Abstract
Background:
Approximately 50–70% of stroke survivors are left with varying degrees of limb paralysis, severely affecting their ability to perform daily activities and engage in rehabilitation. Although conventional rehabilitation interventions, such as task-oriented training and transcranial magnetic stimulation (TMS), have been widely utilized, their efficacy has been constrained by individual differences and limitations in neuroplastic activation. Repetitive peripheral magnetic stimulation (rPMS), a novel non-invasive neuromodulation technique, directly targets peripheral nerves and muscles to potentially facilitate the remodeling of motor pathways. There is a lack of evaluation regarding the effectiveness of rPMS for improving upper limb motor function and spasticity in stroke patients.
Methods:
Randomized controlled trials examining the effects of rPMS in post-stroke patients, published up to 20 February 2025, were searched in PubMed, Embase, the Cochrane Library, and the Web of Science. Methodological quality was evaluated using the Cochrane Collaboration tool. Meta-analyses were performed using RevMan (version 5.4). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method was used to assess the quality of evidence.
Results:
A total of 12 studies involving 492 patients were included. The results of the meta-analysis indicated that, compared to the control group, the subgroup analyses based on disease stage, stimulation frequency, coil type, stimulation duration, and stimulation intensity showed significant improvements, supported by high-quality evidence. The pooled standardized mean differences (SMDs) were as follows: disease stage, SMD = 0.69 (p = 0.006); stimulation frequency, SMD = 0.58 (p = 0.004); coil type, SMD = 0.82 (p = 0.001); stimulation duration, SMD = 0.62 (p = 0.004); and stimulation intensity, SMD = 0.79 (p = 0.002). In addition, rPMS significantly improved patients’ ability to live independently (SMD = 0.66, p <0.0001), supported by moderate-quality evidence. However, rPMS did not demonstrate a significant effect in reducing spasticity [mean difference (MD) = 0.25, p = 0.20], with this finding supported by low-quality evidence.
Systematic review registration:
rPMS improved upper limb motor function, activities of daily living (ADL), and self-care abilities in post-stroke patients, with good acceptability and only mild adverse reactions. Its effect on spasticity was not significant.
Systematic review registration:
www.crd.york.ac.uk/prospero/, CRD420250637455.
1 Introduction
Globally, stroke is the second leading cause of death (11.6% of total deaths) and the third leading cause of disability (5.7% of total DALYs) (1). Stroke could cause a series of complications, such as dysphagia, consciousness dysfunction, limb motor dysfunction, and cognitive dysfunction (2), and approximately 80% of survivors after stroke are left with upper limb dysfunction (3). At present, the effectiveness of traditional treatments for upper limb motor dysfunction after stroke is limited by poor patient compliance, insufficient activation of central plasticity, and a narrow rehabilitation period. These limitations seriously affect patients’ ability to perform daily living and normal activities, causing serious challenges and losses for survivors and their families in terms of quality of life and economy. Repetitive peripheral magnetic stimulation (rPMS), as a non-invasive neuromodulation technique, can affect the excitability and inhibition of the motor cortex by penetrating the deep structures of the brain through painless stimulation. This promotes the plasticity of the motor cortex and further causes changes in brain function (4). Numerous studies have found that rPMS can significantly improve upper limb motor function and daily living abilities after stroke (5–7), providing a mechanism and empirical evidence for exploring its application in the rehabilitation of the upper limb. A growing number of meta-analyses with small sample sizes and non-uniform parameters and outcome indicators have demonstrated the positive effects of rPMS on motor function after stroke (5, 8, 9), limiting the comparability of the assessments of efficacy. This study aimed to comprehensively evaluate the effectiveness of rPMS on upper limb dysfunction after stroke and to provide the latest evidence to guide clinical practice.
2 Materials and methods
This study was registered with PROSPERO under registration number CRD420250637455. It was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10).
2.1 Search strategies
The databases, including Embase, PubMed, the Cochrane Library, the Web of Science, and China National Knowledge Infrastructure (CNKI), were searched to identify studies on the effect of rPMS on post-stroke upper limb motor dysfunction up to 20 February 2025. The English keywords for the database searches included “stroke,” “cerebrovascular accident,” “upper extremity,” “motor function,” “motor performance,” “repetitive peripheral magnetic stimulation,” “peripheral magnetic stimulation,” “magnetic field,” “rPMS,” and “PMS.” The reference lists of the identified articles were checked for potential studies. The detailed search strategies for each database are provided in Supplementary Table S1.
2.2 Inclusion and exclusion criteria
Two reviewers independently conducted the literature screening. Disagreements were recorded and resolved through discussions with a third reviewer. The inclusion criteria were as follows: (1) randomized controlled trial (RCT) studies; (2) studies involving participants who experienced a first-time stroke with upper limb motor dysfunction, confirmed by magnetic resonance imaging or computed tomography; and (3) studies in which the experimental group received rPMS treatment in addition to the control group’s intervention, which could be a placebo, sham, or routine rehabilitation. The exclusion criteria were as follows: (1) animal experiments or studies including healthy volunteers; (2) studies without the target outcome measures; (3) studies for which the full text was not available; and (4) studies lacking complete outcome data. For studies with overlapping data, those with larger or more complete datasets were prioritized. Both published and unpublished studies were considered, and authors were contacted if additional details not reported in the articles were needed.
2.3 Risk of Bias and quality of outcomes assessment
Two reviewers independently evaluated the methodological quality of all included studies. A third reviewer recorded and resolved any disagreements. The Cochrane Collaboration Tool was utilized to assess the risk of bias for each RCT, including adequacy of sequence generation, concealment of allocation, blinding of participants and personnel, blinding of result evaluators, incomplete result data, and selective reporting (11, 12). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidelines for systematic reviews were followed to assess the quality of outcomes (13).
2.4 Data extraction
All included studies were conducted by two independent reviewers. If there was any disagreement, a third reviewer made the final decision. The following data were extracted from the included studies: basic information (study authors, year of publication), participant characteristics (age, time post-stroke, and sample size), rPMS parameters (site, frequency, intensity, and regimen of stimulation), outcome indicators of upper limb motor function, and activities of daily living (ADL).
2.5 Outcome indicators
The primary outcome included the Fugl-Meyer Assessment of the Upper Extremity (FMA-UE). Secondary outcome indicators for efficacy included the Modified Ashworth Scale (MAS), Functional Independence Measure (FIM), and Modified Barthel Index (MBI), as well as dropout rate and adverse effects.
As the first quantitative tool developed to assess the recovery of sensory and motor function after stroke, the FMA has been extensively tested in clinical settings and proven to be both feasible and effective in stroke. The scale is divided into five domains, namely motor function, sensory function, balance, joint range of motion, and joint pain. The FMA-UE evaluates the movement, coordination, and reflexes of the upper limbs (the shoulders, elbows, forearms, wrists, and hands), with its score ranging from 0 (hemiplegia) to 66 (normal motor performance) (14). The MAS is the most commonly used clinical tool for assessing muscle tension and spasticity. It is graded from 0 to 4 (0, 1, 1+, 2, 3, 4), where 0 indicates no resistance and 4 indicates limb stiffness during flexion or extension (15). The FIM, as a scale for evaluating functional independence, assesses 18 kinds of activities of daily living on a 7-point scale, ranging from 1 (completely dependent) to 7 (unassisted independent) (16). The MBI is a five-point scale used to measure activities of daily living, and Tomoko Ohura et al. affirmed its reliability and validity after stroke (17). The outcomes post-intervention from the follow-up phase were selected for meta-analysis if they were reported at multiple time points.
2.6 Statistical analyses
All statistical analyses were performed using RevMan 5.4 software (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Heterogeneity among studies was assessed using the chi-squared (Chi2) test and the I2 statistic. A fixed effects model was applied when heterogeneity was low (I2 <50%), whereas a random effects model was used when heterogeneity was substantial (I2 ≥ 50%). Dichotomous data were presented as risk ratios (RRs) with 95% confidence intervals (CIs), and continuous data were expressed as mean differences (MDs) or standardized mean differences (SMDs) with 95% CIs. Subgroup meta-analyses of the primary outcome were conducted based on predefined variables, such as disease stage, stimulation frequency, coil type, stimulation duration, and stimulation intensity. Combined effect sizes were calculated within each subgroup, and differences between the subgroups were compared.
3 Results
3.1 Search and selection of studies
The selection process of this study is shown in Figure 1. A total of 1,268 potentially relevant studies were screened from four English databases and CNKI using relevant search strategies. Then, 58 duplicates were removed, and 72 studies were excluded because they were too old to obtain the full text. An additional 1,028 studies were removed after screening titles and abstracts. Finally, after reviewing the full texts of the remaining 110 articles, a total of 12 studies were finally included.
Figure 1

PRISMA 2020 flow diagram for new systematic reviews including searches of databases and registers only.
3.2 Characteristics of the included studies
A total of 12 studies with 484 participants were included in this study. The characteristics of the included studies are presented in Table 1. The site, frequency, treatment intensity, number of pulses, on–off ratio, treatment duration, and coil type of rPMS stimulation differed between these studies.
Table 1
| Study | Participants | Sex | Age (mean/median) | Time post-stroke (mean/median) | Targeted points | Frequency intensity pulses | On/off (s) | Treatment time | Coil type | CG intervention | Additional intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Krewer et al. (24) | 63 | 25/38 | EG: 55.00 | EG: 26.00 weeks | Extensors and flexors of the upper arm | 25 Hz, above 10% MCT, 5000 | 1/2 | 20 min, 2 times/day, 2 weeks | Butterfly | Sham | OT | FMA-UE, MTS, BI |
| CG: 54.00 | CG: 37.00 weeks | |||||||||||
| Yang et al. (27) | 30 | 23/7 | EG: 63.67 | EG: 13.87 days | Supraspinatus, deltoid muscles | 5 Hz, 100% RMT | NA | 40 min, 4 weeks | Figure-of-eight | Conventional rehabilitation | NA | FMA-UE |
| CG: 67.20 | CG: 15.47 days | |||||||||||
| Chen et al. (18) | 32 | 23/9 | EG: 49.00 | EG: 37.40 month | From shoulder adductors to extensors, elbow flexors to extensors, wrist flexors to extensors | 5 Hz/20 Hz, 750/5100 | 3/1 | 30 min, one time | Parabola | Sham | NA | FMA-UE, MAS, MTS |
| CG: 45.60 | CG: 22.80 month | 1.5/1 | ||||||||||
| Obayashi and Takahashi (20) | 19 | 13/6 | EG: 64.30 | EG: 9.20 days | Extensor digitorum communis, extensor carpi radialis, flexor digitorum superficialis, triceps brachii, biceps brachii, anterior or middle head of deltoid | 30 Hz, 70% MSO | 2/2 | 15–20 min, until transfer | Round | Standard care | Standard care | FMA-UE, WMFT, FAS, BBT |
| CG: 72.30 | CG: 5.80 days | |||||||||||
| Jiang et al. (6) | 44 | 27/17 | EG: 54.60 | EG: 13.81 weeks | Triceps brachii | 20 Hz, 15–30% MSO, 2400 | 0.5/2 | 20 min, 2 weeks | Round | Untreated | PT | FMA-UE |
| CG: 56.09 | CG: 14.45 weeks | |||||||||||
| Ke et al. (25) | 30 | 14/12 | EG: 58 | EG: 17 days | Axilla | 20 Hz, 40–60% MSO, 1800 | 1/19 | 30 min, 10 days | Figure-of-eight | Sham | Conventional treatments | FMA-UE, MRC |
| CG: 56 | CG: 16 days | |||||||||||
| El Nahas et al. (23) | 42 | 27/9 | EG: 47.88 | NA | Biceps brachii, wrist/finger flexor group | 50 Hz, above MCT, 600 | 2/8 | 1,600 s, 8 days | Figure-of-eight | Sham | NA | MAS |
| CG: 41.60 | ||||||||||||
| Fawaz et al. (19) | 80 | 56/24 | 57.33 | NA | Shoulder abductors, elbow extensors, wrist extensors, supinator muscle | 30 Hz, above 10% MCT, 4500 | 5/1 | 30 min, 3 weeks | Round, butterfly | Sham | OT | FMA-UE, FIM |
| Wu et al. (9) | 30 | 27/3 | EG: 57.00 | EG: 31.89 days | Cervical nerve root | 10 Hz, 80% RMT | 1/5 | 1,000 s, 3 weeks | Round, figure-of-eight | Conventional rehabilitation | PT, OT | FMA-UE, WMFT, BBT |
| CG: 55.33 | CG: 41.58 days | |||||||||||
| Chang et al. (22) | 28 | 15/13 | EG: 51.40 | NA | Arm | 5 Hz, individually adjusted | 2/8 | 2 weeks | Figure-of-eight | Sham | PT, OT, iTBS | FMA-UE, ARAT, FIM |
| CG: 55.60 | ||||||||||||
| Xie et al. (26) | 40 | 29/10 | EG: 61.60 | EG: 33.05 days | Radial nerve (superficial course above the elbow joint) | 25 Hz, 120% RMT, 5000 | 5/15 | 15 min, 2 weeks | Figure-of-eight | Conventional rehabilitation | NA | iEMC, RMS, MF, FMA-UE, ARAT, MBI, MAS |
| CG: 64.00 | CG: 30.25 days | |||||||||||
| Fujimura (41) | 46 | 31/45 | EG: 69.00 | EG: 34.00 days | Shoulder, elbow, forearm, wrist, hand | 30 Hz, 0.65–0.9Tesla, 6,000 | 2/3 | 17 min, 6 weeks | NA | Conventional rehabilitation | NA | FMA-UE, AHI |
| CG: 61.00 | CG: 41.00 days |
Characteristics of the included studies.
EG, experimental group; CG, control group; MCT, muscle contraction threshold; RMT, resting motion threshold; MSO, maximal stimulator output; OT, occupational therapy; PT, physical therapy; iTBS, intermittent theta burst stimulation; FMA-UE, Fugl-Meyer Assessment of the Upper Extremity; MTS, Modified Tardieu Scale; MBI (BI), Modified Barthel Index (Barthel Index); MAS, Modified Ashworth Scale; WMFT, wolf motor function test; FAS, Functional Ability Scale; BBT, Box and Block Test; MRC, medical research council scale; FIM, Functional Independence Measure; ARAT, Action Research Arm Test; iEMC, integrated electromyography; RMS, root mean square; MF, median frequency; AHI, acromiohumeral interval.
A total of eight studies (18–24) stimulated more than two groups of upper limb muscles, with one study (6) stimulating only the triceps brachii muscle and another (25) stimulating the axilla of the affected arm. Furthermore, two studies targeted nerves: one at the cervical nerve root (9) and the other at the radial nerve above the elbow joint (26). The frequency of stimulation was ≤20 Hz in six studies (6, 9, 18, 22, 25, 27) and >20 Hz in six studies (19–21, 23, 24, 26). In addition, six studies (19, 21, 23, 24, 26, 27) determined the intensity of treatment based on the percentage of the maximum output value of the treatment apparatus, and four studies (6, 9, 20, 25) determined it based on the intensity of movement occurring in the wrist at rest. Moreover, one study (22) individualized treatment for patients, while one study (18) did not mention details. In total, five studies had a daily stimulation time of >20 min (18, 19, 23, 25, 27), six studies had a daily stimulation time of ≤20 min (6, 9, 20, 21, 24, 26), and one study (22) did not report the duration. There were seven studies (6, 18, 22–26) with a treatment duration of ≤2 weeks, four studies (9, 19, 21, 27) with a duration of >2 weeks, and one study (20) determined the duration based on the transfer time to the hospital. Six studies (18, 19, 22–25) used sham stimulation as the control, four studies (9, 21, 26, 27) used conventional rehabilitation, one study (20) used standard care, and one study (6) had no treatment in the control group.
3.3 Research quality
As shown in Figures 2, 3, the 12 included studies showed low risk of bias in terms of blinding of outcome assessment (detection bias) and selective reporting (reporting bias), indicating that these studies were relatively standardized in their design and execution. However, a small number of high-risk biases were identified in random sequence generation (selection bias), blinding of participants and personnel (performance bias), and incomplete outcome data (attrition bias), indicating potential statistical errors in these areas. In addition, five studies (6, 9, 19, 23, 24) were assessed as low risk and high quality in terms of methodological quality. There were four studies (18, 21, 26, 27) with high-risk indicators. Among them, Chen et al.’s study (18) showed a high risk of bias in random allocation and allocation concealment, which likely led to selection bias because of non-random allocation. In total, three studies (21, 26, 27) had a high risk of loss of follow-up bias in terms of data integrity. In addition, three studies (20, 22, 25) had unknown risks on a small number of indicators and showed low levels of bias. Overall, the included studies showed high methodological quality and a high evidence level.
Figure 2
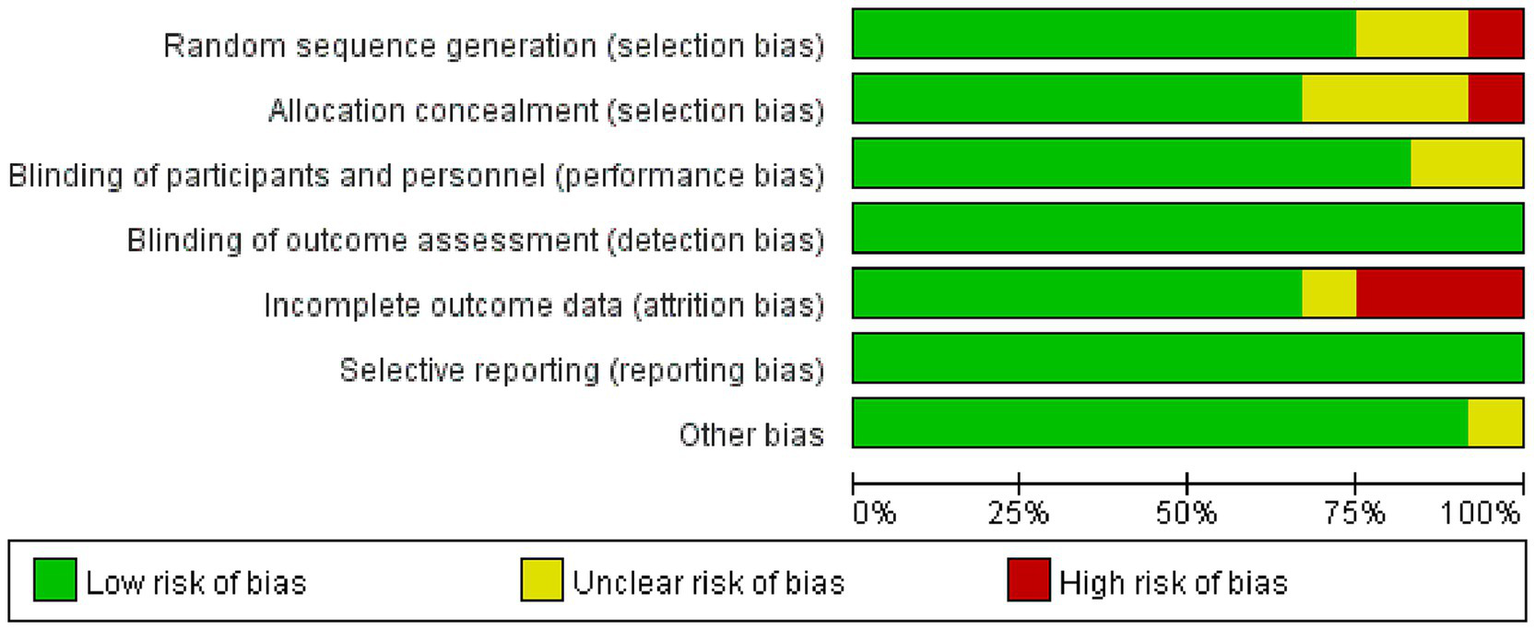
Summary of the risk of bias assessment.
Figure 3

Forest plot of the risk of bias assessment.
3.4 Meta-analysis of the FMA-UE
Furthermore, 11 studies (6, 9, 18–22, 24–27), involving a total of 442 participants, evaluated the effects of rPMS on the FMA-UE. Subgroup analyses were conducted based on disease stage, stimulation frequency, coil type, stimulation duration, and stimulation intensity. The meta-analysis conducted using a random effects model showed the following pooled SMDs: disease stage, SMD = 0.69 (95% CI 0.20–1.17, p = 0.006, I2 = 78%) (Figure 4); stimulation frequency, SMD = 0.58 (95% CI 0.19–0.98, p = 0.004, I2 = 74%) (Figure 5); coil type, SMD = 0.82 (95% CI 0.31–1.32, p = 0.001, I2 = 75%) (Figure 6); stimulation duration, SMD = 0.62 (95% CI 0.19–1.05, p = 0.004, I2 = 76%) (Figure 7); and stimulation intensity, SMD = 0.79 (95% CI 0.29–1.29, p = 0.002, I2 = 78%) (Figure 8).
Figure 4
![Forest plot showing a meta-analysis of studies comparing rPMS and control groups across acute, subacute, and chronic conditions. Each study’s effect size is represented by a green square, with confidence intervals as horizontal lines. Diamond shapes represent pooled effect sizes for each subgroup and overall. The overall effect size favors rPMS, with a standard mean difference of 0.69 [0.20, 1.17]. Test for heterogeneity varies by subgroup, with significant effects noted in subacute and total analyses.](https://www.frontiersin.org/files/Articles/1612490/xml-images/fneur-16-1612490-g004.webp)
Forest plot of the effects of rPMS on upper limb motor function in stroke patients at different disease stages.
Figure 5
![Forest plot showing a meta-analysis of studies comparing rPMS at different frequencies to control groups. Two subgroups, ≤20Hz and >20Hz, display standard mean differences with confidence intervals. The overall effect for ≤20Hz is 0.86 [0.19, 1.52] with significant heterogeneity (I² = 79%). For >20Hz, the effect is 0.27 [-0.09, 0.63] with less heterogeneity (I² = 47%). The total overall effect is 0.58 [0.19, 0.98]. Graphs on the right illustrate effect sizes with diamonds and bars. Heterogeneity and overall effect values are provided at the bottom.](https://www.frontiersin.org/files/Articles/1612490/xml-images/fneur-16-1612490-g005.webp)
Forest plot of the effects of rPMS on upper limb motor function in stroke patients at different stimulation frequencies.
Figure 6
![Forest plot comparing two types of coils, ‘figure-of-eight’ and ‘circular’, in rPMS studies. The diagram includes effect sizes and confidence intervals. For the figure-of-eight coil, the overall standard mean difference favors rPMS with a value of 0.92 and confidence interval [0.15, 1.68]. For the circular coil, the overall difference is 0.73 with a wide confidence interval [-0.03, 1.49]. For the total, the standard mean difference is 0.82 with a confidence interval [0.31, 1.32]. Heterogeneity statistics are provided for each subgroup and the total.](https://www.frontiersin.org/files/Articles/1612490/xml-images/fneur-16-1612490-g006.webp)
Forest plot of the effects of rPMS on upper limb motor function in stroke patients using different coil types.
Figure 7
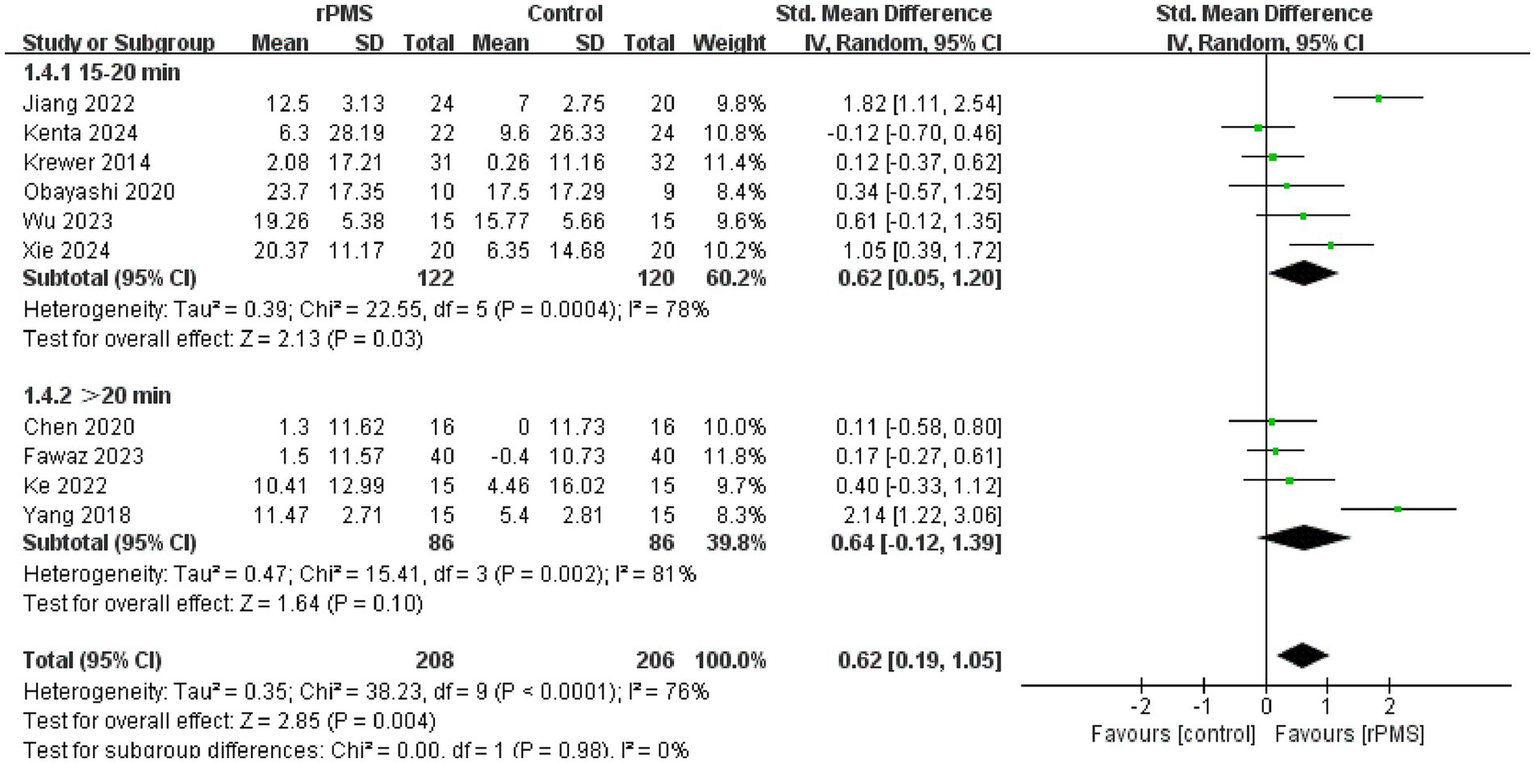
Forest plot of the effects of rPMS on upper limb motor function in stroke patients with different stimulation durations.
Figure 8
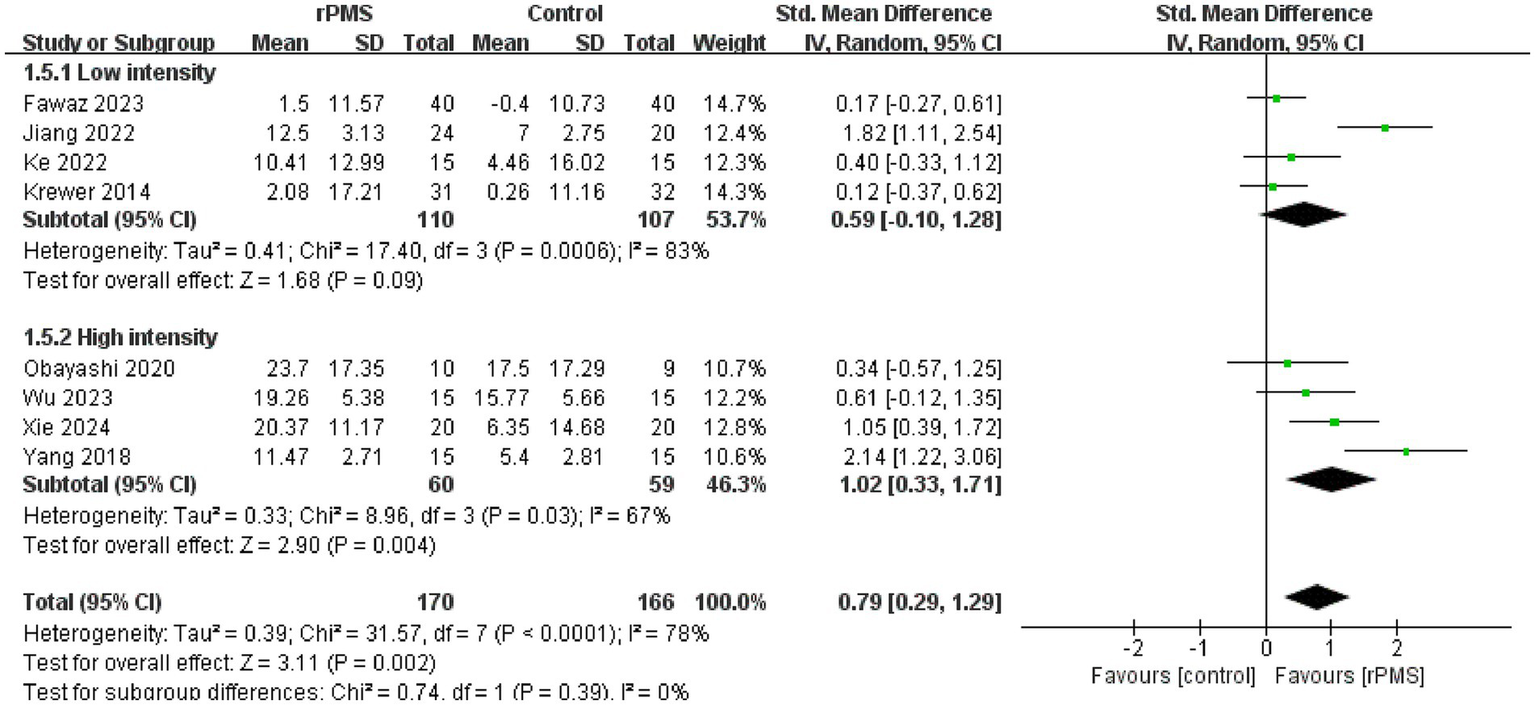
Forest plot of the effects of rPMS on upper limb motor function in stroke patients with different stimulation intensities.
In the disease stage subgroup, rPMS significantly improved upper limb motor function in patients during the subacute phase (SMD = 0.74, 95% CI 0.08–1.40, p = 0.03), while no significant effects were observed in acute-phase (SMD = 1.24, 95% CI −0.52–3.00, p = 0.17) or chronic-phase patients (SMD = 0.12, 95% CI −0.28–0.52, p = 0.56) (Figure 4). Regarding stimulation frequency, the ≤20 Hz subgroup showed significant improvement (SMD = 0.86, 95% CI 0.19–1.52, p = 0.01), whereas the >20 Hz subgroup did not demonstrate significant effects (SMD = 0.27, 95% CI −0.09–0.63, p = 0.14) (Figure 5). In terms of coil type, the figure-eight coil subgroup significantly enhanced FMA-UE scores (SMD = 0.92, 95% CI 0.15–1.68, p = 0.02), while the circular coil subgroup did not reach statistical significance (SMD = 0.73, 95% CI −0.03–1.49, p = 0.06) (Figure 6). Stimulation durations of 15–20 min showed significant effects (SMD = 0.62, 95% CI 0.05–1.20, p = 0.03, I2 = 78%), whereas durations over 20 min did not show significant differences (SMD = 0.64, 95% CI −0.12–1.39, p = 0.10) (Figure 7). For stimulation intensity, the high-intensity subgroup demonstrated significant effects (SMD = 1.02, 95% CI 0.33–1.71, p = 0.004), while the low-intensity subgroup did not reach statistical significance (SMD = 0.59, 95% CI −0.10–1.28, p = 0.09) (Figure 8).
3.5 Analysis of the MAS
A total of three studies (18, 21, 23), involving 114 participants, discussed the influence of rPMS on the MAS. The fixed effects analysis showed no significant improvement in upper limb spasticity compared to the control group (MD = 0.25, 95% CI −0.13−0.63, p = 0.20 > 0.05) (Figure 9).
Figure 9
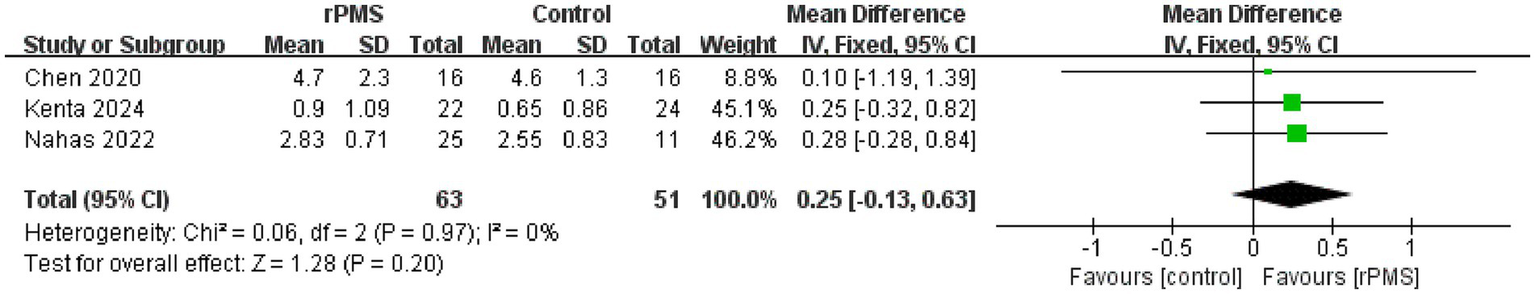
Forest plot of the effects of rPMS on spasticity in stroke patients with upper limb motor impairments.
3.6 Analysis of the FIM and MBI
A total of four studies (9, 19, 22, 26), involving 178 participants, discussed the FIM (19, 22) and MBI (9, 26). The fixed effects analysis showed that rPMS significantly improved patients’ ability to perform ADLs compared to the control group (SMD = 0.66, 95% CI 0.35–0.96, p < 0.0001) (Figure 10).
Figure 10
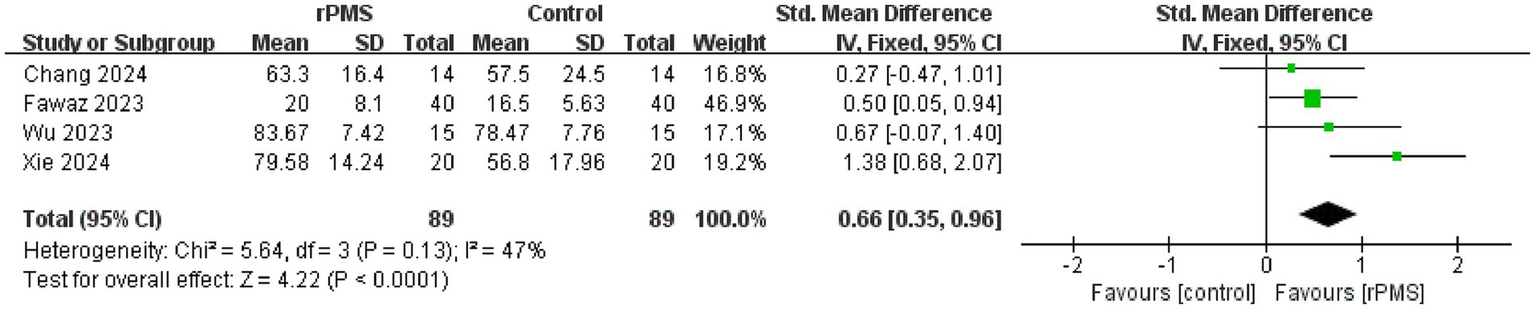
Forest plot of the effects of rPMS on activities of daily living in stroke patients with upper limb motor impairments.
3.7 Meta-analysis of the dropout rate and adverse events
A total of seven studies (18, 21, 23–27), involving 492 participants, evaluated the effects of rPMS on the dropout rate. Heterogeneity among the included studies was low (I2 = 25%, p = 0.24 > 0.05), and therefore a fixed effects model was used for meta-analysis. The results showed no significant difference between the rPMS group and the control group (RR = 0.99, 95% CI 0.65–1.50, p = 0.95) (Figure 11). Four participants in the control group dropped out due to temporary pain, and no adverse events were reported in the rPMS group in the study by Xie et al. (26).
Figure 11

Forest plot of the effects of rPMS on dropout rates in stroke patients.
3.8 GRADE
According to the GRADE assessment, the overall level of evidence for the effect of rPMS was “High” for the FMA-UE, “Low” for the MAS, “Moderate” for the FIM and MBI, and “Low” for the dropout rate. (Table 2).
Table 2
| Outcome indicator | Number of participants | Heterogeneity | Model of analysis | Group effect value | Estimated value | 95% CI | GRADE | |||
|---|---|---|---|---|---|---|---|---|---|---|
| I 2 | P | Z | P | |||||||
| FMA-UE | Disease stage | 334 (9RCT) | 78% | <0.0001 | Random effects | 2.76 | 0.006 | 0.69 (SMD) | 0.20, 1.17 | High |
| Stimulation frequency | 442 (11RCT) | 74% | <0.0001 | Random effects | 2.91 | 0.004 | 0.58 (SMD) | 0.19, 0.98 | High | |
| Coil type | 301 (8RCT) | 75% | 0.0002 | Random effects | 3.19 | 0.001 | 0.82 (SMD) | 0.31, 1.32 | High | |
| Stimulation duration | 414 (10RCT) | 76% | <0.0001 | Random effects | 2.85 | 0.004 | 0.62 (SMD) | 0.19, 1.05 | High | |
| Stimulation intensity | 336 (8RCT) | 78% | <0.0001 | Random effects | 3.11 | 0.002 | 0.79 (SMD) | 0.29, 1.29 | High | |
| MAS | 114 (3RCT) | 0% | 0.97 | Fixed effects | 1.28 | 0.20 | 0.25 (MD) | −0.13,0.63 | Low | |
| FIM&MBI | 178 (4RCT) | 47% | 0.13 | Fixed effects | 4.22 | < 0.0001 | 0.66 (SMD) | 0.35,0.96 | Moderate | |
| Dropout rate | 492 (11RCT) | 25% | 0.24 | Fixed effects | 0.06 | 0.95 | 0.99 (RR) | 0.65,1.50 | Low | |
GRADE quality of evidence profile.
4 Discussion
This study included 12 randomized controlled trials involving a total of 484 participants to systematically evaluate the efficacy of rPMS in treating upper limb dysfunction after stroke. The results demonstrated that rPMS significantly improved upper limb motor function, with particularly notable effects observed in subacute patients receiving treatment protocols characterized by a stimulation frequency of ≤20 Hz, figure-of-eight coils, stimulation durations of 15–20 min, and high intensity. In addition, rPMS effectively enhanced the activities of daily living in patients with upper limb dysfunction. Importantly, rPMS was found to be safe, with no reports of serious adverse effects.
A total of 11 studies (6, 9, 18–22, 24–27) assessed upper limb motor function using the FMA-UE, and the simulated results showed that rPMS could significantly improve upper limb motor function after stroke. A total of eight studies (9, 18, 19, 21, 22, 25–27) revealed that rPMS alone or in combination with physiotherapy and occupational therapy improved upper limb motor function. In addition, two studies (6, 20) suggested that the progress rates of the FMA-UE were significantly different between the two groups. These results are consistent with those of previous systematic reviews (28, 29). Nevertheless, Krewer et al. (24) reported no significant improvement in the motor function of the extensor and flexor muscles of the upper limb after rPMS intervention. There is no statistical evidence suggesting that combining rPMS and transcranial magnetic stimulation (TMS) is more effective than TMS alone in improving motor function (30). Based on the subgroup analysis, these findings may be related to variations in stimulation intensity and frequency, as well as the predominance of chronic-phase patients in the study populations.
The simulated results from the MAS revealed that rPMS had no significant effect on the spasticity of the upper limb. The studies by Chen et al. and Krewe et al. (18, 24) mentioned the use of the Modified Tardieu Scale (MTS) to measure spasticity in patients after stroke, but a meta-analysis could not be completed due to different MTS protocols. Interestingly, Krewe et al. (24) reported a long-term reduction in elbow extensor spasticity after 2 weeks of treatment, but a limited effect on overall upper limb spasticity. In contrast, another study (18) demonstrated an improvement in upper limb spasticity after rPMS, which is inconsistent with our results. Several factors may explain this discrepancy: (1) When focusing on the minimum clinically important difference in a single study, the scores of the MAS may improve by more than 1 point in some patients. However, this statistical difference may not be significant when the effect sizes are combined in a meta-analysis. This suggests that some overall effect sizes, although small yet clinically relevant, may be weakened when simulated. (2) There is variability in patient population and disease severity. There were differences between the studies at baseline in terms of disease duration and the extent of spasticity (baseline MAS score) in the included patients. Patients with higher levels of spasticity are more likely to show improvement with rPMS, while patients with milder spasticity may not show statistically significant changes.
The simulated results showed significant improvements in ADLs compared to the control group, which is consistent with the meta-analysis by Wang et al. (31), indicating that rPMS alone or in combination with rTMS can effectively improve ADLs after stroke. Fine motor movements of the shoulders, elbows, wrists, and fingers are crucial for improving ADL in patients after stroke. Previous studies have shown that, based on improvements in limb motor function in patients after stroke, there are also statistically significant improvements in ADLs (31, 32).
The possible mechanism by which rPMS improves upper limb motor dysfunction after stroke involves multiple neuroplastic regulatory processes. First, rPMS can significantly increase the amplitude of motor evoked potentials via high-frequency stimulation, reduce short-interval intracortical inhibition, and enhance intracortical facilitation. These changes suggest that rPMS can improve the output efficiency of the motor cortex by regulating the balance between inhibitory and excitatory circuits within the cortex (32, 33). For example, Nito et al. reported that 15 min of rPMS at 25 Hz or higher induced an increase in cortical excitability in the relevant area, potentially improving motor output (32). However, this conclusion differs from some subgroup analysis results, possibly due to unidentified underlying factors introducing bias, and therefore requires further research for validation. Second, rPMS treatment can activate neural activity within the superior posterior parietal lobe and premotor cortex, regions closely associated with motor planning and execution. This change may be related to the enhancement of afferent proprioceptive input and the promotion of functional reorganization within the sensorimotor network induced by rPMS, thereby further facilitating motor function recovery (34). Third, rPMS has no significant effect on Hoffmann’s reflex and the maximal M wave, suggesting that its effects are mainly concentrated above the spinal cord level (such as cortical or subcortical structures), rather than directly altering the excitability of spinal motor neurons (32, 33). Finally, animal studies suggest that molecular mechanisms related to rPMS may involve the PHR protein family (e.g., nematode RPM-1), which coordinate the development of motor neural networks by regulating axon termination and synapse formation (35). These underlying mechanisms may provide a molecular basis for the long-term plasticity induced by rPMS (35, 36).
The simulated results of this meta-analysis found that rPMS did not improve the degree of upper limb spasticity in patients after stroke. The possible mechanisms mainly involve three aspects. Firstly, the efficiency of rPMS depends on the frequency, intensity, and location of the stimulation. Low-frequency rPMS (5 Hz) may reduce spasticity by inhibiting spinal reflex arcs. However, if the maintenance of spasticity involves high-frequency abnormal discharges, low-frequency stimulation may not effectively improve spasticity, and the parameters need to be adjusted to match the pathophysiological characteristics (37, 38). In addition, A. Struppler et al. (39) found that rPMS is effective in mild to moderate spasticity but not in severe or fixed muscle contractures, suggesting that structural changes may counteract its neuromodulatory effects. Then, the causes of spasticity are complex and may be related to central sensitization (e.g., imbalance in corticospinal pathway inhibition) or peripheral nerve sensitization (e.g., increased sensitivity of muscle spindle) (38, 40). If spasticity is primarily driven by changes in the peripheral nerve structure (e.g., muscle fibrosis, overactivity of intrafusional γ motor neurons), rPMS may have a limited role in regulating the central sensorimotor network (38, 40). Thirdly, the role of rPMS depends, in part, on the activation of proprioceptive afferent fibers (Class Ia fibers) to regulate central motor control. rPMS may not be able to effectively deliver sensory input to the central nervous system, making it difficult to trigger cortical recombination or supraspinal inhibition with peripheral neuropathy or disturbance of sensory conduction (38, 39).
There are limitations in our meta-analysis. First, some outcome measures, such as the MAS, FIM, and MBI, were based on small sample sizes, which limits the stability and reliability of the effect estimates. Second, none of the included studies clearly reported the implementation of blinding, and some data were extracted from images, which may introduce selection bias. Third, despite conducting subgroup analyses, significant heterogeneity remained, suggesting the presence of unidentified potential confounding factors; therefore, the results should be interpreted with caution. Finally, substantial variability in stimulation sites across the studies made it difficult to perform unified subgroup analyses and is likely a major source of the observed heterogeneity. Future high-quality studies focusing on specific target sites are needed to systematically evaluate the therapeutic effects of different stimulation locations, thereby enhancing the accuracy of the conclusions and their clinical applicability.
5 Conclusion
The results indicate that rPMS can significantly improve upper limb motor function, ADLs, and self-care abilities in stroke patients, but its effect on spasticity relief is limited. Future research should focus on patients in the subacute phase of stroke and consider using protocols with a stimulation frequency ≤20 Hz, a figure-of-eight coil, a stimulation duration of 15–20 min, and high-intensity stimulation to further verify the efficacy of rPMS. To optimize rPMS treatment protocols, more high-quality studies with larger sample sizes and standardized outcome measures are needed to enhance the reliability and generalizability of the findings.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
JL: Data curation, Methodology, Formal analysis, Writing – original draft. MZ: Writing – original draft, Formal analysis, Data curation, Methodology. XL: Conceptualization, Methodology, Data curation, Writing – original draft. WT: Project administration, Writing – original draft, Software, Visualization. YunX: Software, Visualization, Project administration, Writing – original draft. YulX: Conceptualization, Writing – review & editing, Writing – original draft, Funding acquisition. YW: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We express our sincere gratitude to all authors for their valuable contributions to this study. This research was made possible through the collaborative efforts of each team member, who collectively contributed to the study design, data collection, analysis, and manuscript preparation. We appreciate the dedication and hard work of everyone involved in this project. Special thanks are also extended to the institutions that provided support and resources for this research. The successful completion of this study is a testament to the power of teamwork and shared scientific goals. We would like to acknowledge the hard work and dedication of all the staff members who implemented the intervention and evaluation components of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1612490/full#supplementary-material
References
1.
Feigin VL Stark BA Johnson CO Roth GA Bisignano C Abady GG et al . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2.
Hilkens NA Casolla B Leung TW de Leeuw FE . Stroke. Lancet. (2024) 403:2820–36. doi: 10.1016/S0140-6736(24)00642-1
3.
Kitsos GH Hubbard IJ Kitsos AR Parsons MW . The ipsilesional upper limb can be affected following stroke. Sci World J. (2013) 2013:684860. doi: 10.1155/2013/684860
4.
Beaulieu L Massé-Alarie H Camiré-Bernier S Beaulieu L-D Ribot-Ciscar É Schneider C . After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol Clin. (2017) 47:275–91. doi: 10.1016/j.neucli.2017.02.003
5.
Kamo T Wada Y Okamura M Sakai K Momosaki R Taito S . Repetitive peripheral magnetic stimulation for impairment and disability in people after stroke. Cochrane Database Syst Rev. (2022) 9:CD11968. doi: 10.1002/14651858.CD011968.pub4
6.
Jiang Y Zhang D Zhang J Jiang YF Hai H Zhao YY et al . A randomized controlled trial of repetitive peripheral magnetic stimulation applied in early subacute stroke: effects on severe upper-limb impairment. Clin Rehabil. (2022) 36:693–702. doi: 10.1177/02692155211072189
7.
Liang S Wang W Yu F Pan L Xu D Hu R et al . Repetitive peripheral magnetic stimulation combined with transcranial magnetic stimulation in rehabilitation of upper extremity hemiparesis following stroke: a pilot study. J Rehabil Med. (2024) 56:jrm19449. doi: 10.2340/jrm.v56.19449
8.
Momosaki R Yamada N Ota E Abo M . Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database Syst Rev. (2017) 6:CD11968. doi: 10.1002/14651858.CD011968.pub2
9.
Wu X Wang R Wu Q Liao C Zhang J Jiao H et al . The effects of combined high-frequency repetitive transcranial magnetic stimulation and cervical nerve root magnetic stimulation on upper extremity motor recovery following stroke. Front Neurosci. (2023) 17:1100464. doi: 10.3389/fnins.2023.1100464
10.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11.
Corbett MS Higgins JPT Woolacott NF . Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods. (2014) 5:79–85. doi: 10.1002/jrsm.1090
12.
Higgins JP Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al . The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13.
Guyatt GH Oxman AD Vist GE Kunz R Falck-Ytter Y Alonso-Coello P et al . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
14.
Gladstone DJ Danells CJ Black SE . The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. (2002) 16:232–40. doi: 10.1177/154596802401105171
15.
Harb A Kishner S . Modified ashworth scale In: StatPearls. Treasure Island (FL): StatPearls Publishing (2025)
16.
Mackintosh S. Functional independence measure. Aust. J. Physiother. (2009) 55:65. doi: 10.1016/S0004-9514(09)70066-2
17.
Ohura T Hase K Nakajima Y Nakayama T . Validity and reliability of a performance evaluation tool based on the modified Barthel index for stroke patients. BMC Med Res Methodol. (2017) 17:131. doi: 10.1186/s12874-017-0409-2
18.
Chen S Li Y Shu X Wang C Wang H Ding L et al . Electroencephalography mu rhythm changes and decreased spasticity after repetitive peripheral magnetic stimulation in patients following stroke. Front Neurol. (2020) 11:546599. doi: 10.3389/fneur.2020.546599
19.
Fawaz SI Izumi SI Zaki AS Eldiasty SE Saadawy A Saber HGE et al . Repetitive peripheral magnetic stimulation for improving upper limb function in post-stroke hemiparesis. Egypt Rheumatol Rehabil. (2023) 50:35. doi: 10.1186/s43166-023-00204-x
20.
Obayashi S Takahashi R . Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation. (2020) 46:569–75. doi: 10.3233/NRE-203085
21.
Fujimura K Kagaya H Itoh R Endo C Tanikawa H Maeda H . Repetitive peripheral magnetic stimulation for preventing shoulder subluxation after stroke: a randomized controlled trial. Eur J Phys Rehabil Med. (2024) 60:216–24. doi: 10.23736/S1973-9087.24.08264-9
22.
Chang C Chen C Chen R Chang CS Chen CL Chen RS et al . Synergistic efficacy of repetitive peripheral magnetic stimulation on central intermittent theta burst stimulation for upper limb function in patients with stroke: a double-blinded, randomized controlled trial. J Neuroeng Rehabil. (2024) 21:49. doi: 10.1186/s12984-024-01341-w
23.
El Nahas N Kenawy FF Abd Eldayem EH Roushdy TM Helmy SM Akl AZ et al . Peripheral magnetic theta burst stimulation to muscles can effectively reduce spasticity: a randomized controlled trial. J Neuroeng Rehabil. (2022) 19:5. doi: 10.1186/s12984-022-00985-w
24.
Krewer C Hartl S Müller F Koenig E . Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double-blind, sham-controlled study. Arch Phys Med Rehabil. (2014) 95:1039–47. doi: 10.1016/j.apmr.2014.02.003
25.
Ke J Wei J Zheng B Tan T Zhou W Zou X et al . Effect of high-frequency repetitive peripheral magnetic stimulation on motor performance in intracerebral haemorrhage: a clinical trial. J Stroke Cerebrovasc Dis. (2022) 31:106446. doi: 10.1016/j.jstrokecerebrovasdis.2022.106446
26.
Xie Y Lin J Liu Y Cai Y Lian X Ding L et al . Effect of repeated peripheral magnetic stimulations at different sites of upper limbs on wrist motor function in subacute stroke patients: a randomized controlled trial. Chin Gen Pract. (2024) 27:2846–52. doi: 10.12114/j.issn.1007-9572.2024.0081
27.
Yang C Chen P Du W Chen Q Yang H Su M . Musculoskeletal ultrasonography assessment of functional magnetic stimulation on the effect of glenohumeral subluxation in acute poststroke hemiplegic patients. Biomed Res Int. (2018) 2018:1–9. doi: 10.1155/2018/6085961
28.
Fernanda Silva G Campos LF de Aquino Miranda JM Guirro Zuliani F de Souza Fonseca BH de Araújo AET et al . Repetitive peripheral sensory stimulation for motor recovery after stroke: a scoping review. Top Stroke Rehabil. (2024) 31:723–37. doi: 10.1080/10749357.2024.2322890
29.
Liu H Pan J Jia Y . Application of repetitive peripheral magnetic stimulation for recovery of motor function after stroke based on neuromodulation: a narrative review. Brain Netw Modul. (2022) 1:13–9. doi: 10.4103/2773-2398.340140
30.
Qin Y Liu X Zhang Y Wu J Wang X . Effects of transcranial combined with peripheral repetitive magnetic stimulation on limb spasticity and resting-state brain activity in stroke patients. Front Hum Neurosci. (2023) 17:992424. doi: 10.3389/fnhum.2023.992424
31.
Wang Y Fong KNK Sui Y Bai Z Zhang JJ . Repetitive peripheral magnetic stimulation alone or in combination with repetitive transcranial magnetic stimulation in poststroke rehabilitation: a systematic review and meta-analysis. J Neuroeng Rehabil. (2024) 21:181. doi: 10.1186/s12984-024-01486-8
32.
Nito M Katagiri N Yoshida K Koseki T Kudo D Nanba S et al . Repetitive peripheral magnetic stimulation of wrist extensors enhances cortical excitability and motor performance in healthy individuals. Front Neurosci. (2021) 15:632716. doi: 10.3389/fnins.2021.632716
33.
Gallasch E Christova M Kunz A Rafolt D Golaszewski S . Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Front Hum Neurosci. (2015) 9:407. doi: 10.3389/fnhum.2015.00407
34.
Struppler A Binkofski F Angerer B Bernhardt M Spiegel S Drzezga A et al . A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: a PET-H2O15 study. NeuroImage. (2007) 36:T174–86. doi: 10.1016/j.neuroimage.2007.03.033
35.
Schaefer AM Hadwiger GD Nonet ML . Rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. (2000) 26:345–56. doi: 10.1016/S0896-6273(00)81168-X
36.
Opperman KJ Grill B . RPM-1 is localized to distinct subcellular compartments and regulates axon length in GABAergic motor neurons. Neural Dev. (2014) 9:10. doi: 10.1186/1749-8104-9-10
37.
Zschorlich VR Hillebrecht M Tanjour T Qi F Behrendt F Kirschstein T et al . Repetitive peripheral magnetic nerve stimulation (rPMS) as adjuvant therapy reduces skeletal muscle reflex activity. Front Neurol. (2019) 10:930. doi: 10.3389/fneur.2019.00930
38.
Beaulieu LD Schneider C . Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. a review. Neurophysiol Clin. (2013) 43:251–60. doi: 10.1016/j.neucli.2013.05.003
39.
Struppler A Havel P Müller-Barna P . Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS)—a new approach in central paresis. NeuroRehabilitation. (2003) 18:69–82. doi: 10.3233/NRE-2003-18108
40.
Suputtitada A . Emerging theory of sensitization in post-stroke muscle spasticity. Front Rehabil Sci. (2023) 4:1169087. doi: 10.3389/fresc.2023.1169087
41.
Fujimura K Kagaya H Itoh R Endo C Tanikawa H Maeda H . Repetitive peripheral magnetic stimulation for preventing shoulder subluxation after stroke: a randomized controlled trial. Eur. J. Phys. Rehabil. Med. (2024) 60:216–224.
Summary
Keywords
repetitive peripheral magnetic stimulation, stroke rehabilitation, upper limb motor function, systematic review, meta-analysis, stroke
Citation
Liu J, Zhu M, Liu X, Tang W, Xiang Y, Xie Y and Wang Y (2025) Efficacy of repetitive peripheral magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 16:1612490. doi: 10.3389/fneur.2025.1612490
Received
19 May 2025
Accepted
27 August 2025
Published
18 September 2025
Volume
16 - 2025
Edited by
Elisa Kallioniemi, New Jersey Institute of Technology, United States
Reviewed by
Fangling Sun, Capital Medical University, China
Rita Huan-Ting Peng, University of Illinois at Urbana-Champaign, United States
Updates
Copyright
© 2025 Liu, Zhu, Liu, Tang, Xiang, Xie and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulei Xie, xieyulei123@foxmail.com; Yinxu Wang, 34089681@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.