Abstract
Objective:
Our study aimed to evaluate a long-term, in-home, bi-modal wearable device for seizure monitoring in epilepsy patients, assessing its applicability, clinical utility and identifying obstacles in real-life settings.
Methods:
A prospective pilot study involved 14 epilepsy patients at Seoul National University Bundang Hospital from May 26, 2021, to January 31, 2022. Patients used a wearable device developed for the study, featuring four-channel electroencephalogram and accelerometer sensors. Neurologists provided instructions for device usage, and bi-modal signals were recorded during daily activities. Seizures were annotated through comprehensive data review, and clinical suggestion was provided based on annotated daily seizure frequency, identification of different seizure types, and monitoring seizure patterns throughout the day.
Results:
Nine patients (64.3%) used the device for over 100 h, totaling 3,724 h of monitoring and capturing 1,609 seizures. The device successfully recorded various seizure types, including focal, focal with bilateral spread and generalized/bilateral onset which were further annotated by reviewers. Based on the annotated data, we were able to provide clinical suggestions based on number of seizures, identification of ambiguous seizures and monitoring of diurnal seizure pattern. Device discontinuation factors included skin irritation, patients’ unwillingness due to device appearance, and caregivers’ reluctance to use the device.
Conclusion:
This study demonstrates the feasibility and clinical utility of a long-term, in-home, bi-modal wearable device for seizure monitoring in epilepsy patients. The long-term data recorded by the device provided valuable clinical insights, facilitating different treatment suggestions. Addressing issues such as device comfort, appearance, and ease of use is essential for enhancing patient and caregiver adherence. The findings support the potential of wearable technology to improve epilepsy management through seizure monitoring in real-life setting.
1 Introduction
Seizure is a key symptom of epilepsy and are essential for diagnosis and treatment (1, 2). Accurate seizure monitoring is crucial for effective treatment, allowing for adjustments in antiseizure medication (ASM) dosage and type, and assessing the need for alternative treatments. Early identification of prolonged seizures is particularly important to ensure patient safety and prevent potential harm or risks like SUDEP (Sudden Unexpected Death in Epilepsy) (3).
In real-life situations, confirming and accurately monitoring seizures in everyday lives heavily relies on patient and caregiver reports. However, studies have revealed significant discrepancies between actual seizure frequency and the seizure diaries reported by patients and caregivers (4, 5), with many individuals being unaware of these differences (6). As a result, healthcare professionals and patients/caregivers consistently emphasize the need for objective seizure monitoring. The increasing popularity of smartwatches and the growing trend of mobile health management have led to a rise in research on seizure monitoring using wearable devices, with these devices gradually finding their place in clinical settings (7).
The long-term video electroencephalogram (VEEG) examination conducted in hospital settings is widely regarded as the gold standard for diagnosing seizures. However, it has notable limitations in terms of time, cost, and the controlled environment that differs from patients’ everyday lives (8). In response to these limitations, alternative methods for seizure monitoring, such as subcutaneous and ambulatory electroencephalogram (EEG), have been proposed (9, 10). Nonetheless, patients still encounter challenges when it comes to comfortable long-term non-invasive monitoring, leading to recent research focusing on seizure monitoring using wearable devices to address these drawbacks.
Wearable devices exhibit variations in sensitivity and specificity, which are influenced by the characteristics and modality of seizures, posing challenges in ensuring data quality (11, 12). To tackle this issue, studies have consistently utilized bi-modal devices to enhance seizure monitoring ability and capitalize on the strengths of each modality (13–15). However, there is limited documentation on the long-term real-life application of these devices and scarce research on the experiences of patients and healthcare professionals regarding their extended use (16–18).
In this study, we have developed a seizure monitoring system to monitor seizures using a long-term, in-home, bi-modal wearable device with 4 channel electroencephalogram and accelerometer sensors for seizure monitoring in epilepsy patients. A prospective pilot study was performed on 14 epilepsy patients to evaluate clinical utilities, advantages of multi-modal signals and identified obstacles of in-home wearable seizure monitoring device in real-life setting.
2 Methods
2.1 Patient selection
From May 26, 2021, to January 31, 2022, we prospectively enrolled 14 patients diagnosed with epilepsy at Seoul National University Bundang Hospital. Inclusion criteria were as follows: patients diagnosed with epilepsy by our neurologists, capable of integrating wearable systems into their daily routines, experiencing seizures or seizure-like events, and patients or their caregivers able to identify seizures. We further selected individuals with a high seizure frequency who demonstrated strong enthusiasm for participation into the pilot study of the device. We further selected individuals with a high seizure frequency who demonstrated strong enthusiasm for participation into the pilot study of the device. Exclusion criteria included patients and families anticipated to have low digital literacy—defined as an inability to proficiently operate digital applications and wearable devices. In addition, any patient who, due to dermatologic issues, was unable to use the wearable system continuously for at least 1 month was also excluded. Withdrawal criteria were defined as inability to wear the wearable systems for an extended period, difficulties in data transmission due to poor dexterity or technical problems, and determination by neurologists during participation that the patient was ineligible for monitoring based on medical reasons.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-2103-673-311). Informed consent was obtained from all the participants. This study was conducted in accordance with the Declaration of Helsinki.
2.2 Development of seizure monitoring system using long-term, in-home bi-modal seizure monitoring device
The wearable device was developed by SK Biopharmaceuticals Co., Ltd. (hereinafter referred to as SKBP) specifically for this study. The device comprises sensors and control modules. The sensor module features two disposable hydro-gel type electrodes designed for placement behind the ears at the left and right behind-the-ear positions, denoted by LB and RB, respectively. Upon the need to record EEG from frontal lobe, two additional electrodes may be connected to the sensors positioned behind the left ear, with these electrodes placed on the forehead at the left and right forehead positions, denoted by LF and RF, respectively. The number of electrodes were chosen to maximize the applicability to daily lives while ensuring high sensitivity to various types of seizures. All electrodes are secured to the skin using adhesive stickers, eliminating the need for conductive gel. A three-axis accelerometer was positioned behind the right ear sensor and was connected to the sensor module.
The control module, integrated into a Galaxy S10 + Android smartphone (Samsung Electronics, Suwon, Republic of Korea), handles digital signal processing, data upload, and user interaction. It powers the wearable system for up to 12 continuous hours, then requires a 1–2 h recharge. Because data transmission is disabled during charging, recordings obtained in that interval are unavailable for review. The schematic presentation of the device used in our study is shown in Figures 1A,B. Once the sensors and electrodes are in place, patients or caregivers can launch the mobile app. A single tap of the start button initiates recording; tapping it again stops the session, and a subsequent tap immediately resumes recording, enabling quick recovery from any accidental termination. During work hours, users were notified when the device was stopped accidently through telephone call. Seizure events can be logged in real time by pressing the event button during recording. The device was not water-resistant; therefore, users were instructed to pause the recording and remove it before any water exposure, such as showering.
Figure 1

Schematic view of wearable seizure monitoring device used in this study. (A) Overview of the system attached to the smart phone system. (B) Processed image of the device worn by a patient. (C) The example of data recorded from bi-modal wearable device. (D) Scalp EEG of corresponding channel of conventional EEG and on bi-modal wearable device compared in patient 2 and patient 6 (Fp1 for LF, Fp2 for RF). The analysis involves a comparison between the EEG recordings of patients conducted in the hospital, rather than simultaneous recordings. LF, left forehead position; RF, right forehead positions; LB, left behind ear position; RB, right behind ear positions; EEG, electroencephalogram; ACM, accelerometer.
2.3 Data acquisition
To ensure the correct usage of the device and to address potential concerns, two neurologists (HK and YK) provided comprehensive instructions for recording bi-modal signals in home settings. All participants were directed to capture EEG, and ACM signals as part of their bi-modal signal recording, utilizing four electrodes positioned behind the ears and on the forehead.
During recording, all the bi-modal signals were uploaded to the server every 5 min. Once the recording was confirmed, bi-modal signals were downloaded from the server. To facilitate further analysis, all the signals were converted to European data format (EDF) files using EDF browser.1 The bi-modal signals were recorded at a sampling frequency of 250 Hz.
To enhance signal quality, we applied specific filters to each signal type. EEG signals were bandpass filtered between 0.5 and 70 Hz, and accelerometer (ACM) signals (x, y, z axes) between 0.5 and 30 Hz. A 60 Hz notch filter removed powerline interference. This processing yielded four-channel EEG signals (LB, RB, LF, and RF) and three-channel ACM signals (ACM x, ACM y, ACM z) as bi-modal data. Relative to the standard 10–20 montage, the device recorded EEG from F7 (LF), F8 (RF), T3 (LB), and T4 (RB) in a referential configuration. Data were sampled at 250 Hz with 24-bit resolution. The downloaded data were thoroughly analyzed using MATLAB software (MathWorks, Natick, MA, United States) to ensure accurate and standardized processing for further examination and interpretation. To ensure the signal quality, the device continuously checked electrode contact by monitoring for signals < 62.5 Hz on the reference leads. If such a signal was detected, the application alerted the user with an audible tone and pop-up prompt, instructing them to reposition the electrodes until proper contact was restored.
Throughout the recording period, patients and caregivers were instructed to document the times at which they observed or suspected the occurrence of seizures, utilizing the mobile application provided. We referred to this time as event time. All the reported event times were promptly uploaded to the server as soon as the patients or caregivers pressed the confirm button.
2.4 Comparison of EEG quality obtained from the wearable device to conventional EEG
To verify the quality of the EEG recordings obtained from the wearable device, these were evaluated against scalp EEG recordings from the same patient, which were not captured concurrently. Figure 1C displays the processed images from the wearable system, the signals acquired by the device, and a comparison of the EEG quality with that of conventional EEG (Figure 1D). Not all patients go through video EEG monitoring for seizure classifications and conventional 30 min EEGs were chosen as reference EEGs.
2.5 Seizure annotation and clinical suggestion
Two neurologists (H. K. and Y. K.) reviewed all bi-modal recordings to verify seizures in the 14 patients. First, we examined and annotated every seizure reported by patients or caregivers. Next, we used these annotated EEG and bi-modal patterns to review the entire dataset for matching signatures, identifying additional electroclinical events. Further efforts were made to identify non-motor seizures based on patient/caregiver’s semiology notes. The ictal period was defined as the interval from seizure onset to termination. Patients who used the device > 100 h were classified as “dedicated” users; those with < 100 h were deemed “discontinued” users.
Based on the acquired data, annotated daily seizure frequency, identification of different seizure types and seizure distribution throughout the day was analyzed for each patient. Based on the results, clinical suggestions was provided if indicated. The overall process for data acquisition, seizure annotation and clinical suggestion is shown in Figure 2.
Figure 2
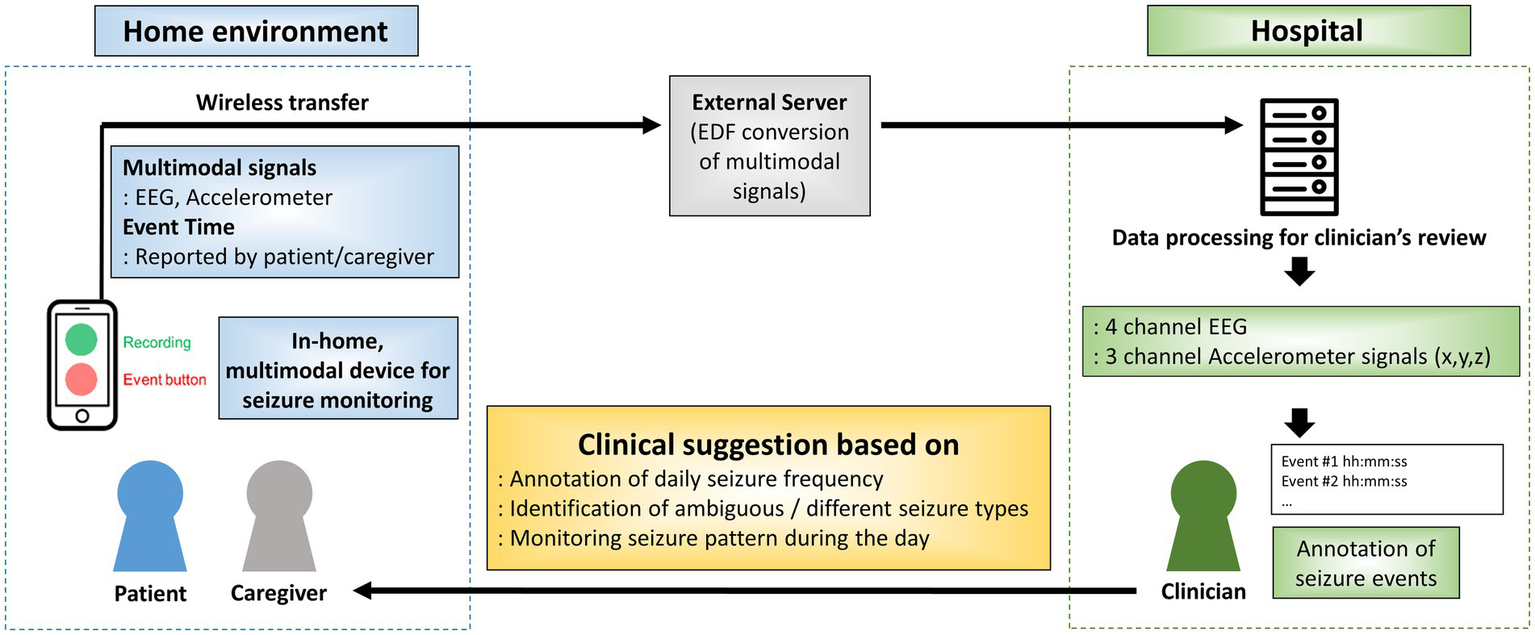
Overall process for data acquisition, seizure annotation and clinical suggestion. EDF: European data format; EEG: electroencephalogram; ACM: accelerometer.
2.6 Hourly seizure frequency analysis
The analysis conducted to focus on the hourly seizure rates, defined as the number of seizures occurring per hour, among patients who engaged in bi-modal signal recording for over a month and experienced at least 10 seizures verified by neurologists. Hourly seizure frequency was annotated to assist patients and caregivers in identifying seizure hotspots during the day, thereby allowing them to recognize periods when they should be more attentive to clinical symptoms.
3 Results
3.1 Patient characteristics and seizure annotations in dedicated users
Fourteen patients (7 male, 7 female) were enrolled. Median age at study entry was 17 years (range 7–28), and median epilepsy onset was 5.5 years (0.4–16). Twelve had focal epilepsy and two had Lennox–Gastaut syndrome; etiology was structural in eight and unknown in six. EEG showed focal epileptiform discharges in 10 patients, multifocal discharges in two, and generalized discharges in two. The clinical characteristics of patients participated in this study is summarized in Table 1.
Table 1
| Sex | Age (yr) | Onset age (yr) | Epilepsy diagnosis | IED | MRI | Etiology | Seizure frequency |
Daily activity |
Number of ASMs | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 20 | 5 | LRE | Focal | NS | Unknown | Several/W | Ambulatory, ID | 4 |
| 2 | M | 23 | 0.4 | LGS | Generalized (Bifrontal) |
Mild atrophy | Unknown | Frequent/D | Bed-ridden | 5 |
| 3 | F | 22 | 1 | LRE | Focal | NS | Unknown | Frequent/D | Bed-ridden | 4 |
| 4 | M | 10 | 4 | LRE | Focal (Multifocal) |
HIE | Structural | Frequent/D | Bed-ridden | 5 |
| 5 | F | 10 | 7 | LRE | Focal | NS | Unknown | Frequent/D | Ambulatory | 3 |
| 6 | F | 9 | 2 | LRE | Focal (Multifocal) |
Band heterotopia | Structural | Frequent/D | Ambulatory, ID | 4 |
| 7 | M | 18 | 6 | LRE | Focal | Infarction | Structural | Several/W | Ambulatory | 1 |
| 8 | F | 12 | 9 | LRE | Focal | Mild atrophy | Structural | Frequent/D | Ambulatory, ID | 4 |
| 9 | M | 13 | 11 | LRE | Focal | PVL | Structural | Several/W | Ambulatory, ID | 2 |
| 10 | M | 7 | 0.5 | LRE | Focal | NS | Unknown | Several/W | Ambulatory | 2 |
| 11 | F | 18 | 15 | LRE | Focal | ACC | Structural | Several/M | Ambulatory, ID | 3 |
| 12 | M | 16 | 7 | LRE | Focal | Tumor (DNET) | Structural | Several/M | Ambulatory | 2 |
| 13 | F | 28 | 16 | LRE | Focal | HS | Structural | Several/M | Ambulatory | 0 |
| 14 | M | 23 | 7 | LGS | Generalized (Bifrontal, GSW, GPFA) | NS | Unknown | Frequent/D | Ambulatory, ID | 5 |
Demographic and clinical characteristics of enrolled patients.
IED, interictal epileptiform discharge; MRI, magnetic resonance imaging; ASM, antiseizure medication; LRE localization-related epilepsy; LGS, Lennox–Gastaut syndrome; GSW, generalized spike–wave complex; GPFA, generalized paroxysmal fast activity; NS, not significant; HIE, hypoxic Ischemic encephalopathy; PVL, periventricular leukomalacia; ACC, agenesis of corpus callosum; DNET, dysembryoplastic neuroepithelial tumor; HS, hippocampal sclerosis; W, week; D, day; M, month; ID, intellectual disability.
Nine of the 14 patients (64.3%) met the “dedicated user” criterion (> 100 h of use). In this group, 1,609 seizures were recorded over 3,724 h, encompassing focal onset, focal-to-bilateral tonic–clonic, tonic, and myoclonic events (Figure 3). These annotated seizures enabled clinical suggestions (Table 2).
Figure 3
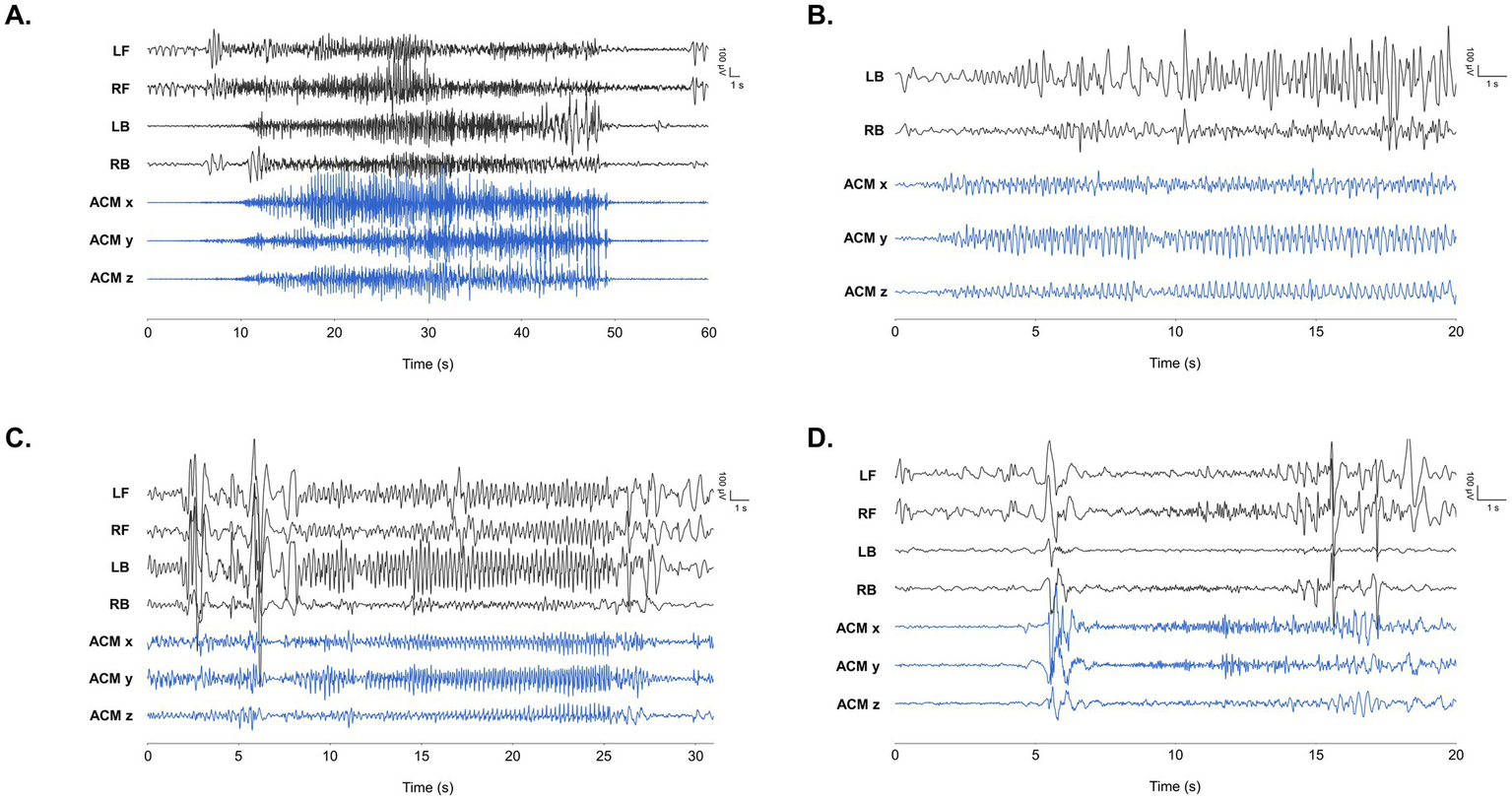
Representative EEG findings during seizure events obtained by the wearable device in our study. LB/RB and LF/RF refers to EEG positioned at left and right behind ears, and left and front, respectively. ACM x/y/z refers to x/y/z axes of the accelerometer positioned at right behind ear. Time frame of each finding is noted on the x-axis of each graph in seconds. (A) Tonic seizure annotated in patient 2. (B) Focal onset seizure with chest tightness in patient 7. (C) Hypomotor seizure with lip cyanosis in patient 8. EEG findings show rhythmic 3.5–4 Hz high voltage spike wave discharges originating from multiple areas. Ictal discharges with shorter time frame is depicted in red dotted box. (D) Myoclonic seizure with generalized discharge in patient 14.
Table 2
| Patient # | # of reported seizures by patient/caregiver | # of annotated seizure events | Monitoring device usage time (hours) | Clinical suggestion | Complaints during device use |
|---|---|---|---|---|---|
| Dedicated users | |||||
| 2 | 61 | 43 (24 Generalized, 19 Focal) |
757.9 | Annotation of daily seizure frequency → clinical suggestion to caregiver |
Skin irritations |
| 3 | 53 | 5 (5 Focal) |
233.9 | Annotation of daily seizure frequency → clinical suggestion to caregiver |
Skin irritations, caregiver’s reluctance |
| 6 | 25 | 13 (13 Focal) |
100.4 | Annotation of daily seizure frequency → clinical suggestion to caregiver |
Skin irritations |
| 7 | 24 | 19 (19 Focal) |
108.8 | Identification of ambiguous seizure types → ASM adjustment led to seizure free state |
|
| 8 | 135 | 1,422 (1,422 Focal) |
957.7 | Annotation of daily seizure frequency → clinical suggestion to caregiver |
|
| 10 | 7 | 0 | 110.2 | Ambiguous symptom monitoring → clinical suggestion of non-epileptic events |
Caregiver’s reluctance |
| 12 | 11 | 4 (4 Focal) |
403.0 | Identification of ambiguous seizure types → ASM adjustment led to seizure free state |
|
| 13 | 7 | 0 | 507.3 | Ambiguous symptom monitoring → clinical suggestion of non-epileptic events |
|
| 14 | 287 | 103 (89 Generalized, 14 Focal) |
414.9 | Identification of ambiguous seizure types → ASM adjustment for better seizure control |
|
| Discontinued users | |||||
| 1 | 0 | 0 | 10.4 | Patient’s unwillingness | |
| 4 | 13 | 0 | 3.4 | Skin irritations, caregiver’s reluctance | |
| 5 | 4 | 0 | 5.6 | Patient’s unwillingness | |
| 9 | 7 | 0 | 64.0 | Patient’s unwillingness | |
| 11 | 5 | 0 | 46.6 | Patient’s unwillingness | |
Summary of clinical outcome of epilepsy patients after using wearable seizure monitoring device.
3.2 Clinical suggestion based on seizure monitoring
3.2.1 Annotation of daily seizure frequency
Patient 8, a 12-year-old girl recovering from FIRES (Febrile Infection Related Epilepsy Syndrome), had daily intractable convulsive seizures. Over 116 days she used the device for 958 h, which captured 1,422 seizures. Her mother’s diary integrated into the app, typically recorded fewer than 10 clinical events, describing motion arrest and head version that evolved into bilateral tonic–clonic seizures (Figure 3C). The marked discrepancy between device-annotated seizures, marked 36.5 seizures/day, and caregiver-reported counts prompted for clinical suggestions and subsequent ASM adjustments.
3.2.2 Identification of ambiguous/different seizure types and utilization of multi-modal signals
Patient 7 is an 18-year-old male diagnosed with lesional focal epilepsy. He is a survivor of Langerhans cell histyocytosis (LCH) and has encephalomalacia in both occipital lobes as a result of hemorrhagic infarction experienced during chemotherapy. The patient frequently experienced episodes of chest tightness followed by decreased consciousness. Over 17 days, he used the device for 109 h. Suspecting the ictal focus was in his brain lesion, we employed a two-channel EEG positioned behind the ear. During these events, ictal EEG changes from the behind-the-ear channels confirmed a focal seizure originating from the encephalomalacia. The recorded data displayed repetitive spike or spike–wave discharges indicative of a seizure (Figure 3B). After confirming an electroclinical seizure, we increased the oxcarbazepine dosage. Following clinical suggestion based on identification of ambiguous seizures, he is now free of seizures with chest tightness.
Patient 14 is 23-year-old male with Lennox–Gastaut syndrome. He is ambulatory but he has severe intellectual disability. As the patient was noted to have multiple seizure types, multi-modal signals and parents’s reports were analyzed together to identify different seizure types. Over a period of 52 days, the device was used for a total of 415 h. Our analysis enabled us to identify 103 seizures, which encompassed various seizure types. Using the caregiver’s descriptions of semiology, we identified three seizure types based on EEG and accelerometer signals. Focal onset hypomotor and tonic seizures were recognized through evolving spike–wave discharges in EEG readings and caregiver annotations. Myoclonic seizures were detected via abrupt changes in accelerometer data accompanied by EEG alterations. Clinical suggestion was provided to the family that the observed motion arrests and myoclonic movements were indeed seizures. After adjusting ASMs, the number of annoted seizures were decreased to 7.8 times/day to 2.5 times a day. Representative EEG findings are shown in Supplementary Figure S1.
Patient 12 is a 16-year-old male who underwent partial surgical removal of a dysembryoplastic neuroepithelial tumor (DNET) in the right temporal uncus. After surgery, his habitual seizures ceased, but he began experiencing episodes of chest pain lasting 20–30 s once or twice a month. Cardiology evaluations failed to identify a cause. Enrolled in our study, we detected spike discharges on EEG correlating with his chest pain episodes, confirming them as epileptic seizures. After adjusting his antiepileptic medications, the patient has been seizure-free for 1 year.
3.2.3 Monitoring seizure pattern throughout the day
We categorized patients’ hourly seizure data to analyze seizure frequency by hour. Despite intermittent device usage and high seizure counts, each patient’s hourly seizure distribution showed distinct patterns. Patient 2 had frequent seizures primarily during sleep. Patient 4 experienced frequent seizures in the morning and afternoon. Patient 8 had fewer seizures during sleep, while patient 14 consistently had seizures throughout the day except during sleep (Supplementary Figure S2). Although ASMs were not adjusted based on these seizure-prone periods, families received clinical suggestions to increase awareness for enhanced patient safety.
3.3 Factors leading to discontinuation or undesirability of wearable device
During the course of the study, a total of 5 patients (35.7%) discontinued the use of wearable devices due to various external factors. Within this group, one patient stopped using the device owing to skin irritations and the caregiver’s reluctance to use the device. Four other patients discontinued use due to their own unwillingness to use the device (Table 2).
3.3.1 Skin irritation
Skin irritation was the primary side effect, occurring only in patients who used the wearable device for over a month (patients 2, 3, 4, and 6). No skin irritation was reported with short-term use. Patient 4 discontinued the device after redness developed, leading to spontaneous improvement. In patient 6, although the device successfully monitored seizure activity from the frontal lobe, continued use was challenging due to discomfort from electrode-induced skin irritation. To address this, only forehead electrodes were used, eliminating the behind-ear electrodes causing irritation. In patients 2 and 4, intermittent use led to spontaneous improvement of skin irritations.
3.3.2 Patients’ unwillingness
Four participants (patients 1, 5, 9, 11) stopped using the device for personal reasons. Each had mild intellectual disability but functioned independently. They found the visible hardware intrusive at school or work, viewing it as a conspicuous marker of their condition; one described feeling “embarrassed” by peers’ questions. This discomfort limited wear time and led to discontinuation.
3.3.3 Caregivers’ reluctance
Caregivers of three bedridden patients found the device and control module burdensome. The intensive care these patients required left little time for electrode application and seizure logging. Consequently, patient 4 discontinued use, whereas patients 3 and 10 persisted and continued to receive clinical suggestions.
4 Discussion
4.1 Enabling effective long-term, in-home seizure monitoring
For effective seizure monitoring using wearable devices, it is essential to ensure comfortable usage by patients in their daily lives and maintain good signal quality. Previous studies have primarily focused on validating results by combining wearable devices with VEEG in a limited controlled hospital environment (13–16, 19). Few studies, including ours, have investigated the extended use of wearable devices for seizure monitoring in everyday home settings. Previous research, while benefiting from VEEG validation, was limited to controlled hospital environments and may not represent data captured during patients’ daily activities. Additionally, these studies faced cost and time constraints, with examination periods typically confined to a maximum of 1 week. Home-based seizure monitoring studies have primarily reported on wrist-worn devices capturing accelerometer data alone or alongside electrodermal activity (20–22).
In this study, we successfully demonstrated that the majority of the patients comfortably used wearable devices with behind-the-ear EEG during extended daily activities, experiencing minimal discomfort or serious adverse events. Utilizing a smartphone as a control module and battery charger allowed for 12 continuous hours of operation, capturing daily activities without battery constraints. By placing up to four electrodes on the forehead and behind the ears based on each patient’s epileptic focus, we were able to perform seizure monitoring with fewer channels (23–26). The implementation of a three-axis accelerometer aided in characterizing different seizure types during annotations. Based on long-term seizure monitoring, clinical suggestions provided to the patients and families further motivated them to use the monitoring device.
4.2 Suggesting clinical utility of long-term seizure monitoring from a pilot study
We demonstrated the possible clinical utility of using wearable devices for seizure monitoring in different epilepsy patients. In clinical practice, accurately assessing seizure frequency is crucial for evaluating and adjusting ASMs. However, previous studies have reported significant discrepancies between patient or caregiver reports and the actual seizure frequency (4, 5, 27). Our study results showed that seizure monitoring using wearable devices can effectively monitor seizures and provide annotation of undetected seizure frequency.
The use of wearable devices provided direct confirmation of ictal EEG changes in patients where the presence of seizures was uncertain. Previous studies have also demonstrated that ambulatory EEG can assist in identifying interictal epileptiform discharges and differentiating seizures in real-life settings of patients (28). However, the inconvenience associated with traditional examination equipment has made it challenging to conduct tests that span several days (8). Considering these findings, we believe that wearable devices can offer patients a comfortable and user-friendly solution for accurate seizure monitoring in their everyday lives.
Using a bi-modal device showed advantages in monitoring various seizure types. We successfully identified myoclonic, tonic, and focal onset seizures with or without impaired awareness. These findings align with prior studies that highlight the superiority of bi-modal wearable devices over single-modality devices, enhancing seizure monitoring rates and effectively identifying diverse forms of seizures (13–15, 29). Although wearable devices emphasizing convenience may exhibit slightly lower sensitivity and specificity in seizure monitoring, the use of bi-modal devices may compensates by increasing the overall monitoring rate. An additional strength of the long-term wearable device was its ability to collect data over extended periods, enabling us to analyze patient-specific seizure patterns at different times throughout the day. Previous studies have reported variations in seizure patterns depending on the time periods and seizure types (8–10). Patients can experience multiple seizure types, and long-term wearable data collection allows for analysis of time-dependent seizure patterns. Some studies suggest seasonal differences in seizures (12, 15). By collecting long-term data, we can examine individual seizure patterns at a more granular level, potentially leading to personalized treatment approaches, including seizure forecasting.
4.3 Discontinuation of wearable device
Among the 14 participants, 9 patients (64.4%) maintained their regular routines and collected bi-modal data—including EEG and accelerometer measurements—for at least 100 h (median 403 h) over more than a month. Five patients discontinued device use; only one stopped due to a direct side effect, specifically skin irritation. Skin rash is a documented potential side effect in wearable ECG and EEG research (17). Skin irritation and rash were the most common side effects observed, consistent with previous wearable EEG studies. In all four patients reporting skin rashes, the irritation spontaneously resolved after electrode removal, indicating it was mild and self-limiting. Only one patient discontinued device use, while the other three remained dedicated. Considering the long-term use of wearable devices, developing electrodes that minimize skin irritation would be advantageous for patients relying on these devices over extended periods.
Studies on wearable device usage among epilepsy patients have suggested that the design and appearance of the devices are less significant for adults than previously anticipated (30). The impact of device design and appearance on usage in pediatric populations remains uncertain. In our study, four adolescents discontinued using wearable devices due to unwillingness to wear exposed, visible designs. Future development of wearable devices for seamless integration into daily activities outside the home should give greater consideration to their external appearance.
Three caregivers struggled to find time to apply the device. Since patients often have disabilities, a simpler and more convenient application process is needed. Strong encouragement from clinicians—emphasizing that using the device could improve the child’s disease management—is essential. Special considerations and improvements are necessary to ensure patients and caregivers can actively and effectively use the wearable device (17, 30–32).
In conclusion, we evaluated the applicability of a long-term, in-home, bi-modal wearable device for seizure monitoring in patients with epilepsy. Overall, the wearable device showed promise in providing sufficient seizure monitoring and valuable information for epilepsy management. The device’s ability to capture bi-modal signals, including EEG and ACM signals, enhances its utility in seizure monitoring. Further research is warranted to explore the long-term real-life application of wearable devices and gather more insights from patients and healthcare professionals regarding their experiences and challenges.
4.4 Limitations
This study has several limitations. First, seizure detection was confined to electrode sites; identifying extra-coverage events would require additional, region-specific leads. Second, poor connectivity and frequent data uploads accelerated battery drain, underscoring the need for on-device storage and analysis. Third, the system operated for up to 12 h on a single charge; extending runtime is essential for multi-day monitoring. Fourth, because not all patients underwent video-EEG monitoring for seizure classification and no simultaneous conventional EEG recordings were obtained, our ability to validate the wearable device’s detected signals is limited—despite efforts to match background activity and interictal discharges from conventional EEG recordings taken at different time points. Fifth, due to lack of various seizure types detected by our monitoring device, we were unable to stratify and analyze various seizure types and their characteristics. Finally, the absence of an ECG channel restricted multimodal assessment, highlighting the need for a skin-friendly ECG module in future designs.
It should be emphasized that this device is not a diagnostic tool and cannot perfectly classify seizure types. Its utility is greatest in patients whose seizure patterns have already been well-characterized through prior EMU admissions or prolonged video EEG monitoring, as it allows for quantification of seizure burden in the home environment. However, its sensitivity and specificity may be lower for non-convulsive seizures and patients with less seizure frequency compared to controlled inpatient environments. Therefore, while the device can provide valuable Supplementary material, it should not be used as the sole basis for diagnosis or treatment decisions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-2103-673-311). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Data curation, Writing – original draft, Investigation, Writing – review & editing, Methodology, Validation, Formal analysis. YK: Validation, Formal analysis, Data curation, Writing – review & editing, Writing – original draft, Methodology, Investigation. YC: Formal analysis, Methodology, Software, Writing – review & editing. AC: Writing – review & editing, Conceptualization. HK: Data curation, Validation, Project administration, Writing – review & editing, Investigation, Methodology, Conceptualization, Writing – original draft, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by SK Biopharmaceuticals Co., Ltd. under Grant No. 06–2021-0147.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from SK Biopharmaceuticals Co., Ltd. under Grant No. 06–2021-0147. The funder had the following involvement in the study: study design, preparation of seizure monitoring devices, and data collection via an external server.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1609838/full#supplementary-material
Footnotes
References
1.
Bruno E Viana PF Sperling MR Richardson MP . Seizure detection at home: do devices on the market match the needs of people living with epilepsy and their caregivers?Epilepsia. (2020) 61:S11–24. doi: 10.1111/epi.16521
2.
Kim H Hwang H . Resting-state electroencephalography (EEG) functional connectivity analysis. Ann Child Neurol. (2018) 26:129–34. doi: 10.26815/jkcns.2018.26.3.129
3.
Atwood AC Drees CN . Seizure detection devices: five new things. Neurol Clin Pract. (2021) 11:367–71. doi: 10.1212/CPJ.0000000000001044
4.
Elger CE Hoppe C . Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet Neurol. (2018) 17:279–88. doi: 10.1016/S1474-4422(18)30038-3
5.
Fisher RS . Bad information in epilepsy care. Epilepsy Behav. (2017) 67:133–4. doi: 10.1016/j.yebeh.2016.10.022
6.
Blachut B Hoppe C Surges R Stahl J Elger CE Helmstaedter C . Counting seizures: the primary outcome measure in epileptology from the patients' perspective. Seizure. (2015) 29:97–103. doi: 10.1016/j.seizure.2015.03.004
7.
Ticci C Luongo T Valvo G Ferrari AR Brovedani P Masi G et al . Clinical and electroencephalographic correlates of psychiatric features in children with frontal lobe epilepsy. Epilepsy Behav. (2019) 92:283–9. doi: 10.1016/j.yebeh.2019.01.008
8.
Baumgartner C Koren JP . Seizure detection using scalp-EEG. Epilepsia. (2018) 59:14–22. doi: 10.1111/epi.14052
9.
Lawley A Evans S Manfredonia F Cavanna AE . The role of outpatient ambulatory electroencephalography in the diagnosis and management of adults with epilepsy or nonepileptic attack disorder: a systematic literature review. Epilepsy Behav. (2015) 53:26–30. doi: 10.1016/j.yebeh.2015.09.032
10.
Weisdorf S Duun-Henriksen J Kjeldsen MJ Poulsen FR Gangstad SW Kjaer TW . Ultra-long-term subcutaneous home monitoring of epilepsy-490 days of EEG from nine patients. Epilepsia. (2019) 60:2204–14. doi: 10.1111/epi.16360
11.
Noh BH . Non-EEG seizure detection using wearable device. Ann Child Neurol. (2017) 25:209–14. doi: 10.26815/jkcns.2017.25.4.209
12.
Ulate-Campos A Coughlin F Gainza-Lein M Fernandez IS Pearl PL Loddenkemper T . Automated seizure detection systems and their effectiveness for each type of seizure. Seizure. (2016) 40:88–101. doi: 10.1016/j.seizure.2016.06.008
13.
Bottcher S Bruno E Epitashvili N Dumpelmann M Zabler N Glasstetter M et al . Intra- and inter-subject perspectives on the detection of focal onset motor seizures in epilepsy patients. Sensors. (2022) 22:318. doi: 10.3390/s22093318
14.
Munch Nielsen J Zibrandtsen IC Masulli P Lykke Sorensen T Andersen TS Wesenberg Kjaer T . Towards a wearable multi-modal seizure detection system in epilepsy: a pilot study. Clin Neurophysiol. (2022) 136:40–8. doi: 10.1016/j.clinph.2022.01.005
15.
Onorati F Regalia G Caborni C Migliorini M Bender D Poh MZ et al . Multicenter clinical assessment of improved wearable multimodal convulsive seizure detectors. Epilepsia. (2017) 58:1870–9. doi: 10.1111/epi.13899
16.
Bruno E Biondi A Bottcher S Lees S Schulze-Bonhage A Richardson MP et al . Day and night comfort and stability on the body of four wearable devices for seizure detection: a direct user-experience. Epilepsy Behav. (2020) 112:107478. doi: 10.1016/j.yebeh.2020.107478
17.
Hadady L Klivenyi P Fabo D Beniczky S . Real-world user experience with seizure detection wearable devices in the home environment. Epilepsia. (2022) 4:S72–7. doi: 10.1111/epi.17189
18.
Simblett SK Biondi A Bruno E Ballard D Stoneman A Lees S et al . Patients' experience of wearing multimodal sensor devices intended to detect epileptic seizures: a qualitative analysis. Epilepsy Behav. (2020) 102:106717. doi: 10.1016/j.yebeh.2019.106717
19.
Johansson D Ohlsson F Krysl D Rydenhag B Czarnecki M Gustafsson N et al . Tonic-clonic seizure detection using accelerometry-based wearable sensors: a prospective, video-EEG controlled study. Seizure. (2019) 65:48–54. doi: 10.1016/j.seizure.2018.12.024
20.
Arends J Thijs RD Gutter T Ungureanu C Cluitmans P Van Dijk J et al . Multimodal nocturnal seizure detection in a residential care setting: a long-term prospective trial. Neurology. (2018) 91:e2010–9. doi: 10.1212/WNL.0000000000006545
21.
Meritam P Ryvlin P Beniczky S . User-based evaluation of applicability and usability of a wearable accelerometer device for detecting bilateral tonic-clonic seizures: a field study. Epilepsia. (2018) 59:48–52. doi: 10.1111/epi.14051
22.
Nasseri M Pal Attia T Joseph B Gregg NM Nurse ES Viana PF et al . Ambulatory seizure forecasting with a wrist-worn device using long-short term memory deep learning. Sci Rep. (2021) 11:21935. doi: 10.1038/s41598-021-01449-2
23.
Frankel MA Lehmkuhle MJ Spitz MC Newman BJ Richards SV Arain AM . Wearable reduced-channel EEG system for remote seizure monitoring. Front Neurol. (2021a) 12:728484. doi: 10.3389/fneur.2021.728484
24.
Frankel MA Lehmkuhle MJ Watson M Fetrow K Frey L Drees C et al . Electrographic seizure monitoring with a novel, wireless, single-channel EEG sensor. Clin Neurophysiol Pract. (2021b) 6:172–8. doi: 10.1016/j.cnp.2021.04.003
25.
Swinnen L Chatzichristos C Jansen K Lagae L Depondt C Seynaeve L et al . Accurate detection of typical absence seizures in adults and children using a two-channel electroencephalographic wearable behind the ears. Epilepsia. (2021) 62:2741–52. doi: 10.1111/epi.17061
26.
Vandecasteele K De Cooman T Dan J Cleeren E Van Huffel S Hunyadi B et al . Visual seizure annotation and automated seizure detection using behind-the-ear electroencephalographic channels. Epilepsia. (2020) 61:766–75. doi: 10.1111/epi.16470
27.
Blachut B Hoppe C Surges R Elger C Helmstaedter C . Subjective seizure counts by epilepsy clinical drug trial participants are not reliable. Epilepsy Behav. (2017) 67:122–7. doi: 10.1016/j.yebeh.2016.10.036
28.
Mikhaeil-Demo Y Gonzalez Otarula KA Bachman EM Schuele SU . Indications and yield of ambulatory EEG recordings. Epileptic Disord. (2021) 23:94–103. doi: 10.1684/epd.2021.1249
29.
Vandecasteele K De Cooman T Chatzichristos C Cleeren E Swinnen L Macea Ortiz J et al . The power of ECG in multimodal patient-specific seizure monitoring: added value to an EEG-based detector using limited channels. Epilepsia. (2021) 62:2333–43. doi: 10.1111/epi.16990
30.
Soroudi A Hernandez N Berglin L Nierstrasz V . Electrode placement in electrocardiography smart garments: a review. J Electrocardiol. (2019) 57:27–30. doi: 10.1016/j.jelectrocard.2019.08.015
31.
Cho J Joo YS Yoon JG Lee SB Kim SY Chae JH et al . Characterizing families of pediatric patients with rare diseases and their diagnostic odysseys: a comprehensive survey analysis from a single tertiary center in Korea. Ann Child Neurol. (2024) 32:167–75. doi: 10.26815/acn.2024.00472
32.
Patel AD Moss R Rust SW Patterson J Strouse R Gedela S et al . Patient-centered design criteria for wearable seizure detection devices. Epilepsy Behav. (2016) 64:116–21. doi: 10.1016/j.yebeh.2016.09.012
Summary
Keywords
epilepsy, seizure monitoring, wearable device, bi-modal signals, clinical utility
Citation
Cho J, Ko YJ, Chung YG, Cho A and Kim H (2025) Improving epilepsy monitoring using long-term, in-home-bi-modal seizure monitoring device: clinical utilities and obstacles from a pilot study. Front. Neurol. 16:1609838. doi: 10.3389/fneur.2025.1609838
Received
11 April 2025
Accepted
27 June 2025
Published
10 July 2025
Volume
16 - 2025
Edited by
Stephan Schuele, Northwestern University, United States
Reviewed by
Mirja Steinbrenner, Charité University Medicine Berlin, Germany
Anna Serafini, University of Illinois Chicago, United States
Updates
Copyright
© 2025 Cho, Ko, Chung, Cho and Kim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hunmin Kim, hunminkim@snubh.org
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.