron.2018.10.009
78. Hu, JS, Vogt, D, Sandberg, M, and Rubenstein, JL. Cortical interneuron development: a tale of time and space. Development. (2017) 144:3867–78. doi: 10.1242/dev.132852
79. Gonzalez-Ramos, A, Waloschkova, E, Mikroulis, A, Kokaia, Z, Bengzon, J, Ledri, M, et al. Human stem cell-derived GABAergic neurons functionally integrate into human neuronal networks. Sci Rep. (2021) 11:22050. doi: 10.1038/s41598-021-01270-x
80. Akerud, P, Canals, JM, Snyder, EY, and Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci. (2001) 21:8108–18. doi: 10.1523/JNEUROSCI.21-20-08108.2001
81. Hoane, MR, Gulwadi, AG, Morrison, S, Hovanesian, G, Lindner, MD, and Tao, W. Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Exp Neurol. (1999) 160:235–43. doi: 10.1006/exnr.1999.7175
82. Varvel, NH, Neher, JJ, Bosch, A, Wang, W, Ransohoff, RM, Miller, RJ, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci USA. (2016) 113:E5665–74. doi: 10.1073/pnas.1604263113
83. Zhang, ZG, Buller, B, and Chopp, M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. (2019) 15:193–203. doi: 10.1038/s41582-018-0126-4
84. Li, Y, Jiang, J, Li, J, Liu, S, Wang, C, Yu, Z, et al. Exosome-derived CDC42 from hypoxia-pretreated neural stem cells inhibits ACSL4-related ferroptosis to alleviate vascular injury in Parkinson's disease mice models. J Neurochem. (2025) 169:e70027. doi: 10.1111/jnc.70027
85. Rybova, J, Sundararajan, T, Kuchar, L, Dlugi, TA, Ruzicka, P, McKillop, WM, et al. Hematopoietic stem cell transplantation leads to biochemical and functional correction in two mouse models of acid ceramidase deficiency. Mol Ther. (2024) 32:3402–21. doi: 10.1016/j.ymthe.2024.08.004
86. de Lanerolle, NC, Kim, JH, Robbins, RJ, and Spencer, DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. (1989) 495:387–95. doi: 10.1016/0006-8993(89)90234-5
87. Miri, ML, Vinck, M, Pant, R, and Cardin, JA. Altered hippocampal interneuron activity precedes ictal onset. eLife. (2018) 7:7. doi: 10.7554/eLife.40750
88. Cunningham, M, Cho, JH, Leung, A, Savvidis, G, Ahn, S, Moon, M, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. (2014) 15:559–73. doi: 10.1016/j.stem.2014.10.006
89. Upadhya, D, Hattiangady, B, Castro, OW, Shuai, B, Kodali, M, Attaluri, S, et al. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci USA. (2019) 116:287–96. doi: 10.1073/pnas.1814185115
90. Stewart, E, Catroppa, C, and Lah, S. Theory of mind in patients with epilepsy: a systematic review and Meta-analysis. Neuropsychol Rev. (2016) 26:3–24. doi: 10.1007/s11065-015-9313-x
91. Hattiangady, B, and Shetty, AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. (2010) 20:97–112. doi: 10.1002/hipo.20594
92. Lenck-Santini, PP, and Scott, RC. Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harb Perspect Med. (2015) 5:a022772. doi: 10.1101/cshperspect.a022772
93. Topolnik, L, and Tamboli, S. The role of inhibitory circuits in hippocampal memory processing. Nat Rev Neurosci. (2022) 23:476–92. doi: 10.1038/s41583-022-00599-0
94. Hattiangady, B, Kuruba, R, Shuai, B, Grier, R, and Shetty, AK. Hippocampal neural stem cell grafting after status epilepticus alleviates chronic epilepsy and abnormal plasticity, and maintains better memory and mood function. Aging Dis. (2020) 11:1374–94. doi: 10.14336/AD.2020.1020
95. UCSD AP. (2023) Pioneering stem cell therapy offers new Hope for epilepsy treatment. Available online at: https://neurosciencenews.com/epilepsy-stem-cells-23703/ (Accessed July 26, 2023).
96. Neurona Therapeutics. Neurona therapeutics presents positive clinical update from NRTX-1001 cell therapy trial in adults with drug-resistant focal epilepsy at American Academy of Neurology (AAN) 2024 annual meeting. (2024). Available online at: https://www.neuronatherapeutics.com/news/press-releases/041524/ (Accessed April 15, 2023).
97. Skog, J, Wurdinger, T, van Rijn, S, Meijer, DH, Gainche, L, Sena-Esteves, M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. (2008) 10:1470–6. doi: 10.1038/ncb1800
98. Beer, L, Mildner, M, and Ankersmit, HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Ann Transl Med. (2017) 5:170. doi: 10.21037/atm.2017.03.50
99. Ma, YN, Hu, X, Karako, K, Song, P, Tang, W, and Xia, Y. Exploring the multiple therapeutic mechanisms and challenges of mesenchymal stem cell-derived exosomes in Alzheimer's disease. Biosci Trends. (2024) 18:413–30. doi: 10.5582/bst.2024.01306
100. El Moshy, S, Radwan, IA, Rady, D, Abbass, MMS, El-Rashidy, AA, Sadek, KM, et al. Dental stem cell-derived Secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cells Int. (2020) 2020:1–29. doi: 10.1155/2020/7593402
101. Pawitan, JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. (2014) 2014:965849. doi: 10.1155/2014/965849
102. Waldau, B, Hattiangady, B, Kuruba, R, and Shetty, AK. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. (2010) 28:1153–64. doi: 10.1002/stem.446
103. Andreska, T, Rauskolb, S, Schukraft, N, Luningschror, P, Sasi, M, Signoret-Genest, J, et al. Induction of BDNF expression in layer II/III and layer V neurons of the motor cortex is essential for motor learning. J Neurosci. (2020) 40:6289–308. doi: 10.1523/JNEUROSCI.0288-20.2020
104. Hattiangady, B, and Shetty, AK. Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl Med. (2012) 1:696–708. doi: 10.5966/sctm.2012-0050
105. Liu, ZZ, Huang, Y, Hong, CG, Wang, X, Duan, R, Liu, JY, et al. Autologous olfactory mucosa mesenchymal stem cells treatment improves the neural network in chronic refractory epilepsy. Stem Cell Res Ther. (2023) 14:237. doi: 10.1186/s13287-023-03458-6
106. Shi, L, Sun, Z, Su, W, Xu, F, Xie, D, Zhang, Q, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. (2021) 54:1527–1542.e8. doi: 10.1016/j.immuni.2021.04.022
107. Shi, Y, Wang, Y, Li, Q, Liu, K, Hou, J, Shao, C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
108. Ela, S, Mager, I, Breakefield, XO, and Wood, MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. (2013) 12:347–57. doi: 10.1038/nrd3978
109. Drommelschmidt, K, Serdar, M, Bendix, I, Herz, J, Bertling, F, Prager, S, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. (2017) 60:220–32. doi: 10.1016/j.bbi.2016.11.011
110. Yang, Y, Ye, Y, Su, X, He, J, Bai, W, and He, X. MSCs-derived exosomes and Neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. (2017) 11:55. doi: 10.3389/fncel.2017.00055
111. Chen, Z, Zhang, C, Fang, Y, Zhang, H, Luo, J, Miao, C, et al. Olfactory mucosa-mesenchymal stem cells with overexpressed Nrf2 modulate angiogenesis and exert anti-inflammation effect in an in vitro traumatic brain injury model. Eur J Med Res. (2025) 30:80. doi: 10.1186/s40001-025-02344-6
112. Si, YL, Zhao, YL, Hao, HJ, Fu, XB, and Han, WD. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. (2011) 10:93–103. doi: 10.1016/j.arr.2010.08.005
113. Xian, P, Hei, Y, Wang, R, Wang, T, Yang, J, Li, J, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. (2019) 9:5956–75. doi: 10.7150/thno.33872
114. Long, Q, Upadhya, D, Hattiangady, B, Kim, DK, An, SY, Shuai, B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA. (2017) 114:E3536–45. doi: 10.1073/pnas.1703920114
115. Hattiangady, B, Rao, MS, and Shetty, AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. (2004) 17:473–90. doi: 10.1016/j.nbd.2004.08.008
116. Hattiangady, B, Rao, MS, and Shetty, AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. (2008) 212:468–81. doi: 10.1016/j.expneurol.2008.04.040
117. Huang, PY, Shih, YH, Tseng, YJ, Ko, TL, Fu, YS, and Lin, YY. Xenograft of human umbilical mesenchymal stem cells from Wharton's jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav Immun. (2016) 54:45–58. doi: 10.1016/j.bbi.2015.12.021
118. Okazaki, MM, Evenson, DA, and Nadler, JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. (1995) 352:515–34. doi: 10.1002/cne.903520404
119. Fukumura, S, Sasaki, M, Kataoka-Sasaki, Y, Oka, S, Nakazaki, M, Nagahama, H, et al. Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Res. (2018) 141:56–63. doi: 10.1016/j.eplepsyres.2018.02.008
120. Shu, X, Du, S, Chen, X, and Li, Z. Transplantation of neural stem cells overexpressing cardiotrophin-1 inhibits sprouting of hippocampal mossy fiber in a rat model of status epilepticus. Cell Biochem Biophys. (2011) 61:367–70. doi: 10.1007/s12013-011-9219-z
121. Cavarsan, CF, Malheiros, J, Hamani, C, Najm, I, and Covolan, L. Is mossy Fiber sprouting a potential therapeutic target for epilepsy? Front Neurol. (2018) 9:1023. doi: 10.3389/fneur.2018.01023
122. Paradiso, B, Zucchini, S, Su, T, Bovolenta, R, Berto, E, Marconi, P, et al. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus. Epilepsia. (2011) 52:572–8. doi: 10.1111/j.1528-1167.2010.02930.x
123. Decimo, I, Bifari, F, Krampera, M, and Fumagalli, G. Neural stem cell niches in health and diseases. Curr Pharm Des. (2012) 18:1755–83. doi: 10.2174/138161212799859611
124. Shetty, AK. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav. (2014) 38:117–24. doi: 10.1016/j.yebeh.2013.12.001
125. Wang, L, Chang, X, She, L, Xu, D, Huang, W, and Poo, MM. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. (2015) 35:8384–93. doi: 10.1523/JNEUROSCI.4682-14.2015
126. Waterhouse, EG, An, JJ, Orefice, LL, Baydyuk, M, Liao, GY, Zheng, K, et al. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. (2012) 32:14318–30. doi: 10.1523/JNEUROSCI.0709-12.2012
127. Fisher, ES, Amarante, MA, Lowry, N, Lotz, S, Farjood, F, Temple, S, et al. Single cell profiling of CD45(+) spinal cord cells reveals microglial and B cell heterogeneity and crosstalk following spinal cord injury. J Neuroinflammation. (2022) 19:266. doi: 10.1186/s12974-022-02627-3
128. Araki, T, Ikegaya, Y, and Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur J Neurosci. (2021) 54:5880–901. doi: 10.1111/ejn.14969
129. Vizoso, FJ, Costa, LA, and Eiro, N. New era of mesenchymal stem cell-based medicine: basis, challenges and prospects. Rev Clin Esp (Barc). (2023) 223:619–28. doi: 10.1016/j.rceng.2023.11.002
130. Mei, R, Wan, Z, Yang, C, Shen, X, Wang, R, Zhang, H, et al. Advances and clinical challenges of mesenchymal stem cell therapy. Front Immunol. (2024) 15:1421854. doi: 10.3389/fimmu.2024.1421854
131. Heris, RM, Shirvaliloo, M, Abbaspour-Aghdam, S, Hazrati, A, Shariati, A, Youshanlouei, HR, et al. The potential use of mesenchymal stem cells and their exosomes in Parkinson's disease treatment. Stem Cell Res Ther. (2022) 13:371. doi: 10.1186/s13287-022-03050-4
132. Wei, L, Yan, W, Shah, W, Zhang, Z, Wang, M, Liu, B, et al. Advancements and challenges in stem cell transplantation for regenerative medicine. Heliyon. (2024) 10:e35836. doi: 10.1016/j.heliyon.2024.e35836
133. Krsek, A, Ostojic, L, Zivalj, D, and Baticic, L. Navigating the Neuroimmunomodulation frontier: pioneering approaches and promising horizons-a comprehensive review. Int J Mol Sci. (2024) 25:25. doi: 10.3390/ijms25179695
134. Alnasser, SM, Alrobian, AS, Alfayez, MS, Almutairi, OT, Almutairi, SS, and Alkeraidees, TS. Pharmacological modulation of stem cells signaling pathway for therapeutic applications. Stem Cell Res Ther. (2025) 16:327. doi: 10.1186/s13287-025-04438-8
135. Guo, L, Lee, HK, Oh, S, Koirala, GR, and Kim, TI. Smart bioelectronics for real-time diagnosis and therapy of body organ functions. ACS Sens. (2025) 10:3239–73. doi: 10.1021/acssensors.5c00024
136. Wang, Z. Assessing Tumorigenicity in stem cell-derived therapeutic products: a critical step in safeguarding regenerative medicine. Bioengineering (Basel). (2023) 10:10. doi: 10.3390/bioengineering10070857
137. Moy, AB, Kamath, A, Ternes, S, and Kamath, J. The challenges to advancing induced pluripotent stem cell-dependent cell replacement therapy. Med Res Arch. (2023) 11:11. doi: 10.18103/mra.v11i11.4784
138. Assen, LS, Jongsma, KR, Isasi, R, Tryfonidou, MA, and Bredenoord, AL. Recognizing the ethical implications of stem cell research: a call for broadening the scope. Stem Cell Reports. (2021) 16:1656–61. doi: 10.1016/j.stemcr.2021.05.021
139. Liu, Z, Pan, C, and Huang, H. The role of axon guidance molecules in the pathogenesis of epilepsy. Neural Regen Res. (2025) 20:1244–57. doi: 10.4103/NRR.NRR-D-23-01620
140. Shetty, AK, and Hattiangady, B. Concise review: prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. (2007) 25:2396–407. doi: 10.1634/stemcells.2007-0313
141. Gonzalez-Ramos, A, Puigsasllosas-Pastor, C, Arcas-Marquez, A, and Tornero, D. Updated toolbox for assessing neuronal network reconstruction after cell therapy. Bioengineering (Basel). (2024) 11:11. doi: 10.3390/bioengineering11050487
142. Trucillo, P. Biomaterials for drug delivery and human applications. Materials (Basel). (2024) 17:456. doi: 10.3390/ma17020456
143. Maestas, MM, Bui, MH, and Millman, JR. Recent progress in modeling and treating diabetes using stem cell-derived islets. Stem Cells Transl Med. (2024) 13:949–58. doi: 10.1093/stcltm/szae059
144. Wang, S, Du, Y, Zhang, B, Meng, G, Liu, Z, Liew, SY, et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell. (2024) 187:6152–6164.e18. doi: 10.1016/j.cell.2024.09.004
145. Miklosz, A, and Chabowski, A. Efficacy of adipose-derived mesenchymal stem cell therapy in the treatment of chronic micro- and macrovascular complications of diabetes. Diabetes Obes Metab. (2024) 26:793–808. doi: 10.1111/dom.15375
146. Zhou, W, Wang, X, Dong, Y, Gao, P, Zhao, X, Wang, M, et al. Stem cell-derived extracellular vesicles in the therapeutic intervention of Alzheimer's disease, Parkinson's disease, and stroke. Theranostics. (2024) 14:3358–84. doi: 10.7150/thno.95953
147. Jiang, S, Wang, H, Yang, C, Feng, F, Xu, D, Zhang, M, et al. Phase 1 study of safety and preliminary efficacy of intranasal transplantation of human neural stem cells (ANGE-S003) in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2024) 95:1102–11. doi: 10.1136/jnnp-2023-332921
148. Barabadi, M, Paton, MCB, Kumar, N, Lim, R, and Payne, NL. Stem cell derived extracellular vesicle therapy for multiple sclerosis, a systematic review and Meta-analysis of preclinical studies. Stem Cells Transl Med. (2024) 13:436–47. doi: 10.1093/stcltm/szae011
149. Matchett, KB, Lynam-Lennon, N, Watson, RW, and Brown, JAL. Advances in precision medicine: tailoring individualized therapies. Cancers (Basel). (2017) 9:9. doi: 10.3390/cancers9110146
150. Tiwari, D, Peariso, K, and Gross, C. MicroRNA-induced silencing in epilepsy: opportunities and challenges for clinical application. Dev Dyn. (2018) 247:94–110. doi: 10.1002/dvdy.24582
151. Carpenter, JC, and Lignani, G. Gene editing and modulation: the holy grail for the genetic epilepsies? Neurotherapeutics. (2021) 18:1515–23. doi: 10.1007/s13311-021-01081-y
152. Ahmad, SR, Zeyaullah, M, AlShahrani, AM, Dawria, A, Ali, H, Mohieldin, A, et al. Deciphering the enigma of neuron-glial interactions in neurological disorders. Front Biosci (Landmark Ed). (2024) 29:142. doi: 10.31083/j.fbl2904142
153. Lybrand, ZR, Goswami, S, and Hsieh, J. Stem cells: a path towards improved epilepsy therapies. Neuropharmacology. (2020) 168:107781. doi: 10.1016/j.neuropharm.2019.107781
154. Stouffer, MA, Golden, JA, and Francis, F. Neuronal migration disorders: focus on the cytoskeleton and epilepsy. Neurobiol Dis. (2016) 92:18–45. doi: 10.1016/j.nbd.2015.08.003
155. Garcia-Penas, JJ. Epilepsy, cognition and ketogenic diet. Rev Neurol. (2018) 66:S71–5. doi: 10.33588/rn.66S01.2017529
156. Tesiye, MR, Gol, M, Fadardi, MR, Kani, SNM, Costa, AM, Ghasemi-Kasman, M, et al. Therapeutic potential of mesenchymal stem cells in the treatment of epilepsy and their interaction with Antiseizure medications. Cells. (2022) 11:11. doi: 10.3390/cells11244129
157. Barzegar, M, Afghan, M, Tarmahi, V, Behtari, M, Rahimi Khamaneh, S, and Raeisi, S. Ketogenic diet: overview, types, and possible anti-seizure mechanisms. Nutr Neurosci. (2021) 24:307–16. doi: 10.1080/1028415X.2019.1627769
158. Vakilna, YS, Chaitanya, G, Hafeez, MU, Ilyas, A, Saranathan, M, Gavvala, J, et al. Pulvinar neuromodulation for seizure monitoring and network modulation in temporal plus epilepsy. Ann Clin Transl Neurol. (2023) 10:1254–9. doi: 10.1002/acn3.51815
159. Zhu, Q, Mishra, A, Park, JS, Liu, D, Le, DT, Gonzalez, SZ, et al. Human cortical interneurons optimized for grafting specifically integrate, abort seizures, and display prolonged efficacy without over-inhibition. Neuron. (2023) 111:e7. doi: 10.1016/j.neuron.2022.12.014
160. Hlebokazov, F, Dakukina, T, Ihnatsenko, S, Kosmacheva, S, Potapnev, M, Shakhbazau, A, et al. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Adv Med Sci. (2017) 62:273–9. doi: 10.1016/j.advms.2016.12.004
161. DaCosta, JC, Portuguez, MW, Marinowic, DR, Schilling, LP, Torres, CM, DaCosta, DI, et al. Safety and seizure control in patients with mesial temporal lobe epilepsy treated with regional superselective intra-arterial injection of autologous bone marrow mononuclear cells. J Tissue Eng Regen Med. (2018) 12:e648–56. doi: 10.1002/term.2334
162. Szczepanik, E, Mierzewska, H, Antczak-Marach, D, Figiel-Dabrowska, A, Terczynska, I, Tryfon, J, et al. Intrathecal infusion of autologous adipose-derived regenerative cells in autoimmune refractory epilepsy: evaluation of safety and efficacy. Stem Cells Int. (2020) 2020:1–16. doi: 10.1155/2020/7104243
163. Milczarek, O, Jarocha, D, Starowicz-Filip, A, Kasprzycki, M, Kijowski, J, Mordel, A, et al. Bone marrow nucleated cells and bone marrow-derived CD271+ mesenchymal stem cell in treatment of encephalopathy and drug-resistant epilepsy. Stem Cell Rev Rep. (2024) 20:1015–25. doi: 10.1007/s12015-023-10673-4
Keywords: stem cell transplantation, refractory epilepsy, modulation of oxidative stress, inhibitory interneurons, clinical translation
Citation: Wang Z, Ma Y, Hu X and Xia Y (2025) Rewiring the seizing brain: stem cell grafts as neuromodulatory architects in epilepsy therapy. Front. Neurol. 16:1596484. doi: 10.3389/fneur.2025.1596484
Edited by:
Neil M. Fournier, Trent University, CanadaReviewed by:
Bharathi Hattiangady, Texas A&M University College of Medicine, United StatesJustin J. Botterill, University of Saskatchewan, Canada
Copyright © 2025 Wang, Ma, Hu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xia, eGlheWluZzAwOEAxNjMuY29t
 Zijie Wang
Zijie Wang Yanan Ma
Yanan Ma Xiqi Hu
Xiqi Hu Ying Xia
Ying Xia