Abstract
Background:
Falls are the leading cause of traumatic brain injury (TBI) in high-income countries, and globally, the incidence of fall-related injuries is projected to rise. In this study, we compare TBI resulting from stair-related falls (SRFs) to falls from standing height (FSH), analyzing their epidemiology and outcomes.
Methods:
In a single-center, registry-based cohort study using the Oslo TBI Registry-Neurosurgery (OTBIR-N), we identified adults (≥18 years) admitted to Oslo University Hospital with TBI from either SRFs or FSH between 2015 and 2022. Epidemiology and outcome measures were compared between the two groups, and a multivariate logistic regression model was used to evaluate the adjusted effect of the fall mechanisms on dichotomized functional outcome (Glasgow outcome score (GOS) 1–3 vs. GOS 4–5).
Results:
A total of 1,432 patients with a median age of 71 years were included. SRFs represented 25%, while FSH represented 52% of all fall-related TBIs. SRF patients were generally younger and healthier, with a higher frequency of moderate to severe TBI than FSH patients (53% vs. 31%; p < 0.001). SRFs also occurred more often during evenings and nights, on weekends, and were more often related to alcohol influence (58% vs. 22%; p < 0.001). Both fall types resulted in unfavorable functional outcomes (GOS 1–3) for a substantial proportion of patients (37% in SRFs and 42% in FSH; p = 0.066). When adjusting for covariates in the multivariable logistic regression model, there was a tendency of SRFs being associated with unfavorable outcomes compared to FSH, but the effect was not statistically significant (OR 1.43, 95%CI 0.97–2.12; p = 0.073).
Conclusion:
SRFs represented a considerable proportion of fall-related TBIs and were associated with poor outcomes in a substantial proportion of patients. Targeted public awareness campaigns addressing the risks associated with staircases, especially when combined with alcohol influence, seem warranted to prevent such injuries.
1 Introduction
Falls are the leading cause of traumatic brain injury (TBI) in high-income countries, where they account for approximately half of all TBIs requiring admission to trauma centers (1–6). The World Health Organization describes falls as a growing and under-recognized public health issue and predicts fall-related injuries to rise globally in the coming decades due to factors such as aging populations, increased urbanization, and sedentary lifestyles (7).
Risk factors for falls can be categorized into person-specific (such as advanced age, comorbidities, ethanol influence, polypharmacy, and functional impairments) and environmental causes (such as slippery floors, lack of stair railings, and poor lighting) (7–9). In older adults, falls often occur at home, where stair-related falls account for 11–22% of these incidents (10, 11). Alcohol intoxication has been shown to affect injury patterns with an increased risk of sustaining a TBI after stair-related falls (SRFs) (12, 13). Compared to falls from standing height (FSH), SRFs imply a higher energy trauma and may therefore be hypothesized to result in more severe injury, including TBI (10, 14–16).
Falls represent the leading cause of TBI and account for a considerable proportion of its overall societal impact. Although many individuals recover after sustaining a TBI, a substantial proportion experience reduced function or disability, often compounded by comorbidity and frailty that further complicate recovery (2, 5, 17, 18). A detailed characterization of fall-related TBIs is essential to tailor healthcare systems to meet current needs. This includes optimizing resources and workflows in trauma centers, refining clinical guidelines, strengthening rehabilitation services, and adopting appropriate preventive measures at the societal level.
In this single-center registry-based cohort study, we compare TBI from SRFs to TBI after falls from FSH, detailing their epidemiology and resulting outcomes.
2 Materials and methods
2.1 Study design and data sources
In this study comparing two common types of falls, we conducted a retrospective, cohort study based on prospectively collected registry data from the Oslo TBI Registry-Neurosurgery (OTBIR-N).
Oslo University Hospital (OUH) serves as the regional neurotrauma center for the Southeast region of Norway, which encompasses a population of approximately 3.1 million and 19 local trauma hospitals covering both urban and rural areas (6). In Norway, trauma care is organized through public hospitals under an equal-access policy and is free of charge. OTBIR-N is a quality control database managed by the Department of Neurosurgery at OUH (6). Prospective registration started in 2015, and data are collected based on available information in electronic medical records. Inclusion criteria for the registry are (i) traumatic brain injury; (ii) cerebral-CT/CTA or cerebral-MRI/MRA with findings of acute trauma (hemorrhage, fracture, traumatic axonal injury, and vascular injury); (iii) admitted to OUH within 7 days post-injury; and (iv) Norwegian social security number.
The current study (24/01340) and OTBIR-N (2016/17569) were approved by the OUH data protection officer.
2.2 Patient inclusion
From OTBIR-N, we identified adults (≥18 years) admitted to OUH between 1 January 2015 and 31 December 2022 who had sustained a TBI confirmed by pathological findings on head CT, either from a stair-related or standing height fall. A fall from standing height is defined in the registry as a fall that begins when a person has his or her feet on the ground, and a stair-related fall is defined as a fall involving one or more steps in a stair. Other categories of falls were not included in the analysis (such as falls from chair/bed, window/balcony, ladder/scaffold, roof, tree, play stand, hillside, and unknown). Patient inclusion is further detailed in the flow chart in Figure 1.
Figure 1

Flow chart of patient inclusion from OTBIR-N.
2.3 Data collection
All variables were retrieved from OTBIR-N. Demographics and details on pre-injury patient status, including functional living status (home independent/home assisted/institution), American Society of Anesthesiologists Physical Status Classification System (ASA-PS) class (19), and the use of antithrombotics, were gathered. Injury details, including place and time of injury and whether the patient was under the influence of alcohol, were collected. TBI severity indicators included the Glasgow coma scale (GCS) score (20) (recorded as the lowest score prior to intubation or arrival at OUH), pupillary dilatation, and Rotterdam CT score (21). Additional TBI characteristics, including details on types of traumatic intracranial hemorrhages and whether the patient had traumatic injuries other than TBI, were also collected.
Outcome measures include mortality (automatically updated in the registry via a link to the national population registry), Glasgow outcome score (GOS) (22) based on available information in electronic patient records 6 months after trauma, and functional living status 6 months post-trauma in the same format as pre-trauma (home-independent/home-assisted/institution).
2.4 Data analysis
The proportion of patients sustaining a TBI from SRFs and FSH to TBI from all fall categories was calculated. Patient characteristics, injury characteristics, TBI severity, and outcome measures were described for the whole cohort and subsequently compared between the SRF group and FSH group using parametric or non-parametric tests as appropriate. Heatmaps were used to describe temporal trends in the incidence of the fall mechanisms, and the proportion of patients who were under the influence of alcohol during the fall was explored and compared between the two groups. UpSet plots were used to describe and compare patterns of TBI subtypes. To evaluate whether the type of fall had an effect on functional outcome, we used a logistic regression model with dichotomized GOS (GOS 1–3 vs. GOS 4–5) as the dependent variable. Age, sex, ASA-PS class, functional living status pre-trauma, GCS, Rotterdam CT score, presence of pupillary dilatation, isolated TBI vs. multitrauma, alcohol influence during the fall, and use of antithrombotics were included as clinically relevant independent variables. Finally, Sankey diagrams were used to illustrate the change in functional living status from pre-trauma to 6 months post-trauma. Statistical analyses were performed using IBM SPSS Statistics version 29, Stata (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.), or R version 4.4.0, and a p-value of <0.05 was considered significant.
3 Results
A total of 1,432 patients were included in the study: 470 patients in the SRF group and 962 patients in the FSH group. These fall mechanisms represented 25 and 52% of all fall-related TBIs in the OTBIR-N in the time period (Figure 2).
Figure 2
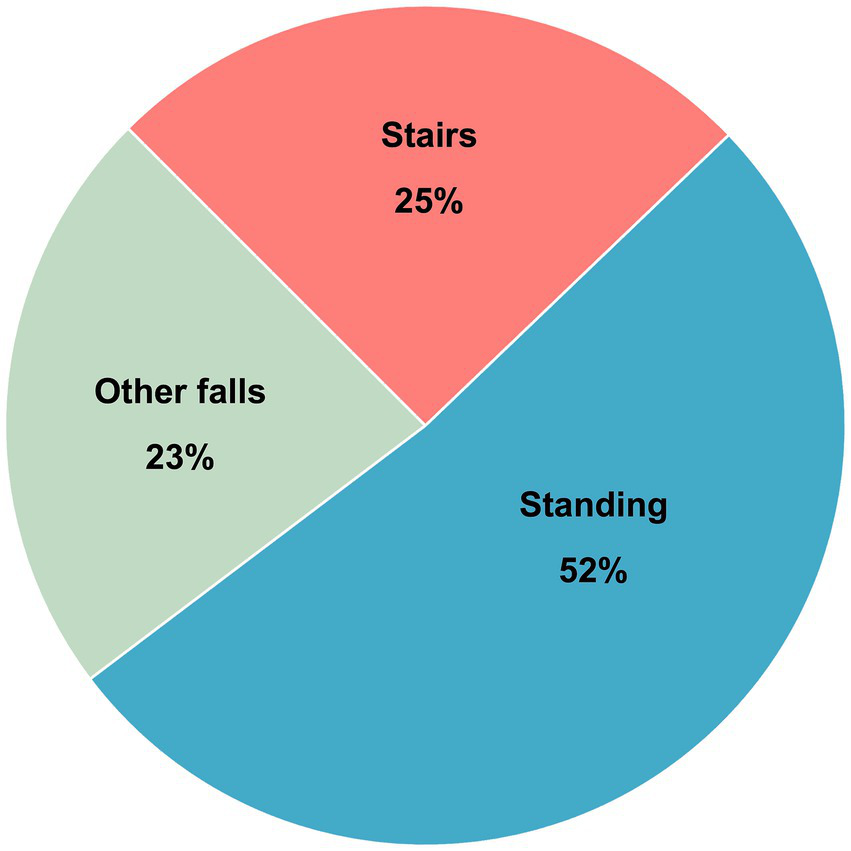
Distribution of fall mechanisms in percent of total in OTBIR-N (N = 2017).
3.1 Patient characteristics
Overall, the median age was 71 years (interquartile range (IQR) 58–81 years); 825/1432 (57%) were male. Patients in the SRF group were younger, had fewer comorbidities, lived more independently, and used less anti-thrombotics compared to patients in the FSH group. Patient characteristics for the whole cohort and the two different fall groups are presented in detail in Table 1.
Table 1
| All (n = 1,432) | SRF (n = 470) | FSH (n = 962) | p-value | |
|---|---|---|---|---|
| Age in years, median (IQR) | 71 (58–81) | 66 (54–76) | 73 (60–83) | <0.001 |
| Age groups | <0.001 | |||
| 18–39 | 113 (8) | 43 (9) | 70 (7) | |
| 40–59 | 270 (19) | 106 (23) | 164 (17) | |
| 60–79 | 655 (46) | 237 (50) | 418 (44) | |
| 80+ | 394 (28) | 84 (18) | 310 (32) | |
| Male | 825 (57) | 280 (60) | 545 (57) | 0.29 |
| Living status | <0.001 | |||
| Home independent | 1,102 (77) | 416 (89) | 686 (71) | |
| Home assisted | 262 (18) | 48 (10) | 214 (22) | |
| Nursing home/Other/unknown | 68 (5) | 6 (1) | 62 (6) | |
| Preinjury ASA | <0.001 | |||
| 1 | 250 (18) | 120 (26) | 130 (14) | |
| 2 | 411 (29) | 144 (31) | 267 (28) | |
| 3 | 731 (51) | 201 (43) | 530 (55) | |
| 4 | 40 (3) | 5 (1) | 35 (4) | |
| Antithrombotic medication: | <0.001 | |||
| None | 785 (55) | 319 (68) | 466 (48) | |
| Single platelet inhibitor | 335 (23) | 91 (19) | 244 (25) | |
| Anticoagulation | 241 (17) | 53 (11) | 188 (20) | |
| Combination | 71 (5) | 7 (2) | 64 (7) | |
| Place of injury | <0.001 | |||
| Private home | 741 (52) | 299 (64) | 442 (46) | |
| Other | 691 (48) | 171 (36) | 464 (54) |
Patient characteristics.
Data are n (%) or median (IQR). p-values are for comparison between the falling down the stairs group and falling from standing height group. FSH, fall from standing height; SRF, stairs-related fall.
3.2 Temporal patterns of injury and alcohol influence
The heat maps (Figure 3) demonstrate an apparent difference in the temporal patterns of injury between the two groups, with SRFs showing a clear preponderance toward evenings and nighttime during the weekends. In contrast, FSH was more evenly distributed with regard to time of day and weekday. Consistent with this, patients in the SRF group were considerably more often under the influence of alcohol compared to patients in the FSH group (274/470 (58%) vs. 213/962 (22%); p < 0.001).
Figure 3

Heat maps visualize the time and day of injury in the two groups. The x-axis denotes the time of day, ranging from 00:00 to 23:59 h, segmented into hourly intervals. The y-axis represents the days of the week, starting from Monday (top) to Sunday (bottom). The color represents the frequency within the time interval, with white colors representing fewer cases and red colors representing higher numbers.
In both groups, the absolute number of falls under alcohol influence peaked in the age group 60–69 years. However, the ratio of patients under the influence of alcohol to those who were not remained highest among those aged <50 years (Figure 4).
Figure 4

Distribution of patients by age groups, type of fall, and alcohol influence during the fall.
3.3 TBI severity and lesion types
When comparing the two groups, SRFs had more severe TBIs, with 53% sustaining a moderate or severe TBI (GCS 3–12) as compared to 31% in the FSH group (p < 0.001). The Rotterdam CT score also indicated more severe radiological TBI in the former group. Additionally, patients in the SRF group more often demonstrated extracranial injuries (232/470 (49%) vs. 207/962 (22%); p < 0.001), were more often admitted to the intensive care unit (ICU), and more frequently had intracranial pressure (ICP) monitored. TBI severity and characteristics are shown in Table 2.
Table 2
| All (n = 1,432) | SRF (n = 470) | FSH (n = 962) | p-value | |
|---|---|---|---|---|
| Glasgow coma scale score | <0.001 | |||
| GCS 13–15 (mild) | 886 (62) | 219 (47) | 667 (69) | |
| GCS 9–12 (moderate) | 240 (17) | 94 (20) | 146 (15) | |
| GCS 3–8 (severe) | 306 (21) | 157 (33) | 149 (16) | |
| Rotterdam CT score | <0.001 | |||
| 1–2 | 449 (31) | 136 (29) | 313 (33) | |
| 3–4 | 844 (59) | 269 (57) | 575 (60) | |
| 5–6 | 139 (10) | 65 (14) | 74 (8) | |
| Pupillary dilatation | 0.097 | |||
| None | 1,336 (93) | 432 (92) | 904 (94) | |
| Unilateral | 57 (4) | 21 (5) | 36 (4) | |
| Bilateral | 35 (2) | 17 (4) | 18 (2) | |
| Unknown | 4 (<1) | 0 | 4 (<1) | |
| Neurosurgical evacuation of mass lesion | ||||
| Any mass lesion type | 176 (12) | 62 (13) | 114 (12) | 0.468 |
| Epidural hematoma | 33 (2) | 13 (3) | 20 (2) | 0.416 |
| Contusion | 32 (2) | 14 (3) | 18 (2) | 0.183 |
| Subdural hematoma | 134 (9) | 49 (10) | 85 (9) | 0.332 |
| ICP monitor | 186 (13) | 97 (21) | 89 (9) | <0.001 |
| ICU admission | 821 (57) | 328 (70) | 493 (51) | <0.001 |
| Multiple trauma | 439 (31) | 232 (49) | 207 (22) | <0.001 |
| In-hospital mortality | 138 (10) | 51 (11) | 87 (9) | 0.276 |
| Discharge destination | <0.001 | |||
| Home | 448 (35) | 123 (29) | 325 (37) | |
| Local hospital | 550 (43) | 202 (48) | 348 (40) | |
| Specialized rehabilitation | 136 (11) | 66 (16) | 70 (8) | |
| Nursing home | 137 (11) | 24 (6) | 113 (13) | |
| Other | 23 (2) | 4 (1) | 19 (2) | |
Injury characteristics, acute treatment, and discharge.
Data are n (%). p-values are for comparison between the stair-related fall group and the standing height fall group. CT, computed tomography; FSH, fall from standing height; ICP, intracranial pressure; ICU, intensive care unit; SRF, stairs-related fall.
Regarding TBI lesion types, isolated acute subdural hematoma (ASDH) or traumatic subarachnoid hemorrhage (SAH) were the most frequent lesions in both groups. In the SRF group, 26% had an isolated lesion type, while in the FSH group, the corresponding proportion was 41%. Conversely, multiple concomitant lesions were more common for SRFs compared to FSH (73% vs. 58%; p < 0.001). The UpSet plot in Figures 5, 6 illustrates the distribution of CT findings in the two groups.
Figure 5
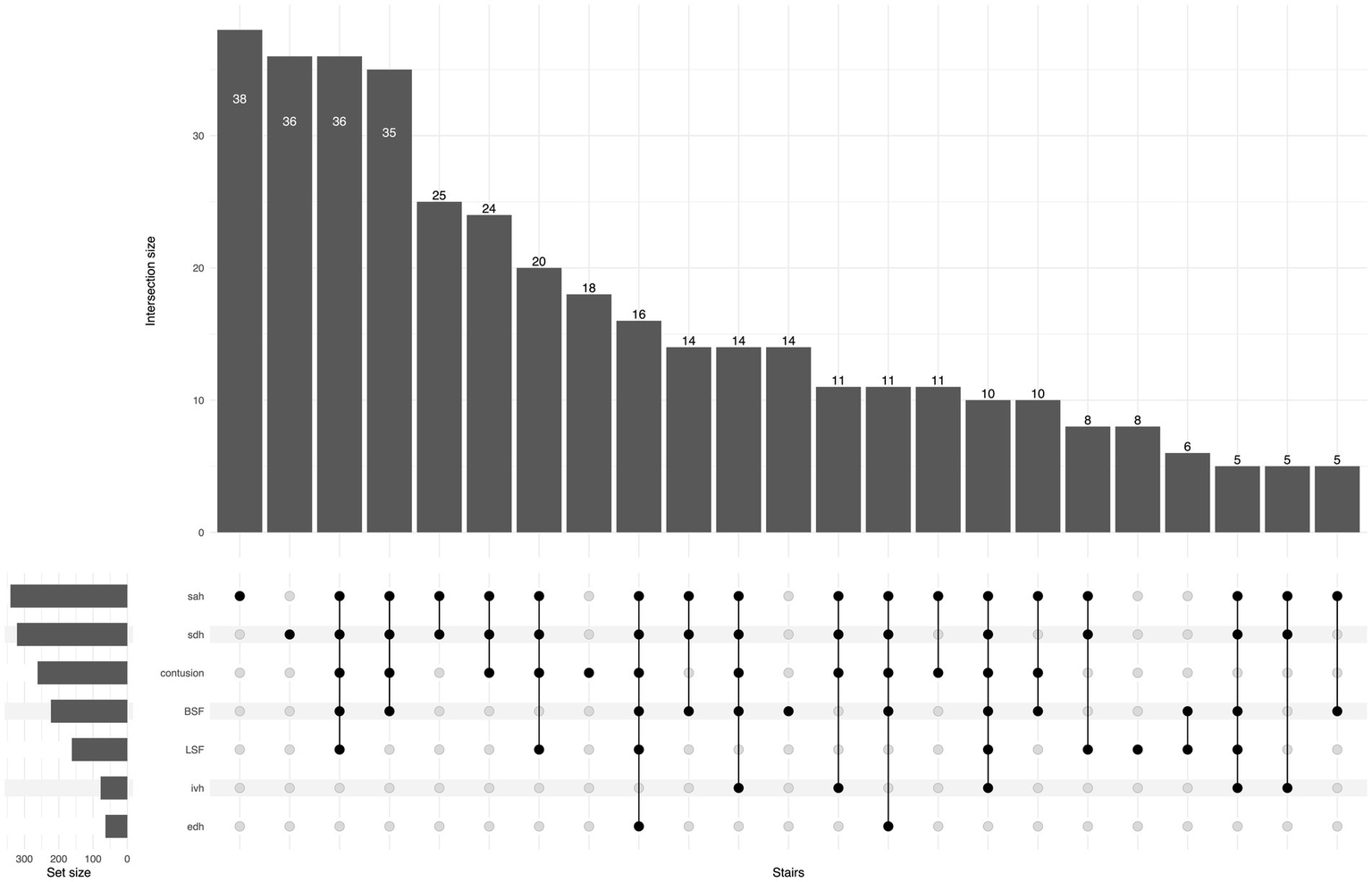
UpSet plot shows traumatic lesions on primary head CT for the SRF group, the number of patients in each lesion/combination is shown at the top of each bar. For ease of reading, the tail in the plot is cut when the number of cases is less than five patients. BSF, basilar skull fracture; EDH, epidural hematoma; IVH, intraventricular hemorrhage; LSF, linear skull fracture; SAH, subarachnoid hemorrhage; SDH, subdural hematoma.
Figure 6
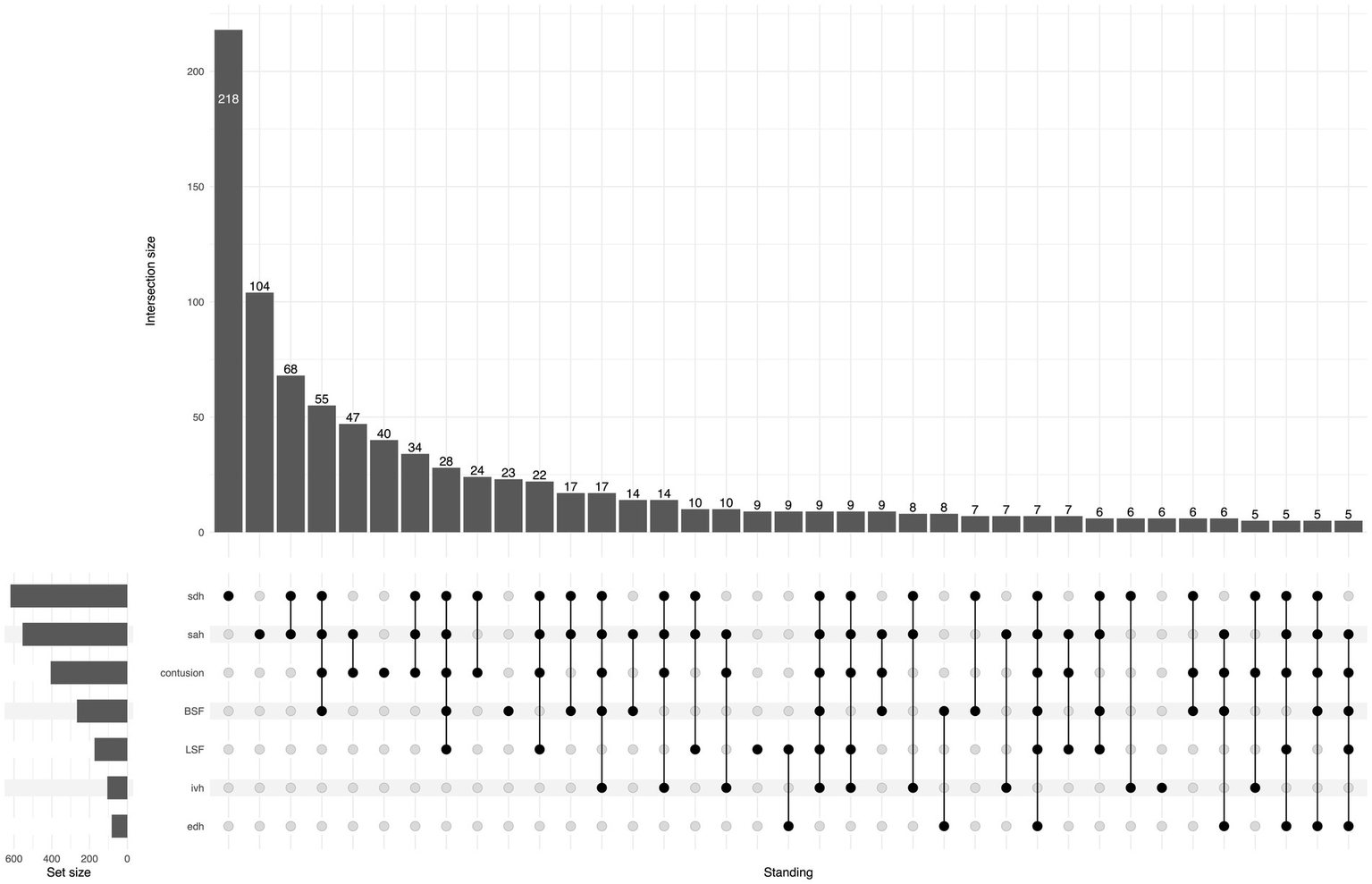
UpSet plot shows traumatic lesions on primary head CT for the FSH group, the number of patients in each lesion/combination is shown at the top of each bar. For ease of reading, the tail in the plot is cut when the number of cases is less than five patients. BSF, basilar skull fracture; EDH, epidural hematoma; IVH, intraventricular hemorrhage; LSF, linear skull fracture; SAH, subarachnoid hemorrhage; SDH, subdural hematoma.
3.4 Outcome at 6 months
At 6 months, GOS was available from 1353/1432 (94%) of the included patients (441/470 (94%) in the SRF group and 912/962 (95%) in the FSH group).
For the whole cohort, 551/1353 (41%) had an unfavorable outcome (GOS 1–3) at 6 months, and mortality was 319/1432 (22%). In the SRF group, 164/441 (37%) had an unfavorable outcome compared to 387/912 (42%) in the FSH group (p = 0.066). Six-month mortality was 86/470 (18%) in the SRF group and 2033/962 (24%) in the FSH (p = 0.012). GOS scores at 6 months are shown in Figure 7.
Figure 7
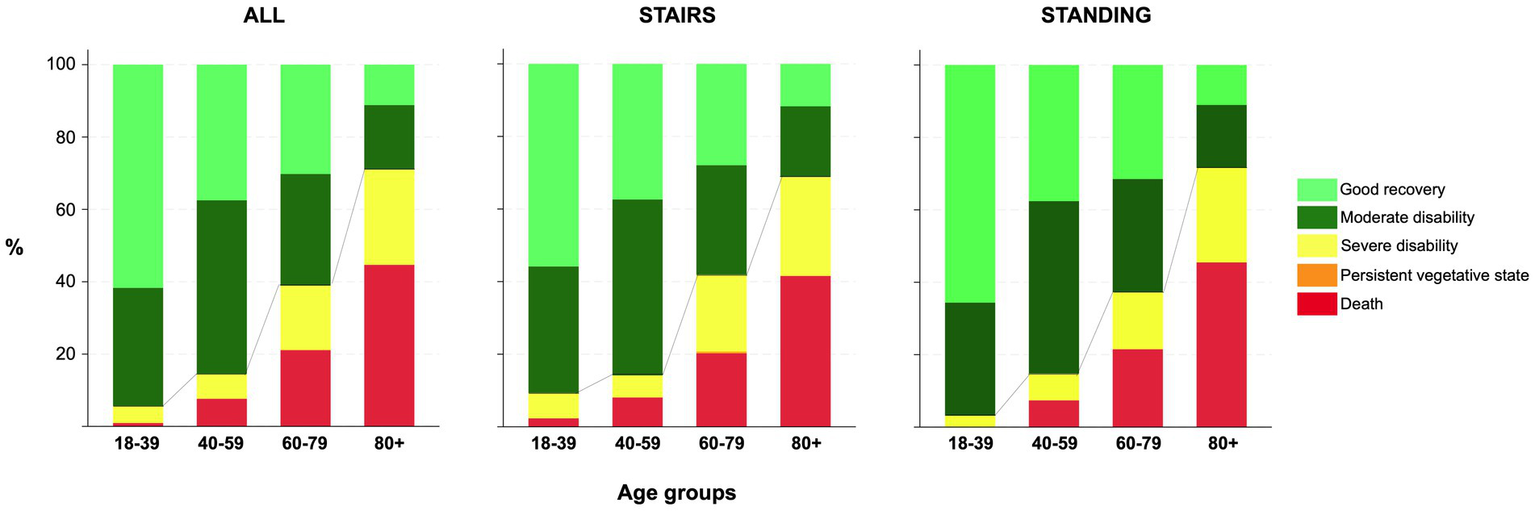
Bar charts for 6-month GOS by age group for all, and the two groups. Line represents proportions of dichotomized GOS and mortality.
When adjusting for covariates in the multivariable logistic regression model for unfavorable outcomes (GOS 1–3), the effect of stair-related fall did not reach statistical significance (OR 1.43, 95%, CI 0.97–2.12; p = 0.073). Covariates and results from the regression model are shown in Figure 8.
Figure 8
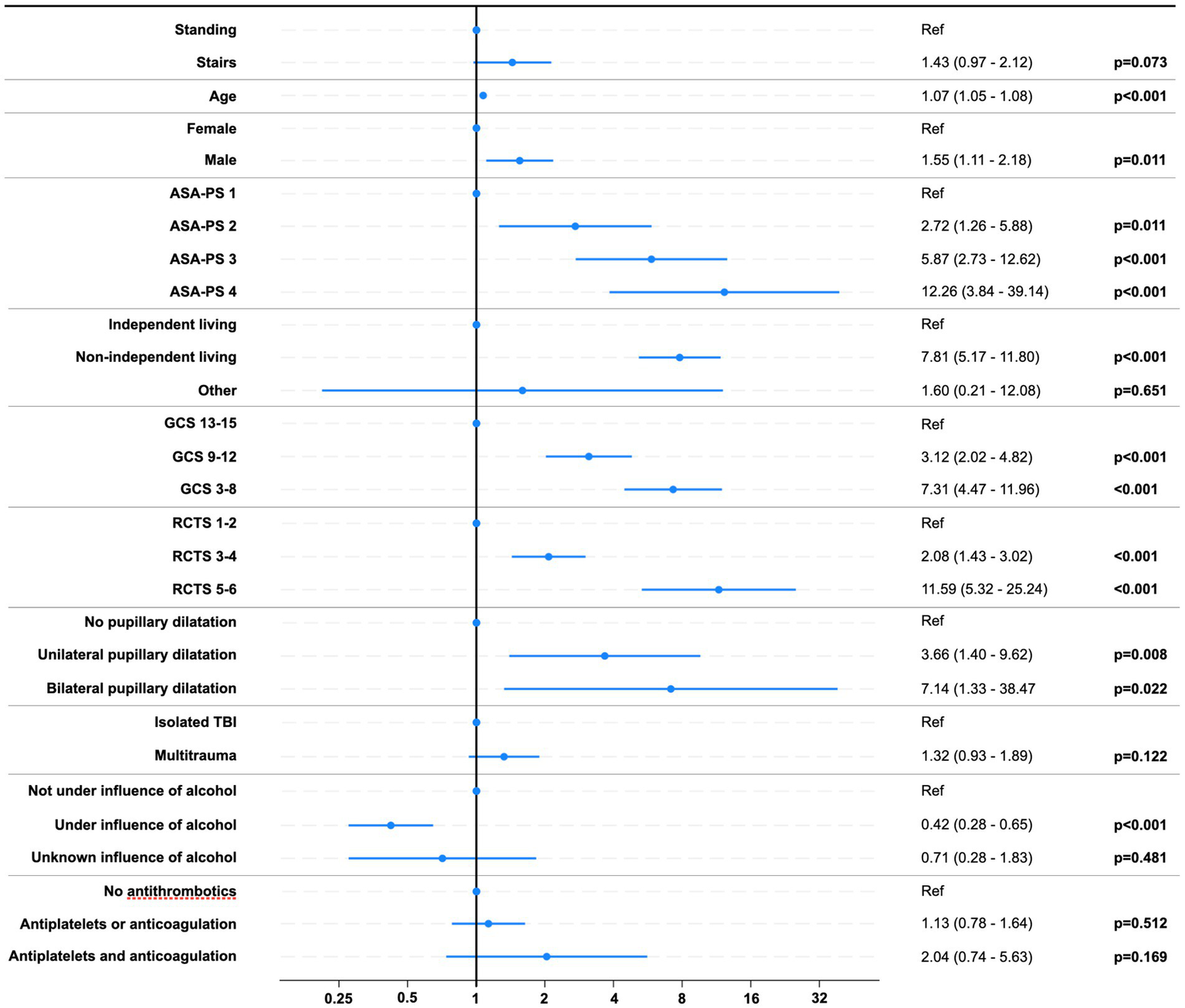
Risk of unfavorable outcome (GOS 1–3). Forest plot of odds ratios with 95% CIs. Abbreviations: ASA-PS, American Society of Anesthesiologists Physical Status Classification System; GCS, Glasgow coma scale score; RCTS, Rotterdam CT score.
The Sankey diagrams in Figures 9, 10 visualize 6-month outcomes for the two groups and changes in living status. In the SRF group, 62% of those who lived independently returned to independent living after 6 months, while 63% returned to independent living in the FSH group (p = 0.8).
Figure 9
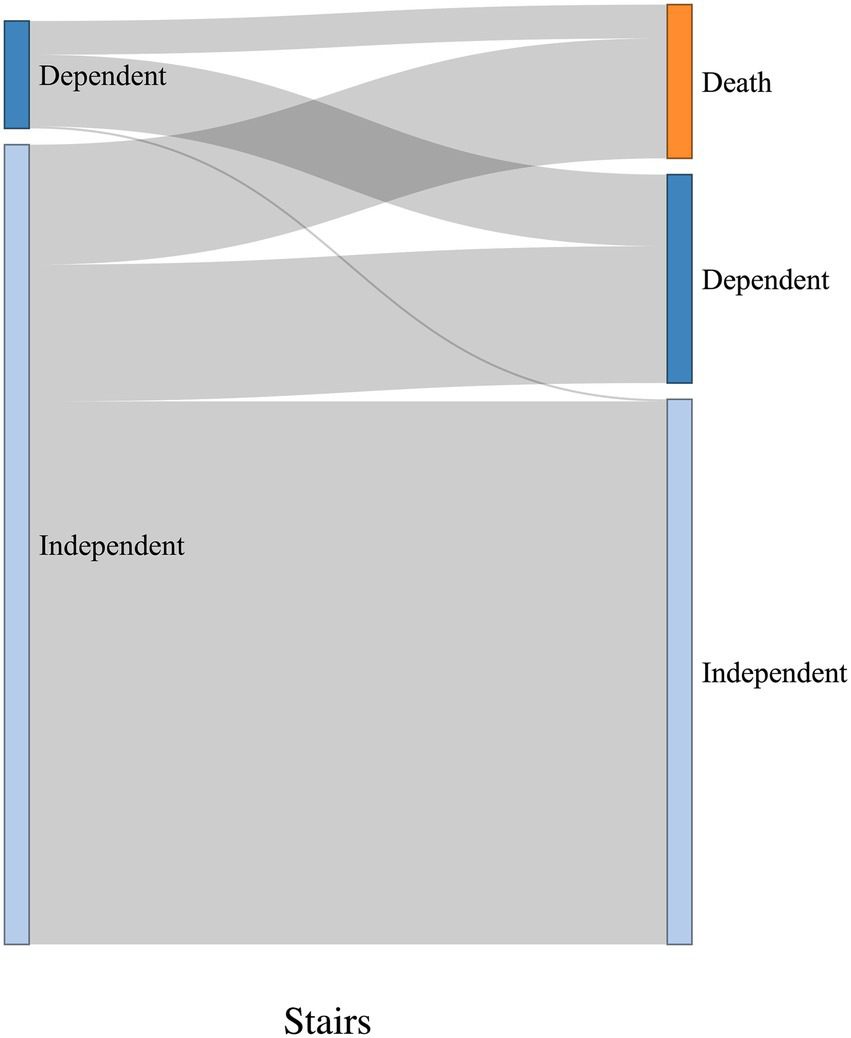
Visualization of change in living status between pre-trauma and 6-month post-trauma for the SRF group. Left side of the diagrams represents pre-trauma, and the right side represents 6-month post-trauma. The height of horizontal columns corresponds to the proportion of patients.
Figure 10
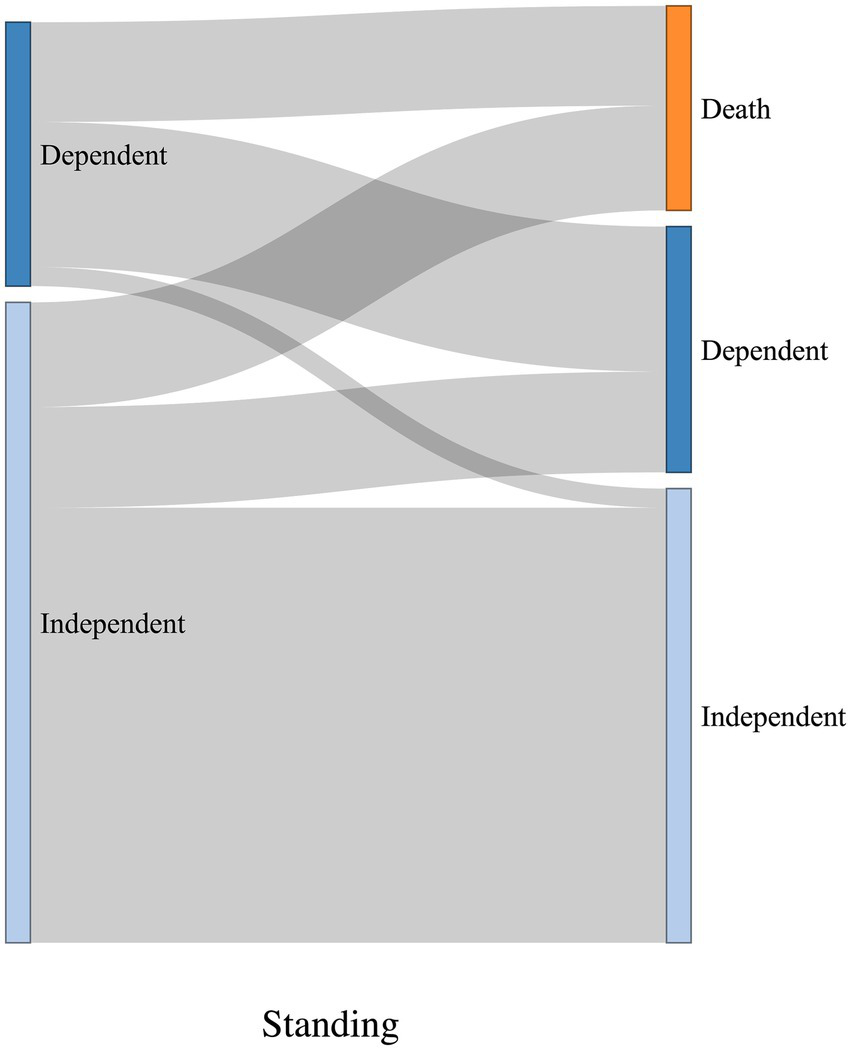
Visualization of change in living status between pre-trauma and 6-month post-trauma for the FSH group. Left side of the diagrams represents pre-trauma, and the right side represents 6-month post-trauma. The height of horizontal columns corresponds to the proportion of patients.
4 Discussion
In this cohort of TBI patients admitted to the regional neurotrauma center with traumatic findings on head CT, we show that the two most common fall mechanisms leading to TBI were falling from standing height- and stair-related falls, with the latter representing a quarter of all registered falls in OTBIR-N. When comparing the two fall mechanisms, patients in the SRF group were younger and had fewer comorbidities before the injury, but also sustained more severe TBIs. Unadjusted unfavorable outcomes were more common in the FSH patients; however, in the adjusted analysis, a signal of falling down the stairs being associated with unfavorable outcomes was observed, and this likely relates to the adjustment for pre-injury patient factors, including age and comorbidities. SRFs leading to TBI showed a predilection for occurring in the evening and nighttime hours and on the weekends and were more often related to alcohol influence compared to TBI from FSH.
Our results indicate two different patterns of patient and situational factors associated with different fall mechanisms. For FSH, the typical patient can be seen as an elderly patient where patient factors such as comorbidities and frailty may dominate as predisposing factors (2, 23). On the other hand, for SRFs, the typical patient can be seen as a middle-aged person where the situational factors of alcohol influence in the evening and night-time at weekends may dominate in their predisposition. Whether these patterns are unique to this cohort remains undetermined; however, other studies on TBI after stair-related falls show similar trends (12–14).
Overall, the results indicate that both fall mechanisms were associated with an unfavorable functional outcome 6 months after injury in a considerable proportion of patients in the present cohort. With falls representing the leading cause of TBI in an aging population in high-income countries, characterization of fall mechanisms is important to facilitate preventive measures. While reducing patient factors of comorbidity and frailty in the population remains something of a general aim of modern medicine, the situational factors associated with falling down the stairs appear more readily attainable for direct preventive measures. In society, having drinks at home on a Saturday night and walking down the stairs will hardly be regarded as even remotely risky behavior; however, the present results indicate that some falls down the stairs are associated with a non-negligible cost for falling. Middle-aged and older people may consider themselves responsible drinkers, which can make them less likely to recognize the risks (24). As such, public information on the risk associated with alcohol influence and staircases seems warranted in the prevention of such falls.
Some limitations of the present study should be noted. First, it is a single-center study, and this may affect its generalizability per se. Second, it is a study on a cohort of TBI patients from a regional referral center, being the sole provider of neurosurgical services in the region. This implies patient selection in the cohort, as regional patients who would not be considered candidates for neurosurgical intervention may not always be transferred from local hospitals. As such, certain categories of patients, for example, older ages and severely comorbid patients, are likely to be under-represented in the cohort as a whole (25, 26); however, this selection bias should expectantly be similar for both fall mechanisms. Third, the level of detail regarding the available information in the registry on fall mechanisms and contributing factors is limited. This includes data on alcohol levels and drug test results, as well as information on stair ascent/descent, the number of steps, and other person-specific and environmental factors. Consequently, this affects the conclusions that can be drawn. Further studies should collect and analyze data on the conditions at the time of the fall in greater detail.
5 Conclusion
SRFs were the second most common fall mechanism leading to TBI after FSH in the present cohort. Compared to FSH, SRFs occurred in younger and less premorbid patients, occurred more often during evening and nighttime hours, and on weekends, were more often associated with alcohol influence, and more commonly resulted in severe TBIs. Public information on the risks associated with the combination of alcohol influence and staircases seems warranted in the prevention of such falls.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets for this study are not publicly available due to privacy and ethical restrictions. Requests to access these datasets should be directed to uxtvec@ous-hf.no.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
CT: Formal analysis, Data curation, Writing – review & editing, Conceptualization, Writing – original draft, Methodology. AR: Formal analysis, Writing – review & editing, Methodology. EH: Supervision, Data curation, Writing – review & editing. TH: Writing – review & editing. UM: Writing – review & editing. MA: Writing – review & editing. KS: Writing – review & editing. PR: Writing – review & editing. DN: Formal analysis, Methodology, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The Department of Neurosurgery and all the neurosurgeons at Ullevål for tracking all patients admitted to OUH with TBI since 2015. Ola Skaansar for substantial contribution to registration when the OTBIR-N was established.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. At a minimal level, I have used GPT-4 Omni, available through the University of Oslo, as an assistant for language improvement during the writing process. However, all text in this submission has been thoroughly worked on by the authors.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
James SL Theadom A Ellenbogen RG Bannick MS Montjoy-Venning W Lucchesi LR et al . Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:56–87. doi: 10.1016/S1474-4422(18)30415-0
2.
Depreitere B Becker C Ganau M Gardner RC Younsi A Lagares A et al . Unique considerations in the assessment and management of traumatic brain injury in older adults. Lancet Neurol. (2025) 24:152–65. doi: 10.1016/S1474-4422(24)00454-X
3.
Lecky FE Otesile O Marincowitz C Majdan M Nieboer D Lingsma HF et al . The burden of traumatic brain injury from low-energy falls among patients from 18 countries in the CENTER-TBI registry: A comparative cohort study. PLoS Med. (2021) 18:e1003761. doi: 10.1371/journal.pmed.1003761
4.
Maas AIR Fitzgerald M Gao G Gupta D Hutchinson P Manley GT et al . Traumatic brain injury over the past 20 years: research and clinical progress. Lancet Neurol. (2022) 21:768–70. doi: 10.1016/S1474-4422(22)00307-6
5.
Steyerberg EW Wiegers E Sewalt C Buki A Citerio G De Keyser V et al . Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. (2019) 18:923–34. doi: 10.1016/S1474-4422(19)30232-7
6.
Tverdal C Aarhus M Andelic N Skaansar O Skogen K Helseth E . Characteristics of traumatic brain injury patients with abnormal neuroimaging in Southeast Norway. Inj Epidemiol. (2020) 7:45. doi: 10.1186/s40621-020-00269-8
7.
WHO . Step safely: Strategies for preventing and managing falls across the life-course. Geneva: World Health Organization (2021).
8.
Ambrose AF Paul G Hausdorff JM . Risk factors for falls among older adults: a review of the literature. Maturitas. (2013) 75:51–61. doi: 10.1016/j.maturitas.2013.02.009
9.
Ohm E Madsen C Alver K . Skader og ulykker i Norge. I: Folkehelserapporten - Helsetilstanden i Norge [In norwegian]. Oslo: Folkehelseinstituttet (2022).
10.
Boyé ND Mattace-Raso FU Van der Velde N Van Lieshout EM De Vries OJ Hartholt KA et al . Circumstances leading to injurious falls in older men and women in the Netherlands. Injury. (2014) 45:1224–30. doi: 10.1016/j.injury.2014.03.021
11.
Moreland BL Kakara R Haddad YK Shakya I Bergen G . A descriptive analysis of location of older adult falls that resulted in emergency department visits in the United States, 2015. Am J Lifestyle Med. (2021) 15:590–7. doi: 10.1177/1559827620942187
12.
Chatha H Sammy I Hickey M Sattout A Hollingsworth J . Falling down a flight of stairs: the impact of age and intoxication on injury pattern and severity. Trauma. (2018) 20:169–74. doi: 10.1177/1460408617720948
13.
Hammig B Haldeman S . Stair-related falls in the USA: traumatic brain injury and the role of alcohol intoxication. J Epidemiol Community Health. (2022) 77:44–8. doi: 10.1136/jech-2022-219396
14.
Hörauf JA Nau C Mühlenfeld N Verboket RD Marzi I Störmann P . Injury patterns after falling down stairs-high ratio of traumatic brain injury under alcohol influence. J Clin Med. (2022) 11:697. doi: 10.3390/jcm11030697
15.
Jacobs JV . A review of stairway falls and stair negotiation: lessons learned and future needs to reduce injury. Gait Posture. (2016) 49:159–67. doi: 10.1016/j.gaitpost.2016.06.030
16.
Zaskey M Seely KD Hansen M Collins HE Burns A Burns B . Outcomes after stairway falls in a rural Appalachian trauma center. Surgery. (2023) 174:626–30. doi: 10.1016/j.surg.2023.05.006
17.
Bryant AM Rose NB Temkin NR Barber JK Manley GT McCrea MA et al . Profiles of cognitive functioning at 6 months after traumatic brain injury among patients in level I trauma centers: A TRACK-TBI study. JAMA Netw Open. (2023) 6:e2349118. doi: 10.1001/jamanetworkopen.2023.49118
18.
Galimberti S Graziano F Maas AIR Isernia G Lecky F Jain S et al . Effect of frailty on 6-month outcome after traumatic brain injury: a multicentre cohort study with external validation. Lancet Neurol. (2022) 21:153–62. doi: 10.1016/S1474-4422(21)00374-4
19.
American Society of Anesthesiologists . ASA Physical Status Classification System. (2014). Available online at: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (Accessed August 11, 2019).
20.
Teasdale G Maas A Lecky F Manley G Stocchetti N Murray G . The Glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. (2014) 13:844–54. doi: 10.1016/S1474-4422(14)70120-6
21.
Maas AI Hukkelhoven CW Marshall LF Steyerberg EW . Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. (2005) 57:1173–82. doi: 10.1227/01.NEU.0000186013.63046.6B
22.
McMillan T Wilson L Ponsford J Levin H Teasdale G Bond M . The Glasgow outcome scale - 40 years of application and refinement. Nat Rev Neurol. (2016) 12:477–85. doi: 10.1038/nrneurol.2016.89
23.
Kiwanuka O Lassarén P Fletcher-Sandersjöö A Tatter C Tjerkaski J Nelson DW et al . ASA score is an independent predictor of 1-year outcome after moderate-to-severe traumatic brain injury. Scand J Trauma Resusc Emerg Med. (2025) 33:25. doi: 10.1186/s13049-025-01338-x
24.
Bareham BK Kaner E Spencer LP Hanratty B . Drinking in later life: a systematic review and thematic synthesis of qualitative studies exploring older people's perceptions and experiences. Age Ageing. (2019) 48:134–46. doi: 10.1093/ageing/afy069
25.
Cuevas-Østrem M Thorsen K Wisborg T Røise O Helseth E Jeppesen E . Care pathways and factors associated with interhospital transfer to neurotrauma centers for patients with isolated moderate-to-severe traumatic brain injury: a population-based study from the Norwegian trauma registry. Scand J Trauma Resusc Emerg Med. (2023) 31:34. doi: 10.1186/s13049-023-01097-7
26.
Rahim S Laugsand EA Fyllingen EH Rao V Pantelatos RI Müller TB et al . Moderate and severe traumatic brain injury in general hospitals: a ten-year population-based retrospective cohort study in Central Norway. Scand J Trauma Resusc Emerg Med. (2022) 30:68. doi: 10.1186/s13049-022-01050-0
Summary
Keywords
traumatic brain injury, epidemiology, falls, stairs, standing height, adults, older
Citation
Tverdal C, Reiner A, Helseth E, Hellstrøm T, Manskow US, Aarhus M, Skogen K, Rønning P and Netteland DF (2025) Comparison of traumatic brain injury resulting from stair-related falls to falls from standing height—a neurotrauma center cohort. Front. Neurol. 16:1599229. doi: 10.3389/fneur.2025.1599229
Received
24 March 2025
Accepted
17 June 2025
Published
04 July 2025
Volume
16 - 2025
Edited by
Chandler Rhodes, National Intrepid Center of Excellence (NICoE), United States
Reviewed by
Clemens Becker, Heidelberg University Hospital, Germany
Albert K. Okrah, Augusta University, United States
Updates
Copyright
© 2025 Tverdal, Reiner, Helseth, Hellstrøm, Manskow, Aarhus, Skogen, Rønning and Netteland.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cathrine Tverdal, uxtvec@ous-hf.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.