- 1State Key Laboratory of Experimental Hematology, Laboratory of Post-Neuroinjury Neurorepair and Regeneration in Central Nervous System Tianjin and Ministry of Education, Department of Neurosurgery, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Neurology, Tianjin Huanhu Hospital, Tianjin, China
- 3Department of Geriatrics, Geriatric Ward of Neurology, Institute of Tianjin Geriatrics, Tianjin Medical University General Hospital, Tianjin, China
- 4Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: Chronic subdural hematoma (CSDH) is a common neurological disorder in the elderly, typically managed through surgical intervention; however, in patients aged 90 years and older, surgery is often not feasible due to comorbidities, anticoagulant use, and other age-related factors. This study evaluates the effects of atorvastatin, either as monotherapy or in combination with dexamethasone, in the conservative treatment of CSDH in patients over 90 years old, while also reviewing the current literature on the management of CSDH in this super-aged population.
Methods: Seventeen super-aged patients diagnosed with CSDH at our neurosurgical department between January 2017 and June 2024, who either refused or were considered unsuitable for surgery, were included in the study. Six patients received atorvastatin monotherapy, while 11 were treated with a combination of atorvastatin and dexamethasone. Head imaging scans were analyzed, and the modified Rankin Scale (mRS) and Markwalder’s Grading Scale/Glasgow Coma Scale (MGS-GCS) scores were assessed before and after treatment.
Results: At the six-month follow-up, all patients showed significant improvement in neurological symptoms, as reflected by lower mRS and MGS-GCS scores. Hematomas were completely absorbed in 10 patients, significantly reduced in five, and unchanged in two patients with calcified hematoma. Three patients developed hyperglycemia, and one patient exhibited transaminitis; these adverse effects were resolved following the discontinuation of dexamethasone and the use of hepatoprotective medications. No mortality was recorded during the six-month follow-up.
Conclusion: Our findings suggest that atorvastatin-based treatment may improve the prognosis of CSDH in super-aged patients and offer a viable therapeutic alternative for those ineligible for surgery.
Introduction
Chronic subdural hematoma (CSDH) is a common neurological disorder in the elderly, typically presenting with headache, hemiparesis, and consciousness disturbances (1, 2). Surgical evacuation is currently the mainstay management for patients with CSDH, including burr-hole drainage, twist-drill craniostomy, and craniotomy (1, 3); however, despite advancements in neurosurgical techniques and operative instruments, management of CSDH in elderly patients remains highly challenging. Advanced age, a primary risk factor for CSDH (4), also correlates with poorer outcomes, likely due to higher comorbidity prevalence and increased use of antithrombotic drugs (5, 6). These factors are associated with higher risks of postoperative recurrence and mortality (5, 7). Moreover, with the population aging, the incidence of CSDH has surged, and the patient demographic is increasingly elderly (8). Therefore, neurosurgeons need to focus more on the treatment of super-aged CSDH patients, especially those over 90 years old.
Surgical intervention has been demonstrated to improve neurological function in CSDH patients over 90 years old who are in good physical condition, but it is associated with a high mortality rate (18.6–26.8% at six-month follow-up) (9, 10). Moreover, many super-aged patients are ineligible for surgery due to complex comorbidities and coagulation disorders, resulting in poor outcomes with conservative care. A study by Hiroyuki Toi, which included 5,414 Japanese CSDH patients over 90 years old, found that more than half had poor neurological outcomes at discharge, and nearly 40% were unable to return home (11). For these patients, particularly those who are ineligible for surgery, there is an urgent need to develop safe and effective conservative treatment strategies.
Several drugs, including dexamethasone, atorvastatin, tranexamic acid, and goreisan, have been investigated for the conservative treatment of CSDH (12). Among these, dexamethasone has been used for over 50 years as either a conservative or postoperative adjunct therapy. However, recent studies suggest that, compared to surgical intervention, dexamethasone treatment results in poorer neurological outcomes and higher complication rates (13), despite its efficacy in preventing postoperative recurrence (14). Nevertheless, it is premature to dismiss dexamethasone’s value in CSDH treatment (15). In contrast, atorvastatin (20 mg daily) has shown significant potential in improving neurological function and promoting hematoma absorption (16), with these effects further enhanced when combined with low-dose dexamethasone (17). As a commonly prescribed lipid-lowering agent, atorvastatin is well-established for preventing cerebrovascular disease in the elderly, with a favorable safety profile.
Therefore, it is reasonable to consider the use of atorvastatin-based conservative treatment for CSDH in super-aged patients, particularly those ineligible for surgery. This case series retrospectively evaluates the clinical outcomes of atorvastatin, either as monotherapy or in combination with dexamethasone, in 17 super-aged CSDH patients who did not undergo surgery. Additionally, we provide a comprehensive review of the existing literature on the management of CSDH in patients aged 90 years and older.
Methods
Case series and treatment procedure
This retrospective case study includes consecutive patients aged 90 years or older, diagnosed with CSDH at the Neurosurgery Center of the General Hospital of Tianjin Medical University between January 2017 and June 2024. Patients who underwent surgery or were in palliative care were excluded. A total of 17 super-aged patients who received atorvastatin monotherapy or combination therapy with low-dose dexamethasone were included. Among them, five were deemed ineligible for surgery due to prolonged anticoagulant use, and six were considered unsuitable for surgery based on poor physical condition, as assessed by experienced neurosurgeons. Additionally, two patients experienced postoperative recurrence, and four patients refused surgery due to fear of the procedure (Supplementary Table 1). All patients were fully informed of the alternative therapeutic processes and signed informed consent forms for atorvastatin monotherapy or combined with dexamethasone.
Six patients received atorvastatin monotherapy, including two who were initially treated with a combination of atorvastatin and dexamethasone but were switched to monotherapy due to severe hyperglycemia. The remaining 11 patients underwent a full course of conservative treatment with a combination of atorvastatin and low-dose dexamethasone. For all patients, atorvastatin was administered orally at 20 mg daily, with a minimum treatment duration of 3 months. Additionally, the patients who received combination therapy were orally administered dexamethasone at a gradually decreasing dose (2.25 mg/day for the first 2 weeks, 1.5 mg/day during the third week, and 0.75 mg/day during the fourth week). Atorvastatin therapy was discontinued upon follow-up confirmation of complete neurological symptom resolution.
Follow-up imaging was performed using computed tomography (CT) or magnetic resonance imaging (MRI) at least once after 2 months of treatment. Additionally, a six-month follow-up was conducted through outpatient visits or telephone interviews, during which clinical outcomes were assessed using the modified Rankin Scale (mRS) and the Markwalder Grading Scale/Glasgow Coma Scale (MGS-GCS) (Supplementary Table 2). The MGS is a clinical grading system for CSDH, with scores ranging from 0 (asymptomatic) to 4 (coma with no motor response to painful stimuli) (18). Liver function and blood glucose levels were regularly monitored to promptly detect and manage potential adverse drug reactions.
Literature review
All relevant articles were identified through PubMed and Web of Science databases using the search terms: “chronic subdural hematoma” or “chronic subdural hemorrhage” and “90 years” or “aged 90” or “nonagenarian.” We reviewed all English-language publications from January 1974 to December 2024. Then, Endnote software was used to remove the duplicate literature between different databases, and a preliminary screening was completed according to the titles and abstracts of the remaining articles. Finally, after excluding literature inconsistent with the review theme through full-text reading, relevant information from eight identified articles was extracted and summarized (Figure 1).
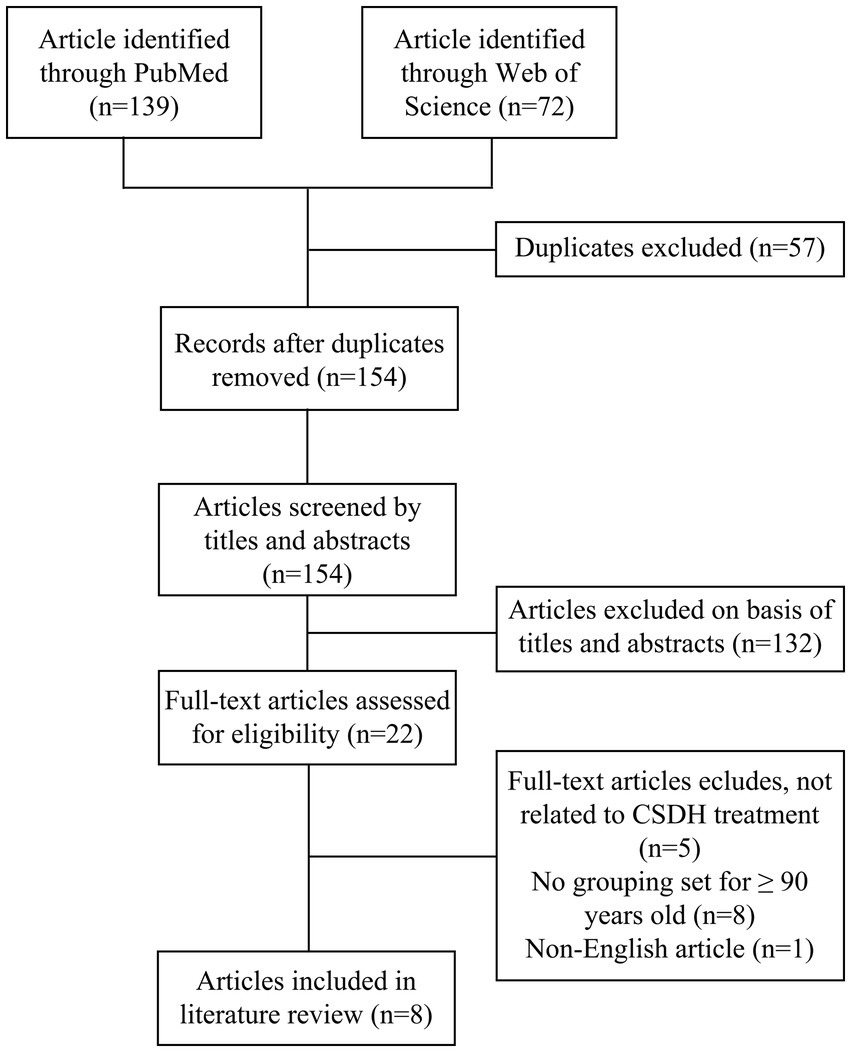
Figure 1. Literature review flowchart. This flowchart delineates the search and review process used to identify and select articles for inclusion in this study.
Results
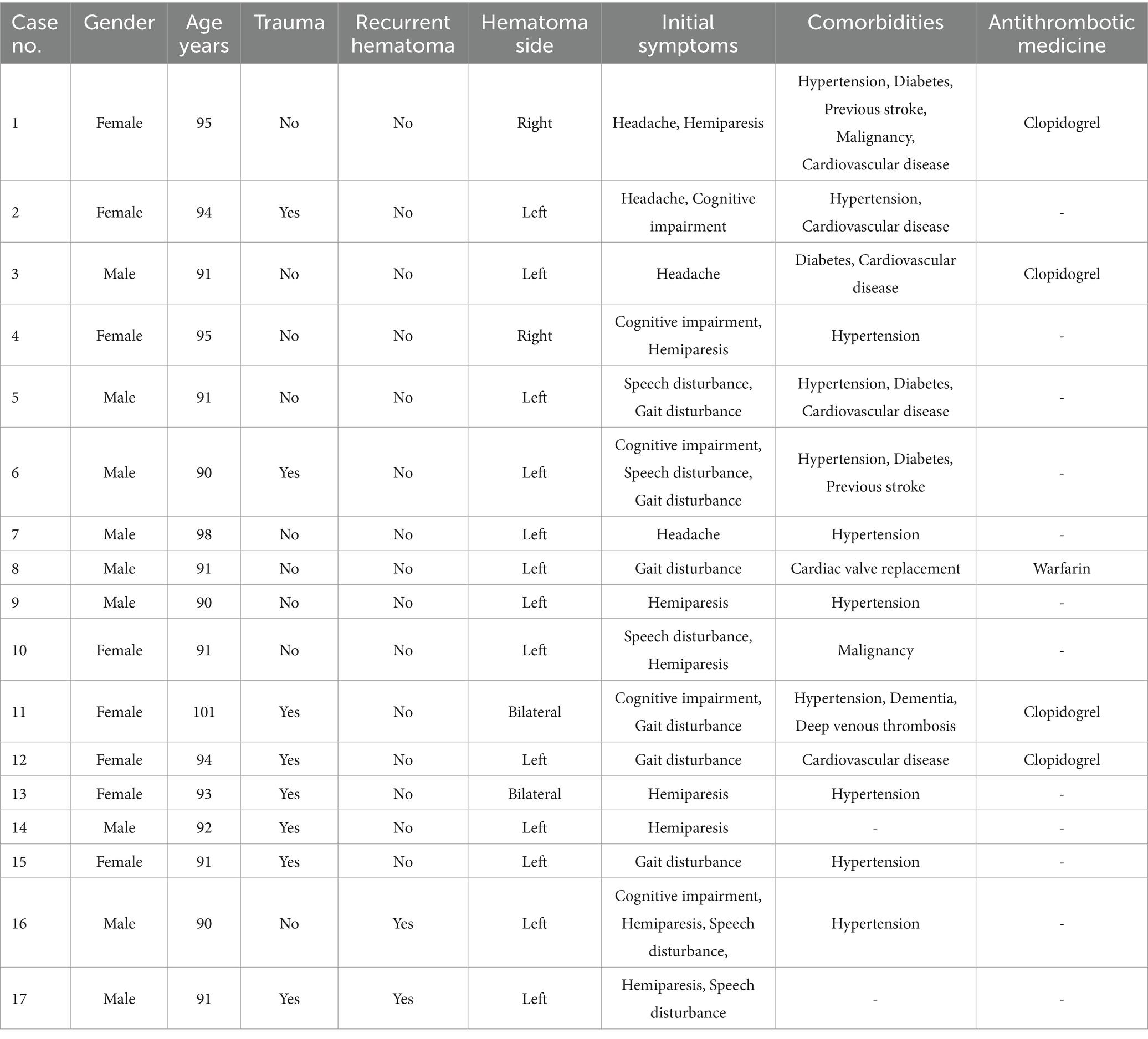
The mean age of the patient sample was 92.82 ± 3.07 years (range: 90–101 years), and the gender distribution was nearly equal (53% male vs. 47% female). Eight patients (47%) had a history of head trauma prior to the diagnosis of CSDH, four patients (24%) were treated with antiplatelet agents (clopidogrel) for heart disease, and one patient had been on warfarin following cardiac valve replacement before the development of CSDH. Hematomas were predominantly located in the left frontotemporal parietal subdural space, with two patients presenting with bilateral hematomas. Eleven patients (65%) exhibited midline shift due to hematoma compression, including two male patients who experienced postoperative recurrence. Most patients presented with mild to moderate symptoms, including headache, hemiplegia, and mild consciousness disturbances, which allowed for the possibility of conservative treatment. The primary comorbidities included hypertension (11 patients, 65%), diabetes (four patients, 24%), and cardiac disease (six patients, 35%), while a few patients had a history of stroke, deep vein thrombosis, dementia, or malignancy (Table 1).
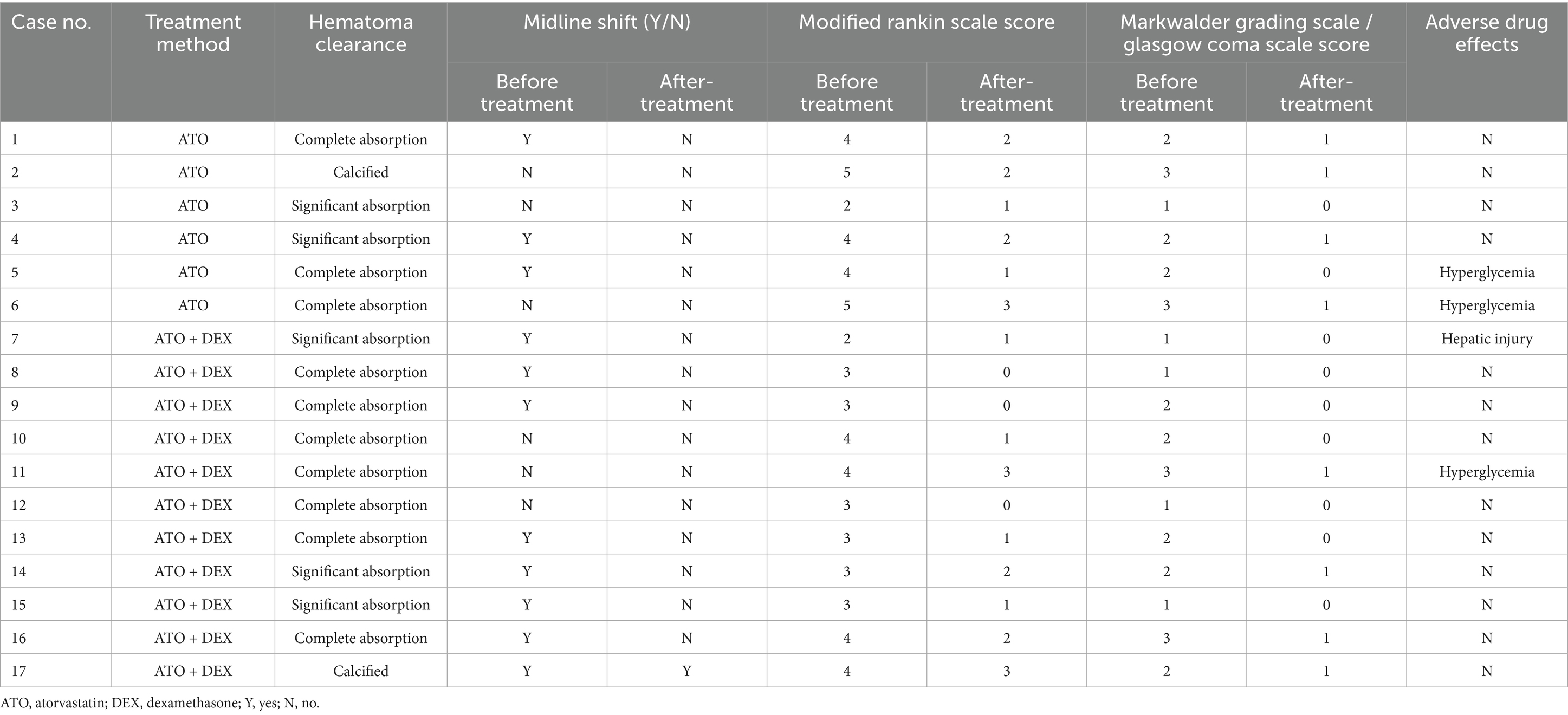
Following atorvastatin-based conservative treatment, CT/MRI imaging revealed complete hematoma absorption in 10 patients and a significant reduction in five (Figure 2). However, two patients with calcified hematomas showed no evidence of CSDH absorption; despite this, their neurological symptoms fully resolved, and atorvastatin was discontinued at the six-month follow-up. Additionally, compared to initial neurological assessments at the time of CSDH diagnosis, both mRS and MGS-GCS scores showed significant improvement following treatment (Table 2). Upon admission, nine patients (53%) presented with severe disability (mRS score 4–5), all of whom exhibited progressive improvement, with mRS scores reducing to 1–3 after treatment. Likewise, MGS-GCS grades decreased in all 17 patients, with a significant relief of neurological symptoms at the six-month follow-up.
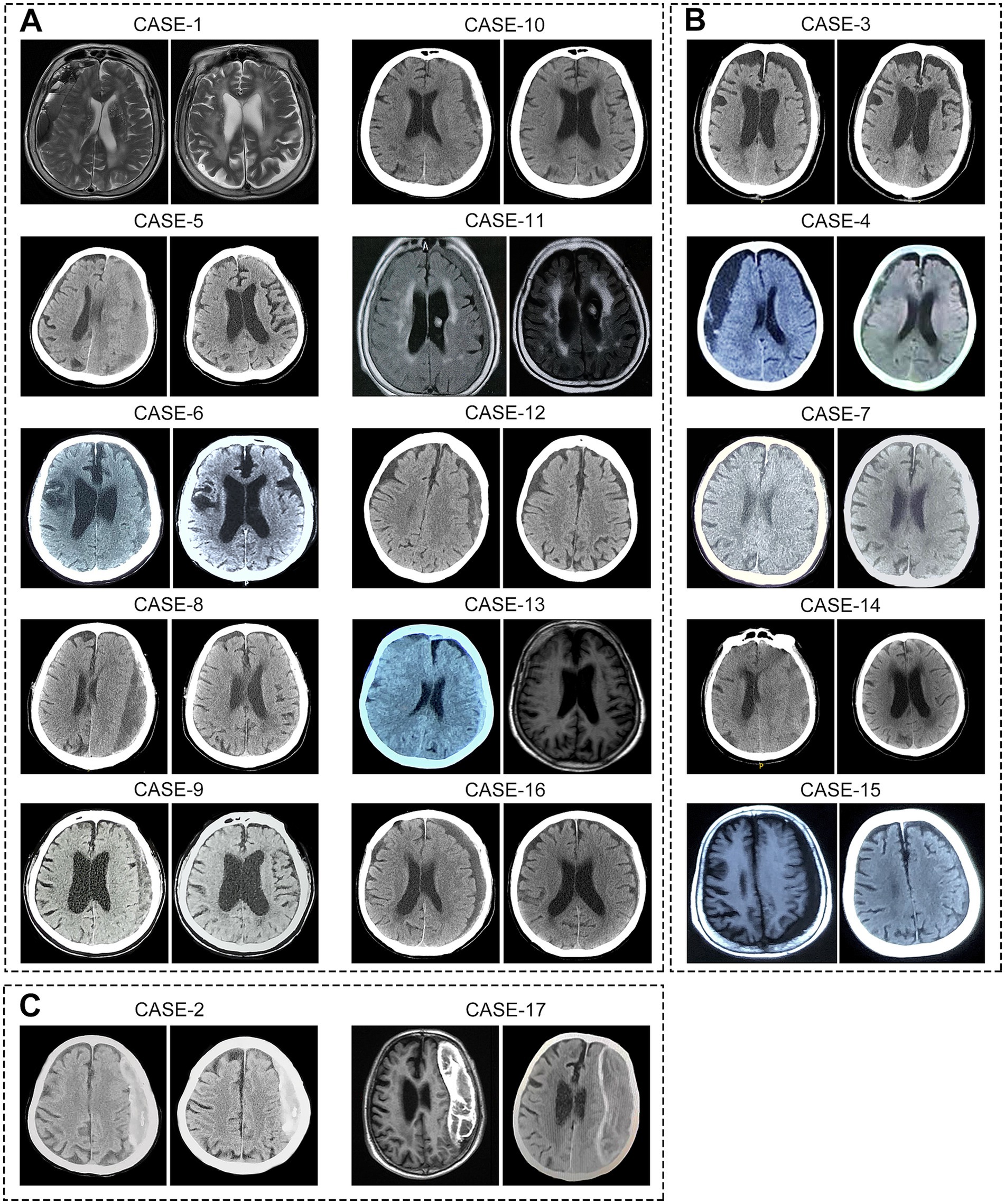
Figure 2. Imaging of hematomas before and after treatment in 17 super-aged CSDH patients. After receiving atorvastatin-based conservative treatment, (A) 10 patients showed complete hematoma absorption, (B) 5 patients exhibited significant reduction in hematoma size, and (C) 2 patients with calcified hematomas showed no apparent absorption.
During atorvastatin therapy, one patient developed elevated transaminase levels, which normalized after treatment with hepatoprotective agents. Additionally, three patients experienced elevated blood glucose levels while receiving concurrent dexamethasone treatment. Of these, two patients with pre-existing diabetes demonstrated poorly controlled glycemia. As a result, dexamethasone was promptly discontinued in these two patients, and atorvastatin monotherapy was continued. Notably, no mortality was recorded during the six-month follow-up period.
A systematic review of the literature identified eight studies on the management of CSDH in patients aged 90 years and older, encompassing 373 patients, 37% of whom were female (Table 3), (9, 10, 19–24). Of these patients, 50–77% had a documented history of head trauma, and 19–64% were on antithrombotic therapy. Despite the lack of established treatment guidelines for this population, the studies suggest that surgical intervention, particularly burr-hole drainage, is safe and recommended for super-aged patients in good physical condition. Compared to conservative care, surgical treatment has been shown to significantly reduce mortality, extend survival, and improve quality of life (9, 19). However, it is associated with higher mortality (8.8–31%) and recurrence rates (10.7–20%) compared to patients under 90 years of age (21–24). Notably, several studies report that regardless of treatment approach, the overall prognosis for super-aged CSDH patients remains poor, with many unable to regain independent living (11, 20). Thus, while surgical intervention is safe and effective for selected super-aged patients in good physical condition, it remains associated with a high mortality rate.
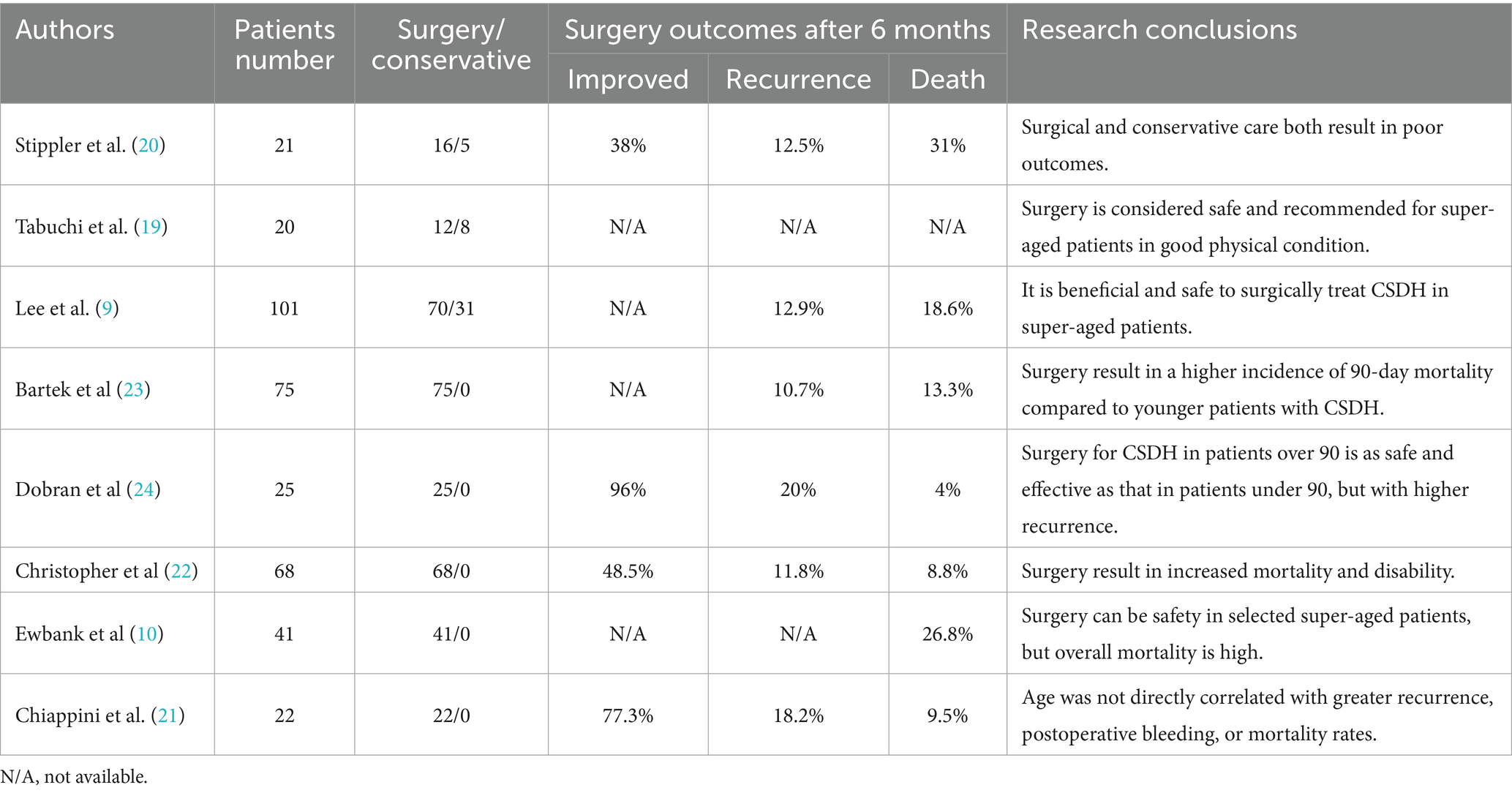
Table 3. Current literature on the management of CSDH in super-aged patients aged 90 years and older.
Discussion
CSDH is a specific type of intracranial hemorrhage, characterized by the accumulation of blood between the dura mater and the arachnoid membrane, resulting in a mass effect that causes headaches, motor deficits, speech difficulties, and cognitive impairments (1). The pathophysiology of CSDH is thought to stem from the rupture of bridging veins following head trauma, leading to hematoma accumulation in the subdural space. This process triggers chronic inflammation, which promotes the formation of hyperpermeable blood vessels and continuous leakage of blood into the subdural space, thereby contributing to CSDH formation (25). This unique pathological mechanism may explain the high recurrence rates of CSDH after surgery and serves as the theoretical basis for using middle meningeal artery embolization (MMAE) in its treatment (26).
Advanced age, anticoagulant use, and severe comorbidities are significant risk factors for recurrent CSDH and are closely associated with poor prognosis (4–7, 27). Notably, complex comorbidities and antithrombotic therapy are prevalent in the super-aged population. In our case series of 17 patients aged 90 years and older, 88% had comorbidities, primarily hypertension, diabetes, and cardiovascular disorders, with 29% receiving antithrombotic therapy. Studies have shown that cardiac and cerebrovascular diseases are the predominant comorbidities in super-aged CSDH patients, with more than half undergoing antithrombotic treatment (10, 23, 24). These factors not only complicate surgical decision-making but also increase patient anxiety regarding surgery, posing significant challenges in the management of CSDH in super-aged patients.
Among the 17 super-aged CSDH patients in our study, 11 were deemed unsuitable for surgery by experienced neurosurgeons due to dependence on antithrombotic therapy or poor physical condition. Additionally, six patients refused surgery, including two who lost confidence following postoperative recurrence and four who were afraid of the procedure. While burr-hole drainage is widely regarded as a safe and effective treatment for CSDH, postoperative recurrence remains a significant challenge in elderly patients. Furthermore, many older patients either cannot tolerate surgery or refuse it, thus being forced into conservative care (28). Compared to surgical intervention, these patients generally have a worse prognosis, with a six-month mortality rate as high as 58.1% (9). Symptomatic supportive therapy alone is insufficient to promote hematoma absorption, and the progression of CSDH further exacerbates neurological deficits, potentially leading to severe complications such as pneumonia, all of which contribute to a worse outcome. However, in this study, 15 patients exhibited significant hematoma absorption and marked improvement in neurological symptoms following atorvastatin-based conservative treatment. A prior randomized controlled trial (RCT) has demonstrated the efficacy of atorvastatin in CSDH management (16). For patients with small hematomas and mild symptoms, atorvastatin-based conservative treatment has gained increasing consensus among neurosurgeons in China (29). This case series is the first to outline the potential effects of atorvastatin monotherapy or its combination with dexamethasone in CSDH patients aged 90 and older.
Dexamethasone has been used as an adjunctive treatment for CSDH for over half a century (30). However, recent studies suggest that, compared to surgery, conservative treatment with dexamethasone is associated with a higher incidence of severe complications, making it less recommended (13). Despite this, dexamethasone has been shown to reduce the recurrence rate of CSDH after surgery significantly (14, 31). This effect likely results from its ability to reduce subdural inflammation, inhibit neovascularization, and decrease blood leakage into the subdural space, thus preventing hematoma reformation (32, 33). Long-term use of high-dose corticosteroids, however, carries risks such as hyperglycemia and immune suppression, which limit their therapeutic effects. Reducing the steroid dosage may provide greater benefits in CSDH treatment. A previous study demonstrated that low-dose dexamethasone (2.25 mg/day, tapered to 0.75 mg/day for 1 month) in combination with atorvastatin yields better results than statin monotherapy in CSDH management (17). Moreover, studies suggested that dexamethasone can promote the absorption of low-density CSDH and improve neurological function (34, 35). Therefore, dismissing the value of corticosteroids in CSDH treatment is premature (15). Given its potential efficacy, further research is needed to assess whether low-dose dexamethasone could be a viable treatment option for super-aged CSDH patients, particularly those ineligible for surgery.
In our study, although two patients experienced postoperative recurrence, their neurological symptoms significantly improved following the initial surgery. In super-aged CSDH patients, burr-hole drainage is currently the primary treatment and markedly improves life expectancy, with six-month survival rates nearly doubling compared to conservative care (9). Despite the clear benefits of surgery, the recurrence rate of 10.7–20% warrants attention (21–24), highlighting the need for optimal perioperative management to prevent recurrence and complications. For patients in good physical condition, local anesthesia may offer better outcomes, as general anesthesia has been associated with a five-fold increase in postoperative complications, primarily pneumonia and cardiovascular events, which are often fatal in elderly patients (36). Furthermore, using body-temperature irrigation fluid during surgery can reduce CSDH recurrence to 6% (37), and 24-h postoperative drainage is more effective in preventing recurrence compared to shorter or longer drainage durations (38, 39). Beyond surgical optimization, postoperative management with atorvastatin or low-dose dexamethasone may further reduce CSDH recurrence (40); however, additional studies are required to establish safe and effective dosing regimens. Given the high prevalence of antithrombotic therapy in super-aged patients, careful consideration is necessary regarding the timing of anticoagulant resumption after surgery. An ongoing pilot RCT investigating the optimal timing for resuming anticoagulation therapy after CSDH surgery may provide valuable insights (41).
Though surgical intervention offers significant benefits, some elderly patients are either ineligible or refuse it. In this cohort of 17 patients receiving conservative treatment, 11 exhibited midline shift due to hematoma compression, yet their neurological deficits were limited to headache, hemiparesis, and mild cognitive dysfunction. Moreover, two patients with calcified hematomas showed substantial neurological improvement at the six-month follow-up, despite no apparent hematoma resolution. These findings suggest that brain atrophy in super-aged patients may alleviate the mass effect, providing a window for conservative treatment. How to promote CSDH absorption? Addressing its pathological mechanisms is crucial. CSDH can be likened to a reservoir, where inflammatory vascular leakage represents the inflow (26), and meningeal lymphatic drainage serves as the outflow (42). Atorvastatin has been shown to preserve endothelial barrier integrity by suppressing inflammation (43, 44). In experimental subdural hematoma models, atorvastatin significantly reduced the expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8, and increased the number of anti-inflammatory regulatory T cells within the subdural cavity, thereby attenuating inflammation and vascular permeability (45, 46). These effects were further enhanced by the addition of dexamethasone (17, 47). Moreover, recent studies suggest that meningeal lymphatic drainage is compromised following hematoma formation due to endothelial disruption, whereas atorvastatin treatment can restore lymphatic function and accelerate hematoma clearance (48). Taken together, atorvastatin may facilitate hematoma absorption through a dual mechanism: reducing inflammatory vascular inflow and enhancing meningeal lymphatic outflow. Similarly, MMAE has demonstrated encouraging effects in accelerating CSDH resolution and reducing recurrence by blocking the inflow. (49, 50) Therefore, MMAE may offer a minimally invasive treatment option for super-aged CSDH patients (51).
The aging population has led to a marked increase in the incidence of CSDH, with a notable rise in super-aged patients, posing significant challenges to healthcare systems (8, 52). Future research should focus on gaining a deeper understanding of the pathophysiology of CSDH and developing strategies to prevent its formation and recurrence. Furthermore, there is a critical need to develop safe and effective conservative treatment options for super-aged patients, particularly those ineligible for surgical intervention.
This case study has several limitations. Firstly, it was non-randomized and lacked a direct comparison between conservative and surgical treatment groups. Although the results are promising, it remains unclear whether atorvastatin-based treatment can serve as an effective alternative to surgical intervention in super-aged patients. Additionally, the limited sample size restricts the ability to assess whether a combination of dexamethasone and atorvastatin is more effective than atorvastatin monotherapy. Lastly, this case study included only a six-month follow-up, lacking an evaluation of the long-term effects of atorvastatin-based conservative treatment. Future clinical trials are needed to address these issues and validate the efficacy of conservative treatment strategies for super-aged CSDH patients.
Conclusion
This retrospective study included 17 super-aged CSDH patients (≥90 years) who were ineligible for surgery. Daily treatment with 20 mg atorvastatin, either alone or in combination with low-dose dexamethasone, led to significant hematoma absorption. Throughout the treatment period, patients underwent close neurological monitoring and cranial imaging. Adverse events included hyperglycemia in three patients and elevated hepatic transaminase levels in one patient, all of which resolved after discontinuation of dexamethasone and initiation of hepatoprotective therapy. At the six-month follow-up, all patients showed notable neurological improvement, with a 100% survival rate. While burr-hole drainage remains the primary treatment for CSDH in super-aged patients, atorvastatin-based conservative therapy may provide a promising alternative for those unable or unwilling to undergo surgery.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. WQ: Data curation, Software, Writing – review & editing. XL: Data curation, Formal analysis, Writing – review & editing. PL: Data curation, Formal analysis, Writing – review & editing. JH: Writing – review & editing. CG: Writing – review & editing. TL: Data curation, Visualization, Writing – review & editing. YZ: Data curation, Writing – review & editing. JZ: Project administration, Supervision, Validation, Writing – review & editing. RJ: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No.82071390, 82071402).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1609514/full#supplementary-material
References
1. Kolias, AG, Chari, A, Santarius, T, and Hutchinson, PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. (2014) 10:570–8. doi: 10.1038/nrneurol.2014.163
2. Feghali, J, Yang, W, and Huang, J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. (2020) 141:339–45. doi: 10.1016/j.wneu.2020.06.140
3. Rodriguez, B, Morgan, I, Young, T, Vlastos, J, Williams, T, Hrabarchuk, EI, et al. Surgical techniques for evacuation of chronic subdural hematoma: a mini-review. Front Neurol. (2023) 14:1086645. doi: 10.3389/fneur.2023.1086645
4. Yang, W, and Huang, J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am. (2017) 28:205–10. doi: 10.1016/j.nec.2016.11.002
5. Abe, Y, Maruyama, K, Yokoya, S, Noguchi, A, Sato, E, Nagane, M, et al. Outcomes of chronic subdural hematoma with preexisting comorbidities causing disturbed consciousness. J Neurosurg. (2017) 126:1042–6. doi: 10.3171/2016.3.Jns152957
6. Baechli, H, Nordmann, A, Bucher, HC, and Gratzl, O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. (2004) 27:263–6. doi: 10.1007/s10143-004-0337-6
7. Uno, M, Toi, H, and Hirai, S. Chronic subdural hematoma in elderly patients: is this disease benign? Neurol Med Chir (Tokyo). (2017) 57:402–9. doi: 10.2176/nmc.ra.2016-0337
8. Stubbs, DJ, Vivian, ME, Davies, BM, Ercole, A, Burnstein, R, and Joannides, AJ. Incidence of chronic subdural haematoma: a single-Centre exploration of the effects of an ageing population with a review of the literature. Acta Neurochir. (2021) 163:2629–37. doi: 10.1007/s00701-021-04879-z
9. Lee, L, Ker, J, Ng, HY, Munusamy, T, King, NK, Kumar, D, et al. Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. J Neurosurg. (2016) 124:546–51. doi: 10.3171/2014.12.Jns142053
10. Ewbank, F, Durnford, A, Akarca, D, Sadek, AR, and Hempenstall, J. Surgical treatment of chronic subdural hematomas in nonagenarians: who to treat? World Neurosurg. (2021) 145:e274–7. doi: 10.1016/j.wneu.2020.10.035
11. Toi, H, Kinoshita, K, Hirai, S, Takai, H, Hara, K, Matsushita, N, et al. Present epidemiology of chronic subdural hematoma in Japan: analysis of 63,358 cases recorded in a national administrative database. J Neurosurg. (2018) 128:222–8. doi: 10.3171/2016.9.Jns16623
12. Wang, X, Song, J, He, Q, and You, C. Pharmacological treatment in the Management of Chronic Subdural Hematoma. Front Aging Neurosci. (2021) 13:684501. doi: 10.3389/fnagi.2021.684501
13. Miah, IP, Holl, DC, Blaauw, J, Lingsma, HF, den Hertog, HM, Jacobs, B, et al. Dexamethasone versus surgery for chronic subdural hematoma. N Engl J Med. (2023) 388:2230–40. doi: 10.1056/NEJMoa2216767
14. Hutchinson, PJ, Edlmann, E, Bulters, D, Zolnourian, A, Holton, P, Suttner, N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. (2020) 383:2616–27. doi: 10.1056/NEJMoa2020473
15. Wells, AJ. Commentary: will dexamethasone ever have a role in the management of chronic subdural hematomas? Brain Behav. (2024) 14:e3446. doi: 10.1002/brb3.3446
16. Jiang, R, Zhao, S, Wang, R, Feng, H, Zhang, J, Li, X, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in Chinese patients: a randomized ClinicalTrial. JAMA Neurol. (2018) 75:1338–46. doi: 10.1001/jamaneurol.2018.2030
17. Wang, D, Gao, C, Xu, X, Chen, T, Tian, Y, Wei, H, et al. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: phase II randomized proof-of-concept clinical trial. J Neurosurg. (2021) 134:235–43. doi: 10.3171/2019.11.Jns192020
18. Markwalder, TM, Steinsiepe, KF, Rohner, M, Reichenbach, W, and Markwalder, H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. (1981) 55:390–6. doi: 10.3171/jns.1981.55.3.0390
19. Tabuchi, S, and Kadowaki, M. Chronic subdural hematoma in patients over 90 years old in a super-aged society. J Clin Med Res. (2014) 6:379–83. doi: 10.14740/jocmr1907w
20. Stippler, M, Ramirez, P, Berti, A, Macindoe, C, Villalobos, N, and Murray-Krezan, C. Chronic subdural hematoma patients aged 90 years and older. Neurol Res. (2013) 35:243–6. doi: 10.1179/1743132813y.0000000163
21. Chiappini, A, Greuter, L, Mariani, L, Guzman, R, and Soleman, J. Mortality and outcome in patients older than 80 years of age undergoing Burr-hole drainage of chronic subdural hematoma. World Neurosurg. (2021) 150:e337–46. doi: 10.1016/j.wneu.2021.03.002
22. Christopher, E, Poon, MTC, Glancz, LJ, Hutchinson, PJ, Kolias, AG, and Brennan, PM. Outcomes following surgery in subgroups of comatose and very elderly patients with chronic subdural hematoma. Neurosurg Rev. (2019) 42:427–31. doi: 10.1007/s10143-018-0979-4
23. Bartek, J Jr, Sjåvik, K, Ståhl, F, Kristiansson, H, Solheim, O, Gulati, S, et al. Surgery for chronic subdural hematoma in nonagenarians: a Scandinavian population-based multicenter study. Acta Neurol Scand. (2017) 136:516–20. doi: 10.1111/ane.12764
24. Dobran, M, Marini, A, Nasi, D, Liverotti, V, Benigni, R, Costanza, MD, et al. Clinical outcome of patients over 90 years of age treated for chronic subdural hematoma. J Korean Neurosurg Soc. (2022) 65:123–9. doi: 10.3340/jkns.2018.0011
25. Edlmann, E, Giorgi-Coll, S, Whitfield, PC, Carpenter, KLH, and Hutchinson, PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. (2017) 14:108. doi: 10.1186/s12974-017-0881-y
26. Kim, H, Choi, Y, Lee, Y, Won, JK, Lee, SH, Suh, M, et al. Neovascularization in outer membrane of chronic subdural hematoma: a rationale for middle meningeal artery embolization. J Korean Neurosurg Soc. (2024) 67:146–57. doi: 10.3340/jkns.2023.0105
27. Kinoshita, S, Ohkuma, H, Fujiwara, N, Katayama, K, Naraoka, M, Shimamura, N, et al. Long-term postoperative prognosis and associated risk factors of chronic subdural hematoma in the elderly. Clin Neurol Neurosurg. (2024) 243:108186. doi: 10.1016/j.clineuro.2024.108186
28. Scerrati, A, Visani, J, Ricciardi, L, Dones, F, Rustemi, O, Cavallo, MA, et al. To drill or not to drill, that is the question: nonsurgical treatment of chronic subdural hematoma in the elderly. A systematic review. Neurosurg Focus. (2020) 49:E7. doi: 10.3171/2020.7.Focus20237
29. Zhang, J. Expert consensus on drug treatment of chronic subdural hematoma. Chin Neurosurg J. (2021) 7:47. doi: 10.1186/s41016-021-00263-z
30. Bender, MB, and Christoff, N. Nonsurgical treatment of subdural hematomas. Arch Neurol. (1974) 31:73–9. doi: 10.1001/archneur.1974.00490380021001
31. Cofano, F, Pesce, A, Vercelli, G, Mammi, M, Massara, A, Minardi, M, et al. Risk of recurrence of chronic subdural hematomas after surgery: a multicenter observational cohort study. Front Neurol. (2020) 11:560269. doi: 10.3389/fneur.2020.560269
32. Edlmann, E, Giorgi-Coll, S, Thelin, EP, Hutchinson, PJ, and Carpenter, KLH. Dexamethasone reduces vascular endothelial growth factor in comparison to placebo in post-operative chronic subdural hematoma samples: a target for future drug therapy? Front Neurol. (2022) 13:952308. doi: 10.3389/fneur.2022.952308
33. Wang, D, Fan, Y, Ma, J, Gao, C, Liu, X, Zhao, Z, et al. Atorvastatin combined with dexamethasone promote hematoma absorption in an optimized rat model of chronic subdural hematoma. Aging. (2021) 13:24815–28. doi: 10.18632/aging.203717
34. Thotakura, AK, and Marabathina, NR. Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg. (2015) 84:1968–72. doi: 10.1016/j.wneu.2015.08.044
35. Miah, IP, Blanter, A, Tank, Y, Zwet, EWV, Rosendaal, FR, Peul, WC, et al. Change in hematoma size after dexamethasone therapy in chronic subdural hematoma subtypes: a prospective study in symptomatic patients. J Neurotrauma. (2023) 40:228–39. doi: 10.1089/neu.2022.0024
36. Wong, HM, Woo, XL, Goh, CH, Chee, PHC, Adenan, AH, Tan, PCS, et al. Chronic subdural hematoma drainage under local anesthesia with sedation versus general anesthesia and its outcome. World Neurosurg. (2022) 157:e276–85. doi: 10.1016/j.wneu.2021.10.074
37. Bartley, A, Bartek, J Jr, Jakola, AS, Sundblom, J, Fält, M, Förander, P, et al. Effect of irrigation fluid temperature on recurrence in the evacuation of chronic subdural hematoma: a randomized clinical trial. JAMA Neurol. (2023) 80:58–63. doi: 10.1001/jamaneurol.2022.4133
38. Hjortdal Grønhøj, M, Jensen, TSR, Miscov, R, Sindby, AK, Debrabant, B, Hundsholt, T, et al. Optimal drainage time after evacuation of chronic subdural haematoma (DRAIN TIME 2): a multicentre, randomised, multiarm and multistage non-inferiority trial in Denmark. Lancet Neurol. (2024) 23:787–96. doi: 10.1016/s1474-4422(24)00175-3
39. Jensen, TSR, Haldrup, M, Hjortdal Grønhøj, M, Miscov, R, Larsen, CC, Debrabant, B, et al. National randomized clinical trial on subdural drainage time after chronic subdural hematoma evacuation. J Neurosurg. (2022) 137:799–806. doi: 10.3171/2021.10.Jns211608
40. Yu, W, Chen, W, Jiang, Y, Ma, M, Zhang, W, Zhang, X, et al. Effectiveness comparisons of drug therapy on chronic subdural hematoma recurrence: a Bayesian network Meta-analysis and systematic review. Front Pharmacol. (2022) 13:845386. doi: 10.3389/fphar.2022.845386
41. Mansouri, A, Nassiri, F, Scales, D, and Pirouzmand, F. Anticoagulation therapy timing in patients with atrial fibrillation after acute and chronic subdural Haematoma (ATTAACH): a pilot randomised controlled trial. BMJ Open. (2024) 14:e090224. doi: 10.1136/bmjopen-2024-090224
42. Liu, X, Gao, C, Yuan, J, Xiang, T, Gong, Z, Luo, H, et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. (2020) 8:16. doi: 10.1186/s40478-020-0888-y
43. Buttmann, M, Lorenz, A, Weishaupt, A, and Rieckmann, P. Atorvastatin partially prevents an inflammatory barrier breakdown of cultured human brain endothelial cells at a pharmacologically relevant concentration. J Neurochem. (2007) 102:1001–8. doi: 10.1111/j.1471-4159.2007.04563.x
44. Ogata, F, Fujiu, K, Matsumoto, S, Nakayama, Y, Shibata, M, Oike, Y, et al. Excess Lymphangiogenesis cooperatively induced by macrophages and CD4(+) T cells drives the pathogenesis of lymphedema. J Invest Dermatol. (2016) 136:706–14. doi: 10.1016/j.jid.2015.12.001
45. Li, T, Wang, D, Tian, Y, Yu, H, Wang, Y, Quan, W, et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J Neurol Sci. (2014) 341:88–96. doi: 10.1016/j.jns.2014.04.009
46. Quan, W, Zhang, Z, Li, P, Tian, Q, Huang, J, Qian, Y, et al. Role of regulatory T cells in atorvastatin induced absorption of chronic subdural hematoma in rats. Aging Dis. (2019) 10:992–1002. doi: 10.14336/ad.2018.0926
47. Fan, YS, Wang, B, Wang, D, Xu, X, Gao, C, Li, Y, et al. Atorvastatin combined with low-dose dexamethasone for vascular endothelial cell dysfunction induced by chronic subdural hematoma. Neural Regen Res. (2021) 16:523–30. doi: 10.4103/1673-5374.293152
48. Yuan, J, Liu, X, Nie, M, Chen, Y, Liu, M, Huang, J, et al. Inactivation of ERK1/2 signaling mediates dysfunction of basal meningeal lymphatic vessels in experimental subdural hematoma. Theranostics. (2024) 14:304–23. doi: 10.7150/thno.87633
49. Ma, L, Hoz, SS, Doheim, MF, Fadhil, A, Sultany, A, Al-Bayati, AR, et al. Middle meningeal artery embolization for "trial-ineligible" chronic subdural hematomas. Neurosurgery. (2025) 96:600–10. doi: 10.1227/neu.0000000000003136
50. Davies, JM, Knopman, J, Mokin, M, Hassan, AE, Harbaugh, RE, Khalessi, A, et al. Adjunctive middle meningeal artery embolization for subdural hematoma. N Engl J Med. (2024) 391:1890–900. doi: 10.1056/NEJMoa2313472
51. Debs, LH, Walker, SE, and Rahimi, SY. Newer treatment paradigm improves outcomes in the most common neurosurgical disease of the elderly: a literature review of middle meningeal artery embolization for chronic subdural hematoma. Geroscience. (2024) 46:6537–61. doi: 10.1007/s11357-024-01173-5
Keywords: chronic subdural hematoma, super-aged patients, atorvastatin, dexamethasone, case series
Citation: Yuan J, Quan W, Liu X, Li P, Huang J, Gao C, Liu T, Zhang Y, Zhang J and Jiang R (2025) Efficacy of atorvastatin-based treatment in super-aged patients with chronic subdural hematoma: a case series and literature review. Front. Neurol. 16:1609514. doi: 10.3389/fneur.2025.1609514
Edited by:
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, ColombiaReviewed by:
Luis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El SalvadorMohamed Arnaout, Zagazig University, Egypt
Copyright © 2025 Yuan, Quan, Liu, Li, Huang, Gao, Liu, Zhang, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianning Zhang, amlhbm5pbmd6aGFuZ0Bob3RtYWlsLmNvbQ==; Rongcai Jiang, amlhbmdyb25nY2FpQHRtdS5lZHUuY24=
†These authors have contributed equally to this work
 Jiangyuan Yuan
Jiangyuan Yuan Wei Quan1†
Wei Quan1† Xuanhui Liu
Xuanhui Liu Pan Li
Pan Li Chuang Gao
Chuang Gao Tao Liu
Tao Liu Jianning Zhang
Jianning Zhang Rongcai Jiang
Rongcai Jiang