- 1College of Sports Science, Jishou University, Jishou, Hunan, China
- 2School of Physical Education and Arts, Hunan University of Medicine, Huaihua, Hunan, China
- 3Faculty of Health Sciences and Sport, Macao Polytechnic University, Macau, Macao SAR, China
- 4Welfare Center (Elderly Care Service Department), Shanghe County Hospital of Traditional Chinese Medicine, Jinan, Shandong, China
Objective: Reduced quality of life is a typical manifestation of Parkinson's disease (PD) patients, and exercise has a significant effect on improving the quality of life of PD patients. However, it is still unclear which exercise is effective in improving quality of life for people with Parkinson's disease. The aim of this study was to compare effective exercises for improving quality of life in patients with Parkinson's disease through a network meta-analysis.
Method: We conducted comprehensive database searches, including PubMed, Cochrane Library, Embase, Web of Science and CNKI. The included studies assessed methodological quality using the Cochrane Bias risk tool, and we collected information from the studies to compare the effects of 25 exercise interventions on quality of life in PD patients.
Results: The results of the network meta-analysis showed that QG is higher than OE (MD, −9.26; 95%CI, −18.25 to −0.27), FAE (MD, −10.77; 95%CI −19.52 to −2.02), VR (MD, −10.65; 95%CI −19.70 to −1.60), TC (MD, −11.06; 95%CI −21.32 to −0.81), CT (MD, −11.42; 95%CI −20.73 to −2.11), RT (MD, −11.60; 95%CI −20.72 to −2.49), CE (MD −12.50; 95%CI −24.47 to −0.52), GT (MD −13.10; 95%CI −24.67 to −1.52) to better improve quality of life in patients with PD. DE is superior to TR (SMD, −2.10; 95%CI −3.75 to −0.45), BT (SMD, −2.63; 95%CI −4.93 to −0.32), BDJ (SMD, −4.14; 95%CI −6.15 to −2.14), RT (SMD, −4.54; 95%CI −6.94 to −2.14), TC (SMD, −5.28; 95%CI −7.73 to −2.84) in reduce depression. ROT is superior to TR (MD, 6.17; 95%CI 0.57 to 11.77), in improving balance capacity.
Conclusion: Our study found that QG improved the quality of life in patients with PD better than other forms of exercise. DE is more effective than other exercises in reducing depression in PD patients. ROT is better at improving the balance of PD patients.
Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder primarily caused by an imbalance of neurotransmitters and a depletion of dopamine in the brain (1). Growing evidence indicates that the incidence of PD has increased in recent years, with reported rates ranging from 3.5 to 42.8% (2). Patients with PD typically present motor symptoms such as progressive motor retardation, myotonia, and abnormal posture and gait. These motor symptoms are often accompanied by non-motor symptoms such as depression, impulse control disorders, anxiety, and insomnia (3). Compared with motor symptoms, non-motor symptoms in patients with PD are more strongly associated with pain severity and hospitalization rates (4). Among these non-motor symptoms, depression and anxiety are common psychiatric conditions that substantially affect the quality of life and contribute to negative psychological states in patients with PD (5). In addition, factors such as self-image, life satisfaction, and social interactions are often negatively affected, which in turn further reduces patients' overall quality of life (6). The World Health Organization (WHO) defines quality of life as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns (7).” Quality of life encompasses physical, psychological, cognitive, and environmental factors, as well as autonomy and social relationships (7, 8). The primary goal of PD treatment is to minimize its negative impact on the patient's functional abilities and overall quality of life. Evaluating how the disease affects quality of life serves as a key indicator of treatment effectiveness (9).
At present, the treatment of Parkinson's disease includes drug therapy DBS surgery (deep brain stimulation) (10), while Western medicine mainly focuses on drugs such as levodopa and dopamine receptor agonists. Drug therapy can temporarily improve the physical disability of patients and control some symptoms through the replacement of dopamine in the brain. However, PD patients taking drugs for a long time will lead to side effects such as depression, anxiety, delusion, hallucination, and even mental disorders, and reduce the quality of life of PD patients. Exercise has been identified as a possible adjunctive treatment for Parkinson's disease, and periodic exercise can effectively improve motor function, depressive symptoms, sleep disturbances, and quality of life in patients (11). In one study, Ferreira et al. (12) found that resistance training was effective in improving the quality of life of PD patients. Moratelli et al. (13) found in a study that Brazilian dance can effectively improve the quality of life of PD patients. In a systematic review and meta-analysis, Song et al. (14) found that Tai Chi and Qigong mind-body exercises effectively improved the quality of life in patients with PD. At present, there is still controversy about what kind of exercise can effectively improve the quality of life of PD patients.
Network meta-analysis (NMA) has become increasingly common for evaluating medical interventions because it estimates the relative effectiveness of all interventions and the ranking of interventions, even in the absence of direct dairy comparisons (15).
However, the current NMA on the improvement of exercise in patients with Parkinson's disease mainly focuses on the movement symptoms of PD patients (16, 17), and there is a lack of research on the quality of life of PD patients. Our study took the quality of life of PD patients as the primary outcome indicator, compared the impact of 25 different exercises on the quality of life of PD patients, determined the best exercise mode to improve the quality of life of PD patients, and guided PD patients to choose the best exercise mode.
Methods and analysis
Registration
This network meta-analysis was designed according to the guidelines for Preferred Reporting Items of Systems Review and Network Meta-Analysis (PRISMA-NMA) (18).
Search strategy
The computer searched PubMed, Web of Science, Embase, Cochrane Library, CNKI, and other databases, and the search period was established until August 20, 2024. The search takes the way of combining subject words and free words. The search strategy uses Pubmed as an example, as shown in Appendix 1.
Study selection
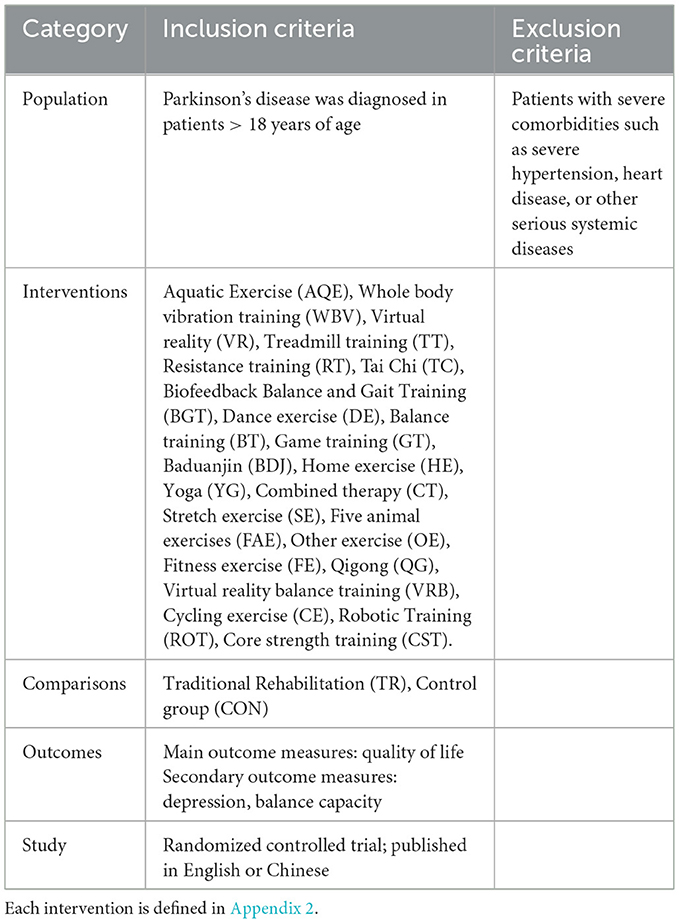
The inclusion criteria for study selection were based on the PICOS methodology (Participants, interventions, comparators, outcomes, and study design) (18), see Table 1.
Data extraction
The following data were extracted independently by two reviewers: first author, year of publication, country, sample size, intervention mode, intervention time, and intervention period. Data are expressed as mean ± standard deviation (mean ± SD). If the outcome indicator reports multiple points, we extract data for the most recent time.
Risk of bias assessment
The risk of bias was assessed independently by two reviewers and by a third reviewer using the tools provided by the Cochrane Collaboration (19), including sequence generation, hidden assignment, blinking, incomplete outcome data, non-selective reporting of results, and other sources of bias. Each criterion was judged to have a low, unclear, or high risk of bias.
Data analysis
The netmeta package of R-4.2.1 software was used to perform mesh meta-analysis. Use the STATA 15.1 “networkplot” feature to draw and generate a network diagram that describes and presents different forms of exercises. We use nodes representing various interventions and edges representing head-to-head comparisons between interventions. Node splitting assesses inconsistencies between direct and indirect comparisons (20). The combined estimates and their 95% confidence intervals (95%CI) were calculated using random effects network analysis. When we are interested in results using the same unit of measure in a study, consider analyzing the results as a therapeutic effect by means difference (MD) or evaluating standardized mean difference (SMD). A pair-to-pair random-effects meta-analysis was used to compare various exercise therapies. The heterogeneity of all pair-to-pair comparisons was assessed using the I2 statistic, and publication bias was evaluated using the P-value of Egger's test and the funnel plot.
Results
Literature selection
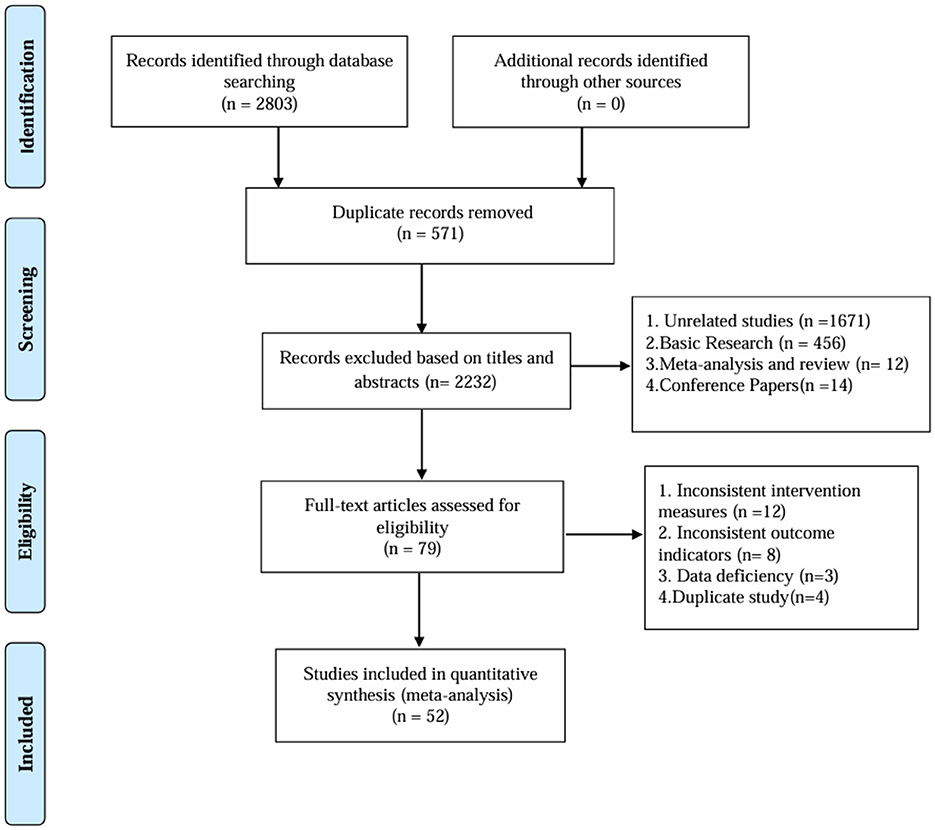
After deleting duplicates, 2,803 records were retrieved, 571 duplicates were removed, 2,232 articles with inconsistent titles were deleted and 52 articles were finally included. The research flow chart is shown in Figure 1.
Study and participant characteristics
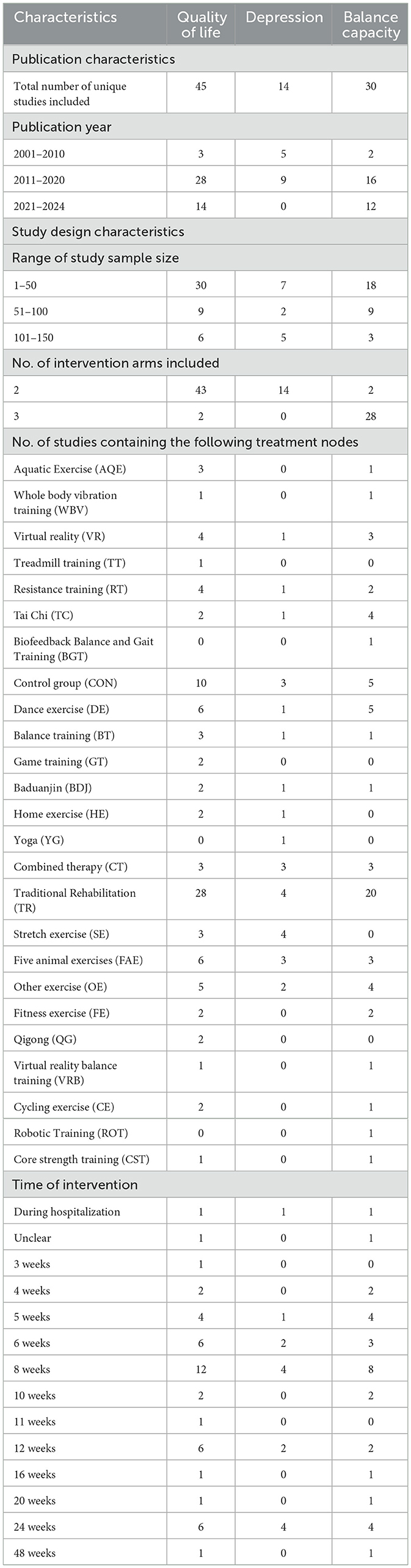
The studies, published between 2009 and 2023, compared the effects of 25 different forms of exercise on quality of life in people with Parkinson's disease. The duration of intervention ranged from 3 weeks to 48 weeks. Of all the included studies, 45 reported quality of life, 14 reported depression, and 30 reported BBS. The characteristics of the studies and participants are shown in Table 2 and Appendix 3. The risk assessment of bias for each study is summarized in Appendix 4 and Figure 2.
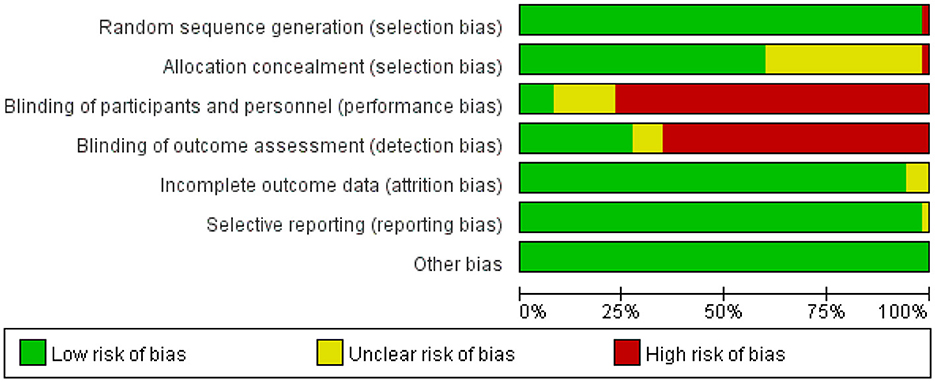
Figure 2. Percentage of studies examining the efficacy of exercise training in patients with non-specific chronic low back pain with low, unclear, and high risk of bias for each feature of the Cochrane Risk of Bias Tool.
Quality of life
A total of 45 studies evaluated Quality of life. We included the following 22 exercise measures in our network meta-analysis (Figure 3A): Aquatic Exercise (AQE), Whole body vibration training (WBV), Virtual reality (VR), Treadmill training (TT), Resistance training (RT), Tai Chi (TC), Control group (CON), Dance exercise (DE), Balance training (BT), Game training (GT), Baduanjin (BDJ), Home exercise (HE), Combined therapy (CT), Traditional Rehabilitation (TR), Stretch exercise (SE), Five animal exercises (FAE), Other exercise (OE), Fitness exercise (FE), Qigong (QG), Virtual reality balance training (VRB), Cycling exercise (CE), Core strength training (CST).Our results show that QG is higher than OE (MD, −9.26; 95%CI, −18.25 to −0.27), FAE (MD, −10.77; 95%CI −19.52 to −2.02), VR (MD, −10.65; 95%CI −19.70 to −1.60), TC (MD, −11.06; 95%CI −21.32 to −0.81), CT (MD, −11.42; 95%CI −20.73 to −2.11), RT (MD, −11.60; 95%CI −20.72 to −2.49), CE (MD −12.50; 95%CI −24.47 to −0.52), GT (MD −13.10; 95%CI −24.67 to −1.52), FE (MD −14.56; 95%CI −26.10 to −3.01), SE (MD −14.83; 95%CI −25.70 to −3.96), CON (MD −15.49; 95%CI −23.94 to −7.04), TR(MD −16.86; 95%CI −24.22 to −9.49), VRB(MD −20.49; 95%CI −40.30 to −0.67), DE(MD −17.58; 95%CI −26.56 to −8.60), HE(MD −18.28; 95%CI −28.34 to −8.22), BT(MD −23.09; 95%CI −34.77 to −11.41), CST(MD −24.84; 95%CI −38.69 to −10.98) to better improve quality of life in patients with Parkinson's disease (Figure 4A). In addition, we performed the Egger's test to assess publication bias (P= 0.352; Appendix 5.1). Heterogeneity and inconsistencies in the mesh meta-analysis were also evaluated (Appendix 6). We conducted a direct comparison of exercise interventions (Appendix 7.1).
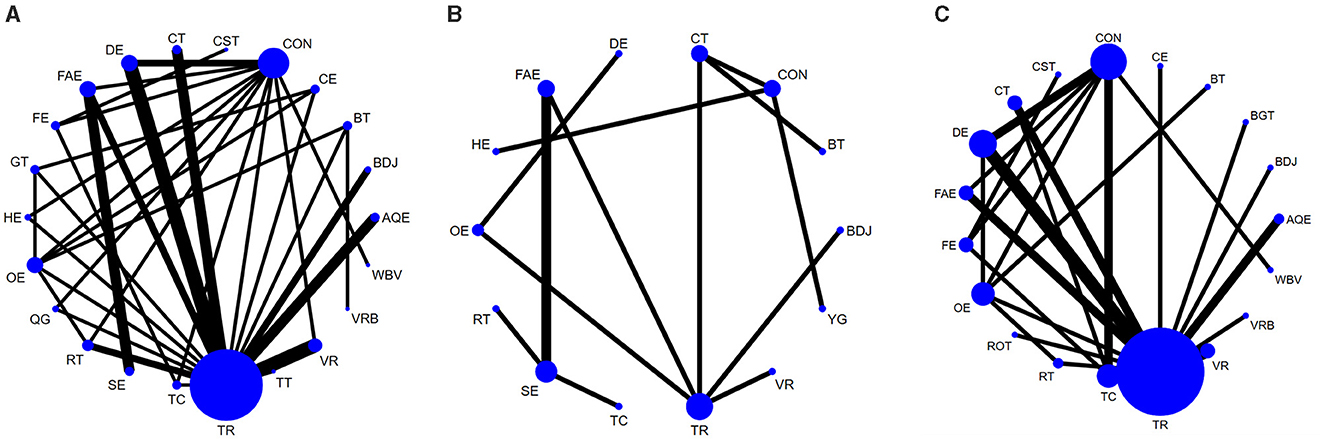
Figure 3. Network diagram of Quality of life (A), Depression (B), Balance capacity (C). The node size represents the number of times the exercise appears in any comparison about that treatment, and the width of the edge represents the total sample size in the comparison it connects. AQE, Aquatic Exercise; WBV, Whole body vibration training; VR, Virtual reality; TT, Treadmill training; RT, Resistance training; TC, Tai Chi; BGT, Biofeedback Balance and Gait Training; CON, Control group; DE, Dance exercise; BT, Balance training; GT, Game training; BDJ, Baduanjin; HE, Home exercise; YG, Yoga; CT, Combined therapy; TR, Traditional Rehabilitation; SE, Stretch exercise; FAE, Five animal exercises; OE, Other exercise; FE, Fitness exercise; QG, Qigong; VRB, Virtual reality balance training; CE, Cycling exercise; ROT, Robotic Training; CST, Core strength training.

Figure 4. Results analysis league chart, Quality of life (A), Depression (B), Balance capacity (C). The data are the mean difference and 95% confidence interval of continuous data. AQE, Aquatic Exercise; WBV, Whole body vibration training; VR, Virtual reality; TT, Treadmill training; RT, Resistance training; TC, Tai Chi; BGT, Biofeedback Balance and Gait Training; CON, Control group; DE, Dance exercise; BT, Balance training; GT, Game training; BDJ, Baduanjin; HE, Home exercise; YG, Yoga; CT, Combined therapy; TR, Traditional Rehabilitation; SE, Stretch exercise; FAE, Five animal exercises; OE, Other exercise; FE, Fitness exercise; QG, Qigong; VRB, Virtual reality balance training; CE, Cycling exercise; ROT, Robotic Training; CST, Core strength training.
Depression
A total of 14 studies evaluated depression. We included the following 14 exercise measures in the network meta-analysis (Figure 3B): Virtual reality (VR), Resistance training (RT), Tai Chi (TC), Control group (CON), Dance exercise (DE), Balance training (BT), Baduanjin (BDJ), Home exercise (HE), Yoga (YG), Combined therapy (CT), Traditional Rehabilitation (TR), Stretch exercise (SE), Five animal exercises (FAE), Other exercise (OE). The results show that DE is superior to TR (SMD, −2.10; 95%CI −3.75 to −0.45), BT (SMD, −2.63; 95%CI −4.93 to −0.32), BDJ (SMD, −4.14; 95%CI −6.15 to −2.14), RT (SMD, −4.54; 95%CI −6.94 to −2.14), TC (SMD, −5.28; 95%CI −7.73 to −2.84) (Figure 4B). In addition, we performed the Egger test to assess publication bias (P = 0.952; Appendix 5.2). We also evaluated the heterogeneity and inconsistencies of the mesh meta-analysis (Appendix 6).
Balance capacity
A total of 30 studies evaluated. We included the following 19 exercise measures in the network meta-analysis (Figure 3C): Aquatic Exercise (AQE), Whole body vibration training (WBV), Virtual reality (VR), Resistance training (RT), Tai Chi (TC), Biofeedback Balance and Gait Training (BGT), Control group (CON), Dance exercise (DE), Balance training (BT), Baduanjin (BDJ), Combined therapy (CT), Traditional Rehabilitation (TR), Five animal exercises (FAE), Other exercise (OE), Fitness exercise (FE), Virtual reality balance training (VRB), Cycling exercise (CE), Robotic Training (ROT), Core strength training (CST). The results show that: ROT is superior to TR (MD, 6.17; 95%CI 0.57 to 11.77) in improving balance capacity (Figure 4C). In addition, we assessed publication bias using the Egger test (P = 0.162; Appendix 5.3). We also evaluated heterogeneity and inconsistencies in the mesh meta-analysis (Appendix 6). We made a direct comparison of exercise interventions (Appendix 7.2).
Discussion
Parkinson's disease is a chronic and progressive neurodegenerative disease, with a high incidence of non-motor symptoms such as insomnia, impulse control disorders, anxiety and depression (21), which seriously affect the quality of life of Parkinson's patients. Therefore, it is significant to identify exercise methods to improve the quality of life of PD patients. Our study included 52 studies exploring 25 exercise methods to clarify the best exercise to improve the quality of life in people with PD.
Our study found that QG is superior to OE, FAE, VR, TC, CT, RT, CE, GT, FE, SE, CON, TR, VRB, DE, HE, BT, and CST in improving the quality of life of PD patients. In our study, PDQ-39 is the evaluation index of PD patients' quality of life. In this study, PDQ-39 was used to evaluate patients' quality of life. Research has proved that PDQ-39 is a reliable and valid tool for assessing the functional impact of PD (22), demonstrating satisfactory internal consistency and stability (23). It offers clinicians a practical and reliable tool for efficiently assessing and standardizing the quality of life of patients with PD. In our study, QG was characterized as a low- to moderate-intensity aerobic exercise that integrates heart, breath, and body regulation. The exercise routine was carefully structured, with a moderate overall intensity. In practice, its focus on guiding qi, calming the mind, and promoting relaxation helps patients clear their thoughts and achieve a tranquil, relaxed state. The coordinated movements of qi and blood can channel and nourish the meridians, enhance circulation, strengthen muscles, and improve the physiological functions of various tissues and organs in the body (24).
QG can effectively enhance telomerase activity in patients with chronic fatigue by modulating the pituitary–thalamic–adrenal axis, reducing oxidative stress levels, and regulating immune responses. In addition, as a low-impact exercise, QG increases the concentration of serum insulin-like growth factor (IGF), which further promotes telomerase activity and helps delay cellular aging (25). Furthermore, studies have demonstrated that QG can enhance the regulatory mechanisms of the neural circuits connecting the cortex, pons, and cerebellum. This improvement contributes to greater precision in fine motor skills and, in turn, enhances the quality of life of patients with PD (26). Apathetic mood is a common symptom in patients with PD. QG, a low-intensity exercise, is well-suited to the physical and mental attributes of older adults. Communities should provide more accessible exercise spaces and enhance guidance on appropriate exercise methods for patients with PD to help improve their quality of life. Compared with VR, RT, and GT, QG is an effective and low-cost form of exercise. Moreover, long-term RT and VR can lead to increased muscle and visual fatigue in patients with PD. Studies have reported mild dizziness after VR, which ultimately increases discomfort for patients with PD (27). Many factors affect the quality of life of patients with PD. Studies have found that the age (28, 29), gender (29), duration of disease (30) and severity of disease (31, 32) course of PD patients can all affect the quality of life of PD patients. Therefore, the influence of other factors should be comprehensively considered when evaluating the impact of exercise interventions on the quality of life of patients with PD. However, our study observed that only QG was superior to other exercises in improving the quality of life of patients with PD. Notably, only two articles on QG were included in this meta-analysis.
Depression is a common negative emotion experienced by patients with PD (33). Depression is characterized by the loss of positive emotions and manifests as a reduced interest or pleasure in daily activities, inattention, and low mood (34). Repeated and persistent emotional stress can also contribute to the progression of neurodegenerative diseases. In our study, DE was more effective than TR, BT, BDJ, RT, and TC in improving depression in patients with PD. The American Dance Therapy Association (35) defines dance therapy as “a psychosomatic therapeutic exercise that promotes emotional, cognitive, physical, and social integration.” Dance therapy can improve social interaction among patients with PD, increase their enthusiasm for exercise, and effectively alleviate emotional disorders (35). Compared with BT and RT, dance is a highly social activity that offers opportunities for non-verbal communication and self-improvement (36, 37). Participating in group dance classes and dancing with partners provides valuable social interaction (38). Therefore, dance serves as movement therapy and improves social engagement, interpersonal relationships, and social support for patients with PD. According to the American Dance Therapy Association, dance therapy promotes emotional, cognitive, physical, and social integration. It helps patients with PD connect with others, improve interpersonal relationships, and prevent social isolation, which in turn eases depression. In addition, DE can reduce the progressive degeneration of neuronal axons, promote dendrite formation of new synapses, establish new neural connections, and activate or create new neural pathways. These improvements enhance the brain's regulatory control over the limbs, improve joint flexibility, and alleviate movement disorders (39). During dancing, external stimuli promote the release of various neurotransmitters, which help patients with PD train motor memory, improve balance and cognitive function, and alleviate depression (40).
However, considering that PD patients are usually elderly and have a stiff gait, they are particularly prone to fall when starting to run, turning around or avoiding obstacles. Therefore, special attention should be paid to the safety of the venue environment, and exercise should be carried out in open Spaces without obstacles as much as possible (41, 42). In addition, for PD patients, warm-up activities should be carried out before exercise to increase the movement of various body parts, posture coordination and breathing and prevent muscle strains (37). During the training process, family members of the patients should accompany and protect them and try to minimize unnecessary injuries. Considering that female PD patients are suspicious and sensitive, they are more likely to be unable to proceed smoothly due to the fear of falling. Psychological communication should be conducted when necessary to alleviate the patients' concerns.
Balance capacity refers to the ability to maintain the body's center of gravity within its base of support during both static (stationary) and dynamic (moving) tasks (43). Good balance control and quick postural adjustment to external disturbances are key factors for stable and efficient walking (44, 45). Our study found that ROT was superior to TR in improving balance in PD patients. ROT is based on the theory of central nervous system plasticity and functional reorganization, and a large number of repetitive and purposefully weight-loss walking training can also improve balance and contribute to the automation of gait and the improvement of pace (46, 47). The closed-chain movement assisted by the G-EOSystem® lower limb rehabilitation robot increased the stimulation of the anti-gravity proprioception of the lower limb with decreased sensitivity. The central nervous system, supplemented by the external visual feedback system, mobilizes the body's postural regulation system and finally realizes the body's balance and stability by effectively exporting the coordinated contraction of the peripheral trunk and lower limb muscles (48). Furthermore, some studies have found that ROT can generate external rhythms through proprioceptive cue effects in a fixed pattern to compensate for the internal rhythm defects caused by abnormal basal ganglion circuits in patients with Parkinson's disease, which is helpful for maintaining normal gait (49, 50), thereby increasing the balance ability of PD patients.
Strengths and limitations
First, this study is the most comprehensive and systematic comparative meta-analysis of the effects of exercise on quality of life in people with Parkinson's disease. In a reasonably large sample size of 52 studies, we included 25 exercise interventions. We compare them directly or indirectly with other interventions to provide new, comprehensive, evidence-based recommendations. In our research, we found that QG can effectively improve the quality of life of PD patients, and DE can improve the depression of PD patients. Overall, this study has specific clinical significance. Overall, our study also has certain limitations, and these results should be treated with caution as the sample size for each intervention is usually relatively small and has some influence on the results. Although we analyzed heterogeneity in the included literature, some factors between studies are unavoidable. The quality of life of PD patients is affected by many factors. We should also consider the influence of other factors when considering the quality of life and depression of PD patients. Unfortunately, different types of exercise set different exercise intensity standards, and many of the included studies did not report exercise intensity. Furthermore, it is important not to overlook the feasibility and accessibility of implementing the most effective physical intervention in clinical or community Settings. During the implementation of QG, DE, and ROT, attention should be paid to the compliance of PD patients, the required infrastructure, and professional training to ensure the best effect of the intervention. The prerequisite for PD patients is to ensure their safety. Therefore, there are requirements for aspects such as the venue, patient attire, and accompanying personnel. In future studies, researchers should report in detail the exercise intensity and exercise cycle and compare the impact of different exercise intensities and exercise cycles on the quality of life of PD patients to determine which exercise intensity is more conducive to improving the quality of life of PD patients.
Conclusion
Our study found that QG improved the quality of life of people with Parkinson's disease better than other forms of exercise. DE can effectively improve depression in patients with Parkinson's disease, and ROT can effectively improve balance in patients with PD. In summary, exercise substantially affects PD's quality of life and negative emotions, but its internal molecular mechanism and the connection and regulation of neural circuits remain to be further explored. In addition, in the quality evaluation of this study, we found that many studies did not use blind methods and randomization, resulting in generally low evidential certainty of research results. Therefore, it is suggested that the survey quality be strictly controlled and the sample size be increased to verify this study's results further. Exercise has positive significance in the prevention, clinical practice and rehabilitation of PD. A standardized therapeutic effect evaluation system should be established for clinical medical staff to clarify the optimal training plan and parameter Settings for different disease courses and types of PD patients. Furthermore, due to the diversity of exercise methods, the focus of intervention for patients by various exercise methods is not the same, and thus the intervention effects are also different. PD patients should adopt a comprehensive intervention form that organically and scientifically combines multiple exercise methods to achieve the best intervention effect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL: Writing – review & editing, Software, Conceptualization, Formal analysis, Writing – original draft. RZ: Writing – original draft, Writing – review & editing, Software. JZ: Writing – original draft, Methodology, Writing – review & editing, Software. XL: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1601080/full#supplementary-material
References
1. Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
2. Kovács M, Makkos A, Pintér D, Juhász A, Darnai G, Karádi K, et al. Screening for problematic internet use may help identify impulse control disorders in Parkinson's disease. Behav Neurol. (2019) 2019:4925015. doi: 10.1155/2019/4925015
3. Reich SG, Savitt JM. Parkinson's disease. Med Clin North Am. (2019) 103:337–50. doi: 10.1016/j.mcna.2018.10.014
4. Lewitt PA, Chaudhuri KR. Unmet needs in Parkinson disease: motor and non-motor. Parkinsonism Relat Disord. (2020) 80:S7–12. doi: 10.1016/j.parkreldis.2020.09.024
5. Ray S, Agarwal P. Depression and anxiety in Parkinson disease. Clin Geriatr Med. (2020) 36:93–104. doi: 10.1016/j.cger.2019.09.012
6. Crispino P, Gino M, Barbagelata E, Ciarambino T, Politi C, Ambrosino I, et al. Gender differences and quality of life in Parkinson's disease. Int J Environ Res Public Health. (2020) 18:198. doi: 10.3390/ijerph18010198
7. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. (1995) 41:1403–9. doi: 10.1016/0277-9536(95)00112-K
8. Zhao N, Yang Y, Zhang L, Zhang Q, Balbuena L, Ungvari GS, et al. Quality of life in Parkinson's disease: a systematic review and meta-analysis of comparative studies. CNS Neurosci Ther. (2021) 27:270–9. doi: 10.1111/cns.13549
9. Jaracz K, Kozubski W. Quality of life in stroke patients. Acta Neurol Scand. (2003) 107:324–9. doi: 10.1034/j.1600-0404.2003.02078.x
10. Martínez-Martín P. An introduction to the concept of “quality of life in Parkinson's disease”. J Neurol. (1998) 245:S2–6. doi: 10.1007/PL00007733
11. Rafferty MR, Schmidt PN, Luo ST, Li K, Marras C, Davis TL, et al. Regular exercise, quality of life, and mobility in Parkinson's disease: a longitudinal analysis of national Parkinson foundation quality improvement initiative data. J Parkinsons Dis. (2017) 7:193–202. doi: 10.3233/JPD-160912
12. Ferreira RM, Alves W, De Lima TA, Alves TGG, Alves Filho PAM, Pimentel CP, et al. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson's disease: a randomized controlled trial. Arq Neuropsiquiatr. (2018) 76:499–506. doi: 10.1590/0004-282x20180071
13. Moratelli JA, Delabary MDS, Curi VS, Passos-Monteiro E, Swarowsky A, Haas AN, et al. An exploratory study on the effect of 2 Brazilian dance protocols on motor aspects and quality of life of individuals with Parkinson's disease. J Dance Med Sci. (2023) 27:153–9. doi: 10.1177/1089313X231178094
14. Song R, Grabowska W, Park M, Osypiuk K, Vergara-Diaz GP, Bonato P, et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. (2017) 41:3–13. doi: 10.1016/j.parkreldis.2017.05.019
15. Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ. (2014) 348:g1741. doi: 10.1136/bmj.g1741
16. Yang Y, Wang G, Zhang S, Wang H, Zhou W, Ren F, et al. Efficacy and evaluation of therapeutic exercises on adults with Parkinson's disease: a systematic review and network meta-analysis. BMC Geriatr. (2022) 22:813. doi: 10.1186/s12877-022-03510-9
17. Zhang SK, Gu ML, Xu H, Zhou WS, Mao SJ, Yang Y. Effects of different exercise modes on gait performance of Parkinson's disease patients: a systematic review and network meta-analysis. Percept Mot Skills. (2023) 130:1524–61. doi: 10.1177/00315125231178669
18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. (2015) 15:58. doi: 10.1186/s12874-015-0060-8
21. Hayes MT. Parkinson's disease and parkinsonism. Am J Med. (2019) 132:802–7. doi: 10.1016/j.amjmed.2019.03.001
22. Margolius A, Cubillos F, He Y, Wu S, Schmidt P, Simuni T. Predictors of clinically meaningful change in PDQ-39 in Parkinson's disease. Parkinsonism Relat Disord. (2018) 56:93–7. doi: 10.1016/j.parkreldis.2018.06.034
23. Hagell P, Whalley D, Mckenna SP, Lindvall O. Health status measurement in Parkinson's disease: validity of the PDQ-39 and nottingham health profile. Mov Disord. (2003) 18:773–83. doi: 10.1002/mds.10438
24. Xumin J, Jiaqi G, Yuanyuan Q, Shanshan W, Bingjie C, Ganoderma S. The inheritance and innovation of guidance techniques can be seen from the comparative study of several modern common guidance techniques. Lishizhen Med Mater Med Res. (2022) 33:444–6. doi: 10.3969/j.issn.1008-0805.2022.02.53
25. Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Pöss J, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. (2008) 52:470–82. doi: 10.1016/j.jacc.2008.04.034
26. Fengchun L, Hong C, Qian M, Jing L, Cuijing L, Yan Y, et al. Nursing quality special management practice based on sports symptoms of patients with Parkinson's disease. Chin Nurs Res. (2019) 33:2832–5. doi: 10.12102/j.issn.1009-6493.2019.16.022
27. Jing L, Zhi Y, Ruisong L, Chao Y, Ming S. The rehabilitation effect of virtual reality technology on the balance function of patients with Parkinson's disease. Chin J Rehabil Med. (2020) 35:682–7. doi: 10.3969/j.issn.1001-1242.2020.06.007
28. Zhou Z, Zhou X, Zhou X, Xiang Y, Zhu L, Qin L, et al. Characteristics of autonomic dysfunction in Parkinson's disease: a large chinese multicenter cohort study. Front Aging Neurosci. (2021) 13:761044. doi: 10.3389/fnagi.2021.761044
29. Simon-Gozalbo A, Rodriguez-Blazquez C, Forjaz MJ, Martinez-Martin P. Clinical characterization of Parkinson's disease patients with cognitive impairment. Front Neurol. (2020) 11:731. doi: 10.3389/fneur.2020.00731
30. Benge JF, Kekecs Z, Encarnacion E, Ainslie M, Herff C, Elkins G, et al. Duration of disease does not equally influence all aspects of quality of life in Parkinson's disease. J Clin Neurosci. (2016) 28:102–6. doi: 10.1016/j.jocn.2015.09.019
31. Chuquilín-Arista F, Álvarez-Avellón T, Menéndez-González M. Impact of depression and anxiety on dimensions of health-related quality of life in subjects with Parkinson's disease enrolled in an association of patients. Brain Sci. (2021) 11:771. doi: 10.3390/brainsci11060771
32. Rosqvist K, Odin P, Lorenzl S, Meissner WG, Bloem BR, Ferreira JJ, et al. Factors associated with health-related quality of life in late-stage Parkinson's disease. Mov Disord Clin Pract. (2021) 8:563–70. doi: 10.1002/mdc3.13186
33. Leite F, Salgado H, Campos O, Carvalho P, Pinto Da Costa M, Queirós P, et al. Clinical aspects of depression in Parkinson's disease. Eur Psychiatry. (2016) 33:S380. doi: 10.1016/j.eurpsy.2016.01.1365
34. Tingting H, Fan S, Shuqin L, Yuhui W. The association between health risk behaviors of medical students and depressive symptoms: a potential shift analysis. Psychol Dev Educ. (2025) 5:730–9. doi: 10.16187/j.cnki.issn1001-4918.2025.05.13
35. Michels K, Dubaz O, Hornthal E, Bega D. “Dance therapy” as a psychotherapeutic movement intervention in Parkinson's disease. Complement Ther Med. (2018) 40:248–52. doi: 10.1016/j.ctim.2018.07.005
36. Bognar S, Defaria AM, O'dwyer C, Pankiw E, Simic Bogler J, Teixeira S, et al. More than just dancing: experiences of people with Parkinson's disease in a therapeutic dance program. Disabil Rehabil. (2017) 39:1073–8. doi: 10.1080/09638288.2016.1175037
37. Rocha PA, Slade SC, Mcclelland J, Morris ME. Dance is more than therapy: qualitative analysis on therapeutic dancing classes for Parkinson's. Complement Ther Med. (2017) 34:1–9. doi: 10.1016/j.ctim.2017.07.006
38. Ashoori A, Eagleman DM, Jankovic J. Effects of auditory rhythm and music on gait disturbances in Parkinson's disease. Front Neurol. (2015) 6:234. doi: 10.3389/fneur.2015.00234
39. Hao H, Yuxiu H. Exercise intervention methods for Parkinson's disease. Chin J Gerontol. (2016) 36:3861–5. doi: 10.3969/j.issn.1005-9202.2016.15.117
40. Ventura MI, Barnes DE, Ross JM, Lanni KE, Sigvardt KA, Disbrow EA, et al. A pilot study to evaluate multi-dimensional effects of dance for people with Parkinson's disease. Contemp Clin Trials. (2016) 51:50–5. doi: 10.1016/j.cct.2016.10.001
41. Heiberger L, Maurer C, Amtage F, Mendez-Balbuena I, Schulte-Mönting J, Hepp-Reymond MC, et al. Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson's disease. Front Aging Neurosci. (2011) 3:14. doi: 10.1016/S1388-2457(11)60292-7
42. Stephan MA, Meier B, Zaugg SW, Kaelin-Lang A. Motor sequence learning performance in Parkinson's disease patients depends on the stage of disease. Brain Cogn. (2011) 75:135–40. doi: 10.1016/j.bandc.2010.10.015
43. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. (2006) 35:ii7–11. doi: 10.1093/ageing/afl077
44. Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. (1995) 50A:M28–34. doi: 10.1093/gerona/50A.1.M28
45. Dietz V, Colombo G. Influence of body load on the gait pattern in Parkinson's disease. Mov Disord. (1998) 13:255–61. doi: 10.1002/mds.870130210
46. Aprile I, Iacovelli C, Goffredo M, Cruciani A, Galli M, Simbolotti C, et al. Efficacy of end-effector robot-assisted gait training in subacute stroke patients: clinical and gait outcomes from a pilot bi-centre study. NeuroRehabilitation. (2019) 45:201–12. doi: 10.3233/NRE-192778
47. Toole T, Maitland CG, Warren E, Hubmann MF, Panton L. The effects of loading and unloading treadmill walking on balance, gait, fall risk, and daily function in parkinsonism. NeuroRehabilitation. (2005) 20:307–22. doi: 10.3233/NRE-2005-20406
48. Klamroth S, Steib S, Devan S, Pfeifer K. Effects of exercise therapy on postural instability in Parkinson disease: a meta-analysis. J Neurol Phys Ther. (2016) 40:3–14. doi: 10.1097/NPT.0000000000000117
49. Picelli A, Capecci M, Filippetti M, Varalta V, Fonte C, DI Censo R, et al. Effects of robot-assisted gait training on postural instability in Parkinson's disease: a systematic review. Eur J Phys Rehabil Med. (2021) 57:472–7. doi: 10.23736/S1973-9087.21.06939-2
Keywords: quality of life, systematic review, Parkinson's disease, meta-analysis, system review
Citation: Li Y, Zhuang R, Zhang J and Liu X (2025) The effect of different exercise training modes on improving quality of life in patients with Parkinson's disease: a network analysis. Front. Neurol. 16:1601080. doi: 10.3389/fneur.2025.1601080
Received: 27 March 2025; Accepted: 12 June 2025;
Published: 02 July 2025.
Edited by:
Haili Pan, Jiangxi Provincial People's Hospital, ChinaReviewed by:
Pedro Chana-Cuevas, University of Santiago, ChileRene Luis Vidal, Universidad Mayor, Chile
Copyright © 2025 Li, Zhuang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Zhang, emloODI4Mjg4QDE2My5jb20=
†These authors share first authorship
 Ying Li
Ying Li Ruixin Zhuang3†
Ruixin Zhuang3†