- 1Shenzhen Frontiers in Chinese Medicine Research Co., Ltd., Shenzhen, China
- 2Heilongjiang University of Chinese Medicine, Harbin, China
- 3Jiangxi University of Chinese Medicine, Nanchang, China
- 4Research Department, Swiss TCM University, Bad Zurzach, Switzerland
- 5Faculty of Chinese Medicine, Macau University of Science and Technology, Macau, China
- 6Department of Specialty Medicine, Ohio University, Athens, OH, United States
- 7Division of CT and MRI, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
- 8Key Laboratory of Acupuncture and Medicine Research of Ministry of Education, Nanjing University of Chinese Medicine, Nanjing, China
- 9The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, China
Background: Primary insomnia (PI) is a prevalent sleep disorder that significantly impacts quality of life. While pharmacological treatments are common, concerns about side effects and dependency have led to increased interest in non-pharmacological alternatives. This study systematically evaluates the efficacy and safety of various non-pharmacological therapies for adult PI through a network meta-analysis, providing evidence-based guidance for clinicians.
Methods: We analyzed 53 randomized controlled trials (RCTs) involving 4,181 adults with PI. The included studies assessed 11 non-pharmacological interventions, such as acupuncture, acupressure, cupping therapy, and cognitive behavioral therapy (CBT), alongside control groups (e.g., placebo, waitlist, and pharmacological comparators). Primary outcomes included the Pittsburgh Sleep Quality Index (PSQI), total sleep time (TST), sleep efficiency (SE), and sleep latency (SL). Data synthesis was performed using STATA 17 software with a random-effects model, and evidence quality was appraised using the GRADE framework.
Results: Pooled analyses revealed that all seven non-pharmacological therapies significantly improved PI outcomes compared to controls. Acupuncture reduced PSQI scores by −2.71 points (95% confidence interval (CI): −4.94 to −0.49) versus waitlist, while acupuncture showed a − 1.81 point reduction (95% CI: −2.93 to −0.68). For SE, acupressure and CBT increased SE by 1.48% (95% CI: 0.56–2.39) and 1.34% (95% CI: 0.70–1.98), respectively, compared to SH. Notably, CBT and acupressure shortened SL by approximately 10 min (e.g., CBT: −10.15 min, 95% CI: −11.79 to −8.52 vs. benzodiazepines), while acupressure extended TST by 2.07 h (95% CI: 0.46–3.68). SUCRA rankings identified CBT as the most effective for reducing SL (85.8% probability) and improving SE (89.2%), whereas acupuncture excelled in increasing TST (84.8%). Adverse events were infrequent and mild, primarily limited to transient localized reactions in acupuncture studies.
Conclusion: This study demonstrates that non-pharmacological therapies are effective and safe in managing PI, with CBT, acupuncture, and acupressure emerging as optimal choices for specific sleep parameters. These findings advocate non-pharmacological interventions into clinical practice and offer clinicians valuable insights for selecting appropriate treatment modalities for PI management. However, study limitations like heterogeneity and small sample sizes highlight the need for larger, well-designed RCTs. Future studies should use standardized measures for more specific insomnia assessment.
1 Introduction
Primary insomnia (PI) is a common sleep disorder characterized by difficulty falling asleep, maintaining sleep, or early morning awakenings, occurring at least three times per week for a minimum of 3 months. These symptoms are frequently accompanied by clinical significant distress and daytime functional impairments, including irritability, fatigue, and decreased productivity. With a global prevalence reaching 22.1% (1), PI substantially compromises health-related quality of life worldwide (2). Although earlier guidelines recommended pharmacological like benzodiazepine (3, 4), their long-term use is limited by adverse effects such as memory impairment, psychological disturbances, and elevated risk of depression/anxiety. Consequently, non-pharmacological interventions like cognitive behavioral therapy (CBT) were historically regarded as adjunctive options.
Recent paradigm shifts are evident in updated guidelines. The European Sleep Research Society now designates non-pharmacological therapies (particularly CBT) as first-line PI treatments, reserving short-term pharmacotherapy only for refractory cases (5). This reflects growing recognition of non-drug approaches as primary management strategies.
Among these interventions, CBT demonstrates robust efficacy in improving psychological outcomes and sleep efficiency (6). Acupuncture, another evidence-based modalities, exerts therapeutic effects through neuroendocrine modulation [e.g., melatonin regulation (7), and autonomic nervous system regulation sympathetic suppression/parasympathetic enhancement (8)]. Clinical study confirms acupuncture’s particular effectiveness in prolonging sleep duration (9). Notably, insomnia severity positively correlates with patients’ pursuit of physical therapies (10). However, existing randomized controlled trials (RCTs) on non-pharmacological treatments frequently exhibit methodological limitations—including small samples and placebo effects—compromising conclusions about their efficacy and safety profiles.
Epidemiological trends reveal increasing PI prevalence among younger populations (11). Adolescents, for instance, average merely 6.25 h of weekday sleep, with 65% experiencing >30-min sleep latency (SL). Despite this demographic shift, most meta-analyses disproportionately focus on elderly cohorts, neglecting younger age groups (12).
To address these gaps, this study conducts a network meta-analysis of RCTs evaluating non-pharmacological PI therapies. By systematically comparing efficacy and safety of various non-pharmacological treatments in adult, this study aims to establish evidence-based clinical recommendations for optimal therapeutic selection.
2 Materials and methods
2.1 Literature search
Two researchers independently performed systematic searches across eight databases (China National Knowledge Infrastructure, VIP, Wanfang, SinoMed, PubMed, EMBASE, Cochrane Library, and Web of Science) from inception through December 24, 2023. The search strategy combined subject terms and free-text keywords using the following terms: (primary insomnia) AND (acupuncture OR electroacupuncture OR acupressure OR cupping OR physical therapy OR cognitive behavioral therapy) AND (randomized controlled trial). The detailed search strategy is presented in Supplementary Tables S1–S8. Manual searches of reference lists from eligible RCTs and meta-analyses supplemented electronic retrieval.
2.2 Inclusion criteria
(1) Study design: Peer-reviewed RCTs published in Chinese or English.
(2) Participants: Adults (≥18 years) meeting DSM-IV-TR criteria (American Psychiatric Association) for PI (13), supplemented by the Chinese guidelines for adult insomnia diagnosis (14). Although DSM-5 represents the current standard, we retained DSM-IV-TR criteria because: (a) 77% of included trials used this framework; (b) core PI diagnostic features remained consistent across editions; and (c) this maintained methodological alignment with most included studies. ICSD-3 criteria (American Academy of Sleep Medicine, 2014) were additionally extracted when reported.
(3) Interventions: Eleven comparators were evaluate: acupuncture, acupressure, cupping therapy (CUP), CBT, sleep hygiene (SH), repetitive transcranial magnetic stimulation (rTMS), relaxation therapy (RT), placebo (sham acupuncture or sham treatment) (PLA), nenzodiazepines (BZD), non-benzodiazepines (NBZD), and waiting list control (Wait List).
(4) Outcome measures: Primary outcomes included Pittsburgh Sleep Quality Index (PSQI), total sleep time (TST), sleep efficiency (SE), and sleep latency (SL), selected based on: (1) consistent reporting in most included studies, and (2) validated psychometric properties in insomnia research. The PSQI is a well-validated self-report questionnaire designed to assess sleep quality over a one-month period, comprising seven components (each scored 0–3; total range: 0–21), with higher scores indicating poorer sleep quality (15). Key sleep parameters, including TST, SE, and SL, were derived from objective measures—either polysomnography (the gold standard for sleep assessment) or actigraphy (a wearable-based method estimating sleep parameters via movement detection)—where available. In cases where objective data were lacking, validated sleep diaries served as an alternative for self-reported TST, SE, and SL. SE was defined as the ratio of actual sleep duration to time spent in bed, while SL represented the duration from lights-out to sleep onset, and TST referred to the total sleep duration per night from sleep initiation to awakening. Although wake after sleep onset (WASO) and early morning awakening are clinically relevant to insomnia, these metrics were excluded from the primary analysis due to inconsistent reporting (available in fewer than 40% of studies) and significant methodological variability across trials.
While the PSQI encompasses a broad range of sleep-related domains—including environmental disturbances and physical symptoms (e.g., nocturnal breathing irregularities)—it remains a widely accepted tool in insomnia research, demonstrating strong correlations with insomnia severity. However, to improve diagnostic specificity, future studies may consider supplementing the PSQI with the Insomnia Severity Index (ISI), which specifically targets core insomnia symptoms, such as difficulties in sleep initiation, maintenance, and associated daytime impairment, thereby enhancing clinical sensitivity and precision in outcome assessment.
2.3 Exclusion criteria
(1) Study types: Excluded studies included case reports, retrospective studies, animal experiments, reviews, cell experiments, and experience summaries.
(2) Participants: Studies were excluded if (a) the diagnostic criteria were absent or unclear, or (b) the participants had concomitant psychiatric disorders or other organic diseases.
(3) Interventions: Studies comparing known effective treatments with experimental therapies were excluded.
(4) Outcome measures: Studies were excluded if (a) they lacked outcome measures or (b) the outcome data could not be obtained, and the authors could not be contacted.
2.4 Literature screening and data extraction
Two researchers independently screened all retrieved records from electronic databases, including clinical trials and the references of relevant systematic reviews or meta-analyses, based on predefined eligibility criteria. Duplicate records and studies available only as abstracts were excluded. Disagreements between researchers were resolved through discussion or, if necessary, by consultation with a third independent researcher.
2.5 Risk of Bias assessment
To assess methodological quality, two researchers independently evaluated the included studies using the Cochrane Risk of Bias (ROB) 2.0 tool (16). This updated version addresses limitations in assessing study design and baseline group comparability by refining evaluation criteria and eliminating ambiguous terminology. ROB 2.0 examines multiple bias domains, including randomization, allocation concealment, baseline imbalances, deviations from intended interventions, missing data, outcome measurement, and selective reporting. Each bias is rated as “low,” “high,” or “unclear.” If disagreements occurred, a third researcher would be consulted to make the final decision.
2.6 Statistical analysis
All analyses were performed using STATA 17 software, with results visualized using relationship plots. For safety outcomes, random-effects models were applied to estimate relative risk (RR) with 95% confidence interval (CI). Treatment effects were quantified as mean differences (MD) or standardized MD, depending on data compatibility. Heterogeneity was evaluated using the I2 statistic, categorized as low (I2 < 30%), moderate (I2, 30–50%), or high (I2 > 50%). Sensitivity analyses were conducted to test result robustness.
For primary outcomes, a network meta-analysis was performed to map associations between interventions. Node sizes reflected participant numbers per intervention, while connecting line widths represented the number of trials comparing paired interventions. Direct and indirect evidence were synthesized to assess comparative effectiveness and safety, expressed as RR with 95% CI. Intervention rankings were derived using the Surface under the Cumulative Ranking (SUCRA) curve, where higher SUCRA values indicated superior efficacy. Ranking probabilities were computed cumulatively to generate a hierarchy of interventions. All tests were two-tailed, with statistical significance set at p < 0.05.
3 Results
3.1 Literature search results
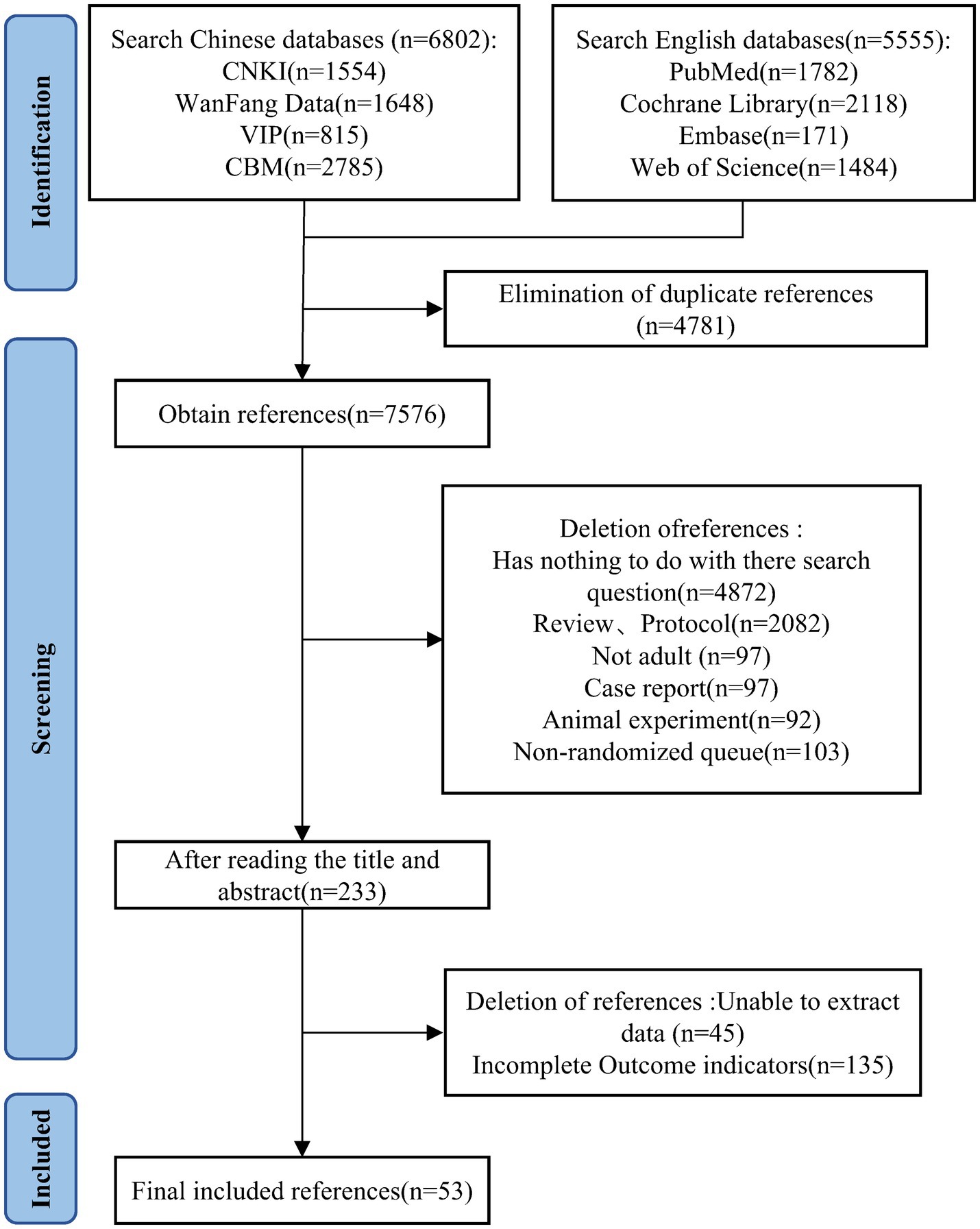
A total of 12,357 studies were systematic retrieved during the literature search. After removing duplicates using Endnote 20, 7,576 records remained. By screening titles and abstracts, 7,343 articles were excluded. A further 180 articles were excluded after full-text evaluation, resulting in the final inclusion of 53 studies (17–69). The detailed literature search process is illustrated in Figure 1.
3.2 Basic characteristics of the included studies
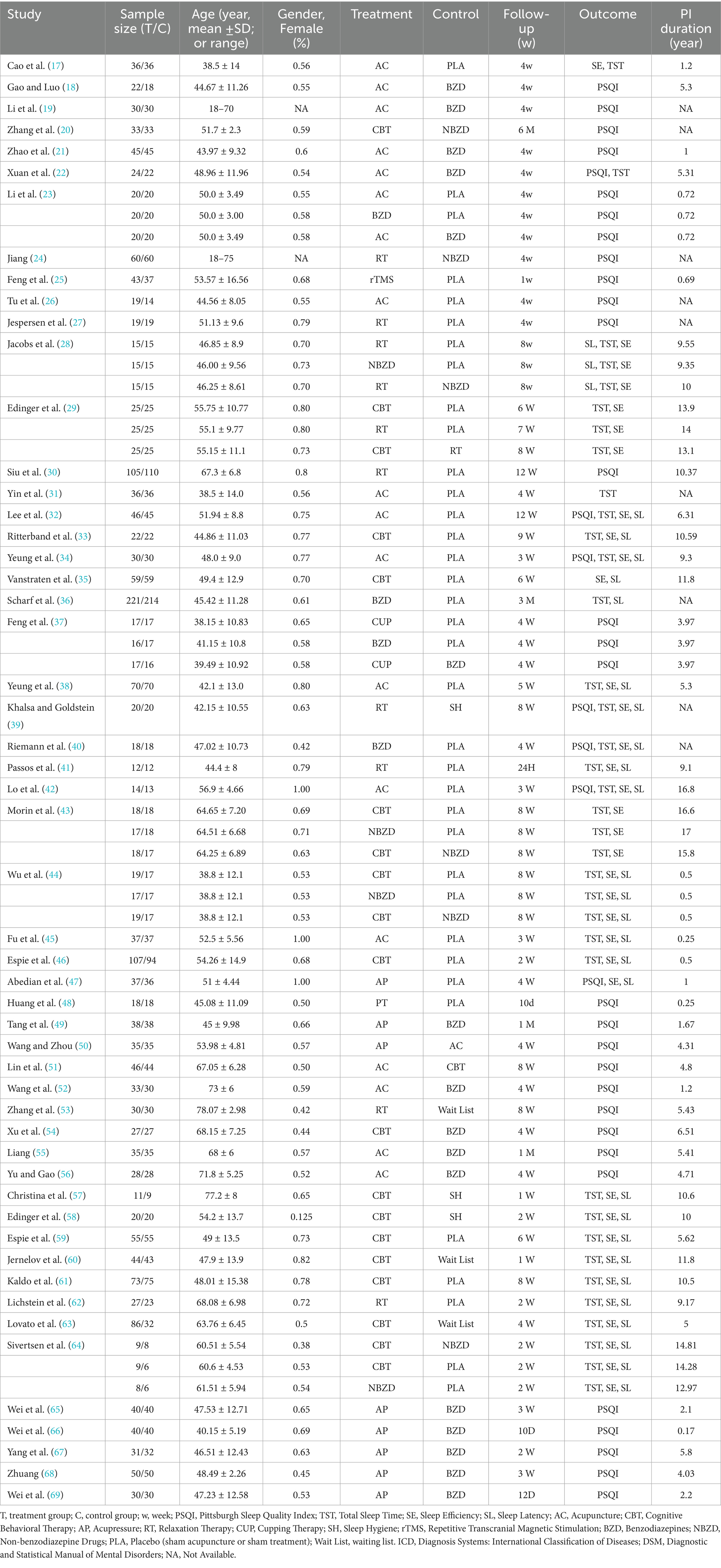
The final analysis included 53 RCTs (total N = 4,181 participants), comprising 46 two-arm and 7 three-arm trials (17–69). These studies evaluated 11 interventions: acupuncture, acupressure, CBT, RT, CUP, SH, rTMS, BZD, NBZD, PLA, and Wait List. Detailed study characteristics are presented in Table 1.
3.3 Risk of Bias assessment using ROB 2.0
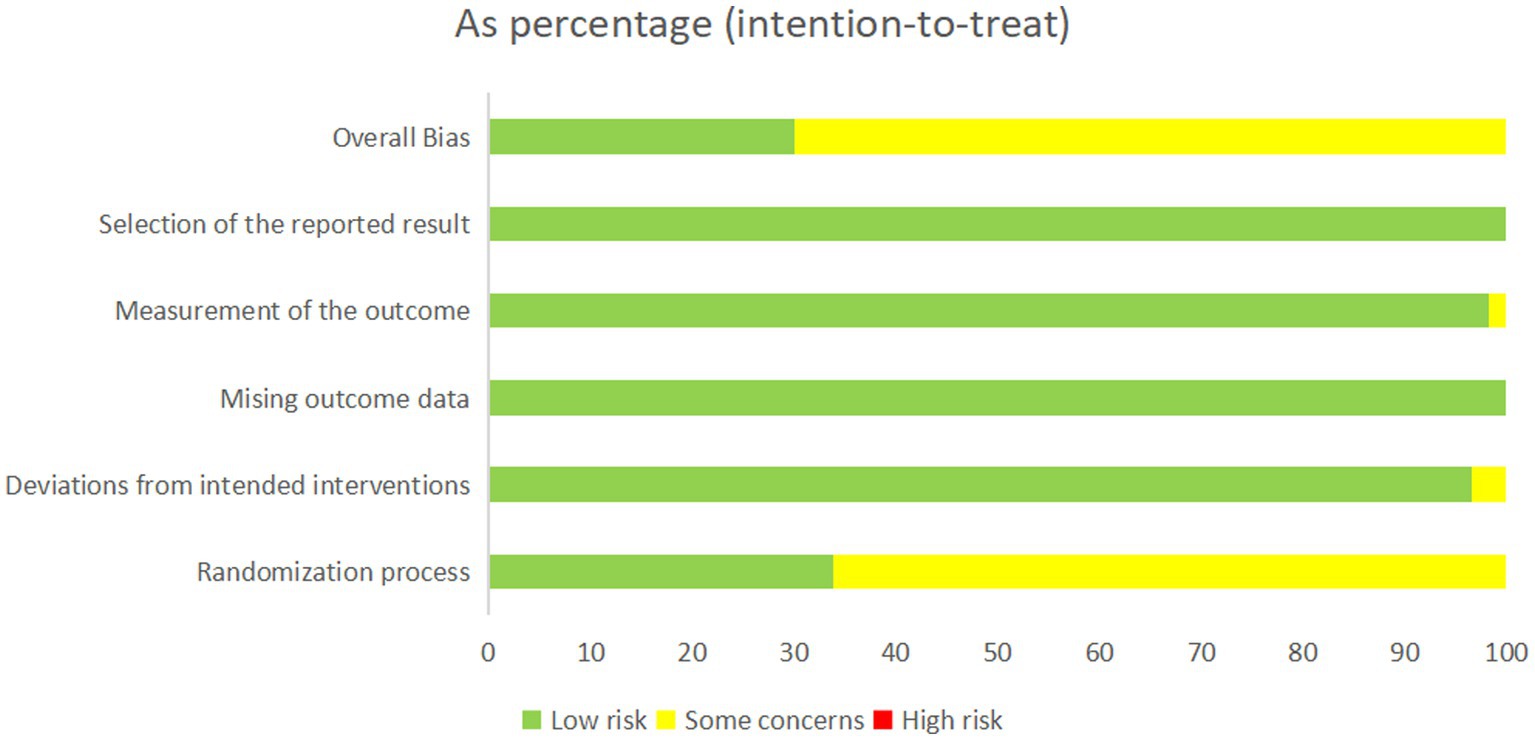
Study quality was evaluated using the Cochrane ROB 2.0 tool. In the randomization domain, 18 studies (33.9%) demonstrated low risk of bias, whereas 35 studies (66.1%) lacked clearly allocation concealment descriptions. All trials showed high adherence to protocol (low risk of deviation bias). Complete outcome data were available for all 53 studies, with appropriate measurement methods for primary outcomes using both subject and objective measures. No studies exhibited high risk of measurement bias. Selective reporting bias was minimal (16 studies, 30.1% low risk; 30 studies, 69.9% potential risk), as detailed in Figure 2 and Supplementary Table S9.
3.4 Evidence quality assessment
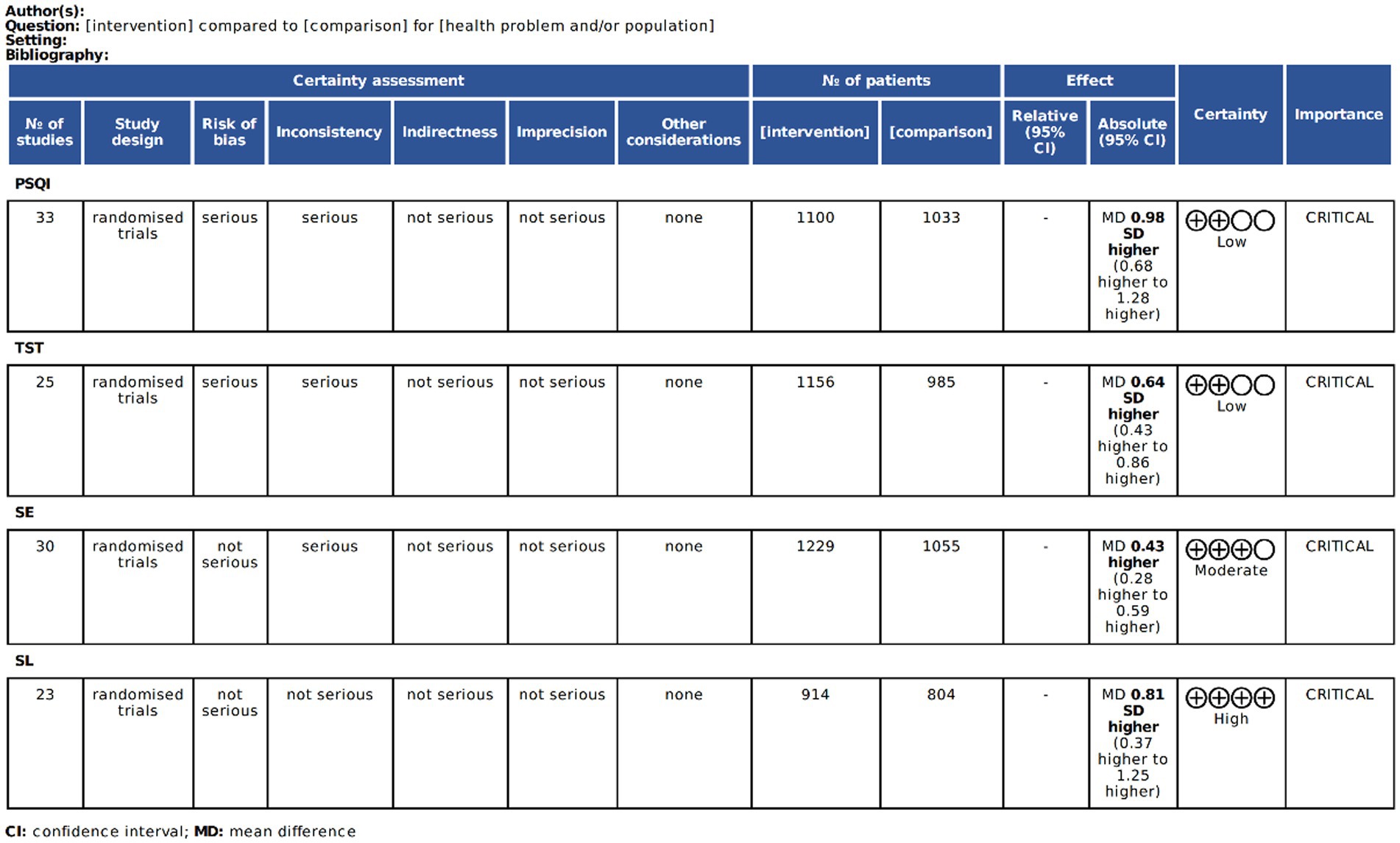
The GRADE system was applied to evaluate evidence quality for all primary outcomes. Final rating reflect comprehensive quality assessments across included studies (17–69) (Figure 3).
3.5 Pairwise meta-analysis results
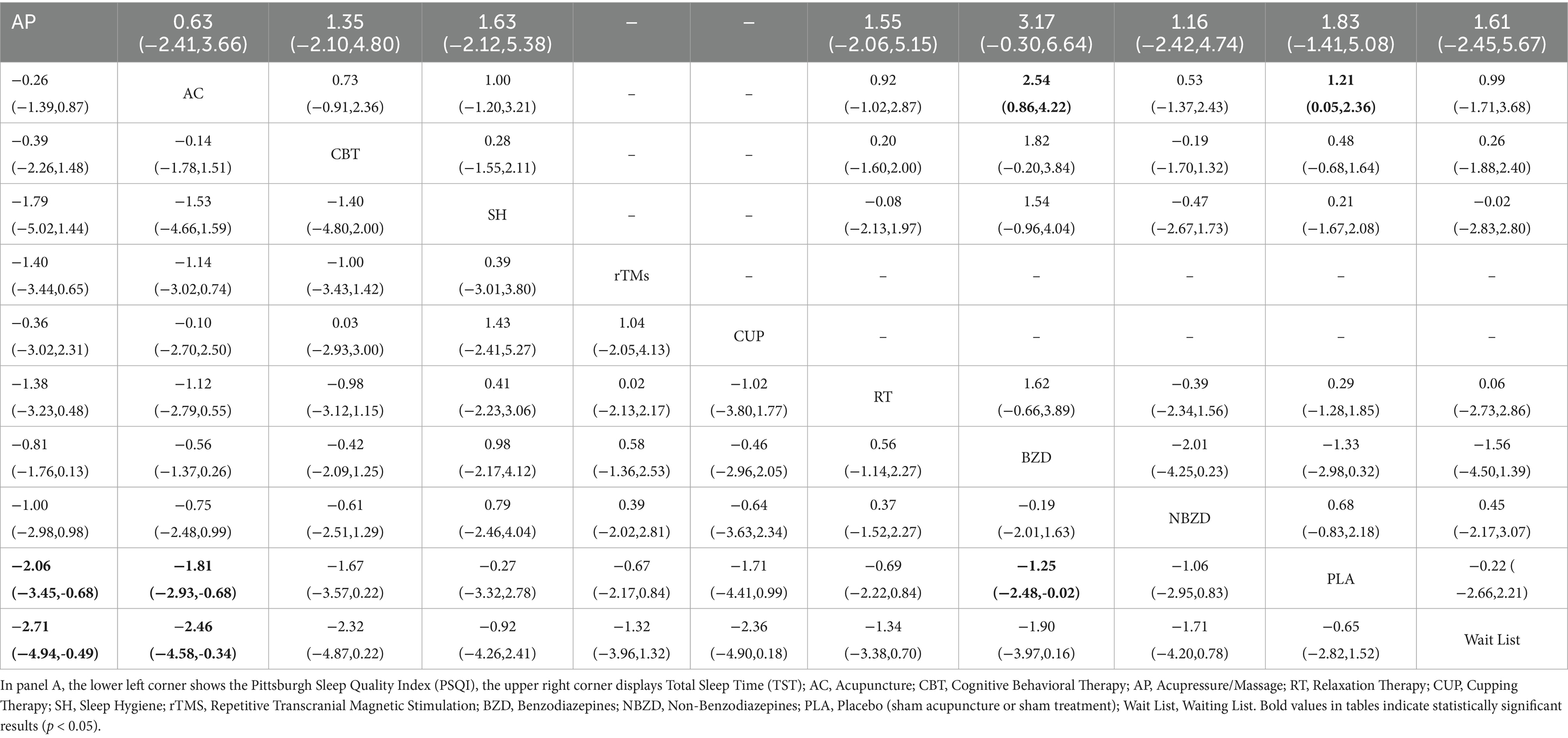
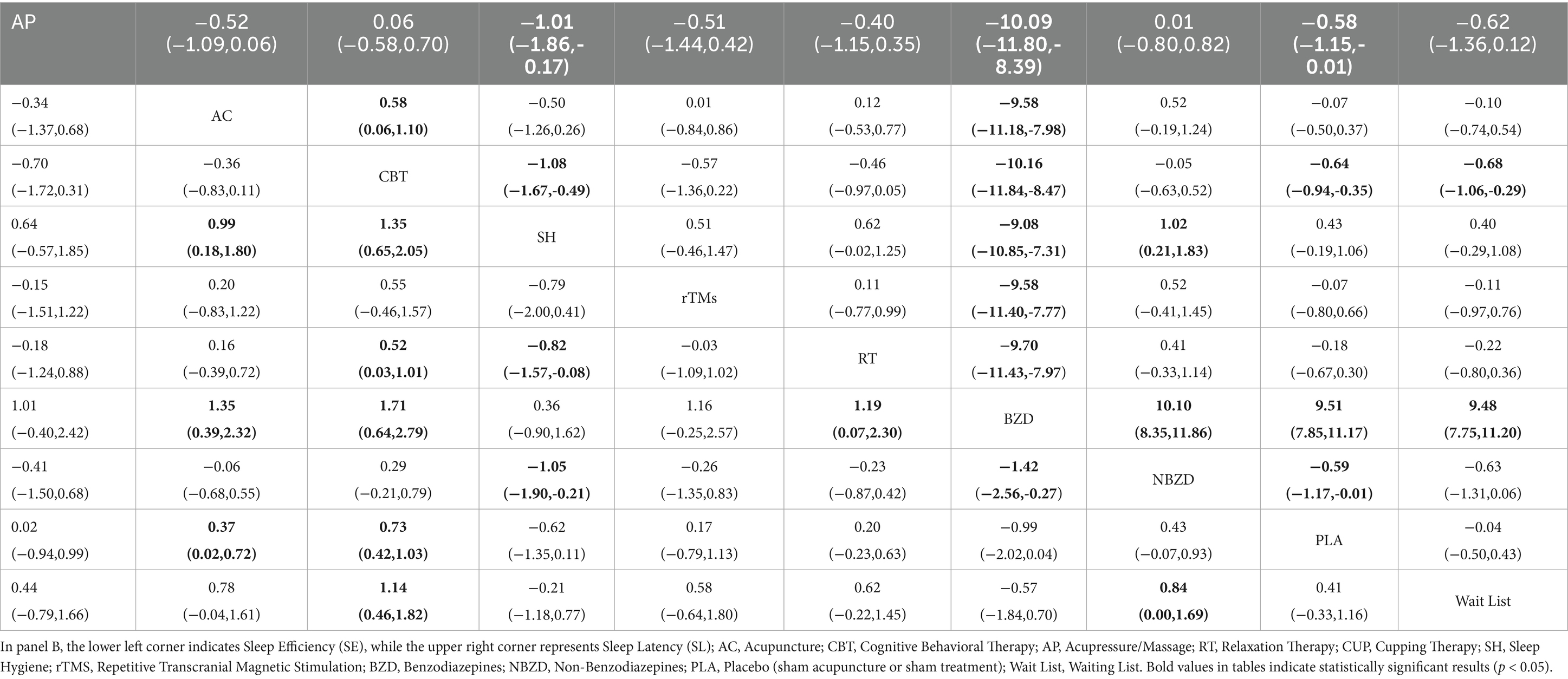
The pairwise meta-analysis results for PSQI, TST, SE, and SL are presented in the Supplementary Figures S1–S4, and Figure 4. Compared to PLA and the Wait List, both BZD (MD = −1.25, 95% CI: −2.48 to −0.02; MD = −1.90, 95% CI: −3.97 to 0.16) and NBZD (MD = −1.06, 95% CI: −2.95 to 0.83; MD = −1.71, 95% CI: −4.20 to 0.78) significantly reduced PSQI scores. Additionally, significant SE improvements were also observed for acupressure (MD = 1.27, 95% CI: 0.37 to 2.18; MD = 1.74, 95% CI: 0.69 to 2.80), acupuncture (MD = 0.88, 95% CI: 0.15 to 0.60; MD = 1.35, 95% CI: 0.51 to 2.20), CBT (MD = 1.14, 95% CI: 0.55 to 1.73; MD = 1.61, 95% CI: 0.67 to 2.55), and BZD (MD = 0.85, 95% CI: 0.10 to 1.59; MD = 1.32, 95% CI: 0.31 to 2.33), with CBT additionally demonstrating significant SL reduction (MD = −0.58, 95% CI: −0.85 to −0.31; MD = −0.63, 95% CI: −0.98 to −0.68).
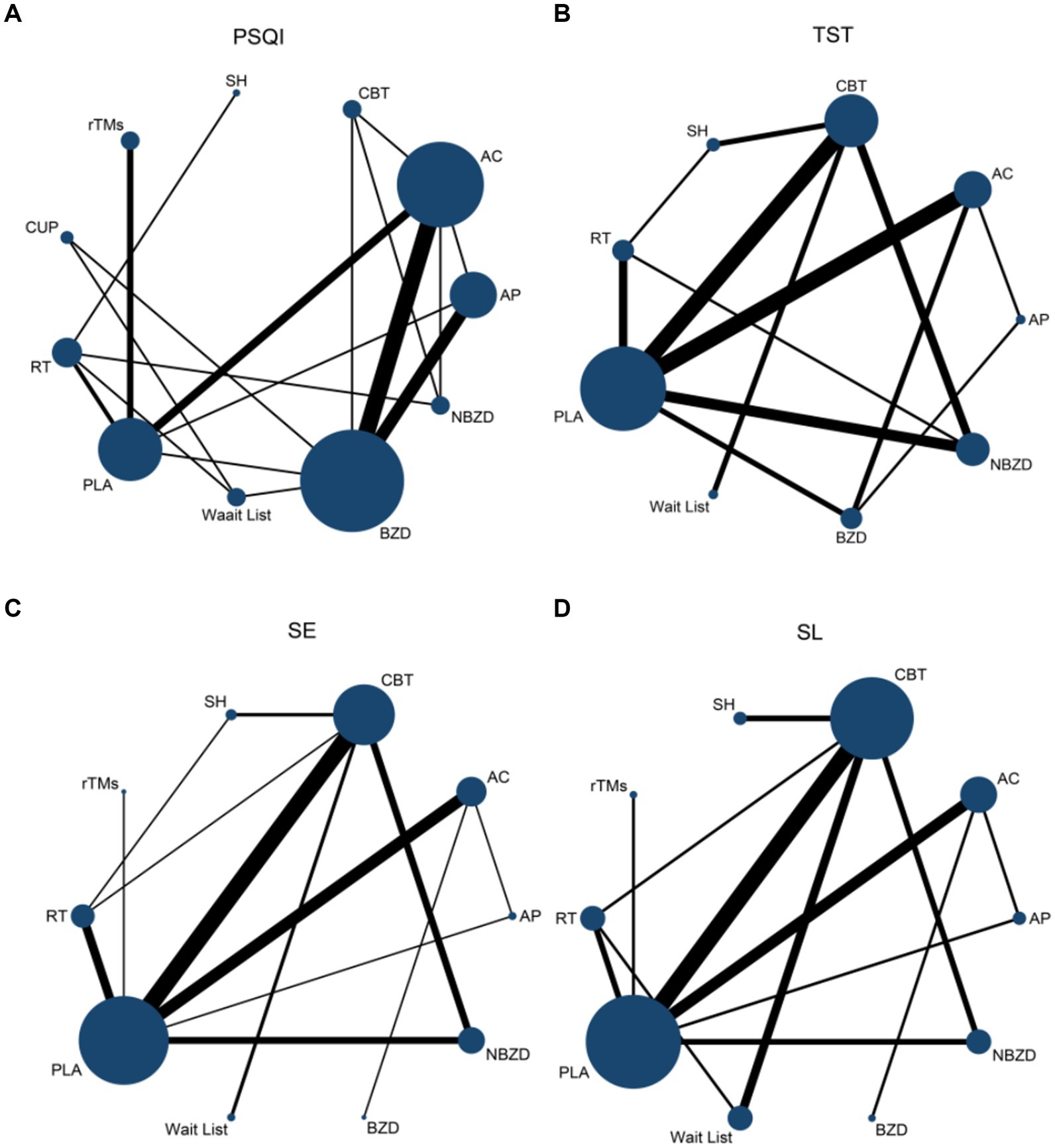
Figure 4. Network evidence map of pairwise comparison on PSQI (A), TST (B), SE (C), and SL (D). PSQI, Pittsburgh Sleep Quality Index; TST, Total Sleep Time; SE, sleep efficiency; SL, sleep latency; AC, Acupuncture; CBT, Cognitive Behavioral Therapy; AP, Acupressure/Massage; RT, Relaxation Therapy; CUP, Cupping Therapy: SH, Sleep Hygiene; rTMS, Repetitive Transcranial Magnetic Stimulation; BZD, Benzodiazepines; NBZD, Non-Benzodiazepines; PLA, Placebo (sham acupuncture or sham treatment); Wait List, Waiting List.
Compared to SH, acupressure (MD = 1.48, 95% CI: 0.56 to 2.39; MD = −1.31, 95% CI: −2.20 to −0.42), CBT (MD = 1.34, 95% CI: 0.70 to 1.98; MD = −1.34, 95% CI: −2.02 to −0.66), CUP (MD = 0.82, 95% CI: 0.15 to 1.50; MD = −1.19, 95% CI: −2.00 to −0.37), and BZD (MD = 1.05, 95% CI: 0.28 to 1.59; MD = −1.32, 95% CI: −2.18 to −0.45) were all effective in improving SE and reducing SL. When compared to RT, acupressure (MD = 0.86, 95% CI: 0.23 to 1.49; MD = −0.55, 95% CI: −1.07 to −0.03) improved SE and shortened SL, while acupuncture showed a significant improvement in SE (MD = 0.47, 95% CI: 0.15 to 0.79), and CBT effectively shortened SL (MD = −0.58, 95% CI: −0.85 to −0.31).
Compared to the Wait List, CUP showed a significant improvement in SE (MD = 1.09, 95% CI: 0.11 to 2.07). Additionally, acupuncture (MD = −9.58, 95% CI: −11.15 to −8.00), acupressure (MD = −10.12, 95% CI: −11.78 to −8.46), CUP (MD = −10.00, 95% CI: −11.67 to −8.33), rTMS (MD = −9.65, 95% CI: −11.39 to −7.90), BZD (MD = −10.13, 95% CI: −11.83 to −8.43), PLA (MD = −9.52, 95% CI: −11.19 to −7.85), and RT (MD = −9.57, 95% CI: −11.19 to −7.56) were all effective in reducing SL. Moreover, acupuncture significantly extended TST compared to PLA, and shortened SL compared to acupressure and CBT.
3.6 Network meta-analysis findings
The network meta-analysis evaluated four efficacy indicators combining objective and subjective sleep measures. Thirty-two studies (n = 2,114) assessed PSQI scores, 25 studies (n = 2,451) measured TST, while SE and SL were evaluated in 2,284 and 1,718 patients, respectively (Table 2, panel A; Table 3, panel B).
Compared to PLA and the Wait List, both acupressure (MD = -2.06, 95% CI -3.45 to −0.68; MD = -2.71, 95% CI -4.94 to −0.49) and acupuncture (MD = -1.81, 95% CI -2.93 to −0.68; MD = -2.46, 95% CI -4.58 to −0.34) significantly decreased PSQI scores. BZD also showed PSQI reduction versus PLA (MD = -1.25, 95% CI -2.48 to −0.02). For TST, acupuncture outperformed BZD (MD = 2.07, 95% CI 0.46–3.68).
For SE improvement, network meta-analysis revealed significant benefits across multiple interventions. Acupressure showed superior efficacy versus PLA (MD = 0.86, 95% CI: 0.23 to 1.49), Wait List (MD = 1.27, 95% CI: 0.37 to 2.18), SH (MD = 1.48, 95% CI: 0.56 to 2.39), and BZD (MD = 1.47, 95% CI: 0.69 to 2.80). CBT demonstrated comparable effects versus PLA (MD = 0.72, 95% CI: 0.45 to 1.00), Wait List (MD = 1.14, 95% CI: 0.55 to 1.73), SH (MD = 1.34, 95% CI: 0.70 to 1.98), and BZD (MD = 1.67, 95% CI: 0.61 to 2.55), as did acupuncture versus PLA (MD = 0.47, 95% CI: 0.15 to 0.79), Wait List (MD = 0.88, 95% CI: 0.15 to 1.60), SH (MD = 1.08, 95% CI: 0.35 to 1.82), and BZD (MD = 1.35, 95% CI: 0.51 to 2.20).
NBZD also significantly improved SE versus Wait List (MD = 0.85, 95% CI: 0.10 to 1.59), SH (MD = 1.05, 95% CI: 0.28 to 1.82), and BZD (MD = 1.32, 95% CI: 0.31 to 2.33). Furthermore, CBT outperformed RT (MD = 0.52, 95% CI: 0.08 to 0.95), while RT itself showed advantages over SH (MD = 0.82, 95% CI: 0.15 to 1.50) and BZD (MD = 1.09, 95% CI: 0.11 to 2.07).
For SL reduction, multiple interventions demonstrated significant efficacy versus comparators. CBT (MD = −1.34, 95% CI: −2.02 to −0.66; MD = −10.15, 95% CI: −11.79 to −8.52), acupressure (MD = −1.31, 95% CI: −2.20 to −0.42; MD = −10.12, 95% CI: −11.78 to −8.46), NBZD (MD = −1.32, 95% CI: −2.18 to −0.45; MD = −10.13, 95% CI: −11.83 to −8.43), RT (MD = −1.19, 95% CI: −2.00 to −0.37; MD = −10.00, 95% CI: −11.67 to −8.33), and PLA (MD = −0.76, 95% CI: −1.49 to −0.03; MD = −9.57, 95% CI: −11.19 to −7.96) all outperformed both SH and BZD.
Compared to acupuncture and PLA, CBT (MD = −0.58, 95% CI: −1.01 to −0.14; MD = −0.58, 95% CI: −0.85 to −0.31) and acupressure (MD = −0.54, 95% CI: −1.06 to −0.02; MD = −0.55, 95% CI: −1.07 to −0.03) showed greater SL reduction. Furthermore, rTMS (MD = −9.65, 95% CI: −11.39 to −7.90), acupuncture (MD = −9.58, 95% CI: −11.15 to −8.00), Wait List (MD = −9.25, 95% CI: −11.19 to −7.85), and SH (MD = −8.81, 95% CI: −10.58 to −7.05) were all superior to BZD for SL reduction. CBT also exceeded Wait List (MD = −0.63, 95% CI: −0.98 to −0.28), while NBZD surpassed PLA (MD = −0.56, 95% CI: −1.10 to −0.01) in shortening SL.
3.7 Inconsistency and heterogeneity analysis
Global inconsistency tests revealed significant heterogeneity across all outcomes (PSQI, TST, SE, and SL; all p < 0.05). Node-splitting analysis confirmed local inconsistencies (p < 0.05) in six triangular loops for PSQI (Supplementary Figure S5). The acupressure-PLA-BZD loop showed the highest inconsistency factor (IF = 5.327, 95% CI: 2.80, 7.86), followed by acupressure-acupuncture-PLA (IF = 3.594, 95% CI: 0.00, 10.62) and acupuncture-CBT-NBZD (IF = 3.392, 95% CI: 2.36, 4.42). Other loops demonstrated lower inconsistency: acupuncture-PLA-BZD (IF = 3.208, 95% CI: 0.00, 7.42), acupressure-acupuncture-BZD (IF = 1.476, 95% CI: 0.00, 3.92), and acupuncture-CBT-BZD (IF = 0.611, 95% CI: 0.00, 4.20). Inconsistency was confirmed in two loops (95% CI excluding 0), while four showed potential consistency (95% CI excluding 0), indicating variability between direct and indirect evidence.
For TST analysis, four triangular loops were formed, showing varying inconsistency levels. The acupuncture-PLA-BZD loop exhibited moderate inconsistency (IF = 4.878, 95% CI: 0.65, 9.11), followed by acupressure-acupuncture -BZD (IF = 3.912, 95% CI: 3.00, 4.82). Lower inconsistency appeared in CBT-PLA-NBZD (IF = 0.187, 95% CI: 0.00, 1.58) and RT-PLA-NBZD (IF = 0.154, 95% CI: 0.00, 2.46). Two loops demonstrated significant inconsistency (95% CI excluding 0), suggesting discordance between direct/indirect comparisons that warrants further investigation.
For SE analysis, four triangular loops were analyzed with distinct inconsistency patterns. The acupressure-acupuncture-RT loop showed the highest inconsistency (IF = 1.868, 95% CI: 0.59, 3.15). Three loops displayed minimal inconsistency: CBT-SH-CUP (IF = 0.451, 95% CI: 0.00, 1.09), CBT-RT-BZD (IF = 0.56, 95% CI: 0.00, 0.85), and CBT-CUP-RT (IF = 0.451, 95% CI: 0.00, 1.09). Two loops exhibited significant inconsistency (95% CI excluding 0), indicating potential divergence between evidence sources.
For SL analysis, four triangular loops were assessed. The acupressure-acupuncture-RT loop showed mild inconsistency (IF = 1.401, 95% CI: 0.21, 2.59), while three loops demonstrated minimal inconsistency: CBT-CUP-RT (IF = 0.654, 95% CI: 0.00, 1.48), CBT-RT-BZD (IF = 0.180, 95% CI: 0.00, 1.09), and CBT-CUP-PLA (IF = 0.062, 95% CI: 0.00, 1.16). Only one loop exhibited significant inconsistency (95% CI excluding 0), suggesting generally consistent direct and indirect comparisons.
Substantial heterogeneity was observed across all outcomes: PSQI (I2 = 91.8%), TST (I2 = 88.3%), SE (I2 = 69.3%), and SL (I2 = 96.6%). Visual inspection of funnel plots revealed symmetrical distributions for all outcomes, indicating low risk of publication bias (Figure 4).
3.8 SUCRA rankings of interventions
The average SUCRA values for each intervention are presented in the Supplementary Figures S6–S9. CBT demonstrated the highest efficacy for both reducing PSQI scores (84.4% probability) and improving SE (91.5%), while ranking first for shortening SL (85.8%). For TST extension, acupuncture showed the highest probability (84.8%), followed by NBZD (62.8%). NBZD also ranked second for SL reduction (82.5% probability). CBT maintained strong performance across all outcomes, ranking third for PSQI reduction (69.9%) and TST extension (54.6%), and second for SE improvement (89.2%). Acupuncture additionally ranked third for SE improvement (70.5% probability).
3.9 Sensitivity and subgroup analyses
The leave-one-out sensitivity analysis confirmed that while excluding individual studies slightly altered effect sizes and p-values (Supplementary Figure S10), the pooled effect direction and statistical significance remained consistent. This consistency indicates robust meta-analytic findings.
Subgroup analyses by treatment duration (Supplementary Figure S11) and disease course (Supplementary Figure S12) revealed substantial heterogeneity across studies (I2 ≥ 69%, p < 0.0001), suggesting significant variability in outcomes. This heterogeneity likely arises from differences in study design (e.g., inclusion criteria, intervention protocols, control groups) or patient characteristics (e.g., disease severity, comorbidities). Between-subgroup heterogeneity was statistically significant for TST and SL (p < 0.05), indicating that treatment duration influences these outcomes. Short-term treatment (0–5 weeks) worsened PSQI and SL scores, whereas long-term treatment (>10 weeks) showed more stable effects, particularly for SE, which exhibited low heterogeneity. Disease duration significantly affected PSQI, TST, and SL (p < 0.05). Shorter disease duration was associated with increased PSQI scores (worsened sleep quality), suggesting greater challenges in improving sleep during early disease stages. TST and SL varied across subgroups, with shorter disease duration linked to reduced TST or prolonged SL, highlighting prominent sleep initiation difficulties. In contrast, SE did not differ significantly between subgroups, implying that disease duration has a weaker impact on sleep efficiency than on other metrics, though the overall trend remained unfavorable.
3.10 Safety analysis
Among the 53 included studies, 23 (43.4%) reported adverse events. Medication-related adverse effects (6 studies) included hepatorenal dysfunction, cardiovascular events, xerostomia, and daytime somnolence. Acupuncture-related adverse events (2 studies) comprised transient local pain, bruising, and hand paresthesia, all resolving with continued treatment. One study reported sleep restriction in the CBT group. Four studies documented unspecified adverse events, while 10 studies reported no safety concerns. Overall, adverse event incidence was low, with acupuncture demonstrating particularly favorable safety due to its transient effects.
4 Discussion
PI remains a significant global public health challenge. This systematic review of 53 RCTs demonstrates that seven non-pharmacological interventions—compared against sham controls or pharmacotherapies—significantly improved core sleep parameters, including PSQI scores, TST, SE, and SL, supporting their role as first-line treatments for PI. These findings, while compelling, are tempered by the low-to-moderate certainty of evidence, primarily due to methodological limitations in the included studies.
Pairwise meta-analyses demonstrated that acupressure, acupuncture, and CBT were particularly effective in improving specific sleep parameters associated with PI, including PSQI scores, SE, SL, and TST. Among these, CBT showed the most pronounced effects in reducing PSQI scores and SL while enhancing SE, whereas acupuncture yield superior outcomes in extending TST. These results are consistent with previous findings (70), reinforcing CBT’s status as the benchmark non-pharmacological intervention for the treatment of PI. While PSQI, TST, SE, and SL are validated measures of sleep architecture, they do not assess key insomnia domains like cognitive hyperarousal or daytime impairment—a limitation inherent to our focus on sleep-specific outcomes. The PSQI’s inclusion of non-specific factors (e.g., sleep environment) may reduce its sensitivity for pure insomnia assessment compared to the ISI (71), which directly measures core symptoms. We recommend future trials adopt ISI to standardize insomnia-specific evaluation while maintaining PSQI for broader sleep quality assessment.
In contrast, BZDs showed limited efficacy, performing worse than waitlist controls in most sleep outcomes, with the exception of PSQI scores. This inferior performance likely reflects the well-documented risks of BZDs, such as tolerance, dependence, and withdrawal symptoms (72). Although NBZDs were effective in increasing TST and shortening SL, concerns remain regarding their adverse effect profile. Notably, non-pharmacological interventions—particularly CBT—were linked to significantly fewer side effects and demonstrated better safety and long-term tolerability, making them more appropriate for chronic PI, where pharmacological treatments may exacerbate cognitive impairment, mood disturbances, or rebound insomnia.
Subgroup and sensitivity analyses further validated the robustness of the findings. Specifically, patients with long-standing PI (duration >10 years) and those undergoing prolonged treatment exhibited greater therapeutic benefits alongside reduced heterogeneity, suggesting that non-pharmacological interventions may offer superior efficacy in chronic PI management. These results support a paradigm shift in clinical practice, advocating for the reclassification of non-pharmacological therapies—traditionally regarded as adjunctive—to first-line treatment status in evidence-based guidelines.
Nevertheless, this review focused solely on monotherapy approaches. Future studies should investigate synergistic effects of combined interventions (e.g., CBT plus acupuncture) to optimize clinical outcomes. Additionally, pragmatic trials focusing on implementation are needed to evaluate real-world barriers like cost, availability, and provider training—that may influence accessibility and adherence. These studies will be essential for developing practical, scalable care models for PI.
4.1 Strengths and limitations
The strengths of this study include the use of guideline-recommended therapeutic approaches as the basis for literature searches, offering a strong basis for clinical decision-making when selecting appropriate treatment options. Sensitivity analyses confirmed the robustness of the results, as no directional changes were observed, supporting the reliability of the findings. However, substantial heterogeneity remained despite performing subgroup analyses based on treatment duration and disease course.
Variability in study design, inclusion and exclusion criteria, baseline characteristics (e.g., average age and geographic location), and trial duration likely contributed to these inconsistencies. Furthermore, the use of different scoring systems across the included RCTs limited our ability to fully resolve the observed heterogeneity.
While network meta-analysis provides a powerful framework for comparing multiple interventions indirectly, its validity depends critically on the quality and methodological consistency of included studies. Several limitations should be acknowledged. First, the analysis included 53 eligible RCTs, limiting statistical power and generalizability. Many trials had small sample sizes and exhibited methodological heterogeneity, particularly in intervention protocols, blinding procedures, and allocation concealment. Although most studies employed adequate randomization, the inherent challenges of blinding in non-pharmacological research—coupled with a lack of validation regarding its success—may have introduced performance and detection biases, potentially compromising outcome objectivity. Further limitations arise from inconsistencies in outcome measurement and reporting. Critical metrics of sleep maintenance, such as WASO and early morning awakening, were excluded due to heterogeneous definitions and insufficient standardized data across studies. The PSQI, while a widely accepted tool, does not specifically quantify WASO, thereby limiting its sensitivity in detecting sleep fragmentation. These discrepancies underscore the pressing need for uniform outcome measures in insomnia research to facilitate cross-study comparisons and enhance clinical relevance. To address these gaps, future investigations should prioritize large-scale, rigorously RCTs featuring robust blinding procedures, transparent reporting standards, and harmonized outcome assessments. Such efforts would strengthen the evidence base and yield more definitive guidance for clinical practice.
5 Conclusion
Non-pharmacological interventions demonstrated superior efficacy compared with pharmacological approaches for improving multiple sleep parameters in patients with PI. These therapies significantly increased TST, enhanced SE, reduced SL, and lowered PSQI scores. Additionally, they exhibited excellent safety profiles and high patient acceptability. Among all modalities examined, CBT, acupuncture, and acupressure emerged as the most effective options and should be considered first-line treatments.
Despite the promising results, several limitations should be acknowledged that may affect the generalizability and interpretation of the findings. Most of the included trials used the PSQI as the primary outcome measure. Although widely used, the PSQI assesses a broad range of sleep-related domains and may lack specificity for core insomnia symptoms. Notably, the PSQI does not directly evaluate key features such as cognitive hyperarousal or daytime impairment. In addition, other clinically relevant indicators—such as WASO and early morning awakening—were excluded due to inconsistent reporting and methodological heterogeneity. These limitations may reduce both the interpretability and generalizability of the results.
To enhance measurement precision and comparability across studies, future trials should employ more insomnia-specific instruments, such as the ISI, which directly assesses the severity of insomnia symptoms and their impact on daytime functioning. Moreover, large-scale, rigorously designed RCTs using standardized outcome measures are needed to strengthen the evidence base and support future guideline development.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Q-hZ: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition, Resources. Y-jL: Conceptualization, Data curation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GY: Conceptualization, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. M-yZ: Conceptualization, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Jia-hY: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Data curation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. X-mY: Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Q-lL: Conceptualization, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Jin-hY: Conceptualization, Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. X-lL: Conceptualization, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Y-mL: Conceptualization, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. T-cX: Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. FJ: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Scientific Research Projects of Medical and Health Institutions of Longhua District, Shenzhen (2022010). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank the China-Swiss TCM Centre for their support of the publication fee.
Conflict of interest
Q-hZ and J-hY were employed by Shenzhen Frontiers in Chinese Medicine Research Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1607903/full#supplementary-material
References
1. Roth, T, Coulouvrat, C, Hajak, G, Lakoma, MD, Sampson, NA, Shahly, V, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; ICD-10; and research diagnostic criteria/ICSD-2 criteria: results from the American insomnia survey. Biol Psychiatry. (2011) 69:592–600. doi: 10.1016/j.biopsych.2010.10.023
2. Aernout, E, Benradia, I, Hazo, JB, Sy, A, Askevis-Leherpeux, F, Sebbane, D, et al. International study of the prevalence and factors associated with insomnia in the general population. Sleep Med. (2021) 82:186–92. doi: 10.1016/j.sleep.2021.03.028
3. Qaseem, A, Kansagara, D, Forciea, MA, Cooke, M, and Denberg, TDfor the Clinical Guidelines Committee of the American College of Physicians*. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2016) 165:125–33. doi: 10.7326/M15-2175
4. Wilt, TJ, Macdonald, R, Brasure, M, Olson, CM, Carlyle, M, Fuchs, E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. (2016) 165:103–12. doi: 10.7326/M15-1781
5. Riemann, D, Espie, CA, Altena, E, Arnardottir, ES, Baglioni, C, Bassetti, CLA, et al. The European insomnia guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res. (2023) 32:e14035. doi: 10.1111/jsr.14035
6. Hertenstein, E, Trinca, E, Wunderlin, M, Schneider, CL, Züst, MA, Fehér, KD, et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: a systematic review and meta-analysis. Sleep Med Rev. (2022) 62:101597. doi: 10.1016/j.smrv.2022.101597
7. Spence, DW, Kayumov, L, Chen, A, Lowe, A, Jain, U, Katzman, MA, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatry Clin Neurosci. (2004) 16:19–28. doi: 10.1176/jnp.16.1.19
8. Li, Z, Jiao, K, Chen, M, and Wang, C. Effect of magnitopuncture on sympathetic and parasympathetic nerve activities in healthy drivers – assessment by power spectrum analysis of heart rate variability. Eur J Appl Physiol. (2003) 88:404–10. doi: 10.1007/s00421-002-0747-5
9. Wang, SC, Jiang, YM, Lai, DD, Liu, LM, and Cao, YN. Clinical observation on the effect of acupuncture at Shenmen and Sishencong on polysomnography in 150 patients with primary insomnia. Chin J Integr Med. (2018) 33:3203–5.
10. Pfeiffer, AM, Triplett, C, and Siengsukon, CF. Examining the prevalence of sleep disturbances in patients seeking physical therapy services. Physiother Theory Pract. (2024) 40:556–64. doi: 10.1080/09593985.2022.2134754
11. Hysing, M, Pallesen, S, Stormark, KM, Lundervold, AJ, and Sivertsen, B. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. (2013) 22:549–56. doi: 10.1111/jsr.12055
12. Kwon, CY, Lee, B, Cheong, MJ, Kim, TH, Jang, BH, Chung, SY, et al. Non-pharmacological treatment for elderly individuals with insomnia: a systematic review and network meta-analysis. Front Psych. (2021) 11:608896. doi: 10.3389/fpsyt.2020.608896
13. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association (2013).
14. Zhao, ZX, Zhang, P, and Huang, LQ. Guidelines for the diagnosis and treatment of insomnia in Chinese adults. Chin J Neurol. (2012) 45:11. doi: 10.3760/cma.j.issn.1006-7876.2012.07.022
15. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
16. Zhirong, Y, Feng, S, and Siyan, Z. Risk of bias assessment: (2) revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB 2.0). Chin J Endemiol. (2017) 38:1285–91.
17. Cao, Y, Yin, X, Yue, HY, Li, SS, and Xu, SF. Effects of Acupuncture Based on Regulating Du Meridian and Calming Mind on Sleep Quality in Patients with Primary Insomnia Liaoning Zhongyi Zazhi. (2020) 47:157–60. doi: 10.13192/j.issn.1000-1719.2020.12.046
18. Gao, SF, and Luo, YJ. Clinical observation on the intervention of auricular acupuncture for primary insomnia. Zhongyiyao Daobao. (2016) 22:94–5. doi: 10.13862/j.cnki.cn43-1446/r.2016.02.037
19. Li, YJ, Xie, M, Ruan, Q, Feng, CY, and Tao, HS. Evaluation of clinical efficacy of abdominal acupuncture for chronic primary insomnia with heart-kidney disharmony and its effect on plasma 5-HT. Liaoning Zhongyi Zazhi. (2019) 46:2412–5. doi: 10.13192/j.issn.1000-1719.2019.11.048
20. Zhang, HJ, Yao, Y, and Zhang, JW. A controlled study of cognitive-behavioral therapy and combined pharmacotherapy for primary insomnia. Chin J Pract Neurol. (2010) 13:6–9.
21. Zhao, J, Hong, QY, Yang, Y, Yao, J, Zhou, XP, and Wang, GL. Effects of He’s three connections method on sleep-wake patterns and sleep quality in patients with primary insomnia. Hebei Zhongyi. (2020) 42:1241–4.
22. Xuan, YB, Guo, J, Wang, LP, and Wu, X. The effect of acupuncture on sleep quality in patients with primary insomnia: a randomized controlled study. Zhongguo Zhen Jiu. (2007) 12:886–8.
23. Li, L, Liu, R, Zhang, T, Guo, J, and Chen, H. Effects of acupuncture on sleep quality and excessive wakefulness in patients with primary insomnia. Shanghai Zhenjiu Zazhi. (2019) 38:973–7. doi: 10.13460/j.issn.1005-0957.2019.09.0973
24. Jiang, L. Clinical study on the treatment of primary insomnia with traditional Chinese medicine pillow combined with five-tone therapy. Sichuan Zhongyi. (2020) 38:210–2.
25. Feng, XJ, Gai, HJ, and Wang, XY. Observational study on the efficacy of repetitive transcranial magnetic stimulation for primary insomnia. Clin Psychiatr J. (2017) 27:415–7.
26. Tu, JH, Chung, WC, Yang, CY, and Tzeng, DS. A comparison between acupuncture versus zolpidem in the treatment of primary insomnia. Asian J Psychiatry. (2012) 5:231–5. doi: 10.1016/j.ajp.2011.12.003
27. Jespersen, KV, Otto, M, Kringelbach, M, van Someren, E, and Vuust, P. A randomized controlled trial of bedtime music for insomnia disorder. J Sleep Res. (2019) 28:e12817. doi: 10.1111/jsr.12817
28. Jacobs, GD, Pace-Schott, EF, Stickgold, R, and Otto, MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. (2004) 164:1888–96. doi: 10.1001/archinte.164.17.1888
29. Edinger, JD, Wohlgemuth, WK, Radtke, RA, Marsh, GR, and Quillian, RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. (2001) 285:1856–64. doi: 10.1001/jama.285.14.1856
30. Siu, PM, Yu, AP, Tam, BT, Chin, EC, Yu, DS, Chung, KF, et al. Effects of tai chi or exercise on sleep in older adults with insomnia: a randomized clinical trial. JAMA Netw Open. (2021) 4:e2037199. doi: 10.1001/jamanetworkopen.2020.37199
31. Yin, X, Gou, M, Xu, J, Dong, B, Yin, P, Masquelin, F, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. (2017) 37:193–200. doi: 10.1016/j.sleep.2017.02.012
32. Lee, MRG, Breitstein, J, Hoyt, T, Stolee, J, Baxter, T, Kwon, H, et al. Cognitive behavioral therapy for insomnia among active duty military personnel. Psychol Serv. (2021) 18:42–50. doi: 10.1037/ser0000340
33. Ritterband, LM, Thorndike, FP, Gonder-Frederick, LA, Magee, JC, Bailey, ET, Saylor, DK, et al. Efficacy of an internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. (2009) 66:692–8. doi: 10.1001/archgenpsychiatry.2009.66
34. Yeung, W, Chung, K, Zhang, S, Yeung, WF, Chung, KF, Zhang, SP, et al. Electroacupuncture for primary insomnia: a randomized controlled trial. Sleep. (2009) 32:1039–47. doi: 10.1093/sleep/32.8.1039
35. Van Straten, A, Emmelkamp, J, De Wit, J, Lancee, J, Andersson, G, van Someren, EJ, et al. Guided internet-delivered cognitive behavioural treatment for insomnia: a randomized trial. Psychol Med. (2014) 44:1521–32. doi: 10.1017/S0033291713002249
36. Scharf, MB, Black, J, Hull, S, Landin, R, and Farber, R. Long-term nightly treatment with indiplon in adults with primary insomnia: results of a double-blind, placebo-controlled, 3-month study. Sleep. (2007) 30:743–52. doi: 10.1093/sleep/30.6.743
37. Schramm, PJ, Zobel, I, Mönch, K, Schramm, E, and Michalak, J. Sleep quality changes in chronically depressed patients treated with mindfulness-based cognitive therapy or the cognitive behavioral analysis system of psychotherapy: a pilot study. Sleep Med. (2016) 17:57–63. doi: 10.1016/j.sleep.2015.09.022
38. Yeung, WF, Yu, BYM, Yuen, JWM, Ho, JYS, Chung, KF, Zhang, ZJ, et al. Semi-individualized acupuncture for insomnia disorder and oxidative stress: a randomized, double-blind, sham-controlled trial. Nat Sci Sleep. (2021) 13:1195–207. doi: 10.2147/NSS.S318874
39. Khalsa, SBS, and Goldstein, MR. Treatment of chronic primary sleep onset insomnia with kundalini yoga: a randomized controlled trial with active sleep hygiene comparison. J Clin Sleep Med. (2021) 17:1841–52. doi: 10.5664/jcsm.9320
40. Riemann, D, Voderholzer, U, Cohrs, S, Rodenbeck, A, Hajak, G, Rüther, E, et al. Trimipramine in primary insomnia: results of a polysomnographic double-blind controlled study. Pharmacopsychiatry. (2002) 35:165–74. doi: 10.1055/s-2002-34119
41. Passos, GS, Poyares, D, Santana, MG, Garbuio, SA, Tufik, S, and Mello, MT. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med. (2010) 6:270–5. doi: 10.5664/jcsm.27825
42. Lo, C, Liao, WC, Liaw, JJ, Hang, LW, and Lin, JG. The stimulation effect of auricular magnetic press pellets on older female adults with sleep disturbance undergoing polysomnographic evaluation. Evid Based Complement Alternat Med. (2013) 2013:1–8. doi: 10.1155/2013/530438
43. Morin, CM, Colecchi, C, Stone, J, Sood, R, and Brink, D. Behavioral and pharmacological therapies for late-life insomnia. JAMA. (1999) 281:991. doi: 10.1001/jama.281.11.991
44. Wu, R, Bao, J, Zhang, C, Deng, J, and Long, C. Comparison of sleep condition and sleep-related psychological activity after cognitive-behavior and pharmacological therapy for chronic insomnia. Psychother Psychosom. (2006) 75:220–8. doi: 10.1159/000092892
45. Fu, C, Zhao, N, Liu, Z, Yuan, LH, Xie, C, Yang, WJ, et al. Acupuncture improves peri-menopausal insomnia: A randomized controlled trial. Sleep. (2017) 40:153. doi: 10.1093/sleep/zsx153
46. Espie, CA, MacMahon, KMA, Kelly, HL, MacMahon, KM, Broomfield, NM, Douglas, NJ, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. (2007) 30:574–84. doi: 10.1093/sleep/30.5.574
47. Abedian, Z, Eskandari, L, Abdi, H, and Ebrahimzadeh, S. The effect of acupressure on sleep quality in menopausal women: a randomized control trial. Complement Ther Clin Pract. (2015) 40:328–34.
48. Huang, Z, Li, Y, Bianchi, MT, Zhan, S, Jiang, F, Li, N, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. (2018) 11:1103–9. doi: 10.1016/j.brs.2018.05.016
49. Tang, HL, Chen, Z, Pang, J, and Mo, Q. Shujing tuina therapy for insomnia: a randomized controlled study. Zhongguo Zhen Jiu. (2015) 35:816–8. doi: 10.13703/j.0255-2930.2015.08.017
50. Wang, N, and Zhou, XY. Effects of the three-part Tuina technique on monoamine neurotransmitters and sleep quality in patients with primary insomnia. Zhongyi Xuebao. (2024) 39:11. doi: 10.16368/j.issn.1674-8999.2024.03.112
51. Lin, JZ, Zhang, ML, Qu, LM, Wang, JC, and Ye, RF. Long-term efficacy of acupuncture combined with biofeedback relaxation therapy for chronic insomnia in elderly patients. Chin J Integr Med. (2012) 27:2222–4.
52. Wang, JP, Wang, JB, Wang, LC, and Zhang, Y. Combined acupuncture and medication therapy for senile insomnia: a randomized controlled study. Zhongguo Zhen Jiu. (2015) 35:544–8. doi: 10.13703/j.0255-2930.2015.06.004
53. Zhang, J, Liu, X, Xie, X, Zhao, D, Shan, MS, Zhang, XL, et al. Mindfulness-based stress reduction for chronic insomnia in adults older than 75 years: a randomized, controlled, single-blind clinical trial. Explore (NY). (2015) 11:180–5. doi: 10.1016/j.explore.2015.02.005
54. Xu, P, Ji, WD, and Pan, YS. Effects of cognitive behavioral therapy combined with medication on serum cytokines and cortisol in elderly patients with sleep disorders. Pract Geriatr Med. (2015) 29:137–41.
55. Liang, XM. Clinical observation on auricular acupressure treatment for primary insomnia in elderly patients. Shanghai Zhenjiu Zazhi. (2017) 36:719–22. doi: 10.13460/j.issn.1005-0957.2017.06.0719
56. Yu, XP, and Gao, QC. Clinical study on the effects of acupuncture on sleep quality and cognitive function in elderly patients with primary insomnia. Jiangsu Zhongyi Yao. (2019) 51:62–4.
57. McCrae, CS, McGovern, R, Lukefahr, R, and Stripling, AM. Research evaluating brief behavioral sleep treatments for rural elderly (RESTORE): a preliminary examination of effectiveness. Am J Geriatr Psychiatry. (2007) 15:979–82. doi: 10.1097/JGP.0b013e31813547e6
58. Edinger, JD, Olsen, MK, Stechuchak, KM, Means, MK, Lineberger, MD, Kirby, A, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. (2009) 32:499–510. doi: 10.1093/sleep/32.4.499
59. Espie, CA, Kyle, SD, Williams, C, Ong, JC, Douglas, NJ, Hames, P, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. (2012) 35:769–81. doi: 10.5665/sleep.1872
60. Jernelöv, S, Lekander, M, Blom, K, Rydh, S, Ljótsson, B, Axelsson, J, et al. Efficacy of a behavioral self-help treatment with or without therapist guidance for co-morbid and primary insomnia: a randomized controlled trial. BMC Psychiatry. (2012) 12:5. doi: 10.1186/1471-244X-12-5
61. Kaldo, V, Jernelöv, S, Blom, K, Ljótsson, B, Brodin, M, Jörgensen, M, et al. Guided internet cognitive behavioral therapy for insomnia compared to a control treatment – a randomized trial. Behav Res Ther. (2015) 71:90–100. doi: 10.1016/j.brat.2015.06.001
62. Lichstein, KL, Riedel, BW, Wilson, NM, Lester, KW, and Aguillard, RN. Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J Consult Clin Psychol. (2001) 69:227–39. doi: 10.1037/0022-006X.69.2.227
63. Lovato, N, Lack, L, Wright, H, and Kennaway, DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. (2014) 37:117–26. doi: 10.5665/sleep.3320
64. Sivertsen, B, Omvik, S, Pallesen, S, Bjorvatn, B, Havik, OE, Kvale, G, et al. Cognitive behavioral therapy vs Zopiclone for treatment of chronic primary insomnia in older adults. JAMA. (2006) 295:2851–8. doi: 10.1001/jama.295.24.2851
65. Wei, M, Cao, RF, Gu, F, Gu, F, and Lv, Q. Observation of therapeutic effect of neigong Yi Zhi Chan Tuina for insomnia. Shanghai J Tradit Chin Med. (2013) 47:60–1.
66. Wei, DM, Liu, JZ, Li, Y, Chu, R, Liu, YL, and Wang, Z. Clinical observation on the efficacy of adult and pediatric Tuina combined with acupoint pressing for primary insomnia. Hebei Zhongyi. (2022) 44:460–3.
67. Yang, HR, Wang, YX, Zhou, B, Lei, Y, Yang, T, Wang, JM, et al. Randomized controlled study of the three-part Tuina technique for insomnia based on the theory of regulating Qi. Chin J Integr Med. (2024) 39:10.
68. Zhuang, QX. Clinical efficacy and safety of traditional Chinese Tuina therapy for insomnia. Clin Rational Drug Use J. (2020) 13:11. doi: 10.15887/j.cnki.13-1389/r.2020.22.051
69. Wei, M, Cao, RF, Gu, F, and Lv, Q. Clinical observation of the efficacy of Cao Renfa’s three-step Tuina method for insomnia. Shanghai J Tradit Chin Med. (2017) 51:61–3. doi: 10.16305/j.1007-1334.2017.01.018
70. Gao, L, Kong, DZ, Wang, SJ, and Zhang, Z. Network meta-analysis of the efficacy of external TCM therapies in improving sleep quality for insomnia. World Chin Med. (2022) 17:2753–8.
71. Bastien, CH, Vallières, A, and Morin, CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
72. Xiang, XJ, Liu, TB, Wang, CY, Liu, TQ, Zhou, YF, and Ruan, CJ. Adverse reactions and management of benzodiazepines. Chin J Drug Abuse Prev Treat. (2017) 23:256–60. doi: 10.15900/j.cnki.zylf1995.2017.05.003
Glossary
PI - Primary insomnia
RCTs - randomized controlled trials
CBT - cognitive behavioral therapy
PSQI - Pittsburgh Sleep Quality Index
TST - total sleep time
SE - sleep efficiency
SL - sleep latency
ROB - Cochrane Risk of Bias
RR - relative risk
CI - confidence interval
MD - Mean difference
SUCRA - Surface under the Cumulative Ranking
RT - Relaxation Therapy
CUP - Cupping Therapy
SH - Sleep Hygiene
rTMS - Repetitive Transcranial Magnetic Stimulation
BZD - Benzodiazepines
NBZD - Non-Benzodiazepines
PLA - Placebo (sham acupuncture or sham treatment)
Wait List - Waiting List
IF - inconsistency factor
Keywords: primary insomnia, cognitive behavioral therapy, acupuncture, acupressure, cupping therapy
Citation: Zhang Q-h, Liu Y-j, Yang G, Zhu M-y, Yang J-h, Li L, Yan X-m, Liu Q-l, Yue J-h, Li X-l, Li Y-m, Xu T-c and Jiang F (2025) Efficacy and safety of non-pharmacological therapies for primary insomnia: a network meta-analysis. Front. Neurol. 16:1607903. doi: 10.3389/fneur.2025.1607903
Edited by:
Stephen Sheldon, Northwestern University, United StatesReviewed by:
Leisha Cuddihy, University of Rochester, United StatesNimit Khara, Bhaikaka University, India
Copyright © 2025 Zhang, Liu, Yang, Zhu, Yang, Li, Yan, Liu, Yue, Li, Li, Xu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Jiang, amlhbmdmYW4xMjIxQGp4dXRjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Qin-hong Zhang
Qin-hong Zhang Yong-jian Liu
Yong-jian Liu Guanhu Yang
Guanhu Yang Mei-yi Zhu3
Mei-yi Zhu3 Jin-huan Yue
Jin-huan Yue Xiao-ling Li
Xiao-ling Li Fan Jiang
Fan Jiang