- 1Department of Rehabilitation, Jilin University China-Japan Union Hospital, Changchun, China
- 2Department of Rehabilitation, Zhejiang Provincial People's Hospital, Hangzhou, China
Background: Parkinson’s disease (PD), the second most prevalent neurodegenerative disorder, leads to lower extremity dysfunction that critically contributes to falls and disability, yet effective rehabilitation remains limited.
Objective: Systematic assessment of the effects of treadmill training on lower limb motor performance in patients with PD.
Methods: As of March 1, 2024, a systematic search was conducted in PubMed, Web of Science, Embase, and the Cochrane Library to gather randomized controlled trials (RCTs) that report the effects of treadmill training on patients with PD. Data on the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III), the Timed Up and Go test (TUG), the Berg Balance Scale (BBS),6-Minute Walk Test (6MWT),10 Meter Walk Test (10MWT), and the Parkinson’s Disease Questionnaire-39 (PDQ-39) outcome metrics, as well as general characteristics of the studies, participant demographics, and details regarding the intervention and control groups, were extracted. The Cochrane Risk of Bias tool was employed to evaluate the quality of articles at risk, while the funnel plot and Egger’s test were utilized to assess publication bias.
Results: 16 RCTs comprising 582 participants were included. The meta-analysis indicated that treadmill training (TT) produced significantly better outcomes than conventional therapy (CT) in the post-intervention assessments of motor symptoms (UPDRS-III: SMD: -0.45; 95% CI: −0.73 to −0.17), and gait performance (6MWT: SMD 0.53; 95% CI: 0.08 to 0.97; 10MWT: 0.93; 95% CI: 0.54 to 1.32). Body Weight Supporting Treadmill (BBS) for Better Healing However, quality of life (PDQ-39: SMD: -0.35; 95% CI: −0.95 to 0.25), balance (BBS: SMD SMD: -0.35; 95% CI: −0.95 to 0.25; TUG: SMD: -0.35; 95% CI: −0.95 to 0.25), and treatment effects were comparable.
Conclusion: TT (especially weight-supported) vs. conventional training demonstrates superior efficacy in enhancing lower limb mobility for Parkinson’s disease, improving muscular endurance and short-term gait speed, but requires enhanced dynamic balance integration.
Systematic trial registration: https://www.crd.york.ac.uk/prospero/, identifier, CRD42021256958.
1 Introduction
PD is the second most prevalent neurodegenerative disorder globally, affecting approximately 3.6% of individuals over the age of 60 (1). The hallmark motor symptoms include resting tremor, rigidity, bradykinesia, and gait abnormalities, which progressively deteriorate as the disease advances, resulting in a substantial loss of functional independence (2).
Lower limb motor decline is a primary concern impacting patients’ quality of life (3). This condition is characterized by reduced muscle strength, restricted joint mobility, loss of gait symmetry, and impaired balance (4). These dysfunctions not only elevate the risk of falls—which can result in fractures, hospitalization, and even death, even in the early stages of the disease—but also further restrict physical activity, increase dependence, and contribute to psychological issues such as anxiety and depression (5).
Lower limb dysfunction is particularly pronounced in elderly patients with PD. This population frequently presents with comorbidities such as osteoporosis and arthritis, which can exacerbate the decline in lower limb motor function and compromise the safety of rehabilitation interventions (6). Additionally, the combined effects of age-related neuromuscular deterioration and the pathophysiology of PD further impair lower limb motor control (7). Consequently, developing targeted intervention strategies for elderly patients with PD is essential for enhancing their functional independence and overall quality of life.
The clinical management of PD is primarily dominated by pharmacological and neurosurgical treatments; however, physical therapy plays a crucial role in enhancing motor function (8, 9). In recent years, treadmill training has garnered attention for its dual mechanism: it not only improves lower limb muscle strength but also optimizes parameters such as stride length and step frequency through weight-bearing rhythmic exercise (10).
Additionally, it may promote neural plasticity in the motor cortex, thereby enhancing coordination (11). Although studies have shown improvements in gait and static balance, there are notable limitations in the existing evidence: (1) a lack of sufficient studies involving elderly patients with PD, which overlooks the impact of comorbidities on outcomes; (2) a fragmented assessment of lower limb capacity, which lacks an integrated analysis of muscle strength, gait, and functional activities.
Therefore, this study comprehensively assessed the effects of conventional/weight-loss treadmill training on lower limb motor abilities (e.g., lower limb muscle strength, endurance, balance and functional mobility) in elderly PD patients through systematic review and meta-analysis to provide an evidence-based basis for optimizing their rehabilitation strategies.
2 Methods
2.1 Protocol and registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12) guidelines. The protocol has been registered with the International Prospective Register for Systematic Reviews (PROSPERO; registration number CRD42021256958).
2.2 Literature search and study selection
A systematic search was conducted on March 1, 2025, across the following electronic databases: PubMed, Web of Science, Embase, and the Cochrane Library. The search strategy utilized the following keywords: (“Parkinson’s disease” OR “idiopathic Parkinson’s disease” OR “Lewy body Parkinson’s disease” OR “paralysis agitans” OR “primary parkinsonism”) AND (“treadmill training”). Only full-text articles published in English were included in the search.
2.3 Eligibility criteria
Two independent reviewers, Yin and Hu, screened the titles and abstracts of all retrieved records in a blinded manner. Any discrepancies were resolved by consulting a third reviewer, Li. Studies that met the screening criteria were subsequently evaluated in full text. All studies were included based on the following criteria:
Inclusion criteria: (1) Study design: randomized controlled trials (RCTs); (2) Population: Patients with Parkinson’s disease (Hoehn-Yahr stages I-III) aged ≥55 years; (3) Population: Disease duration ≥1 year, MMSE ≥24, and medically stable; (4) Intervention: conventional or body-weight-supported treadmill; (5) Comparison: Usual care, gait training, or standard comprehensive training; (6) Outcome: Includes UPDRS-III, BBS and TUG test, gait performance as assessed by 6MWT, 10MWT and quality of life as evaluated by PDQ-39.
Exclusion criteria: (1) Intervention: Combined with additional interventions; (2) Intervention: Short intervention duration (single or few sessions); (3) Comparison: Baseline data imbalance (Supplementary Table 1).
2.4 Data extraction
Data extraction was conducted independently by two reviewers, Yin and Hu. Any disagreements were resolved through discussion with a third reviewer, Li.
The extracted data included the following: (1) the general characteristics of each study (Authorship, Study design, Country, and Date of publication); (2) Characteristics of Participants (Age, Groups and Sample Size, Number of Men and Women, Parkinson’s Stage); (3) Characteristics of the intervention and control groups (treatment regimen, type of treadmill equipment): (4) Post-intervention quantitative data for the UPDRS-III, 6MWT, 10MWT, TUG, BBS, and PDQ-39, including mean and standard deviation.
Only data collected immediately after the intervention were included; no follow-up data were considered.
2.5 Risk of bias
Two reviewers (Yin and Hu) independently assessed potential bias using an improved Cochrane Risk of Bias Tool across five domains: randomization, intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was categorized into one of three levels: high risk of bias, cause for concern, or low risk of bias. The reviewers consulted a third author, Li, to resolve any discrepancies.
2.6 Level of evidence
2.6.1 Grading of recommendations, assessment, development, and evaluation (GRADE) rating of evidence quality
The GRADE system categorizes the quality of evidence based on five factors: bias. Risk, inconsistency, imprecision, indirectness, and publication bias are significant factors that can affect the quality of evidence. Quality was classified as high, moderate, low, or very low. The recommendations were divided into strong and weak levels.
2.6.2 Oxford centre for evidence-based medicine: levels of evidence
Evidence levels were determined based on the latest guidelines from the Oxford Centre for Evidence-Based Medicine’s Evidence-Based Medicine Charts.
2.7 Statistical analysis
Statistical analyses were conducted using STATA version 15.1 (STATA Corp., College Station, TX, USA). The standardized mean difference (SMD) was employed to combine data, and Hedge’s g and 95% confidence intervals (CIs) served as effect size measures for continuous data. Heterogeneity among studies was evaluated using the χ2 test. An I2 ≤ 50% and p > 0.1 indicated no significant heterogeneity, while an I2 > 50% and p < 0.1 indicated significant heterogeneity. Potential sources of heterogeneity were investigated through subgroup analysis, and publication bias was assessed using funnel plots and Egger’s test.
3 Results
3.1 Search results
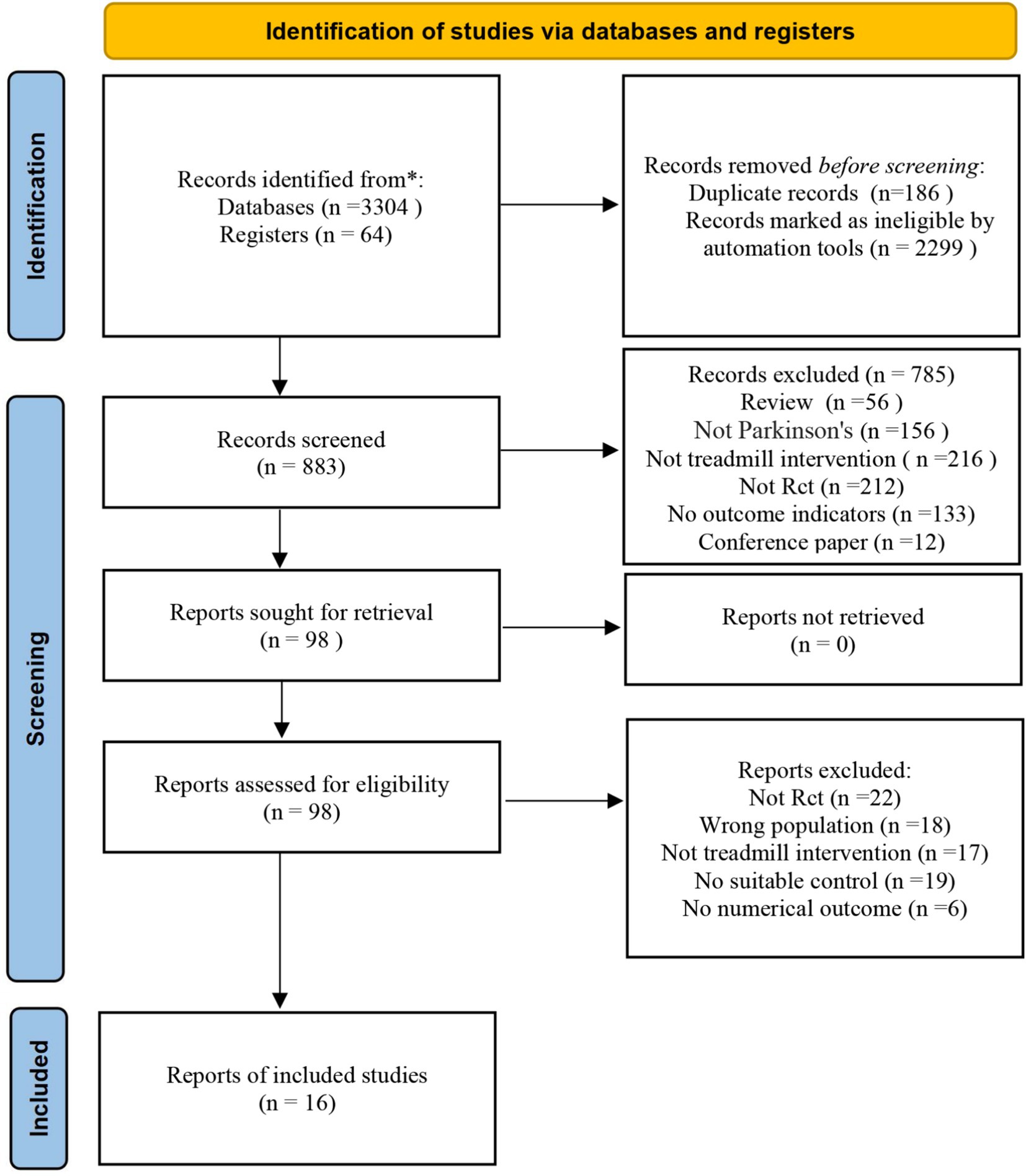
The initial search yielded 3,368 potential articles. Of these, 186 duplicates were excluded from the analysis. Additionally, 2,299 records were marked as ineligible by automation tools. After screening the titles and abstracts,98 studies were identified for further evaluation. Of these, 82 articles did not meet the inclusion criteria and were excluded from the study, leaving 16 studies for the meta-analysis (13–26) (Figure 1).
3.2 Study characteristics
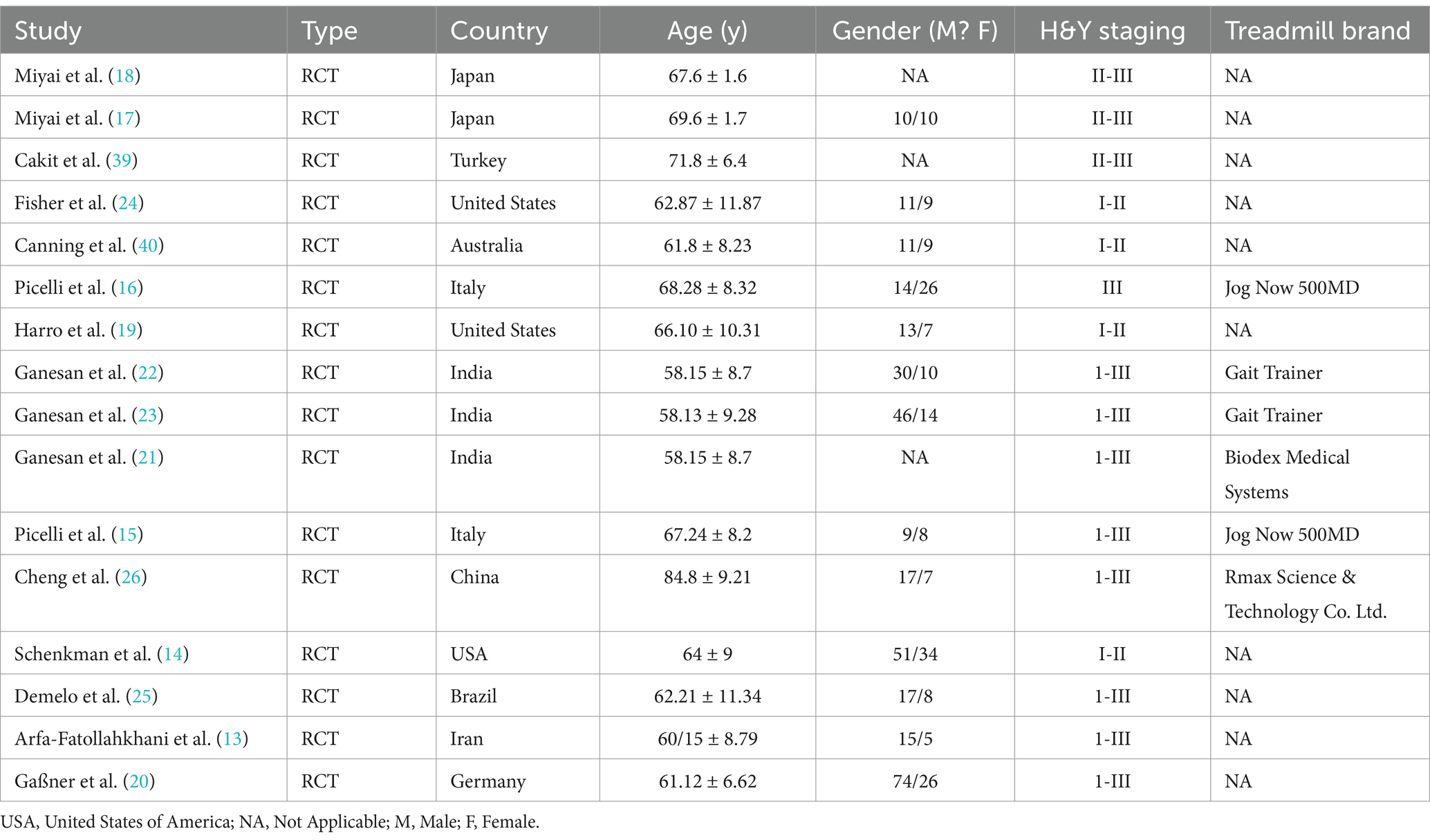
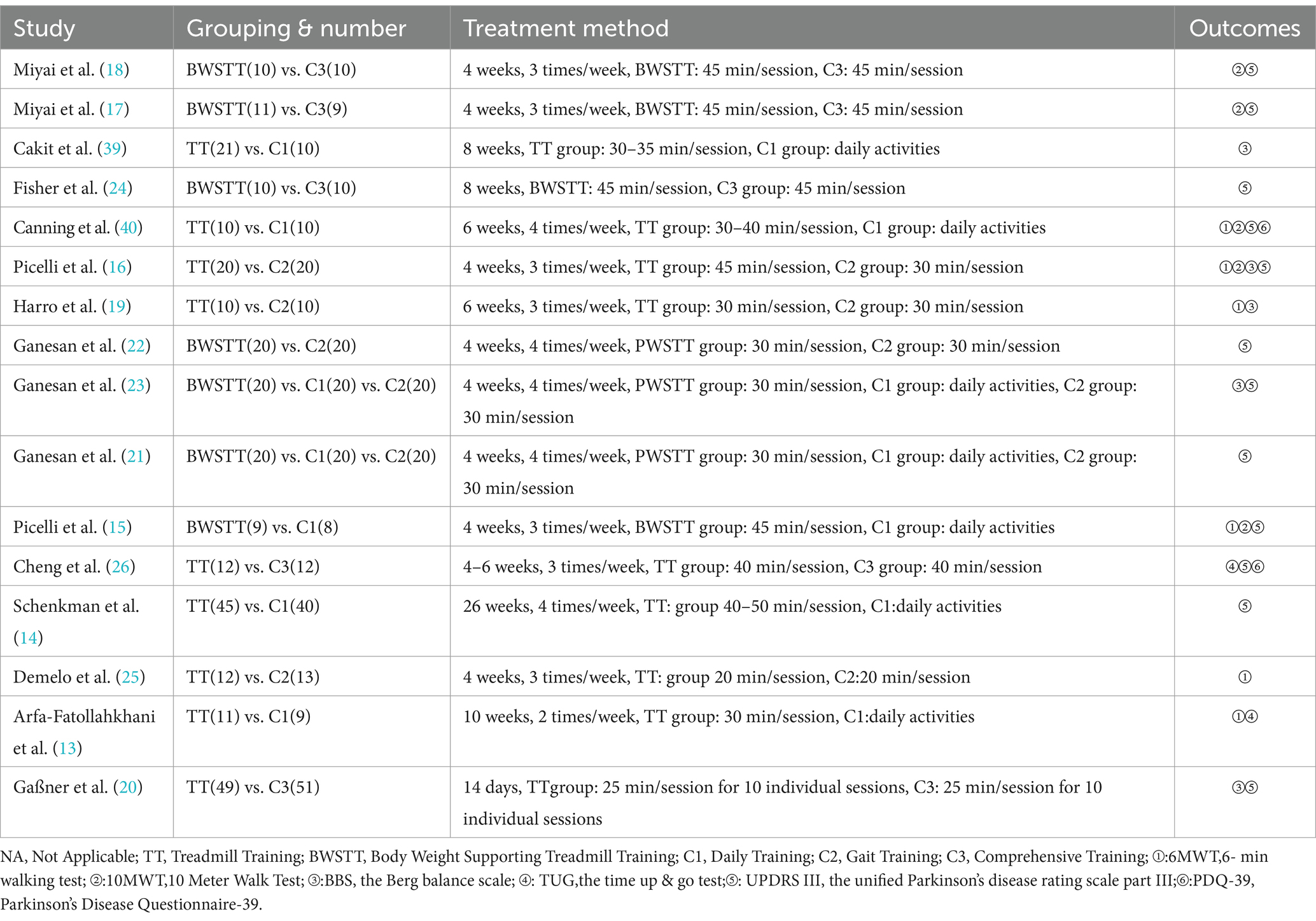
A total of 16 RCTs (2000–2022) involving 582 participants were included. The treadmill group comprised 274 participants, while the control group included 308 participants. The study populations were from Japan, Turkey, the United States, Australia, Italy, India, China, Brazil, Iran, and Germany. The majority of participants were elderly aged 60 and above, with Hoehn-Yahr stages ranging from I to III. The treadmill group underwent either treadmill training (TT) or body-weight-supported treadmill training (BWSTT), while the control group primarily engaged in routine activities, gait training, or comprehensive training. The intervention duration ranged from 2 to 10 weeks, with a frequency of 2–4 sessions per week, each lasting 25–45 min (As shown in Tables 1, 2).
3.3 The risk-of-bias assessment
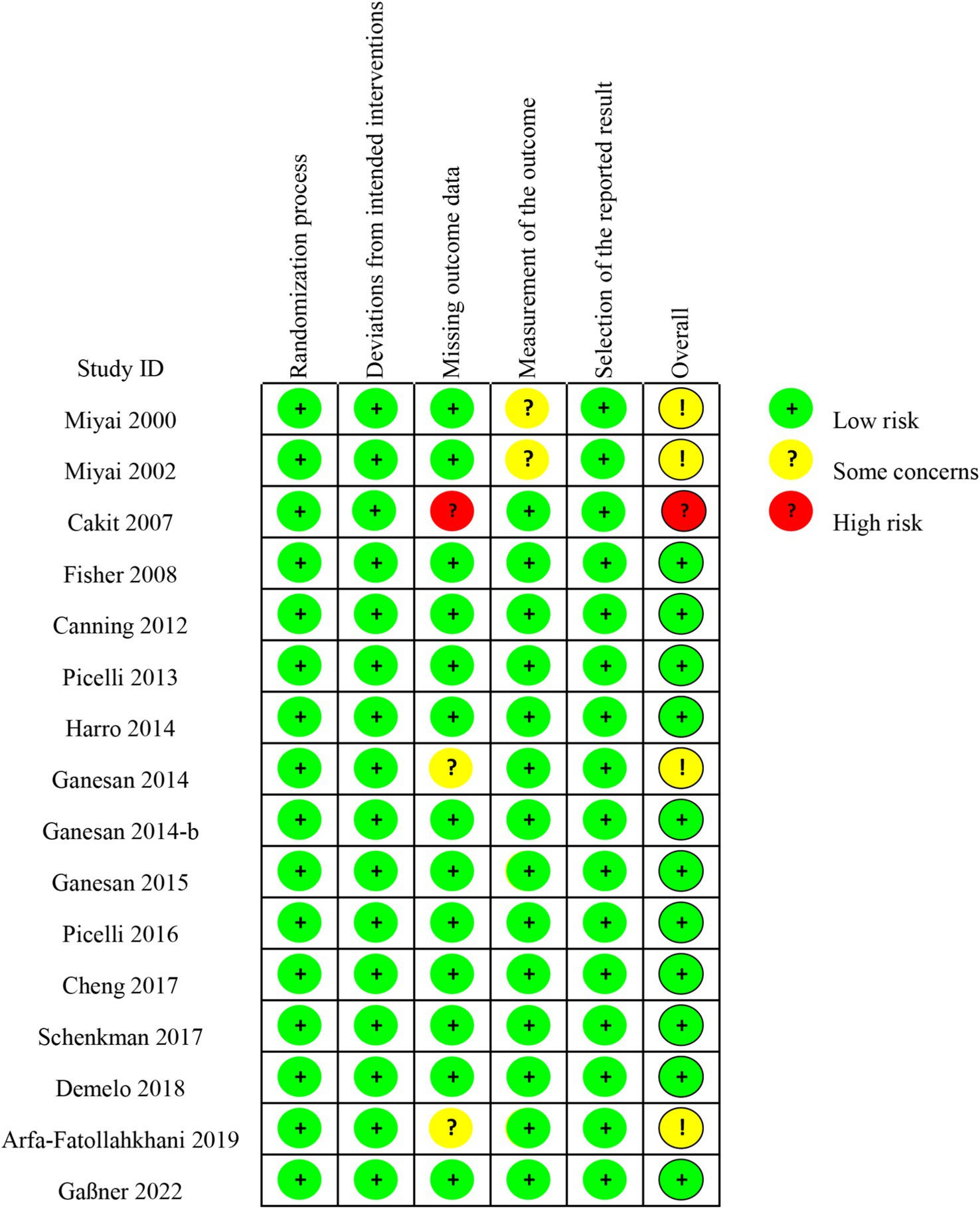
The risk of bias assessment for the included studies indicated that out of the 16 RCT studies11 were considered to be at low risk of bias, 4 had somed some concerns and 1 had a high risk of bias (Figure 2, Supplementary Figure 2).
3.4 Outcomes
3.4.1 UPDRS III score
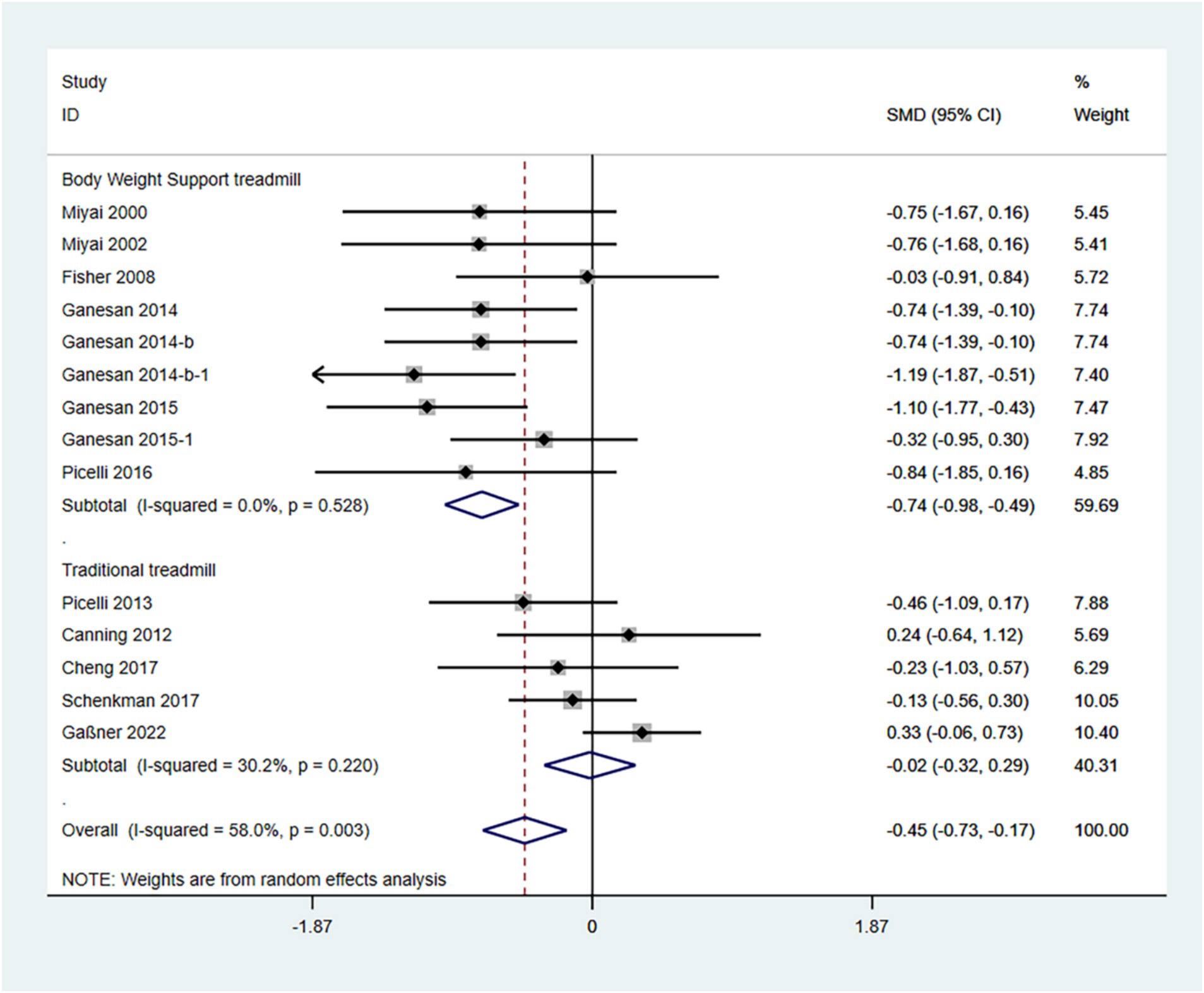
The UPDRS III score is a standardized tool used to evaluate motor function in patients with Parkinson’s disease, where lower scores (SMD) indicate less severe symptoms. Therefore, an SMD (95% CI) < 0 suggests an improvement in symptoms.
In the random effects model, treadmill training significantly reduced the degree of Parkinson’s symptoms compared to conventional training (SMD: -0.45; 95% CI: −0.73 to −0.17), but with greater heterogeneity (I2 = 80.2%, p = 0.003).
The analysis revealed that the type of treadmill intervention resulted in a high degree of heterogeneity. Subgroup analyses showed that in a random-effects model, BWSTT significantly reduced Parkinson’s symptoms compared with conventional training (SMD: -0.74; 95% CI: −0.98 to −0.49), with low heterogeneity (I2 = 0.0%, p = 0.528); treadmill training did not show a significant difference in the degree of Parkinsonian symptoms when compared to conventional training (SMD: -0.02; 95% CI: −0.32 to 0.29), and the heterogeneity was moderate (I2 = 30.0%, p = 0.220) (Figure 3).
The funnel plot and Egger’s test (p > 0.05) indicate no publication bias (Supplementary Figures 1–3).
3.4.2 6-MWT
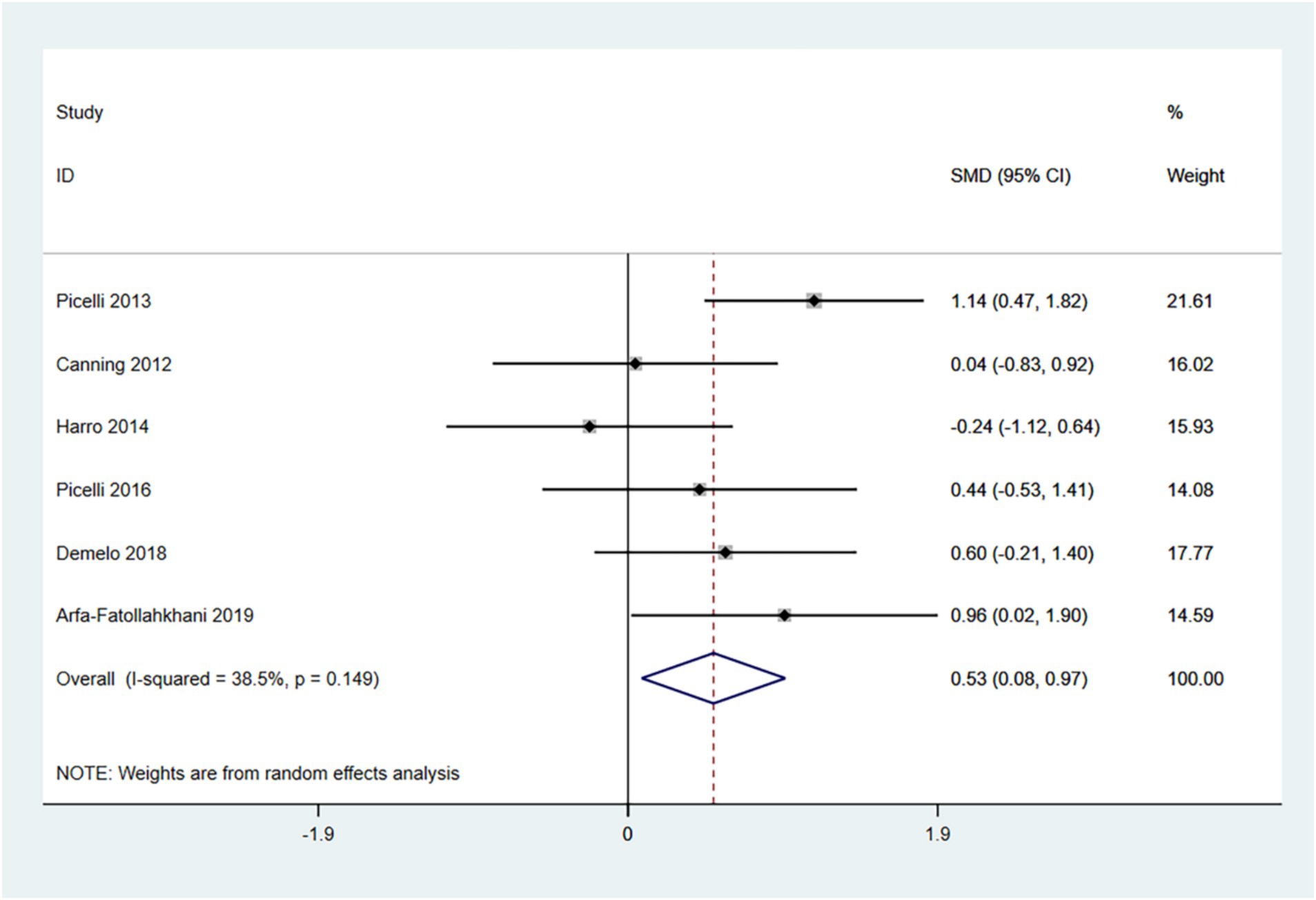
The 6-MWT is primarily used to measure the maximum distance a patient can walk in 6 min and is a widely utilized tool for assessing functional mobility. Higher scores indicate better exercise tolerance. Therefore, an SMD (95% CI) > 0 suggests an improvement in symptoms.
In the random effects model, treadmill training significantly increased walking distance compared to conventional training (SMD: 0.53; 95% CI: 0.08 to 0.97), with moderate heterogeneity (I2 = 38.5%, p = 0.149) (Figure 4).
The funnel plot and Egger’s test (p > 0.05) indicate no publication bias (Supplementary Figures 4–6).
3.4.3 10-MWT
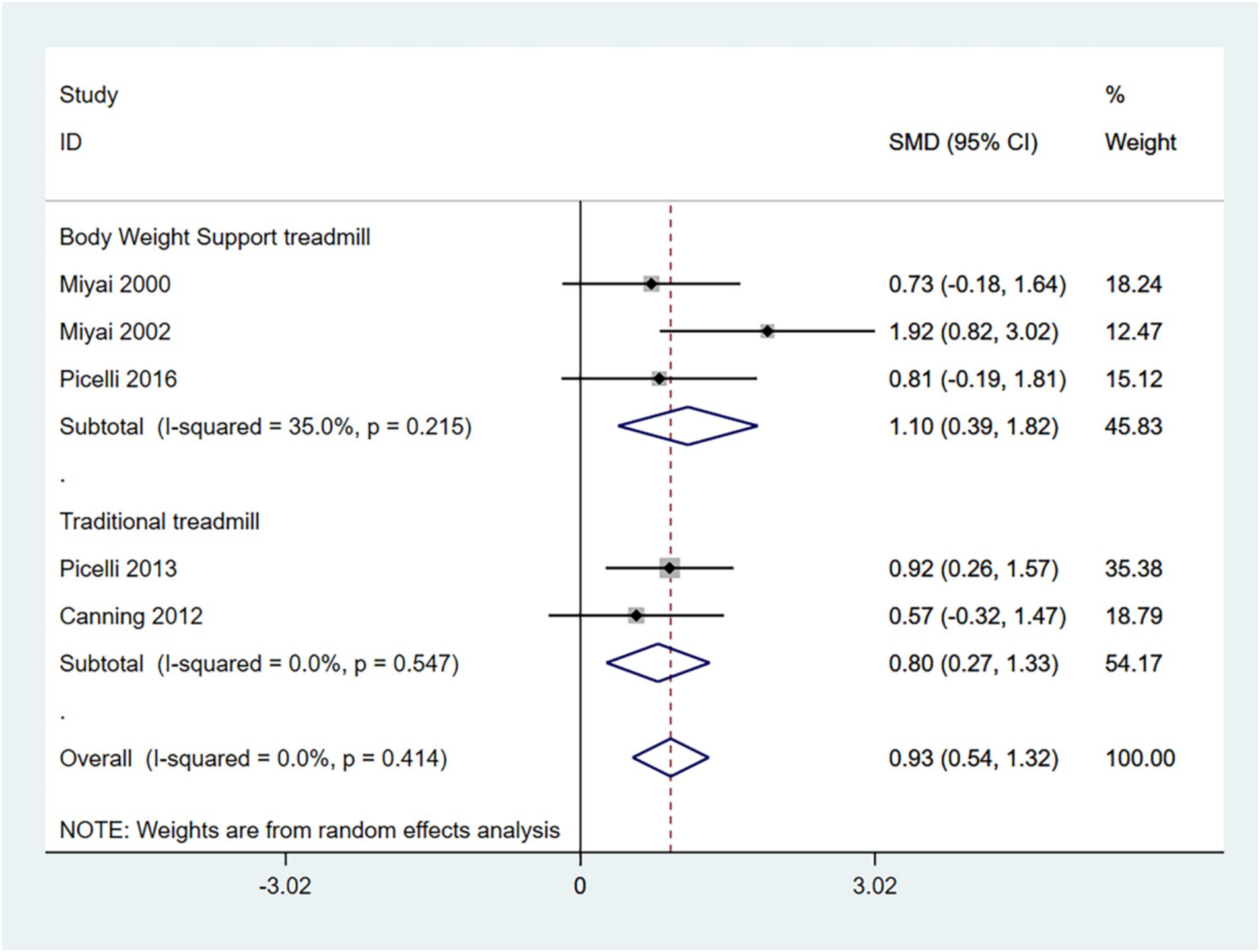
The 10-MWT is primarily used to assess short-distance walking speed, balance, and functional mobility. Higher scores indicate better walking ability. Therefore, an SMD (95% CI) > 0 suggests an improvement in symptoms.
In the random effects model, treadmill training significantly increased walking speed compared to conventional training (SMD: 0.93; 95% CI: 0.54 to 1.32), with low heterogeneity (I2 = 0.0%, p = 0.421).
In addition, the grouping of interventions revealed that both conventional treadmills and weight-loss treadmills significantly increased walking speed in the 10MWT compared to conventional training. In the random effects model, BWSTT significantly increased walking speed compared to conventional training (SMD: 1.10; 95% CI: 0.39 to 1.82), with moderate heterogeneity (I2 = 35.0%, p = 0.215); traditional treadmill training significantly increased walking speed compared to conventional training (SMD: 0.80 95% CI: 0.27 to 1.33), with low heterogeneity (I2 = 0.0%, p = 0.547) (Figure 5). BWSTT is somewhat more effective.
The funnel plot and Egger’s test (p > 0.05) indicate no publication bias (Supplementary Figures 7–9).
3.4.4 BBS
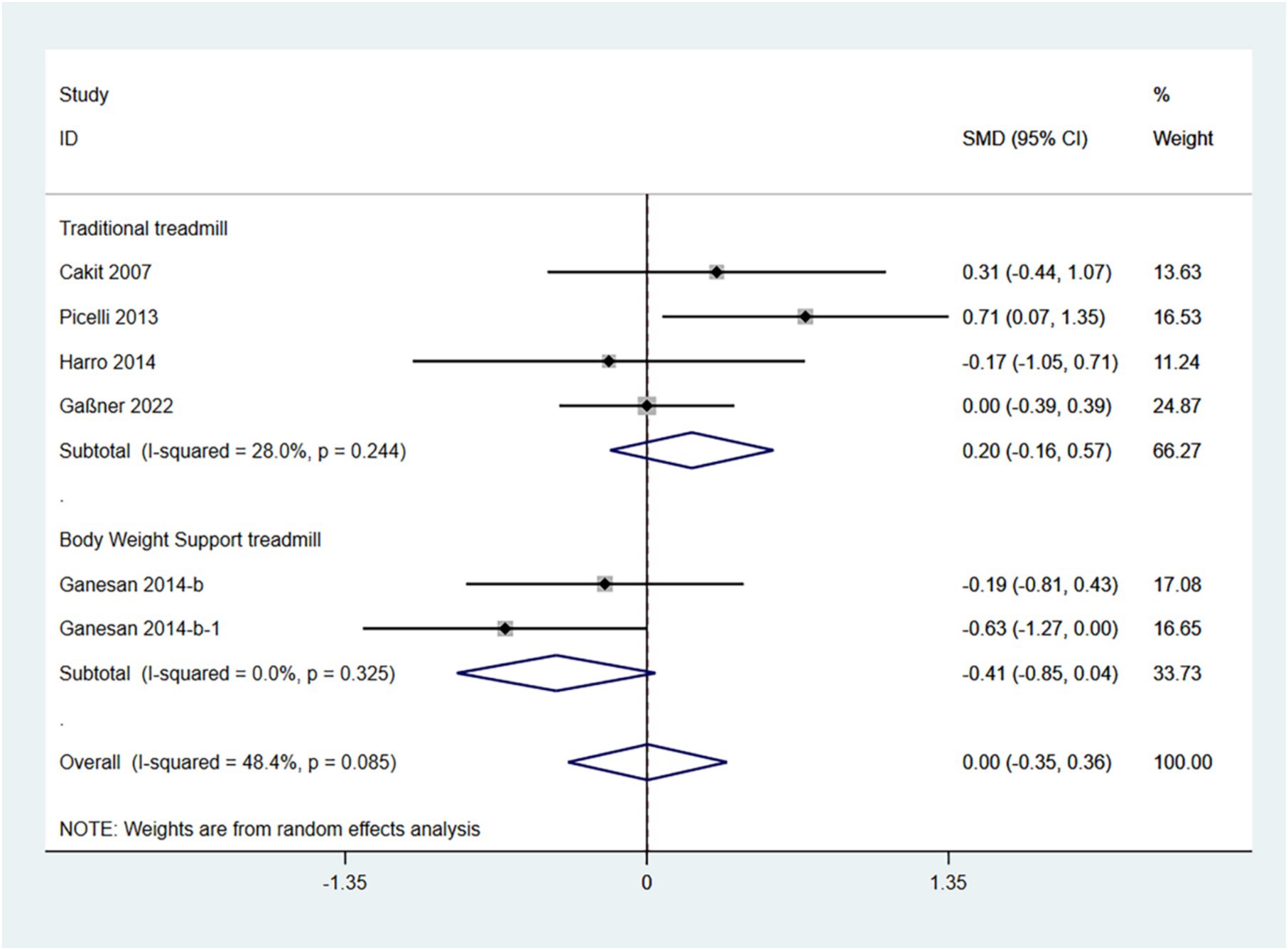
The BBS is a comprehensive balance assessment tool that evaluates a patient’s static, dynamic, and functional balance. Higher scores indicate better balance. Therefore, an SMD (95% CI) > 0 suggests an improvement in symptoms.
In the random effects model, treadmill training did not show a significant difference in BBS compared to conventional training (SMD: 0.00; 95% CI: −0.35 to 1.36), with moderate heterogeneity (I2 = 48.4%, p = 0.085).
The analysis revealed that the type of treadmill intervention resulted in a high degree of heterogeneity. Subgroup analyses showed that in a random-effects model, traditional treadmill training did not show a significant difference in BBS compared to conventional training (SMD: 0.20; 95% CI: −0.16 to 0.57), with moderate heterogeneity (I2 = 28.0%, p = 0.244); BWSTT did not show a significant difference in BBS compared to conventional training (SMD: -0.41; 95% CI: −0.85 to −0.04), with low heterogeneity (I2 = 0.0%, p = 0.325) (Figure 6). Traditional treadmill training is somewhat more effective.
The funnel plot and Egger’s test (p > 0.05) indicate no publication bias (Supplementary Figures 10–12).
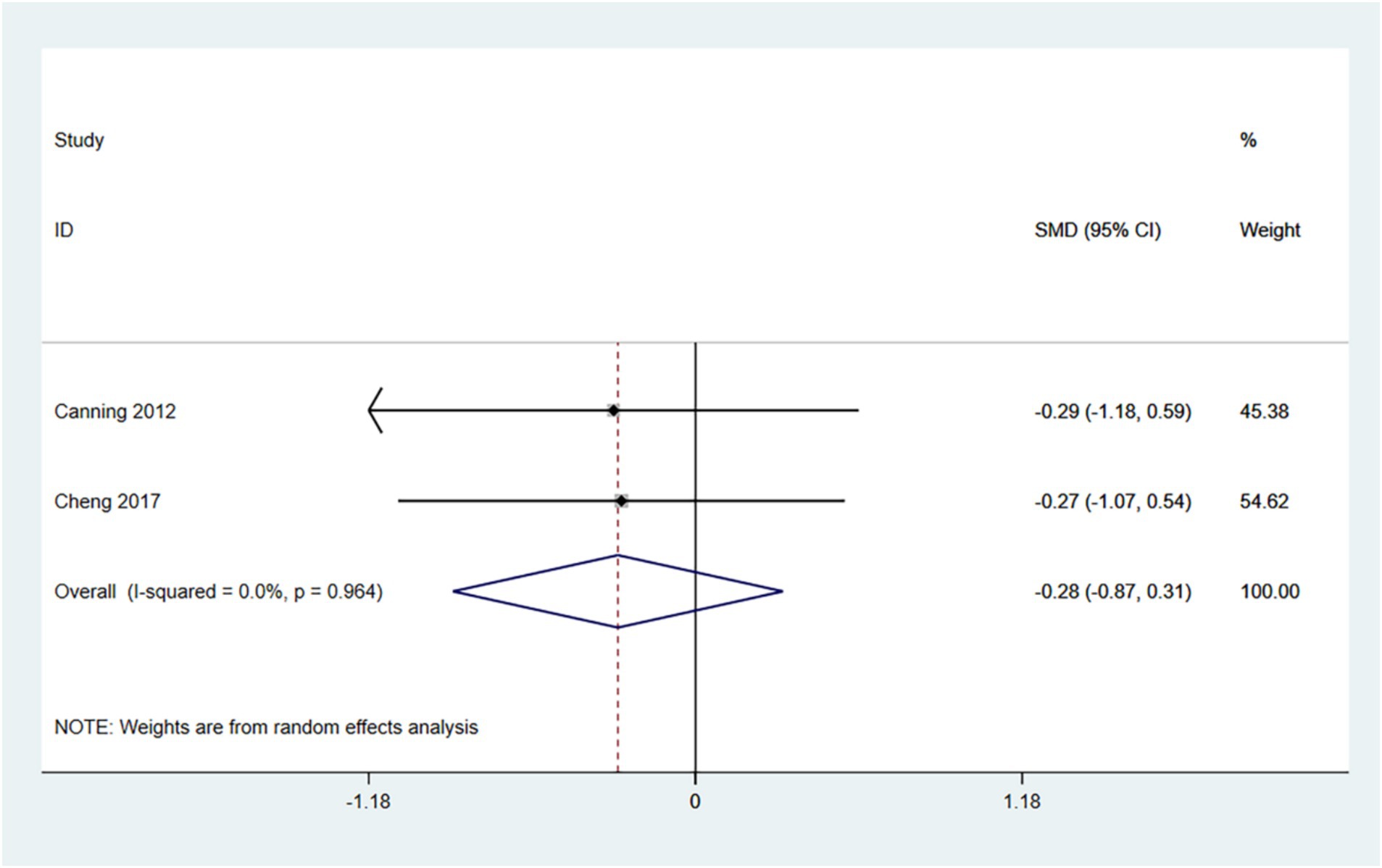
3.4.5 TUG
The TUG comprehensively evaluates an individual’s mobility, balance, and risk of falling. Lower scores (i.e., shorter times to complete the test) indicate that the patient is more mobile, has better balance, and is at a reduced risk of falls. Therefore, an SMD (95% CI) < 0 suggests an improvement in symptoms.
In the random effects model, treadmill training did not show a significant difference in TUG times compared to conventional training (SMD: -0.35; 95% CI: −0.95 to 0.25), with low heterogeneity (I2 = 0.0%, p = 0.678). Treadmill training has a tendency to reduce TUG times.
The number of articles is insufficient for conducting a funnel plot and Egger’s test (see Figure 7).
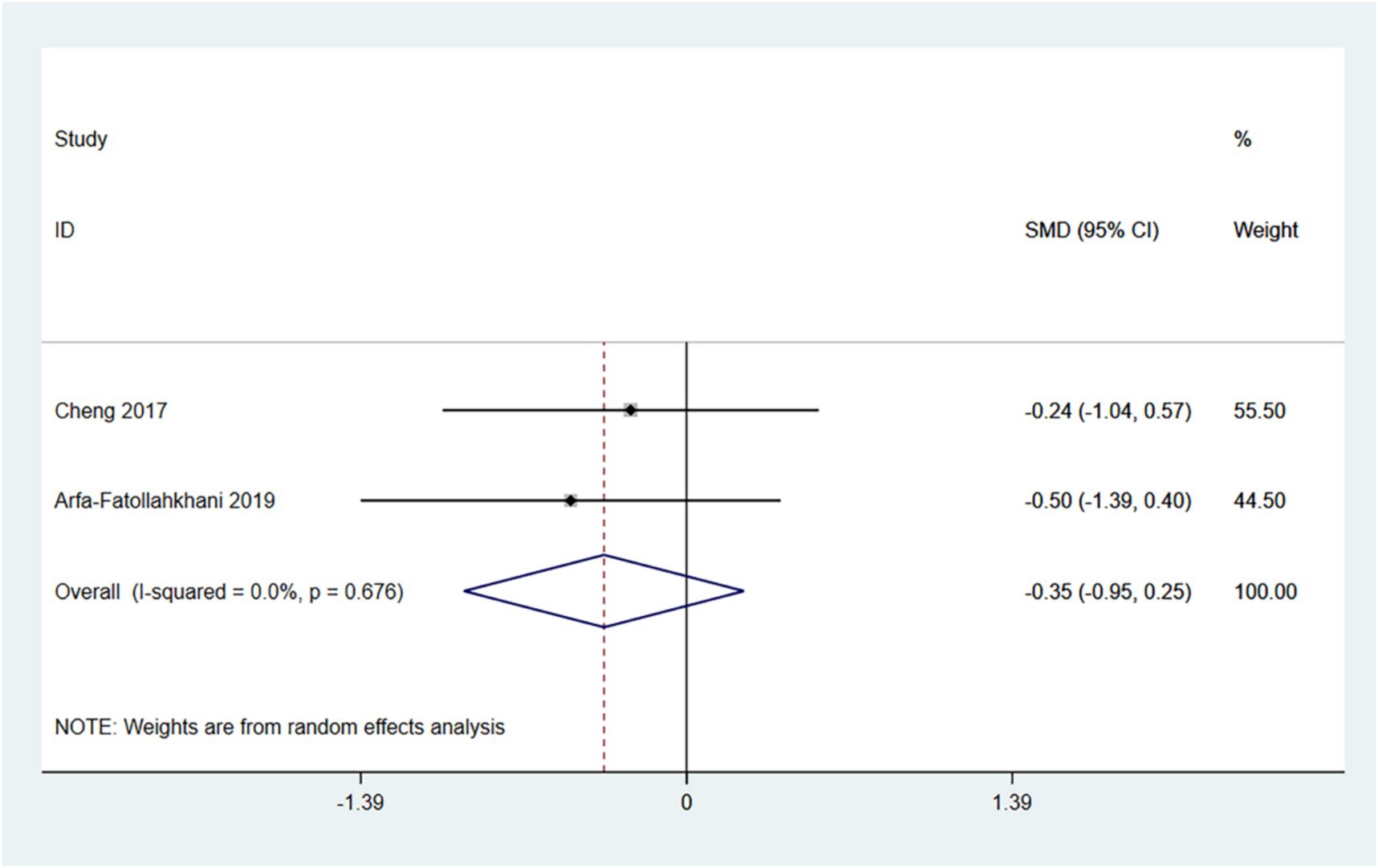
3.4.6 PDQ-39
The PDQ-39 provides a comprehensive assessment of the impact of Parkinson’s disease on patients’ daily lives, emotional well-being, and social support. A lower score indicates a better quality of life for the patient. Therefore, an SMD (95% CI) < 0 suggests an improvement in symptoms.
In the random effects model, treadmill training did not show a significant difference in PDQ-39 score compared to conventional training (SMD: -0.35; 95% CI: −0.95 to 0.25), with low heterogeneity (I2 = 0.0%, p = 0.678). Treadmill training has a tendency to reduce PDQ-39 score.
The number of articles is insufficient for conducting a funnel plot and Egger’s test (see Figure 8).
3.5 Level of evidence
3.5.1 GRADE system recommendation evaluation
Based on the quality assessment and meta-analysis, the GRADE system was employed to evaluate the findings. The results indicated that approximately 66.7% of the evidence was of high quality, while the remaining 33.3% was of moderate quality (Supplementary Table 3).
3.5.2 Oxford Centre for Evidence-Based Medicine: evaluation of the level of evidence
The prevalence of the problem was at level 2, whereas the evaluation of diagnosis, prognosis, treatment benefits, treatment harms, and screening were all assessed at level 1 (Supplementary Table 4).
4 Discussion
4.1 Discussion of the Main findings
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder characterized by motor dysfunction, impaired balance, and gait abnormalities, which significantly impact patients’ quality of life and independence (27). In recent years, treadmill training has emerged as an effective rehabilitation intervention, demonstrating potential benefits in enhancing motor function and functional capacity in individuals with Parkinson’s disease (28). However, there are still several methodological limitations (e.g., small sample sizes, inconsistent intervention protocols, etc.) and pressing research priorities (e.g., long-term outcome assessment and the optimization of individualized training protocols) in the field. Therefore, this study enhanced its quality through meta-analysis. The results of this meta-analysis indicate that treadmill training, particularly BWSTT, may positively impact lower extremity muscular endurance, cardiorespiratory fitness, functional mobility, and balance coordination in patients with Parkinson’s disease. This improvement may contribute to a reduced risk of falls and enhanced patient independence.
We first analyzed the impact of treadmill training on the enhancement of overall motor abilities in patients with Parkinson’s disease, using the UPDRS III score as a primary index to assess motor function. The results indicated that treadmill training significantly reduced the UPDRS III score compared to conventional training; however, there was a high degree of heterogeneity (I2 > 50%). To further investigate the source of this heterogeneity, we conducted a subgroup analysis, categorizing treadmill training into conventional and BWSTT groups. The findings revealed that the BWSTT group significantly reduced the UPDRS III score, accompanied by a notable decrease in heterogeneity. In contrast, the conventional treadmill group did not demonstrate a significant difference in the improvement of the UPDRS III score when compared to conventional therapy. These results suggest that BWSTT is more effective than conventional therapy in enhancing the overall motor function of patients with Parkinson’s disease, while the effects of conventional treadmill training are comparable to those of conventional therapy. By reviewing and analyzing the literature, it appears that the weight-loss treadmill is more effective at reducing the load on the lower limbs during walking (29). This reduction may facilitate walking training for patients, decrease their fear of falling, and boost their motivation and confidence in participating in such training (30). Additionally, it may enhance sensory feedback and promote neuroplasticity by stimulating the motor cortex (31), and such effects could be further augmented by integrating advanced technologies like robotic-assisted gait training and virtual reality tools (32).
This study first assessed the ability of patients with Parkinson’s disease to engage in moderate-intensity exercise over extended periods, as an indicator of their muscular endurance and cardiorespiratory fitness, using the 6-MWT. The results showed that treadmill training significantly increased the walking distance in the 6-MWT compared to conventional rehabilitation methods. Furthermore, the study evaluated short-term walking speed, balance, and functional mobility using the 10-MWT. Treadmill training was associated with a significant improvement in walking speed compared to standard approaches, with the effects of BWSTT appearing even more pronounced.
This study focused on assessing the balance abilities of patients with Parkinson’s disease using BBS. The results indicated that the therapeutic effects of treadmill training and traditional conventional training were comparable in enhancing the balance of these patients. Even after further categorizing treadmill training into conventional and weight-loss groups, the treatment effects in each group remained similar to those observed with conventional routine training. Balance improvements depend not only on lower limb strength but also on sensory systems like proprioception, vision, and vestibular functions (33). As Lena et al. note, rehabilitation interventions target these systems to enhance balance in Parkinson’s disease (34). While treadmill training can effectively enhance gait and lower limb strength, the stimulation of other sensory systems, such as the visual and vestibular systems, may be more limited (35), though recent VR-based interventions have shown promising improvements in these areas (36).
The TUG is utilized to evaluate the functional mobility of patients with Parkinson’s disease. It primarily measures the time required for patients to perform a series of movements, including standing up, walking, turning around, and sitting down, all within a brief period. Analyses revealed no significant difference between treadmill training and traditional conventional training in reducing TUG times. Possible explanations for this finding may include the following: treadmill training primarily focuses on enhancing gait and walking ability, which may have a limited impact on dynamic balance (e.g., turning and one-legged standing) (37). Additionally, the existing literature on this topic is sparse, and the small sample sizes may contribute to a deviation of the results from reality.
Finally, this study utilized the PDQ-39 to assess the quality of life in patients with Parkinson’s disease. The results indicated that treadmill training was comparable to traditional conventional training in enhancing patients’ quality of life. It is hypothesized that this outcome may be attributed to the limited research literature related to this indicator and the insufficient sample size, which may have led to some deviation from the actual results.
4.2 Advantages and limitations
This systematic review and meta-analysis offers several advantages:
(1) The substantial number of included studies, the extensive sample size, and the diverse range of countries involved more accurately highlight the advantages of treadmill training over traditional conventional training in enhancing motor function in patients with Parkinson’s disease; (2) Revealed that BWSTT may have superior therapeutic effects; (3) No publication bias was identified in the included studies; (4) revealed potential shortcomings of treadmill training in stimulating the visual and vestibular balance systems; (5) highlighted the potential limitations of treadmill training in enhancing dynamic balance (e.g., turning and one-legged standing; (6) demonstrated the potential benefits of treadmill training in enhancing muscular endurance and short-term walking speed.
This study has several limitations: (1) The study population primarily consists of elderly individuals, which may limit the applicability of the findings to other demographic groups; (2) Regarding the two indicators, TUG test and PDQ-39, the literature included and the sample sizes are relatively small, potentially introducing bias into the results. Further research is necessary to enhance and validate these findings; (3) There are potential shortcomings in the discussion of treadmill training, which are based on a review of the literature. Future experiments should be designed to verify these results; (4) Treadmill training currently lacks a standardized treatment protocol.
4.3 Directions for future research
Future research should focus on the following areas: First, efforts should be directed toward further enhancing the treadmill to better meet patients’ needs for visual stimulation and dynamic balance functions, such as turning and one-legged standing. While some studies have started to explore this area, the current research remains insufficient in both breadth and depth. Second, to address the limitations of the small sample size in the current study, future research should include larger cohorts or consider multicenter collaborations to enhance statistical power. it is essential to design large-scale randomized controlled trials to establish standardized protocols. Additionally, research that addresses the humanistic aspects of patient care, such as quality of life, should be incorporated.
Moreover, when considering the practical implementation of treadmill training programs in community or rural settings, it is important to acknowledge several potential barriers. These may include limited access to specialized equipment, a lack of trained professionals, and financial constraints. In rural areas, transportation issues could also pose significant challenges, making it difficult for patients to attend regular sessions. To overcome these obstacles, strategies such as community-based initiatives, telemedicine solutions, and the development of more cost-effective training equipment could enhance the feasibility and effectiveness of treadmill training for Parkinson’s disease patients in these settings, especially when combined with customizable features such as self-selected speeds and immersive VR environments, which have shown clinical benefits (38).
5 Conclusion
Compared to traditional training methods, treadmill training offers greater benefits in enhancing lower limb mobility for patients with Parkinson’s disease. It is effective in improving muscular endurance and accelerating short-term walking speed, with weight-loss treadmills demonstrating superior therapeutic efficacy. However, to further enhance the therapeutic effects, improvements in visual stimulation and dynamic balance training on the treadmill are necessary. Additionally, the long-term sustainability and adherence to treadmill training programs should be considered for lasting benefits.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XY: Writing – review & editing, Writing – original draft. PP: Formal analysis, Software, Writing – review & editing. HZ: Writing – review & editing, Data curation, Visualization. JH: Writing – review & editing, Data curation, Investigation. YW: Writing – review & editing, Conceptualization. PL: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1609912/full#supplementary-material
Abbreviations
BBS, alance as measured by the Berg Balance Scale; BWSTT, body-weight-supported treadmill training; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; PD, Parkinson’s disease; PDQ-39, the Parkinson’s Disease Questionnaire-39; PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized controlled trials; TT, treadmill training; TUG, the Timed Up and Go test; UPDRS-III, the Unified Parkinson’s Disease Rating Scale Part III; 6MWT, the 6-Minute Walk Test; 10MWT, 10 Meter Walk Test.
References
1. Marino, BLB, de Souza, LR, Sousa, KPA, Ferreira, JV, Padilha, EC, da Silva, CHTP, et al. Parkinson's disease: a review from pathophysiology to treatment. Mini Rev Med Chem. (2020) 20:754–67. doi: 10.2174/1389557519666191104110908
2. Cattaneo, C, and Jost, WH. Pain in Parkinson's disease: pathophysiology, classification and treatment. J Integr Neurosci. (2023) 22:132. doi: 10.31083/j.jin2205132
3. Skinner, JW, Christou, EA, and Hass, CJ. Lower extremity muscle strength and force variability in persons with Parkinson disease. J Neurol Phys Ther. (2019) 43:56–62. doi: 10.1097/NPT.0000000000000244
4. Durmus, B, Baysal, O, Altinayar, S, Altay, Z, Ersoy, Y, and Ozcan, C. Lower extremity isokinetic muscle strength in patients with Parkinson's disease. J Clin Neurosci. (2010) 17:893–6. doi: 10.1016/j.jocn.2009.11.014
5. Li, X, He, J, Yun, J, and Qin, H. Lower limb resistance training in individuals with Parkinson's disease: an updated systematic review and meta-analysis of randomized controlled trials. Front Neurol. (2020) 11:591605. doi: 10.3389/fneur.2020.591605
6. Metta, V, Sanchez, TC, and Padmakumar, C. Osteoporosis: a hidden nonmotor face of Parkinson's disease. Int Rev Neurobiol. (2017) 134:877–90. doi: 10.1016/bs.irn.2017.05.034
7. Roeder, L, Boonstra, TW, and Kerr, GK. Corticomuscular control of walking in older people and people with Parkinson's disease. Sci Rep. (2020) 10:2980. doi: 10.1038/s41598-020-59810-w
8. Lee, DH, Woo, BS, Park, YH, and Lee, JH. General treatments promoting independent living in Parkinson's patients and physical therapy approaches for improving gait-a comprehensive review. Medicina. (2024) 60:711. doi: 10.3390/medicina60050711
9. Scelzo, E, Beghi, E, Rosa, M, Angrisano, S, Antonini, A, Bagella, C, et al. Deep brain stimulation in Parkinson's disease: a multicentric, long-term, observational pilot study. J Neurol Sci. (2019) 405:116411. doi: 10.1016/j.jns.2019.07.029
10. Yao, J, Guo, N, Xiao, Y, Li, Z, Li, Y, Pu, F, et al. Lower limb joint motion and muscle force in treadmill and over-ground exercise. Biomed Eng Online. (2019) 18:89. doi: 10.1186/s12938-019-0708-4
11. Bishnoi, A, Lee, R, Hu, Y, Mahoney, JR, and Hernandez, ME. Effect of treadmill training interventions on spatiotemporal gait parameters in older adults with neurological disorders: systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:2824. doi: 10.3390/ijerph19052824
12. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGfor the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
13. Arfa-Fatollahkhani, P, Safar Cherati, A, Habibi, SAH, Shahidi, GA, Sohrabi, A, and Zamani, B. Effects of treadmill training on the balance, functional capacity and quality of life in Parkinson’s disease: a randomized clinical trial. J Complement Integr Med. (2019) 17:20180245. doi: 10.1515/jcim-2018-0245
14. Schenkman, M, Moore, CG, Kohrt, WM, Hall, DA, Delitto, A, Comella, CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. (2018) 75:219–26. doi: 10.1001/jamaneurol.2017.3517
15. Picelli, A, Varalta, V, Melotti, C, Zatezalo, V, Fonte, C, Amato, S, et al. Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson's disease: a pilot, single-blind, randomized controlled trial. Funct Neurol. (2016) 31:25–31. doi: 10.11138/fneur/2016.31.1.025
16. Picelli, A, Melotti, C, Origano, F, Neri, R, Waldner, A, and Smania, N. Robot-assisted gait training versus equal intensity treadmill training in patients with mild to moderate Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. (2013) 19:605–10. doi: 10.1016/j.parkreldis.2013.02.010
17. Miyai, I, Fujimoto, Y, Yamamoto, H, Ueda, Y, Saito, T, Nozaki, S, et al. Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. (2002) 83:1370–3. doi: 10.1053/apmr.2002.34603
18. Miyai, I, Fujimoto, Y, Ueda, Y, Yamamoto, H, Nozaki, S, Saito, T, et al. Treadmill training with body weight support: its effect on Parkinson's disease. Arch Phys Med Rehabil. (2000) 81:849–52. doi: 10.1053/apmr.2000.4439
19. Harro, CC, Shoemaker, MJ, Frey, O, Gamble, AC, Harring, KB, Karl, KL, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson's disease: a randomized controlled trial. NeuroRehabilitation. (2014) 34:541–56. doi: 10.3233/NRE-141048
20. Gaßner, H, Trutt, E, Seifferth, S, Friedrich, J, Zucker, D, Salhani, Z, et al. Treadmill training and physiotherapy similarly improve dual task gait performance: a randomized-controlled trial in Parkinson's disease. J Neural Transm (Vienna). (2022) 129:1189–200. doi: 10.1007/s00702-022-02514-4
21. Ganesan, M, Sathyaprabha, TN, Pal, PK, and Gupta, A. Partial body weight-supported treadmill training in patients with Parkinson disease: impact on gait and clinical manifestation. Arch Phys Med Rehabil. (2015) 96:1557–65. doi: 10.1016/j.apmr.2015.05.007
22. Ganesan, M, Sathyaprabha, TN, Gupta, A, and Pal, PK. Effect of partial weight-supported treadmill gait training on balance in patients with Parkinson disease. PM R. (2014) 6:22–33. doi: 10.1016/j.pmrj.2013.08.604
23. Ganesan, M, Pal, PK, Gupta, A, and Sathyaprabha, TN. Treadmill gait training improves baroreflex sensitivity in Parkinson's disease. Clin Auton Res. (2014) 24:111–8. doi: 10.1007/s10286-014-0236-z
24. Fisher, BE, Wu, AD, Salem, GJ, Song, J, Lin, CH(J), Yip, J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. (2008) 89:1221–9. doi: 10.1016/j.apmr.2008.01.013
25. de Melo, GEL, Kleiner, AFR, Lopes, JBP, Dumont, AJL, Lazzari, RD, Galli, M, et al. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson's disease. NeuroRehabilitation. (2018) 42:473–80. doi: 10.3233/NRE-172355
26. Cheng, FY, Yang, YR, Wu, YR, Cheng, SJ, and Wang, RY. Effects of curved-walking training on curved-walking performance and freezing of gait in individuals with Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. (2017) 43:20–6. doi: 10.1016/j.parkreldis.2017.06.021
27. Rinalduzzi, S, Trompetto, C, Marinelli, L, Alibardi, A, Missori, P, Fattapposta, F, et al. Balance dysfunction in Parkinson's disease. Biomed Res Int. (2015) 2015:434683. doi: 10.1155/2015/434683
28. Tsai, SY, Tai, CH, and Lee, YY. Exploring potential predictors of treadmill training effects in people with Parkinson disease. Arch Phys Med Rehabil. (2024) 105:525–30. doi: 10.1016/j.apmr.2023.09.008
29. Kristiansen, M, Odderskær, N, and Kristensen, DH. Effect of body weight support on muscle activation during walking on a lower body positive pressure treadmill. J Electromyogr Kinesiol. (2019) 48:9–16. doi: 10.1016/j.jelekin.2019.05.021
30. Nilsson, L, Carlsson, J, Danielsson, A, Fugl-Meyer, A, Hellström, K, Kristensen, L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. (2001) 15:515–27. doi: 10.1191/026921501680425234
31. Anggelis, E, Powell, ES, Westgate, PM, Glueck, AC, and Sawaki, L. Impact of motor therapy with dynamic body-weight support on functional Independence measures in traumatic brain injury: an exploratory study. NeuroRehabilitation. (2019) 45:519–24. doi: 10.3233/NRE-192898
32. Spanakis, M, Xylouri, I, Patelarou, E, and Patelarou, A. A literature review of high-tech physiotherapy interventions in the elderly with neurological disorders. Int J Environ Res Public Health. (2022) 19:9233. doi: 10.3390/ijerph19159233
33. Roytman, S, Paalanen, R, Carli, G, Marusic, U, Kanel, P, van Laar, T, et al. Multisensory mechanisms of gait and balance in Parkinson's disease: an integrative review. Neural Regen Res. (2025) 20:82–92. doi: 10.4103/NRR.NRR-D-23-01484
34. Lena, F, Modugno, N, Greco, G, Torre, M, Cesarano, S, Santilli, M, et al. Rehabilitation interventions for improving balance in Parkinson's disease: a narrative review. Am J Phys Med Rehabil. (2023) 102:270–4. doi: 10.1097/PHM.0000000000002077
35. Shin, J, and Chung, Y. The effects of treadmill training with visual feedback and rhythmic auditory cue on gait and balance in chronic stroke patients: a randomized controlled trial. NeuroRehabilitation. (2022) 51:443–53. doi: 10.3233/NRE-220099
36. Calabrò, RS, Naro, A, Cimino, V, Buda, A, Paladina, G, di Lorenzo, G, et al. Improving motor performance in Parkinson's disease: a preliminary study on the promising use of the computer assisted virtual reality environment (CAREN). Neurol Sci. (2020) 41:933–41. doi: 10.1007/s10072-019-04194-7
37. Steib, S, Klamroth, S, Gaßner, H, Pasluosta, C, Eskofier, B, Winkler, J, et al. Perturbation during treadmill training improves dynamic balance and gait in Parkinson's disease: a single-blind randomized controlled pilot trial. Neurorehabil Neural Repair. (2017) 31:758–68. doi: 10.1177/1545968317721976
38. Fundarò, C, Maestri, R, Ferriero, G, Chimento, P, Taveggia, G, and Casale, R. Self-selected speed gait training in Parkinson's disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. (2019) 55:456–62. doi: 10.23736/S1973-9087.18.05368-6
39. Cakit, BD, Saracoglu, M, Genc, H, Erdem, HR, and Inan, L. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson’s disease. Clin Rehabil. (2007) 21:698–705. doi: 10.1177/0269215507077269
Keywords: Parkinson’s disease, treadmill training, lower extremity function, gait rehabilitation, elderly patients, meta-analysis
Citation: Yin X, Peng P, Zhang H, Hu J, Wei Y and Li P (2025) Treadmill training for gait rehabilitation in elderly patients with mild-to-moderate Parkinson’s disease: a systematic review and meta-analysis. Front. Neurol. 16:1609912. doi: 10.3389/fneur.2025.1609912
Edited by:
Anthony Pak Hin Kong, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Marios Spanakis, University of Crete, GreeceFrancesco Lena, Mediterranean Neurological Institute Neuromed (IRCCS), Italy
Copyright © 2025 Yin, Peng, Zhang, Hu, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: PinMei Li, bGlwbUBqbHUuZWR1LmNu
 XiaoTing Yin1
XiaoTing Yin1 PinMei Li
PinMei Li