- Department of Stroke Center, Affiliated Hospital of Nantong University, Nantong, China
Background: Hemorrhagic transformation (HT) and symptomatic intracranial hemorrhage (sICH) are common complications of endovascular thrombectomy (EVT) in acute ischemic stroke (AIS) patients. The role of peripheral immune inflammation in HT after EVT is unclear. This study aimed to evaluate the relationship between immune inflammatory factor levels and HT and sICH occurrence, and to develop predictive models.
Methods: We included 81 AIS patients who underwent EVT. Peripheral blood samples were collected immediately post-EVT to measure immunoinflammatory markers. Least absolute shrinkage and selection operator (LASSO) regression was used to select variables, and backward stepwise multivariable logistic regression identified independent predictors and predictive models for HT and sICH. The models’ discrimination was assessed using the area under the receiver operating characteristic curve (AUC), and calibration was evaluated using the Hosmer–Lemeshow test. Logistic regression models were used to evaluate the impact of HT or sICH on 90-day functional outcomes and mortality.
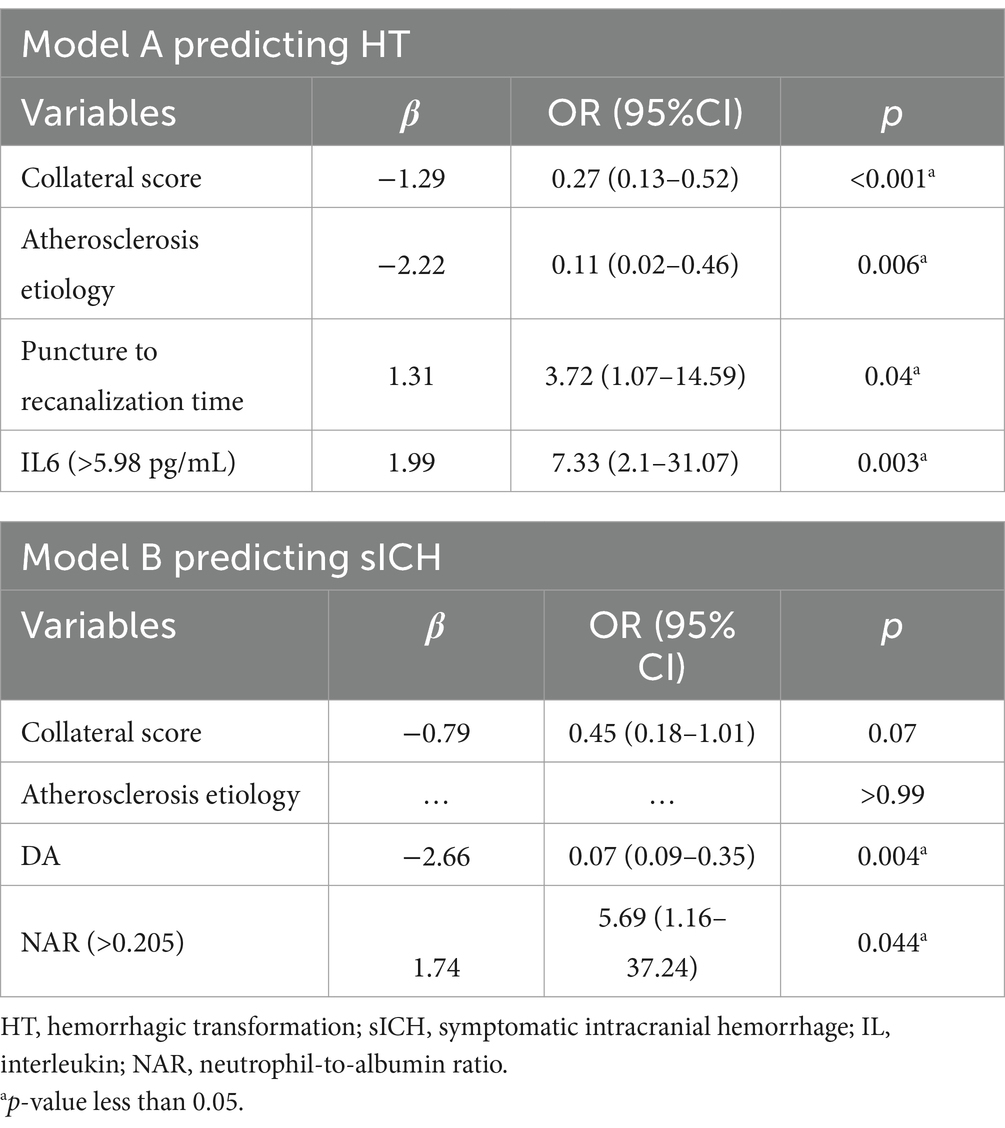
Results: The HT rate was 39.51% (32/81), and the sICH rate was 17.07% (14/81). Multivariate analysis revealed that HT after EVT was significantly associated with collateral score [OR 0.27 (95% CI 0.13–0.52), p < 0.001], arteriosclerosis etiology [OR 0.11 (95% CI 0.02–0.46), p = 0.006], puncture to recanalization time [OR 3.72 (95% CI 1.07–14.59), p = 0.04], and levels of IL-6 [OR 7.33 (95% CI 2.1–31.07), p = 0.003; AUC 0.696 (95% CI 0.593–0.799)]. sICH was independently related to direct aspiration (DA) techniques [OR 0.07 (95% CI 0.09–0.35), p = 0.004] and neutrophil-to-albumin ratio (NAR) values [OR 5.69 (95% CI 1.16–37.24), p = 0.044; 0.676 (95% CI 0.550–0.803)]. Both predictive models for HT [AUC 0.898 (95% CI 0.831–0.965)] and sICH [AUC 0.925 (0.853–0.997)] exhibited good discrimination and calibration.
Conclusion: IL-6 and NAR are potential biomarkers for predicting HT and sICH in AIS patients after EVT. This study developed simple and effective predictive models for HT and sICH based on immunoinflammatory factors. Future research should explore the spatiotemporal effects of immune inflammation on prognosis in AIS patients undergoing EVT.
Introduction
Acute ischemic stroke (AIS) is characterized by high incidence, disability, and mortality rates, thereby imposing a significant burden on healthcare systems globally (1, 2). Endovascular thrombectomy (EVT) has emerged as an effective and widely adopted reperfusion therapy for AIS due to large vessel occlusion (LVO) (3–5). Nonetheless, the common and serious complication of EVT, hemorrhagic transformation (HT), is strongly correlated with poor clinical outcomes and can substantially undermine the therapeutic advantages of EVT (6, 7). Consequently, HT is frequently employed as a safety outcome in clinical research. Therefore, identifying risk factors for HT is crucial for optimizing clinical decision-making and enhancing postoperative management strategies.
Recent studies have identified several risk factors for HT following EVT, including the National Institutes of Health Stroke Scale (NIHSS) score, admission blood glucose levels, hyperdense middle cerebral artery sign, and symptom onset to puncture time (8–11). Furthermore, HT f post-EVT is strongly linked to blood-brain barrier (BBB) disruption, which is induced by reperfusion-related inflammation (12). Reperfusion increases reactive oxygen species (ROS) generation and heightens BBB permeability, facilitating the recruitment and activation of resident brain immune cells, such as microglia and astrocytes, as well as infiltrating blood-derived immune cells, including neutrophils and lymphocytes (13, 14). These immune cells release a diverse array of immunoinflammatory cytokines that modulate the balance between pro-inflammatory and anti-inflammatory responses, which is essential for maintaining BBB integrity (15, 16). However, there is a paucity of evidence regarding the relationship between peripheral blood immunoinflammatory cytokine levels and HT following EVT. This prospective study aimed to investigate the impact of immediate post-EVT peripheral blood immunoinflammatory cytokine levels on HT and symptomatic intracranial hemorrhage (sICH) in AIS patients, with the goal of facilitating early prediction of HT and sICH, thereby guiding treatment strategies.
Methods
Study design and management
From January 2024 to March 2025, consecutive adult AIS patients who underwent EVT for large vessel occlusion (LVO) at our center were recruited. The exclusion criteria included: (1) absence of post-EVT non-contrast computed tomography (NCCT) data of the head; (2) lack of immediate post-EVT intravenous blood samples; (3) patients with active infection, or those who had received antibiotics or immunosuppressive therapy within 2 weeks prior to stroke; (4) NCCT evidence of intracranial hemorrhage (ICH) before EVT; (5) procedure-related ICH, such as vascular perforation or arterial dissection; (6) patients with brain tumors, intracranial aneurysms, vascular malformations, or other intracranial space-occupying lesions; (7) patients with coagulopathy, hemorrhagic diseases, or a history of malignancy. All inclusion and exclusion criteria were determined by two trained senior neurologists. This study was approved by the Institutional Review Board (2021-Q094-02), and written informed consent was obtained from all enrolled patients.
All included patients underwent EVT under general anesthesia following the current AIS treatment guidelines, performed by experienced neurointerventionists. The selection of endovascular device [e.g., long sheath (NeuronMax, Penumbra, United States; Ballast, Balt, France; Gmax, Genesis, China), aspiration catheter (Ace, Penumbra, United States; React, Medtronic, United States), distal access catheter (Zenith, China; Soft, TONBRIDGE, China; Easyport, YIJIE, China; Navien, EV3, United States; Wellthrough, INT MEDICAL, China), microcatheter (Rebar, Medtronic, United States; Prowler Select plus, Codma, United States), microwire (Synchro SELECT, Stryker, United States; Avigo, Medtronic, United States; Traxcess, MicroVention, United States), and stents (Solitaire; Medtronic, United States; EmboTrap, Cerenovus, United States)], changes in thrombectomy strategy or device, and decisions regarding any salvage procedures (e.g., intracranial angioplasty with or without stenting and intra-arterial drug therapy), were determined by the operator, considering the pathogenesis of occlusion, the patient’s clinical condition, and other relevant factors.
Post-procedure, patients were closely monitored and managed in the stroke center. NCCT was performed 24–36 h post-EVT or promptly if neurological deterioration occurred, to exclude HT. Imaging assessments were conducted by experienced neurologists and radiologists blinded to clinical information. In cases of HT on NCCT, repeat NCCT was performed to assess the hemorrhage expansion or absortion, and to differentiate HT from contrast leakage. Systolic blood pressure was controlled below 140 mmHg in patients with successful reperfusion and below 160 mmHg in others.
Data collection and outcomes
We collected demographic characteristics, medical history, pre-stroke modified Rankin Scale (mRS) score, and stroke characteristics, including the NIHSS score at admission, stroke etiology by TOAST criteria (17), blood glucose level at admission, systolic blood pressure at admission, Alberta Stroke Program Early CT Score (ASPECTS) at admission, the occlusion site, and collateral circulation score based on the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) classification (18). Treatment details included symptom onset to puncture time, the modified thrombolysis in cerebral infarction (mTICI) score at the final intracranial angiogram, puncture to recanalization time, the use of intravenous tissue plasminogen activator (IV tPA), EVT technique [direct aspiration (DA), stent retriever (SR), or combined DA and SR], and the number of passes.
Venous blood samples required for laboratory tests (including complete blood counts, biochemical markers, blood lipids, and immunoinflammatory cytokines) were collected immediately after the final angiography according to standard institutional guidelines. The neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and neutrophil-to-albumin ratio (NAR) were defined as described in previous studies (19–21). A Navios flow cytometer (Beckman Coulter, California, United States) was used to measure the plasma levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFN-γ, IFN-α, and TNF-α.
Safety outcomes were HT indicated by NCCT performed 24–36 h after EVT and sICH. HT and sICH were identified and classified according to the ECASS II (European Cooperative Acute Stroke Study) classification (22): HI1 was defined as small petechiae along the margins of the infarct; HI2 as confluent petechiae within the infarcted area without space-occupying effect; PH1 as blood clots in 30% of the infarcted area with slight space-occupying effect; and PH2 as blood clots ≥30% of the infarcted area with substantial space-occupying effect. Any ICH visible on NCCT with neurological deterioration (NIHSS score increase ≥4 points) was defined as sICH. Functional outcomes were the 90-day good outcome, defined as mRS scores of 0–2.
Statistical analysis
Statistical analyses were conducted using R Studio (version 4.4.2). The Shapiro–Wilk test was applied to assess the normality of continuous variables. Normally distributed continuous variables were presented as mean ± standard deviation (SD); non-normally distributed continuous variables were presented as median (IQR); categorical variables were presented as frequency (percentage). Univariate comparisons for continuous variables were conducted using the independent t-test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. For categorical variables, differences were assessed using the chi-squared test or Fisher’s exact test, depending on the expected cell counts. In multivariable analyses, continuous variables were dichotomized based on their median values to simplify interpretation. To address multicollinearity and overfitting, variable selection was performed using least absolute shrinkage and selection operator (LASSO) regression (23). The optimal penalty parameter (λ) was determined using λmin + 1SE approach. Variables with non-zero coefficients from the LASSO regression were then included in a backward stepwise logistic regression based on the Akaike information criterion (AIC) to identify the best-fitting predictive models and independent predictors for HT and sICH. The discriminative performance of the selected models and independent predictors was assessed using the area under the receiver operating characteristic (ROC) curve (AUC). Model calibration was assessed using the Hosmer–Lemeshow test. A two-sided p-value of <0.05 was considered statistically significant.
Results
A total of 108 AIS patients receiving EVT were enrolled for this study. Five patients were excluded due to infection within 2 weeks prior to the stroke; seven patients were excluded for receiving antibiotics within 2 weeks prior to the stroke; one patient was excluded due to procedure-related ICH caused by vascular perforation; 12 patients were excluded due to the absence of NCCT within 24–36 h post-EVT; and two patients were excluded due to the ipsilateral intracranial aneurysms. Ultimately, 81 patients were included in this study. Complete data were successfully obtained for venous blood samples immediately following EVT, along with clinical characteristics, imaging and follow-up outcomes (Figure 1).
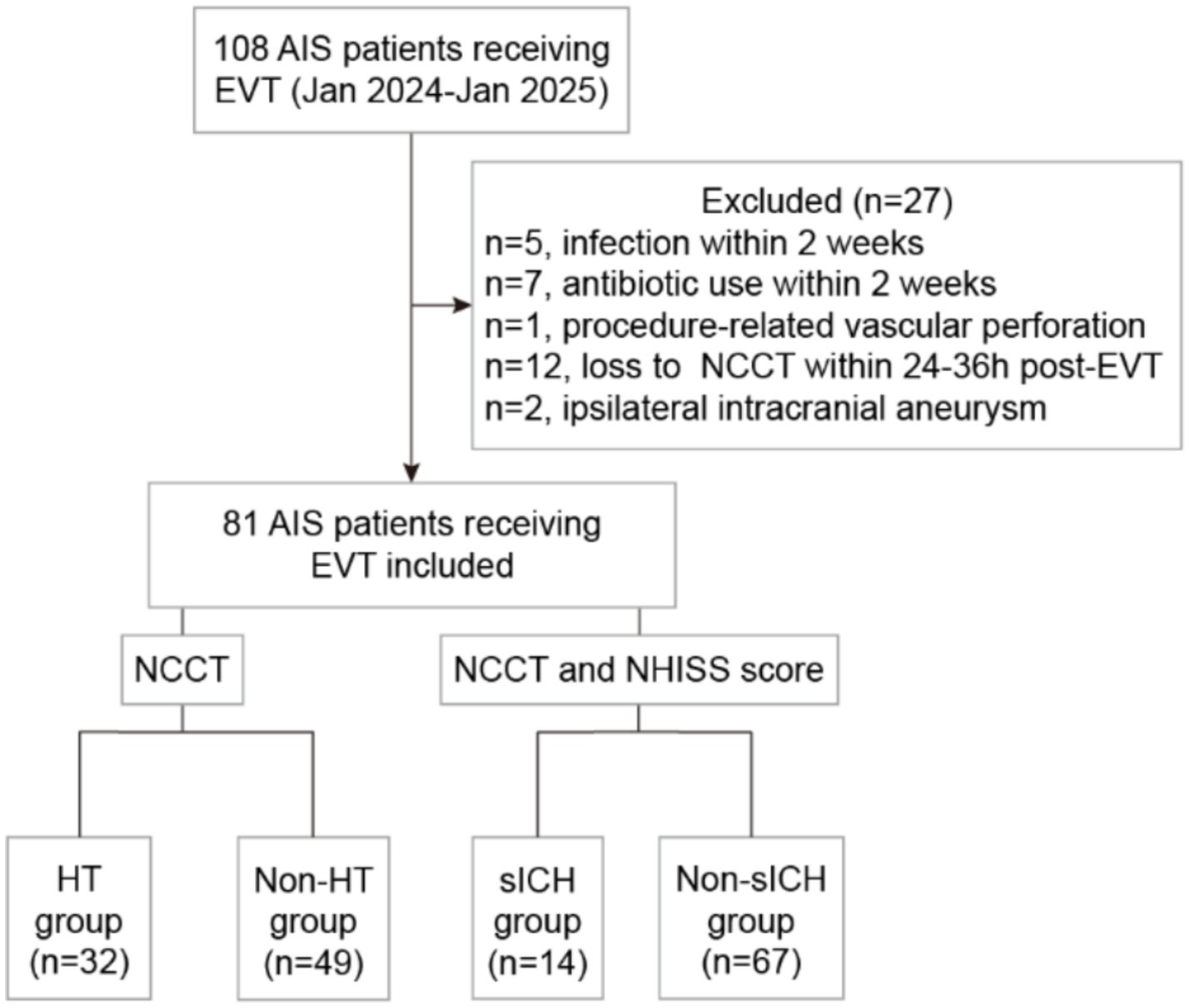
Figure 1. Study flowchart. AIS, acute ischemic stroke; EVT, endovascular thrombectomy; NCCT, non-contrast computed tomography; NHISS, National Institutes of Health Stroke Scale; HT, hemorrhagic transformation; sICH, symptomatic intracranial hemorrhage.
In the overall cohort, the median age was 72 years (range 65–76), with males comprising 62.96% (51/81). The rate of reperfusion (mTICI ≥2b) was 91.36% (74/81). The percentage of HT patients was 39.51% (32/81), including 3 HI1, 8 HI2, 13 PH1 and 8 PH2. The incidence of sICH was 17.07% (14/81). Among patients receiving IV-tPA combined with EVT, the rates of HT and sHT were 47.83% (11/23) and 30.43% (7/23), respectively; whereas in patients receiving EVT alone, the rates of HT and sHT were 36.21% (21/58) and 12.07% (7/58), respectively. At the 90-day follow-up, 45 patients (55.56%) achieved a good functional outcome (mRS 0–2). The 90-day mortality rate was 12.35% (10/81). Patients were divided into two groups: the HT group (n = 32) and non-HT group (n = 49), or the sICH group (n = 14) and non-sICH group (n = 67). Clinical characteristics, treatment details, functional outcomes, and univariate analysis results were presented in Table 1.
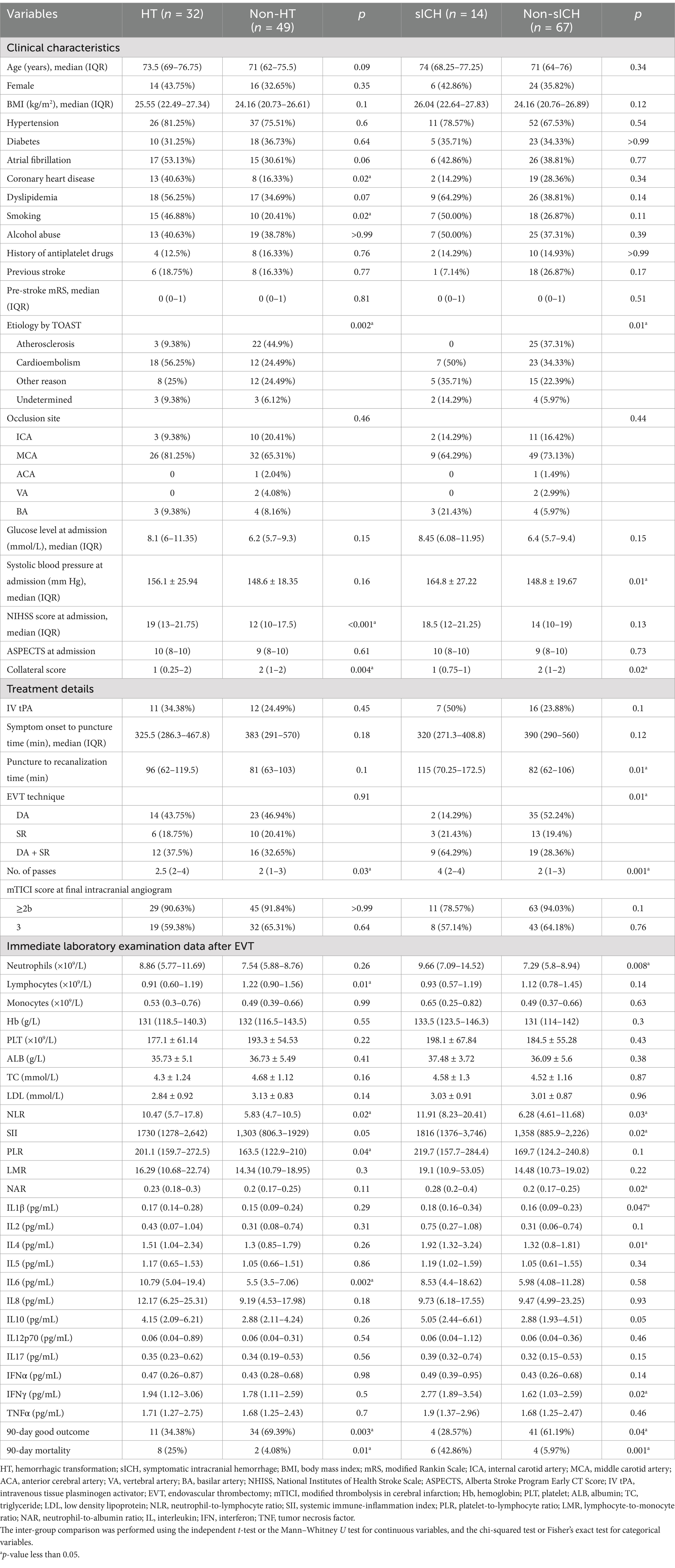
Table 1. Univariate analysis in clinical characteristics, treatment details and functional outcomes.
Univariate analysis
Compared with the non-HT group, the HT group exhibited a significantly higher proportion of patients with coronary artery disease (40.63% vs. 16.33%, p = 0.02) and smoking patients (46.88% vs. 20.41%, p = 0.02). The stroke etiology analysis revealed significant differences between the HT group, sICH group, and the negative control group (p = 0.002). In the positive groups, cardioembolism was the predominant etiology (56.25% for HT group and 50% for sICH group), whereas atherosclerosis was the primary cause in the negative control group (44.9%). The NHISS score at admission was significantly higher in the HT group compared to the non-HT group [19 (13–21.75) vs. 12 (10–17.5), p < 0.001]. sICH patients had significantly higher mean systolic blood pressure at admission (164.8 ± 27.22 vs. 148.8 ± 19.67, p = 0.01). The occurrence of HT or sICH after EVT was significantly associated with worse collateral circulation (p = 0.004 for HT and p = 0.02 for sICH). No other differences were observed in demographic, clinical, or stroke characteristics between the groups.
In terms of treatment details, the number of passes attempting for thrombectomy was significantly associated with the occurrence of HT and sICH [2.5 (2–4) vs. 2 (1–3), p = 0.03 for HT; 4 (2–4) vs. 2 (1–3), p = 0.001 for sICH]. Furthermore, the puncture to recanalization time in the sICH group was significantly longer than in the non-sICH group [115 (70.25–172.55) vs. 82 (62–106), p = 0.01]. Notably, EVT technique also differed significantly between the sICH and non-sICH groups (p = 0.01), with DA + SR being the predominant technique in sICH patients (64.29%) and DA being more common in non-sICH patients (52.24%).
In laboratory findings, we observed that HT patients had lower lymphocyte counts (p = 0.01), higher NLR values (p = 0.02), and higher IL-6 levels (p = 0.002) in immediate post-EVT venous blood samples; sICH patients exhibited higher neutrophil counts (p = 0.008), elevated values of NLR (p = 0.03), SII (p = 0.02) and NAR (p = 0.02), increased levels of IL-1β (p = 0.047), IL-4 (p = 0.01), and IFN-γ (p = 0.02).
Compared to the non-HT group, the HT group demonstrated a significantly lower rate of 90-day good functional outcomes (34.38% vs. 69.39%, p = 0.003) and a higher mortality rate (25% vs. 4.08%, p = 0.01). Similarly, sICH was significantly associated with worse 90-day functional outcomes (p = 0.04) and higher 90-day mortality (p = 0.001).
Variable selection, multivariate regression analysis and model performance evaluation
The optimal λ was selected using λmin + 1SE criterion (λ = 0.0916). Using LASSO regression, five variables with non-zero coefficients associated with HT and six variables with non-zero coefficients associated with sICH were identified (Figure 2). These selected variables and the use of IV-tPA were then included in a backward stepwise logistic regression model to establish the optimal predictive model for HT and sICH, along with their independent predictive factors (Table 2). In Model A, which predicted HT (AIC = 73.06), all included variables were identified as independent predictors, including collateral score [OR 0.27 (95% CI 0.13–0.52), p < 0.001], arteriosclerosis etiology [OR 0.11 (95% CI 0.02–0.46), p = 0.006], puncture to recanalization time [OR 3.72 (95% CI 1.07–14.59), p = 0.04], and IL-6 [OR 7.33 (95% CI 2.1–31.07), p = 0.003]. In the Model B (AIC = 49.44) predicting sICH, DA [OR 0.07 (95% CI 0.09–0.35), p = 0.004] was identified as an independent protective factor for sICH, while NAR [OR 5.69 (95% CI 1.16–37.24), p = 0.044] was an independent risk factor.
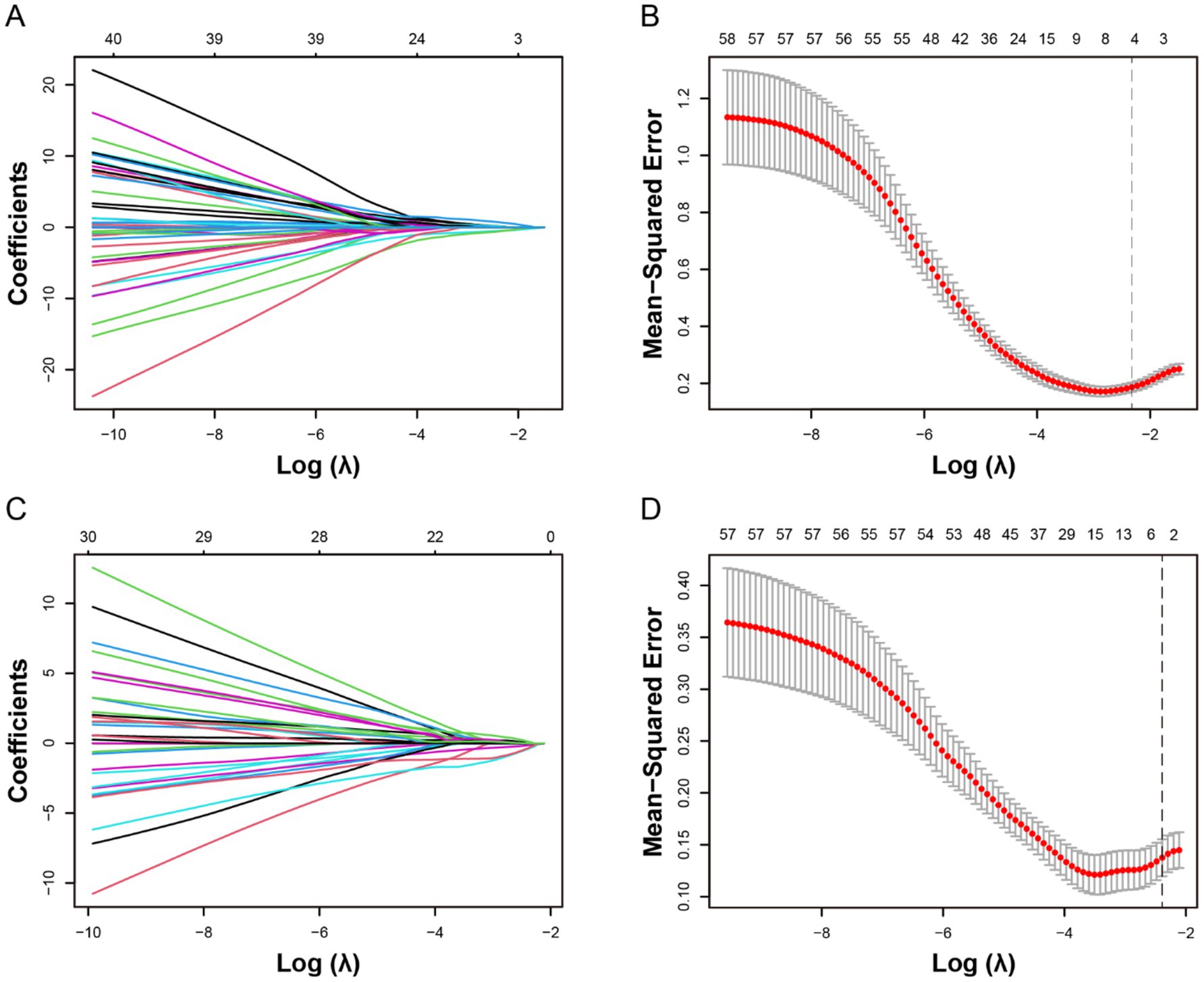
Figure 2. Selection of potential predictive factors for hemorrhagic transformation (HT) and symptomatic intracranial hemorrhage (sICH) using the least absolute shrinkage and selection operator (LASSO) regression. The LASSO coefficient plots for the dependent variables HT (A) and sICH (C) were shown. The 10-fold cross-validation for parameter selection (λ) in the LASSO Logistic regression models for HT (B) and sICH (D) was presented. The optimal λ is selected as λmin + 1SE, with λ = 0.0916 and log(λ) = −2.3226. The black vertical dashed line indicates the optimal value determined by the λmin + 1SE criterion.
After confirming no multicollinearity among the variables (Figure 3A), the AUC for Model A in predicting HT was 0.898 (95% CI 0.831–0.965), indicating strong discriminatory performance (Figure 3B). The AUC value for IL-6 was 0.696 (95% CI 0.593–0.799). The Hosmer–Lemeshow test statistic was not significant (χ2 = 4.91, p = 0.84), indicating that Model A had good calibration. Similarly, after ensuring no multicollinearity among the variables in Model B (Figure 3C), the ROC curve demonstrated that Model B, which was associated with sICH, had excellent discrimination [AUC = 0.925 (0.853–0.997)], with the AUC for NAR alone being 0.676 (95% CI 0.550–0.803) (Figure 3D). Model B also exhibited good calibration (χ2 = 6.46, p = 0.69, as per the Hosmer–Lemeshow Test).
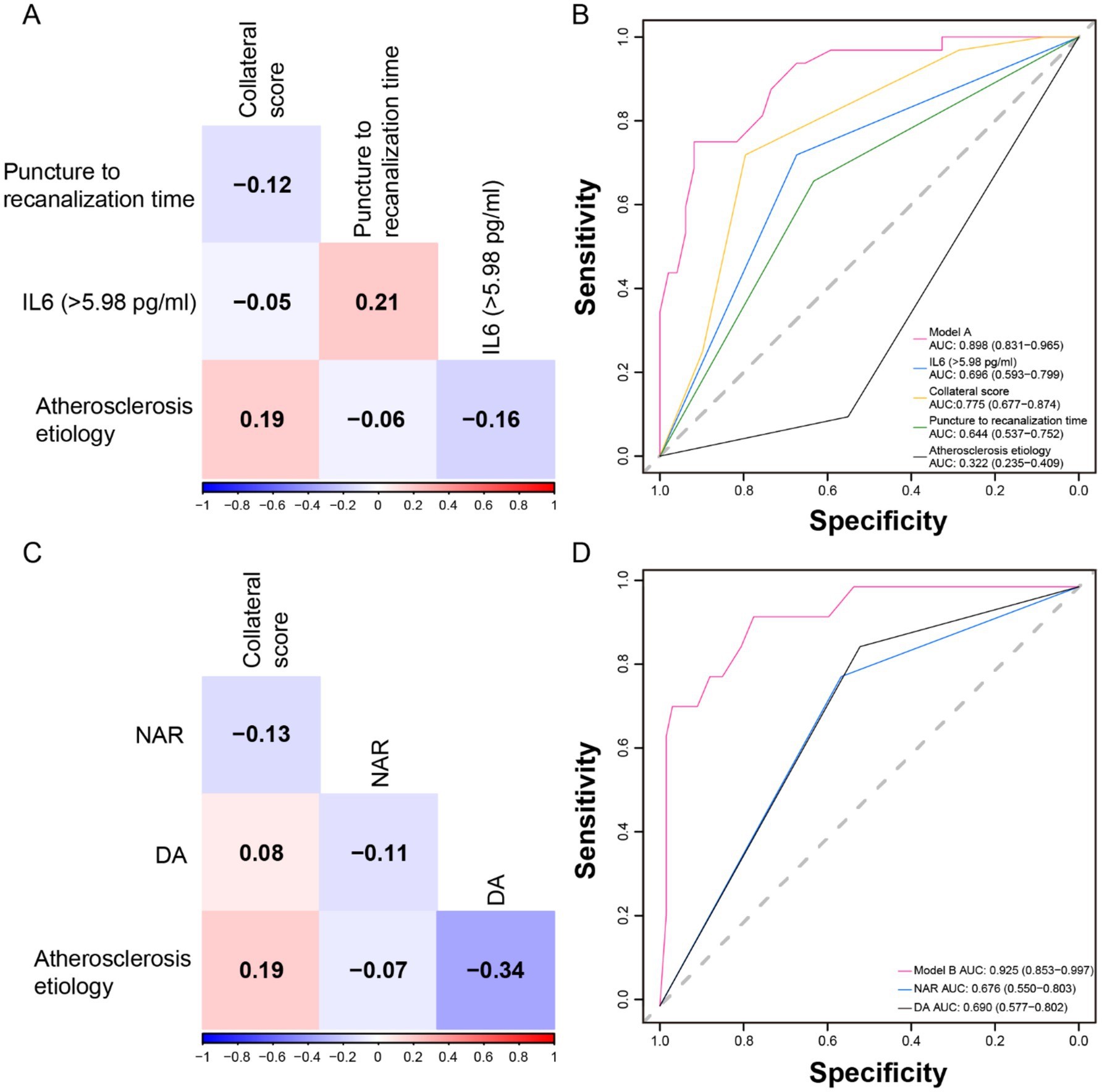
Figure 3. Multicollinearity test of variables, and receiver operating characteristic (ROC) curves of models and independent predictors. The correlation matrix indicated that there was no multicollinearity among the variables in Model A (A) and Model B (C). (B) Model A and its independent predictor ROC curves. (D) Model B and its independent predictor ROC curves.
Discussion
This study observed a post-EVT HT incidence of 39.51%, consistent with previous studies (9, 24, 25). The incidence of sICH was slightly higher (17.07%) compared to the large meta-analysis report (4.4%) (3), possibly due to the longer symptom onset to puncture time, higher proportion of hypertension, and higher admission blood glucose levels in our study cohort. Through LASSO and multivariate regression analysis, we found that the occurrence of HT within 24–36 h after EVT was independently associated with lower collateral score, non-atherosclerotic etiology, longer symptom to puncture time, and higher IL-6 levels. The occurrence of sICH was independently related to the use of non-DA thrombectomy techniques and higher NAR values. The predictive models for HT and sICH exhibited good discrimination and calibration. To our knowledge, this is the first study to explore the factors influencing the occurrence of HT and sICH after EVT incorporating peripheral immunoinflammatory cytokines.
EVT restored blood flow, which increased oxidative stress and promoted the release of inflammatory cytokines, triggering a cascade of pathological events. These events involved the activation of apoptotic pathways, BBB disruption, cerebral edema, and HT. Ultimately, ischemia–reperfusion injury exacerbated brain damage and neurological deficits (16, 26). IL-6 activates and recruits neutrophils and monocytes, stimulating endothelial cells to secrete adhesion molecules and other inflammatory mediators, thereby enhancing the local and general inflammatory response (27). Circulating and local IL-6 induces a pre-thrombotic state, stimulating the production of platelet-derived growth factor, fibroblast growth factor, and macrophage colony-stimulating factor, which in turn promotes smooth muscle cell proliferation (28). Large meta-analyses have demonstrated a dose–response relationship between peripheral IL-6 levels and the risk of ischemic stroke (29). Elevated peripheral IL-6 levels were significantly associated with lower first-pass effect rates (defined as achieving complete or near-complete reperfusion after first thrombectomy) (30) and futile EVT reperfusion (31). Peripheral serum IL-6 levels were considered a poor prognostic factor in AIS patients (32), correlating with imaging parameters such as the mean volume of diffusion weighted imaging lesions at admission, perfusion defects, and final infarct area (33). Recent studies indicated that IL-6 levels in intracranial ischemic arterial bed samples is associated with a lower 90-day mRS score (34). Our study found that IL-6 levels exceeding 5.98 pg/mL were significantly independently associated with HT after EVT. IL-6 alone demonstrated 69.6% accuracy in predicting HT, whereas the predictive model incorporating IL-6 achieved 89.8% accuracy. Therefore, IL-6 played a critical role throughout the AIS course, influencing onset, EVT efficiency, hemorrhagic complications, and functional recovery. Preclinical studies, however, suggested that IL-6 had a dual role, promoting neurogenesis, angiogenesis, and neuronal differentiation (35, 36). The uncertain neurotrophic effects of IL-6 may explain why IL-6 in our study predicted HT but not sICH (worsening neurological deficits). There are currently two registry clinical trials on cytokines in AIS patients (NCT05004389; NCT03297827). The value of the spatiotemporal distribution of IL-6 as an inflammatory marker in AIS deserves further investigation. Timely adjustment of high-risk factors for HT could be an effective measure to enhance post-EVT management, for example, the early use of IL-6 receptor inhibitors (37).
An increase in NAR reflected a worsening systemic inflammatory response, which might exacerbate brain edema and tissue damage by increasing BBB permeability. Albumin plays a key role in osmoregulation, antioxidation, and anti-inflammation (38). Low serum albumin levels are associated with an increased risk of ischemic stroke and ICH (39). High NAR values have been shown to be positively correlated with stroke severity (40), increasing the need for intensive care in AIS patients (41), and significantly associated with poor functional outcomes at 90 days (42). Recent studies have demonstrated that combining NAR with triglyceride levels into a noval index was significantly correlated with spontaneous HT in AIS patients (43). NAR has proven to be a promising biomarker value in prognostic studies of other cardiovascular and cerebrovascular diseases, such as subarachnoid hemorrhage and acute myocardial infarction without ST-segment elevation (44, 45). However, this study did not find a predictive effect of NAR on HT, possibly because the levels of peripheral neutrophils and albumin immediately after reperfusion had not yet reached their extreme values (46, 47). The primary contributors to HT, which promote neuroinflammation and disrupt the BBB, are the inflammatory products of multiple immune cells (such as IL-6). Neutrophils, as one of the “producers,” may have an insignificant role. The positive correlation between NAR and sICH (AUC = 0.676) suggested that in HT patients, once accompanied by neurological deterioration, the intense systemic immune-inflammatory response is closely associated with secondary brain cell injury. Therefore, in sICH patients with a strong neuroinflammatory response, the NAR levels in the early stage after reperfusion are often elevated, as can be observed from the intergroup comparison. This simple, cost-effective, and efficient inflammatory marker offers clinicians a dynamic, long-term tool to assess patient risk. For individualized postoperative management decisions in patients with high NAR levels, consideration can be given to the use of Cl-amidine liposomal nanocarriers (targeting the inhibition of central neutrophil extracellular traps), which have been shown to effectively reduce ischemic and reperfusion injury (48), as well as albumin-assisted therapy (49).
Consistent with previous studies on clinical and imaging predictors of HT (50–52), we also found that poorer collateral circulation scores and longer symptom onset to puncture time were significantly associated with HT. Both factors are related to prolonged BBB damage and the expansion of the core infarct. AIS patients with large artery atherosclerosis often present with well-developed collateral circulation. However, the pathological vascular calcification limits the initial reperfusion improvement, thereby attenuating reperfusion injury. Furthermore, we observed a negative correlation between DA as a first-line thrombectomy technique and the occurrence of sICH. A meta-analysis of 19 studies revealed that the combined DA + SR technique is associated with a higher risk of sICH (53). SR-induced endothelial injury may be a potential cause of increased blood-brain barrier permeability (54).
This study has several limitations. First, as a single-center study with a relatively small sample size, selection bias was inherent. Under the background of high heterogeneity of serum biomarkers, the retrospective nature of the study and the small sample size may impact the reliability and generalizability of the results. Second, although NCCT was used for dynamic evaluation of HT, the extravasation of contrast agents inevitably influenced the outcome observations. Moreover, the study population predominantly consisted of individuals with a high prevalence of large artery atherosclerosis, which limited the generalizability of the findings. Finally, dynamic assessment of peripheral immune inflammatory cytokine levels is essential for further research into their role, particularly at key time points such as admission, pre-procedure, and discharge.
Conclusion
This study provided evidence that immunoinflammatory factor IL-6 in AIS patients after EVT was positively correlated with HT, and NAR was positively associated with sICH. Additionally, both HT and sICH were negatively correlated with prognosis. The study explored straightforward and effective biomarkers and predictive models for HT and sICH. These findings could provide valuable support for clinical decision-making and interventions designed to minimize the incidence of EVT-related complications such as HT and sICH, while offering new insights into optimizing treatment strategies for patients. Future research should further investigate the spatiotemporal dynamics of immune inflammation, its regulatory mechanisms, and advancements in EVT techniques to reduce the occurrence of these severe complications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Affiliated Hospital of Nantong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LB: Investigation, Writing – review & editing, Project administration, Supervision, Conceptualization, Data curation, Validation, Formal analysis, Writing – original draft, Software, Methodology, Visualization, Resources. YW: Investigation, Data curation, Writing – review & editing. SH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feigin, VL, Stark, BA, and Johnson, CO. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Saini, V, Guada, L, and Yavagal, DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. (2021) 97:S6–S16. doi: 10.1212/WNL.0000000000012781
3. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
4. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
5. Goyal, M, Demchuk, AM, Menon, BK, Eesa, M, Rempel, JL, Thornton, J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
6. van Kranendonk, KR, Treurniet, KM, Boers, AMM, Berkhemer, OA, van den Berg, LA, Chalos, V, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg. (2019) 11:464–8. doi: 10.1136/neurintsurg-2018-014141
7. Constant Dit Beaufils, P, Preterre, C, De Gaalon, S, Labreuche, J, Mazighi, M, Di Maria, F, et al. Prognosis and risk factors associated with asymptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke: a prospective multicenter cohort study. Eur J Neurol. (2021) 28:229–37. doi: 10.1111/ene.14539
8. Shen, H, Ma, Q, Jiao, L, Chen, F, Xue, S, Li, J, et al. Prognosis and predictors of symptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke. Front Neurol. (2022) 12:730940. doi: 10.3389/fneur.2021.730940
9. Tian, B, Tian, X, Shi, Z, Peng, W, Zhang, X, Yang, P, et al. Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular thrombectomy. Stroke. (2022) 53:1674–81. doi: 10.1161/STROKEAHA.121.035425
10. Kang, Z, Wu, L, Sun, D, Zhou, G, Wu, X, Qiu, H, et al. Proximal hyperdense middle cerebral artery sign is associated with increased risk of asymptomatic hemorrhagic transformation after endovascular thrombectomy: a multicenter retrospective study. J Neurol. (2023) 270:1587–99. doi: 10.1007/s00415-022-11500-5
11. Lee, YB, Yoon, W, Lee, YY, Kim, SK, Baek, BH, Kim, JT, et al. Predictors and impact of hemorrhagic transformations after endovascular thrombectomy in patients with acute large vessel occlusions. J Neurointerv Surg. (2019) 11:469–73. doi: 10.1136/neurintsurg-2018-014080
12. Yang, C, Hawkins, KE, Doré, S, and Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. (2019) 316:C135–53. doi: 10.1152/ajpcell.00136.2018
13. Anrather, J, and Iadecola, C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
14. De Meyer, SF, Denorme, F, Langhauser, F, Geuss, E, Fluri, F, and Kleinschnitz, C. Thromboinflammation in stroke brain damage. Stroke. (2016) 47:1165–72. doi: 10.1161/STROKEAHA.115.011238
15. Lakhan, SE, Kirchgessner, A, and Hofer, M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. (2009) 7:97. doi: 10.1186/1479-5876-7-97
16. Jurcau, A, and Simion, A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. (2021) 23:14. doi: 10.3390/ijms23010014
17. Amarenco, P, Bogousslavsky, J, Caplan, LR, Donnan, GA, and Hennerici, MG. Classification of stroke subtypes. Cerebrovasc Dis. (2009) 27:493–501. doi: 10.1159/000210432
18. Higashida, RT, Furlan, AJ, Roberts, H, Tomsick, T, Connors, B, Barr, J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09
19. Bao, J, Li, Y, Zhang, Y, Ma, M, Wang, J, Liu, Y, et al. Development and validation of a novel nomogram model predicting the unfavorable outcome based on NAR and collaterals status for patients with AIS. J Stroke Cerebrovasc Dis. (2024) 33:107855. doi: 10.1016/j.jstrokecerebrovasdis.2024.107855
20. Güneş, M, and Büyükgöl, H. A novel predictive marker for in-hospital mortality in acute cerebral infarction: low-density lipoprotein cholesterol to lymphocyte ratio. Cureus. (2020) 12:e9986. doi: 10.7759/cureus.9986
21. Zhu, X, Cheang, I, Xu, F, Gao, R, Liao, S, Yao, W, et al. Long-term prognostic value of inflammatory biomarkers for patients with acute heart failure: construction of an inflammatory prognostic scoring system. Front Immunol. (2022) 13:1005697. doi: 10.3389/fimmu.2022.1005697
22. Hacke, W, Kaste, M, Fieschi, C, von Kummer, R, Davalos, A, Meier, D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
23. Huang, YQ, Liang, CH, He, L, Tian, J, Liang, CS, Chen, X, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. (2016) 34:2157–64. doi: 10.1200/JCO.2015.65.9128
24. Semerano, A, Laredo, C, Zhao, Y, Rudilosso, S, Renú, A, Llull, L, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. (2019) 50:3456–64. doi: 10.1161/STROKEAHA.119.026743
25. van der Steen, W, van der Ende, NAM, Luijten, SPR, Rinkel, LA, van Kranendonk, KR, van Voorst, H, et al. Type of intracranial hemorrhage after endovascular stroke treatment: association with functional outcome. J Neurointerv Surg. (2023) 15:971–6. doi: 10.1136/jnis-2022-019474
26. Jurcau, A, and Ardelean, IA. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J Integr Neurosci. (2021) 20:727–44. doi: 10.31083/j.jin2003078
27. Uyama, N, Tsutsui, H, Wu, S, Yasuda, K, Hatano, E, Qin, XY, et al. Anti-interleukin-6 receptor antibody treatment ameliorates postoperative adhesion formation. Sci Rep. (2019) 9:17558. doi: 10.1038/s41598-019-54175-1
28. Zimmermann, M, Aguilera, FB, Castellucci, M, Rossato, M, Costa, S, Lunardi, C, et al. Chromatin remodelling and autocrine TNFα are required for optimal interleukin-6 expression in activated human neutrophils. Nat Commun. (2015) 6:6061. doi: 10.1038/ncomms7061
29. Papadopoulos, A, Palaiopanos, K, Björkbacka, H, Peters, A, de Lemos, JA, Seshadri, S, et al. Circulating interleukin-6 levels and incident ischemic stroke: a systematic review and meta-analysis of prospective studies. Neurology. (2022) 98:e1002–12. doi: 10.1212/WNL.0000000000013274
30. Mechtouff, L, Bochaton, T, Paccalet, A, Crola Da Silva, C, Buisson, M, Amaz, C, et al. A lower admission level of interleukin-6 is associated with first-pass effect in ischemic stroke patients. J Neurointerv Surg. (2022) 14:248–51. doi: 10.1136/neurintsurg-2021-017334
31. Mechtouff, L, Bochaton, T, Paccalet, A, Da Silva, CC, Buisson, M, Amaz, C, et al. Association of interleukin-6 levels and futile reperfusion after mechanical thrombectomy. Neurology. (2021) 96:e752–7. doi: 10.1212/WNL.0000000000011268
32. Jenny, NS, Callas, PW, Judd, SE, LA, MC, Kissela, B, Zakai, NA, et al. Inflammatory cytokines and ischemic stroke risk: the REGARDS cohort. Neurology. (2019) 92:e2375–84. doi: 10.1212/WNL.0000000000007416
33. Hotter, B, Hoffmann, S, Ulm, L, Meisel, C, Fiebach, JB, and Meisel, A. IL-6 plasma levels correlate with cerebral perfusion deficits and infarct sizes in stroke patients without associated infections. Front Neurol. (2019) 10:83. doi: 10.3389/fneur.2019.00083
34. Dargazanli, C, Blaquière, M, Moynier, M, de Bock, F, Labreuche, J, Ter Schiphorst, A, et al. Inflammation biomarkers in the intracranial blood are associated with outcome in patients with ischemic stroke. J Neurointerv Surg. (2025) 17:159–66. doi: 10.1136/jnis-2023-021365
35. Yao, H, Zhang, Y, Shu, H, Xie, B, Tao, Y, Yuan, Y, et al. Hyperforin promotes post-stroke neuroangiogenesis via astrocytic IL-6-mediated negative immune regulation in the ischemic brain. Front Cell Neurosci. (2019) 13:201. doi: 10.3389/fncel.2019.00201
36. Zhu, H, Zhang, Y, Zhong, Y, Ye, Y, Hu, X, Gu, L, et al. Inflammation-mediated angiogenesis in ischemic stroke. Front Cell Neurosci. (2021) 15:652647. doi: 10.3389/fncel.2021.652647
37. Chu, X, Ma, Z, Liu, Y, Sun, J, Wang, N, Li, C, et al. IRIS, a randomised, double-blind, placebo-controlled trial of interleukin-6 receptor inhibition undergoing endovascular treatment in acute anterior circulation ischaemic stroke: study rationale and design. Stroke Vasc Neurol. (2024). doi: 10.1136/svn-2024-003574
38. Akirov, A, Masri-Iraqi, H, Atamna, A, and Shimon, I. Corrigendum to ‘Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients’ American Journal of Medicine 2017:130(12):1465.e11-1465.e19. Am J Med. (2017) 133:646. doi: 10.1016/j.amjmed.2020.02.001
39. Li, J, Imano, H, Yamagishi, K, Cui, R, Muraki, I, Umesawa, M, et al. Serum albumin and risks of stroke and its subtypes-the circulatory risk in communities study (CIRCS). Circ J. (2021) 85:385–92. doi: 10.1253/circj.CJ-20-0384
40. Mao, S, Hu, Y, Zheng, X, Yang, C, Yang, M, Li, X, et al. Correlation analysis of neutrophil/albumin ratio and leukocyte count/albumin ratio with ischemic stroke severity. Cardiol Cardiovasc Med. (2023) 7:32–8. doi: 10.26502/fccm.92920305
41. Zawiah, M, Khan, AH, Farha, RA, Usman, A, Al-Ashwal, FY, and Akkaif, MA. Assessing the predictive value of neutrophil percentage to albumin ratio for ICU admission in ischemic stroke patients. Front Neurol. (2024) 15:1322971. doi: 10.3389/fneur.2024.1322971
42. Bao, J, Zhang, Y, Ma, M, Wang, J, Jiang, X, Guo, J, et al. Neutrophil-to-albumin ratio as a prognostic factor in patients with acute ischemic stroke. Curr Neurovasc Res. (2024) 21:300–9. doi: 10.2174/0115672026328594240614080241
43. Bao, J, Ma, M, Wu, K, Wang, J, Zhou, M, Guo, J, et al. Integrating neutrophil-to-albumin ratio and triglycerides: a novel indicator for predicting spontaneous hemorrhagic transformation in acute ischemic stroke patients. CNS Neurosci Ther. (2024) 30:e70133. doi: 10.1111/cns.70133
44. Karasu, M, Karaca, Y, Yıldırım, E, Kobat, MA, and Er, F. Neutrophil-to-albumin ratio: a promising tool for CAD assessment in non-ST elevation AMI. Eur Rev Med Pharmacol Sci. (2023) 27:11832–9. doi: 10.26355/eurrev_202312_34781
45. Zhang, R, Zhang, Y, Liu, Z, Pei, Y, He, Y, Yu, J, et al. Association between neutrophil-to-albumin ratio and long-term mortality of aneurysmal subarachnoid hemorrhage. BMC Neurol. (2023) 23:374. doi: 10.1186/s12883-023-03433-x
46. Makris, K, Koniari, K, Spanou, L, Gialouri, E, Evodia, E, and Lelekis, M. Prognostic significance of serum albumin level changes in acute ischemic stroke: the role of biological and analytical variation. Clin Chem Lab Med. (2016) 54:143–50. doi: 10.1515/cclm-2015-0281
47. Jickling, GC, and Dziedzic, T. Neutrophil count is related to stroke outcome following endovascular therapy. Neurology. (2019) 93:194–5. doi: 10.1212/WNL.0000000000007851
48. Sun, S, Lv, W, Li, S, Zhang, Q, He, W, Min, Z, et al. Smart liposomal nanocarrier enhanced the treatment of ischemic stroke through neutrophil extracellular traps and cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway inhibition of ischemic penumbra. ACS Nano. (2023) 17:17845–57. doi: 10.1021/acsnano.3c03390
49. Pan, S, Du, K, Liu, S, Wang, S, Luo, L, Xu, Y, et al. Albumin adjuvant therapy for acute ischemic stroke with large vessel occlusion (AMASS-LVO): rationale, design, and protocol for a phase 1, open-label, clinical trial. Front Neurol. (2024) 15:1455388. doi: 10.3389/fneur.2024.1455388
50. Kuang, Y, Zhang, L, Ye, K, Jiang, Z, Shi, C, and Luo, L. Clinical and imaging predictors for hemorrhagic transformation of acute ischemic stroke after endovascular thrombectomy. J Neuroimaging. (2024) 34:339–47. doi: 10.1111/jon.13191
51. van Kranendonk, KR, Treurniet, KM, AMM, B, Berkhemer, OA, van den Berg, LA, Chalos, V, et al. Clinical and imaging markers associated with hemorrhagic transformation in patients with acute ischemic stroke. Stroke. (2019) 50:2037–43. doi: 10.1161/STROKEAHA.118.024255
52. Honig, A, Molad, J, Horev, A, Simaan, N, Sacagiu, T, Figolio, A, et al. Predictors and prognostic implications of hemorrhagic transformation following cerebral endovascular thrombectomy in acute ischemic stroke: a multicenter analysis. Cardiovasc Intervent Radiol. (2022) 45:826–33. doi: 10.1007/s00270-022-03115-0
53. Texakalidis, P, Giannopoulos, S, Karasavvidis, T, Rangel-Castilla, L, Rivet, DJ, and Reavey-Cantwell, J. Mechanical thrombectomy in acute ischemic stroke: a meta-analysis of stent retrievers vs direct aspiration vs a combined approach. Neurosurgery. 86:464–77. doi: 10.1093/neuros/nyz258
Keywords: hemorrhagic transformation (HT), acute ischemic stroke (AIS), endovascular thrombectomy (EVT), symptomatic intracranial hemorrhage (sICH), IL-6, neutrophil-to-albumin ratio, immunoinflammatory biomarkers
Citation: Bao L, Wang Y and He S (2025) Immunoinflammatory biomarkers as predictors of hemorrhagic transformation in acute ischemic stroke patients after endovascular thrombectomy. Front. Neurol. 16:1606563. doi: 10.3389/fneur.2025.1606563
Edited by:
Rony Abdi Syahputra, University of North Sumatra, IndonesiaReviewed by:
Jan Hendrik Schaefer, Goethe University, GermanyOzge Altintas Kadirhan, Kırklareli University, Türkiye
Copyright © 2025 Bao, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang He, NjcxMDU3MDRAcXEuY29t
 Li Bao
Li Bao Yuhang Wang
Yuhang Wang Shuang He
Shuang He