Abstract
Background:
Episodic migraine is a prevalent and disabling neurological disorder with a significant impact on quality of life and productivity. Preventive treatment aims to reduce the frequency, intensity, and disability associated with migraine attacks. However, the comparative efficacy and safety of available preventive strategies remain insufficiently addressed in the literature, especially in low- and middle-income countries.
Objective:
To evaluate the efficacy and safety of pharmacological and non-pharmacological preventive treatments for episodic migraine through a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods:
Following PRISMA guidelines, a comprehensive literature search was conducted across Wiley Online, BVS, MEDLINE, and OVID databases through November 2024. Eligible studies were RCTs comparing preventive treatments with placebo or active comparators in adults with episodic migraine. This review was not registered in PROSPERO due to institutional constraints at the time of project initiation. Primary outcomes included changes in monthly migraine days (MMD), monthly headache days (MHD), acute medication days (AMD), adverse events (AE) and serious adverse events (SAE). Meta-analysis was performed using fixed- or random-effects models depending on heterogeneity.
Results:
Thirty-nine RCTs involving over 15,000 patients were included. Anti-CGRP monoclonal antibodies and gepants demonstrated the most consistent reduction in MMD (−3.2 to −4.4 days) with favorable tolerability. Traditional agents such as topiramate and propranolol showed modest efficacy with higher AE rates. Combination therapies offered superior MMD reductions (up to −5.1 days) but were associated with increased side effects. Non-pharmacological interventions (e.g., neuromodulation, acupuncture) showed promising results but lacked standardization. Meta-analysis of allopathic treatments revealed a significant MMD reduction vs. placebo (−1.25 days; 95% CI − 1.47 to −1.04; p < 0.001).
Conclusion:
CGRP-targeted therapies and gepants are effective first-line options for episodic migraine prevention. Combinations may enhance efficacy but at the cost of tolerability. Non-pharmacological treatments represent useful adjuncts. These findings support individualized, multimodal preventive strategies, particularly in resource-limited settings. However, interpretation should consider potential publication and language bias, as well as the short follow-up duration in many included trials.
1 Introduction
Episodic migraine is a highly prevalent and disabling neurological disorder, particularly among women, affecting millions worldwide (1). It imposes a considerable socioeconomic burden, including lost workdays, decreased productivity, increased healthcare utilization with indirect costs related to caregiving, and diminished quality of life (2, 3). From a clinical standpoint, episodic migraine is characterized by recurrent attacks lasting 4–72 h and occurring on fewer than 15 days per month. These attacks are often unpredictable in onset and severity, resulting in significant physical and emotional distress (2). Effective management requires both acute treatments for symptom relief and preventive strategies aimed at reducing attack frequency and severity over time (4).
Preventive strategies, which range from pharmacological agents like antiseizure medications, beta-blockers, calcitonin gene-related peptide (CGRP) inhibitors, and (anti-CGRP) gepants to non-pharmacological approaches such as cognitive-behavioral therapy and neuromodulation, focus on decreasing the overall burden of the condition (5, 6, 7). Despite significant advances in treatment options, there remains considerable variability in individual responses, highlighting the need for personalized treatment regimens (8).
Although many studies have assessed individual acute and preventive treatments for episodic migraine (4), comprehensive analyses comparing efficacy and safety across multiple pharmacological and non-pharmacological modalities are still lacking, especially in underserved populations (9). Furthermore, global treatment guidelines remain fragmented and inconsistent regarding the integration of emerging therapies, particularly non-pharmacological approaches. This highlights the need for updated evidence-based recommendations applicable across both high- and low-resource healthcare systems. The present study aims to systematically evaluate the efficacy and safety of preventive treatments for episodic migraine in adults, using randomized controlled trials (RCTs) comparing pharmacological or non-pharmacological interventions with placebo or active comparators. A pairwise meta-analysis was performed following PRISMA guidelines, focusing on key outcomes such as monthly migraine days (MMD), monthly headache days (MHD), acute medications days (AMD), and adverse events (AE). Through this work, we aim to inform clinical decision-making and support more equitable guideline development, with a particular focus on relevance for developing countries such as Mexico.
2 Materials and methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This systematic review and meta-analysis was not registered in PROSPERO due to institutional limitations at the time of project initiation. It was part of a broader systematic analysis of preventive treatments for episodic migraine available in Mexico. The study protocol was developed with input from clinical and research experts in headache management. A panel of six neurologists specializing in preventive strategies was assembled to guide protocol development.
Numerous systematic reviews have evaluated the efficacy of various pharmacological treatments, both specific (gepants or monoclonal antibodies) and non-specific (beta-blockers, anti-seizure medications, antidepressants, and others), either individually or in combination, as well as device-based therapies for the prevention of episodic migraine. A systematic review of RCTs was conducted to synthesize the existing evidence on pharmacological and non-pharmacological interventions for episodic migraine.
2.1 Data sources and searches
A systematic search was conducted across Wiley Online, BVS, MEDLINE, and OVID from their inception until November 2, 2024. Additional searches included clinical trial registries, government databases and websites, conference proceedings, patient advocacy group websites, systematic reviews/meta-analyses, and medical society websites, though these were ultimately excluded. The technical expert panel assisted in identifying relevant literature. A medical reference librarian designed and executed the search strategy, which was peer-reviewed by a second librarian and validated by coauthors MK, V-J, and I R-L.
2.2 Study selection
Eligible studies (1) included adult patients (≥18 years) with episodic migraine; (2) evaluated preventive pharmacologic and non-pharmacological treatments; (3) involved randomized clinical trials (RCTs) (phase II/phase III) comparisons of the intervention with placebo, usual care, another pharmacologic therapy, or no treatment (4) reported outcome of interest as reduction of monthly migraine days (MMD), monthly headache days (MHD) and acute medications days (AMD), (5) adverse events (AE) and serious adverse events (SAE). We excluded in vitro, phase I clinical trials, nonrandomized, open-labeled trials, studies without original data, and single-group studies. Therapies in development or intravenous administration terminated development or unavailable in the global market were excluded. Additionally, studies on patients diagnosed with tension headaches or other headache disorders and treated with NSAIDs, triptans, or ergot alkaloids therapies were excluded. Case reports, case series, reviews, post-hoc analyses, or multiple reports of the same study were excluded.
The original study definitions were retained despite evolving migraine criteria, provided they aligned with the current International Classification of Headache Disorders, Third Edition (ICHD-3) standards for episodic migraine (10), characterized by headaches occurring on ≤14 days per month in individuals with migraine. Studies were restricted to those published in English or Spanish.
2.3 Data extraction
An extraction form was developed to standardize data collection. Two reviewers independently extracted study characteristics. Inter-rater agreement was assessed using Cohen’s kappa (κ = 0.82), indicating substantial agreement. Discrepancies were resolved through consensus discussion. A third reviewer (K.V.) was consulted when necessary. Authors were contacted for clarifications when data were missing or unclear. The extracted data included the generic name of the drug or device, author, year, study design, sample size, intervention details, administration route, dose, frequency, adverse effects, efficacy and safety outcomes, time frame, and availability in Mexico. Treatments were categorized as monotherapy or combination regimens in migraine prevention.
2.4 Search strategy
A comprehensive literature search used detailed search terms and Boolean operators to identify relevant studies. The search focused on RCTs that were double-blind AND placebo-controlled interventions. The following pharmacological and non–pharmacological treatment options were included: Erenumab OR CGRP antagonist OR fremanezumab OR galcanezumab OR Eptinezumab OR gepants OR rimegepant OR atogepant OR topiramate OR propranolol OR beta-blocker OR venlafaxine OR valproate OR oxcarbazepine OR candesartan OR amitriptyline OR antiepileptics, OR antidepressants, OR melatonin OR lanepitant OR aspirin OR NSAIDs OR memantine OR neuromodulation OR nerve blockers OR vestibular treatments OR acupuncture. Additionally, the search incorporated studies involving herbal supplements, oils, and combinations using Rayyan© Software, Cambridge, MA, USA.
2.5 Outcome measures
The primary efficacy outcome included reducing MMD in the active study group compared with the placebo. The secondary efficacy outcomes were MHD and reduction of AMD, which include specific and non-specific substances. When data on reduction in days or standard deviations were not directly reported in the articles, they were estimated based on comparisons between baseline and final values, reported percentage changes, or visual inspection of figures. Standard deviations were calculated from reported standard errors or visually estimated when necessary. The primary safety outcome included the presence and frequency or percentage of adverse effects and SAE; type, and severity of adverse effects using the Common Terminology Criteria for grading from Grade 1 (mild) to Grade 5 (death), and availability in Mexico.
2.6 Risk of bias assessment
The risk of bias was evaluated using the Cochrane Risk of Bias Tool for Randomized Trials (RoB 2, v2) (11). This assessment covered five key domains: (1) bias in randomization procedures, (2) bias from deviations in intended interventions, (3) bias due to incomplete outcome data, (4) bias in outcome measurement, and (5) bias in selective reporting. Each domain was rated as “low risk,” “some concerns,” or “high risk,” with an overall bias judgment assigned per study. For this analysis, MMD were the primary outcome to determine bias. Two independent reviewers (D.S. and M.A.M.M.) conducted the assessments.
2.7 Statistical analysis
Data analysis was performed with SPSS software (v.31; IBM Corp., Armonk, NY, USA). Heterogeneity across studies was evaluated using the Chi-square test and quantified via the I2 statistic. A fixed-effect model was applied for analyses with low heterogeneity (I2 < 50%), while a random-effects model was used when I2 ≥ 50%. No subgroup or sensitivity analyses were pre-specified due to the limited number of homogeneous studies available. Funnel plots were generated to assess publication bias for the primary efficacy comparison (allopathic pharmacological treatments vs. placebo for MMD). Egger’s test was applied and showed no evidence of publication bias (p = 0.27). Continuous outcomes were expressed as mean differences (MD) with 95% confidence intervals (CIs). Statistical significance was defined as p < 0.05. The meta-analyses conducted in this review were limited to efficacy outcomes, specifically MMD, due to heterogeneity in the reporting and classification of AE and SAE across studies.
3 Results
3.1 Characteristics of the included studies
Our search until November 30, 2024, identified 605 scientific papers through database and trial registry screening; after removing duplicates or illegible by automation tools, 202 records remained. Figure 1 shows the flowchart of the clinical studies screened and excluded, and finally, 39 RCTs were included and analyzed. All studies were published between 1987 and 2024. All studies were randomized double-blinded clinical trials classified in pharmacological treatments and non-pharmacological interventions.
Figure 1

Flowchart of the randomized clinical trials analyzed.
3.2 Study designs and frequencies
The included studies predominantly employed RCT designs, with the majority (27) utilizing double-blind, placebo-controlled methodologies. Additionally, 12 studies implemented double-blind RCTs with active comparators, directly comparing the efficacy and safety of different pharmacological treatments.
3.3 Sample size distribution
The included studies demonstrated considerable variability in sample sizes ranging from 28 to 1,001 participants, with a distinct trend toward larger trials (>300 participants) evaluating newer therapeutic classes such as anti-CGRP monoclonal antibodies (e.g., erenumab, fremanezumab) and gepants (e.g., rimegepant, atogepant). In contrast, smaller-scale trials were more frequently observed for established drug classes, including anti-seizure medications (e.g., topiramate, valproate) and beta-blockers (e.g., propranolol), as well as non-pharmacologic approaches such as acupuncture.
3.4 Study settings
All interventions were administered in outpatient or clinical trial environments, with routes including oral, subcutaneous (SC), intravenous (IV), transcutaneous, and topical.
3.5 Efficacy and safety of pharmacologic therapies
Table 1 summarizes the study design, sample size, interventions, clinical outcomes, adverse events profile, and availability in Mexico. This section presents a structured narrative synthesis of the efficacy and safety findings of each pharmacologic class used for episodic migraine prevention.
Table 1
| Episodic migraine preventive treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Generic drug name, formulation | Author, year, and ref* | Study design and sample size | Main intervention (route, dose & frequency) | Outcome | |||||
| MMD (monthly migraine days) | MHD (monthly headache days) | AMD (acute medication days) | AE (adverse events) | SAEs (serious adverse events) | Available in Mexico (yes/no) | ||||
| CGRP antagonist | |||||||||
| Erenumab, SC | Goadsby, P. J. et al. (2017) (12) | RCT double-blind, placebo-controlled, 955 participants 317 erenumab 70 mg 319 erenumab 140 mg 319 placebo |
Erenumab, SC 70 mg 140 mg Placebo SC |
At week 12 −70 mg: −3.2 (SD ± 3.5) −140 mg: −3.7 (SD ± 3.5) Placebo: −1.8 (SD ± 3.5) |
No reported | At week 12 −70 mg: −1.1 (SD ± 1.7) −140 mg: −1.6 (SD ± 1.7) Placebo: −0.2 (SD ± 1.7) |
Erenumab: −70 mg: 57% −140 mg: 55% -upper respiratory tract infection -nasopharyngitis -sinusitis Placebo: 63% -upper respiratory tract infection -nasopharyngitis -sinusitis |
Erenumab: −70 mg: 2.5% −140 mg: 1.9% -upper respiratory tract infection -nasopharyngitis -sinusitis Placebo: 2.2% -upper respiratory tract infection -nasopharyngitis -sinusitis |
Yes |
| Fremanezumab, SC | Dodick et al., 2018 (13) | RCT double-blind, placebo-controlled, 875 participants 290 fremanezumab monthly 291 fremanezumab single dose 294 placebo |
SC 225 mg monthly 675 mg single dose |
At week 12 -Monthly: −3.7 (SD ± 4.0) -Single dose: −3.4 (SD ± 4.0) Placebo: −2.2 (SD ± 4.0) |
No reported | At week 12 -Monthly: −3.0 (SD ± 2.5) -Single dose: −2.9 (SD ± 2.5) Placebo: −1.6 (SD ± 2.5) |
Fremanezumab: -Monthly: 47% -Single dose: 44% Placebo: 40% -Injections site reactions |
Fremanezumab: 1.3% -Depression -Anxiety Placebo: 2% -Injections site erythema -Injection site induration |
Yes |
| Galcanezumab, SC | Skljarevski et al., 2018 (14) | RCT double-blind, placebo-controlled, 915 participants |
SC 120 mg 240 mg monthly |
At 6 months −120 mg: −4.3 (SD ± 4.5) −240 mg: −4.2 (SD ± 4.4) placebo: −2.3 (SD ± 4.2) |
No reported | At 6 months −120 mg: −3.7 (SD ± 3.0) −240 mg: −3.6 (SD ± 2.9) placebo: −1.9 (SD ± 4.2) |
Galcanezumab: 58% -Injections site reactions Placebo: 56% -Injections site reactions |
Galcanezumab: −120 mg: 2.2% −240 mg: 4.0% -Injection site reactions -pruritic rash -bronchiectasis Placebo: 1.7% -Injection site reactions |
Yes |
| Eptinezumab, IV | Smith et al., 2020 (15) | RCT double-blind, placebo-controlled, 888 participants 223 eptinezumab 30 mg 221 eptinezumab 100 mg 222 eptinezumab 300 mg 222 placebo |
IV 30 mg 100 mg 300 mg every 12 weeks |
At week 12 Eptinezumab 30 mg: −4.0 (SD ± 8.0) Eptinezumab 100 mg: −3.9 (SD ± 8.0) Eptinezumab 300 mg: −4.3 (SD ± 8.0) Placebo: −3.2 (SD ± 8.0) |
No reported | No reported | Eptinezumab 100 mg: 21.2% Eptinezumab 300 mg: 18.5% -upper respiratory tract infections -sinusitis -fatigue -Nausea Placebo: 17.3% -upper respiratory tract infections -sinusitis -fatigue -Nausea |
No reported | Yes |
| Gepants | |||||||||
| Rimegepant, oral | Croop et al., 2021 (16) | RCT double-blind, placebo-controlled, 747 participants 373 rimegepant 374 placebo |
Oral 75 mg once daily |
At week 12 Rimegepant: −4.3 (SD ± 4.6) Placebo: −3.5 (SD ± 4.6) |
No reported | At week 12 Rimegepant: −3.7 (SD ± 5.2) Placebo: −4.0 (SD ± 5.2) |
Rimegepant: 35.6% -Nausea -fatigue -upper respiratory tract infections Placebo: 34.9% -Nausea -fatigue -upper respiratory tract infections |
Rimegepant: 1.2% -AMI -CVD -allergic reaction Placebo: 1.5% -AMI -CVD -allergic reaction |
Yes |
| Atogepant, oral | Goadsby et al., 2020 (17) | RCT double blind placebo controlled 825 participants 93 atogepant 10 mg 183 atogepant 30 mg 186 atogepant 60 mg 86 atogepant 30 mg twice 91 atogepant 60 mg twice 186 placebo |
Oral 10 mg 30 mg 60 mg once daily |
At week 12 Atogepant: 10 mg: −4.0 (SD ± 2.8) 30 mg: −3.8 (SD ± 2.7) 60 mg: −3.6 (SD ± 2.6) 30 mg (twice): −4.2 (SD ± 3.5) 60 mg (twice): −4.1 (SD ± 3.5) Placebo: −2.9 (SD ± 2.6) |
At week 12 Atogepant: 10 mg: −4.3 (SD ± 3.8) 30 mg: −4.2 (SD ± 4.0) 60 mg: −3.9 (SD ± 3.9) 30 mg (twice): −4.2 (SD ± 3.5) 60 mg (twice): −4.3 (SD ± 3.5) Placebo: −2.9 (SD ± 4.00) |
At week 12 Atogepant: 10 mg: −3.7 (SD ± 2.8) 30 mg: −3.9 (SD ± 2.7) 60 mg: −3.5 (SD ± 2.6) 30 mg (twice): −3.8 (SD ± 2.6) 60 mg (twice): −3.6 (SD ± 2.6) Placebo: −2.4 (SD ± 2.6) |
Atogepant: 26% -nausea, -fatigue - Constipation Placebo: 16% -nausea, -fatigue - Constipation |
No reported | Yes |
| Atogepant, oral | Ailani et al., 2021 (18) | RCT double-blind, placebo-controlled, 873 participants 214 atogepant 10 mg 223 atogepant 30 mg 222 atogepant 60 mg 214 placebo |
Oral 10 mg 30 mg 60 mg once daily |
At week 12 Atogepant: 10 mg: −3.7 (SD ± 2.9) 30 mg: −3.9 (SD ± 2.9) 60 mg: −4.2 (SD ± 2.9) Placebo: −2.5 (SD ± 2.9) |
At week 12 Atogepant: 10 mg: −3.9 (SD ± 2.9) 30 mg: −4.0 (SD ± 2.9) 60 mg: −4.2 (SD ± 2.9) Placebo: −2.5 (SD ± 2.9) |
At week 12 Atogepant: 10 mg: −3.7 (SD ± 2.9) 30 mg: −3.7 (SD ± 2.9) 60 mg: −3.9 (SD ± 2.9) Placebo: −2.4 (SD ± 2.9) |
Atogepant: 10 mg: 63.8% 30 mg: 61.5% 60 mg: 62.3% -Nausea -Constipation Placebo: 54.6% -Nausea -Constipation |
No reported | Yes |
| Atogepant, oral | Tassorelli et al., 2024 (19) | RCT double-blind, placebo-controlled, 309 participants 154 atogepant 155 placebo |
Oral 60 mg once daily |
At week 12 Atogepant: −4.2 (SD ± 4.9) Placebo: −1.9 (SD ± 4.9) |
No reported | No reported | Atogepant: 10% -Constipation -Fatigue -Nausea Placebo: 3% -Constipation -Fatigue -Nausea |
Atogepant: 2% -Constipation Placebo: 1% -Nausea |
Yes |
| Atogepant, oral | Schwedt et al., 2022 (20) | RCT double-blind, placebo-controlled, 873 participants 214 atogepant 10 mg 223 atogepant 30 mg 222 atogepant 60 mg 214 placebo |
Oral 10 mg 30 mg 60 mg once daily |
At week 12 Atogepant: 10 mg: −4.2 (SD ± 2.9) 30 mg: −4.3 (SD ± 3.0) 60 mg: −4.4 (SD ± 2.9) Placebo: −3.0 (SD ± 2.9) |
At week 12 Atogepant: 10 mg: −4.2 (SD ± 2.9) 30 mg: −4.2 (SD ± 3.0) 60 mg: −4.4 (SD ± 3.0) Placebo: −3.0 (SD ± 1.9) |
At week 12 Atogepant: 10 mg: −3.3 (SD ± 2.9) 30 mg: −3.4 (SD ± 3.0) 60 mg: −3.7 (SD ± 3.0) Placebo: −1.7 (SD ± 2.9) |
Atogepant: 53.7% -Constipation -Fatigue -Nausea Placebo: 56.8% -Constipation -Fatigue -Nausea |
Atogepant: 4.1% -Nausea -Fatigue Placebo: 2.7% -Fatigue |
Yes |
| Anti-seizure medications | |||||||||
| Topiramate vs. propranolol, oral | Ashtari, Shaygannejad and Akbari, 2008 (21) | RCT double-blind, 60 participants 30 Topiramate 30 Propranolol |
Oral topiramate: 25–50 mg daily propranolol: 40–80 mg daily |
At week 8 Topiramate: −4.2 (SD ± 1.2) Propranolol: −3.6 (SD ± 0.9) |
No reported | No reported | Topiramate: 60% -Paresthesia, -weight loss -somnolence -dizziness Propranolol: 50% -bradycardia, −hypotension -dizziness. |
No reported | Yes |
| Valproate extended-release (Divalproex), oral vs. placebo | Freitag et al., 2002 (22) | RCT double-blind, placebo-controlled, 237 participants |
Oral Valproate extended release 500–1,000 mg daily |
At week 4 Valproate extended release: −1.7 (SD ± 0.4) Placebo: −0.7 (SD ± 0.4) |
At week 4 Valproate extended release: −1.2 (SD ± 0.2) Placebo: −0.6 (SD ± 0.2) |
No reported | Valproate extended release: 68% -Infection -Nausea -Asthenia -Flu -Dyspepsia -Diarrhea Placebo: 45% -Nausea -Diarrhea -Flu |
Valproate extended release: 2% -Nausea Placebo: 1% -Nausea |
Yes |
| Oxcarbazepine, oral | Silberstein et al., 2008 (23) | RCT double-blind, placebo-controlled, 170 participants 85 oxcarbazepine 85 placebo |
Oral Oxcarbazepine 300–1,200 mg daily |
At week 12 Oxcarbazepine: −1.3 (SD ± 2.6) Placebo: −1.7 days (SD ± 2.6) |
No reported | At week 12 Oxcarbazepine: −1.2 (SD ± 3.7) Placebo: −2.1 (SD ± 3.7) |
Oxcarbazepine: 80% -Fatigue -Dizziness - Nause. Placebo: 65% -Fatigue -Dizziness -Somnolence |
Oxcarbazepine: 10.6% -Acute vestibulopathy Placebo: 4.7% days -Depression |
Yes |
| Topiramate, oral | Storey et al., 2001 (24) | RCT double-blind, placebo-controlled, 40 participants 19 Topiramate 21 Placebo |
Oral 25–200 mg daily |
At week 20 Topiramate: −1.8 (SD ± 2.2) Placebo: −0.5 (SD ± 2.8) |
No reported | No reported | Topiramate: 72% -paresthesia -weight loss -memory impairment -emotional lability -abnormal vision Placebo: 40% -Drowsiness -Nausea -Gastrointestinal intolerance |
Topiramate: 15% -Nausea -Emotional lability Placebo: No reported |
Yes |
| Beta-blockers | |||||||||
| Propranolol long-acting, oral | Pradalier et al., 1989 (25) | RCT double-blind, placebo-controlled, 74 participants 40 Propranolol 34 Placebo |
Oral 160 mg daily |
At week 12 160 mg: −2.9 (SD ± 1.2) Placebo: −0.4 (SD ± 2.1) |
No reported | No reported | Propranolol: 80 mg: 50% 160 mg: 60% -Tiredness -Dizziness placebo: 40% -Tiredness -Dizziness |
No reported | Yes |
| Propranolol vs. N-alpha methyl histamine, oral | Millán-Guerrero et al., 2014 (26) | RCT is controlled with another active arm, 60 participants 30 Propranolol 30 placebo |
Oral propranolol: 80 mg n-alpha methyl histamine: 10 mg |
At week 12 N-alpha Methyl Histamine: −2.0 (SD ± 0.2) Propranolol: −4.2 (SD ± 0.1) |
No reported | No reported | N-alpha Methyl Histamine: 45% -Reactions in the injection site Propranolol: 55% -Reactions in the injection site |
No reported | Yes |
| Metoprolol vs. placebo | Steiner et al., 1987 (27) | RCT double-blind placebo-controlled 59 participants 28 Metoprolol 31 placebo |
Oral Metoprolol 50–100 mg BID |
At week 4 Metoprolol: −1.2 (SD ± 0.6) Placebo: −0.4 (SD ± 0.2) |
No reported | No reported | Metoprolol: 17.9% -Nightmares -Weight increase -Disenea Placebo: 12.9% -Drowsiness -Vertigo -Pruritis |
Metoprolol: 3.5% -Heartburn Placebo: No reported |
Yes |
| Metoprolol vs. placebo | Kangasniemi et al., 1987 (28) | RCT double-blind placebo-controlled cross-over | Oral Metoprolol slow-release 200 mg daily |
At week 8 Metoprolol: −2.0 Placebo: −1.3 |
No reported | No reported | Metoprolol: 36% -fatigue -gastrointestinal disturbances -sleep disturbances Placebo: 18% -fatigue -gastrointestinal disturbances |
No reported | Yes |
| Metoprolol vs. propranolol | Olsson et al., 2009 (29) | RCT double-blind cross-over study 56 participants |
Oral Metoprolol 50 mg BID Propranolol 40 mg BID daily |
At week 8 Metoprolol: −1.2 (SD ± 1,4) Placebo: −1.2 (SD ± 1.4) |
No reported | No reported | Metoprolol: 36% -fatigue -gastrointestinal disturbances -sleep disturbances Propranolol: 18% -fatigue -gastrointestinal disturbances -sleep disturbances |
No reported | Yes |
| Metoprolol vs. nevibolol | Schellenberg et al., 2007 (30) | RCT double-blind 30 participants 14 metoprolol 16 nebivolol |
Oral Metoprolol 47.5–95 mg Nevibolol 5 mg daily |
At week 14 Metoprolol: −2.1 (SD ± 1.4) Nevibolol: −1.7 (SD ± 2.1) |
No reported | No reported | Metoprolol: 93% -fatigue -bradycardia Nevibolol: 69% -fatigue -bradicardia |
Metoprolol: 7.1% -migraine deterioration Nevibolol:6.2% -sleep disturbances |
Yes |
| Angiotensin receptor blocker | |||||||||
| Candesartan vs. propranolol slow release vs. placebo | Stovner et al., 2013 (31) | RCT triple-blind placebo-controlled, double cross-over study 72 participants 59 candesartan 61 propranolol 61 placebo |
Oral Candesartan: 60 mg Propranolol: 160 mg daily |
At week 12 Candesartan: −1.8 (SD ± 4.1) Propranolol slow release: −1.9 (SD ± 4.2) Placebo: −1.2 (SD ± 4.2) |
At week 12 Candesartan: −2.9 (SD ± 4.1) Propranolol slow release: −1.9 (SD ± 4.2) Placebo: −2.2 (SD ± 4.2) |
No reported | Candesartan: 50% -Respiratory tract infections -Dizziness -Bodily pain -Sleep problems Propranolol slow release: 58% -Respiratory tract infections -Bodily pain -Dizziness -Sleep problems Placebo: 33% -Respiratory tract infections -Bodily pain -Sleep problems -Diarrhoea |
No reported | Yes |
| Antidepressants | |||||||||
| Amitriptyline vs. valproate, oral | Kalita, Bhoi and Misra, 2013 (32) | RCT double-blind 300 participants 150 amitriptyline 150 valproate |
Oral amitriptyline: 20 mg twice a day valproate: 500 mg daily |
At week 12 amitriptyline: −7.8 (SD ± 0.5) Divalproate: −6.9 (SD ± 2.7) |
No reported | No reported | Amitriptyline: 22% -Drowsiness -Dry mouth Divalproate: 18% -Weight gain -Nausea |
Amitriptyline: 1% -Severe sedation Divalproate: 3% -Liver enzyme elevation |
Yes |
| Amitriptyline ER, oral | Lampl et al., 2009 (33) | RCT double-blind 132 participants 66 low dose 66 high dose |
Oral amitriptyline: 25 mg daily amitriptyline: 50 mg daily |
At week 12 Amitriptyline low dose: −1.0 (SD ± 2.0) Amitriptyline high dose: −1.0 (SD ± 2.0) |
No reported | No reported | Amitriptyline low dose: 12% -Mild sedation Amitriptyline high dose: 18% -Mild sedation -Dry mouth -Weight gain |
Amitriptyline low doses: No reported Amitriptyline high dose: 1% -Sedation |
No |
| Venlafaxine vs. placebo | Ozyalcin et al., 2005 (34) | RCT double-blind placebo-controlled 60 participants 21 venlafaxine 150 mg 20 venlafaxine 75 mg 19 placebo |
Oral venlafaxine 75 mg venlafaxine 150 mg daily |
At week 8 venlafaxine 75 mg: −1.8 (SD ± 1.3) venlafaxine 150 mg: −2.0 (SD ± 1.5) placebo: −0.9 (SD ± 1.9) |
No reported | No reported | venlafaxine 75 mg:100% -Nausea -somnolence -fatigue venlafaxine 150 mg: 95.2% -Nausea -somnolence -fatigue placebo: 55.6% -Nausea -fatigue -dizziness |
No reported | Yes |
| Venlafaxine vs. escitalopram | Tarlaci et al., 2009 (35) | RCT double-blind 93 participants 35 venlafaxine 58 escitalopram |
Oral venlafaxine 72.8 mg escitalopram 12.4 mg daily |
At week 12 venlafaxine: −3.8 (SD not available) escitalopram: −2.6 (SD not available) |
No reported | No reported | Venlafaxine: 28.6% -Nausea -somnolence -dizziness Escitalopram: 0% |
Venlafaxine: 34.3% -Nausea -somnolence -dizziness |
Yes |
| Venlafaxine vs. amitriptyline | Bulut et al., 2004 (36) | RCT double-blind cross-over 52 participants 26 venlafaxine 26 amitriptyline |
Oral venlafaxine amitriptyline |
At week 12 venlafaxine: −2.3 (SD ± 1.8) amitriptyline: −1.7 (SD ± 1.9) |
No reported | No reported | Venlafaxine: 23% -Nausea -tachycardia -others Amitriptyline: 80% -hypersomnia -dry mouth |
Venlafaxine: 3.2% -nausea -taquicardia -others Amitriptyline: 19.2% -hypersomnia -orthostatic hypotension |
Yes |
| Hormonal therapy | |||||||||
| Melatonin, oral | Alstadhaug et al., 2010 (37) | RCT double-blind cross over placebo-controlled, 48 participants |
Oral 2 mg daily |
At week 8 Melatonin: −2.8 (SD ± 1.6) Placebo: −2.9 (SD ± 1.4) |
No reported | No reported | Melatonin: 2.8% -Fatigue -Dizziness -Nervousness Placebo: 4.7% -Eczema -Fatigue -Dry mouth |
No reported | Yes |
| NK-1 receptor antagonist | |||||||||
| Lanepitant, oral | Goldstein et al., 2001 (38) | RCT double-blind placebo-controlled 84 participants 42 lanepitant 42 placebo |
Oral 200 mg daily |
At week 12 Lanepitant: −0.9 (SD ± 3.7) Placebo: −0.5 (SD ± 3.4) |
No reported | No reported | Lanepitant: 52.4% -Headache -Back pain -Diarrhea Placebo: 40.5% -Headache -Back pain -Diarrhea |
Lanepitan: 2.3% -Nausea -Heart plapitations Placebo: 4.7% -Insomnia -Confusion |
No |
| NSAIDs | |||||||||
| Acetylsalicylic acid, oral | Benseñor et al., 2001 (39) | RCT double-blind, placebo-controlled, 1,001 participants |
Oral 81 mg daily |
At 36 months Aspirin: −1.3 (SD ± 2.0) Placebo: −1.5 (SD ± 1.9) |
No reported | No reported | No Reported | No reported | Yes |
| NMDA antagonist | |||||||||
| Memantine, oral | Noruzzadeh et al., 2016 (40) | Double-blind RCT 52 participants 25 memantine 27 placebo |
Oral 10 mg daily |
At week 12 Memantine −3.5 (SD ± 1.6) Placebo: −0.8 (SD ± 2.1) |
No reported | No reported | Memantine: 13% -Dizziness -Fatigue -Nausea Placebo: 3.7% -Nausea |
Memantine: No reported Placebo: 3.7% -Vertigo |
Yes |
| Neuromodulator | |||||||||
| Occipital nerve stimulation vs. sham | Liu et al., 2017 (41) | RCT 110 participants 22 tONS 2 Hz 22 tONS 100 Hz 22 tONS 2/100 Hz 22 sham 22 Topiramato |
Transcutaneous frequencies: 2 Hz 100 Hz 2/100 Hz daily |
No reported | At 1 month: 2 Hz: −2.0 (SD ± 1.4) 100 Hz: −5.5 (SD ± 1.4) 2/100 Hz: −3.0 (SD ± 1.4) Sham: −0.5 (SD ± 1.4) TPM: −6.0 (SD ± 1.4) |
No reported | tONS 2 Hz: 25% -Pain and hematoma tONS 100 Hz: 20% -Pain and hematoma tONS 2/100 Hz: 22% -Pain and hematoma -Sham: 18% -Pain and hematoma |
No reported | No |
| Caloric vestibular stimulation | Wilkinson et al., 2017 (42) | RCT 81 participants |
30 min each daily |
At week 12 CVS: −3.9 (SD ± 2.7) Placebo: −1.1 (SD ± 3.9) |
No reported | At week 12 CVS: −3.9 (SD ± 3.2) Placebo: −1.7 (SD ± 6.1) |
CVS: -Nausea -Dizziness -Ear discomfort -Tinnitus Placebo: -Nausea -Dizziness -Ear discomfort -Tinnitus |
No reported | Yes |
| Acupuncture | Alecrim-Andrade et al., 2006 (43) | RCT 28 participants 14 real acupuncture 14 sham acupuncture |
Acupuncture sessions twice weekly |
At week 12 Acupuncture: −2.5 (SD ± 2.6) Sham acupuncture: −1.0 (SD ± 3.3) |
No reported | No reported | Acupuncture: 25% -Pain -Hematoma Sham acupuncture: 20% -Pain |
No reported | Yes |
| Herbal supplements and oil | |||||||||
| Tanacetum parthenium, oral | Pfaffenrath et al., 2002 (44) | Double-blind, RCT 147 participants 37 Tanacetum 2.08 mg 36 Tanacetum 6.25 mg 39 tanacetum 18.75 mg 35 placebo |
Oral 2.08 mg 6.25 mg 18.75 mg three times daily |
At week 12 Tanacetum parthenium 2.08 mg: −0.2 (SD ± 1.2) 6.25 mg: −0.9 (SD ± 1.7) 18.75 mg: −0.4 (SD ± 1.7) Placebo: −0.7 (SD ± 1.8) |
No reported | No reported |
Tanacetum parthenium: 35% -Nausea -Diarrhea Placebo: 35% -Nausea -Diarrhea |
No reported | No |
| Ginger, oral | Martins et al., 2020 (45) | Double-blind RCT 107 participants 53 Ginger 54 placebo |
Oral 200 mg three times daily |
At week 12 Ginger: −0.9 (SD ± 2.1) Placebo: −0.7 (SD ± 2.2) |
At week 12 Ginger: −08 (SD ± 2.9) Placebo: −0.5 (SD ± 2.9) |
At week 12 Ginger: −0.9 (SD ± 2.1) Placebo: −0.6 (SD ± 1.4) |
Ginger: 30% Placebo: 14.8% -Heartburn -Nausea -Constipation |
Ginger: 7.5% Placebo: 1.8% -Heartburn -Nausea -Constipation |
No |
| Basil Essential Oil, topic | Ahmadifard et al., 2020 (46) | Triple-blind, RCT 144 participants 36 basil oil 2% 36 basil oil 4% 36 basil oil 6% 36 placebo |
Topic 2, 4, 6% 3 times daily |
At week 12 Basil essential oil 2%: −2.8 (SD ± 1.8) 4%: −3.0 (SD ± 1.8) 6%: −3.2 (SD ± 1.8) Placebo: −1.0 (SD ± 1.8) |
No reported | No reported | Oil: 8.3% -Skin irritation Placebo: 2.7% |
No reported | No |
| Combinations | |||||||||
| Topiramate and amitriptyline (alone or in combination), oral | Keskinbora and Aydinli, 2008 (47) | Double-blind, RCT 63 participants 20 Topiramate 22 Amitriptyline 21 Combination |
Oral topiramate: 50–200 mg daily vs. amitriptyline: 10–150 mg daily |
At week 12 Topiramate: −5.6 (SD ± 3.3) Amitriptyline: −5.1 (SD ± 2.7) Combination: −5.1 (SD ± 2.8) |
No reported | No reported | Topiramate: 30% -Paresthesia -Fatigue -Loss of appetite Amitriptyline: 25% -Sedation -Dry mouth Combination: 35% -Dizziness -Weight gain -Fatigue |
Topiramate: 10% -Paresthesia -Loss of appetite Amitriptyline: 8% -Drowsiness Combination: 4.3% -Sedation -Dizziness |
Yes |
| Topiramate and flunarizine (alone or in combination), oral | Luo et al., 2012 (48) | Double-blind, RCT 126 participants 39 Flunarizine 44 Topiramate 43 Combination |
Oral topiramate: 50–10 mg daily flunarizine: 5–10 mg daily |
At week 12 Topiramate: −3.4 (SD ± 1.6) Flunarizine: −3.1 (SD ± 1.5) Combination: −2.6 (SD ± 0.8) |
No reported | No reported | Topiramate: 25% -Memory disturbances -Paresthesia -Fatigue -Weight loss Flunarizine: 20.5% -Drowsiness -Weight gain -Gastrointestinal disturbances Combination: 23.3% -Sedation -Fatigue |
No reported | Yes |
| Topiramate plus nortriptyline, oral | Krymchantowski, Da Cunha Jevoux, and Bigal, 2012 (49) | RCT double-blind, placebo-controlled, 80 participants 17 Topiramate 19 Nortriptyline 44 Combination |
Oral topiramate: 50 mg 100 mg daily nortriptyline: 25–75 mg daily |
At week 6 Topiramate: −3.5 (SD ± 2.3) Nortriptyline: −3.2 (SD ± 2.3) Combination: −4.6 (SD ± 1.9) |
No reported | No reported | Combination: 65.9% -Weight loss -Dry mouth -Paresthesia -Somnolence Placebo: 41.2% -Weight loss -Weight gain |
No reported | Yes |
| Propranolol and nortriptyline (alone or in combination), oral | Domingues et al., 2009 (50) | Double-blind RCT 44 participants 14 Propranolol 14 Nortriptyline 16 Combination |
Oral propranolol: 40 mg daily nortriptyline: 25 mg daily |
At week 8 Propranolol: −4.0 (SD ± 3.9) Nortriptyline: −1.0 (SD ± 4.3) Combination: −4.0 (SD ± 4.1) |
No reported | No reported | Propranolol: 18% -Fatigue Nortriptyline: 22% -Drowsiness -Dry mouth Combination: 15% -Dizziness -Mild sedation |
Propranolol: 5% -Fatigue Nortriptyline: 6% -Drowsiness Combination: 3% -Dizziness -Sedation |
Yes |
Summary of the efficacy and safety of randomized clinical trials in preventing episodic migraine.
3.5.1 Anti-CGRP monoclonal antibodies
Anti-CGRP monoclonal antibodies (erenumab, fremanezumab, galcanezumab, eptinezumab) showed consistent reductions in MMD, ranging from −3.2 to −4.3 days, mostly evaluated at 12 weeks, except for galcanezumab which was assessed at 6 months. AMD was reduced between −1.1 and −3.7 days within the same timeframe. MHD was not consistently reported. AE rates ranged from 18.5 to 58%, with upper respiratory infections, injection site reactions, constipation, and fatigue being most common. SAEs occurred in 1.3–4%, including abdominal pain, asthenia, and bronchiectasis.
3.5.2 Gepants
Gepants (rimegepant and atogepant) demonstrated significant reductions in MMD (−3.6 to −4.4 days), MHD (−3.9 to −4.4 days), and AMD (−3.3 to −3.9 days), all consistently evaluated at 12 weeks. AE ranged widely (10–63.8%), mainly nausea, constipation, and fatigue. SAE occurred in 2–4.1%, including allergic reactions and fatigue.
3.5.3 Antiseizure medications
Topiramate, valproate, and oxcarbazepine showed heterogeneous evaluation periods: topiramate was assessed at 8–20 weeks, valproate at 4 weeks, and oxcarbazepine at 12 weeks. MMD reductions ranged from −1.3 to −4.2 days. Valproate showed a −1.2-day reduction in MHD at 4 weeks, and oxcarbazepine showed a −1.2-day reduction in AMD at 12 weeks. AE were frequent (60–80%), particularly paresthesia, weight loss, dizziness, and cognitive effects. SAE ranged from 2 to 15%.
3.5.4 Beta-blockers
Propranolol, metoprolol, and nebivolol showed MMD reductions from −1.2 to −4.2 days over evaluation periods ranging from 4 to 14 weeks. MHD and AMD were not reported. AE frequency varied significantly (17.9–93%), with fatigue, dizziness, and gastrointestinal issues being most common. SAE ranged from 3.5 to 7.1%.
3.5.5 Angiotensin receptor blockers
Candesartan showed a reduction in MMD (−1.8 days) and MHD (−2.9 days), both measured at 12 weeks. AMD data was not reported. AE frequency reached 50%, mainly respiratory infections, dizziness, and sleep problems. No SAE were specified.
3.5.6 Antidepressants
Amitriptyline, venlafaxine, and escitalopram were evaluated over 8–12 weeks. MMD reductions ranged from −1.0 to −7.8 days. MHD and AMD were not reported. AE incidence ranged widely (0–100%), often including drowsiness, fatigue, nausea, and weight gain. SAE ranged from 1 to 34.3%, with severe sedation and cardiovascular symptoms in some cases.
3.5.7 Hormonal therapy
Melatonin was evaluated over 8 weeks, showing an MMD reduction of −2.8 days. MHD and AMD were not reported. AE occurred in 2.8% of cases, including fatigue and dizziness. No SAE were reported.
3.5.8 NK-1 receptor antagonists
Lanepitant was evaluated over 12 weeks and showed a modest MMD reduction of −0.9 days. No data was available for MHD or AMD. AE were reported in 52.4% of participants, including headache and gastrointestinal symptoms. SAE occurred in 2.3% of participants.
3.5.9 NSAIDs
Aspirin (acetylsalicylic acid) showed a −1.3-day reduction in MMD after long-term evaluation at 36 months. MHD, AMD, AE, and SAE data were not reported.
3.5.10 NMDA antagonists
Memantine was evaluated over 12 weeks, achieving an MMD reduction of −3.5 days. AMD remained unchanged (0 days), and MHD was not reported. AE (13%) included dizziness and fatigue. SAE were not specified.
3.5.11 Herbal supplements and oils
Tanacetum, ginger, and basil oil were studied over 12 weeks. MMD reductions ranged from −0.2 to −3.2 days. Ginger also led to a reduction in MHD (−0.8 days) and AMD (−0.9 days). AE frequencies varied (8.3–35%) and included nausea, diarrhea, and skin irritation. Ginger was associated with a 7.5% SAE rate.
3.5.12 Combination therapies
Combinations such as topiramate with amitriptyline, flunarizine, or nortriptyline were evaluated between 6 and 12 weeks. MMD reductions ranged from −2.6 to −5.1 days. MHD and AMD data were mostly unavailable. AE frequency ranged from 15 to 65.9%, and SAE reached 4.3%, including sedation, weight gain, and dizziness.
Among the evaluated pharmacological groups, Gepants (Rimegepant, Atogepant) demonstrated one of the most substantial reductions in both Monthly Migraine Days (MMD) and Acute Medication Days (AMD), with MMD decreasing between −3.6 to −4.4 days and AMD showing a reduction of −3.3 to −3.9 days at 12 weeks. Similarly, anti-CGRP monoclonal antibodies (Erenumab, Fremanezumab, Galcanezumab, Eptinezumab) exhibited notable efficacy, achieving MMD reductions ranging from −3.2 to −4.3 days and AMD reductions of −1.1 to −3.7 days across various studies. Additionally, combination therapies such as Topiramate + Amitriptyline or Propranolol + Nortriptyline presented the most significant MMD reduction, with values reaching up to −5.1 days, though AMD data was not reported for this group. These findings highlight the potential of these medication classes in effectively reducing migraine frequency and medication use.
3.6 Meta-analysis of efficacy of the pharmacological treatments
3.6.1 Allopathic medications
We analyzed the RCTs that compared the intervention with placebo at the 12 weeks, using MMD as the primary outcome, as it is the most consistent measure of efficacy. To reduce heterogeneity in the timing of outcome evaluations, we excluded RCTs with different times for the assessment of the outcomes, active and heterogeneous comparators, and study arms that involved drug combinations previously mentioned in Table 1. Figure 2 of allopathic treatment showed a global −1.25 mean difference (95%, confidence interval CI −1.47, −1.04, p = 0.001) to favor the active treatments, except in some negative RCTs using eptinezumab, oxcarbazepine, candesartan, propranolol, and lanepitant. Although the pooled mean reduction in monthly migraine days (MMD) was −1.25 days, this effect should be interpreted in light of the baseline MMD observed in the placebo groups, which typically ranged from 4.5 to 7.5 days across the included trials. This corresponds to a relative reduction of approximately 17–28%, indicating a potentially meaningful clinical benefit despite the modest absolute value. Figure 3 displays the funnel plot corresponding to this meta-analysis of allopathic treatments versus placebo for MMD. Visual inspection showed no significant asymmetry, and Egger’s test did not detect publication bias. It is important to note that the quantitative meta-analysis focused exclusively on efficacy outcomes (MMD), and pooled estimates for AE or SAE were not calculated due to significant variability in reporting methods and definitions among included studies.
Figure 2

Forest plot of the randomized clinical trials placebo-controlled using allopathic treatments for the prevention of episodic migraine.
Figure 3

Funnel plot of the randomized clinical trials, placebo-controlled using allopathic treatments to prevent episodic migraine.
3.6.2 Homeopathic medications
We analyzed the RCTs that compared the intervention with placebo at 12 weeks, using MMD as the primary outcome. Figure 4 shows that homeopathic treatments had a global mean difference of −0.79 (95% confidence interval [CI]: −1.65 to 0.07, p = 0.07), which was not significant compared with the placebo. The funnel plot, shown in Figure 5 corresponds to the meta-analysis of homeopathic treatments. Visual inspection showed no major asymmetry, and Egger’s test did not indicate significant publication bias.
Figure 4

Forest plot of the randomized clinical trials placebo-controlled using homeopathic treatments for the prevention of episodic migraine.
Figure 5
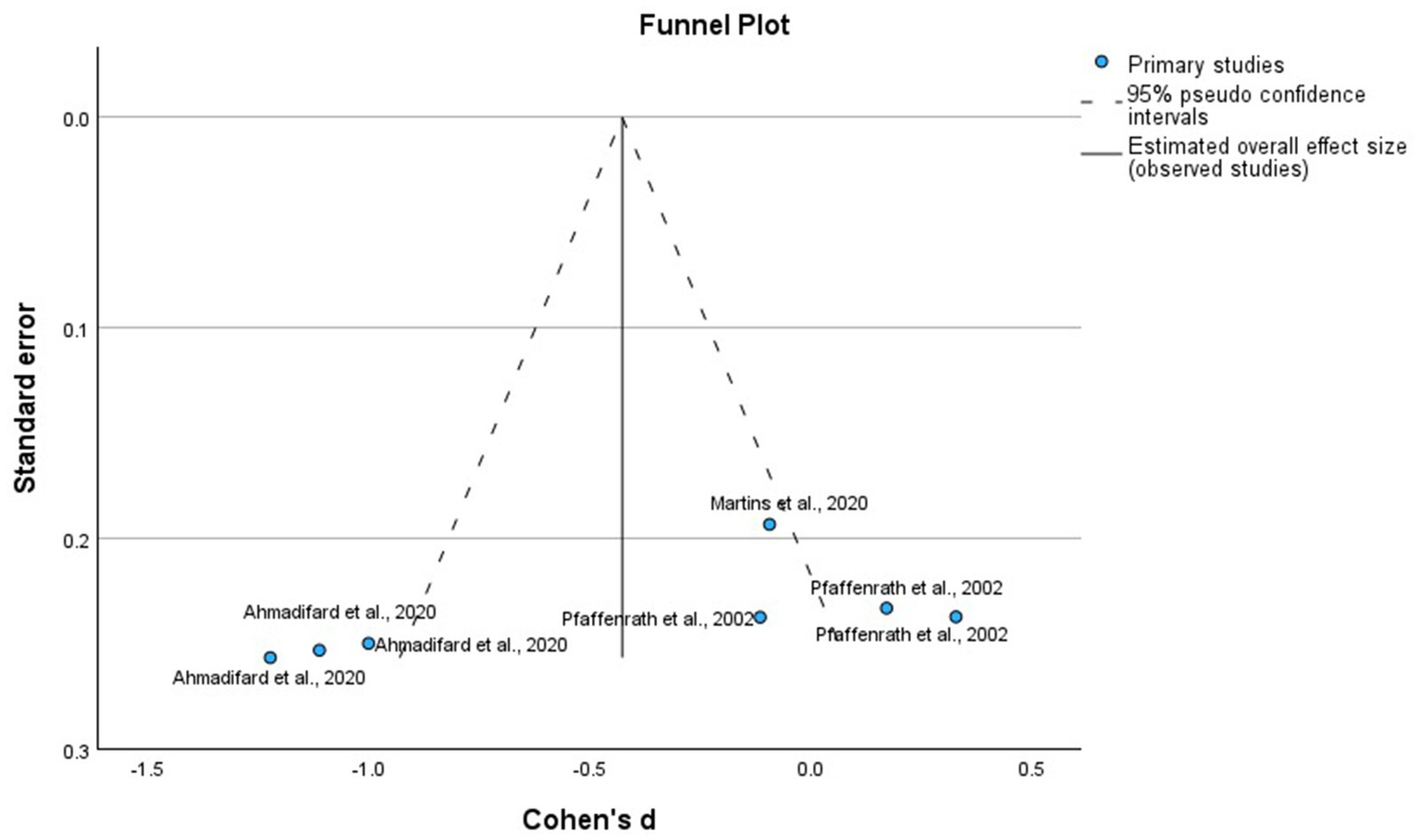
Funnel plot of the randomized clinical trials placebo-controlled using homeopathic treatments for the prevention of episodic migraine.
3.7 Efficacy and safety of non-pharmacologic therapies
Table 2 summarizes a structured narrative synthesis of non-pharmacological. This section provides a structured narrative synthesis of non-pharmacological interventions for episodic migraine prevention.
Table 2
| Intervention | Evaluation period | MMD (days) | MHD (days) | AMD (days) | AE | SAE |
|---|---|---|---|---|---|---|
| Occipital Nerve Stimulation (ONS) | 1 month | Not reported | −2.0 to −5.5 | Not reported | Pain, hematoma, nausea, dizziness (up to 25%) | Not reported |
| Caloric Vestibular Stimulation (CVS) | 12 weeks | −3.9 | −3.9 | −3.9 | Nausea, dizziness, ear discomfort, tinnitus | Not reported |
| Acupuncture | 12 weeks | −2.5 | Not reported | Not reported | Pain and hematoma at puncture sites (25%) | Not reported |
Summary of non-pharmacological therapies.
Non-pharmacological interventions included neuromodulation techniques such as occipital nerve stimulation, caloric vestibular stimulation, and acupuncture. These modalities were assessed primarily over a 12-week period, except for occipital nerve stimulation, which reported outcomes at 1 month.
Occipital nerve stimulation showed reductions in MHD ranging from −2.0 to −5.5 days at 1 month, depending on the stimulation frequency. MMD also improved, although the data were not consistently reported across all frequency subgroups. AE were reported in up to 25% of patients and included local pain, hematoma at the stimulation site, nausea, and dizziness. SAE were not reported in these trials.
Caloric vestibular stimulation was evaluated over 12 weeks and demonstrated a reduction in both MMD and MHD of −3.9 days. The same intervention showed a reduction in AMD of −3.9 days. The most frequent AE included nausea, dizziness, ear discomfort, and tinnitus. No SAE were reported.
Acupuncture, evaluated over a 12-week period, showed a reduction of −2.5 days in MMD compared to a −1.0-day reduction in the sham acupuncture control group. MHD and AMD were not reported. AE occurred in 25% of patients and included local pain and hematoma at the puncture sites. No SAE were reported.
Although these interventions yielded promising effects in reducing migraine frequency and associated medication use, the wide variability in protocols, outcome definitions, and follow-up times hindered direct comparison and aggregation of results. Nonetheless, the generally favorable safety profile across studies supports the potential role of these non-pharmacological strategies as adjunctive treatments in individualized preventive regimens.
Unfortunately, the meta-analysis of the efficacy of the non-pharmacological treatments was not feasible for the heterogeneity of the outcome measurements used in each study.
3.8 GRADE evidence profile
A structured GRADE assessment was conducted to determine the certainty of evidence for the main pharmacological comparisons included in this review. This approach complements the narrative synthesis and meta-analysis by addressing potential limitations in risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Table 3 summarizes the GRADE evidence profiles for anti-CGRP monoclonal antibodies, gepants, and combination therapies, using monthly migraine days (MMD) as the primary outcome.
Table 3
| Comparison | Outcome | Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Certainty | Effect estimate | Reason for downgrading |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CGRP monoclonal antibodies vs placebo | MMD at 12 weeks | 4 RCTs | Not serious | Not serious | Not serious | Serious (some wide CIs) | Unlikely | Moderate | −3.2 to −4.3 days | Imprecision due to variability in sample size and confidence intervals |
| Gepants vs placebo | MMD at 12 weeks | 5 RCTs | Not serious | Not serious | Not serious | Not serious | Unlikely | High | −3.6 to −4.4 days | None |
| Combination therapy vs monotherapy | MMD | 3 RCTs | Some concerns | Serious (high heterogeneity) | Serious (limited generalizability) | Serious (small sample size) | Possible | Low | −2.6 to −5.1 days | Small sample sizes and variation in comparator drugs |
GRADE evidence profiles.
The certainty of evidence was rated as moderate for anti-CGRP therapies due to some imprecision across trials, high for gepants based on robust and consistent findings, and low for combination treatments, mainly due to heterogeneity, indirect comparisons, and small sample sizes. These ratings provide a useful framework for interpreting the strength and applicability of the observed clinical effects.
3.9 Risk of bias
Bias was evaluated following the guidelines of the Cochrane Handbook for Systematic Reviews. The details are illustrated in Figures 6, 7. We did not find any risk of bias in the RCTs using the intention-to-treat modality. However, in the Per-protocol approach, participants were randomly assigned to groups using computer-generated random sequences through an interactive web-response system in all two studies. One trial noted that pharmacists were unblinded; however, their role was limited to drug preparation and inventory management. Another trial did not provide details on allocation concealment and blinding of outcome assessment. All two trials reported patient follow-up losses; each predefined outcome was clearly described. Studies meeting the inclusion criteria were included in this meta-analysis.
Figure 6

Risk of bias for the randomized clinical trials included intention-to-treat.
Figure 7

Risk of bias for the randomized clinical trials included per protocol.
4 Discussion
This systematic meta-analysis provides a comprehensive evaluation of the efficacy and safety of preventive interventions for episodic migraine, with a particular focus on those available in Mexico. Although the allopathic treatment analysis demonstrated an overall favorable effect for active interventions (mean difference: –1.25; 95% CI: −1.47 to −1.04; p = 0.001), several agents—including eptinezumab, oxcarbazepine, candesartan, propranolol, and lanepitant—showed non-significant effects in individual RCTs, highlighting variability in therapeutic response. The findings confirm that, despite the wide range of therapeutic options, there remains significant heterogeneity in clinical outcomes regarding reduction in MMD and treatment tolerability.
Anti-CGRP antagonists—including erenumab, fremanezumab, galcanezumab, and eptinezumab—demonstrated clinically relevant reductions in MMD with acceptable safety profiles (51). These results are consistent with international literature, where anti-CGRP monoclonal antibodies have shown superiority over traditional treatments regarding specificity and treatment adherence.
Similarly, gepants (rimegepant and atogepant), as oral CGRP receptor modulators, showed comparable efficacy to monoclonal antibodies, albeit with variability in adverse events, particularly gastrointestinal side effects (52).
Regarding conventional therapies, antiepileptic drugs and beta-blockers, despite their widespread use, showed more modest efficacy and higher rates of adverse events, limiting their applicability to specific patient profiles (53). Notably, pharmacological combinations—such as topiramate with amitriptyline or propranolol with nortriptyline—achieved the most pronounced reductions in MMD, although with lower tolerability, underscoring the need for individualized risk–benefit assessment (54, 55).
Non-pharmacological interventions, including occipital nerve stimulation, vestibular stimulation, and acupuncture, demonstrated beneficial effects on some clinical outcomes. However, the lack of uniformity in outcome measures and the limited number of controlled studies hindered their inclusion in the quantitative meta-analysis. Nevertheless, their favorable safety profile and potential utility as adjunctive therapies warrant further exploration through studies with robust methodological design (56).
In light of the limited availability of certain pharmacological agents and therapies in Mexico, it is imperative to outline strategic steps for incorporating newer evidence-based treatments into national formularies, ensuring equitable access and alignment with international standards of care.
From a methodological perspective, the risk of bias analysis using the Cochrane RoB 2 tool revealed an overall low risk of bias, particularly in studies that used intention-to-treat analysis (57). However, some limitations persisted, especially in studies with limited information on allocation concealment or blinding of outcome assessors.
The limitation of the present analysis is the exclusion of patients over 65 years of age, pregnant women, and individuals with cardiovascular or cerebrovascular conditions, which restricts the generalizability of the findings. Furthermore, variability in follow-up periods (ranging from 3 to 6 months), potential publication bias, and the lack of access to unpublished or incomplete data may have influenced the aggregate results (58).
4.1 Limitations of the meta-analysis
The present review also has some limitations. First, the present study was restricted to eligibility criteria, in which merely “Number of studies” were included in the analysis. Some unpublished and missing data from studies also influence aggregate results. Furthermore, some of the studies were completed by the same researchers, which may lead to publication bias. In addition, the double-blind period of these included studies ranged from 3 to 6 months, and the difference might result in heterogeneity. To reduce heterogeneity, only studies with 12-week follow-up were included in the final quantitative synthesis. While this approach improved comparability across trials, it also excluded a significant number of potentially relevant studies and may have limited the scope of the analysis, particularly with regard to long-term efficacy and safety outcomes.
Additionally, pharmacological treatments were grouped into broad therapeutic classes (e.g., antidepressants, anti-seizure medications), despite marked differences in their mechanisms of action and clinical profiles. For example, amitriptyline and venlafaxine, although both classified as antidepressants, have distinct pharmacodynamic properties; similarly, topiramate and valproate differ substantially in their molecular targets and tolerability. This classification may oversimplify treatment effects and obscure clinically meaningful differences between individual agents. Greater granularity, as reflected in the compound-specific data provided in Supplementary material, is likely to be more informative for guiding clinical decision making. Also, the analysis excluded older, non-specific pharmacological therapies commonly used in migraine prevention—such as other beta-blockers and certain calcium channel blockers—owing to the lack of recent or high-quality RCTs meeting inclusion criteria. Some of them are treatments that remain widely prescribed in routine practice in some countries, and their omission from the current synthesis may limit the generalizability of the findings.
In addition, non-pharmacological and nutraceutical interventions were grouped into heterogeneous categories, despite having distinct therapeutic mechanisms and varying levels of supporting evidence. This broad classification complicates interpretation and precludes firm conclusions about the relative efficacy of individual non-drug strategies.
Moreover, this review included only studies published in English or Spanish, which may have introduced language bias and limited the inclusion of potentially relevant trials from other regions. Otherwise, Meta-analyses were conducted using SPSS due to software availability at the institution. While SPSS is appropriate for basic fixed- and random-effects models, it does not offer the advanced options or flexibility of specialized platforms such as RevMan or R-based packages like meta or metafor. This may limit some statistical nuance in modeling or subgroup analysis.
Finally, due to the exclusion of patients older than 65 years, pregnant individuals, and those with significant cardiovascular or cerebrovascular comorbidities, the generalizability of the results is limited. These populations, which are frequently encountered in clinical practice, remain underrepresented in current trials. Moreover, future studies should prioritize the investigation of subgroup-specific responses to preventive treatments, including stratification by migraine frequency (e.g., high-frequency episodic vs. chronic migraine), sex, and age group. Such analyses are essential to advancing a more tailored and equitable approach to migraine management.
Furthermore, studies with longer follow-ups and larger sample sizes should be performed to identify the confirmative safety profile of gepants and monoclonal antibodies and determine the duration of its therapeutic effects.
Taken together, these limitations highlight the need for further high-quality, head-to-head trials of both pharmacological (e.g., gepants vs. monoclonal antibodies) and non-pharmacological treatments, with mechanistic specificity, standardized outcomes, and longer follow-up durations, and evaluations of cost-effects of the treatment to better inform personalized approaches to migraine prevention.
4.2 Bullet points
Preventive therapy for episodic migraine should be individualized.
Combined strategies (pharmacological + non-pharmacological) are recommended.
Decision-making should consider comorbidities, adverse effect profiles, and patient preferences.
5 Conclusion
This systematic meta-analysis highlights the efficacy and safety of preventive treatments for episodic migraine, with a focus on their applicability in Mexico. While active treatments showed an overall favorable effect (mean difference: –1.25; 95% CI: −1.47 to −1.04; p = 0.001). with a variability in response. Anti-CGRP monoclonal antibodies and gepants were associated with clinically meaningful reductions in MDD and acceptable safety, offering advantages over conventional therapies. Traditional agents, including beta-blockers and antiepileptics, showed more modest efficacy and tolerability, while pharmacological combinations, though effective, were limited by side effects. Non-pharmacological strategies showed promise but lacked consistent evidence.
The limited availability of newer therapies in Mexico highlights the need for national strategies to expand formulary access and align with international treatment standards. Methodological limitations—including the exclusion of older adults and pregnant individuals, short follow-up periods, and variability in drugs. Future research should prioritize inclusive, long-term, and head-to-head trials to better inform personalized, evidence-based migraine prevention.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
M-KV-J: Funding acquisition, Validation, Writing – review & editing. AM-M: Data curation, Writing – review & editing. IR-L: Project administration, Resources, Validation, Writing – review & editing. MMe: Data curation, Writing – review & editing. MR-Á: Data curation, Writing – review & editing. JP-G: Data curation, Writing – review & editing. RV-G: Data curation, Writing – review & editing. DS: Conceptualization, Methodology, Supervision, Writing – original draft. MP-P: Methodology, Writing – review & editing. EG: Data curation, Formal analysis, Software, Writing – review & editing. MMo: Data curation, Formal analysis, Software, Writing – review & editing. CT: Formal analysis, Software, Writing – review & editing. MG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Pfizer Mexico. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1611303/full#supplementary-material
References
1.
Ahmad SR Rosendale N . Sex and gender considerations in episodic migraine. Curr Pain Headache Rep. (2022) 26:505–16. doi: 10.1007/s11916-022-01052-8
2.
Riggins N Ehrlich A . Episodic migraine and older adults. Curr Pain Headache Rep. (2022) 26:331–5. doi: 10.1007/s11916-022-01029-7
3.
Lanteri-Minet M . Economic burden and costs of chronic migraine. Curr Pain Headache Rep. (2014) 18:385. doi: 10.1007/s11916-013-0385-0
4.
Orlova YY Mehla S Chua AL . Drug safety in episodic migraine management in adults part 1: acute treatments. Curr Pain Headache Rep. (2022) 26:481–92. doi: 10.1007/s11916-022-01057-3
5.
Hubig LT Smith T Chua GN Lloyd AJ Powell L Johnston K et al . A stated preference survey to explore patient preferences for novel preventive migraine treatments. Headache. (2022) 62:1187–97. doi: 10.1111/head.14386
6.
Raggi A Leonardi M Arruda M Caponnetto V Castaldo M Coppola G et al . Hallmarks of primary headache: part 1 – migraine. J Headache Pain. (2024) 25:189. doi: 10.1186/s10194-024-01889-x
7.
Pellesi L Do TP Hougaard A . Pharmacological management of migraine: current strategies and future directions. Expert Opin Pharmacother. (2024) 25:673–83. doi: 10.1080/14656566.2024.2349791
8.
Thorlund K Toor K Wu P Chan K Druyts E Ramos E et al . Comparative tolerability of treatments for acute migraine: a network meta-analysis. Cephalalgia. (2016) 37:965–78. doi: 10.1177/0333102416660552
9.
Lampl C MaassenVanDenBrink A Deligianni CI Gil-Gouveia R Jassal T Sanchez-Del-Rio M et al . The comparative effectiveness of migraine preventive drugs: a systematic review and network meta-analysis. J Headache Pain. (2023) 24:56. doi: 10.1186/s10194-023-01594-1
10.
Headache Classification Committee of the International Headache Society (IHS) . The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
11.
Higgins JP Altman DG Gøtzsche PC Juni P Moher D Oxman AD et al . The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:1–9. doi: 10.1136/bmj.d5928
12.
Goadsby PJ Reuter U Hallström Y Broessner G Bonner JH Zhang F et al . A controlled trial of Erenumab for episodic migraine. N Engl J Med. (2017) 377:2123–32. doi: 10.1056/NEJMoa1705848
13.
Dodick DW Silberstein SD Bigal ME Yeung PP Goadsby PJ Blankenbiller T et al . Effect of Fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. (2018) 319:1999–2008. doi: 10.1001/jama.2018.4853
14.
Skljarevski V Matharu M Millen BA Ossipov MH Kim B-K Yang JY . Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. (2018) 38:1442–54. doi: 10.1177/0333102418779543
15.
Smith TR Janelidze M Chakhava G Cady R Hirman J Allan B et al . Corrigendum to "Eptinezumab for the prevention of episodic migraine: sustained effect through 1 year of treatment in the PROMISE-1 study" [Clin Therapeut 42 (12) (2020) 2254-65]. Clin Ther. (2021) 43:791. doi: 10.1016/j.clinthera.2021.01.019
16.
Croop R Lipton RB Kudrow D Stock DA Kamen L Conway CM et al . Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. (2021) 397:51–60. doi: 10.1016/S0140-6736(20)32544-7
17.
Goadsby PJ Dodick DW Ailani J Trugman JM Finnegan M Lu K et al . Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. (2020) 19:727–37. doi: 10.1016/S1474-4422(20)30234-9
18.
Ailani J Lipton RB Goadsby PJ Guo H Miceli R Severt L et al . Atogepant for the preventive treatment of migraine. N Engl J Med. (2021) 385:695–706. doi: 10.1056/NEJMoa2035908
19.
Tassorelli C Nagy K Pozo-Rosich P Lanteri-Minet M Sacco S Nežádal T et al . Safety and efficacy of atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): a randomised, placebo-controlled, phase 3b trial. Lancet Neurol. (2024) 23:382–92. doi: 10.1016/S1474-4422(24)00025-5
20.
Schwedt TJ Lipton RB Ailani J Silberstein SD Tassorelli C Guo H et al . Time course of efficacy of atogepant for the preventive treatment of migraine: results from the randomized, double-blind ADVANCE trial. Cephalalgia. (2022) 42:3–11. doi: 10.1177/03331024211042385
21.
Ashtari F Shaygannejad V Akbari M . A double-blind, randomized trial of low-dose topiramate vs propranolol in migraine prophylaxis. Acta Neurol Scand. (2008) 118:301–5. doi: 10.1111/j.1600-0404.2008.01087.x
22.
Freitag FG Collins SD Carlson HA Goldstein J Saper J Silberstein S et al . A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. (2002) 58:1652–9. doi: 10.1212/WNL.58.11.1652
23.
Silberstein S Saper J Berenson F Somogyi M McCague K D'Souza J . Oxcarbazepine in migraine headache: a double-blind, randomized, placebo-controlled study. Neurology. (2008) 70:548–55. doi: 10.1212/01.wnl.0000297551.27191.70
24.
Storey JR Calder CS Hart DE Potter DL . Topiramate in migraine prevention: a double-blind, placebo-controlled study. Headache. (2001) 41:968–75. doi: 10.1046/j.1526-4610.2001.01190.x
25.
Pradalier A Serratrice G Collard M Hirsch E Feve J Masson M et al . Long-acting propranolol in migraine prophylaxis: results of a double-blind, placebo-controlled study. Cephalalgia. (1989) 9:247–53. doi: 10.1046/j.1468-2982.1989.904247.x
26.
Millán-Guerrero RO Isais-Millán R Guzmán-Chávez B Castillo-Varela G . N alpha methyl histamine versus propranolol in migraine prophylaxis. Can J Neurol Sci. (2014) 41:233–8. doi: 10.1017/s0317167100016632
27.
Steiner TJ Joseph R Hedman C Rose FC . Metoprolol in the prophylaxis of migraine: parallel-groups comparison with placebo and dose-ranging follow-up. Headache. (1988) 28:15–23. doi: 10.1111/j.1365-2524.1988.hed2801015.x
28.
Kangasniemi P Andersen AR Andersson PG Gilhus NE Hedman C Hultgren M et al . Classic migraine: effective prophylaxis with metoprolol. Cephalalgia. (1987) 7:231–8. doi: 10.1046/j.1468-2982.1987.0704231.x
29.
Olsson JE Behring HC Forssman B Hedman C Hedman G Johansson F et al . Metoprolol and propranolol in migraine prophylaxis: a double-blind multicentre study. Acta Neurol Scand. (1984) 70:160–8. doi: 10.1111/j.1600-0404.1984.tb00815.x
30.
Schellenberg R Lichtenthal A Wöhling H Graf C Brixius K . Nebivolol and metoprolol for treating migraine: an advance on beta-blocker treatment?Headache. (2008) 48:118–25. doi: 10.1111/j.1526-4610.2007.00785.x
31.
Stovner LJ Linde M Gravdahl GB Tronvik E Aamodt AH Sand T et al . A comparative study of candesartan versus propranolol for migraine prophylaxis: a randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia. (2014) 34:523–32. doi: 10.1177/0333102413515348
32.
Kalita J Bhoi SK Misra UK . Amitriptyline vs divalproate in migraine prophylaxis: a randomized controlled trial. Acta Neurol Scand. (2013) 128:65–72. doi: 10.1111/ane.12081
33.
Lampl C Huber G Adl J Luthringshausen G Franz G Marecek S et al . Two different doses of amitriptyline ER in the prophylaxis of migraine: long-term results and predictive factors. Eur J Neurol. (2009) 16:943–8. doi: 10.1111/j.1468-1331.2009.02631.x
34.
Ozyalcin SN Talu GK Kiziltan E Yucel B Ertas M Disci R . The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. (2005) 45:144–52. doi: 10.1111/j.1526-4610.2005.05029.x
35.
Tarlaci S . Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. (2009) 32:254–8. doi: 10.1097/WNF.0b013e3181a8c84f
36.
Bulut S Berilgen MS Baran A Tekatas A Atmaca M Mungen B . Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover study. Clin Neurol Neurosurg. (2004) 107:44–8. doi: 10.1016/j.clineuro.2004.03.004
37.
Alstadhaug KB Odeh F Salvesen R Bekkelund SI . Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. (2010) 75:1527–32. doi: 10.1212/WNL.0b013e3181f9618c
38.
Goldstein DJ Offen WW Klein EG Phebus LA Hipskind P Johnson KW et al . Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia. (2001) 21:102–6. doi: 10.1046/j.1468-2982.2001.00161.x
39.
Benseñor IM Cook NR Lee IM Chown MJ Hennekens CH Buring JE . Low-dose aspirin for migraine prophylaxis in women. Cephalalgia. (2001) 21:175–83. doi: 10.1046/j.0333-1024.2001.00194.x
40.
Noruzzadeh R Modabbernia A Aghamollaii V Ghaffarpour M Harirchian MH Salahi S et al . Memantine for prophylactic treatment of migraine without aura: a randomized double-blind placebo-controlled study. Headache. (2016) 56:95–103. doi: 10.1111/head.12732
41.
Liu Y Dong Z Wang R Ao R Han X Tang W et al . Migraine prevention using different frequencies of transcutaneous occipital nerve stimulation: a randomized controlled trial. J Pain. (2017) 18:1006–15. doi: 10.1016/j.jpain.2017.03.012
42.
Wilkinson D Ade KK Rogers LL Attix DK Kuchibhatla M Slade MD et al . Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. (2017) 57:1065–87. doi: 10.1111/head.13120
43.
Alecrim-Andrade J Maciel-Júnior JA Cladellas XC Correa-Filho HR Machado HC . Acupuncture in migraine prophylaxis: a randomized sham-controlled trial. Cephalalgia. (2006) 26:520–9. doi: 10.1111/j.1468-2982.2006.01062.x
44.
Pfaffenrath V Diener HC Fischer M Friede M von Henneicke- Zepelin HH Investigators . The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. (2002) 22:523–32. doi: 10.1046/j.1468-2982.2002.00396.x
45.
Martins LB Rodrigues AMDS Monteze NM Tibaes JRB Amaral MHA Gomez RS et al . Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia. (2020) 40:88–95. doi: 10.1177/0333102419869319
46.
Ahmadifard M Yarahmadi S Ardalan A Ebrahimzadeh F Bahrami P Sheikhi E . The efficacy of topical basil essential oil on relieving migraine headaches: a randomized triple-blind study. Complement Med Res. (2020) 27:310–8. doi: 10.1159/000506349
47.
Keskinbora K Aydinli I . A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. (2008) 110:979–84. doi: 10.1016/j.clineuro.2008.05.025
48.
Luo N Di W Zhang A Wang Y Ding M Qi W et al . A randomized, one-year clinical trial comparing the efficacy of topiramate, flunarizine, and a combination of flunarizine and topiramate in migraine prophylaxis. Pain Med. (2012) 13:80–6. doi: 10.1111/j.1526-4637.2011.01295.x
49.
Krymchantowski AV da Cunha Jevoux C Bigal ME . Topiramate plus nortriptyline in the preventive treatment of migraine: a controlled study for nonresponders. J Headache Pain. (2012) 13:53–9. doi: 10.1007/s10194-011-0395-4
50.
Domingues RB Silva AL Domingues SA Aquino CC Kuster GW . A double-blind randomized controlled trial of low doses of propranolol, nortriptyline, and the combination of propranolol and nortriptyline for the preventive treatment of migraine. Arq Neuropsiquiatr. (2009) 67:973–7. doi: 10.1590/s0004-282x2009000600002
51.
Soni P Chawla E . Efficacy and safety of anti-calcitonin gene-related peptide monoclonal antibodies for treatment of chronic migraine: a systematic review and network meta-analysis. Clin Neurol Neurosurg. (2021) 209:106893. doi: 10.1016/j.clineuro.2021.106893
52.
Silvestro M Orologio I Siciliano M Trojsi F Tessitore A Tedeschi G et al . Emerging drugs for the preventive treatment of migraine: a review of CGRP monoclonal antibodies and gepants trials. Expert Opin Emerg Drugs. (2023) 28:79–96. doi: 10.1080/14728214.2023.2207819
53.
Rushendran R Vellapandian C . Advances in migraine treatment: a comprehensive clinical review. Curr Protein Pept Sci. (2025) 26:422–35. doi: 10.2174/0113892037329429241123095325
54.
Chowdhury D Bansal L Duggal A Datta D Mundra A Krishnan A et al . TOP-PRO study: a randomized double-blind controlled trial of topiramate versus propranolol for prevention of chronic migraine. Cephalalgia. (2022) 42:396–408. doi: 10.1177/03331024211047454
55.
Alex A Armand CE . Rational polypharmacy for migraine. Pract Neurol. (2022):30–4
56.
Han X Yu S . Non-pharmacological treatment for chronic migraine. Curr Pain Headache Rep. (2023) 27:663–72. doi: 10.1007/s11916-023-01162-x
57.
Nejadghaderi SA Balibegloo M Rezaei N . The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: a perspective on the pros and cons. Health Sci Rep. (2024) 7:e2165. doi: 10.1002/hsr2.2165
58.
Cho L Vest AR O’Donoghue ML Ogunniyi MO Sarma AA Denby KJ et al . Increasing participation of women in cardiovascular trials: JACC council perspectives. J Am Coll Cardiol. (2021) 78:737–51. doi: 10.1016/j.jacc.2021.06.022
Summary
Keywords
episodic migraine, migraine prevention, CGRP monoclonal antibodies, gepants, non-pharmacological therapy, meta-analysis
Citation
Vélez-Jiménez M-K, Martínez-Mayorga AP, Rodriguez-Leyva I, Figueroa-Medina MJ, Reyes-Alvarez MT, Pérez-García JC, Vargas-García RD, San-Juan D, Pierdant-Perez M, Gómez EG, Morales Morales MA, Trenado C and Martínez-Gurrola MA (2025) Comprehensive preventive treatments for episodic migraine: a systematic review of randomized clinical trials. Front. Neurol. 16:1611303. doi: 10.3389/fneur.2025.1611303
Received
14 April 2025
Accepted
15 July 2025
Published
18 August 2025
Volume
16 - 2025
Edited by
Claudia Altamura, Fondazione Policlinico Campus Bio-Medico, Italy
Reviewed by
Lanfranco Pellesi, University of Southern Denmark, Denmark
Cinzia Aurilia, IRCCS San Raffaele Roma srl, Italy
Francesca Boscain, Azienda ULSS 6 Euganea, Italy
Updates
Copyright
© 2025 Vélez-Jiménez, Martínez-Mayorga, Rodriguez-Leyva, Figueroa-Medina, Reyes-Alvarez, Pérez-García, Vargas-García, San-Juan, Pierdant-Perez, Gómez, Morales, Trenado and Martínez-Gurrola.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel San-Juan, dsanjuan@innn.edu.mx
†ORCID: Rodriguez-Leyva, orcid.org/0000-0002-3316-1471
Daniel San Juan Orta, orcid.org/0000-0001-6685-5851
García Gómez, orcid.org/0009-0009-9737-0529
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.