- 1Stroke and Ageing Research (STAR), School of Clinical Sciences at Monash Health, Monash University, Clayton, Melbourne, VIC, Australia
- 2Monash Health, Diagnostic Imaging, Monash Health, Clayton, Melbourne, VIC, Australia
Introduction
Sanctuary sites (1) is a term used to describe regions of brain parenchyma with a low probability of infarction compared to other areas, due to the compensatory capacity of leptomeningeal anastomoses (LMA) following vessel occlusion (2, 3). The importance of LMA has been evident since the experiments by Heubner in 1874 (4). Heubner witnessed the filling of the entire arterial tree of the cerebral cortex after injecting colored liquid into a single artery, proximally ligated so there was no connection with the Circle of Willis (CoW) (4). However, the significance of LMA has been questioned. Cohnheim (5) disregarded them and believed in the concept of “end-arteries.” Duret (6) recognized their existence, but he and Charcot (7) questioned their functionality due to their small caliber. Beevor (8) acknowledged their presence anatomically and described variabilities in arterial territories but did not make the connection between the two (9–11). In 1925, Fay (12) attempted to prove Cohnheim's (5) theory of “end-arteries,” but found comparable results to Heubner (4) with mercury injection filling all cortical arteries despite the branches of the CoW being tied (12). The first detailed anatomy of LMA was provided by Vander Eecken and Adams (13) in 1953. Similar observations have been documented by Wollschlaeger and Wollschlaeger (14) and Lazorthes et al. (15). These arterial connections have also been extensively confirmed angiographically (16–23). The small diameter of these vessels and the substantial inter and intra-individual variability may account for the initial lack of recognition (9, 13, 17, 23–26). Current evidence for LMA maintaining the ischemic penumbra and reducing infarct growth is apparent in the extended time window reperfusion trials (27, 28). Collateral status is now recognized as a key determinant of reperfusion and clinical outcome following endovascular clot retrieval (28–31). However, a comprehensive understanding of the anatomy, location and prevalence of leptomeningeal collaterals (9), sanctuary sites (1) and their importance in modifying stroke deficits is still in development.
The concept of sanctuary sites in ischemic stroke
Sanctuary sites are regions of potentially salvageable penumbral tissue predominantly present within the frontal, parietal and occipital cortex (1). Figure 1 is a schematic representation of sanctuary sites (1) created using published digital maps of cortical stroke with documented vessel occlusion (3, 32–34). Segmented infarcts from T2-weighted magnetic resonance images were averaged to generate a probability of infarction at a voxel level for each arterial territory. The probability of infarction at each voxel (Pi) for the combined arterial territories was calculated as previously described (1). The probability of sanctuary sites was calculated using the formula probability of sanctuary sites = 1–Pi. Sanctuary sites were identified as regions with a probability of infarction (Pi) < 0.1 (1).
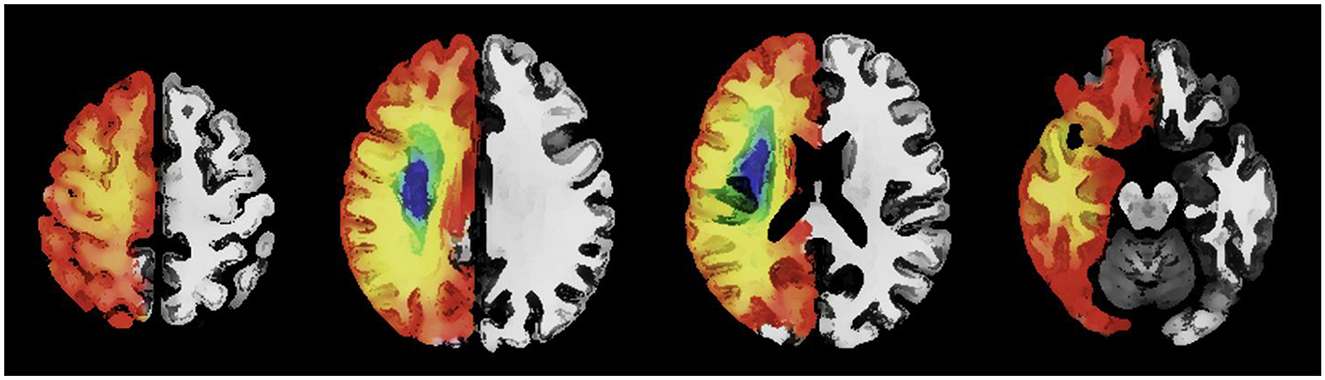
Figure 1. An axial image with a schematic representation of the topography of sanctuary sites created using published digital maps of cortical stroke with documented vessel occlusion. Leptomeningeal collaterals may support blood flow to the superficial compartment to maintain penumbra. Regions (red/orange) have a low probability of infarction and a high probability of sanctuary sites. Regions (blue/green) have a high probability of infarction and a low probability of sanctuary sites.
Historical data and current works have alluded to the presence of sanctuary sites (1, 3, 13, 17, 33, 35–38). Following proximal MCA occlusion it is well recognized that the highest probability of infarction centers around the striatocapsular region and the centrum semiovale, followed by the insular (24, 33, 35, 37, 39, 40). Compensatory flow from posterior cerebral artery (PCA) and anterior cerebral artery (ACA) to MCA through LMA may enable sparing of cortical regions of the frontal and parietal lobes from infarction (1, 13, 17, 33, 35–38), following reperfusion. Although we acknowledge inter-individual variability in LMA, we propose that knowledge of the detailed anatomy of the anastomoses as described by Vander Eecken and Adams (13), and others (14–16, 26) may be useful to increase our understanding of the potential locations of sanctuary sites (1, 3, 32–34). Table 1 describes the possible locations of sanctuary sites using data from previously published digital atlas of probability of infarction (3, 32–34) and the sanctuary sites map (1). To assist with anatomic interpretation, a database Talairach Daemon was used to relate the voxel coordinate in the X, Y, and Z planes (available at http://www.talairach.org/daemon.html).
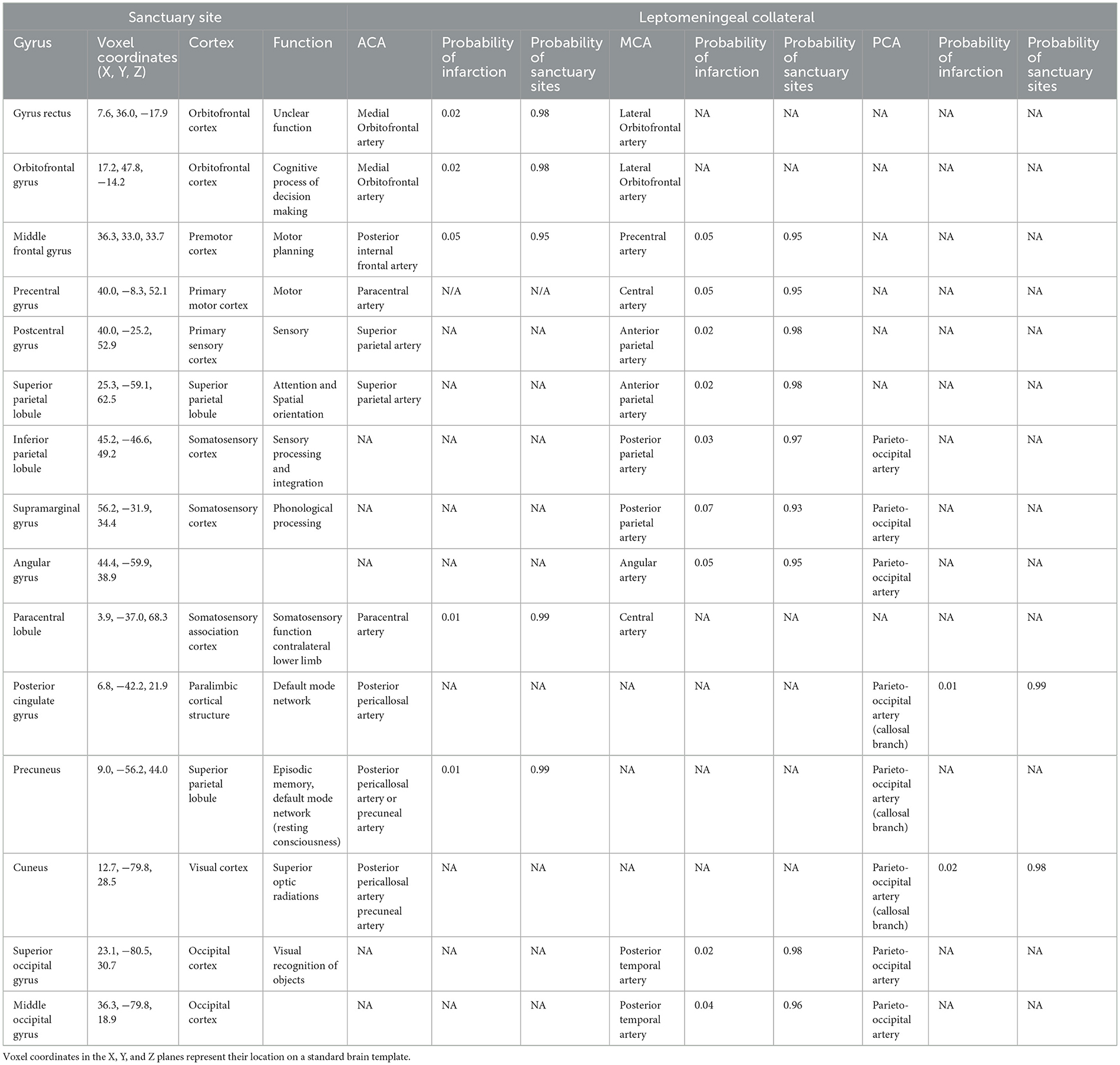
Table 1. Location of possible sanctuary sites (regions of low probability of infarction due to blood supply from leptomeningeal anastomoses).
Vascular anatomy of collateral systems
During the embryo and fetal stages of ontogenesis, the vascular supply for the brain is exclusively from the internal carotid artery (ICA) (41). Pial collaterals develop in early fetal life, connecting branches of the rostral and caudal trunk of the ICA. The rostral trunk of the ICA becomes the ACA medially and MCA laterally and the caudal trunk becomes the PCA (41). The anterior communicating artery (AcomA) forms an anastomotic connection between the two ACA and the posterior communicating artery (PcomA) joins the ICA to the PCA. Following progressive atrophy of the PcomA the vertebral system develops (41). In proximal vessel occlusion, the CoW redistributes blood from posterior to anterior circulation via the PcomA or between hemispheres via the AcomA. In imaging studies, a complete configuration of the circle is reported in <42% of people (42, 43) and its ability to rescue in occlusions distal to CoW via the PcomA has been previously overstated (44). Following MCA occlusion, the CoW is unable to salvage penumbra (2). Perfusion to the MCA territory is instead maintained via recruitment of LMA (2). These arteriole-arteriole anastomoses connect select distal branches of the ACA, MCA, and PCA (13, 21, 25) as either end-to-end or candelabra anastomoses (13, 21). Luminal size and number are important factors which determine the ability of LMA to maintain cerebral perfusion (45). In agreement with Heubner (4), Van der Zwan and Hillen (10, 11) reported diameters as large as 1 mm. A computer model of the cerebral circulation has also shown that similar sizes of the LMA were necessary to keep cerebral blood flow above 30% (2). Furthermore, animal models have also shown that LMAs which connect branches of ACA and MCA, have larger luminal size and reduced basal tone and myogenic reactivity compared to pial arterioles which do not (46).
Location and impact of sanctuary sites
LMA between ACA and MCA
Most LMA occur between the ACA and the MCA (9, 13, 18, 21–23). These anastomoses are crucial to supporting the metabolic needs of important motor and somatosensory areas (see Table 1) (1, 3). A frequent occurrence is the presence of double anastomotic channels in the pre-central, central and post-central regions (14). The posterior internal frontal artery, a branch of the callosomarginal artery forms end-end anastomosis in the pre-central sulcus with the precentral artery (13, 16, 17) (from the MCA) to supply the anterior border of the precentral gyrus (1, 14). In the central sulcus, the paracentral artery and the central artery (13, 16, 17) form connections to supply the posterior border of the precentral gyrus (14), the anterior border of the postcentral gyrus (1, 14) and the paracentral lobule (3, 47, 48). Anastomoses of the superior parietal branch (also known as precuneal branch) with the anterior parietal artery (13, 14, 16, 17) lie within the postcentral sulcus to supply the posterior border of the postcentral gyrus (1) and superior parietal lobule (1) above the intraparietal sulcus. In ACA stroke, these anastomoses limit ACA infarct topography and result in sparing of M1 fiber tracts (3, 48) which may impact motor outcome. These LMA may also be important following MCA occlusion (49). Inferomedially, sanctuary sites exist in the orbital gyrus and gyrus rectus (1, 3, 36) due to anastomoses from the medial and lateral orbitofrontal arteries (13, 14). The low probability of infarction of the rostral aspect of the superior frontal gyrus (3) may be due to compensation from fine transverse or oblique connections between the frontopolar branch (of the ACA) (16) with the orbito-frontalis branch (of the MCA) (13, 16). Following occlusion of the MCA (33) or ACA, (3) the low probability of infarction of the middle frontal gyrus (3, 33) (see Table 1) is likely due to anastomoses between the anterior and middle internal frontal arteries (16, 21, 26) (from the ACA) and the superior branches of the orbitofrontal artery (from the MCA) (9, 13, 16, 21, 26) which lie in the superior frontal sulcus.
LMA between ACA and PCA
Medially, LMA between the terminal branches of the pericallosal artery (14) or precuneal artery (13, 14, 16, 17) (from the ACA) and the posterior callosal branch of the parieto-occipital artery (from the PCA) (13, 14, 16, 17, 21) may account for the low probability of infarction in the precuneus (1, 3) and posterior cingulate gyrus (1, 3) following ACA stroke and the cuneus (1, 34, 50) and splenium (34, 51) following PCA stroke.
LMA between MCA and PCA
End to end anastomosis between the posterior parietal (16), posterior temporal (13) or angular artery (13, 16, 17, 21) (from the MCA) with the parieto-occipital artery (13, 16, 17, 21) (from the PCA) lie within the inferior portion of the intraparietal sulcus or superior portion on the parieto-occipital sulcus (9, 13). Following occlusion of the MCA, they may account for the low probability of infarction seen in the supramarginal and angular gyrus (1, 33) and following PCA occlusion they may explain the presence of sanctuary sites in the superior and middle occipital gyrus (1, 50) and the inferior parietal lobule (1) (Table 1).
LMA between ACA and contralateral ACA
Finally, in almost two thirds of cases (52), the distal branches of the ACA extend to the medial surface of the contralateral hemisphere and can form anastomoses with branches of the pericallosal (16, 21) and callosomarginal arteries (13, 21) including the precuneal and paracentral arteries (9, 13). These branches may further support blood flow to the precuneus, paracentral lobule, posterior cingulate gyrus (1, 3) and splenium (52).
Haemodynamic factors affecting LMA
Based on simulation studies, in the absence of vessel occlusion, low flow within LMA occurs due to the lack of pressure difference between arterial territories. Following obstruction of an artery, the subsequent drop in blood flow and a fall in pressure in downstream vessels results in reversal in flow direction and hemodynamic recruitment of LMA surrounding the occluded vessel (53). This retrograde flow produces an increase in blood flow through LMA via an increase in lumen diameter and a fall in vascular resistance. This helps to maintain cerebral perfusion and support the penumbra until recanalization and reperfusion (53). LMA give rise to penetrating arterioles which pierce the cortical surface and enter the brain substance to supply the capillaries in the microvascular sub-surface bed (26, 54, 55). Each penetrating arteriole forms a vascular unit. As these arterioles lack anastomoses (56), they can leave subcortical structures vulnerable to profound ischemia if they are occluded (54). In animal models, flow reversal in LMA and active dilation of penetrating arterioles lying close to an LMA restores blood flow to regions of ischemia following MCA occlusion (55). In contrast, regions further away from LMA are less able to provide compensatory flow, resulting stasis of blood in penetrating arterioles with an increased risk of infarction (54). A combination of genetic and vascular risk factors likely accounts for the significant inter-individual variation seen in leptomeningeal collaterals. We will review these in the following paragraph.
Genetic and vascular risk factors affecting LMA
In mice, genetic background is a major determinant of this variation, with approximately 80% found localized to the Rabep2 gene (57). Collaterogenesis, can occur secondary to hypoxia and vessel occlusion (58) and was abolished in mice lacking Rabep2 gene (57). Lower arterial oxygen levels in pial watershed areas, is thought to stimulate collaterogenesis through increased expression of Rabep2 gene and increased signaling of vascular endothelial growth factor (VEGF-A) (57, 59). It is unclear at present whether similar genetic polymorphisms will be discovered which account for the variation in collaterals seen in humans.
Several vascular risk factors have been shown to influence the development of collaterals. Age is the most important risk factor for ischemic stroke (60), and its influence on collaterals has been studied in both humans (61–64) and animal models (65, 66). In mice models, with increasing age, a process known as collateral “rarefaction” can occur (65). This results in a reduction in the number and luminal diameter of collaterals leading to increased vascular resistance. Additionally, animal models have shown that aging is believed to impair the capacity of pial arteries to dilate (65, 66). Collateral rarefaction can also occur in the presence of vascular endothelial dysfunction and cardiovascular risk factors (66, 67). Hypertension, has been shown to reduce the development and compensatory capacity of collaterals following vessel occlusion in both animal models (46, 66, 68, 69) and humans (64, 70–72). In spontaneously hypertensive rat models, LMA responded with increased myogenic vasoconstriction in response to elevated pressure (46, 69). The resulting vascular dysfunction and vasoconstriction compromises collateral flow and can increase susceptibility to ischemic injury. An important system in the pathogenesis of hypertension is the renin-angiotensin system. Treatment with an angiotensin converting enzyme inhibitor and subsequent lowering of ANG II levels prevented vasoconstriction of LMA and reversed vascular dysfunction, independent of blood pressure lowering (73).
Hyperlipidemia through the formation of atherosclerosis, is also believed to stimulate ischemic preconditioning and collaterogenesis (74). Favorable collaterals have been associated with hyperlipidemia in humans (61). In mouse models, statins have been shown to upregulate endothelial nitric oxide synthetase resulting in increased cerebral blood flow and reduction in infarct size (75–77). However, the presence of prior statin use has shown conflicting results in humans, with some studies suggesting statin treatment prior to stroke resulted in the presence of higher collaterals grades on angiography (78), even in those with cardioembolic stroke (79). Whilst other studies have shown poorer collaterals in stroke patients with prior-stroke statin use (61, 62).
Poor collateral status and faster evolution from penumbra to infarct core has also been associated with acute and chronic hyperglycemia (64, 71, 72, 80, 81), in patients with type 2 diabetes (82, 83), and in smokers (61). Improving our understanding of clinical factors which impact collaterogenesis or collateral rarefaction may enable us to develop effective therapies in the future.
Collateral therapeutics
Despite a plethora of studies investigating neuroprotection agents in stroke, successful augmentation of collateral hemodynamics following vessel occlusion has provided mixed results, with some interventions showing limited success (84, 85). Early trials of head positioning to improve cerebral perfusion in ischaemic stroke were neutral, but showed that the fully supine position was safe (86). More recently pre-clinical trials (87) and clinical trials (88, 89) investigating head positioning in large artery occlusion have shown more promise. In a pre-clinical randomized trial, positioning with head down using a 15° tilt resulted in increased cerebral blood flow, reduced infarct volume and improved clinical outcome (87) in rodent models with middle cerebral artery occlusion. Unsurprisingly this benefit was greater in subjects with good collaterals, but even a mild improvement was seen in cases with poor collaterals (90). In humans, studies have shown head-down positioning (−20o Trendelenburg position) was well tolerated and resulted in a modest improvement in cerebral perfusion on imaging (91). A randomized multicenter trial of prolonged head down positioning in patients with large artery atherosclerosis of the anterior circulation not suitable for reperfusion therapies, also showed that it was safe but was statistically neutral for the primary outcome of 90 day functional independence (modified Rankin scale 0–2) (88). In contrast, head positioning when used prior to endovascular clot retrieval in patients with large vessel occlusion, has been shown in a small randomized clinical trial to reduce neurological deterioration, defined as an National Institutes of Health Stroke Scale (NIHSS) of 2 or more (89).
Pre-clinical studies in animal models have shown that induced hypertension can improve cerebral blood flow through collaterals and reduce infarct volume (92, 93). In a multicenter randomized clinical trial, therapeutic induced hypertension using phenylephrine in patients with non-cardioembolic stroke ineligible for reperfusion therapies was associated with early neurological improvement and functional independence at 90 days (94). Blood pressure augmentation may be beneficial in selected patients (94) but further randomized controlled trials are needed to define its safety profile and optimal use.
Implications and future directions
The presence of LMA between cortical branches of the ACA and MCA may support blood flow to the superficial compartment to maintain penumbra (see Figure 1). Therefore, the concept of sanctuary sites (1) may provide a framework for identifying patients with a large core (95–98) who have the ability to reach a good functional outcome following treatment with clot retrieval. Imaging studies with MRI suggest that in some cases penumbra can exist up to 48 h (99). Development of adjuvant medical therapies which augment blood flow through LMA (100) may enable extension of current time windows for reperfusion therapies and allow successful treatment of patients who require long distance transfer to a comprehensive stroke center for thrombectomy (101). Pharmacological augmentation of blood flow through established LMA may also be sufficient to improve clinical outcome in cases of large and medium vessel occlusion not suitable for endovascular clot retrieval (100, 102–105) or where thrombolysis is contraindicated (100).
Conclusion
We have identified regions with a low probability of infarction due to the functionality of LMA. In the modern era of reperfusion therapy, knowledge of the locations of sanctuary sites may help guide therapeutic management. Augmenting collaterals to support blood flow to sanctuary sites may be beneficial when used in conjunction with endovascular clot retrieval, especially if there are delays to treatment.
Author contributions
TT: Writing – original draft, Writing – review & editing. HM: Conceptualization, Writing – review & editing. JV: Writing – review & editing. SS: Writing – review & editing. L-AS: Writing – review & editing. TP: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACA, anterior cerebral artery; AcomA, anterior communicating artery; CoW, Circle of Willis; ICA, internal carotid artery ; LMA, leptomeningeal anastomoses; MCA, middle cerebral artery; PCA, posterior cerebral artery; PcomA, posterior communicating artery.
References
1. Thirugnanachandran T, Ma H, Donnan GA, Reutens DC, Phan TG. Introducing the concept of sanctuary sites in ischemic stroke. Stroke. (2024) 55:1405–8. doi: 10.1161/STROKEAHA.123.046083
2. Phan TG, Hilton J, Beare R, Srikanth V, Sinnott M. Computer modeling of anterior circulation stroke: proof of concept in cerebrovascular occlusion. Front Neurol. (2014) 5:176. doi: 10.3389/fneur.2014.00176
3. Thirugnanachandran T, Beare R, Mitchell M, Wong C, Vuong J, Singhal S, et al. Anterior cerebral artery stroke: role of collateral systems on infarct topography. Stroke. (2021) 52:2930–8. doi: 10.1161/STROKEAHA.120.032867
4. Heubner O. Die luetischen Erkrankungen der Hirnarterien. Leipzig, Germany: FC Vogel (1874). p. 170–214.
6. Duret H. Recherches anatomiques sur la circulation de l'encéphale. Arch physiol norm path I. (1874) 60−91.
7. Charcot JM. Lectures on the Localisation of Cerebral and Spinal Diseases. London: New Sydenham Society (1883).
8. Beevor C. On the distribution of different arteries supplying the human brain. Philos Trans R Soc Lond B Biol Sci. (1909) 200:1–55. doi: 10.1098/rstb.1909.0001
9. Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. (2003) 34:2750–62. doi: 10.1161/01.STR.0000095791.85737.65
10. Van Der Zwan A, Hillen B. Araldite F as injection material for quantitative morphology of cerebral vascularization. Anat Rec. (1990) 228:230–6. doi: 10.1002/ar.1092280215
11. van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg. (1992) 77:927–40. doi: 10.3171/jns.1992.77.6.0927
12. Fay T. The cerebral vasculature. preliminary report of study by means of roentgen ray. J Am Med Assoc. (1925) 84:1727–30. doi: 10.1001/jama.1925.02660490019008
13. Vander Eecken HM, Adams RD. The anatomy and functional significance of the meningeal arterial anastomoses of the human brain. J Neuropathol Exp Neurol. (1953) 12:132–57. doi: 10.1097/00005072-195304000-00002
14. Wollschlaeger G, Wollschlaeger PB. Arterial anastomoses of the human brain. A radiographic-anatomic study. Acta Radiol Diagn. (1966) 5:604–14. doi: 10.1177/02841851660050P165
15. Lazorthes G, Gouazé A, Salamon G. Vascularisation et circulation de l'encéphale. Paris: Masson (1976).
16. Mount LA, Taveras JM. Arteriographic demonstration of the collateral circulation of the cerebral hemispheres. AMA Arch Neurol Psychiatry. (1957) 78:235–53. doi: 10.1001/archneurpsyc.1957.02330390017003
17. Rosegay H, Welch K. Peripheral collateral circulation between cerebral arteries; a demonstration by angiography of the meningeal arterial anastomoses. J Neurosurg. (1954) 11:363–77. doi: 10.3171/jns.1954.11.4.0363
18. Ethelberg S. On changes in circulation through the anterior cerebral artery; a clinico-angiographical study. Acta Psychiatr Neurol Scand Suppl. (1951) 75:1–211.
19. Mount LA, Taveras JM. A study of the collateral circulation of the brain following ligation of the internal carotid artery. Trans Am Neurol Assoc. (1953) 3:47–9.
20. Welch K, Stephens J, Huber W, Ingersoll C. The collateral circulation following middle cerebral branch occlusion. J Neurosurg. (1955) 12:361–8. doi: 10.3171/jns.1955.12.4.0361
21. Hawkins TD. The collateral anastomoses in cerebro-vascular occlusion. Clin Radiol. (1966) 17:203–19. doi: 10.1016/S0009-9260(66)80026-0
22. Tatelman M. Pathways of cerebral collateral circulation. Radiology. (1960) 75:349–62. doi: 10.1148/75.3.349
23. Raney R, Raney AA, Sanchez-Perez JM. The role of complete cerebral angiography in neurosurgery. J Neurosurg. (1949) 6:222–37. doi: 10.3171/jns.1949.6.3.0222
24. De Reuck J, Vander Eecken H. The topography of cerebral infarcts. Acta Clin Belg. (1977) 32:112–8. doi: 10.1080/17843286.1977.11717848
25. Vander Eecken HM. The Anastomoses Between the Leptomeningeal Arteries of the Brain: Their Morphological, Pathological, and Clinical Significance. Springfield, Ill: Charles C Thomas (1959).
26. Van Den Bergh R, Van Der Eecken H. Anatomy and embryology of cerebral circulation. In:Luyendijk W, , editor. Progress in Brain Research. Elsevier (1968). p. 1–25. doi: 10.1016/S0079-6123(08)61433-8
27. Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. (2013) 33:1168–72. doi: 10.1038/jcbfm.2013.77
28. Leng X, Fang H, Leung TW, Mao C, Miao Z, Liu L, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2016) 87:537–44. doi: 10.1136/jnnp-2015-310965
29. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. (2011) 42:693–9. doi: 10.1161/STROKEAHA.110.595256
30. Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, et al. Differential effect of baseline computed tomographic angiography collaterals on clinical outcome in patients enrolled in the interventional management of stroke III trial. Stroke. (2015) 46:1239–44. doi: 10.1161/STROKEAHA.115.009009
31. Al-Dasuqi K, Payabvash S, Torres-Flores GA, Strander SM, Nguyen CK, Peshwe KU, et al. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke. (2020) 51:e193–202. doi: 10.1161/STROKEAHA.120.029892
32. Phan TG, Donnan GA, Srikanth V, Chen J, Reutens DC. Heterogeneity in infarct patterns and clinical outcomes following internal carotid artery occlusion. Arch Neurol. (2009) 66:1523–8. doi: 10.1001/archneurol.2009.259
33. Phan TG, Donnan GA, Wright PM, Reutens DC. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. (2005) 36:986–91. doi: 10.1161/01.STR.0000163087.66828.e9
34. Phan TG, Fong AC, Donnan G, Reutens DC. Digital map of posterior cerebral artery infarcts associated with posterior cerebral artery trunk and branch occlusion. Stroke. (2007) 38:1805–11. doi: 10.1161/STROKEAHA.106.477000
35. Sperber C, Karnath H-O. Topography of acute stroke in a sample of 439 right brain damaged patients. Neuroimage Clin. (2016) 10:124–8. doi: 10.1016/j.nicl.2015.11.012
36. Kim DE, Park JH, Schellingerhout D, Ryu WS, Lee SK, Jang MU, et al. Mapping the supratentorial cerebral arterial territories using 1160 large artery infarcts. JAMA Neurol. (2019) 76:72–80. doi: 10.1001/jamaneurol.2018.2808
37. Cheng B, Golsari A, Fiehler J, Rosenkranz M, Gerloff C, Thomalla G. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J Cereb Blood Flow Metab. (2011) 31:36–40. doi: 10.1038/jcbfm.2010.185
38. Wu O, Cloonan L, Mocking SJ, Bouts MJ, Copen WA, Cougo-Pinto PT, et al. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke. (2015) 46:2438–44. doi: 10.1161/STROKEAHA.115.009643
39. Boers AMM, Berkhemer OA, Slump CH, van Zwam WH, Roos YBWEM, van der Lugt A, et al. Topographic distribution of cerebral infarct probability in patients with acute ischemic stroke: mapping of intra-arterial treatment effect. J Neurointerv Surg. (2017) 9:431–6. doi: 10.1136/neurintsurg-2016-012387
40. Stoeckel MC, Wittsack HJ, Meisel S, Seitz RJ. Pattern of cortex and white matter involvement in severe middle cerebral artery ischemia. J Neuroimaging. (2007) 17:131–40. doi: 10.1111/j.1552-6569.2007.00102.x
41. Dragoi G, Melinte P, Radu L, Mihaela M, Dinca I, Catrina M. The consequences of the epigensis of the encephala anastomotic arterial system, Implications in pathology. Rom J Leg Med. (2012) 20:163–72. doi: 10.4323/rjlm.2012.163
42. Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. (1999) 30:2671–8. doi: 10.1161/01.STR.30.12.2671
43. Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, de Groot JC, Algra A, Hillen B, et al. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. (1998) 207:103–11. doi: 10.1148/radiology.207.1.9530305
44. Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. (1994) 330:1565–70. doi: 10.1056/NEJM199406023302204
45. Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. (2010) 30:923–34. doi: 10.1038/jcbfm.2010.10
46. Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke. (2016) 47:1618–25. doi: 10.1161/STROKEAHA.116.013392
47. Thirugnanachandran T, Ma H, Singhal S, Slater LA, Davis SM, Donnan GA, et al. Refining the ischemic penumbra with topography. Int J Stroke. (2018) 13:277–84. doi: 10.1177/1747493017743056
48. Thirugnanachandran T, Ma H, Vuong J, Mitchell M, Wong C, Singhal S, et al. Topographic evolution of anterior cerebral artery infarction and its impact on motor impairment. Cerebrovasc Dis. (2022) 51:248–58. doi: 10.1159/000519134
49. Portera-Cailliau C, Doherty CP, Buonanno FS, Feske SK. Middle cerebral artery territory infarction sparing the precentral gyrus: report of three cases. J Neurol Neurosurg Psychiatry. (2003) 74:510–2. doi: 10.1136/jnnp.74.4.510
50. Thirugnanachandran T, Vuong J, Kong Y, Chen J, Chen C, Clissold B, et al. The anatomy of infarcts causing hemianopia and quadrantanopia in posterior cerebral artery stroke. Cerebrovasc Dis. (2025) 1–7. doi: 10.1159/000547444
51. Benke T, Dazinger F, Pechlaner R, Willeit K, Clausen J, Knoflach M. Lesion topography of posterior cerebral artery infarcts. J Neurol Sci. (2021) 428:117585. doi: 10.1016/j.jns.2021.117585
52. Rhoton AL Jr. The supratentorial arteries. Neurosurgery. (2002) 51:S53–120. doi: 10.1097/00006123-200210001-00003
53. Liebeskind DS. Collateral circulation. Stroke. (2003) 34:2279–84. doi: 10.1161/01.STR.0000086465.41263.06
54. Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA. (2007) 104:365–70. doi: 10.1073/pnas.0609551104
55. Baran U, Li Y, Wang RK. Vasodynamics of pial and penetrating arterioles in relation to arteriolo-arteriolar anastomosis after focal stroke. Neurophotonics. (2015) 2:25006. doi: 10.1117/1.NPh.2.2.025006
56. Wilson DF, Matschinsky FM. Cerebrovascular blood flow design and regulation; vulnerability in aging brain. Front Physiol. (2020) 11:584891. doi: 10.3389/fphys.2020.584891
57. Lucitti JL, Sealock R, Buckley BK, Zhang H, Xiao L, Dudley AC, et al. Variants of Rab GTPase-effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke. (2016) 47:3022–31. doi: 10.1161/STROKEAHA.116.014160
58. Faber JE, Storz JF, Cheviron ZA, Zhang H. High-altitude rodents have abundant collaterals that protect against tissue injury after cerebral, coronary and peripheral artery occlusion. J Cereb Blood Flow Metab. (2021) 41:731–44. doi: 10.1177/0271678X20942609
59. Zhang H, Rzechorzek W, Aghajanian A, Faber JE. Hypoxia induces de novo formation of cerebral collaterals and lessens the severity of ischemic stroke. J Cereb Blood Flow Metab. (2020) 40:1806–22. doi: 10.1177/0271678X20924107
60. Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (oxford vascular study). Lancet. (2005) 366:1773–83. doi: 10.1016/S0140-6736(05)67702-1
61. Nannoni S, Sirimarco G, Cereda CW, Lambrou D, Strambo D, Eskandari A, et al. Determining factors of better leptomeningeal collaterals: a study of 857 consecutive acute ischemic stroke patients. J Neurol. (2019) 266:582–8. doi: 10.1007/s00415-018-09170-3
62. Malik N, Hou Q, Vagal A, Patrie J, Xin W, Michel P, et al. Demographic and clinical predictors of leptomeningeal collaterals in stroke patients. J Stroke Cerebrovasc Dis. (2014) 23:2018–22. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.018
63. Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. (2011) 32:1640–5. doi: 10.3174/ajnr.A2564
64. Li K, Jiang H, Yu J, Liu Y, Zhang L, Ma B, et al. Determinants of leptomeningeal collateral status in acute ischemic stroke: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. (2024) 13:e034170. doi: 10.1161/JAHA.124.034170
65. Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. (2011) 31:1748–56. doi: 10.1161/ATVBAHA.111.227314
66. Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. (2015) 18:265–81. doi: 10.1007/s10456-015-9465-6
67. Faber JE, Chilian WM, Deindl E, Royen Nv, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. (2014) 34:1854–9. doi: 10.1161/ATVBAHA.114.303929
68. Omura-Matsuoka E, Yagita Y, Sasaki T, Terasaki Y, Oyama N, Sugiyama Y, et al. Hypertension impairs leptomeningeal collateral growth after common carotid artery occlusion: restoration by antihypertensive treatment. J Neurosci Res. (2011) 89:108–16. doi: 10.1002/jnr.22522
69. Cipolla MJ, Chan S-L. Impact of acute and chronic hypertension on changes in pial collateral tone in vivo during transient ischemia. Hypertension. (2020) 76:1019–26. doi: 10.1161/HYPERTENSIONAHA.120.15356
70. Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. (2019) 50:1751–7. doi: 10.1161/STROKEAHA.119.025142
71. Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. (2013) 74:241–8. doi: 10.1002/ana.23906
72. Liebeskind DS, Jahan R, Nogueira RG, Zaidat OO, Saver JL. Impact of collaterals on successful revascularization in solitaire FR with the intention for thrombectomy. Stroke. (2014) 45:2036–40. doi: 10.1161/STROKEAHA.114.004781
73. Li Z, Lindner DP, Bishop NM, Cipolla MJ. ACE (angiotensin-converting enzyme) inhibition reverses vasoconstriction and impaired dilation of pial collaterals in chronic hypertension. Hypertension. (2020) 76:226–35. doi: 10.1161/HYPERTENSIONAHA.119.14315
74. Rocha M, Desai S, Son J, Tonetti DA, Jovin T, Jadhav AP. Clinical characteristics of fast and slow progressors of infarct growth in anterior circulation large vessel occlusion stroke. J Cereb Blood Flow Metab. (2021) 41:271678x211015068. doi: 10.1177/0271678X211015068
75. Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. (2012) 110:727–38. doi: 10.1161/CIRCRESAHA.111.253419
76. Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. (2002) 942:23–30. doi: 10.1016/S0006-8993(02)02649-5
77. Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. (2010) 106:1870–81. doi: 10.1161/CIRCRESAHA.109.212746
78. Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, et al. Statin enhancement of collateralization in acute stroke. Neurology. (2007) 68:2129–31. doi: 10.1212/01.wnl.0000264931.34941.f0
79. Lee MJ, Bang OY, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Role of statin in atrial fibrillation-related stroke: an angiographic study for collateral flow. Cerebrovasc Dis. (2014) 37:77–84. doi: 10.1159/000356114
80. Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R, Rovira A, et al. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab. (2007) 27:1616–22. doi: 10.1038/sj.jcbfm.9600460
81. van Seeters T Biessels GJ Kappelle LJ van der Graaf Y Velthuis BK on on behalf of the Dutch acute stroke study I. Determinants of leptomeningeal collateral flow in stroke patients with a middle cerebral artery occlusion. Neuroradiology. (2016) 58:969–77. doi: 10.1007/s00234-016-1727-5
82. Liebeskind DS, Saber H, Xiang B, Jadhav AP, Jovin TG, Haussen DC, et al. Collateral circulation in thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke. (2022) 53:742–8. doi: 10.1161/STROKEAHA.121.034471
83. Ferrari F, Moretti A, Villa RF. Hyperglycemia in acute ischemic stroke: physiopathological and therapeutic complexity. Neural Regen Res. (2022) 17:292–9. doi: 10.4103/1673-5374.317959
84. Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, et al. Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. (2019) 50:2108–17. doi: 10.1161/STROKEAHA.118.024582
85. Hammer MD, Schwamm L, Starkman S, Schellinger PD, Jovin T, Nogueira R, et al. Safety and feasibility of NEUROFLO use in eight- to 24-hour ischemic stroke patients. Int J Stroke. (2012) 7:655–61. doi: 10.1111/j.1747-4949.2011.00719.x
86. Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarria VV, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. (2017) 376:2437–47. doi: 10.1056/NEJMoa1615715
87. Beretta S, Versace A, Carone D, Riva M, Dell'Era V, Cuccione E, et al. Cerebral collateral therapeutics in acute ischemic stroke: a randomized preclinical trial of four modulation strategies. J Cereb Blood Flow Metab. (2017) 37:3344–54. doi: 10.1177/0271678X16688705
88. Chen HS, Zhang NN, Cui Y, Li XQ, Zhou CS, Ma YT, et al. A randomized trial of trendelenburg position for acute moderate ischemic stroke. Nat Commun. (2023) 14:2592. doi: 10.1038/s41467-023-38313-y
89. Alexandrov AW, Shearin AJ, Mandava P, Torrealba-Acosta G, Elangovan C, Krishnaiah B, et al. Optimal head-of-bed positioning before thrombectomy in large vessel occlusion stroke: a randomized clinical trial. JAMA Neurol. (2025) 82:905–14. doi: 10.1001/jamaneurol.2025.2253
90. Mariani J, Beretta S, Diamanti S, Versace A, Martini B, Viganò M, et al. Head down tilt 15° in acute ischemic stroke with poor collaterals: a randomized preclinical trial. Neuroscience. (2023) 523:1–6. doi: 10.1016/j.neuroscience.2023.05.011
91. Goh R, Cheong E, Dodd L, Hampton C, Cagi L, Chia N, et al. Head positioning for stroke blood flow augmentation assisting reperfusion therapies (HEAD-START) study. Cerebrovasc Dis. (2025) 1–13. doi: 10.1159/000547306
92. Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. (2008) 39:1548–55. doi: 10.1161/STROKEAHA.107.499483
93. Cole DJ, Drummond JC, Osborne TN, Matsumura J. Hypertension and hemodilution during cerebral ischemia reduce brain injury and edema. Am J Physiol-Heart Circ Physiol. (1990) 259:H211–7. doi: 10.1152/ajpheart.1990.259.1.H211
94. Bang OY, Chung JW, Kim SK, Kim SJ, Lee MJ, Hwang J, et al. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurology. (2019) 93:e1955–63. doi: 10.1212/WNL.0000000000008520
95. Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N Engl J Med. (2023).
96. Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. (2023) 388:1272–83. doi: 10.1056/NEJMoa2213379
97. Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. (2022) 386:1303–13. doi: 10.1056/NEJMoa2118191
98. Bendszus M, Fiehler J, Subtil F, Bonekamp S, Aamodt AH, Fuentes B, et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet. (2023) 402:1753–63. doi: 10.1016/S0140-6736(23)02032-9
99. Ma H, Wright P, Allport L, Phan TG, Churilov L, Ly J, et al. Salvage of the PWI/DWI mismatch up to 48 h from stroke onset leads to favorable clinical outcome. Int J Stroke. (2015) 10:565–70. doi: 10.1111/ijs.12203
100. Cipolla MJ. Therapeutic induction of collateral flow. Transl Stroke Res. (2023) 14:53–65. doi: 10.1007/s12975-022-01019-2
101. Garcia-Esperon C, Wu TY, Carraro do Nascimento V, Yan B, Kurunawai C, Kleinig T, et al. Ultra-long transfers for endovascular thrombectomy-mission impossible?: the Australia-New Zealand experience. Stroke. (2023) 54:151–8. doi: 10.1161/STROKEAHA.122.040480
102. Salim HA, Yedavalli V, Musmar B, Adeeb N, E l Naamani K, Henninger N, et al. Endovascular therapy versus best medical management in distal medium middle cerebral artery acute ischaemic stroke: a multinational multicentre propensity score-matched study. J Neurol Neurosurg Psychiatry. (2024) 96:239–48. doi: 10.1136/jnnp-2024-333669
103. Goyal M, Ospel JM, Ganesh A, Dowlatshahi D, Volders D, Möhlenbruch MA, et al. Endovascular treatment of stroke due to medium-vessel occlusion. N Engl J Med. (2025) 392:1385–95. doi: 10.1056/NEJMoa2411668
104. Clarençon Clarençon F, Durand-Zaleski I, Premat K, Baptiste A, Chabert E, Ferrier A, et al. Evaluation of mechanical thrombectomy in acute ischemic stroke related to a distal arterial occlusion: a randomized controlled trial. Int J Stroke. (2024) 19:367–72. doi: 10.1177/17474930231205213
Keywords: sanctuary sites, leptomeningeal anastomoses, ischemic penumbra, topography, stroke
Citation: Thirugnanachandran T, Ma H, Vuong J, Singhal S, Slater L-A and Phan T (2025) Sanctuary sites in cortical stroke. Front. Neurol. 16:1606666. doi: 10.3389/fneur.2025.1606666
Received: 06 April 2025; Accepted: 08 September 2025;
Published: 30 September 2025.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Simone Beretta, San Gerardo Hospital, ItalyCopyright © 2025 Thirugnanachandran, Ma, Vuong, Singhal, Slater and Phan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanh Phan, VGhhbmguUGhhbkBtb25hc2guZWR1
 Tharani Thirugnanachandran
Tharani Thirugnanachandran Henry Ma
Henry Ma Jason Vuong
Jason Vuong Shaloo Singhal
Shaloo Singhal Lee-Anne Slater
Lee-Anne Slater Thanh Phan
Thanh Phan