- 1Unit of Neurorehabilitation, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
- 2Santa Cecilia Conservatory of Music, Rome, Italy
- 3Euterpe APS Cultural Association, Rome, Italy
- 4Insieme Association, Sahel Alma, Lebanon
- 5School of Music and Performing Arts, Holy Spirit University of Kaslik, Jounieh, Lebanon
- 6Faculty of Law, Université La Sagesse, Furn El Chebbak, Lebanon
- 7Neurorehabilitation Research Area, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
Neurodevelopmental disorders (NDD), as defined by DSM-5-TR and CDDR, comprise heterogeneous early-onset conditions involving executive dysfunction, motor planning deficits, language impairments, and socio-emotional dysregulation. Evidence from neuroimaging and clinical studies suggests that music-based interventions may engage distributed neural networks—including fronto-striatal, temporo-parietal, limbic, and brainstem circuits—through predictive timing, cross-modal synchronization, and adaptive plasticity. However, clinical translation has been hindered by methodological heterogeneity, insufficient standardization, and reduced reproducibility, together with limited integration of clinical, functional, and neurophysiological indicators, absence of unified protocols combining individualized and orchestral modules with explicit transfer mechanisms, and insufficient monitoring of fidelity and multisite feasibility. This perspective proposes the IncluSive Orchestral mUsic therapy accordiNg to the euterpe methoD (I-SOUND), a clinically adapted orchestral framework structured to integrate three complementary modules: Individual Music Therapy (IMT), an Orchestral Music Therapy module (OMT), and a Multidirectional and Iterative Transfer Process (MIT-P). Developed from the progressive refinement of the Euterpe Method and the pediatric EM Active algorithm, the model is intended to target specific neurofunctional domains and to explore generalization to everyday contexts. A two-phase evaluation—comprising an observational study followed by a randomized controlled trial—is planned to assess feasibility, fidelity, sustainability, and clinical applicability in heterogeneous NDD populations. Particular attention is given to the methodological challenge of balancing ethical inclusion with internal validity. No efficacy claims are advanced, as the framework requires empirical verification before clinical conclusions can be drawn.
1 Introduction
Neurodevelopmental disorders (NDD), as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) (1) and the Clinical Descriptions and Diagnostic Requirements for ICD-11 Mental, Behavioral and Neurodevelopmental Disorders (CDDR) (2), are early-onset, heterogeneous conditions affecting executive function, motor planning, language, and socio-emotional regulation. Prevalence estimates vary owing to differences in diagnostic criteria, assessment methods, and systemic disparities, underscoring the need for rigorous, inclusive research designs (3).
From a neurofunctional perspective, the orchestra can be framed as a multisensory relational system characterized by temporal synchronization, hierarchical coordination, and functional differentiation. When clinically contextualized, it may engage motor, cognitive, and socio-affective systems in line with adaptive plasticity (4). Neuroimaging and neurophysiology indicate that individual and ensemble practice recruit distributed cortical–subcortical networks mediating auditory, motor, and affective integration (5–8), consistent with predictive timing, sensorimotor coupling, interpersonal synchronization, and neuromodulatory processes. Because most findings derive from neurotypical samples, these mechanisms remain hypotheses requiring targeted verification in NDD.
Clinically adapted orchestral music-making may operate as a multimodal enriched therapeutic environment supporting combined sensory, motor, and social stimulation (9, 10). However, persistent gaps include protocol heterogeneity, limited standardization and reproducibility, and insufficient integration of clinical, functional, and neurophysiological indicators. Designs must also balance ethical inclusion with internal validity where baseline variability and comorbidities complicate interpretation. Current literature lacks unified protocols combining individual and orchestral music therapy with explicit transfer mechanisms, fidelity monitoring, and multisite feasibility assessments (11–13).
The present framework results from progressive refinement of the Euterpe Method in pediatric and adolescent NDD cohorts, where diagnostic criteria, outcome indicators, and modular structures were operationalized. Prior studies reported adaptability in home-based telerehabilitation (14), targeted interventions for cerebral palsy (CP) (15), and methodological structuring of neurofunctional algorithms (16), leading to the EM Active procedural model and related algorithms for diverse contexts. Building on this platform, individual and orchestral modules were clinically expanded for adolescents and young adults with NDD. The IncluSive Orchestral mUsic therapy accordiNg to the euterpe methoD (I-SOUND) model is thus proposed as a methodological framework intended to: reconcile inclusion with methodological integrity, integrate multimodal neurofunctional targets, and explore reproducibility in heterogeneous clinical populations.
I-SOUND comprises: (i) Individual Music Therapy (IMT), targeting domain-specific outcomes; (ii) Orchestral Music Therapy (OMT), an ensemble-based module promoting interpersonal synchronization and hierarchical coordination; and (iii) the Multidirectional and Iterative Transfer Process (MIT-P), a regulatory mechanism for cross-context generalization and consolidation. The model is evaluated through a two-phase design: Phase 1, a longitudinal observational protocol adhering to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (17); and Phase 2, a randomized controlled trial (RCT) aligned with the Consolidated Standards of Reporting Trials (CONSORT) (18, 19); interventions are described using the Template for Intervention Description and Replication (TIDieR) (20).
2 State of the art in music-based interventions for neurodevelopmental disorders
2.1 Neurofunctional rationale
Ensemble music-making constitutes a temporally structured, multimodal environment with differentiated roles and hierarchical coordination (9, 10). Computational and theoretical models suggest engagement of predictive coding for social synchronization and sensorimotor coupling (8, 21). In typically developing populations, activity spans fronto-striatal, temporo-parietal, limbic, and brainstem circuits contributing to entrainment and alignment (5–7, 22).
Structured music training has been associated with experience-dependent plasticity and enriched-environment effects across motor, cognitive, and socio-affective systems (4, 10, 12, 23, 24). Reported neurostructural adaptations include enhanced interhemispheric connectivity, reorganization of motor regions, and strengthened audio–motor coupling in pediatric and adult cohorts (7, 25, 26). These processes are salient during the 9–25-year developmental window, when sensorimotor, executive, and socio-affective systems show heightened susceptibility to experience-driven modulation (27–29).
Overall, these findings support the hypothesis that clinically adapted orchestral practice could function as a multimodal enriched environment during sensitive developmental windows. Within this perspective, I-SOUND is introduced as an exploratory framework to examine such hypotheses in NDD through sequential individual–ensemble modules and regulatory transfer mechanisms aimed at ecological validity and reproducibility (15, 16).
2.2 Neurobiological substrates of musical interaction
Evidence on neural substrates of musical interaction in NDD is limited; most data derive from neurotypical or mixed samples. Neuroimaging and electrophysiology suggest that ensemble music can enhance audio–motor coupling, engage mirror neuron systems, and modulate dopaminergic and serotonergic pathways, although current evidence derives predominantly from cross-sectional studies and remains preliminary (4–7). Predictive coding accounts posit minimization of prediction errors in melody, rhythm, and harmony, potentially improving synchrony (8). Groove-rich music and salient visual cues may promote motor engagement and alignment (21), recruiting fronto-striatal circuits, insula, and anterior cingulate cortex—regions implicated in timing, emotion, and social regulation. Group-based musical practices have also been associated with changes in neurotrophic and stress-related indices, but these findings remain exploratory and are not the focus of the present program, which prioritizes clinically validated functional outcomes. These mechanisms remain plausible targets requiring structured testing in NDD, which I-SOUND is designed to explore.
2.3 Ensemble-based approaches without controlled clinical validation
Several ensemble programs were conceived primarily for psychosocial inclusion rather than as trial-ready clinical protocols; their evidence base is largely observational or pilot-level, including El Sistema (22, 30–33), Nordoff-Robbins (34), Strokestra (35, 36), Esagramma (37), AllegroModerato (38), and community-oriented frameworks such as Community Music Therapy (39–45). These initiatives prioritize expressive and relational aims and provide useful observations—for example, groove-rich repertoires and salient visual interaction may support coordination and prosocial behaviors (21). Yet most lack standardized eligibility criteria, fidelity thresholds, prespecified endpoints, or multisite procedures with blinded assessment in pediatric or transitional-age NDD cohorts (46, 47).
In contrast, I-SOUND may be described as a clinical framework that proposes to translate these inclusive premises into a trial-ready structure, organized around the modular sequence IMT-OMT-MIT-P. Its design is intended to align with DSM-5-TR/CDDR diagnostic criteria, to incorporate predefined fidelity metrics with an intraclass correlation coefficient (ICC) ≥ 0.80, and to follow a two-phase methodology (Phase 1 STROBE; Phase 2 CONSORT) aimed at enhancing transparency, reproducibility, and clinical applicability. In this context, I-SOUND is introduced as a tentative clinical framework differing by: (i) a standardized IMT–OMT sequence regulated by MIT-P; (ii) TIDieR-compliant specification with fidelity thresholds (ICC ≥ 0.80) and replication materials; (iii) a two-phase design (Phase 1 STROBE; Phase 2 CONSORT) with stratified randomization and blinded outcomes; and (iv) multicenter feasibility through predefined adaptations and accredited provider training (20, 46, 47).
2.4 Evidence in neurodevelopmental disorders
Music-based interventions (MBI) in NDD have reported effects in motor, language, and socio-emotional domains, particularly in autism spectrum disorder (ASD), CP, and selected genetic syndromes (46, 48–50). Modalities include Rhythmic Auditory Stimulation (RAS), therapeutic singing, and instrumental training, with substantial heterogeneity in dosage, duration, and complexity. Reviews indicate that music may support functional and structural adaptations, and that ensemble formats could contribute to socially mediated plasticity (10, 23). Nonetheless, most investigations are modality-specific and seldom integrate individualized and collective modules within stratified cohorts. No controlled studies have validated an orchestral framework explicitly combining IMT, OMT, and MIT-P in NDD, nor examined multisite feasibility with blinded assessment and fidelity monitoring.
2.5 Gap analysis
Despite growing interest, the MBI literature in NDD is marked by methodological variability, short follow-up, and limited standardization (46, 51, 52). Incorporating predictive-processing models and groove-mediated engagement within structured designs may clarify ensemble-driven plasticity. Yet interventions rarely include predefined fidelity metrics, stratification strategies, or blinded assessments. To our knowledge, no published protocol integrates IMT, OMT, and MIT-P within a unified, developmentally calibrated orchestral framework with systematic clinical monitoring and multicenter evaluation. I-SOUND was therefore developed as a cautious methodological proposal aligned with DSM-5-TR/CDDR, operationalizing neurofunctional premises through MIT-P cycles and sequencing modules to accommodate developmental transitions. Generalization is conceptualized as near and far transfer, operationalized through time-defined MIT-P cycles to support consolidation and cross-context application.
3 Methods and study design
This two-phase program investigates I-SOUND in individuals with NDD (DSM-5-TR, CDDR) (1, 2), aged 9–25 years in Phase 1 and 18–25 years in Phase 2, eligible if functionally able to join structured sessions. Exclusion criteria include uncorrected sensory deficits, unstable conditions, contraindications to group participation, and profiles incompatible with standardized testing as detailed in Table 1. The developmental window was chosen for sensitivity to experience-dependent plasticity across motor, cognitive, and socio-affective systems (27–29).
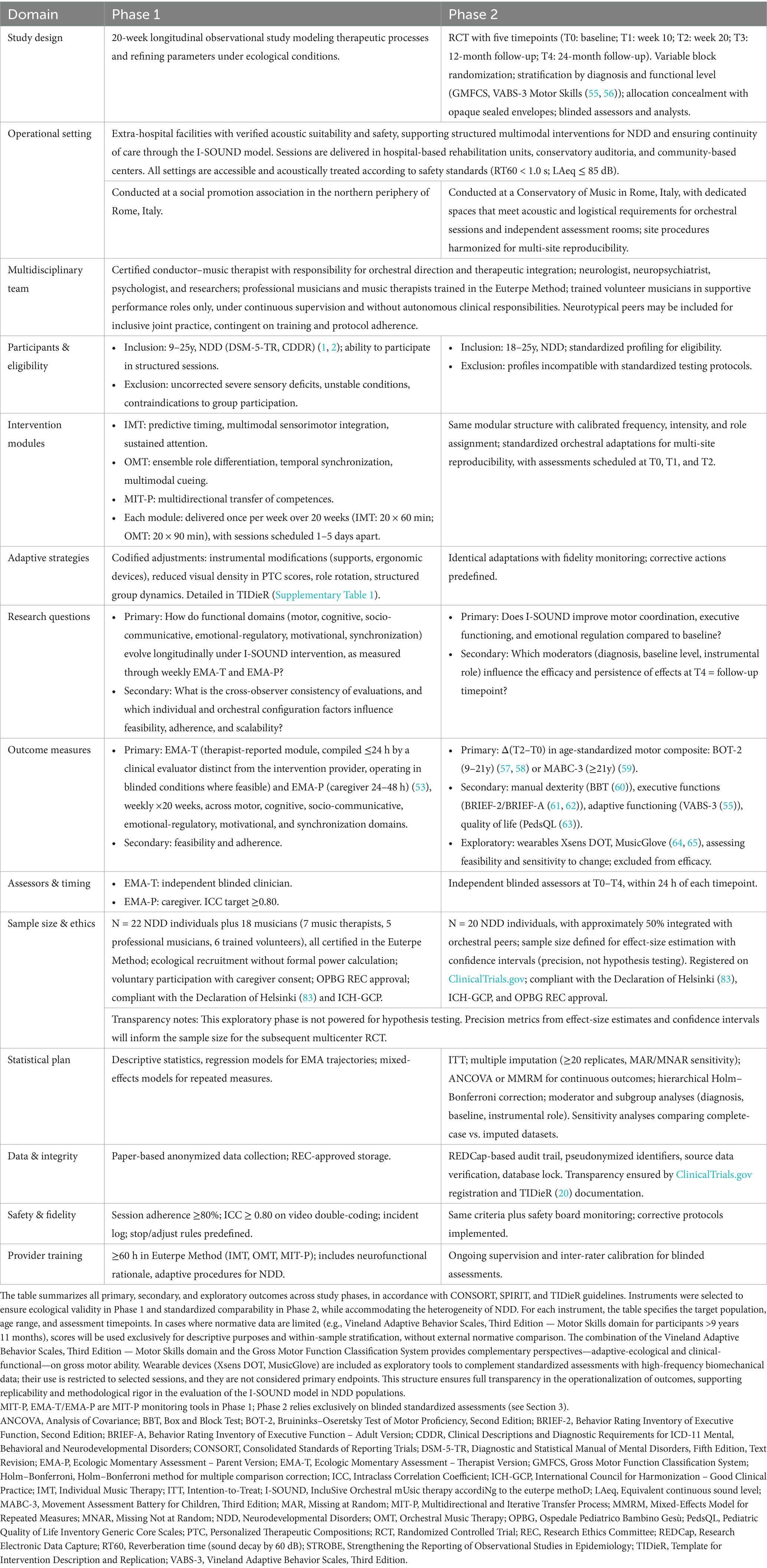
Phase 1, a 20-week longitudinal observational study aligned with STROBE (17), employs weekly Ecological Momentary Assessments (EMA) (53), developed by clinical experts: therapist-reported (EMA-T), compiled within 24 h by an evaluator distinct from the intervention provider under blinded conditions, and caregiver-reported (EMA-P), completed within 48 h, encompassing motor, cognitive, socio-communicative, emotional-regulatory, motivational, and synchronization domains. An independent co-rating is performed on a 20–25% sample of sessions; fidelity requires an ICC ≥ 0.80. Each weekly cycle comprised one IMT session (60 min) and one OMT session (90 min), scheduled 1–5 days apart, resulting in a total of 20 sessions per module across the 20-week program. Descriptive and regression analyses map trajectories; procedures and fidelity safeguards appear in Supplementary materials 1, 2 and Table 1.
Phase 2 is an RCT aligned with CONSORT (18, 19), with assessments scheduled at T0 (baseline), T1 (week 10), T2 (week 20), T3 (12-month follow-up), and T4 (24-month follow-up). The choice of 10- and 20-week intervals allows detection of short- and medium-term changes, while annual and biennial follow-ups provide information on maintenance and long-term trajectories, consistent with literature on outcome monitoring in NDD (54). Randomization uses variable blocks stratified by diagnosis and functional level through the Gross Motor Function Classification System and the Motor Skills domain of the Vineland Adaptive Behavior Scales, Third Edition (55, 56). Allocation concealment is performed with independent opaque envelopes; assessors and analysts remain blinded. Outcomes include the Bruininks–Oseretsky Test of Motor Proficiency, Second Edition for ages 9–21 years (57, 58) or the Movement Assessment Battery for Children, Third Edition for ages above 21 years (59) as the age-standardized motor composite, which represents the primary endpoint Δ(T2–T0). Secondary outcomes include the Box and Block Test (60), the Behavior Rating Inventory of Executive Function, Second Edition and Behavior Rating Inventory of Executive Function–Adult Version (61, 62), the Vineland Adaptive Behavior Scales, Third Edition (55), and the Pediatric Quality of Life Inventory (63). Exploratory wearables such as Xsens DOT and MusicGlove (64, 65) provide parallel monitoring of feasibility and sensitivity to change but are excluded from efficacy testing. Further implementation details are in Table 1.
Variability and bias are managed through age-calibrated tools, stratification, blinded assessments, and a Research Electronic Data Capture (REDCap) system with role-based access control and complete audit trail. Adaptive strategies including instrumental modifications, role rotation, reduced visual density of Personalized Therapeutic Compositions (PTC) scores, and group management are pre-specified for replicability and codified in Table 1 and the TIDieR’s Supplementary Table 1. Analyses include intention-to-treat, multiple imputation with at least 20 replicates and both missing-at-random and missing-not-at-random sensitivity, analysis of covariance or mixed-effects model for repeated measures, hierarchical Holm–Bonferroni, as well as moderator and subgroup analyses by diagnosis, baseline profile, and instrumental role. Sensitivity analyses will compare complete-case and imputed datasets. Safety monitoring includes predefined stop and adjust rules, adverse-event logs, fidelity thresholds of at least 80% adherence, and inter-rater reliability with ICC ≥ 0.80 on double coded video material. Providers receive at least 60 h of training in the Euterpe Method across IMT, OMT, and MIT-P to ensure intervention fidelity. Transparency is reinforced by registration on ClinicalTrials.gov and TIDieR documentation in Table 1 and the Supplementary Table 1.
3.1 Modular structure and clinical adaptation
I-SOUND, a clinically intensive extension of the Euterpe Method, is structured according to TIDieR (20) and integrates principles of experience-dependent plasticity (29), stratified functional targeting, and adaptive musical codification (Figure 1). IMT and OMT are derived from the EM Active algorithm, initially designed for pediatric cohorts and later adapted for adolescents and young adults with NDD. EM Active functions as a clinical device supporting adaptive resilience and longitudinal functional targeting, maintaining methodological continuity across developmental phases (15, 16).
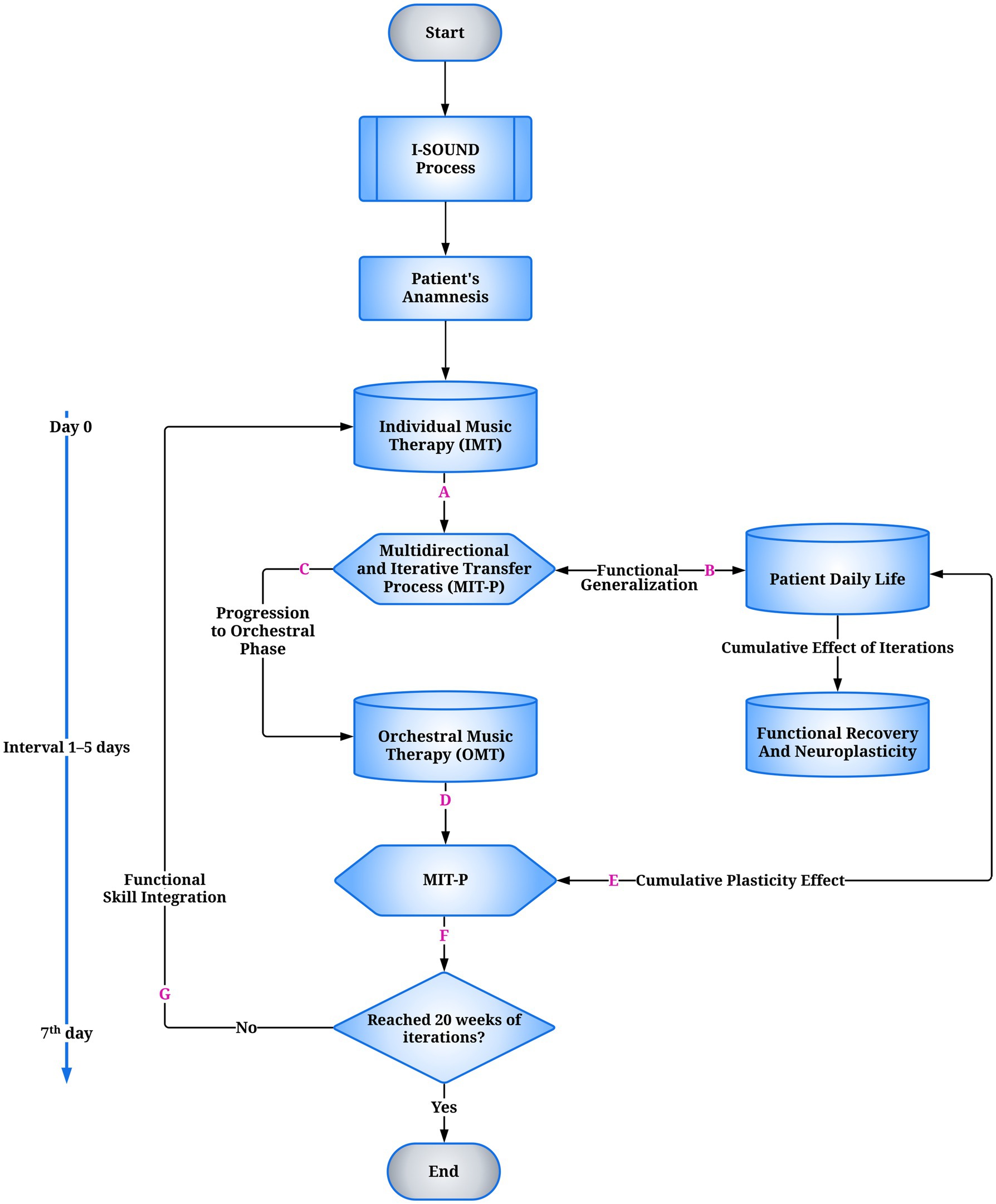
Figure 1. Flowchart of the I-SOUND model for neurodevelopmental disorders (NDD). The process begins with the clinical-functional assessment, which guides the individualized intervention. This is followed by Individual Music Therapy (IMT), targeting predictive timing, multimodal sensorimotor integration, and sustained attention. At the end of each cycle, the Multidirectional and Iterative Transfer Process (MIT-P) (A) regulates functional outcomes: if transferable, they are functionally transposed into ecological daily-life contexts (B); if adequate, they allow progression to the orchestral phase (C). Orchestral Music Therapy (OMT) sessions are not co-delivered on the same day as IMT but are scheduled exclusively after an inter-session latency of 1–5 days, during which MIT-P mediates the stabilization and inter-contextual transfer of functional gains. Within OMT, MIT-P supports intermodular coherence and skill integration (D). Both trajectories converge toward functional recovery and experience-dependent neuroplasticity (E). A decision node (F) verifies the predefined temporal threshold of 20 weeks of iterative cycles: if met, the process concludes (End); if not, the loop restarts (G). The temporal structure includes micro cycles (Day 0, 1–5-day interval) and weekly macrocycles (7th day). IMT, Individual Music Therapy; OMT, Orchestral Music Therapy; MIT-P, Multidirectional and Iterative Transfer Process.
3.1.1 Modules and methodological chronology
• IMT: 60-min individual sessions targeting predictive timing, multimodal sensorimotor integration, and sustained attention.
• OMT: 90-min orchestral sessions extending IMT-acquired skills into hierarchical ensemble structures. Clinically adapted conducting techniques—gestural segmentation, temporal modulation, multimodal cueing, and role distribution—are combined with therapeutic materials, including Compositional Sound Interventions (CSI) and PTC, to align orchestral execution with individualized neurofunctional objectives (16). Replicable adaptations include adjustable chin/hand supports, low-density color-coded PTC staves, programmed role rotation, and scheduled ‘quiet breaks’ for attention regulation, all formalized in Table 1 and Supplementary Table 1.
• MIT-P: a transversal regulatory mechanism sustaining inter-modular coherence and consolidation through cycles of active practice, consistent with adaptive motor-learning models and therapeutic context modulation (66). Evidence indicates transfer to untrained tasks even without spatiotemporal similarity (67). MIT-P integrates automatic and reflective components (low/high-road transfer) (68–70), supported by experience-modulated cortical plasticity and transient disinhibition dynamics (24).
4 Discussion
The present framework derives from the progressive refinement of the Euterpe Method in pediatric and adolescent NDD cohorts, where clinical criteria, assessment tools, and modular structures were delineated. Its adaptability has been documented in telerehabilitation protocols (14), targeted interventions for CP (15), and the methodological formalization of neurofunctional procedures (16). This formalization evolved into four complementary algorithms—EM Hospital-based, EM Active, EM Receptive, and EM Telerehabilitation—that, despite different contexts and aims, provided a unified platform for clinical translation. On this foundation, orchestral adaptations were developed through clinically oriented conducting techniques and dedicated compositional materials, forming the basis for OMT. Within this modular progression, the I-SOUND model is framed as a structured extension designed to address persistent methodological challenges: balancing ethical inclusion with internal validity (STROBE, CONSORT), integrating individual and orchestral domains through MIT-P mechanisms, and pursuing reproducibility in heterogeneous NDD populations, consistent with TIDieR criteria (17–20).
4.1 Theoretical framing and methodological positioning
I-SOUND is a modular, cyclic framework addressing three recurrent gaps: limited integration of individual and ensemble modalities, absence of a formalized transfer mechanism, and lack of standardized, reproducible procedures. Its architecture aligns with neurofunctional substrates implicated in adaptive plasticity, predictive timing, and cross-modal synchronization (71). The design seeks to balance ethical inclusion with internal validity through adaptive eligibility and implementation strategies. Within this framework, MIT-P coordinates transfer between IMT and OMT via temporally defined practice sequences, supporting learning, consolidation, and fidelity across heterogeneous profiles. Distinct from community-oriented programs, I-SOUND incorporates prespecified fidelity thresholds (e.g., ICC ≥ 0.80), stratified randomization, blinded assessment, multicenter planning, and systematic video coding by multiple trained raters to mitigate observational bias (72).
4.2 Scientific and clinical implications
IMT targets predictive timing and multimodal sensorimotor integration; OMT extends these capacities in structured ensemble contexts. MIT-P ensures inter-modular coherence through repeated cycles (66), with potential improvements in untrained tasks (67), conceptualized as near and far transfer and integrating automatic and reflective components (68–70), hypothetically engaging cortical plasticity and transient disinhibition (24). Collectively, modules could activate bilateral audio–motor networks, including supplementary motor area, premotor cortex, basal ganglia, and cerebellum, consistent with beat-based mechanisms (73, 74). Hierarchical synchronization and role differentiation, interpreted within predictive-coding models, may reduce error signals and support emotional regulation and interpersonal coordination (8). Observations from collective music-making in neurotypical cohorts point to improvements in motor control, emotion regulation, and plasticity, which require empirical verification in NDD. High-groove music and visual social cues have been associated with increased movement energy and coordination in ensembles (21). Plasticity studies suggest that instrumental practice can modulate fronto-parietal and cerebellar activity, with auditory and striatal measures predicting learning rate; preliminary evidence indicates modulation of neurotrophic and stress-related biomarkers, although findings remain exploratory (10, 23, 75, 76). Caregiver involvement, ecological momentary assessment, and wearable sensors are positioned to increase ecological validity, personalize parameters, and enhance sensitivity to change; wearables remain exploratory and excluded from efficacy analyses.
4.3 Methodological strengths and core limitations
The biphasic design—observational modeling followed by RCT—aims to balance ecological validity with controlled hypothesis testing (77, 78). Strengths include validated multidomain assessments (79), fidelity controls, and modular implementation adaptable to diverse contexts (80). Preliminary Italy–Lebanon experience may support transcultural replication, pending empirical confirmation. The inclusion of EMA-T/EMA-P (Supplementary materials 1, 2) and TIDieR (Supplementary Table 1) contributes to reproducibility, sensitivity to change, and transparency, aligning with STROBE, CONSORT, and TIDieR.
Limitations include the absence of harmonized international protocols (81), resource demands (specialized personnel, adapted instruments, flexible spaces) (82), and the methodological challenge of balancing ethical inclusion with internal validity. Another limitation is the lack of accredited training for the conductor–music therapist role, requiring structured pathways. Current mitigation—modular equipment, inter-institutional collaborations, and training initiatives—remains partial.
4.4 Operational challenges and safety management
Safety is addressed through pre-session health checks, continuous therapist monitoring, and post-session debriefings, with adaptive procedures allowing modification or suspension in cases of instability (78, 83). Incidents are centrally logged and reviewed weekly by the clinical team. To limit bias, systematic video recording with multi-rater cross-coding improves reliability, although resource-intensive. Operational constraints persist (specialized personnel, adapted instruments, institutional variability) (82); current mitigation via modular setups and shared facilities provides only partial relief. Accredited training and harmonized protocols remain prerequisites for scaling.
4.5 Future directions
The modular structure may be adapted to specific NDD subgroups (71, 84). Wearable technologies and longitudinal EMA could enhance ecological monitoring. Given that the current program already includes structured follow-up assessments up to 24 months, subsequent research should investigate whether extending monitoring beyond this timeframe is clinically informative (85). In future applications, if the intervention is requested for periods longer than 20 weeks, additional follow-up assessments will be planned according to treatment course and outcomes observed at earlier timepoints, allowing data-driven adaptation of longitudinal monitoring (86). Furthermore, planned developments include multicenter implementation with harmonized standards, accredited training for the conductor–music therapist role, and a standardized starter-kit (instrument set, compositional/therapeutic library, fidelity manual). International collaborations may facilitate scalability while maintaining cultural adaptability.
5 Conclusion
I-SOUND is a theory-based, clinically adaptable framework integrating IMT, OMT, and MIT-P. Its biphasic evaluation—observational modeling followed by RCT—seeks to balance inclusion with internal validity and scalability. Feasibility, sustainability, and applicability will be assessed to develop standardized multimodal strategies for NDD. In essence, I-SOUND is a structured clinical hypothesis requiring validation through controlled studies; the present framework provides a coherent basis for such verification without inferring outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TL: Visualization, Project administration, Investigation, Supervision, Methodology, Writing – review & editing, Conceptualization, Writing – original draft. FD'A: Writing – original draft, Investigation, Data curation, Writing – review & editing, Visualization, Conceptualization. SS: Supervision, Writing – review & editing. RS: Project administration, Writing – review & editing. MH: Writing – review & editing, Project administration. MT: Project administration, Writing – review & editing. RG: Writing – review & editing, Project administration. TC: Writing – review & editing, Project administration. DL: Supervision, Writing – review & editing, Project administration. EC: Investigation, Writing – review & editing, Supervision, Conceptualization, Writing – original draft, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Italian Ministry of Health with Current Research funds. The authors also wish to acknowledge Venusto Alluigi and Carmine Armento for their financial contributions, which further supported the project. This study received partial funding support from Fondazione CARICIV -Cassa di Risparmio di Civitavecchia (Italy), in the form of a micro-grant supporting preliminary clinical implementation activities of the Euterpe Method. The funder had no role in the design, analysis, or writing of the manuscript.
Acknowledgments
The authors wish to express their gratitude to the Municipal Administration of Ladispoli for the logistical support provided throughout the project. Appreciation is also extended to the volunteers and music therapists of the Euterpe APS Cultural Association for their qualified and ongoing commitment to the therapeutic activities. Special thanks are due to the Piccolo Fiore APS Association and Aps Nuove Frontiere Onlus/Ets, composed of families of individuals with special needs, for their significant contribution through the active participation of the children in the orchestral music therapy sessions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1612955/full#supplementary-material
Abbreviations
I-SOUND, IncluSive Orchestral mUsic therapy accordiNg to the euterpe methoD; IMT, Individual Music Therapy; OMT, Orchestral Music Therapy; MIT-P, Multidirectional and Iterative Transfer Process.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, DC: American Psychiatric Publishing (2022).
2. World Health Organization. Clinical descriptions and diagnostic requirements for ICD-11 mental, behavioral and neurodevelopmental disorders (CDDR). Geneva: World Health Organization (2024).
3. Francés, L, Ruiz, A, Soler, CV, Francés, J, Caules, J, Hervás, A, et al. Prevalence, comorbidities, and profiles of neurodevelopmental disorders according to the DSM-5-TR in children aged 6 years old in a European region. Front Psych. (2023) 14:1260747. doi: 10.3389/fpsyt.2023.1260747
4. Olszewska, AM, Gaca, M, Herman, AM, Jednoróg, K, and Marchewka, A. How musical training shapes the adult brain: predispositions and neuroplasticity. Front Neurosci. (2021) 15:630829. doi: 10.3389/fnins.2021.630829
5. Koelsch, S. Towards a neural basis of music-evoked emotions. Trends Cogn Sci. (2010) 14:131–7. doi: 10.1016/j.tics.2010.01.002
6. Zatorre, RJ, and Salimpoor, VN. From perception to pleasure: music and its neural substrates. Proc Natl Acad Sci USA. (2013) 110:10430–7. doi: 10.1073/pnas.1301228110
7. Schlaug, G. Musicians and music making as a model for the study of brain plasticity. Prog Brain Res. (2015) 217:37–55. doi: 10.1016/bs.pbr.2014.11.020
8. Vuust, P, Heggli, OA, Friston, KJ, and Kringelbach, ML. Music in the brain. Nat Rev Neurosci. (2022) 23:287–305. doi: 10.1038/s41583-022-00578-5
9. Han, Y, Yuan, M, Guo, YS, Shen, XY, Gao, ZK, and Bi, X. The role of enriched environment in neural development and repair. Front Cell Neurosci. (2022) 16:890666. doi: 10.3389/fncel.2022.890666
10. Chatterjee, D, Hegde, S, and Thaut, M. Neural plasticity: the substratum of music-based interventions in neurorehabilitation. NeuroRehabilitation. (2021) 48:155–66. doi: 10.3233/NRE-208011
11. Jaschke, AC, Howlin, C, Pool, J, Greenberg, YD, Atkinson, R, Kovalova, A, et al. Study protocol of a randomized control trial on the effectiveness of improvisational music therapy for autistic children. BMC Psychiatry. (2024) 24:637. doi: 10.1186/s12888-024-06086-3
12. Sharda, M, Tuerk, C, Chowdhury, R, Jamey, K, Foster, N, Custo-Blanch, M, et al. Music improves social communication and auditory-motor connectivity in children with autism. Transl Psychiatry. (2018) 8:231. doi: 10.1038/s41398-018-0287-3
13. Fan, Q, Ding, M, Cheng, W, Su, L, Zhang, Y, Liu, Q, et al. The clinical effects of Orff music therapy on children with autism spectrum disorder: a comprehensive evaluation. Front Neurol. (2024) 15:1387060. doi: 10.3389/fneur.2024.1387060
14. Bompard, S, Liuzzi, T, Staccioli, S, D’Arienzo, F, Khosravi, S, Giuliani, R, et al. Home-based music therapy for children with developmental disorders during the COVID-19 pandemic. J Telemed Telecare. (2021) 29:211–6. doi: 10.1177/1357633X20981213
15. Liuzzi, T, Bompard, S, Raponi, M, D’Arienzo, F, Staccioli, S, Napoli, E, et al. Euterpe music therapy method for children with cerebral palsy. Front Neurol. (2024) 15:1388712. doi: 10.3389/fneur.2024.1388712
16. Liuzzi, T, D’Arienzo, F, Raponi, M, De Bartolo, P, Tarabay, M, Giuliani, R, et al. Euterpe music therapy methodology and procedure algorithms. Front Neurol. (2024) 15:1443329. doi: 10.3389/fneur.2024.1443329
17. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
18. Schulz, KF, Altman, DG, and Moher, DCONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c332. doi: 10.1136/bmj.c332
19. Hopewell, S, Chan, A-W, Collins, GS, Hróbjartsson, A, Moher, D, Schulz, KF, et al. CONSORT 2025 statement: updated guideline for reporting randomised trials. PLoS Med. (2025) 22:e1004587. doi: 10.1371/journal.pmed.1004587
20. Hoffmann, TC, Glasziou, PP, Boutron, I, Milne, R, Perera, R, Moher, D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
21. Dotov, D, Bosnyak, D, and Trainor, LJ. Collective music listening: movement energy is enhanced by groove and visual social cues. Q J Exp Psychol. (2021) 74:1037–53. doi: 10.1177/1747021821991793
22. Hedayati, N, Schibli, K, and D’Angiulli, A. El Sistema-inspired ensemble music training is associated with changes in children’s neurocognitive functional integration: preliminary ERP evidence. Neurocase. (2016) 22:538–47. doi: 10.1080/13554794.2016.1241885
23. Zaatar, MT, Alhakim, K, Enayeh, M, and Tamer, R. The transformative power of music: insights into neuroplasticity, health, and disease. Brain Behav Immun. (2024) 35:100716. doi: 10.1016/j.bbih.2023.100716
24. Ribic, A. Stability in the face of change: lifelong experience-dependent plasticity in the sensory cortex. Front Cell Neurosci. (2020) 14:76. doi: 10.3389/fncel.2020.00076
25. Habibi, A, Damasio, A, Ilari, B, Elliott Sachs, M, and Damasio, H. Music training and child development: a review of recent findings from a longitudinal study. Ann N Y Acad Sci. (2018) 1423:73–81. doi: 10.1111/nyas.13606
26. Choi, US, Sung, YW, and Ogawa, S. Brain plasticity reflects specialized cognitive development induced by musical training. Cereb Cortex Commun. (2021) 2:37. doi: 10.1093/texcom/tgab037
27. Fuhrmann, D, Knoll, LJ, and Blakemore, SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. (2015) 19:558–66. doi: 10.1016/j.tics.2015.07.008
28. Paus, T, Keshavan, M, and Giedd, JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. (2008) 9:947–57. doi: 10.1038/nrn2513
29. Ismail, FY, and Ljubisavljevic, MR. Neuroplasticity in neurodevelopmental disorders In: FY Ismail, PJ Accardo, and BK Shapiro, editors. Capute and Accardo’s neurodevelopmental disabilities in infancy and childhood. Cambridge, MA: Academic Press (2025). 71–84.
30. Fasano, MC, Semeraro, C, Cassibba, R, Kringelbach, ML, Monacis, L, de Palo, V, et al. Short-term orchestral music training modulates hyperactivity and inhibitory control in school-age children: a longitudinal behavioral study. Front Psychol. (2019) 10:750. doi: 10.3389/fpsyg.2019.00750
31. Majno, M. From the model of El Sistema in Venezuela to current applications: learning and integration through collective music education. Ann N Y Acad Sci. (2012) 1252:56–64. doi: 10.1111/j.1749-6632.2012.06498.x
32. Merati, N, Siedlikowski, S, Puzhko, S, Hamzeh, J, Wary, N, Clark, R, et al. In their words: children’s perspectives on an El Sistema music program’s effects on their well-being. Prog Community Health Partnersh. (2019) 13:359–69. doi: 10.1353/cpr.2019.0069
33. Ilari, BS, Keller, P, Damasio, H, and Habibi, A. The development of musical skills of underprivileged children over the course of 1 year: a study in the context of an El Sistema-inspired program. Front Psychol. (2016) 7:62. doi: 10.3389/fpsyg.2016.00062
34. Guerrero, N, Turry, A, Geller, DA, and Raghavan, P. From historic to contemporary: Nordoff-Robbins music therapy in collaborative interdisciplinary rehabilitation. Music Ther Perspect. (2014) 32:38–46. doi: 10.1093/mtp/miu014
35. Syczyk, O, Clare, L, Rutley, R, Aries, A, Hunter, S, and O'Mara, M. CSP2023: 497 – service evaluation of STROKESTRA®-stoke, a collaborative community-based music-making stroke therapy programme: the therapists' perspective. Physiotherapy. (2024) 123:e259–60. doi: 10.1016/j.physio.2024.04.327
36. King, E, Presicce, G, Dunn, R, Prior, H, and White, C. The STROKESTRA® community Programme: research report. England: University of Hull (2025).
37. Sbattella, L. La mente orchestra: elaborazione della risonanza e autismo. Milano, IT: Vita e Pensiero (2008).
38. AllegroModerato. (2025). Metodo. Available online at: https://allegromoderato.it/metodo/ (Accessed February 18, 2025).
39. Stige, B, Ansdell, G, Elefant, C, and Pavlicevic, M. Where music helps: Community music therapy in action and reflection. Farnham, UK: Ashgate (2010).
40. Tiszai, L. Consonante, the barrier-free method: orchestral work with individuals with severe disabilities. J Art Life. (2016) 8:1–17.
41. Tiszai, L. Community music therapy and intellectual disability: Theory and practice from a Hungarian perspective. Szeged, HU: Szegedi Egyetemi Kiadó – Juhász Gyula Felsőoktatási Kiadó (2019).
42. Pavlicevic, M. Groups in music: Strategies from music therapy. London, UK: Jessica Kingsley Publishers (2003).
43. Ansdell, G. Reflection: where performing helps: processes and affordances of performance in community music therapy In: B Stige, G Ansdell, C Elefant, and M Pavlicevic, editors. Where music helps: community music therapy in action and reflection. Abingdon, UK: Routledge (2016). 161–88.
44. Pavlicevic, M, and Ansdell, G. Community music therapy. London, UK: Jessica Kingsley Publishers (2004).
45. Rickson, DJ. Stepping into the spotlight: collaborative efforts towards musical inclusion. Approaches. (2014) 6:99–112. doi: 10.56883/aijmt.2014.423
46. Geretsegger, M, Fusar-Poli, L, Elefant, C, Mössler, KA, Vitale, G, and Gold, C. Music therapy for autistic people. Cochrane Database Syst Rev. (2022) 5:CD004381. doi: 10.1002/14651858.CD004381.pub4
47. Grau-Sánchez, J, Jamey, K, Paraskevopoulos, E, Dalla Bella, S, Gold, C, Schlaug, G, et al. Putting music to trial: consensus on key methodological challenges investigating music-based rehabilitation. Ann N Y Acad Sci. (2022) 1518:12–24. doi: 10.1111/nyas.14892
48. Santonja-Medina, CS, Marrades-Caballero, E, Santonja-Medina, F, and Sanz-Mengibar, JM. Neurologic music therapy improves participation in children with severe cerebral palsy. Front Neurol. (2022) 13:795533. doi: 10.3389/fneur.2022.795533
49. Ghai, S, Ghai, I, and Effenberg, AO. Effect of rhythmic auditory cueing on gait in cerebral palsy: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. (2018) 14:43–59. doi: 10.2147/NDT.S148053
50. Braun Janzen, T, Koshimori, Y, Richard, NM, and Thaut, MH. Rhythm and music-based interventions in motor rehabilitation: current evidence and future perspectives. Front Hum Neurosci. (2022) 15:789467. doi: 10.3389/fnhum.2021.789467
51. Jiang, D, Liu, X, Lin, Q, Wang, G, Wang, G, and Zhang, D. Music intervention for neurodevelopment in the pediatric population: a systematic review and meta-analysis. Sci Rep. (2025) 15:10388. doi: 10.1038/s41598-025-93795-8
52. Milcent Fernandez, E, and Newman, CJ. Music therapy and music-based interventions in pediatric neurorehabilitation. Children. (2025) 12:773. doi: 10.3390/children12060773
53. Shiffman, S, Stone, AA, and Hufford, MR. Ecological momentary assessment. Annu Rev Clin Psychol. (2008) 4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415
54. Mulraney, M, de Silva, U, Joseph, A, Sousa Fialho, MDL, Dutia, I, Munro, N, et al. International consensus on standard outcome measures for neurodevelopmental disorders: a consensus statement. JAMA Netw Open. (2024) 7:e2416760. doi: 10.1001/jamanetworkopen.2024.16760
55. Sparrow, SS, Cicchetti, DV, and Saulnier, CA. Vineland adaptive behavior scales, third edition (Vineland-3). San Antonio, TX: Pearson (2016).
56. Palisano, R, Rosenbaum, P, Walter, S, Russell, D, Wood, E, and Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. (1997) 39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x
57. Deitz, JC, Kartin, D, and Kopp, K. Review of the Bruininks-Oseretsky test of motor proficiency, second edition (BOT-2). Phys Occup Ther Pediatr. (2007) 27:87–102. doi: 10.1080/J006v27n04_06
58. Wuang, YP, and Su, CY. Reliability and responsiveness of the Bruininks–Oseretsky test of motor proficiency–second edition in children with intellectual disability. Res Dev Disabil. (2009) 30:847–55. doi: 10.1016/j.ridd.2008.12.002
59. Pearson Clinical. Movement assessment battery for children, Third Edition (MABC-3). Sydney: Pearson Assessment Australia (2023).
60. Mathiowetz, V, Volland, G, Kashman, N, and Weber, K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. (1985) 39:386–91. doi: 10.5014/ajot.39.6.386
61. Gioia, GA, Isquith, PK, Guy, SC, and Kenworthy, L. Behavior rating inventory of executive function, second edition (BRIEF-2). Lutz, FL: PAR Inc (2015).
62. Roth, R, Isquith, PK, and Gioia, GA. Behavior rating inventory of executive function – Adult version (BRIEF-A). Lutz, FL: Psychological Assessment Resources (2005).
63. Varni, JW, Seid, M, and Kurtin, PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. (2001) 39:800–12. doi: 10.1097/00005650-200108000-00006
64. Nguyen, T, Choromanski, L, Kreuzer, T, and Stroppini, J. Exploring the feasibility of a virtual, home-based MusicGlove® protocol for children with hemiparetic cerebral palsy. Open J Occup Ther. (2022) 10:1–15. doi: 10.15453/2168-6408.1836
65. den Hartog, D, van der Krogt, MM, van der Burg, S, Aleo, I, Gijsbers, J, Bonouvrie, LA, et al. Home-based measurements of dystonia in cerebral palsy using smartphone-coupled inertial sensor technology and machine learning: a proof-of-concept study. Sensors. (2022) 22:4386. doi: 10.3390/s22124386
66. Kleynen, M, Beurskens, A, Olijve, H, Kamphuis, J, and Braun, S. Application of motor learning in neurorehabilitation: a framework for health-care professionals. Physiother Theory Pract. (2020) 36:1–20. doi: 10.1080/09593985.2018.1483987
67. Schaefer, SY, Patterson, CB, and Lang, CE. Transfer of training between distinct motor tasks after stroke: implications for task-specific approaches to upper-extremity neurorehabilitation. Neurorehabil Neural Repair. (2013) 27:602–12. doi: 10.1177/1545968313481279
68. Salomon, G, and Perkins, DN. Rocky roads to transfer: rethinking mechanisms of a neglected phenomenon. Educ Psychol. (1989) 24:113–42. doi: 10.1207/s15326985ep2402_1
69. Hallam, S. The power of music: its impact on the intellectual, social and personal development of children and young people. Int J Music Educ. (2010) 28:269–89. doi: 10.1177/0255761410370658
70. Hallam, S, and Himonides, E. The power of music: An exploration of the evidence. Cambridge: Open Book Publishers (2022).
71. Altenmüller, E, and Lee, A. Neuroplasticity as a driver of beneficial effects in music interventions in children with developmental disorders. Music Med. (2023) 15:207–13. doi: 10.47513/mmd.v15i4.937
72. Bakeman, R, and Quera, V. Sequential analysis and observational methods for the behavioral sciences. Cambridge: Cambridge University Press (2011).
73. Pranjić, M, Braun Janzen, T, Vukšić, N, and Thaut, M. From sound to movement: mapping the neural mechanisms of auditory–motor entrainment and synchronization. Brain Sci. (2024) 14:1063. doi: 10.3390/brainsci14111063
74. Herholz, SC, Coffey, EB, Pantev, C, and Zatorre, RJ. Dissociation of neural networks for predisposition and for training-related plasticity in auditory-motor learning. Cereb Cortex. (2016) 26:3125–34. doi: 10.1093/cercor/bhv138
75. Pantev, C, and Herholz, SC. Plasticity of the human auditory cortex related to musical training. Neurosci Biobehav Rev. (2011) 35:2140–54. doi: 10.1016/j.neubiorev.2011.06.010
76. Kunikullaya, UK, Pranjić, M, Rigby, A, Pallás-Ferrer, I, Anand, H, Kunnavil, R, et al. The molecular basis of music-induced neuroplasticity in humans: a systematic review. Neurosci Biobehav Rev. (2025) 175:106219. doi: 10.1016/j.neubiorev.2025.106219
77. Craig, P, Dieppe, P, Macintyr, S, Michie, S, Nazareth, I, Petticrew, M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. (2008) 337:a1655. doi: 10.1136/bmj.a1655
78. Chan, AW, Tetzlaff, JM, Altman, DG, Laupacis, A, Gøtzsche, PC, Krleža-Jerić, K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
79. Lezak, MD, Howieson, DB, Bigler, ED, and Tranel, D. Neuropsychological assessment. Oxford: Oxford University Press (2012).
80. Boutron, I, Page, MJ, Higgins, JPT, Altman, DG, Lundh, A, and Hróbjartsson, A. Considering bias and conflicts of interest among the included studies. In: Higgins, JPT, J Thomas, J Chandler, and M Cumpston, et al., editors. Cochrane handbook for systematic reviews of interventions. London: Cochrane (2022).
81. Moher, D, Hopewell, S, Schulz, KF, Montori, V, Gøtzsche, PC, Devereaux, PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c869. doi: 10.1136/bmj.c869
82. Eldridge, SM, Lancaster, GA, Campbell, MJ, Thabane, L, Hopewell, S, Coleman, CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One. (2016) 11:e0150205. doi: 10.1371/journal.pone.0150205
83. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
84. Thapar, A, Cooper, M, and Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry. (2017) 4:339–46. doi: 10.1016/S2215-0366(16)30376-5
85. Antolini, G, and Colizzi, M. Where do neurodevelopmental disorders go? Casting the eye away from childhood towards adulthood. Healthcare. (2023) 11:1015. doi: 10.3390/healthcare11071015
Keywords: inclusive orchestral music therapy, Euterpe Method, auditory-motor, neurodevelopmental disorders, cerebral palsy, autism spectrum disorder, inclusion
Citation: Liuzzi T, D’Arienzo F, Staccioli S, Slaïby RF, Harb MBS, Tarabay M, Giuliani R, Chirico T, Lettori D and Castelli E (2025) Inclusive orchestral music therapy according to the Euterpe Method: a multimodal framework for neurodevelopmental disorders. Front. Neurol. 16:1612955. doi: 10.3389/fneur.2025.1612955
Edited by:
Veronica Rivi, University of Modena and Reggio Emilia, ItalyReviewed by:
Roziah Sidik, National University of Malaysia, MalaysiaCopyright © 2025 Liuzzi, D’Arienzo, Staccioli, Slaïby, Harb, Tarabay, Giuliani, Chirico, Lettori and Castelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tommaso Liuzzi, bGl1enppLnRvbW1hc29AZ21haWwuY29t
 Tommaso Liuzzi
Tommaso Liuzzi Fiammetta D’Arienzo
Fiammetta D’Arienzo Susanna Staccioli
Susanna Staccioli Rita Faraj Slaïby
Rita Faraj Slaïby Maroun Bou Sleiman Harb4,6
Maroun Bou Sleiman Harb4,6