Abstract
Background and aim:
Wilson’s disease (WD), an autosomal recessive copper metabolism defect, causes pathological copper deposition in hepatic and neurological systems, culminating in cirrhosis and neuropsychiatric manifestations. Our understanding of neurological deterioration in neurological WD patients following sodium dimercaptopropanesulfonate (DMPS) treatment is limited. Thus, this study aims to analyze the phenotypic spectrum and predictors of DMPS-induced neurological deterioration in neurological WD.
Methods:
Demographic (age, gender, weight), clinical (K-F ring, duration of illness), and biochemical parameters [alanine aminotransferase, aspartate aminotransferase, albumin, serum ceruloplasmin, blood urea nitrogen, serum creatinine, 24 h urinary copper, lactate, homocysteine (HCY)] were systematically evaluated alongside neuroimaging data, followed by receiver operating characteristic (ROC) curve analysis to identify predictive biomarkers for neurological deterioration in DMPS-induced neurological WD patients.
Results:
A total of 277 neurological WD patients were enrolled, among whom 24.5% (68/277) developed neurological deterioration. Notably, 70.6% (48/68) of the patients experiencing neurological worsening were male. Among the patients, 91.2% (62/68) exhibited mild deterioration, while 8.8% (6/68) experienced severe deterioration. Multivariate logistic regression analysis indicated that sex [odds ratio (OR) = 0.41[95% confidence interval (CI) = 0.18–0.94], p = 0.035], brain Magnetic Resonance Imaging (MRI) score (OR = 2.89[95% CI = 1.99–4.21], p < 0.001), and HCY (OR = 1.45[95% CI = 1.27–1.65], p < 0.001) were associated with neurological deterioration. Subgroup analysis revealed statistically significant differences in male proportion (36/19 vs. 75/84, p = 0.019), brain MRI score (median: 5 vs. 4, p < 0.001), and HCY levels (mean: 20.75 vs. 17.77, p < 0.001) between the deterioration and non-deterioration groups within the under-35 cohort. ROC analysis of composite biomarkers demonstrated significant predictive capacity for neurological deterioration in DMPS-induced neurological WD (AUC = 0.862).
Conclusion:
Neurological deterioration in DMPS-induced neurological WD patients is not rare and predominantly occurs in males. We identified three independent risk factors for this deterioration: sex, brain MRI score, and HCY. A composite risk model incorporating these parameters achieved superior predictive accuracy compared to individual biomarker.
Introduction
Wilson’s disease (WD), an autosomal recessive disorder resulting from pathogenic ATP7B mutations, disrupts copper homeostasis with toxic hepatic, cerebral, and corneal accumulation. The prevalence of this disorder in the Chinese population is approximately 5.87 in 100,000, with a higher incidence observed in younger individuals (1). Clinically, the disease primarily manifests with symptoms of hepatic impairment and neurological deficits, the latter of which include dystonia, tremors, bradykinesia, ataxia, cognitive impairments, and chorea-like movements. The use of copper chelators to reduce copper overload has been shown to be an effective method for improving neurological symptoms in WD patients (2). However, approximately 50% of patients with neurological WD continue to experience neurological symptoms during the copper chelation process (3, 4), and more than 30% of these patients experience neurological deterioration during the early stages of chelation therapy (5, 6).
Sodium dimercaptopropanesulfonate (DMPS) is a first-line copper chelator widely used among Chinese patients, exhibiting a copper-chelating efficacy 2.6 times greater than that of D-penicillamine (7). DMPS therapy has demonstrated efficacy in ameliorating hepatic dysfunction and neuropsychiatric manifestations, leading to its incorporation into the Clinical Practice Guidelines for WD in China (2022 edition) as an evidence-based recommendation for therapeutic decision-making. However, a proportion of WD patients experience paradoxical neurological decline following DMPS treatment, which can even be life-threatening. Zhang et al. (6) previously reported that WD patients undergoing DMPS therapy exhibited neurological deterioration, including tremors and speech disorders. This adverse reaction significantly affects the applicability of the intervention in specific populations. Furthermore, there is limited research on neurological deterioration associated with DMPS therapy. Therefore, the identification of predictive risk factors is essential for formulating individualized therapeutic approaches and mitigating the risk of neurological deterioration in WD patients.
This study explores clinical characteristics and risk factors of DMPS-induced neurological deterioration in neurological WD patients, offering an objective reference for clinical strategies. Identifying these risk factors can guide clinical decisions and patient management, emphasizing the need for personalized approaches in complex conditions.
Materials and methods
Study population
This retrospective study analyzed 277 neurologically predominant WD patients undergoing DMPS therapy at the First Affiliated Hospital of Anhui University of Chinese Medicine (Sept 2021–Oct 2023). The inclusion criteria were as follows: (a) Satisfaction with the Leipzig scoring system diagnostic criteria for WD, the Leipzig score ≥4 (8); (b) Neurological symptoms as the initial presentation, neurological score ≥ 1 regarded as neurological WD (9, 10); (c) The clinical data were complete. The exclusion criteria were as follows: (a) Severe hepatic or renal dysfunction; (b) Switching of chelation agents during treatment; (c) Undergoing splenectomy or splenic artery embolization during treatment; (d) Severe psychiatric disorders encompassing severe depressive episodes (with psychomotor impairment), bipolar affective disorder (manic or mixed episodes), and profound cognitive impairment that significantly impacted daily functioning (11); (e)Forced cessation of chelation due to significant disease exacerbation during treatment; (f) Allergy to the trial medicine.
This study was designed as case–control study and was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine (Approval No.: 2021AH-60).
Data collection
The clinical characteristics and laboratory biochemical indicators of patients were retrospectively collected using the Hospital Information System (HIS) system of the Information Center. These included age, weight, gender, duration of illness, Kayser-Fleischer (K-F) ring, serum ceruloplasmin (CER), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum creatinine (Scr), albumin (ALB), 24-h urinary copper, lactate, and homocysteine (HCY). Additionally, electronic medical records regarding clinical symptoms, signs, medication history, imaging data, and disease progression before and after treatment were also recorded.
In this study, we conducted a follow-up assessment of the recovery of patients with neurological deterioration. The follow-up began after the patients were discharged and lasted for 6 months, with assessments conducted every 3 months. Data were collected through telephone interviews, video calls via WeChat, and outpatient clinic visits, including patients’ survival status and disease recovery. Loss to follow-up was defined as the inability to contact the patient during the follow-up period. Definition of Recovery: “Recovery” was defined as a return to the patient’s pre-deterioration neurological baseline status, as assessed by the same neurologist using the Modified Young Scale (MYS) (12). Post-deterioration Management: Following neurological deterioration, the standard protocol involved immediate temporary suspension of DMPS, initiation of supportive therapy, and subsequent cautious reintroduction of chelation therapy at a reduced dosage once the patient had stabilized.
Deterioration and non-deterioration identification
Clinical data were prospectively collected and independently assessed by two board-certified neurologists using standardized functional assessment scales at both admission and discharge time points. Based on changes in total scores, the patients were classified into deterioration group and non-deterioration group. The study employed the MYS to evaluate neurological function changes. The MYS has proven to be a valuable instrument in the clinical assessment of WD, effectively serving as a tool for monitoring disease progression and treatment response. This scale contains eight domains: language, oropharyngeal muscular dystonia, appendicular muscular dystonia, ataxia, tremors, chorea-like movements, gait status, and higher cortical functions. Each domain consists of two items, with a five-point scoring system for each item based on the severity of symptoms, ranging from 0 (mild) to 4 (severe). The total score was derived by summing the scores of all items. Treatment efficacy was assessed using changes in the total score of MYS compared to baseline, with an increase of more than 2 points indicating neurological deterioration (13). In the present study, an increase in total score of no more than 4 points was classified as mild deterioration, while an increase exceeding 4 points was classified as severe deterioration.
Clinical management
Both groups of patients had completed diagnostic evaluation and subsequently received standardized therapeutic management for a period exceeding 2 weeks, with each treatment cycle consisting of continuous copper chelation therapy for 5 days followed by a 2-day drug cessation period to mitigate adverse reactions (7). During this period, zinc and calcium supplements were administered. All neurological WD patients strictly adhered to a low-copper diet. DMPS (No.: H31021514, each 2 mL contains 0.125 g, Shanghai Hefeng Pharmaceutical Co., Ltd., Shanghai) was initiated intravenously at a dose of 5–10 mg·kg−1·d−1, gradually increased to 15–20 mg·kg−1·d−1 within 1 week, with administration once daily (14), this dosage was maintained throughout the treatment period.
Quantitative assessment of lesioned brain regions
Based on the quantification standards for Magnetic Resonance Imaging (MRI) abnormal signals established by Sinha et al., abnormal signals in WD were classified into high and low signals on T2-weighted images. The semi-quantitative evaluation of T2 sequence MRI findings can reflect and assess the pathological stages and severity of brain involvement in WD patients to a certain extent (15, 16). Using a 3.0 T MRI system, imaging specialists scored the regions of interest based on the extent of high signal (graded 0–3) and signal intensity (scaled 0–3) in WD patients. The sum of these two scores yielded brain MRI score, with higher scores indicating more severe damage (17).
Statistical analysis
SPSS 26.0 (SPSSInc., Chicago, Illinois, United States) was used for data analysis. All results were presented as the mean ± standard deviation, as percentage, or as the median [P25, P75]. Differences between groups for continuous variables and categorical data were assessed using the t-test, Mann–Whitney U test, and Chi-square test, respectively. Univariate analyses were performed to identify candidate variables related to DMPS-induced neurological deterioration in WD patients (p < 0.05). Subsequently, multivariate logistic regression analysis was conducted to quantify the independent effects of significant predictors. Odds ratios (OR) and 95% confidence interval (CI) were calculated, and the results were considered statistically significant at p < 0.05. A forest plot was generated using GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, California, United States) to assess the differences among the parameters. Simultaneously, a subgroup analysis based on age characteristics was conducted for all WD patients to assess the credibility and accuracy of the study results. And we performed a 6-month follow-up on the deterioration group to assess the recovery rate after deterioration. Additionally, we employed multivariable logistic regression to develop a composite diagnostic model integrating key biomarkers, subsequently validating its discriminative capacity through receiver operating characteristic (ROC) curve analysis. The predictive accuracy was quantified by calculating the area under the curve (AUC) with corresponding 95% CI, alongside determination of optimal cutoff value maximizing the Youden index (J = sensitivity + specificity − 1). Diagnostic performance strata were defined as follows: AUC > 0.90 (excellent discrimination), 0.70–0.90 (moderate clinical utility), 0.50–0.70 (limited predictive value), and <0.50 (no better than chance prediction).
Results
Baseline data and clinical characteristics
As shown in Table 1, a total of 68 neurological WD patients with DMPS-induced neurological deterioration were included in the present study, accounting for 24.5% of the study population. Among the patients, 91.2% (62/68) exhibited mild deterioration, while 8.8% (6/68) experienced severe deterioration. Aged 10–52 years (mean, 26.38 ± 9.02 years), the 68 patients included 70.6% (48/68) males. Controls (n = 209) were WD patients without neurological deterioration, aged 11–52 years (mean, 28.51 ± 7.97 years), 45.9% (96/209) of whom were male. Both groups exhibited consistent biomarker profiles, characterized by positive K-F ring and serum CER levels below 200 mg/L. Moreover, the two groups demonstrated comparable characteristics with respect to age, ALT, AST, BUN, Scr, and ALB (p > 0.05).
Table 1
| Variable | Deterioration group (n = 68) | Non-deterioration group (n = 209) | t/X2 | p value |
|---|---|---|---|---|
| Age (year) | 26.38 ± 9.02 | 28.51 ± 7.97 | 1.85 | 0.065 |
| Sex (n, %) | 12.50 | <0.001 | ||
| Male | 48 (70.6%) | 96 (45.9%) | ||
| Female | 20 (29.4%) | 113 (54.1%) | ||
| Weight (kg) | 62.63 ± 11.27 | 59.79 ± 9.94 | 0.12 | 0.049 |
| Duration of illness (year) | 5.97 ± 4.32 | 4.26 ± 3.31 | 3.16 | 0.002 |
| ALT (IU/L) | 23.21 ± 9.88 | 25.52 ± 13.29 | 1.32 | 0.188 |
| AST (IU/L) | 24.24 ± 10.14 | 23.87 ± 10.13 | 0.26 | 0.796 |
| BUN (mmol/L) | 4.80 ± 1.43 | 4.91 ± 1.78 | 0.47 | 0.640 |
| Scr (umol/L) | 48.62 ± 8.41 | 50.50 ± 7.98 | 1.67 | 0.096 |
| ALB (g/L) | 39.95 ± 5.21 | 38.87 ± 5.79 | 1.37 | 0.172 |
| Brain MRI score (point) | 5.00 ± 0.93 | 3.94 ± 0.94 | 8.14 | <0.001 |
| 24-h urinary copper (ug) | 794.91 ± 304.68 | 720.46 ± 249.08 | 2.02 | 0.044 |
| Lactate (mmol/L) | 0.66 ± 0.13 | 0.61 ± 0.16 | 2.16 | 0.032 |
| HCY (umol/L) | 20.85 ± 3.56 | 17.73 ± 2.82 | 7.39 | <0.001 |
Baseline data and clinical characteristics between the two groups (x̅ ± s).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Scr, serum creatinine; HCY, homocysteine. Bold values indicate statistical significance at p < 0.05.
However, the deterioration group exhibited a higher proportion of male (48/20 vs. 96/113, p < 0.001), as well as elevated levels in weight (mean: 62.63 vs. 59.79, p = 0.049), duration of illness (mean: 5.97 vs. 4.26, p = 0.002), brain MRI scores (mean: 5.00 vs. 3.94, p < 0.001), 24-h urinary copper (mean: 794.91 vs. 720.46, p = 0.044), lactate (mean: 0.66 vs. 0.61, p = 0.032), and HCY (mean: 20.85 vs. 17.73, p < 0.001). Additionally, the common neurological deterioration symptoms observed in patients with neurological WD included dysarthria (77.9%, 53/68), dysphagia (70.6%, 48/68), postural abnormalities (47.1%, 32/68), and limb tremors (44.1%, 30/68), choreic involuntary movements (30.9%, 21/68), ataxia (29.4%, 20/68), and psychiatric disorders (2.9%, 2/68).
In this study, we conducted follow-up assessments for WD patients with neurological deterioration over a period of 6 months. At 3 months, the recovery rate in the deterioration group was 76.5% (52/68). By 6 months, this rate increased to 89.7% (61/68). The four most frequent symptoms of improvement were as follows: tremor of the hands and feet, abnormal limb posture, gait abnormalities, and psychiatric disorders. Notably, there were no reported deaths by the end of the follow-up period.
Multivariable analysis
Univariate analysis identified 7 parameters (including sex, weight, duration of illness, brain MRI score, 24-h urinary copper, lactate, and HCY) that were significantly associated with neurological deterioration (p < 0.05). These parameters were included in multivariate logistic regression model. Multivariate logistic regression analysis indicated that sex (OR, 0.41; 95% CI, 0.18–0.94; p = 0.035), brain MRI score (OR, 2.89; 95% CI, 1.99–4.21; p < 0.001), and HCY (OR, 1.45; 95% CI, 1.27–1.65; p < 0.001) were associated with neurological deterioration (Table 2; Figure 1).
Table 2
| Variable | Deterioration group (n = 68) | Non-deterioration group (n = 209) | OR (95% CI) | P value |
|---|---|---|---|---|
| Sex (n, %) | 0.41 (0.18–0.94) | 0.035 | ||
| Male | 48 (70.6%) | 96 (45.9%) | ||
| Female | 20 (29.4%) | 113 (54.1%) | ||
| Weight (kg) | 62.63 ± 11.27 | 59.79 ± 9.94 | 1.01 (0.97–1.04) | 0.784 |
| Duration of illness (year) | 5.97 ± 4.32 | 4.26 ± 3.31 | 1.06 (0.97–1.16) | 0.195 |
| Brain MRI score (point) | 5.00 ± 0.93 | 3.94 ± 0.94 | 2.89 (1.99–4.21) | <0.001 |
| 24-h urinary copper (ug) | 794.91 ± 304.68 | 720.46 ± 249.08 | 1.00 (0.99–1.01) | 0.256 |
| Lactate (mmol/L) | 0.66 ± 0.13 | 0.61 ± 0.16 | 0.87 (0.07–2.69) | 0.914 |
| HCY (umol/L) | 20.85 ± 3.56 | 17.73 ± 2.82 | 1.45 (1.27–1.65) | <0.001 |
Multivariable logistic regression analysis of neurological deterioration risk factors (x̅ ± s).
HCY, homocysteine; 95% CI, 95% confidence interval; OR, odds ratio. Bold values indicate statistical significance at p < 0.05.
Figure 1

Presents a forest plot of each factor. The left column shows the factors. The odds ratio for each study is indicated by a solid circle, with the confidence intervals shown by horizontal lines. HCY, homocysteine.
Subgroup analyses
Table 3 presented the baseline characteristics of WD patients in this subgroup. In the subgroup of patients aged <35 years, the deterioration group had a significantly higher male proportion, brain MRI score, and HCY level than the non-deterioration group (p < 0.05). For patients aged ≥35 years, brain MRI score and HCY level still showed significant differences between the two groups (p < 0.05).
Table 3
| Variable | <35 years | ≥35 years | ||||
|---|---|---|---|---|---|---|
| Deterioration group (n = 55) | Non-deterioration group (n = 159) | P value | Deterioration group (n = 13) | Non-deterioration group (n = 50) | P value | |
| Age (year) | 23 (18, 30) | 26 (21, 30) | 0.059 | 38 (36, 41) | 38 (36, 41) | 0.693 |
| Sex (n, %) | 0.019 | 0.461 | ||||
| Male | 36 (65.5%) | 75 (47.2%) | 4 (30.8%) | 21 (42.0%) | ||
| Female | 19 (34.5%) | 84 (52.8%) | 9 (69.2%) | 29 (58.0%) | ||
| Weight (kg) | 61.63 ± 11.60 | 59.67 ± 10.25 | 0.240 | 66.84 ± 8.92 | 62.16 ± 8.97 | 0.061 |
| Duration of illness (year) | 4 (1.5, 7) | 3 (2, 6) | 0.588 | 8 (6, 14) | 4 (2, 6) | 0.001 |
| ALT (IU/L) | 23.02 ± 10.09 | 24.87 ± 12.17 | 0.313 | 24.02 ± 9.25 | 27.60 ± 12.33 | 0.451 |
| AST (IU/L) | 22.94 ± 10.67 | 23.99 ± 9.81 | 0.505 | 29.73 ± 4.62 | 23.50 ± 11.17 | 0.055 |
| BUN (mmol/L) | 4.83 ± 1.41 | 4.74 ± 1.52 | 0.695 | 4.68 ± 1.59 | 5.47 ± 2.32 | 0.259 |
| Scr (umol/L) | 48.64 ± 8.09 | 50.74 ± 7.97 | 0.094 | 48.54 ± 9.99 | 49.74 ± 8.01 | 0.649 |
| ALB (g/L) | 40.29 ± 5.11 | 39.16 ± 5.77 | 0.199 | 38.51 ± 5.58 | 37.95 ± 5.78 | 0.758 |
| Brain MRI score (point) | 5 (4, 6) | 4 (3, 5) | <0.001 | 5 (4, 6) | 4 (3, 5) | 0.004 |
| 24-h urinary copper (ug) | 799.42 ± 318.81 | 730.71 ± 261.85 | 0.115 | 775.80 ± 245.85 | 687.85 ± 202.09 | 0.186 |
| Lactate (mmol/L) | 0.7 (0.6, 0.7) | 0.6 (0.5, 0.7) | 0.013 | 0.62 ± 0.16 | 0.60 ± 0.16 | 0.793 |
| HCY (umol/L) | 20.75 ± 3.77 | 17.77 ± 2.81 | <0.001 | 21.27 ± 2.57 | 17.61 ± 2.89 | <0.001 |
Characteristics associated with neurological deterioration in WD subgroups by age (x̅ ± s).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Scr, serum creatinine; HCY, homocysteine.
Receiver operating characteristic curves for variables
The ROC curves (Figure 2) for sex, brain MRI score, HCY, and combined predictive indicators (CPI) showed the AUC values of 0.377, 0.778, 0.758, and 0.862, respectively (Table 4). The result indicated that CPI had moderate clinical utility. In the ROC curve, the closer a point is to (0, 1), the better the model’s discriminatory ability. The predictive value of CPI for diagnosing DMPS-induced neurological deterioration in WD patients was superior to that of other individual indicators.
Figure 2
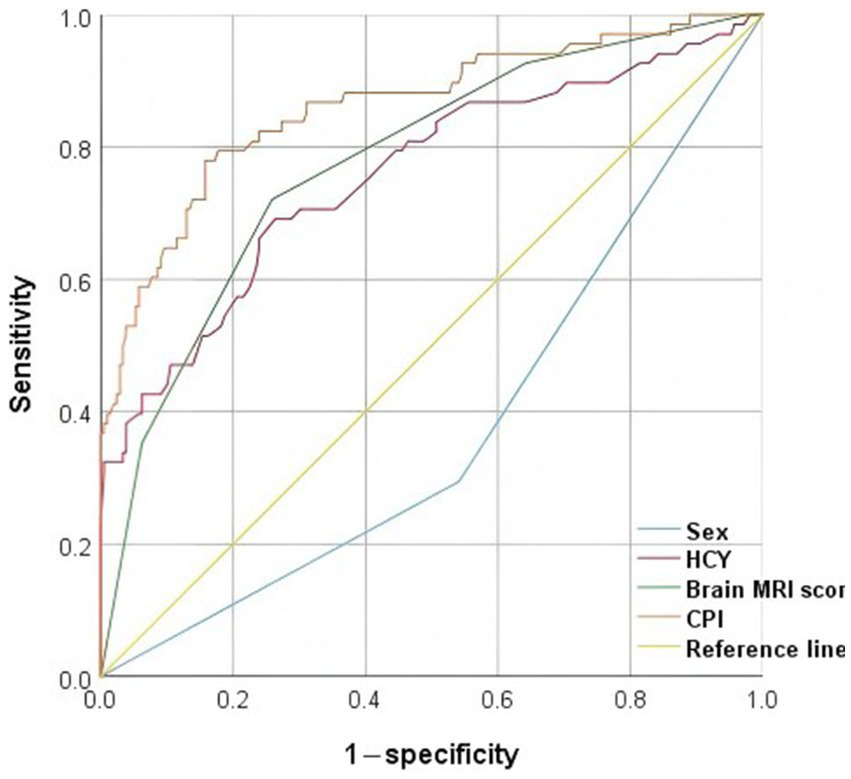
Presents an ROC curve evaluating the predictive performance of different variables for neurological deterioration, with true positive rate (sensitivity) plotted on the y-axis against false positive rate (1 − specificity) on the x-axis. The AUC quantifies the overall discriminative ability of each model. HCY, homocysteine; CPI, combined predictive indicators.
Table 4
| Variable | AUC | Cut-off value | Sensitivity | Specificity | Asymptotic significance | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Sex | 0.377 | – | – | – | 0.002 | 0.302 | 0.452 |
| HCY | 0.758 | 19.25 | 0.691 | 0.737 | <0.001 | 0.686 | 0.830 |
| Brain MRI score | 0.778 | 4.5 | 0.721 | 0.742 | <0.001 | 0.714 | 0.842 |
| CPI | 0.862 | – | – | – | <0.001 | 0.806 | 0.917 |
Receiver operating characteristic curves for candidate variables.
HCY, homocysteine; CPI, combined predictive indicators.
Discussion
In this retrospective study, we conducted a comprehensive analysis of 68 patients with WD who exhibited neurological deterioration following DMPS therapy, comparing their clinical profiles with 209 matched controls without neurological deterioration. Our findings demonstrated an overall incidence rate of 24.5% for DMPS-induced neurological deterioration, with mild neurological deterioration constituting the predominant presentation (91.2%), while severe deterioration was observed in only 8.8% of patients. Notably, the cohort with neurological deterioration showed statistically significant demographic distinctions: these patients presented a higher male predominance (male:female ratio 2.4:1 vs. 0.8:1 in controls) compared to the non-deterioration group. Our findings demonstrated a statistically significant concordance with the previous study by Cai et al. (18). It is speculated that the difference in estrogen levels may be the reason. Emerging evidence positions estrogen as a multifaceted neuroprotectant through three principal mechanisms: (a) potentiating endogenous antioxidant defenses via MAPK/ERK and PI3K/Akt signaling pathway activation; (b) suppressing neuroinflammatory responses through microglial deactivation mediated by TLR4/NF-κB signaling pathway; and (c) epigenetic modulation of neuroprotective gene networks involving pro-inflammatory cytokines, antioxidant enzymes, and anti-apoptotic proteins expression (19–21). These pleiotropic actions not only underlie its therapeutic potential in neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases but may also inform novel therapeutic strategies for mitigating neurological deterioration in WD, particularly given the observed gender disparity in disease progression trajectories.
Clinical analysis showed dystonia, movement disorders, and limb tremors were most frequent in WD patients with neurological deterioration, consistent with prior literature on neurological deterioration in WD patients treated with penicillamine (2, 22, 23). This indicates extrapyramidal symptoms are a main sign of neurological deterioration, closely linked to copper ions’ selective damage to the basal ganglia and its connecting fibers (24–26). Building upon the compelling evidence linking WD-associated neurological progression with copper dyshomeostasis, we hypothesize that neurological WD patients undergo pathogenic copper flux during clinical deterioration, which consequently triggers compartment-specific redistribution of copper ions through dysregulated transmembrane transport mechanisms. Therefore, we recommend a more cautious and individualized medication strategy and closer attention for neurological WD patients, which can improve their prognosis and quality of life.
Longitudinal follow-up data showed that in WD patients receiving DMPS therapy, the rate of neurological function recovery improved significantly in a time-dependent manner. By the 6-month follow-up point, the recovery rate reached 89.7%, with no treatment-related fatalities during the study period. Notably, compared to penicillamine, the continuous neurological deterioration rate was significantly lower with DMPS (27), indicating that DMPS-induced neurological deterioration may be partially reversible.
Multivariable logistic regression analysis revealed three independent predictors of neurological deterioration in neurological WD: sex (OR = 0.41, p = 0.035), brain MRI score (OR = 2.89, p < 0.001), and HCY (OR = 1.45, p < 0.001). While our analysis identified these biomarkers as independent risk factors, their association with underlying disease severity cannot be entirely ruled out. We further acknowledge that the initial neurological deterioration observed in some severe cases may represent a ‘therapeutic lag effect’, where neurological improvement is delayed despite successful biochemical decoppering. This finding is not consistent with the inclusion criteria applied in our study. Notably, in the subgroup of patients aged <35 years, the deterioration group had a significantly higher male proportion (36/19 vs. 75/84, p = 0.019), brain MRI score (median: 5 vs. 4, p < 0.001), and HCY level (mean: 20.75 vs. 17.77, p < 0.001) than the non-deterioration group. Meanwhile, for patients aged ≥35 years, brain MRI score (median: 5 vs. 4, p = 0.004) and HCY level (mean: 21.27 vs. 17.61, p < 0.001) still showed significant differences between the two groups. The absence of statistically significant sex-based differences in the ≥35-year age subgroup (p = 0.461) may be attributable to the limited sample size. While previous epidemiological studies have identified 5–35 years as the peak age range for WD onset (28, 29), critical gaps persist in type-specific epidemiological characterization. Motivated by this evidence gap, our study used 35 years as the age cutoff to analyze the clinical characteristics and risk factors of neurological deterioration in neurological WD patients.
The neurological deterioration in WD involves complex pathophysiological mechanisms, where the mislocalization, redistribution, and neurotoxicity of copper are closely linked (30–32). Copper accumulation in astrocytes compromises the blood–brain barrier, leading to neuronal and oligodendroglial damage and diverse neurological symptoms (33–35). Neuroimaging modalities, capable of directly quantifying end-organ neurological involvement through structural and functional correlates, serve as valuable objective biomarkers. This capability enables both guiding chelation therapy intensity based on real-time pathological burden assessment and predicting therapeutic responsiveness through baseline imaging signatures (25). T2-weighted imaging shows characteristic hyperintensity in the basal ganglia, thalamus, and/or brainstem in WD, seen in 90% of WD patients with neurological manifestations (36, 37). Building on this foundation, Dusek et al. demonstrated that WD primarily causes central atrophy through deformation and surface-based morphometry, with overall brain atrophy correlating with neurological severity (17). Although our study differs in perspective, it corroborates that quantitative MRI analysis offers further insights into WD-related brain damage and susceptibility changes.
HCY, a key mediator of oxidative stress, DNA epigenetic regulation, and cellular homeostasis, has been established as a biomarker for vascular and neurodegenerative disorders (38–40). While direct evidence linking HCY to neurological WD remains scarce, clinical studies have demonstrated significantly elevated serum HCY levels (p < 0.01) in WD patients with hepatic steatosis compared to those without fatty liver involvement (41). Mechanistic study using the Jackson toxic milk mouse model of WD reveals that copper accumulation disrupts hepatic methionine metabolism through inhibition of S-adenosylhomocysteine hydrolase, leading to pathological S-adenosylhomocysteine accumulation and inducing systemic hypomethylation, which triggers inflammatory cascades that ultimately contribute to neural tissue damage (42). These findings collectively suggest that HCY elevation may serve as a predictive indicator of neurological deterioration in WD, potentially reflecting copper-induced neuroinflammatory activation. Further investigations are required to delineate the molecular mechanisms linking HCY dysregulation to copper metabolism perturbations, with emphasis on their pathogenic crosstalk in WD progression.
Additionally, ROC curve analysis revealed that a multivariable-adjusted biomarker panel incorporating sex, brain MRI score, and HCY, demonstrated superior predictive performance for neurological deterioration in neurological WD (AUC = 0.862, p < 0.001). Therefore, timely assessment of these indicators is crucial for early identification of disease deterioration and improved prognosis. This not only deepens our understanding of the clinical features of neurological WD but also provides a more refined monitoring tool for clinical practice, thereby enhancing the precision of clinical interventions.
Limitations
This study has several limitations. First, the retrospective design may introduce bias, and the single-center nature of the research could limit the generalizability of the findings to a broader population. Furthermore, while the sample size is adequate for preliminary analysis, it may not fully capture the spectrum of neurological manifestations in WD patients receiving DMPS treatment across different environmental factors influencing disease expression. The 6-month follow-up period, while aligned with standard early assessment windows for WD, may limit detection of delayed neurological events such as late-onset paradoxical deterioration or therapeutic lag effect. Future research should aim for multi-center, prospective studies with larger cohorts and extended follow-up durations to validate these findings. Additionally, exploring the potential mechanisms of neurological deterioration during chelation therapy and the long-term impact of DMPS treatment on neurological outcomes in WD patients is essential.
Conclusion
Neurological deterioration in DMPS-induced neurological WD patients is not rare and predominantly occurs in males. We identified three independent risk factors for this deterioration: sex, brain MRI score, and HCY. A composite risk model incorporating these parameters achieved superior predictive accuracy compared to individual biomarker. Additionally, our findings not only provide clinicians with crucial data on neurological deterioration in WD but also revealed specific biomarkers for disease severity assessment, thus bolstering clinical decision-making.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine (Approval number: 2021AH-60). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
YG: Writing – original draft, Writing – review & editing. JZ: Resources, Methodology, Software, Investigation, Writing – review & editing, Conceptualization, Data curation. LT: Methodology, Data curation, Investigation, Software, Writing – review & editing, Resources. SJ: Software, Investigation, Resources, Writing – review & editing, Methodology, Data curation, Supervision. GY: Funding acquisition, Conceptualization, Writing – review & editing, Writing – original draft, Resources. WY: Conceptualization, Resources, Writing – review & editing, Writing – original draft, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Grants from the National Natural Science Foundation of China [grant number: U22A20366], Anhui University Collaborative Innovation Project Plan [grant number: GXXT-2020-025], and the Anhui Provincial Science and Technology Major Special Project for Traditional Chinese Medicine [grant number: 202303a07020004].
Acknowledgments
We extend our gratitude to all doctors for their contributions in providing clinical data on the patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Cheng N Wang K Hu W Sun D Wang X Hu J et al . Wilson disease in the south Chinese Han population. Can J Neurol Sci. (2014) 41:363–7. doi: 10.1017/s0317167100017315
2.
Litwin T Dusek P Antos A Członkowska A Bembenek J . Tackling the neurological manifestations in Wilson's disease - currently available treatment options. Expert Rev Neurother. (2023) 23:1249–59. doi: 10.1080/14737175.2023.2268841
3.
Mulligan C Bronstein JM . Wilson disease: an overview and approach to management. Neurol Clin. (2020) 38:417–32. doi: 10.1016/j.ncl.2020.01.005
4.
Litwin T Dušek P Członkowska A . Symptomatic treatment of neurologic symptoms in Wilson disease. Handb Clin Neurol. (2017) 142:211–23. doi: 10.1016/b978-0-444-63625-6.00018-5
5.
Ziemssen T Smolinski L Członkowska A Akgun K Antos A Bembenek J et al . Serum neurofilament light chain and initial severity of neurological disease predict the early neurological deterioration in Wilson's disease. Acta Neurol Belg. (2023) 123:917–25. doi: 10.1007/s13760-022-02091-z
6.
Zhang J Xiao L Yang W . Combined sodium Dimercaptopropanesulfonate and zinc versus D-penicillamine as first-line therapy for neurological Wilson's disease. BMC Neurol. (2020) 20:255. doi: 10.1186/s12883-020-01827-9
7.
Chen D Zhou X Hou H Feng L Liu J Liang Y et al . Clinical efficacy of combined sodium dimercaptopropanesulfonate and zinc treatment in neurological Wilson's disease with D-penicillamine treatment failure. Ther Adv Neurol Disord. (2016) 9:310–6. doi: 10.1177/1756285616641598
8.
Ferenci P Caca K Loudianos G Mieli-Vergani G Tanner S Sternlieb I et al . Diagnosis and phenotypic classification of Wilson disease. Liver Int. (2003) 23:139–42. doi: 10.1034/j.1600-0676.2003.00824.x
9.
Zhu XQ Li LY Yang WM Wang Y . Combined dimercaptosuccinic acid and zinc treatment in neurological Wilson's disease patients with penicillamine-induced allergy or early neurological deterioration. Biosci Rep. (2020) 40:BSR20200654. doi: 10.1042/bsr20200654
10.
Merle U Schaefer M Ferenci P Stremmel W . Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut. (2007) 56:115–20. doi: 10.1136/gut.2005.087262
11.
First MB . Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. (2013) 201:727–9. doi: 10.1097/NMD.0b013e3182a2168a
12.
Zhou X Liao J Liu Y Qin H Xiao X . Symptom aggravation after withdrawal of metal chelating agent therapy in patients with Wilson's disease. Brain Behav. (2023) 13:e3170. doi: 10.1002/brb3.3170
13.
Hou H Chen D Liu J Feng L Zhang J Liang X et al . Clinical and genetic analysis in neurological Wilson's disease patients with neurological worsening following Chelator therapy. Front Genet. (2022) 13:875694. doi: 10.3389/fgene.2022.875694
14.
Xu SQ Li XF Zhu HY Liu Y Fang F Chen L . Clinical efficacy and safety of chelation treatment with typical penicillamine in cross combination with DMPS repeatedly for Wilson's disease. J Huazhong Univ Sci Technolog Med Sci. (2013) 33:743–7. doi: 10.1007/s11596-013-1190-z
15.
Zhao TN Lian YS Hu XM Tan W Deng G Liu H et al . Clinical and MRI analysis of hepatic and cerebral hepatolenticular degeneration Chinese. J Magn Reson Imaging. (2021) 12:1–5. doi: 10.12015/issn.1674-8034.2021.03.001
16.
Mahale RR Stezin A Prasad S Kamble N Holla VV Netravathi M et al . Clinical spectrum, radiological correlation and outcome of movement disorders in Wilson's disease. Tremor Other Hyperkinet Mov (N Y). (2023) 13:37. doi: 10.5334/tohm.794
17.
Dusek P Smolinski L Redzia-Ogrodnik B Golebiowski M Skowronska M Poujois A et al . Semiquantitative scale for assessing brain MRI abnormalities in Wilson disease: a validation study. Mov Disord. (2020) 35:994–1001. doi: 10.1002/mds.28018
18.
Cai L Huang X Ye Y Yang D Xie L Fu D et al . Role of gender and age in features of Wilson's disease. Front Neurol. (2023) 14:1176946. doi: 10.3389/fneur.2023.1176946
19.
Mills ZB Faull RLM Kwakowsky A . Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease?Int J Mol Sci. (2023) 24:3205. doi: 10.3390/ijms24043205
20.
Villa A Vegeto E Poletti A Maggi A . Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. (2016) 37:372–402. doi: 10.1210/er.2016-1007
21.
Schaffner SL Tosefsky KN Inskter AM Appel-Cresswell S Schulze-Hentrich JM . Sex and gender differences in the molecular etiology of Parkinson's disease: considerations for study design and data analysis. Biol Sex Differ. (2025) 16:7. doi: 10.1186/s13293-025-00692-w
22.
Mohr I Pfeiffenberger J Eker E Merle U Poujois A Ala A et al . Neurological worsening in Wilson disease - clinical classification and outcome. J Hepatol. (2023) 79:321–8. doi: 10.1016/j.jhep.2023.04.007
23.
Kumar M Murugan TP Lionel AP Thomas MM Mannam P Yoganathan S . Management of Children and Adolescents with Wilson disease and neurological worsening following D-Penicillamine therapy: a single Centre experience. Ann Indian Acad Neurol. (2022) 25:698–702. doi: 10.4103/aian.aian_519_21
24.
Pinto C Malaquias MJ Miranda HP Temudo T Silva E Ramos C et al . Brain MRI in the decision for liver transplantation in pediatric neurological Wilson's disease. Mov Disord Clin Pract. (2022) 9:941–8. doi: 10.1002/mdc3.13547
25.
Shribman S Poujois A Bandmann O Czlonkowska A Warner TT . Wilson's disease: update on pathogenesis, biomarkers and treatments. J Neurol Neurosurg Psychiatry. (2021) 92:1053–61. doi: 10.1136/jnnp-2021-326123
26.
Su D Zhang Z Zhang Z Gan Y Zhang Y Liu X et al . Microstructural and functional impairment of the basal ganglia in Wilson's disease: a multimodal neuroimaging study. Front Neurosci. (2023) 17:1146644. doi: 10.3389/fnins.2023.1146644
27.
Litwin T Dzieżyc K Karliński M Chabik G Czepiel W Członkowska A . Early neurological worsening in patients with Wilson's disease. J Neurol Sci. (2015) 355:162–7. doi: 10.1016/j.jns.2015.06.010
28.
Kathawala M Hirschfield GM . Insights into the management of Wilson's disease. Ther Adv Gastroenterol. (2017) 10:889–905. doi: 10.1177/1756283x17731520
29.
European Association for Study of Liver . EASL clinical practice guidelines: Wilson's disease. J Hepatol. (2012) 56:671–85. doi: 10.1016/j.jhep.2011.11.007
30.
Zhou XX Xiao X Qin H Chen D Wu C . Study on different pathogenic factors in different disease stages of patients with Wilson disease. Neurol Sci. (2021) 42:3749–56. doi: 10.1007/s10072-020-04973-7
31.
Bandmann O Weiss KH Kaler SG . Wilson's disease and other neurological copper disorders. Lancet Neurol. (2015) 14:103–13. doi: 10.1016/s1474-4422(14)70190-5
32.
Sun CL Cheng N Hu JY . Clinical significance and progress of detection methods for serum free copper detection in Wilson disease. Int J Lab Med. (2018) 39:1756–9. doi: 10.3969/j.issn.1673-4130.2018.14.026
33.
Rędzia-Ogrodnik B Członkowska A Bembenek J Antos A Kurkowska-Jastrzębska I Skowrońska M et al . Brain magnetic resonance imaging and severity of neurological disease in Wilson's disease - the neuroradiological correlations. Neurol Sci. (2022) 43:4405–12. doi: 10.1007/s10072-022-06001-2
34.
Lutsenko S Washington-Hughes C Ralle M Schmidt K . Copper and the brain noradrenergic system. J Biol Inorg Chem. (2019) 24:1179–88. doi: 10.1007/s00775-019-01737-3
35.
Lorincz MT . Neurologic Wilson's disease. Ann N Y Acad Sci. (2010) 1184:173–87. doi: 10.1111/j.1749-6632.2009.05109.x
36.
Litwin T Gromadzka G Członkowska A Gołębiowski M Poniatowska R . The effect of gender on brain MRI pathology in Wilson's disease. Metab Brain Dis. (2013) 28:69–75. doi: 10.1007/s11011-013-9378-2
37.
Kozić D Svetel M Petrović B Dragasević N Semnic R Kostić VS . MR imaging of the brain in patients with hepatic form of Wilson's disease. Eur J Neurol. (2003) 10:587–92. doi: 10.1046/j.1468-1331.2003.00661.x
38.
Bonetti F Brombo G Zuliani G . The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener Dis Manag. (2016) 6:133–45. doi: 10.2217/nmt-2015-0008
39.
Bhattacharjee N Borah A . Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson's disease. Neurochem Int. (2016) 101:48–55. doi: 10.1016/j.neuint.2016.10.001
40.
Kaplan P Tatarkova Z Sivonova MK Racay P Lehotsky J . Homocysteine and mitochondria in cardiovascular and cerebrovascular systems. Int J Mol Sci. (2020) 21:21. doi: 10.3390/ijms21207698
41.
Jia SP Wang MX Tao Z Gao YN Yu GR Yang WM . Analysis of risk factors for fatty liver disease in children with Wilson's disease. Eur J Gastroenterol Hepatol. (2024) 36:1046–53. doi: 10.1097/meg.0000000000002801
42.
Mordaunt CE Shibata NM Kieffer DA Czlonkowska A Litwin T Weiss KH et al . Epigenetic changes of the thioredoxin system in the tx-j mouse model and in patients with Wilson disease. Hum Mol Genet. (2018) 27:3854–69. doi: 10.1093/hmg/ddy262
Summary
Keywords
Wilson’s disease, neurological deterioration, risk factors, clinical characteristics, sodium dimercaptopropanesulfonate
Citation
Gao Y, Zhang J, Tang L, Jia S, Yu G and Yang W (2025) DMPS-induced neurological deterioration in neurological Wilson’s disease patients: a retrospective case-control study on clinical characteristics and risk factors. Front. Neurol. 16:1599209. doi: 10.3389/fneur.2025.1599209
Received
27 March 2025
Accepted
08 September 2025
Published
29 September 2025
Volume
16 - 2025
Edited by
Sara Samadzadeh, Charité University Medicine Berlin, Germany
Reviewed by
Abilo Tadesse, University of Gondar, Ethiopia
Agnieszka Antos, Institute of Psychiatry and Neurology in Warsaw, Poland
Updates
Copyright
© 2025 Gao, Zhang, Tang, Jia, Yu and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guran Yu, ygrwyyxx@163.comWenming Yang, ywmwyyx@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.