Abstract
Background:
The translation of standard-of-care in acute ischemic stroke reperfusion interventions into practice is well established, but multifactorial obstacles exist in complete adoption, which has led to inequities in access and delivery of services. The objective of this study was to improve access and efficiency of ischemic stroke treatment across four Atlantic Canadian Provinces.
Methods:
A stepped-wedge cluster trial was conducted over 30 months with 3 clusters covering 34 sites. The trial was conducted across all 4 Atlantic Canadian provinces: Nova Scotia (NS), New Brunswick (NB), Prince Edward Island (PE), and Newfoundland and Labrador (NL). The design was quasi-randomized, with each cluster associated with one or more provinces: cluster 1—NS; cluster 2—NB and PE; and cluster 3—NL. The patient population was all ischemic stroke patients across all 4 provinces. The intervention was a 6-month modified Quality Improvement Collaborative (mQIC), which was modified from the Breakthrough Series Collaborative to be half of the 1-year period and conducted virtually. The intervention consisted of assembling an interdisciplinary improvement team, 2 full-day workshops, webinars, and virtual site visits. Suggested changes included 6 process improvement strategies.
Results:
Over the trial period, 8,594 ischemic stroke patients were included, out of which 1,599 patients received acute reperfusion treatment. The proportion of patients that received treatment did not increase significantly with the intervention [0.4% increase for patients that received thrombolysis and/or EVT (p = 0.68)]. Median door-to-needle time was reduced by 9.8 min with the intervention (p = 0.006). Cluster 3 saw the greatest improvements in both access and efficiency.
Conclusion:
A mQIC intervention resulted in improvement of process measures like door-to-needle time. Quality improvement initiatives may need to be longer to allow full implementation and tailored for each health system to ensure that each system sees improvement. In-person activities might be critical to ensure fidelity of the intervention.
Clinical trial registration:
ISRCTN11109800, https://www.isrctn.com/ISRCTN11109800.
Introduction
Revolutionary advances in stroke treatment have occurred over the past two decades from thrombolysis using intravenous (IV) alteplase in the mid-1990’s (1–3) and Tenecteplase (4) to the most recent ground-breaking trials in 2015 showing the high efficacy of endovascular thrombectomy (EVT) (5, 6). These treatments can reduce disability due to stroke (7), thus reducing the need for assistance with daily living tasks (8), increase return to the workplace (9), and reduce hospital costs (10).
Although treatment of acute ischemic stroke patients with alteplase and EVT are part of guideline care in Canada and around the world (11, 12), less than optimal utilization rates for both of these treatments are observed. This lag in the translation of best standard of care is well known, which has led to increasing research in implementation science (13). In acute ischemic stroke treatment, the substantial economic and societal benefits of these therapies continue to make it critical to pursue optimal uptake. The lag in the uptake of best standard of care is exacerbated by inequities due to geographic, economic and demographic disparities, which has created a split between urban and rural access to treatment. Furthermore, access to EVT in rural areas is even more challenging because the treatment is relatively new and requires specialized equipment and personnel. Although both of these therapies mitigate disability, their effectiveness has been shown to be highly time dependent. In stroke treatment, minutes matter (5, 14), bringing to light the mantra, “time is brain” (15) Therefore, there have been calls to reduce hospital thrombolysis treatment times to 30 min from hospital arrival (10, 16), and to create seamless transfer processes for more efficient access to EVT for patients living outside major urban centers (17, 18). Atlantic Canada is comprised of four provinces: Nova Scotia (NS), New Brunswick (NB), Prince Edward Island (PE), and Newfoundland and Labrador (NL). Stroke treatment in Atlantic Canada is particularly challenging because of small populations with a significant proportion that live in rural areas.
Using a stepped-wedge cluster trial design, this study [ACTEAST (Atlantic Canada Together Enhancing Acute Stroke Treatment)] aimed to evaluate if access to and efficiency of acute ischemic stroke treatment with IV thrombolysis and EVT across Atlantic Canada would improve with a modified Quality Improvement Collaborative (mQIC) intervention (19, 20). The objective of the cluster trial is to increase the proportion of ischemic stroke patients that receive reperfusion therapy and reduce the time to treatment.
Methods
ACTEAST project overview
The ACTEAST Project was a 30-month stepped-wedge cluster trial from May 1 2020 to October 31 2022. The trial was quasi-randomized in-so-far as the sites were not randomized to a cluster for pragmatic reasons; rather, each cluster was associated with a province. A quasi-randomization methodology was used because of the close network of all stroke centers across each province, which would make it difficult to not contaminate each cluster if sites were to be randomized to different clusters. Each cluster had a single center that was capable of EVT treatment with the remainder of sites only capable of thrombolysis treatment; patients at these thrombolysis-only sites who were eligible for EVT were transferred to the EVT-capable center in their province. There were a total of 34 sites with 10 sites in cluster 1, 12 sites in cluster 2, and 12 sites in cluster 3. The stepped-wedge cluster trial design is shown in Figure 1. This trial took place entirely during the COVID-19 pandemic where restriction and additional processes were required including testing all patients for COVID-19 infection. These restrictions also limited the administration of intervention activities to being entirely virtual.
Figure 1

Stepped-wedge trial design and timeline. NS, Nova Scotia; NB, New Brunswick; PE, Prince Edward Island; NL, Newfoundland and Labrador; n denotes the number of sites in each cluster; N(IS), number of all ischemic stroke patients; N(treated), number of community onset ischemic stroke patients treated with thrombolysis and/or Endovascular Thrombectomy.
ACTEAST was conducted across all four Atlantic Canadian provinces, which are smaller and less populated provinces as compared to the other Canadian provinces and have a combined population of approximately 2.5 million people. The population (21), geographic size, number of stroke centres by type, and age/sex standardized stroke admission rates per 100,000 population by province (22) is provided in Table 1. The stroke centre type of Primary Stroke Centre (PSC) indicates hospitals that only capable of thrombolysis treatment, and the stroke centre type of Comprehensive Stroke Centre (CSC) indicates hospitals that are capable of both thrombolysis and EVT. The population is balanced in clusters 1 (NS) and 2 (NB & PE); however, cluster 3 (NL) has approximately half the population of the other two clusters. Furthermore, cluster 3 (NL) has a much larger land area than the other two clusters, where 27.5% of the area is in the more populated area of the island of Newfoundland; the large mainland of Labrador only has 5.2% of the province’s population. Figure 2 shows the geography and distribution of stroke centres. The age and sex adjusted stroke admission rates per 100,000 population are the highest in cluster 2 with PE and NB having 182 and 163, respectively (22). The age and risk standardized stroke incidence rate is greater in Atlantic Canada than in other provinces: up to 140 strokes/100,000 people/year in the Atlantic provinces compared to 113 strokes/100,000 people/year in Ontario (22). The Atlantic provinces have a larger percent of its population living in rural areas (41 to 53%), which is more than double other Canadian provinces; for example, only 14, 17, 14, and 19% live in rural areas for Ontario, Alberta, British Columbia, and Quebec, respectively (23). Atlantic Canada also has an older population (24). All provinces have a provincially administered universal healthcare system.
Table 1
| Province | Cluster | Population (2022 Estimate) (21) | Size (km2) | Number of PSCs | Number of CSCs | Number of Alteplase Capable Centres (bypass) | Age/Sex standardized stroke admission rates per 100,000 population (2012) (22) |
|---|---|---|---|---|---|---|---|
| Nova Scotia (NS) | 1 | 1,002,586 | 55,284 | 9 | 1 | 1 | 139 |
| New Brunswick (NB) | 2 | 797,102 | 72,907 | 9 | 1 | 0 | 163 |
| Prince Edward Island (PE) | 2 | 166,331 | 5,660 | 2 | 0 | 0 | 182 |
| Newfoundland and Labrador (NL) | 3 | 522,453 | 405,720 | 10 | 1* | 1 | 159 |
Population, geographic size, number of stroke centres by type, and age/sex standardized stroke admission rates of each of each Canadian Atlantic provinces with their cluster allocation.
The CSC in Newfoundland and Labrador began performing EVT treatment on June 20, 2022.
PSC, Primary Stroke Centre; CSC, Comprehensive Stroke Centre; EVT, Endovascular Thrombectomy.
Figure 2
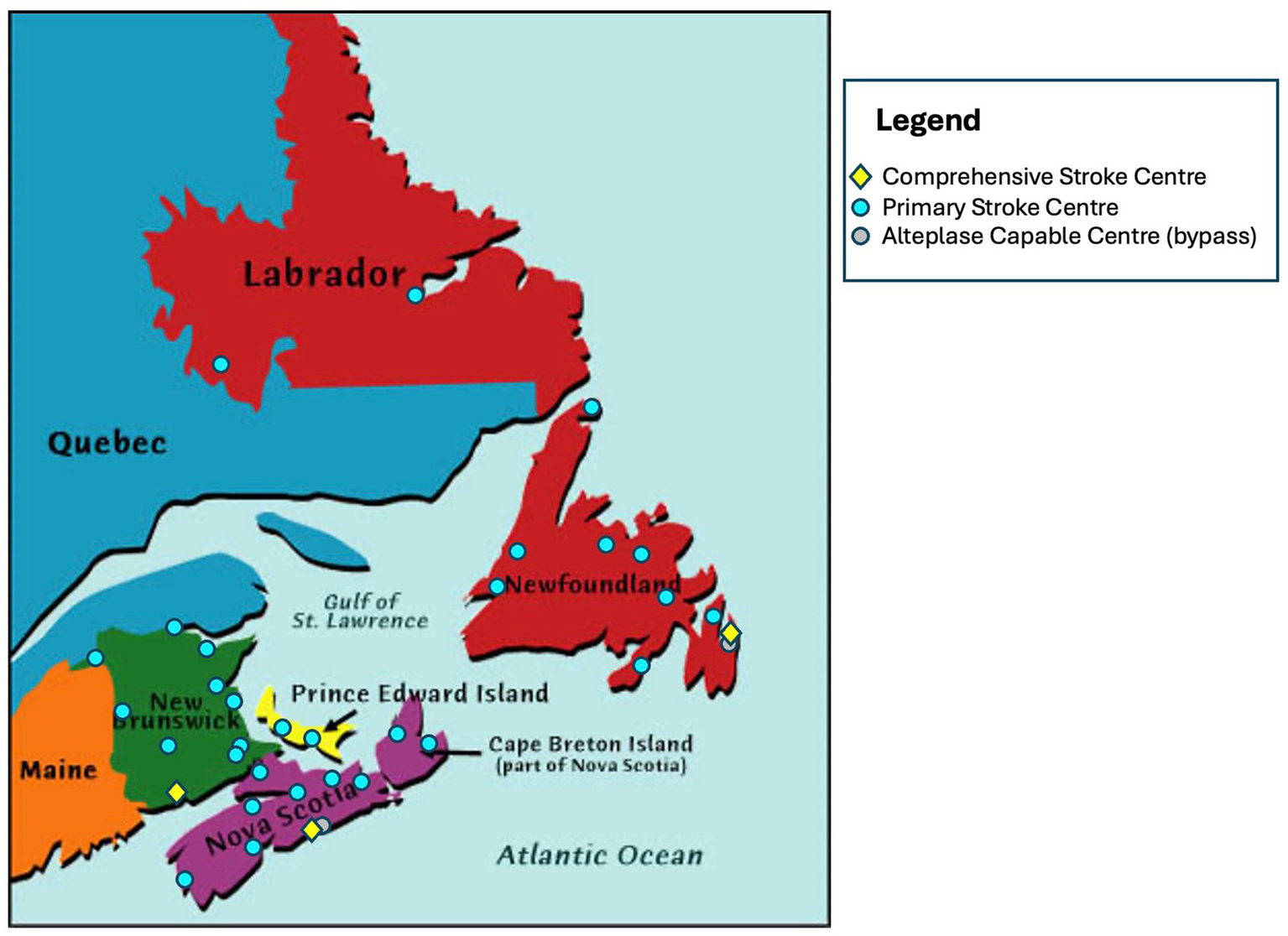
Geographic distribution of stroke centres across all 4 participating provinces.
Each province has developed a provincial stroke system, where specific hospitals have been designated as stroke centers. Prior to the intervention, each province had a centralized provincial emergency medical services (EMS) to transport patients to hospitals. EMS in these provinces use the Cincinnati Pre-hospital stroke screen (CPSS) to identify stroke patients, and they take suspected stroke patients to the nearest stroke centre. The time-window from onset for transporting the patient to a stroke centre when the CPSS was positive varied by province. All designated stroke centers have CT (computed tomography) scanners, CTA (CT angiogram) capabilities, and expertise to treat patients with thrombolysis. Patients with an LVO at the PSCs were identified using a CTA; however, prior to the mQIC intervention, the CTA was not universally completed immediately after the CT. PSCs across all provinces have access to stroke neurology expertise through telehealth. Ambulances bypass closer hospitals to take suspected stroke patients directly to a designated stroke center, which are distributed across each province to ensure optimal access for the population in each province.
EVT services were available in NS and NB in the cities of Halifax and Saint John, respectively. PE has set-up cross-provincial transfer agreement with the EVT-capable hospital in Halifax, NS. NL did not have EVT services until June 20, 2022, which was completed during the trial period. Initially, only local patients had access to EVT, so there were no patients transferred for EVT in NL during the trial period. None of the provinces were using a pre-hospital LVO screening tool during the trial period, and all centers have access to CTA for determination of LVO. The utilization rates for ambulance use by acute ischemic stroke patients for each province is not specially known, but previous study found that 66% of all admitted stroke and TIA (transient ischemic attack) patients arrived by ambulance in Canada (25).
Intervention
Site enrolment and patient population
The intervention used a mQIC methodology adapted from the Institute for Healthcare Improvement’s Breakthrough Series Collaborative (19). The mQIC began with each site formally enrolling and assembling an interdisciplinary team. Enrolment into the ACTEAST trial was done at a site level. The Principal Investigator (NK) developed relationships with key medical and administrative personnel in all provinces to ensure that all designated stroke centers enrolled. All enrolled sites were required to set-up an interdisciplinary improvement team that consisted of paramedics, emergency department nurses, emergency physicians, stroke physicians (if applicable), stroke nurses (if applicable), CT technologists, radiologists, and administrators. Each team member was enrolled into the study and consented their participation.
Full-day workshop
The mQIC consisted of two full-day workshops, which the enrolled team from each site attended. The first workshop highlighted evidence on treatment of ischemic stroke and strategies for improving efficiency of treatment. It included a presentation by one of the participating site’s improvement team on changes that they have implemented and are working on to improve treatment efficiency at their hospital. The afternoon consisted of each team planning their improvements and reporting it back to the entire group. The second workshop was conducted 6–8 weeks after the first, where teams presented their changes, and the afternoon also had a planning period with report back. This second workshop had presentation from four participating sites where they presented the improvements that they are working towards. Both of the workshops included a model of sharing individual site’s improvement activities, which promoted peer-to-peer learning rather than just didactive learning from experts.
Virtual site visits
During the 6-month intervention period, the sites were supported with virtual site visits to each participating site. The purpose of the virtual site visits was to determine the site’s improvement teams progress towards implementing changes. Site visits were completed virtually with all sites via a 1-h Zoom meeting, and they were led by the Principal Investigator (NK) with attendance from as many members from the site’s improvement team and others from the site who were able to attend. During the site visit, the objectives of ACTEAST were reviewed, and the site provided an update of their activities towards implementing changes. The mean attendance (SD) for each site visit were: 8.8 (4.5) for cluster 1, 7.0 (2.8) for cluster 2, and 8.3 (5.7) for cluster 3.
Webinars
Five webinars were held during the 6-month intervention, where approximately 40–60% of the webinar presentations were delivered by a participating site showing their improvement efforts. The webinars had significant sharing of learning including successes and failures, activities, and action plans; additionally, they also provided presentations on evidence and best-practice. Full details of the mQIC design and engagement of all sites and personnel have previously been reported (20).
Change package
All enrolled sites and their improvement teams were provided with a change package at the first workshop. This outlined the following changes for each team to trial, adapt, and implement at their site:
-
Prenotification and stroke team activation: paramedics pre-notify the hospital of an incoming stroke patient, and paramedics ensure two IV lines are in place; the hospital creates a single call activation of the stroke team.
-
Rapid registration process: the hospital adopts a “registration as unknown” (similar for trauma patients), pre-registration process by using information from paramedics, or a quick registration process where only minimal information is entered initially.
-
Patient moved to CT scanner on Emergency Medical Services (EMS) stretcher: this includes having a thrombolysis kit ready and conducting a quick neurological exam on the way to the scanner.
-
Not waiting for bloodwork when not indicated for the patient
-
Thrombolysis administered in imaging area
-
Rapid transfer process for EVT: adopting a heads-up call to the transport system when presented with a severe stroke.
Measures and data collection
To measure access to treatment, the proportion of all ischemic stroke patients who received treatment was used. There were 3 separate measures for this: proportion that received IV thrombolysis only; proportion that received EVT only; and proportion that received IV thrombolysis and/or EVT (any treatment). We also assessed the proportion of patients that were transferred for EVT from a thrombolysis-only center.
To assess speed at which treatment was administered (treatment efficiency), all community onset ischemic stroke patients that received treatment were included and stroke that occurred in-hospital were excluded. Thrombolysis efficiency was assessed using door-to-needle time (time from hospital arrival to the start of thrombolysis treatment) and 911-to-needle time (time from 911 call to start of thrombolysis treatment). EVT efficiency was assessed using door-to-arterial-access time (time from hospital arrival to the start of EVT treatment) and 911-to-arterial-access time (time from 911 call to start of EVT treatment). Transfer efficiency was assessed using door-in-door-out time, which is the time from arrival at the thrombolysis-only center to departure from this center to the EVT-capable center.
Patient outcomes were measured using a pragmatic approach by utilizing hospital discharge disposition. The outcome measures included proportion of patients discharged home and proportion of patients that died in-hospital. This was done for all ischemic stroke patients and for all treated stroke patients (excluding in-hospital stroke patients).
Data were collected by different approaches for each province. All ischemic stroke patients that received treatment with thrombolysis (alteplase and Tenecteplase) and/or EVT were included in the study for each province. NS and PE have provincial registries that contained all the necessary data. The identification of patients for inclusion (ischemic stroke patients that received acute treatment) was done in NS and PE by extracting records from their provincial stroke registries of patients that received treatment. Data is collected for the registries by a stroke coordinator through chart reviews. EMS data were linked to the registry data to obtain the date and time of the 911 call. In NB and NL, the identification of patients for inclusion was done by reviewing the dispensing of thrombolytic drug through the hospital’s pharmacy and the use of the angiosuite usage for the EVT procedure. Data from NB was collected by conducting chart reviews, which were done either by the data analyst or by the health authority’s research office. Data from NL was collected by chart review conducted either by the local stroke coordinator or through Memorial University’s Quality of Care NL organization. Although the data collection methods were heterogenous, as two provinces have a provincial registry that we were able to use for this study, data collection was done through chart reviews across all four provinces, as the provincial registries in NS and PE are populated through chart reviews. The patient chart at all participating hospitals included information on patient sex, patient age at time of stroke, onset time or last seen normal time, NIHSS, time thrombolysis was started, time that the patient left the primary stroke center for EVT, and time of arterial-access. Time of CT scan was collected through PACS (picture archiving and communication system). Data quality checks were conducted by the research team once the data was received to ensure data validity especially pertaining to the date and time fields. If there were errors discovered, they were sent back to the province for further validation. There were no exclusions made in the data collection phase. All strokes that occurred in hospital were excluded from efficiency measures: 911-to-needle, door-to-needle, 911-to-arterial-access, door-to-arterial-access, and door-in-door-out times.
Statistical analysis
The data for this trial includes all ischemic stroke patients in the entire province regardless of the number of sites that formally enrolled. The trial is therefore a population based study; no sample size was calculated. All missing values for age, sex, and National Institutes of Health Stroke Scale (NIHSS) were imputed using the K-Nearest Neighbor algorithm (k-NN) (26). The imputation method of kNN was chosen because it provides more accurate results than simpler methods such as median imputation for complex datasets such as this one; furthermore, kNN is suitable for high missingness at it considers other data features to capture patterns and correlations in the data (27). The missing data must be missing completely at random (MCAR), so we will conduct further comparisons with missing data and not missing data to ensure they are MCAR (28). The full ischemic stroke cohort was used to determine if the proportion of ischemic stroke patients that received treatment increased; this cohort does not have data on age, sex and National Institutes of Health Stroke Scale (NIHSS); therefore, it was only adjusted for cluster assignment. The treated patient cohort was used to determine the reduction in treatment time. This cohort includes age and sex, but NIHSS values were anticipated to be largely incomplete due to the variation in expertise on this measure across the centres. Ideally, adjustments will be made with age, sex, and NIHSS; however, if the NIHSS missing values are determined to be not missing completely at random, they will only be adjusted for age, sex and cluster. For the comparison analysis related to all ischemic stroke patients, unadjusted proportion comparison is conducted using χ2 analysis, while a mixed effects logistic regression with cluster as random effects variable is used for adjusted analysis. For analysis related to all treated patients, unadjusted analysis comparison is conducted using χ2 for proportions and Wilcoxon rank-sum test for continuous variables. The comparison analysis uses a mixed effects logistic regression for binary/categorical variables and a mixed effects quantile regression for continuous variables. In these analyses, age, sex, and NIHSS are fixed effects variables while cluster was a random effects variable. We only adjusted for cluster for the entire ischemic stroke cohort as we did not have patient level data on age, sex, and NIHSS. For the treated cohort, we adjusted for age, sex, NIHSS and cluster. We did not include the time of the intervention as cluster captures the temporality of the stepped-wedge trial, and the overall intervention period is short. When analyzing results by cluster, we used a simple quantile regression model.
Ethical considerations and trial registration
Ethical approval for this study was obtained from the NS Health Research Ethics Board (REB# 1025460), Health PEI Ethics Board, Horizon Health Network Research Ethics Board (ROMEO File #: 100906 and RS#: 2020–2893), Vitalité Health Network Ethics Office (ROMEO file number: 101305), and Newfoundland and Labrador Health Research Ethics Board (Researcher Portal File #: 20210255 and Reference #: 2020.074). The trial is registered with the ISRCTN registry (ISRCTN11109800, https://www.isrctn.com/ISRCTN11109800).
Results
Over 30 months from May 12020 to October 312022, 8,594 ischemic stroke patients were included in the analysis for this trial, out of which 1,599 (18.6%) patients received acute treatment. There were 3065 ischemic stroke patients in the pre-intervention period of whom 547 (17.8%) received treatment, and 3803 ischemic stroke patients were in the post-intervention period of whom 718 (18.9%) patients were treated. These treated number include in-hospital stroke treatments, but excludes extended treatment times. The full details of patient allocation by cluster and period are shown in Figure 1.
Figure 3 shows the flow diagram for patient allocation in the pre-intervention and post-intervention periods, as well as the number of patients in the intervention period. Strokes that occurred in-hospital were excluded from the treated patient cohort, as they did not have a relevant door time thus the process measures (door-to-needle, door-to-arterial-access, and door-in-door-out time) were not relevant for these patients. The total number of in-hospital stroke patients that were excluded were 19 in the pre-intervention group, 12 in the intervention group, and 45 in the post-intervention group for total of 76. Additionally, there were some community onset treated patients that had treatment times that were beyond typical standard of care, and were removed to prevent these outliers from affecting the group comparisons. These exclusions were door-to-needle time of greater than 4.5 h; door-to-arterial-access times greater than 6 h; and door-in-door-out times of greater than 16 h. The reason for the 16-h door-in-door-out threshold was based on the results of the DEFUSE 3 (29) and DAWN trail (30), which showed the efficacy of EVT in the extended time window as late as 16 to 24 h, thus we used an intermediary DIDO time of 16 h, and an additional of 1.5 h prior to arrival at the PSC from onset, an average of 1 h transport time, and 1 h for the door-to-arterial-access at the CSC, we get an onset-to-arterial-access time of 19.5 h. The total number of exclusions due to a door-to-needle time of greater than 4.5 h were 16 in the pre-intervention group and 4 in the post-intervention group. Additionally, the total number of patients excluded due to a door-in-door-out time of greater than 960 min was 2, which only occurred in the pre-intervention group. There was 1 patient excluded due to a door-to-arterial-access time greater than 360 min, which occurred in the post-intervention group. The total number of exclusions in the treated cohort was 99 with 37 in the pre-intervention group, 12 in the intervention period, and 50 in the post-intervention group.
Figure 3
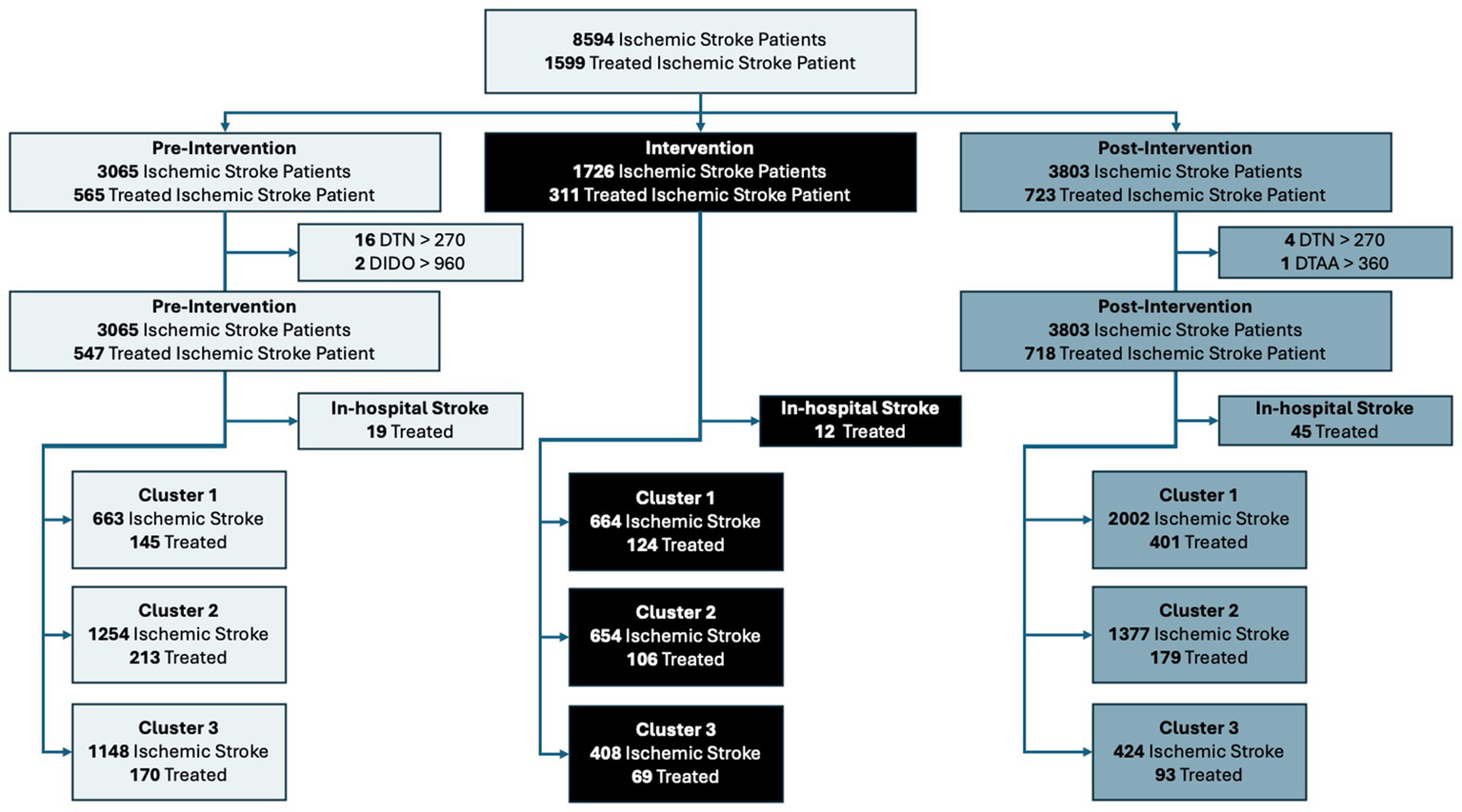
Flow diagram of patient allocation across each period and cluster. DTN, Door-to-needle time; DTAA, Door-to-arterial-access time’ DIDO, Door-in-door-out time.
The results for all ischemic stroke patients are shown in Table 2. The adjusted proportion of patients that received treatment did not increase significantly with a 0.4% increase for patients that received thrombolysis and/or EVT (p = 0.68). Cluster 3 showed the most significant improvements compared to the other clusters in proportion of patients treated with a 6.52% increase (p = 0.003) in the proportion receiving treatment. Cluster 3 also saw a 39.35% increase (p < 0.0001) in the proportion of patients discharged home. The trends for the proportion of ischemic stroke patients that received treatment is shown in Figure 4. Cluster 1 and 3 showed upward trends while cluster 2 showed a downward trend.
Table 2
| Entire Cohort | Pre-intervention period n = 3065 | Post-intervention period n = 3803 | p (significance) |
|---|---|---|---|
| N (%) | N (%) | ||
| Received Thrombolysis$ | 474 (15.46%) | 620 (16.30%) | 0.36 |
| Received Thrombolysis (adjusted)* | 495 (16.15%) | 647 (17.02%) | 0.3 |
| Received EVT$ | 115 (3.75%) | 196 (5.15%) | 0.006 |
| Received EVT (adjusted)* | 123 (4.00%) | 151 (3.98%) | 0.9 |
| Received either thrombolysis and/or EVT$ | 547 (17.85%) | 718 (18.87%) | 0.28 |
| Received either thrombolysis and/or EVT (adjusted) | 570 (18.60%) | 723 (19.00%) | 0.68 |
| Transferred for EVT@$ | 118 (3.84%) | 193 (5.07%) | 0.017 |
| Transferred for EVT (adjusted)* | 127 (4.15%) | 145 (3.80%) | 0.14 |
| Discharged home | 1015 (33.10%) | 1569 (41.25%) | < 0.0001 |
| Discharged home (adjusted)* | 1016 (33.15%) | 1768 (46.50%) | < 0.0001 |
| Died | 324 (10.57%) | 570 (14.98%) | < 0.0001 |
| Died (adjusted)* | 349 (11.39%) | 464 (12.20%) | 0.3 |
| Cluster 1 | N = 663 | N = 2002 | |
| Received Thrombolysis$ | 132 (19.90%) | 383 (19.10%) | 0.70 |
| Received EVT$ | 40 (6.03%) | 122 (6.09%) | 1 |
| Received either thrombolysis and/or EVT$ | 155 (23.37%) | 444 (22.17%) | 0.55 |
| Transferred for EVT@$ | 43 (6.40%) | 114 (5.60%) | 0.51 |
| Discharged home | 236 (35.59%) | 699 (34.91%) | 0.78 |
| Died | 117 (17.64%) | 360 (17.98%) | 0.89 |
| Cluster 2 | N = 1254 | N = 1377 | |
| Received Thrombolysis$ | 165 (13.15%) | 147 (10.67%) | 0.056 |
| Received EVT$ | 75 (5.98%) | 71 (5.15%) | 0.40 |
| Received either thrombolysis and/or EVT$ | 215 (17.14%) | 181 (13.14%) | 0.004 |
| Transferred for EVT@$ | 75 (5.98%) | 79 (5.73%) | 0.85 |
| Discharged home | 543 (43.30%) | 616 (44.73%) | 0.48 |
| Died | 165 (13.15%) | 189 (13.72%) | 0.71 |
| Cluster 3 | N = 1148 | N = 424 | |
| Received Thrombolysis$ | 177 (15.41%) | 90 (21.22%) | 0.0081 |
| Received EVT$ | 0 | 3 (0.70%) | 0.02 |
| Received either thrombolysis and/or EVT$ | 177 (15.41%) | 93 (21.93%) | 0.003 |
| Transferred for EVT | 0 | 0 | - |
| Discharged home | 236 (20.55%) | 254 (59.90%) | < 0.0001 |
| Died | 42 (3.65%) | 21 (4.95%) | 0.30 |
Proportion of patients treated and discharge disposition for all ischemic stroke patients; adjustments are only made for cluster allocation; treated patients include both community and hospital onset stroke patients.
Excludes hospital onset stroke patients.
Includes in-hospital stroke treatments, but excludes extended treatment times.
Adjustments for cluster allocation only.
Figure 4
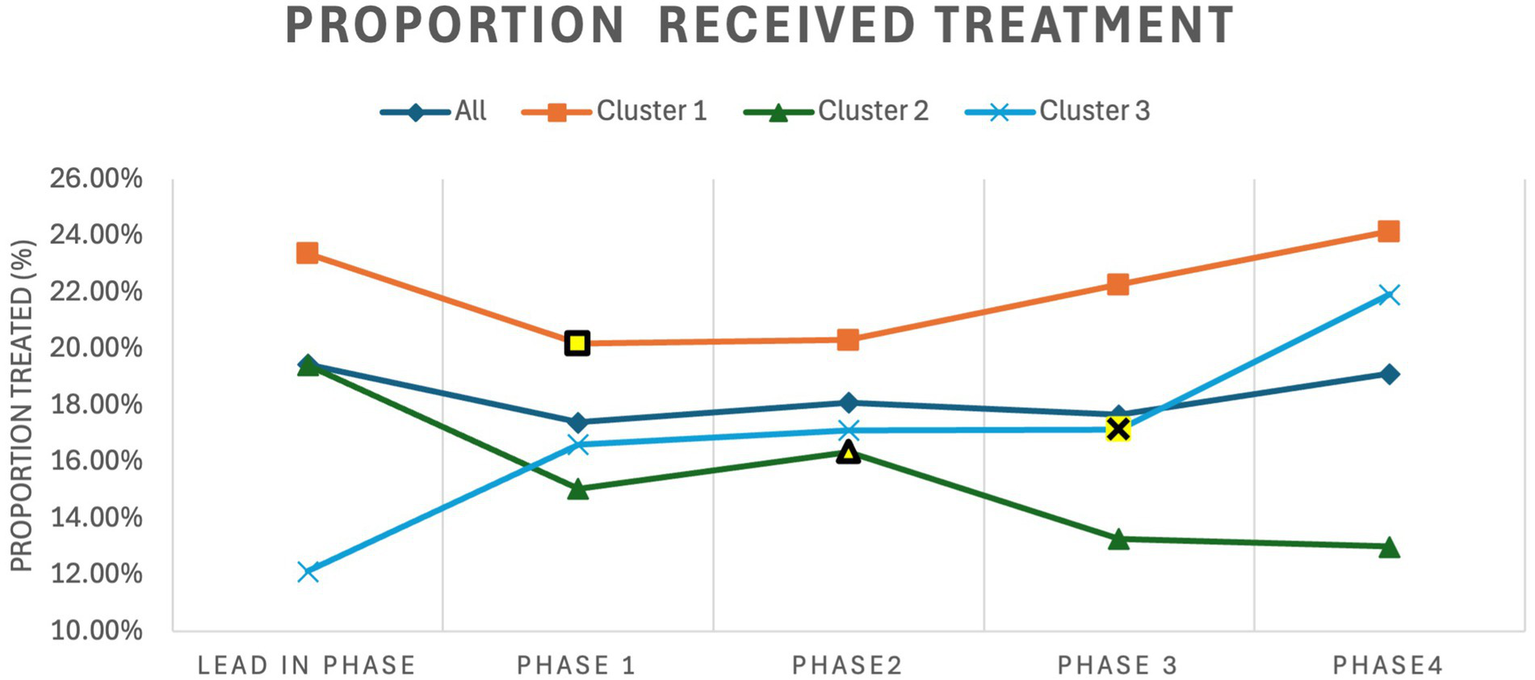
Trends for proportion of ischemic stroke patients that received treatment for the entire cohort and for each cluster by trial phase. The intervention period for each cluster is highlighted in yellow. Treated refers to ischemic stroke patients treated with thrombolysis and/or Endovascular Thrombectomy.
For the results of all acutely treated ischemic stroke patients, Table 3 shows the comparison of baseline characteristics. Overall the pre-intervention and post-intervention periods are similar except the median time from onset to 911 call was 5 min faster (p = 0.001) in the post-intervention period. These results show a high number of missing NIHSS across all clusters. In order to ensure MCAR, a sub-group analysis of only those cases with NIHSS was conducted along with the full cohort (see Supplementary Table S1), these results show significant departure from the full dataset showing that NIHSS is not MCAR; therefore, we did not include NIHSS in the adjustments.
Table 3
| All clusters | Pre-intervention period | Post-intervention period | Significance | ||
|---|---|---|---|---|---|
| N (missing) | Value | N (missing) | Value | ||
| Age mean (SD) | 528 (0) | 70.8 (13.2) | 669 (4) | 70.4 (13.6) | 0.64 |
| Sex, women (%) | 528 (0) | 236 (44.7%) | 670 (3) | 303 (45.2%) | 0.90 |
| NIHSS, median (IQR) | 230 (298) | 10 (6–14) | 242 (428) | 10 (5–15) | 0.60 |
| Onset to door, median (IQR) | 493 | 85.0 (52.0–146.0) | 629 | 85.0 (57.0–130.0) | 0.70 |
| Onset to 911, median (IQR) | 364 | 22.0 (8.7–68.0) | 434 | 17.0 (6.7–45.0) | 0.001 |
| Cluster 1 | May 2020 to Oct 2020 | May 2021 to Oct 2022 | |||
| Age, mean (SD) | 145 (0) | 73.0 (13.3) | 401 (0) | 71.01 (13.0) | 0.13 |
| Sex, women (%) | 145 (0) | 68 (46.8%) | 401 (0) | 188 (46.8%) | 1 |
| NIHSS, median (IQR) | 73 (72) | 9 (5–12) | 147 (254) | 8 (5–13) | 0.84 |
| Onset to door, median (IQR) | 144 | 84.8 (54.5–134.2) | 381 | 85.1 (60.0–127.0) | 0.94 |
| Onset to 911, median (IQR) | 99 | 14.2 (7.6–35.7) | 279 | 13.2 (6.0–35.1) | 0.40 |
| Cluster 2 | May 2020 to Apr 2021 | Nov 2021 to Oct 2022 | |||
| Age, mean (SD) | 213 (0) | 69.8 (13.7) | 179 (0) | 68.8 (14.2) | 0.48 |
| Sex, women (%) | 213 (0) | 95 (44.6%) | 179 (0) | 67 (37.4%) | 0.18 |
| NIHSS, median (IQR) | 102 (111) | 11 (7–15) | 90 (89) | 12 (7–17) | 0.62 |
| Onset to door, median (IQR) | 192 | 82.5 (52.0–142.8) | 159 | 88.0 (55.0–135.0) | 0.71 |
| Onset to 911, median (IQR) | 167 | 25.0 (7.0–92.5) | 116 | 20.0 (7.0–63.25) | 0.36 |
| Cluster 3 | May 2020 to Oct 2021 | May 2022 to Oct 2022 | |||
| Age, mean (SD) | 170 (0) | 70.5 (12.2) | 89 (4) | 70.8 (14.3) | 0.64 |
| Sex, women (%) | 170 (0) | 73 (42.94%) | 90 (3) | 48 (53.33%) | 0.14 |
| NIHSS, median (IQR) | 55 (115) | 10 (6–14) | 6 (87) | 5 (5–9.75) | 0.20 |
| Onset to door, median (IQR) | 157 | 88.0 (51.0–150.0) | 90 | 78.5 (45.0–134.0) | 0.19 |
| Onset to 911, median (IQR) | 98 | 37.5 (13.0–85.0) | 39 | 32.0 (19.0–74.0) | 0.88 |
Baseline characteristics for all community onset ischemic stroke patients that received treatment with thrombolysis and/or endovascular thrombectomy (excludes in-hospital onset stroke patients and extended treatment and transfer times).
The process and outcomes results for all community onset ischemic patients treated with thrombolysis and/or EVT are shown in Table 4. The only time measure to see a significant reduction is door-to-needle time, which went down to a median of 62.0 min (IQR: 38.7–85.9 min) from 71.2 min (IQR: 43.5–99.5 min) (p = 0.01). The results broken down by cluster is shown in the Supplementary Tables S2–S4. Cluster 1 showed no significant improvements. Cluster 2 showed a 7.5 min reduction in the median door-to-CT time (p < 0.001), a 13.2 min reduction in the median door-to-needle time (p < 0.001), and a 6.0 min reduction in 911-to-needle time (p = 0.04). Cluster 3 showed the most improvement, and their post-intervention median door-to-needle times was the lowest of all 3 clusters; they went from a median door-to-needle time of 55.6 min (IQR: 49.7–61.6 min) to a median of 49.5 min (IQR: 41.8–57.3 min) (p = 0.05). This improvement also carried through to the median 911-to-needle time, where the median time dropped by 7.6 min (p = 0.04). Cluster 3 also saw a 5.81% increase (p = 0.0081) in the proportion of ischemic stroke patients that received thrombolysis, and a 6.52% increase (p = 0.003) in the proportion of ischemic stroke patients that received thrombolysis and/or EVT.
Table 4
| Pre-intervention period | Post-intervention period | Significance | |||
|---|---|---|---|---|---|
| n | Values | n | Value | ||
| Door to CT (min), median (IQR) (unadjusted) | 464 | 17.0 (9.0–36.2) | 580 | 17.0 (10.0–28.0) | 0.80 |
| Door to CT (min), median (IQR) (adjusted) | 464 | 17.3 (15.0–19.6) | 580 | 16.8 (15.1–18.4) | 0.06 |
| Door to Needle (min), median (IQR) (unadjusted) | 416 | 73.0 (49.0–109.0) | 566 | 62.5 (40.0–93.0) | < 0.0001 |
| Door to Needle (min), median (IQR) (adjusted) | 416 | 72.0 (66.1–77.9) | 566 | 62.2(58.3–66.9) | 0.006 |
| Door-in-door-out (min), median (IQR) (unadjusted) | 55 | 148.0 (116.0–189.5) | 91 | 155.5 (127.0–188.5) | 0.62 |
| Door-in-door-out (min), median (IQR) (adjusted) | 55 | 153 (135.0–172.0) | 91 | 159.0 (148.0–169.0) | 0.59 |
| Door-to-arterial-access (min), median (IQR) (unadjusted) | 106 | 58 (11.2–101.7) | 178 | 70.0 (30.0–100.0) | 0.11 |
| Door-to-arterial-access (min), median (IQR) (adjusted) | 106 | 55.1 (41.8–68.4) | 178 | 50.4 (42.6–58.2) | 0.50 |
| 911-to-needle (min), median (IQR) (unadjusted) | 330 | 129.6 (97.0–164.6) | 413 | 125.0 (93.0–157.0) | 0.14 |
| 911-to-needle (min), median (IQR) (adjusted) | 330 | 131.0 (123.0–139.0) | 413 | 123.0 (116.0–130.0) | 0.15 |
| 911-to-arterial-access (min), median (IQR) (unadjusted) | 69 | 270.0 (147.0–356.0) | 88 | 273.0 (160.0–349.0) | 0.96 |
| 911-to-arterial-access (min), median (IQR) (adjusted) | 69 | 239.0 (190.0–288.0) | 88 | 244.0 (205.0–282.0) | 0.86 |
| Length of Stay& (days) median (IQR) (unadjusted) | 423 | 6.0 (3.0–13.0) | 520 | 6.0 (3.0–14.0) | 0.30 |
| Length of Stay& (days) median (IQR) (adjusted) | 423 | 5.6 (4.9–6.2) | 520 | 6.3 (5.7–7.0) | 0.08 |
| Discharged home, n (%)(unadjusted) | 528 | 272 (51.5%) | 673 | 282 (41.9%) | 0.001 |
| Discharged home, n (%) (adjusted) | 528 | 265 (50.2%) | 673 | 291 (43.2%) | 0.018 |
| Discharged to rehabilitation, n (%) (unadjusted) | 528 | 64 (12.1%) | 673 | 107 (15.8%) | 0.07 |
| Discharged to rehabilitation, n (%) (adjusted) | 528 | 76 (14.4%) | 673 | 92 (13.7%) | 0.68 |
| Mortality at discharge, n (%) (unadjusted) | 528 | 84 (15.9%) | 673 | 121 (17.9%) | 0.40 |
| Mortality at discharge, n (%) (adjusted) | 528 | 90 (17.0%) | 673 | 115 (17.1%) | 0.94 |
Treatment times and outcome measures for all community onset ischemic stroke patients treated with thrombolysis and/or endovascular thrombectomy.
LOS does not include patients that died in hospital.
Adjustments were made for cluster, age, and sex. IQR: interquartile range, min: minutes.
The trends for all acutely treated ischemic stroke patients are shown in Figure 5. Panel A show the trends for median door to needle time for all thrombolysed patients with cluster 2 and 3 showing a downward trend. Panel B shows the trend for median door-in-door-out times with phase 2 showing longer times across all clusters. Panel C shows the trend for median door-to-arterial-access times with cluster 1 showing a downward trend. Note that the trends for door-in-door-out and door-to-arterial-access times do not include cluster 3, as they only started their EVT service in the final phase. Panel D on Figure 4 show the trends for proportion of treated ischemic stroke patients that are discharged home. Site level improvement analysis was conducted across all three clusters, and these are shown in Supplementary Figure S1. This reveals that there was similar proportion of sites that improved across the clusters, and likely the ability of high volume sites to improve was the key modifying factor in seeing improvement across the cluster.
Figure 5
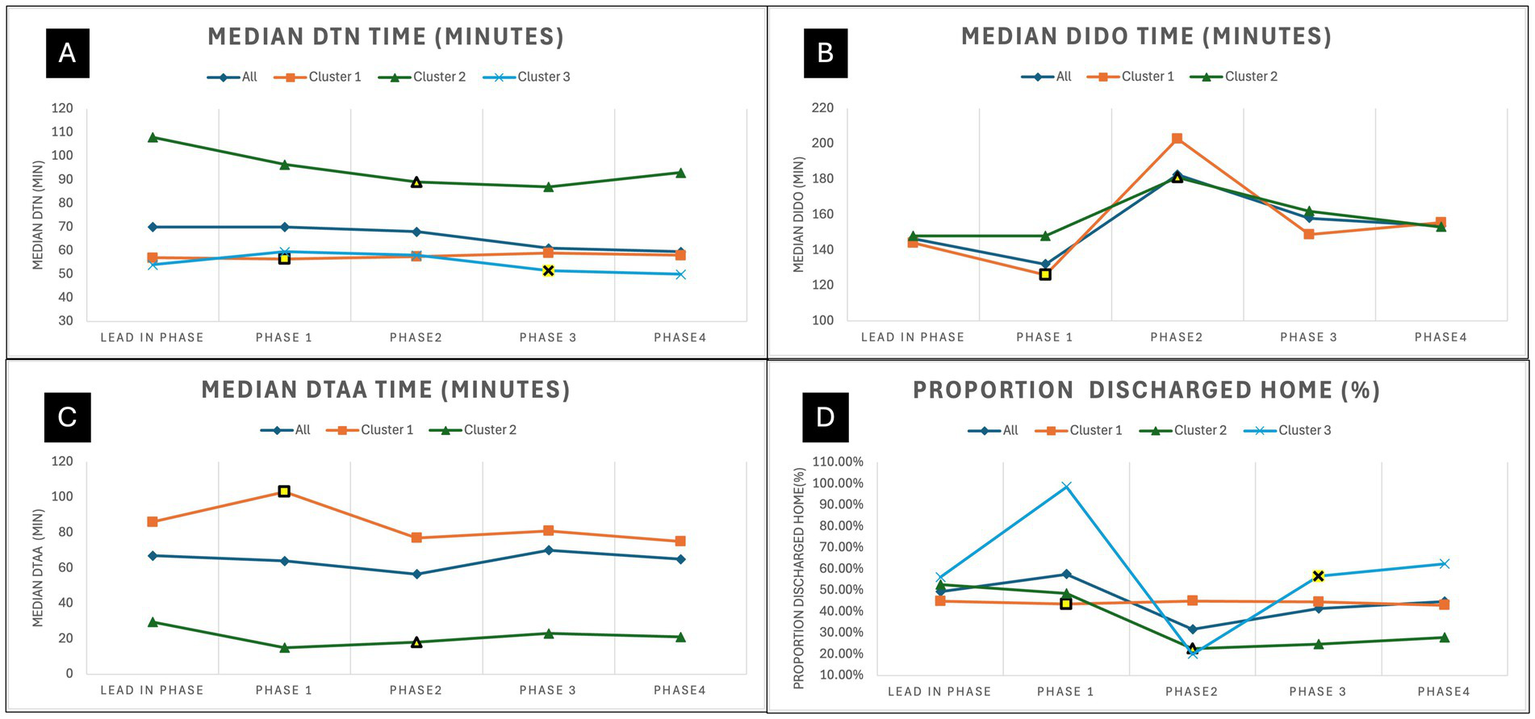
Trends for community onset ischemic stroke patients that received treatment for each phase. (A) Shows the trends for median door-to-needle time for all thrombolysed ischemic stroke patients. (B) Shows the trends for median door-in-door-out time for all patients transferred for EVT. (C) Shows the trends for median door to Arterial Access time for all patients treated with EVT. (D) Shows the trends for proportion of community onset treated ischemic stroke patients discharged home from acute care. The intervention period for each cluster is highlighted in yellow. EVT, Endovascular thrombectomy; DTN, Door-to-needle time; DTAA, Door-to-arterial-access time; DIDO, Door-in-door-out time.
Discussion
These results show that improvements were minimal and highly dependent on the cluster. Although there were improvements to the door-to-needle times, these improvements are only present in clusters 2 and 3. However, the trends reveals additional details, as Figure 4 shows that cluster 1 and 2 showed an increasing trends in the proportion of ischemic stroke patients that received treatment after the intervention, which suggests that the intervention had an positive effect in improving proportion treated for these two clusters; however, cluster 2 showed a downward trend, which resulted in no overall affect across the clusters. It is difficult to determine why cluster 2 showed this negative affect in the proportion treated, as this cluster also has the lowest proportion of ischemic stroke patients treated across the clusters.
The results for the efficiency of treatments showed the overall reduction in median door-to-needle time was 9.2 min. The trends show that cluster 2 and 3 trended downwards. However, cluster 2 is showing that the last period did trend upwards, so the improvements may not be sustained. The door-in-door-out time times showed no difference across clusters or impact of the intervention on this measure. Additionally, phase 2 show a stark increase in door-in-door-out times across the two clusters that were transferring patients, which may have been due to increased COVID-19 measures in the region at that time. Interestingly, cluster 1 showed a downward trend after the intervention for door-to-arterial-access time, which appears to have been sustained, which was not statistically significant, but a potential positive impact of the intervention.
The overall reduction in door-to-needle time of 9.2 min is much less compared to previous studies that used a similar intervention (31–33). The door-to-needle initiative in Alberta saw approximately 30-min reduction in median times (31); the California initiative resulted in approximately 20-min reduction (32); and the Chicago initiative saw a 15.5 min reduction (33). None of these studies used a stepped-wedge trial design, which may account for some of the lower impact, as their improvements may have been overstated due to secular trends and other factors. The settings in Alberta, California, and Chicago studies are much more urban than Atlantic Canada; even in the population study for Alberta Canada, where 17% of their population lives in rural areas compared to approximately 50% for Atlantic Canada (23). Additionally, the intervention period was much shorter in this study, which may suggest that more time is needed to allow sites to implement their changes especially if more patients are being seen outside of urban centers.
In addition to the intervention period being too short, the pre-intervention and post-intervention periods should be longer. The trend charts shown in Figures 4, 5, show significant variation, which is indicative of an unstable system. Having a longer comparison periods will allow us to determine if the gains observed in cluster 2 and 3 will be sustained over time. Cluster 2 had longer DTN times as their baseline, so a longer post-intervention period would have allowed us to observe if their gains are held. Further, a longer the post-intervention period for cluster 3 would have allowed us to see the number of patients being treated with EVT and trends for DTAA and DIDO times.
Furthermore, the entire trial took place during a time of COVID-19 restrictions. Hospitals had additional tasks such as conducting a COVID-19 test on all patients; furthermore, personnel were participating in projects related to mitigating spread of COVID-19, which created a significant distraction from implementing changes to improve acute stroke treatment (34). In addition, the mQIC was held entirely virtually due to the pandemic, and these results may signal that virtual Improvement Collaboratives are less efficacious, and there is a need for face-to-face workshops and site visits. Previous studies have noted that virtual quality improvement require a longer lead time (35), so a shorter implementation period combined with virtual delivery in our study may have had a compounding effect on the impact of the intervention. Moreover, the stressors due to the COVID-19 pandemic to the healthcare system varied over the trial period, which can explain some of the variability in the impact of the intervention across each cluster, as the pre- and post-periods were conducted during different pandemic phases. Atlantic Canada has some of the strictest restrictions in Canada to try to minimize the spread of the novel virus. The worst outbreaks did not occur until the second quarter of 2021, and health system staffing issues were realized in early 2022 (36). The temporal progression of the pandemic phases may have affected each cluster differently, as the timing of each cluster occurred in a different pandemic phase: cluster 1’s post-period occurred during a period of peak hospitalizations and infections; cluster 2’s post period was occurred during staffing issues, and the intervention period occurred during peak hospitalization; and cluster 3 had less effects of the pandemic during its post period.
A significant limitation of the intervention was that key process data was not available during the intervention period for data feedback to the teams. Data was collected for the purpose of this trial after the intervention period due to the significant effort to extract the data from the charts. Even the provinces with stroke registries had significant delays in the recording of the data. This resulted in improvement efforts being conducted without data feedback, which is critical for improvement. This may have been another reason why there was lower impact of the intervention from previous studies that used a similar intervention, as one of the previous studies developed a provincial registry for quality improvement efforts during their QIC (31). Additionally, there were other limitations related to data. We did not have patient level demographic (age and sex) and stroke severity for the entire ischemic stroke cohort, this led to inconsistencies in the adjustments for the entire ischemic stroke cohort (adjustments for cluster only) and the treated ischemic stroke cohort (adjustments for age, sex, and cluster), which may lead to inaccurate adjusted values. Although, cluster was a key modifier, the patient demographic and stroke severity may also have affected the results. Furthermore, due to the high number of missing NIHSS, stroke severity could not be included in the adjusted analysis, which is a key limitation, and future studies should aim to improve NIHSS data completeness for more robust adjustment.
These results show that the intervention also needs to be tailored for each health system. This study shows that cluster 3 was able to improve the proportion of patients that receive treatment and efficiency of treatment, cluster 2 was able to improve door-to-needle times, and cluster 1 was not able to make any changes. Since this stepped-wedge trial was designed such that each intervention was similar with limited customization, this may have had variable impact on each health system. This shows the need to assess each health system and tailor the implementation strategy for each health system based on their barriers (37). Furthermore, additional customized supports to each site may have been necessary, which was not done in this intervention.
The process changes were observed in the door-to-needle metric with the trends showing a positive impact for door-to-arterial-access for cluster 1. This is likely because the improvement strategies were based on improving this single metric. Additional evidence-based strategies to improve door-to-arterial-access and door-in-door-out times are needed to see improvements in these metrics. Furthermore, Improvement Collaboratives that focus on a single metric are shown to be more successful than those that focus on multiple metrics and improvements (31, 38).
To summarize, the mQIC intervention should be enhanced to observe greater improvements. The suggested enhancements are: (1) the intervention period should be a minimum of 1-year long to allow teams enough time to make the changes; (2) the workshops and site visits should be in-person rather than virtual; (3) data feedback needs to occur throughout the intervention; (4) pre-intervention readiness assessments should be completed for each cluster to tailor the intervention for each cluster; and (5) change suggestions should include ways to lower door-in-door-out times and door-to-arterial-access times.
Although the results showed significant improvements to the proportion of patients discharged home, it is unlikely that the intervention had this level of impact on this pragmatic outcome measures. Although there was an increase in the proportion of patients that received treatment in cluster 3 and improvement in thrombolysis time for clusters 2 and 3, these improvements are unlikely to have seen a 13% improvement in proportion of ischemic stroke patients discharged home. Additionally, sub-group analysis by stroke severity could not be done on proportion of ischemic stroke patients discharged home and proportion died due to lack of NIHSS data for all ischemic stroke patients, but this would reveal additional details about the effect that is being seen for these measures. Furthermore, the discharge home of the treated ischemic stroke patient cohort showed a reduction, which is counter intuitive to the aim of the study. The trends shown on Figure 5 reveal that this decrease was most apparent in cluster 2 and highly variable in cluster 3. This result is more hypothesis generating, and could be due to the effects of COVID protocols in these regions during the various phases of virus containment measures. This shows the need to have true outcomes measures such as 90-day mRS (modified Rankin Score) even for quality improvement initiatives. The use of administrative data to assess outcomes has significant limitations, which are difficult to interpret especially when there is turbulence in the system due to a global pandemic, thus investment in the collection of 90-day mRS is critical to fully interpret the impact on outcomes.
Conclusion
The intervention to improve access and efficiency of acute ischemic stroke treatment in four Atlantic Canadian provinces made a 9.2-min improvement in median door-to-needle times. A longer intervention period that is 1-year long and tailored to each health system with in-person workshops and sites visits may result in more improvements.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical approval for this study was obtained from the NS Health Research Ethics Board (REB# 1025460), Health PEI Ethics Board, Horizon Health Network Research Ethics Board (ROMEO File #: 100906 and RS#: 2020-2893), Vitalité Health Network Ethics Office (ROMEO file number: 101305), and Newfoundland and Labrador Health Research Ethics Board (Researcher Portal File #: 20210255 and Reference #: 2020.074). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NK: Visualization, Funding acquisition, Conceptualization, Project administration, Writing – review & editing, Investigation, Writing – original draft, Supervision. EC: Project administration, Methodology, Writing – review & editing, Data curation. SiA: Writing – review & editing, Formal Analysis, Visualization. JG: Conceptualization, Funding acquisition, Project administration, Investigation, Writing – review & editing, Data curation. DV: Conceptualization, Funding acquisition, Writing – review & editing. ShA: Writing – review & editing, Investigation, Funding acquisition. HW: Project administration, Writing – review & editing, Funding acquisition, Conceptualization, Investigation. PF: Project administration, Conceptualization, Writing – review & editing, Investigation, Funding acquisition. EL: Investigation, Writing – review & editing, Funding acquisition, Project administration. TH-N: Project administration, Writing – review & editing, Investigation, Funding acquisition. RC: Writing – review & editing, Supervision, Project administration, Data curation. BrM: Investigation, Writing – review & editing, Project administration, Funding acquisition. JS: Investigation, Project administration, Data curation, Writing – review & editing. WS: Data curation, Investigation, Funding acquisition, Writing – review & editing, Project administration. CC: Funding acquisition, Writing – review & editing, Data curation, Project administration, Investigation. MH: Funding acquisition, Conceptualization, Validation, Writing – review & editing, Methodology. BiM: Investigation, Writing – review & editing, Conceptualization, Funding acquisition, Methodology. SP: Writing – review & editing, Project administration, Investigation, Methodology, Conceptualization, Funding acquisition.
Group members of [The ACTEAST Collaborators]
Shahram Abootalebi, Brian Archer, Leslie Austin-Smith, Dylan Blacquiere, Ashley Boyce, Greg Browne, Alix Carter, Tania Chandler, Fraser Clift, Jamie Drapeau, Charles Duffy, Marsha Eustace, Rachel Goss, Kelly Goudey, Edgar Goulette, Gordon Gubitz, Jens Heidenreich, Milan Jurga, Nadia LeBreton, Michelle MacGrath, Carolyn MacPhail, Alier Marrero, Gregory McNally, Jennifer McVey, Matthew Murphy, Susannah Piercey, Amer Qureshi, Nicole Tupper, Scott Theriault, Lindsay Richards, Shauna Sommerville, Janel Swain.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Canadian Institutes for Health Research (CIHR) Project Grant (PJT-169124). A small grant was also received from Medtronic.
Acknowledgments
Owen Parfrey at Memorial University’s Quality of Care NL assisted with facilitating data procurement and collection across NL. Jody Rhodenizer at Horizon Health Network was key in obtaining data for all Horizon hospital in NB. We thank the research office at Vitalité Health Network for visiting hospitals in the Vitalité Health Authority in NB. Dr. Jessalyn Holodinsky guided us in understanding the best methodology for statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1610307/full#supplementary-material
References
1.
Wardlaw JM Murray V Berge E Del Zoppo GJ . Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. (2009) 4:1–142. doi: 10.1002/14651858.CD000213.pub3
2.
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–8. doi: 10.1056/NEJM199512143332401
3.
Emberson J Lees KR Lyden P Blackwell L Albers G Bluhmki E et al . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
4.
Menon BK Buck BH Singh N Deschaintre Y Almekhlafi MA Coutts SB et al . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. (2022) 400:161–9. doi: 10.1016/S0140-6736(22)01054-6
5.
Goyal M Menon BK Van Zwam WH Dippel DW Mitchell PJ Demchuk AM et al . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
6.
Goyal M Demchuk AM Menon BK Eesa M Rempel JL Thornton J et al . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
7.
Adamson J Beswick A Ebrahim S . Is stroke the most common cause of disability?J Stroke Cerebrovasc Dis. (2004) 13:171–7. doi: 10.1016/j.jstrokecerebrovasdis.2004.06.003
8.
Claesson L Lindén T Skoog I Blomstrand C . Cognitive impairment after stroke–impact on activities of daily living and costs of care for elderly people. Cerebrovasc Dis. (2005) 19:102–9. doi: 10.1159/000082787
9.
Daniel K Wolfe CD Busch MA McKevitt C . What are the social consequences of stroke for working-aged adults?: a systematic review. Stroke. (2009) 40:e431–40. doi: 10.1161/STROKEAHA.108.534487
10.
Mittmann N Seung SJ Hill MD Phillips SJ Hachinski V Coté R et al . Impact of disability status on ischemic stroke costs in Canada in the first year. CJNS. (2012) 39:739–800. doi: 10.1017/S0317167100015638
11.
Boulanger JM Lindsay MP Gubitz G Smith EE Stotts G Foley N et al . Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke. (2018) 13:949–84. doi: 10.1177/1747493018786616
12.
Rubin R . It takes an average of 17 years for evidence to change practice—the burgeoning field of implementation science seeks to speed things up. JAMA. (2023) 329:1333–6. doi: 10.1001/jama.2023.4387
13.
Mallonee S Fowler C Istre GR . Bridging the gap between research and practice: a continuing challenge. INJ Prev. (2006) 12:357–9. doi: 10.1136/ip.2006.014159
14.
Saver JL Goyal M Van der Lugt AA Menon BK Majoie CB Dippel DW et al . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. (2016) 316:1279–89. doi: 10.1001/jama.2016.13647
15.
Saver JL . Time is brain—quantified. Stroke. (2006) 37:263–6. doi: 10.1161/01.STR.0000196957.55928.ab
16.
Kamal N Benavente O Boyle K Buck B Butcher K Casaubon LK et al . Good is not good enough: the benchmark stroke door-to-needle time should be 30 minutes. CJNS. (2014) 41:694–6. doi: 10.1017/cjn.2014.41
17.
Kamal N Jeerakathil T Demchuk AM Smith EE Mann B Buck B et al . Door-in-door-out times across a Canadian province for optimal access to endovascular therapy. Eur Stroke J. (2018) 3:3–586. doi: 10.1177/2396987318770127
18.
Kamal N Demchuk AM Hill MD Yu AY Stotts G Zagorski B et al . Canadian trends in door-in-door-out times for endovascular treatment. Int J Stroke. (2017) 12:1569. doi: 10.1177/1747493017721569
19.
Institute for Healthcare Improvement . The breakthrough series: IHI’S collaborative model for achieving breakthrough improvement. In: IHI innovation series white paper. Boston: Institute for Healthcare Improvement (2003). Available online at: https://www.ihi.org/library/white-papers/breakthrough-series-ihis-collaborative-model-achieving-breakthrough
20.
Kamal N Aljendi S Carter A Cora EA Chandler T Clift F et al . Improving access and efficiency of ischemic stroke treatment across four Canadian provinces using a stepped wedge trial: methodology. Front Stroke. (2022) 1:1014480. doi: 10.3389/fstro.2022.1014480
21.
Statistic Canada . (2025). Population Estimates, quarterly. Available online at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 (Accessed May 27, 2025)
22.
Kamal N Lindsay MP Côté R Fang J Kapral MK Hill MD . Ten-year trends in stroke admissions and outcomes in Canada. Can J Neurol Sci. (2015) 42:168–75. doi: 10.1017/cjn.2015.20
23.
Statistics Canada . (2024). Canada Goes Urban. Available online at: https://www150.statcan.gc.ca/n1/pub/11-630-x/11-630-x2015004-eng.htm (Accessed May 27, 2025).
24.
Statistics Canada . (2016). Chart 3 proportion of the population aged 15 to 64 within the total population, Canada, provinces and territories. Available online at: https://www150.statcan.gc.ca/n1/daily-quotidien/170503/cg-a003-eng.htm (Accessed May 27, 2025).
25.
Kapoor A Lindsay MP Amy YX Goia C Cheskes S Verbeek PR et al . Call 911: lower ambulance utilization among young adults, especially women, with stroke. Can J Neurol Sci. (2020) 47:764–9. doi: 10.1017/cjn.2020.119
26.
Zhang S . Nearest neighbor selection for iteratively kNN imputation. J Syst Softw. (2012) 85:2541–52. doi: 10.1016/j.jss.2012.05.073
27.
Gautam R Latifi S . Comparison of simple missing data imputation techniques for numerical and categorical datasets. J Res Eng Appl Sci. (2023) 8:468–75. doi: 10.46565/jreas.202381468-475
28.
Dettori JR Norvell DC Chapman JR . The sin of missing data: is all forgiven by way of imputation?Glob Spine J. (2018) 8:892–4. doi: 10.1177/2192568218811922
29.
Albers GW Marks MP Kemp S Christensen S Tsai JP Ortega-Gutierrez S et al . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
30.
Nogueira RG Jadhav AP Haussen DC Bonafe A Budzik RF Bhuva P et al . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
31.
Kamal N Jeerakathil T Stang J Liu M Rogers E Smith EE et al . Provincial door-to-needle improvement initiative results in improved patient outcomes across an entire population. Stroke. (2020) 51:2339–46. doi: 10.1161/STROKEAHA.120.029734
32.
Nguyen-Huynh MN Klingman JG Avins AL Rao VA Eaton A Bhopale S . Novel telestroke program improves thrombolysis for acute stroke across 21 hospitals of an integrated healthcare system. Stroke. (2018) 49:133–9. doi: 10.1161/STROKEAHA.117.018413
33.
Prabhakaran S Lee J O’Neill K . Regional learning collaboratives produce rapid and sustainable improvements in stroke thrombolysis times. Circ Cardiovasc Qual Outcomes. (2016) 9:585–92. doi: 10.1161/CIRCOUTCOMES.116.003222
34.
Aljendi S Mrklas KJ Kamal N . Qualitative evaluation of a quality improvement collaborative implementation to improve acute ischemic stroke treatment in Nova Scotia, Canada. Healthcare. (2024) 12:1–25. doi: 10.3390/healthcare12181801
35.
Khurshid Z De Brún A Moore G McAuliffe E . Virtual adaptation of traditional healthcare quality improvement training in response to COVID-19: a rapid narrative review. Hum Resour Health. (2020) 18:81–8. doi: 10.1186/s12960-020-00527-2
36.
Canadian Institute for Health Information . (n.d.). COVID-19 hospitalization and emergency department statistics. Available online at: https://www.cihi.ca/en/covid-19-hospitalization-and-emergency-department-statistics (Accessed May 27, 2025).
37.
Powell BJ Beidas RS Lewis CC Aarons GA McMillen JC Proctor EK et al . Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. (2017) 44:177–94. doi: 10.1007/s11414-015-9475-6
38.
Schouten LM Hulscher ME van Everdingen JJ Huijsman R Grol RP . Evidence for the impact of quality improvement collaboratives: systematic review. BMJ. (2008) 336:1491–4. doi: 10.1136/bmj.39570.749884.BE
Summary
Keywords
ischemic stroke, thrombolysis (for acute ischaemic stroke), endovascular thrombectomy, door-to-needle (DTN) time, door-in-door-out time, door to arterial access time, stepped-wedge cluster design, quality improvement collaborative
Citation
Kamal N, Cora EA, Alim S, Goldstein J, Volders D, Aljendi S, Williams H, Fok PT, van der Linde E, Helm-Neima T, Cashin R, Metcalfe B, Savoie J, Simpkin W, Chisholm C, Hill MD, Menon BK and Phillips S (2025) Improving access and efficiency of acute ischemic stroke treatment across four Canadian provinces: a stepped-wedge trial. Front. Neurol. 16:1610307. doi: 10.3389/fneur.2025.1610307
Received
11 April 2025
Accepted
23 July 2025
Published
18 August 2025
Volume
16 - 2025
Edited by
Cheng-Yang Hsieh, Tainan Sin Lau Hospital, Taiwan
Reviewed by
Pi-Shan Sung, National Cheng Kung University, Taiwan
Tsang-Shan Chen, Sin-Lau Christian Hospital, Taiwan
Updates
Copyright
© 2025 Kamal, Cora, Alim, Goldstein, Volders, Aljendi, Williams, Fok, van der Linde, Helm-Neima, Cashin, Metcalfe, Savoie, Simpkin, Chisholm, Hill, Menon and Phillips.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noreen Kamal, Noreen.Kamal@dal.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.