Abstract
Objective:
The purpose of this meta-analysis was to investigate the effectiveness and safety of repetitive transcranial magnetic stimulation (rTMS) in the treatment of patients with post-stroke aphasia (PSA).
Methods:
The PubMed, PEDro, Embase, Cochrane Library, CNKI, Wanfang Data and Web of Science databases were systematically searched from inception until January 30, 2024. Eligible randomized controlled trials (RCTs) contained information on the population (PSA), intervention (rTMS), and outcomes (Western Aphasia Battery, Aphasia Quotient, Aphasia Battery in Chinese, Boston Diagnostic Aphasia Examination, Aachener Aphasie Test, Concise Chinese Aphasia Test and Computerized Picture Naming Test). Participants in the rTMS intervention group were compared with those in sham or other control groups. Two independent researchers searched for, screened, and qualified the articles. Two independent researchers extracted key information from each eligible study. The authors’ names, year of publication, setting, total sample size, rTMS parameters, baseline/mean difference (MD), and 95% confidence interval (CI) were extracted using a standardized form, and the methodological quality was assessed using the Cochrane Risk of Bias tool (Revman 5.40, Nordic Cochrane Center) and GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system.
Results:
Thirty relevant RCTs were included, involving a total of 1,597 patients. The analysis turned out that rTMS combined with speech and language therapy (SLT) resulted in significant improvements in auditory comprehension, naming, repetition, and spontaneous speech in patients with PSA compared with sham stimulation combined with SLT or SLT alone in the control group. (auditory comprehension, MD = 1.94, 95%CI = [1.16, 2.17], p < 0.001; naming, MD = 1.53, 95%CI = [0.82, 2.24], p < 0.001; repetition, MD = 1.79, 95%CI = [1.20, 2.38], p < 0.001; spontaneous speech, MD = 1.97, 95%CI = [1.65, 2.29], p < 0.001).
Conclusion:
This meta-analysis showed that rTMS can safely and effectively promote the recovery of speech function in patients with PSA.
Clinical trial registration:
The study has been registered with Prospero https://www.crd.york.ac.uk/PROSPERO/search, (CRD42022363899).
1 Introduction
Post-stroke aphasia (PSA) refers to impaired or permanent loss of the ability to express and understand speech symbols caused by cerebrovascular disease, which results in a variety of language dysfunction, including listening, speaking, reading, and writing (1, 2). The incidence of PSA is high, with more than a third of stroke patients suffering from aphasia (3, 4). Due to the inability to communicate correctly, PSA patients are more prone to be depressed and anxious, which can seriously affect their quality of rehabilitation and life (5–7). Recent data show that stroke patients with aphasia incur significantly higher hospitalization costs for medical treatment, nursing, and related medical services than those without, which puts a huge burden on patients, their families, and society (8). The pathogenesis of PSA is not fully understood. However, some researchers have proposed the hypothesis that aphasia is related to the degree of lesions in the left hemisphere. When the lesions in the left hemisphere are small, the cortical area around the lesions in the ipsilateral hemisphere can play a role in compensating for ischemia. The right hemisphere’s corresponding speech-motor and language areas can functionally compensate for ischemia when the left hemisphere is extensively diseased (9, 10). Recently, researchers have also analyzed the mechanism of PSA from the perspective of neuroplasticity and explored possible intervention directions (11).
Common clinical treatment modalities for PSA include medication and speech training. Commonly used pharmacological treatments include dopaminergic, acetylcholinesterase inhibitors, and amino acid neurotransmitters (12). But of note, medication can only be used to improve some of the clinical symptoms of PSA patients. Due to the lack of uniform clinical standards for therapy, the effectiveness of speech and language training also varies from person to person (13–15). Early rehabilitation interventions include promoting communication outcome, functional restructuring, and blockade removal. Speech and language training (SLT) is highly recommended by the United State Stroke Foundation and the Australian Stroke Foundation as a significant treatment throughout aphasia (level 1A evidence) (15). In addition, the effectiveness of traditional SLT varies from person to person and may be due to a variety of factors, such as the patient’s level of aphasia, the technician’s individual nursing skills, communication strategies, and many other factors. Exploring novel, easy-to-implement, and efficient rehabilitation methods is still urgently needed to improve the clinical outcome of PSA further.
In recent years, with the development of non-invasive brain stimulation techniques, neuromodulation techniques such as transcranial direct current stimulation(tDCS) and repetitive transcranial magnetic stimulation (rTMS) have been widely used in the treatment of various clinical disorders, including depression (16), cognitive disorders (17), motor dysfunction (18), post-stroke dysphagia (19), PSA (20) and so on. Therefore, neuromodulation techniques are being used as novel therapeutic modalities that can complement the treatment of PSA. In addition, a recent net meta-analysis (21) showed that rTMS, a commonly used clinical neuromodulation technique, has better efficacy than tDCS in treating people with PSA. In most clinical settings, rTMS is rarely used as a stand-alone treatment for post-stroke aphasia. Instead, rTMS is typically administered in combination with speech and language therapy (SLT), which remains the gold standard rehabilitation approach. Some trials also paired rTMS with pharmacological treatments or cognitive training interventions. This concurrent use is based on the rationale that neuromodulation may enhance neuroplasticity, thereby amplifying the effects of behavioral therapies. Understanding these treatment pairings is essential for interpreting differences in efficacy across studies. This net meta-analysis demonstrated that therapeutic effects in the naming domain were moderated by the mean period of each therapy condition and the first language, while significant associations with age, therapy period, and number of sessions were observed for spontaneous speech. Overall, LF-rTMS is the most prioritized NIBS mode to alleviate global severity.
rTMS is a safe, painless, and easy-to-manipulate noninvasive neuromodulation technique that can modulate the excitability of cortical neurons on a temporal scale that exceeds the stimulation time course and on a spatial scale that exceeds the stimulation site (22). rTMS works by generating an induced magnetic field in order to induce secondary electrical currents in the adjacent neural tissues, which activate the cerebral cortex and changes the brain tissue-related physiological processes to achieve localization of cortical functions; At the same time, it can also improve local blood rheology and cortical metabolism by regulating the excitability of local brain tissues, affecting the release and transmission of neurotransmitters within the brain, and promoting the repair of damaged brain cells (22–24), and thus has been widely used in clinical rehabilitation.
Although previous review (20, 25) have discussed the therapeutic application of rTMS in patients with PSA, the clinical efficacy of rTMS in treating PSA patients, the optimal intervention parameters of rTMS and the safety of rTMS in the clinical treatment of PSA are still worthy of further analysis and exploration. And, recently, a number of new evidences of randomized controlled trials (RCTs) of rTMS for PSA have emerged. Thus, this meta-analysis aims to further explore the clinical efficacy and optimal intervention parameters of rTMS for PSA, and to provide a clinical evidence-based basis for the effective application of rTMS for PSA.
2 Methods
2.1 Protocol and registration
Our systematic review was designed and implemented based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline (26). The study has been registered with Prospero (CRD42022363899).
2.2 Search strategy
In the initial screening, two researchers (CG and YXD) independently searched RCTs related to the topic in seven databases: Web of Science, PubMed, Embase, Cochrane Library, CNKI, Wanfang Data, and PEDro. Search for studies published between the date of database creation and September 28, 2022. We searched for standardized disease names in the International Classification of Diseases, 11th edition (ICD − 11). Ultimately, we identified the keywords for this study as “Stroke,” “Aphasia,” “Language Expression Disorder,” “Listening Comprehension Disorder,” and “Repetitive Transcranial Magnetic Stimulation.” In addition, we manually searched other relevant literature, such as studies included in some systematic reviews and meta-analyses, to broaden the search for eligible articles. As an example, the search strategy for the PubMed database is as follows (Table 1).
Table 1
| No. search items | |
|---|---|
| #1 | Stroke [MESH] |
| #2 | (Cerebrovascular Accident) OR (Brain Vascular Accident) OR (Cerebrovascular Accidents) OR (Strokes) OR (CVA) OR (CVAs) OR (Cerebrovascular Apoplexy) OR (Apoplexy, Cerebrovascular) OR (Vascular Accident, Brain) OR (Brain Vascular Accident) OR (Vascular Accidents, Brain) OR (Brain Vascular Accidents) OR (Cerebrovascular Stroke) OR (Cerebrovascular Strokes) OR (Stroke, Cerebrovascular) OR (Strokes, Cerebrovascular) OR (Apoplexy) OR (Cerebral Stroke) OR (Cerebral Strokes) |
| #3 | #1 OR #2 |
| #4 | Aphasia [MESH] |
| #5 | (Mixed Aphasia) OR (Global Aphasia) OR (Language Expression Disorder) OR (Listening Comprehension Disorder) OR (Motor Aphasia) OR (Broca Aphasia) OR (Wernicke Aphasia) OR (Alogia) OR (Alogia Acquired) OR (Aphasia) OR (Dysphasia) |
| #6 | #4 OR #5 |
| #7 | Transcranial Magnetic Stimulation [MESH] |
| #8 | (Repetitive Transcranial Magnetic Stimulation) OR(Magnetic Stimulation, Transcranial) OR (Magnetic Stimulations, Transcranial) OR (Stimulation, Transcranial Magnetic) OR (Stimulations, Transcranial Magnetic) OR (Transcranial Magnetic Stimulations) OR (Transcranial Magnetic Stimulation, Single Pulse) OR (Transcranial Magnetic Stimulation, Paired Pulse) OR (Transcranial Magnetic Stimulation, Repetitive) OR (TMS) OR (rTMS) OR (iTBS) |
| #9 | #7 OR #8 |
| #10 | #3 AND #6 AND #9 |
The specific search strategy of PubMed database.
2.3 Inclusion and exclusion criteria of the study
Included studies were required to follow our pre-defined inclusion and exclusion criteria strictly. According to the PICOS principles, the inclusion criteria of our review were as follows: (1) participants: patients diagnosed with post-stroke aphasia; (2) interventions: rTMS; (3) comparison: experimental group (rTMS) versus control group (placebo or no treatment) condition; (4) outcomes: Western Aphasia Battery (WAB), Aphasia Quotient (AQ), Aphasia Battery in Chinese (ABC), Boston Diagnostic Aphasia Examination (BADE), Aachener Aphasie Test (AAT), Concise Chinese Aphasia Test (CCAT) and Computerized Picture Naming Test (CPNT); (5) type of studies: RCT; (6) studies published in English or Chinese. Exclusion criteria for the literature: (1) duplicate data; (2) full-text content not available; (3) data not extractable.
2.4 Study selection
After completing the database search, we imported all retrieved studies into Endnote 20’s document management system (Endnote 20, United States) and removed duplicate studies using the software management function. Two researchers (CG and LX) then read the title and abstract of each study simultaneously and screened studies based on the inclusion and exclusion criteria we had previously developed. For initially screened studies, the two researchers would downloaded and read through the full text, removing articles that do not meet the inclusion criteria and discussed them to confirm their eligibility. If the two researchers disagree on the screening process of a study, the principal investigator (ZYD) was asked to provide advice and reach an agreement.
2.5 Data extraction
Two researchers (MH and LX) independently extracted the following data and items from the included literature: first author of the study, year of publication, the sample size of participating studies, age, gender, duration of disease, interventions tested, outcome indicators, and adverse effects. In addition, when the two researchers encountered difficulties in understanding or extracting the complete literature data during the data extraction process, the original authors of the literature would be contacted by sending an email to obtain the full trial data. When no response was received from the original author after three consecutive contacts, the study will be defined as missing data. Suppose two researchers disagree during the data extraction process. In that case, both would be placed in a research team with the Principal Investigator to discuss and resolve the issue. If the two researchers disagree on the screening process of a study, the principal investigator (ZYD) would be asked to provide advice and reach an agreement. The principal investigator will convene a meeting of the research team to discuss the reasons for any disagreements; once the sources of conflict are resolved, consensus will be reached.
2.6 Quality assessment
The quality assessment of the literature studies was completed independently by two researchers (MH and JHH), then discussed to produce consistent results. Risk bias was assessed using the Cochrane Risk of Bias tool (Revman 5.40, Nordic Cochrane Center). A total of seven items were considered: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The risk bias assessment was mapped, and different colors differentiated the results into three levels: high risk—red, unknown risk—yellow and low risk—green. Heterogeneity between studies was statistically analyzed by Revman 5.40. The magnitude of heterogeneity was expressed as I2, with heterogeneity judged as high risk when I2 ≥ 75%, moderate risk when 75% > I2 ≥ 50%, low heterogeneity when 50% > I2 ≥ 25%, and no heterogeneity if I2 = 0% (27). I2 quantifies the extent of heterogeneity between studies. On the one hand, we select the appropriate effect model for the forest plot according to the magnitude of I2 to minimize the impact of high heterogeneity on the pooled results. On the other hand, during the assessment of evidence quality, we also use I2 to grade the strength of the evidence. The quality of evidence for outcome indicators was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, which examines study limitations, intermittency, inconsistency, and imprecision of results (28). The results were assessed by grading the evidence for the outcome indicators as “high,” “moderate,” “low,” or “very low,” and the strength of the recommendations was divided into two levels: “strong” and “weak” (29).
2.7 Statistical analysis
The extracted study data were entered into Revman 5.40 softwarea for statistical and analytical purposes. The decision to use a fixed or a random effects model for the meta-analysis was based on the magnitude of heterogeneity. A random effects model was used when I2 ≥ 50%, and a fixed effects model was used when I2 < 50%. Mean difference (MD) and 95% confidence interval (CI) were used to express the effect size for studies using the same measure. Vice versa, standardized mean difference (SMD) and 95%CI were used to express the effect size for studies using different measures. p < 0.05, statistically significant.
2.8 Safety assessment
The number, type, and duration of adverse events that occurred during the rTMS intervention were counted in all the studies concerned. And statistically analyze the patients who experienced adverse events, as a percentage of the number of subjects. Record whether there were any intolerable or even life-threatening adverse reactions that caused the subjects to withdraw from the experiment. And, to track whether the adverse events in each study, still persisted during the follow-up time.
3 Results
3.1 Literature search findings
A total of seven databases were searched for literature, and the initial search resulted in 994 studies. Duplicate studies were screened out and removed by software, leaving 628 studies. Two researchers (YZL and CG) read the titles and abstracts of these studies and screened out 574 that were irrelevant to the topic. The remaining 54 studies were downloaded in full and read through, and 24 studies were still excluded (8 studies were screened for not using the internationally accepted aphasia ratings listed in the inclusion criteria or data on outcome indicators were not fully available; 12 studies were screened for not strictly using a randomized controlled trial design; four studies were screened for not using rTMS as the primary intervention in an experimental comparison study). Finally, 30 eligible studies were included (30–59) (Figure 1).
Figure 1

Flow graph of selection and exclusion.
3.2 Characteristics of included studies
Across the included randomized controlled trials, rTMS was most commonly applied in conjunction with SLT, whereas a smaller number of trials compared rTMS plus SLT with SLT alone or with sham stimulation plus SLT. Only a few studies combined rTMS with pharmacological agents. This variability in study design illustrates that rTMS is more appropriately viewed as an adjunctive rather than independent therapy for PSA. To improve clarity, we have minimized use of acronyms in the Results section, spelling out the assessment tools (e.g., Western Aphasia Battery instead of WAB on first mention). Table 2 summarizes the basic data of the 30 RCTs. A total of 1,597 patients with PSA were included, with sample sizes ranging from 12 to 120, of which 819 patients with PSA were treated with rTMS. Subjects’ aphasia types had non-fluent aphasia, Broca aphasia, Motor aphasia, Global aphasia, and Various aphasia. Among the included RCTs, the outcome indicators for rating aphasia in post-stroke patients included Western Aphasia Battery (WAB), Aachener Aphasia Test (AAT), Aphasia Battery in Chinese (ABC), Aphasia Quotient (AQ); Concise Chinese Aphasia Test (CCAT); Computerized Picture Naming Test (CPNT) and Boston Diagnostic Aphasia Examination (BDAE).
Table 2
| Study | Gender (M/F) |
Age (years) | Stroke duration | Aphasia type | Interventions | Outcome measures | Total time | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1. Barwood et al. (59) | G1:2/4 G2: 1/5 |
G1: 60.8 ± 5.98 G2: 67 ± 13.11 |
3.49 ± 1.27 years 3.46 ± 1.53 years |
Non-fluent aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
BDAE | 10 days | 2 months |
| 2. Chang (46) | G1;35/28 G2:33/30 |
67.3 ± 19.9 G2:66.4 ± 15.8 |
6.9 ± 3.1 days 7.3 ± 3.5 days |
Broca aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
WAB | 15 days | No |
| 3. Chen et al. (58) | G1;3/5 G2:3/4 |
65.7 66.5 |
<7 days | Broca aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
ABC | 10 days | 2 weeks |
| 4. Fan (48) | G1;25 G2:25 |
≥18 | NR | NR | G1: SLT + rTMS G2: SLT + sham rTMS |
ABC; AQ | 20 days | No |
| 5. Guo et al. (49) | G1:11/9 G2:12/8 |
62.1 ± 10.6 64.4 ± 8.5 |
33.1 ± 8.6 days 30.6 ± 9.4 days |
Broca aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
WAB; AQ | 24 days | No |
| 6. Haghighi et al. (47) | G1:3/3 G2:2/4 |
61.67 ± 7.06 60.50 ± 11.85 |
4–8 weeks | Broca aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
WAB | 10 days | No |
| 7. Heiss et al. (55) | G1:15 G2:14 |
68.5 ± 8.19 69.0 ± 6.33 |
50.1 ± 23.96 days 39.7 ± 18.43 days |
NR | G1: SLT + rTMS G2: SLT + sham rTMS |
AAT | 10 days | No |
| 8. Hu et al. (4) | G1: 7/3 G2: 6/4 G3: 5/5 G4: 6/4 |
46.5 ± 12.1 48.5 ± 11.2 50.7 ± 10.4 47.3 ± 9.8 |
7.1 ± 2.7 months 7.5 ± 3.2 months 6.8 ± 2.3 months 7.7 ± 3.4 months |
Non-fluent aphasic | G1: SLT + rTMS G1: SLT + rTMS G3: SLT + sham rTMS G4: SLT |
WAB | 2 weeks | 2 months |
| 9. Lai et al. (30) | G1:21/16 G2:20/17 |
62.01 ± 6.29 61.49 ± 6.36 |
1 ~ 3 months | Various | G1: SLT + rTMS G2: SLT + sham rTMS |
AQ | 8 months | No |
| 10. Li et al. (43) | G1:9/6 G2:7/8 |
65.3 ± 5.6 68.3 ± 5.8 |
47.5 ± 7.4 days 51.0 ± 9.6 days |
Motor aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
AQ; WAB | 3 weeks | 3 weeks |
| 11. Liu et al. (31) | G1:24/16 G2:26/14 |
54.1 ± 6.2 53.3 ± 5.4 |
58.4 ± 15.6 days 60.2 ± 14.3 days |
NR | G1: SLT + rTMS G2: SLT + sham rTMS |
AQ; WAB | 4 weeks | No |
| 12. Peng and Zhou (37) | G1:26/14 G2:27/13 G3:24/16 |
59.79 ± 5.58 59.80 ± 5.91 59.73 ± 5.82 |
10.4 ± 2.83 days 10.4 ± 2.76 days 10.37 ± 2.8 days |
NR | G1: SLT + rTMS G2: SLT + sham rTMS G3: SLT |
AQ; WAB | 4 weeks | No |
| 13. Qiu et al. (36) | G1:19/1 G2:18/2 |
55.00 ± 10.72 52.25 ± 15.00 |
2.12 ± 1.8 months 1.56 ± 1.6 months |
Non-fluent aphasia | G1: SLT + rTMS G2: SLT + sham rTMS | WAB | 4 weeks | No |
| 14. Qu et al. (35) | G1:13/7 G2:14/6 |
68.60 ± 7.78 67.80 ± 7.32 |
26.5 ± 12.5 days 25.8 ± 11.8 days |
Non-fluent aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
AQ; WAB | 2 weeks | No |
| 15. Ren et al. (38) | G1:12/6 G2: 7/6 G3:9/6 |
65.95 ± 8.53 62.46 ± 10.95 63.60 ± 16.71 |
55.9 ± 19.4 days 50.6 ± 23.8 days 61.2 ± 22.7 days |
Global aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
WAB | 3 weeks | No |
| 16. Rubi-fessen et al. (50) | G1:5/10 G2:9/6 |
67.9 ± 8.12 69.6 ± 6.67 |
41.5 ± 21.5 days 48.7 ± 21.6 days |
NR | G1: SLT + rTMS G2: SLT + sham rTMS |
AAT | 10 days | No |
| 17. Seniów et al. (54) | G1:8/12 G2:10/10 |
61.8 ± 11.8 59.7 ± 10.7 |
33.5 ± 24.1 days 39.9 ± 28.9 days |
Various | G1: SLT + rTMS G2: SLT + sham rTMS |
BDAE | 3 weeks | 15 weeks |
| 18. Shen (41) | G1:16/14 G2:17/13 |
57.31 ± 2.51 57.28 ± 2.35 |
3.75 ± 1.32 days 3.25 ± 1.25 days |
NR | G1: SLT + rTMS G2: SLT |
ABC | 4 weeks | No |
| 19. Tao (40) | G1:20/11 G2:18/13 |
60.2 ± 5.1 59.3 ± 4.5 |
NR | NR | G1: SLT + rTMS G2: SLT |
AQ; ABC | 4 weeks | No |
| 20. Thiel et al. (53) | G1:13 G2:11 |
69.8 ± 7.96 71.2 ± 7.78 |
37.5 ± 18.5 days 50.6 ± 22.6 days |
Various | G1: SLT + rTMS G2: SLT + sham rTMS |
AAT | 10 days | 3 weeks |
| 21. Tsai et al. (52) | G1:24/9 G2:17/6 |
62.3 ± 12.1 11.6 ± 4.3 |
17.8 ± 7.2 months 18.3 ± 8.2 months |
Non-fluent aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
CCAT | 10 days | 3 months |
| 22. Waldowski et al. (56) | G1:6/7 G2:7/6 |
62.31 ± 11.03 60.15 ± 10.58 |
28.9 ± 19.4 days 48.5 ± 32.33 days |
Various | G1: SLT + rTMS G2: SLT + sham rTMS |
CPNT; BDAE | 3 weeks | 15 weeks |
| 23. Wang et al. (51) | G1:14/1 G2:13/2 |
61.3 ± 13.2 60.4 ± 11.9 |
16.8 ± 6.4 months 16.1 ± 7.3 months |
Non-fluent aphasia | G1: SLT + rTMS G2: SLT + sham rTMS |
CCAT | 2 weeks | 3 months |
| 24. Wang et al. (39) | G1:23/3 G2:11/4 G3:9/6 |
59.53 ± 1.37 57.00 ± 1.24 47.07 ± 1.37 |
< 3 months | NR | G1: SLT + rTMS G2: SLT + sham rTMS |
WAB | 2 weeks | No |
| 25. Weiduschat et al. (57) | G1:1/5 G2:4/0 |
66.67 ± 8.26 63.75 ± 3.83 |
45.2 ± 21.0 days 57.5 ± 23.3 days |
Various | G1: SLT + rTMS G2: SLT + sham rTMS |
AAT | 2 weeks | 7 weeks |
| 26. Fang et al. (45) | G1:28/20 G2:30/22 |
64.3 ± 15.7 63.5 ± 16.5 |
10.7 ± 3.5 days 10.7 ± 3.7 days |
NR | G1: SLT + rTMS G2: SLT |
AQ; WAB | 4 weeks | No |
| 27. Yang et al. (42) | G1:11/9 G2:10/10 |
46.34 ± 11.5 47.64 ± 13.6 |
6 months | NR | G1: SLT + rTMS G3: SLT |
WAB | 4 weeks | No |
| 28. Yin et al. (33) | G1:24/26 G2:25/25 |
58.45 ± 3.50 57.35 ± 4.20 |
≤7 days | Various | G1: SLT + rTMS G3: SLT |
AQ; ABC | 4 weeks | No |
| 29. Zhang et al. (34) | G1:30 G2:30 |
63.2 ± 10.3 | NR | Motor aphasia | G1: SLT + rTMS G3: SLT |
ABC | 10 days | No |
| 30. Zhou et al. (32) | G1:30/23 G2:28/25 |
61.25 ± 8.41 59.87 ± 7.64 |
9.35 ± 3.27 weeks 8.91 ± 2.36 weeks |
Motor aphasia | G1: SLT + rTMS G3: SLT |
AQ; WAB | 4 weeks | No |
The characteristic of the included studies.
M, male; F, Female; G1, group 1; G2, group 2; G3, group 3; G3, group4; SLT, speech and language training; rTMS, repetitive transcranial magnetic stimulation; NR, not report; AAT, Aachener Aphasie Test; CCAT, Concise Chinese Aphasia Test; CPNT, Computerized Picture Naming Test; AQ, Aphasia Quotient; ABC, Aphasia Battery in Chinese; WAB, Western Aphasia Battery; BADE, Boston Diagnostic Aphasia Examination.
In addition, Table 3 summarizes the intervention parameters of rTMS for post-stroke patients in each study, such as stimulation site, intensity, frequency, number of pulses, and stimulation time. Among the studies we included, 2 studies used rTMS at 0.5 Hz to treat PSA patients, 28 studies used rTMS at 1 Hz to treat PSA patients, and 3 studies used rTMS at 10 Hz to treat PSA patients. Overall, there are more clinical studies using low-frequency rTMS to treat PSA than high-frequency rTMS. And low-frequency rTMS treatment is mainly 1 Hz rTMS.
Table 3
| Study | Parameters | Adverse events and rates | ||||
|---|---|---|---|---|---|---|
| Frequency | Stimulation location | Intensity | Number of pulses a day | Stimulation time | ||
| 1. Barwood et al. (59) | 1 Hz | The anterior portion of homolog to right pars triangularis in Broca’s area | 90% RTM | 1,200 pulses | 20 min a day, 10 days | No |
| 2. Chang (46) | 1 Hz | Broca’s area in the right hemisphere | 80% RTM | 500 pulses | 20 min a day, 15 days | No |
| 3. Chen et al. (58) | 1 Hz | Broca’s area in the right hemisphere | 80% RTM | 500 pulses | 20 min a day, 5 days a week, 2 weeks | No |
| 4. Fan (48) | 1 Hz | No report | 90% RTM | 1,200 pulses | 20 min a day, 5 days a week, 4 weeks | No |
| 5. Guo et al. (49) | 1 Hz | Right side hemispheric language mirror area | 70%RTM | 1,800 pulses | 30 min a day, 6 days a week, 4 weeks | Headache; nausea <24 h (n = 2/40) |
| 6. Haghighi et al. (47) | 1 Hz | The inferior posterior frontal gyrus | 100%RTM | No report | 20 min a day, 5 days a week, 2 weeks | No |
| 7. Heiss et al. (55) | 1 Hz | Contralesional inferior frontal gyrus | 90%RTM | No report | 20 min a day, 5 days a week, 2 weeks | No |
| 8. Hu et al. (44) | G1: 1 Hz G2: 10 Hz |
Mirror area within Broca’s area | 80%RTM | 600 pulses | 10 min a day, 5 days a week, 2 weeks | Dizziness <24 h (n = 1/20) |
| 9. Lai et al. (30) | 1 Hz | Broca or Wernicke area in the right hemisphere | 90%RTM | 1,200 pulses | 20 min a day, 5 days a week, 8 weeks | No |
| 10. Li et al. (43) | 1 Hz | Broca’s mirror area in the right hemisphere | 80%RTM | 1,200 pulses | 20 min a day, 5 days a week, 3 weeks | No |
| 11. Liu et al. (31) | 10 Hz | Broca’s and Wernicke’s zones in the left hemisphere | 90%RTM | 1,200 pulses | 10 min a day, 5 days a week, 4 weeks | No |
| 12. Peng and Zhou (37) | 1 Hz | Broca’s area in the right hemisphere | 80%RTM | 960 pulses | 20 min a day, 5 days a week, 4 weeks | No |
| 13. Qiu et al. (36) | 1 Hz | Broca’s mirror area in the right hemisphere | 80%RTM | 1,200 pulses | once a day, 5 days a week, 4 weeks | Dizziness <24 h (n = 1/20) |
| 14. Qu et al. (35) | 1 Hz | Broca’s area in the right hemisphere | 100% RTM |
1,200 pulses | once a day, 5 days a week, 2 weeks | No |
| 15. Ren et al. (38) | 1 Hz | G1: The homolog of the left Broca’s area; G2: The homolog of the left Wernicke’s area | 80%RTM | 1,200 pulses | 20 min a day, 5 days a week, 3 weeks | No |
| 16. Rubi-fessen et al. (50) | 1 Hz | The right triangular part of the inferior frontal gyrus | 90%RTM | No report | 20 min a day, 5 days a week, 2 weeks | No |
| 17. Seniów et al. (54) | 1 Hz | The right-hemisphere homolog of Broca’s area | 90%RTM | 1800 pulses | 20 min a day, 5 days a week, 3 weeks | No |
| 18. Shen (41) | 0.5 Hz | Language mirror area of the cerebral hemisphere | 80%RTM | 600 pulses | 22 min a day, 5 days a week, 4 weeks | No |
| 19. Tao (40) | 1 Hz | No report | No report | 1,200 pulses | 23 min a day, 7 days a week, 4 weeks | No |
| 20. Thiel et al. (53) | 1 Hz | The right triangular part of the posterior inferior frontal gyrus | 90%RTM | No report | 20 min a day, 5 days a week, 2 weeks | No |
| 21. Tsai et al. (52) | 1 Hz | The contralesional pars triangularis | 90%RTM | 600 pulses | 10 min a day, 5 days a week, 2 weeks | No |
| 22. Waldowski et al. (56) | 1 Hz | Two parts of Broca’s area homologs: the anterior part and posterior part | 90%RTM | No report | 30 min a day, 5 days a week, 3 weeks | No |
| 23. Wang et al. (51) | 1 Hz | The contralesional target area | 90%RTM | 1,200 pulses | 20 min a day, 5 days a week, 2 weeks | No |
| 24. Wang et al. (39) | G1: 1 Hz G2: 0.5 Hz |
Broca’s area of the left cerebral hemisphere | 90%RTM | 1,200 pulses | 20 min a day, 5 days a week, 2 weeks 40 min a day, 5 days a week, 2 weeks |
No |
| 25. Weiduschat et al. (57) | 1 Hz | The right triangular part of the inferior frontal gyrus | 90%RTM | No report | 20 min a day, 5 days a week, 2 weeks | No |
| 26. Fang et al. (45) | G1: 10 Hz G2:1 Hz |
G1: Broca’s area of the left cerebral hemisphere G2: Broca’s area of the right cerebral hemisphere |
80%RTM | 1,000 pulses | 20 min a day, 5 days a week,4 weeks | No |
| 27. Yang et al. (42) | 1 Hz | Right inferior frontal gyrus triangle | 80%RTM | 480 pulses | 20 min a day, 5 days a week,4 weeks | No |
| 28. Yin et al. (33) | 1 Hz | Broca’s and Wernicke’s zones in the right hemisphere | 40% ~ 90%RTM | 800 pulses | 20 min a day, 5 days a week,2 weeks | No |
| 29. Zhang et al. (34) | 1 Hz | Broca’s area of the right cerebral hemisphere | 80%RTM | 500 pulses | 30 min a day, 10 consecutive days | No |
| 30. Zhou et al. (32) | 1 Hz | Broca’s area of the right cerebral hemisphere | 90%RTM | 1,200 pulses | 20 min a day, 5 days a week, 4 weeks | No |
Main parameters of rTMS.
3.3 Quality assessment result
The risk of bias assessment showed that in all included RCTs, four RCTs (31, 40, 49, 55) did not use blinding for the assessment of outcome indicators and had a high risk of detection bias. And nine studies (31, 33, 34, 37, 41, 45, 50, 52, 58) did not explicitly report blinding for assessing outcome indicators, and the risk of detection bias was unclear. The risk of bias was low for all RCTs in the other items evaluated for risk of bias. Overall, the risk of bias was low in our included studies (Figures 2, 3).
Figure 2

Risk of bias summary of included studies.
Figure 3

Risk of bias graph of included studies.
We evaluated the level of evidence for the outcome indicators of the included studies by GRADE. Three outcome indicators were rated as intermediate, (AQ, ABC and WAB) due to high heterogeneity between studies (I2 > 80%) and were therefore downgraded in the inconsistency assessment. Four outcome indicators (AAT, BADE, CCAT and CPNT) were rated as intermediate because the sample sizes were too small (n < 100), which tended to influence the imprecision of the study results, and were downgraded in the imprecision assessment. The remaining outcome indicators were not found to be downgraded factors in each of the GRADE assessments. Overall, the GRADE recommended evidence level for the outcome indicator was “strong” (Table 4).
Table 4
| Assessment content | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| AAT | ABC | AQ | BADE | CCAT | CPNT | WAB | |
| Number of studies | 4 | 7 | 16 | 2 | 2 | 3 | 12 |
| Design | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
| Study limitations | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inconsistency | 0 | −1* | −1* | 0 | 0 | 0 | −1* |
| Indirectness | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imprecision | −1# | 0 | 0 | −1# | −1# | −1# | 0 |
| Publication bias | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Effect size | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GRADE quality | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Symbolic expression | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ | ⊕ ⊕ ⊕⊖ |
Grading of recommendations assessment, development, and evaluation (GRADE) quality of evidence.
AAT, Aachener Aphasie Test; ABC, Aphasia Battery in Chinese; AQ, Aphasia Quotient; BADE, Boston Diagnostic Aphasia Examination. CCAT, Concise Chinese Aphasia Test; CPNT, Computerized Picture Naming Test; WAB, Western Aphasia Battery. *High heterogeneity (I2 > 80%); #the sample size was too small (n < 100).
3.4 Results of statistical analysis
There are 12 studies (31, 32, 35, 36, 38, 39, 43–47, 49) rated the speech function of PSA patients by WAB with I2 > 50% between studies and therefore used a random-effects model for data analysis. The results of the forest plot analysis showed that patients treated with rTMS had greater improvements in areas of verbal comprehension and expression. The specific improvement results were as follows: rTMS was more effective in improving auditory comprehension in PSA patients compared to control group (MD = 1.94, 95% CI = [1.16, 2.17], I2 = 79%, p < 0. 001, Figure 4); rTMS was more effective in improving naming ability in PSA patients compared to control group (MD = 1.53, 95% CI = [0.82, 2.24], I2 = 77%, p < 0. 001, Figure 5); rTMS was more effective in improving verbal repetition in PSA patients compared with the control group (MD = 1.79, 95% CI = [1.20, 2.38], I2 = 50%, p < 0.001, Figure 6); and rTMS was more effective in improving PSA patients’ spontaneous speech (MD = 1.97, 95% CI = [1.65, 2.29], I2 = 0%, p < 0.001, Figure 7).
Figure 4

Forest plot for auditory comprehension (Western Aphasia Battery).
Figure 5
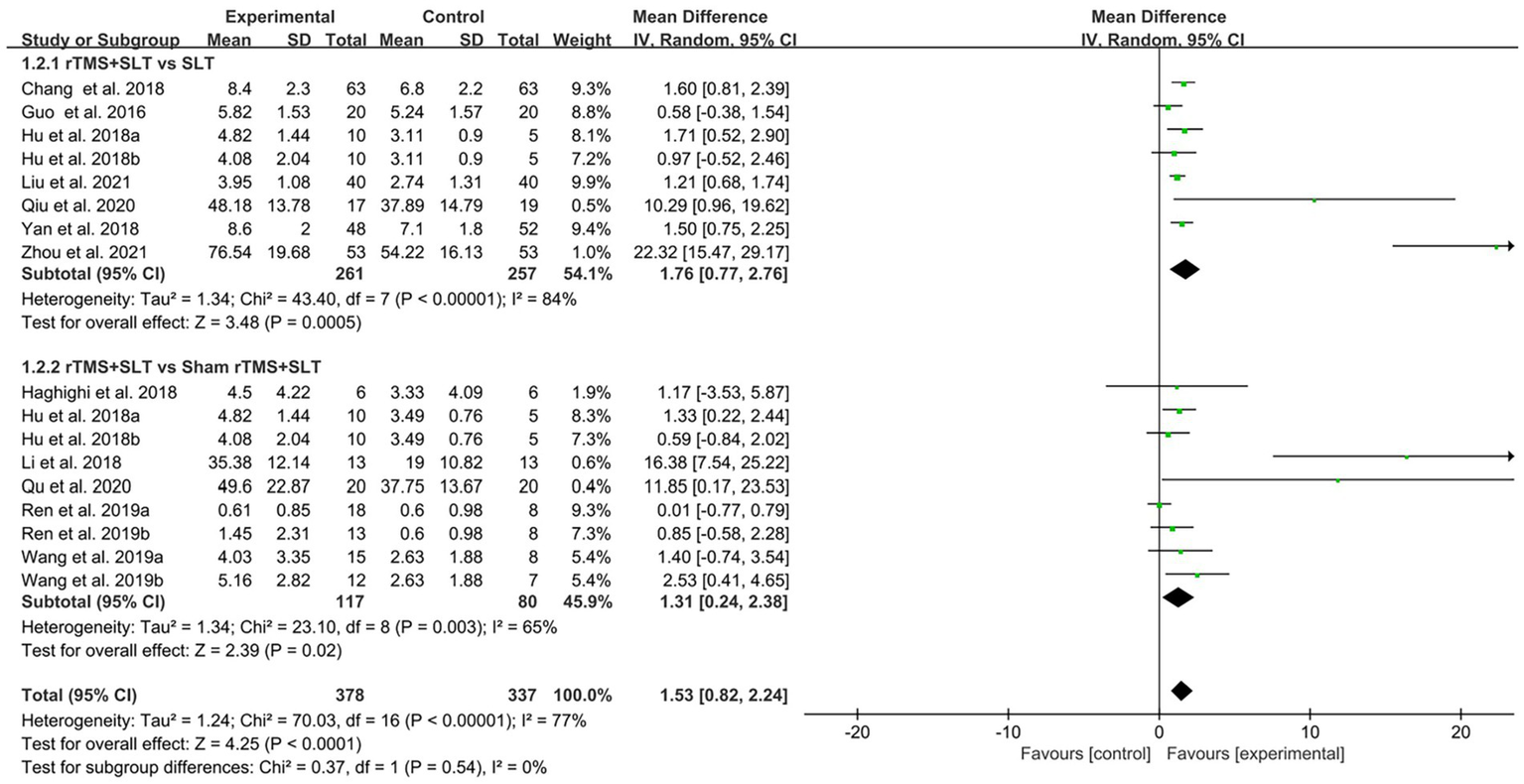
Forest plot for naming (Western Aphasia Battery).
Figure 6
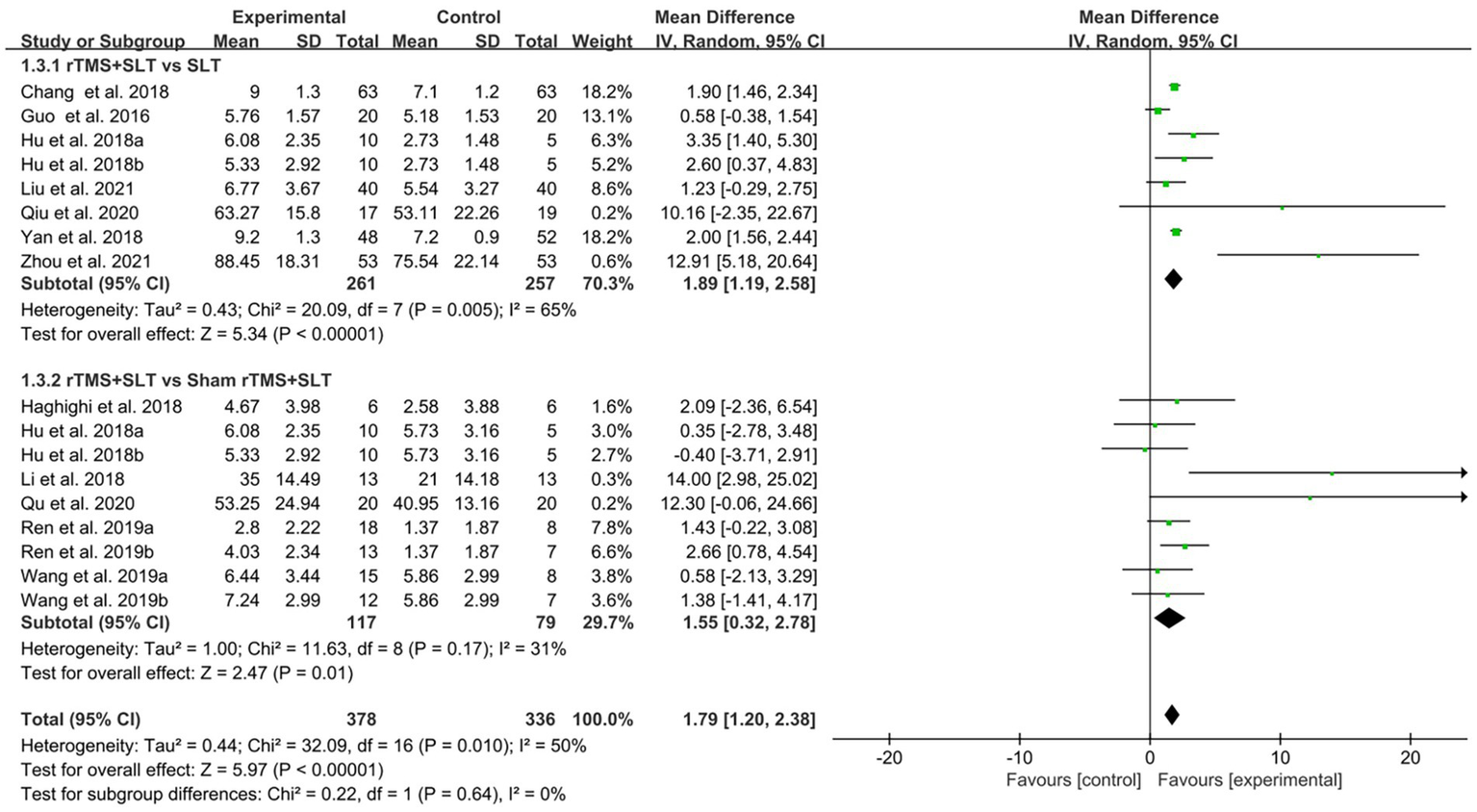
Forest plot for repetition (Western Aphasia Battery).
Figure 7

Forest plot for spontaneous speech (Western Aphasia Battery).
The degree of impairment in PSA patients was assessed in 16 studies (30, 32, 33, 35–40, 43–49) using AQ scores with an I2 > 50% between studies, so the data were analyzed using a random effects model. The results of data analysis showed that compared to the control group, the experimental group showed better improvement in AQ scores than the control group (MD = 13.82, 95% CI = [11.68, 15.97], I2 = 52%, p < 0.001; Figure 8).
Figure 8

Forest plot for Aphasia Quotient (AQ).
The degree of language loss in PSA patients was assessed by ABC in 7 studies (33, 34, 40–42, 48, 58) with I2 > 50% between studies, and we analyzed the data using a random-effects model. The results of the forest plot analysis showed that compared to the control group, the experimental group had better outcomes in ABC scores (MD = 24.79, 95% CI = [17.80, 31.77], I2 = 95%, p < 0.001; Figure 9).
Figure 9
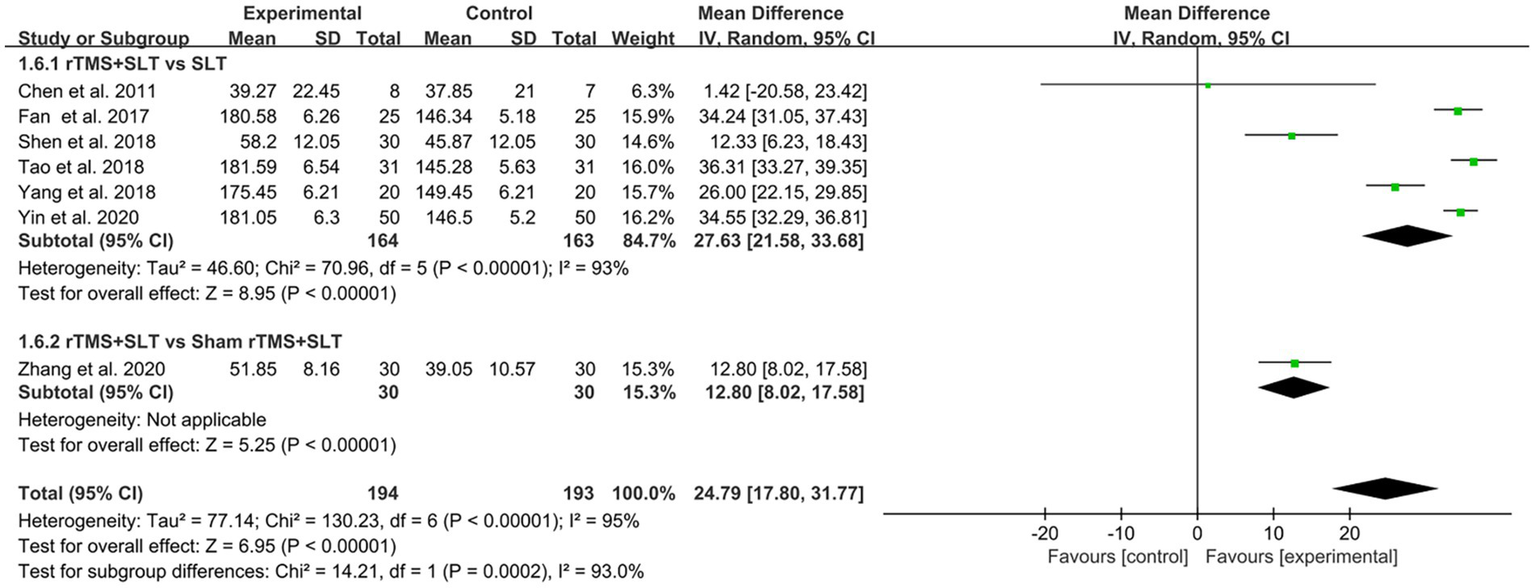
Forest plot for Aphasia Battery in Chinese (ABC).
In 4 studies (50, 53, 55, 56), the verbal function of PSA patients was assessed using the AAT scale, with an I2 < 50% between studies, and we analyzed the data using a fixed effects model. The results of the forest plot analysis showed that compared to the control group, the experimental group showed a significant improvement in AAT scores compared with the control group (MD = 13.74, 95% CI = [9.43, 18.06], I2 = 0%, p < 0.001; Figure 10).
Figure 10

Forest plot for Aachener Aphasie Test (AAT).
The severity of aphasia in PSA patients was assessed in two studies (54, 59) using the BADE scale, with an I2 < 50% between studies, and we analyzed the data using a fixed effects model. The results of the analysis of the forest plot showed that the experimental group had a better improvement than the control group in terms of BADE scores in patients with PSA (MD = 38.37, 95% CI = [6.32, 70.42], I2 = 22%, p = 0.02; Figure 11).
Figure 11

Forest plot for Boston Diagnostic Aphasia Examination (BADE).
Two studies (51, 52) used the CCAT scale to assess language function in PSA patients, and the I2 value between studies was 0%, thus the data were analyzed using a fixed effects model. The results of the analysis of the forest plot showed that the experimental group had a more positive contribution in improving the CCAT scores of PSA patients compared to the control group (MD = 1.39, 95% CI = [0.25, 2.53], I2 = 0%, p = 0.02; Figure 12).
Figure 12

Forest plot for Concise Chinese Aphasia Test (CCAT).
In addition, 3 studies (51, 52, 56) tested the naming function of PSA patients by CPNT alone, with an I2 < 50% between studies, so the data were analyzed using a fixed effects model. The results of the forest plot analysis showed that the experimental group was more able to improve the naming ability of PSA patients and promote the recovery of verbal function compared to the control group (MD = 3.95, 95% CI = [0.84, 7.06], I2 = 8%, p = 0.01; Figure 13).
Figure 13

Forest plot for Computerized Picture Naming Test (CPNT).
3.5 Adverse event reporting results
Of the 30 studies we included, only three studies reported the occurrence of adverse events. One of these studies (49) reported that two participants treated with rTMS experienced transient headache and nausea with a duration of <24 h, which accounted for 2/819 of the total number of participants in the experimental group of our study. Two other studies (36, 44) reported transient dizziness in two subjects treated with rTMS for <24 h, which accounted for 2/819 of the total number of participants in the experimental group of our study. In summary the number of patients with PSA treated with rTMS who developed adverse events as a proportion of the total number of participants in the experimental group was 4/819. In addition, based on the results reported in all studies, no patients withdrew from the experimental studies due to exhibited excessive adverse reactions. Moreover, only three of the 30 included studies reported adverse events (reporting rate 10%), and all RCTs had small sample sizes (n < 100). Therefore, we must consider the possibility of publication bias arising from unrecorded or unreported adverse events, which could underestimate the true risks of rTMS and thereby overstate its safety. We recommend that future studies continue to adhere strictly to established rTMS safety guidelines to ensure rigorous practice.
4 Discussion
The aim of this meta-analysis was to determine the efficacy of rTMS on the rehabilitation of speech function in patients with PSA. In the analysis obtained so far, we found that rTMS can effectively promote the recovery of speech function in PSA patients, which is consistent with the partial results of previous studies (60–62). Previous studies used rTMS as the intervention in PSA patients and employed speech-function scales (WAB, AQ, ABC, etc.) as outcome measures; they likewise demonstrated that rTMS can effectively improve language abilities in this population, but none assessed the safety of rTMS for PSA. The analysis with WAB as the assessment outcome showed that rTMS combined with SLT treatment was more effective than SLT alone in treating patients with PSA, as evidenced by the improvement in patients’ language abilities such as auditory comprehension, naming, repetition, and spontaneous speech. Compared with previous meta-analyses (20, 25), we have not only added recently published RCTs but also widened the spectrum of stimulation frequencies employed across studies and incorporated a broader array of outcome measures to provide more comprehensive assessments. Meanwhile, the improvement of the results assessed by CCAT and AAT indicated that rTMS could effectively enhance the speech function of PSA patients. In addition, the improvement of the assessment results by AQ, ABC and BADE showed that rTMS could effectively reduce the degree of aphasia impairment in PSA patients. In addition, to provide a higher level of evidence support, we conducted a more in-depth analysis and discussion of the mechanism of action of rTMS in treating PSA patients and the treatment effects of different intervention parameters.
Currently, more researchers prefer the “hemispheric balance theory” for the treatment rationale of rTMS in stroke patients (63, 64). An important factor influencing treatment efficacy is the site of stimulation. In the majority of trials, rTMS was delivered to the contralesional hemisphere, most often the right inferior frontal gyrus or its homolog of Broca’s area. Several studies, however, applied stimulation to lesioned hemisphere regions or adopted bilateral protocols. While our data were not sufficient to conduct subgroup meta-analysis of stimulation site, existing evidence suggests that site-specific modulation may differentially affect language outcomes in patients with Broca-type, global, or motor aphasia. Likewise, pairing rTMS with behavioral interventions such as SLT appears to maximize recovery potential compared to rTMS alone. Future large-scale trials should stratify patients according to stimulation target and aphasia profile to clarify whether specific protocols yield superior outcomes. In our brain, the bilateral hemispheres are in a state of equilibrium of mutual inhibition under normal physiological conditions, usually called “transcallosal mutual inhibition.” However, the hemispheric equilibrium of mutual inhibition can be disrupted in stroke patients with brain damage. For example, motor aphasia occurs in patients with damage to the Broca’s area in the left hemisphere, resulting in a decrease in the inhibitory capacity of the right hemisphere, which in turn leads to an activation of the right hemisphere and an increase in the inhibitory effect of the right hemisphere on the left hemisphere, thus breaking the balance of bilateral hemispheric inhibition and affecting the recovery of speech function in patients with post-stroke aphasia (63, 64). In order to correct the imbalance between the two hemispheres, we need to regulate the excitability of both cortices, and rTMS can do just this. It has been shown that rTMS can produce an electric field in the brain based on the principle of electromagnetic induction, which induces depolarized neurons to regulate cortical excitability (65). It has been found that high-frequency (>1 Hz) rTMS increases cortical excitability and low-frequency (≤1 Hz) rTMS decreases cortical excitability (66–68). and through this mechanism, rTMS can regulate the imbalance in both hemispheres (69–71) and cause plasticity changes in the cerebral cortex, thus promoting the recovery of speech function in post-stroke aphasic patients (50, 72). Thiel et al. (53) investigated the mechanism of rTMS using fMRI and found that rTMS could inhibit the hyperactivation of the healthy hemisphere, which led to a decrease in the inhibitory ability of the healthy hemisphere on the language control area of the affected hemisphere and promoted the rebalancing of the bilateral hemispheres, thus improving the language function of patients with post-stroke aphasia.
Among the 30 RCTs included, only three employed high-frequency rTMS; the remainder used low-frequency stimulation, and no uniform outcome measures were adopted. Thus, the available data are insufficient for a subgroup analysis comparing the efficacy of high- versus low-frequency rTMS. And the results of forest plot data show that both high-frequency rTMS and low-frequency rTMS can have a positive therapeutic effect on aphasia in stroke patients. Combined with the balance theory of both human hemispheres (73), there are good reasons to try the combination of high-frequency rTMS and low-frequency rTMS and to conduct a comparative efficacy study with low-frequency rTMS or high-frequency rTMS alone to explore the best treatment option of rTMS for post-stroke aphasia treatment. Yan et al. (45) reported in the previous study that combining high-frequency rTMS with low-frequency rTMS can effectively promote the recovery of speech function in stroke patients. Moreover, Hu et al. (44) also proven that low-frequency rTMS had superior and longer-lasting therapeutic effects than high-frequency rTMS on the recovery of speech function in patients with non-fluent aphasia, especially in the areas of spontaneous speech, aphasia quotient, and auditory comprehension function. However, a study by Wang et al. (39) showed that there was no difference in the therapeutic effect of low-frequency rTMS of different frequencies on patients with PSA. It can be seen that more, multicenter, RCTs with large sample sizes of high-frequency rTMS in combination with low-frequency rTMS are still needed to further approach the optimal intervention parameters of rTMS for post-stroke aphasia in the future.
Wang et al. (39) and Shen (41) expanded the selection of parameters of the commonly used rTMS and conducted a comparative study of the efficacy of 0.5-Hz rTMS and 1-Hz rTMS on PSA patients. The results found that patients in the sham stimulation group, both 0.5 Hz group, and 1 Hz group all had better WAB scores after treatment. Moreover, there was no statistically significant difference in the efficacy between the 0.5 Hz group and the 1 Hz group in treating patients with PSA, nor was there a significant difference in the improvement of WAB scores in PSA patients. However, the two groups were not identical regarding improvement in speech function. With the extension of treatment time, the 0.5 Hz group showed better progress than the 1 Hz group in auditory comprehension indexes.
In comparison, the 1 Hz group showed better improvement than the 0.5 Hz group in spontaneous speech indexes. The results of Wang et al. suggest that 0.5 Hz and 1 Hz rTMS can produce respective more advantageous therapeutic effects on different aphasic symptoms, so should different frequencies of rTMS should be selected for targeted treatment to enhance the therapeutic effects of rTMS on PSA patients corresponding to various symptoms of aphasia. More RCTs with different stimulation frequencies of rTMS for PSA need to be conducted in the future to expand the selection of treatment parameters so that we can provide individualized treatment for PSA patients with different symptoms in the clinical treatment of PSA patients.
An expert guideline published in 2021 (74) addresses the safety and recommendations for the use of rTMS in healthy subjects and patient populations. This guideline provides the most up-to-date information on the possible induction of seizures, which are theorized to be the most serious risk of rTMS. It has become apparent that such a risk is low, even in patients taking drugs acting on the central nervous system, at least with the use of traditional stimulation parameters and focal coils for which large data sets are available. However, in this study, we included a total of 819 subjects, but only 4 subjects experienced symptoms such as transient dizziness and nausea, and no patient experienced any seizure symptoms. First, we were not direct participants in this RCT and cannot be certain that these side effects necessarily came from the therapeutic effects of rTMS, and second, if these adverse effects did come as a result of the rTMS intervention, they did not result in any persistent, irreversible changes in the condition of the PSA patients. Finally, compared to the total number of participants in the trial, the number of patients experiencing adverse effects was only 0.5% of the total, making the probability of adverse events extremely low. Considering the clinical application of rTMS, the treatment of PSA patients with rTMS is indeed highly safe.
4.1 Study limitations
However, our meta-analysis also has several limitations that should be considered. First, the sample size of our included RCTs was small (n < 100), and too small a sample size tends to bias the assessment of treatment effects and overestimate the efficacy of rTMS. Second, although our studies all showed the positive impact of transcranial magnetic stimulation (TMS) in patients with post-stroke aphasia, most did not report patient follow-ups further to confirm the long-term effects of TMS. Third, the studies we included differed regarding the stimulation sites and the number of pulses. The number of available studies did not allow for more detailed subgroup analysis. Fourth, the absence of gray reports may lead to bias in comprehensive analysis results. Meaningful research is more likely to be accepted for publication, making us cautious about jumping to conclusions. Fifth, this study lack of patient-level data and possible cultural/geographical biases (majority of studies from China). Therefore, more multicenter follow-up, double-blind RCTs should be conducted to facilitate longitudinal and cross-sectional comparisons of different stimulation parameters of rTMS, to determine the optimal treatment protocol, and to improve the clinical efficacy of rTMS in patients with PSA. Moreover, heterogeneity in the site of stimulation across studies limits the generalizability of pooled results. Most studies targeted contralesional areas, but some used ipsilesional or bilateral protocols, which may lead to distinct therapeutic trajectories. The absence of detailed subgroup analyses also prevents us from assessing whether different aphasia phenotypes (e.g., Broca, global, motor) respond differently to rTMS. This remains an important future direction for tailoring interventions.
5 Conclusion
This study shows that rTMS can safely and effectively improve speech function in patients with post-stroke aphasia (PSA), particularly in auditory comprehension, naming, repetition, and spontaneous speech, which aligns with the findings of Gholami et al. (20) Transient adverse events such as headache, nausea, and dizziness were observed during treatment, but the incidence was very low (0.49%) and the symptoms resolved within 24 h. Furthermore, our systematic review and analysis indicate that different rTMS frequencies produce distinct therapeutic benefits for specific aphasic symptoms: 0.5 Hz rTMS outperforms 1 Hz rTMS in improving auditory comprehension, whereas 1 Hz rTMS is more advantageous for enhancing spontaneous speech. Future multicenter, large-sample randomized controlled trials using different rTMS frequencies are needed to determine the optimal stimulation parameters for PSA.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LX: Methodology, Conceptualization, Writing – review & editing. YD: Data curation, Conceptualization, Writing – review & editing, Project administration. CG: Formal analysis, Methodology, Project administration, Writing – original draft. JH: Methodology, Conceptualization, Writing – review & editing, Project administration, Data curation. MH: Methodology, Project administration, Writing – review & editing, Investigation. ZD: Conceptualization, Writing – original draft, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to Ganzhou People’s Hospital, Gannan Medical University, and Jiangxi Provincial People’s Hospital for their invaluable support and contributions to this research. Their resources and collaboration significantly enhanced the quality of our study. We also thank our colleagues for their insightful discussions and assistance throughout the research process, which greatly aided our efforts.
Conflict of interest
The authors declare that this research was conducted without any commercial or financial relationships that could be perceived as potential conflicts of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1614586/full#supplementary-material
References
1.
LaPointe LL . Foundations: adaptation, accommodation, aristos In: Aphasia and related neurogenic language disorders. 3rd ed. New York, NY: Thieme (2005)
2.
Plowman E Hentz B Ellis C Jr . Post-stroke aphasia prognosis: a review of patient-related and stroke-related factors. J Eval Clin Pract. (2012) 18:689–94. doi: 10.1111/j.1365-2753.2011.01650.x
3.
Benjamin EJ Blaha MJ Chiuve SE Cushman M Das SR Deo R et al . Heart disease and stroke Statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
4.
Engelter ST Gostynski M Papa S Frei M Born C Ajdacic-Gross V et al . Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke. (2006) 37:1379–84. doi: 10.1161/01.STR.0000221815.64093.8c
5.
Sheppard SM Sebastian R . Diagnosing and managing post-stroke aphasia. Expert Rev Neurother. (2021) 21:221–34. doi: 10.1080/14737175.2020.1855976
6.
Murray J Maloney S Underdown K Doeltgen S . Patient suitability for free water protocols in acute stroke and general medicine: a qualitative study of clinician perceptions. Int J Lang Commun Disord. (2022) 57:630–44. doi: 10.1111/1460-6984.12713
7.
Naeser MA Martin PI Ho M Treglia E Kaplan E Bashir S et al . Transcranial magnetic stimulation and aphasia rehabilitation. Arch Phys Med Rehabil. (2012) 93:S26–34. doi: 10.1016/j.apmr.2011.04.026
8.
Brogan EL Kim J Grimley RS Wallace SJ Baker C Thayabaranathan T et al . The excess costs of hospitalization for acute stroke in people with communication impairment: a Stroke123 data linkage substudy. Arch Phys Med Rehabil. (2023) 104:942–9. doi: 10.1016/j.apmr.2023.01.015
9.
Liu Q Li W Yin Y Zhao Z Yang Y Zhao Y et al . The effect of music therapy on language recovery in patients with aphasia after stroke: a systematic review and meta-analysis. Neurol Sci. (2022) 43:863–72. doi: 10.1007/s10072-021-05743-9
10.
Berthier ML . Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging. (2005) 22:163–82. doi: 10.2165/00002512-200522020-00006
11.
Crosson B Rodriguez AD Copland D Fridriksson J Krishnamurthy LC Meinzer M et al . Neuroplasticity and aphasia treatments: new approaches for an old problem. J Neurol Neurosurg Psychiatry. (2019) 90:1147–55. doi: 10.1136/jnnp-2018-319649
12.
Zhang X Shu B Zhang D Huang L Fu Q Du G . The efficacy and safety of pharmacological treatments for post-stroke aphasia. CNS Neurol Disord Drug Targets. (2018) 17:509–21. doi: 10.2174/1871527317666180706143051
13.
Nouwens F de Lau LM Visch-Brink EG van de Sandt-Koenderman WM Lingsma HF Goosen S et al . Efficacy of early cognitive-linguistic treatment for aphasia due to stroke: a randomised controlled trial (Rotterdam aphasia therapy Study-3). Eur Stroke J. (2017) 2:126–36. doi: 10.1177/2396987317698327
14.
Meinzer M Darkow R Lindenberg R Flöel A . Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain. (2016) 139:1152–63. doi: 10.1093/brain/aww002
15.
Burton B Isaacs M Brogan E Shrubsole K Kilkenny MF Power E et al . An updated systematic review of stroke clinical practice guidelines to inform aphasia management. Int J Stroke. (2023) 18:17474930231161454. doi: 10.1177/17474930231161454
16.
Yanyu S Ying L Kexin L Jin W . Non-invasive brain stimulation for treating post-stroke depression: a network meta-analysis. Int J Geriatr Psychiatry. (2023) 38:e5941. doi: 10.1002/gps.5941
17.
O'Donoghue M Leahy S Boland P Galvin R McManus J Hayes S . Rehabilitation of cognitive deficits poststroke: systematic review and meta-analysis of randomized controlled trials. Stroke. (2022) 53:1700–10. doi: 10.1161/STROKEAHA.121.034218
18.
Chen JM Li XL Pan QH Yang Y Xu SM Xu JW . Effects of non-invasive brain stimulation on motor function after spinal cord injury: a systematic review and meta-analysis. J Neuroeng Rehabil. (2023) 20:3. doi: 10.1186/s12984-023-01129-4
19.
Pisegna JM Kaneoka A Pearson WG Jr Kumar S Langmore SE . Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol. (2016) 127:956–68. doi: 10.1016/j.clinph.2015.04.069
20.
Gholami M Pourbaghi N Taghvatalab S . Evaluation of rTMS in patients with poststroke aphasia: a systematic review and focused meta-analysis. Neurol Sci. (2022) 43:4685–94. doi: 10.1007/s10072-022-06092-x
21.
Ding X Zhang S Huang W Zhang S Zhang L Hu J et al . Comparative efficacy of non-invasive brain stimulation for post-stroke aphasia: a network meta-analysis and meta-regression of moderators. Neurosci Biobehav Rev. (2022) 140:104804. doi: 10.1016/j.neubiorev.2022.104804
22.
Siebner HR Rothwell J . Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. (2003) 148:1–16. doi: 10.1007/s00221-002-1234-2
23.
Lefaucheur JP André-Obadia N Antal A Ayache SS Baeken C Benninger DH et al . Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. (2014) 125:2150–206. doi: 10.1016/j.clinph.2014.05.021
24.
Rossini PM Burke D Chen R Cohen LG Daskalakis Z Di Iorio R et al . Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee Clin Neurophysiol. (2015) 126:1071–107. doi: 10.1016/j.clinph.2015.02.001
25.
Keser Z Ikramuddin S Shekhar S Feng W . Neuromodulation for post-stroke motor recovery: a narrative review of invasive and non-invasive tools. Curr Neurol Neurosci Rep. (2023) 23:893–906. doi: 10.1007/s11910-023-01319-6
26.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JP et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
27.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
28.
Goldet G Howick J . Understanding GRADE: an introduction. J Evid Based Med. (2013) 6:50–4. doi: 10.1111/jebm.12018
29.
Guyatt G Oxman AD Akl EA Kunz R Vist G Brozek J et al . GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
30.
Lai ZF Huang L Fan P . Clinical observation of repeated transcranial magnetic stimulation combined with speech rehabilitation training in the treatment of speech dysfunction after stroke. Contemp Med. (2022) 28:42–4. doi: 10.3969/j.issn.1009-4393.2022.07.014
31.
Liu C Liu AX Zhen QX Qi L . Effect of conventional speech rehabilitation combined with transcranial magnetic stimulation on rehabilitation and healing of aphasia patients. Chin Prim Health Care. (2021) 35:92–4. doi: 10.3969/i.issn.1001-568X.2021.05.0030
32.
Zhou H Yuan L Wen Y Jiang W Yang L Chen H et al . Rehabilitation efficacy of low-frequency repetitive transcranial magnetic stimulation combined with speech training on post-stroke aphasia. Neural Injury And Functional Reconstruction. (2021) 16:614–6. doi: 10.21037/apm21-1055
33.
Yin Z . Study on the efficacy of low-frequency repetitive transcranial magnetic stimulation in the treatment of post-stroke lateral peripheral aphasia. J Mod Med Health. (2020) 36:2781–4.
34.
Xiaoling Z Weiliang H Xueping Z Haiqing S Haiying G Aimin Y et al . Effect of repetitive transcranial magnetic stimulation on aphasia in patients with ischemic stroke. J Brain Nerv Dis. (2020) 28:331–3.
35.
Qu YZ Zhong GL Lv J Lan LK . Observation on the efficacy of low frequency repetitive transcranial magnetic stimulation in the treatment of non-fluent aphasia after subacute stroke. CHINA MODERN DOCTOR. (2020) 58:112–5. Available at: https://d.wanfangdata.com.cn/periodical/ChvQZXJpb2RpY2FsSQOhJMlAyNTA2MjISFnp3a2p6bG1sLXI5d3MyMDIwMTcwMjkaCblwMnlVOejg1
36.
Qiu LF Cai YF Jiang YJ Lin WQ Li XY . Effects of response elaboration training combined with rTMS in patients with non-fluent aphasia. Chin J Rehabil Med. (2020) 35:1192–7. doi: 10.3969/i.issn.1001-1242.2020.10.007
37.
Peng H Zhou C . Clinical study of repetitive transcranial magnetic stimulation combined with speech rehabilitation training in the treatment of aphasia after cerebral infarction. J Clin Med Pract. (2020) 24:36–40. doi: 10.7619/jcmp.202017009
38.
Ren C Zhang G Xu X Hao J Fang H Chen P et al . The effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: a randomized sham-controlled study. Biomed Res Int. (2019) 2019:1–7. doi: 10.1155/2019/4589056
39.
Wang L Zhu Y Li X Wu X Yang Y Ji M et al . Efficacy and safety of low‑frequency repetitive transcranial magnetic stimulation for non‑fluent aphasia in the subacute phase after stroke. Chin J Phys Med Rehabil. (2019) 41:662–7. doi: 10.3760/cma.j.issn.0254‑1424.2019.09.005
40.
Tao JYL . Evaluation on the language training combined with transcranial magnetic stimulation in the treatment of aphasia of stroke. Chin Community Doctors. (2018) 34:152–4. doi: 10.3969/j.issn.1007-614x.2018.12.091
41.
Shen S . Effects and mechanisms of repetitive transcranial magnetic stimulation for treatment of left hemisphere cerebral infarction aphasia. Syst Med. (2018) 3:42–44+52. doi: 10.19368/j.cnki.2096-1782.2018.09.042
42.
Yang N . Efficacy of YRD transcranial magnetic stimulation in post stroke aphasia. Chin J Rehabil Med. (2018) 27:922–3.
43.
Li ZH Zhao YP Ren CL Cai DL Wu SY Fang H . Mechanism in the treatment of subacute motor aphasia with low frequency repetitive transcranial magnetic stimulation by quantitative electroencephalography. Chin J Rehabil Med. (2018) 33:794–9. doi: 10.3969/j.issn.1001-1242.2018.07.008
44.
Hu XY Zhang T Rajah GB Stone C Liu LX He JJ et al . Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res. (2018) 40:459–65. doi: 10.1080/01616412.2018.1453980
45.
Fang Y Weizhou Z Jiewen Z Yong Y Jun X . Clinical study of bilateral rTMS on patients with aphasia after cerebral infarction. Chin J Pract Nerv Dis. (2018) 21:129–32. doi: 10.12083/SYSJ.2018.02.033
46.
Chang L . Clinical observation of low frequency repetitive transcranial magnetic stimulation for the treatment of motor aphasia after acute cerebral infarction. Neural InjuryAnd Functional Reconstruction. (2018) 13:53–4. doi: 10.16780/j.cnki.sjssgncj.2018.01.020
47.
Haghighi M Mazdeh M Ranjbar N Seifrabie MA . Further evidence of the positive influence of repetitive transcranial magnetic stimulation on speech and language in patients with aphasia after stroke: results from a double-blind intervention with sham condition. Neuropsychobiology. (2017) 75:185–92. doi: 10.1159/000486144
48.
Fan C . Observation on curative effect of repetitive transcranial magnetic stimulation combined with language training for patients with stroke aphasia. Nurs Res. (2017) 31:1783–4. doi: 10.3969/j.issn.1009-6493.2017.14.037
49.
Guo C Zhu GP Deng WY Ma T Du AM Zhao ZY . Ffect of repetifive transcraulal magnetic stimulation combined with memantine hydrochloride and speech therapy on the treatment of motor aphasia after cerebral infarction. Chin Med Herald. (2016) 13:83–6.
50.
Rubi-Fessen I Hartmann A Huber W Fimm B Rommel T Thiel A et al . Add-on effects of repetitive transcranial magnetic stimulation on subacute aphasia therapy: enhanced improvement of functional communication and basic linguistic skills. A randomized controlled study. Arch Phys Med Rehabil. (2015) 96:1935–1944.e2. doi: 10.1016/j.apmr.2015.06.017
51.
Wang CP Hsieh CY Tsai PY Wang CT Lin FG Chan RC . Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke. (2014) 45:3656–62. doi: 10.1161/STROKEAHA.114.007058
52.
Tsai PY Wang CP Ko JS Chung YM Chang YW Wang JX . The persistent and broadly modulating effect of inhibitory rTMS in nonfluent aphasic patients: a sham-controlled, double-blind study. Neurorehabil Neural Repair. (2014) 28:779–87. doi: 10.1177/1545968314522710
53.
Thiel A Hartmann A Rubi-Fessen I Anglade C Kracht L Weiduschat N et al . Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. (2013) 44:2240–6. doi: 10.1161/STROKEAHA.111.000574
54.
Seniów J Waldowski K Leśniak M Iwański S Czepiel W Członkowska A . Transcranial magnetic stimulation combined with speech and language training in early aphasia rehabilitation: a randomized double-blind controlled pilot study. Top Stroke Rehabil. (2013) 20:250–61. doi: 10.1310/tsr2003-250
55.
Heiss WD Hartmann A Rubi-Fessen I Anglade C Kracht L Kessler J et al . Noninvasive brain stimulation for treatment of right- and left-handed poststroke aphasics. Cerebrovasc Dis. (2013) 36:363–72. doi: 10.1159/000355499
56.
Waldowski K Seniów J Leśniak M Iwański S Członkowska A . Effect of low-frequency repetitive transcranial magnetic stimulation on naming abilities in early-stroke aphasic patients: a prospective, randomized, double-blind sham-controlled study. ScientificWorldJournal. (2012) 2012:518568. doi: 10.1100/2012/518568
57.
Weiduschat N Thiel A Rubi-Fessen I Hartmann A Kessler J Merl P et al . Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke. (2011) 42:409–15. doi: 10.1161/STROKEAHA.110.597864
58.
Chen F Wang XM Sun XR Ke S Wang YX Zhao XQ et al . Therapeutical effect of low-frequency repetitive transcranial magnetic stimulation on cerebral infarction aphasia and its effect on brain electrical activity. Chin J Cerebrovasc Dis. (2011) 5:96–101. doi: 10.3969/j.issn.1672-9248.2011.02.003
59.
Barwood CH Murdoch BE Whelan BM Lloyd D Riek S O'Sullivan J et al . The effects of low frequency repetitive transcranial magnetic stimulation (rTMS) and sham condition rTMS on behavioural language in chronic non-fluent aphasia: short term outcomes. NeuroRehabilitation. (2011) 28:113–28. doi: 10.3233/NRE-2011-0640
60.
Li T Zeng X Lin L Xian T Chen Z . Effects of repetitive transcranial magnetic stimulation with different frequencies on post-stroke aphasia: a PRISMA-compliant meta-analysis. Medicine (Baltimore). (2020) 99:e20439. doi: 10.1097/MD.0000000000020439
61.
Zheng Y Zhong D Huang Y He M Xiao Q Jin R et al . Effectiveness and safety of repetitive transcranial magnetic stimulation (rTMS) on aphasia in cerebrovascular accident patients: protocol of a systematic review and meta-analysis. Medicine. (2019) 98:e18561. doi: 10.1097/MD.0000000000018561
62.
Yao L Zhao H Shen C Liu F Qiu L Fu L . Low-frequency repetitive transcranial magnetic stimulation in patients with Poststroke aphasia: systematic review and meta-analysis of its effect upon communication. J Speech Lang Hear Res. (2020) 63:3801–15. doi: 10.1044/2020_JSLHR-19-00077
63.
Postman-Caucheteux WA Birn RM Pursley RH Butman JA Solomon JM Picchioni D et al . Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci. (2010) 22:1299–318. doi: 10.1162/jocn.2009.21261
64.
Heath S McMahon KL Nickels LA Angwin A MacDonald AD van Hees S et al . An fMRI investigation of the effects of attempted naming on word retrieval in aphasia. Front Hum Neurosci. (2015) 9:291. doi: 10.3389/fnhum.2015.00291
65.
Thibaut A Schiff N Giacino J Laureys S Gosseries O . Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. (2019) 18:600–14. doi: 10.1016/S1474-4422(19)30031-6
66.
Hara T Abo M Kobayashi K Watanabe M Kakuda W Senoo A . Effects of low-frequency repetitive transcranial magnetic stimulation combined with intensive speech therapy on cerebral blood flow in post-stroke aphasia. Transl Stroke Res. (2015) 6:365–74. doi: 10.1007/s12975-015-0417-7
67.
de Aguiar V Paolazzi CL Miceli G . tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex. (2015) 63:296–316. doi: 10.1016/j.cortex.2014.08.015
68.
Fitzgerald PB Fountain S Daskalakis ZJ . A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. (2006) 117:2584–96. doi: 10.1016/j.clinph.2006.06.712
69.
Julkunen P Säisänen L Danner N Niskanen E Hukkanen T Mervaala E et al . Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage. (2009) 44:790–5. doi: 10.1016/j.neuroimage.2008.09.040
70.
Takechi U Matsunaga K Nakanishi R Yamanaga H Murayama N Mafune K et al . Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol. (2014) 125:2055–69. doi: 10.1016/j.clinph.2014.01.034
71.
Pastuszak Ż Stępień A Piusińska-Macoch R Brodacki B Tomczykiewicz K . Evaluation of repetitive transcranial magnetic stimulation effectiveness in treatment of psychiatric and neurologic diseases. Pol Merkur Lekarski. (2016) 40:388–92.
72.
Li Y Qu Y Yuan M Du T . Low-frequency repetitive transcranial magnetic stimulation for patients with aphasia after stoke: a meta-analysis. J Rehabil Med. (2015) 47:675–81. doi: 10.2340/16501977-1988
73.
Calautti C Leroy F Guincestre JY Marié RM Baron JC . Sequential activation brain mapping after subcortical stroke: changes in hemispheric balance and recovery. Neuroreport. (2001) 12:3883–6. doi: 10.1097/00001756-200112210-00005
74.
Rossi S Antal A Bestmann S Bikson M Brewer C Brockmöller J et al . Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
Summary
Keywords
repetitive transcranial magnetic stimulation, post-stroke aphasia, systematic review, noninvasive brain stimulation, meta-analysis
Citation
Xie L, Diao Y, Gong C, Huang J, Huang M and Dong Z (2025) Efficacy and safety of repetitive transcranial magnetic therapy for post-stroke aphasia: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 16:1614586. doi: 10.3389/fneur.2025.1614586
Received
19 April 2025
Accepted
15 September 2025
Published
20 October 2025
Volume
16 - 2025
Edited by
Nicholas Aderinto, Ladoke Akintola University of Technology, Nigeria
Reviewed by
Georgios Mikellides, University of Nicosia, Cyprus
Mark H. Myers, University of Tennessee Health Science Center (UTHSC), United States
Updates
Copyright
© 2025 Xie, Diao, Gong, Huang, Huang and Dong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenying Dong, x7u03k@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.