Abstract
Introduction:
Aneurysmal subarachnoid hemorrhage (SAH) carries a high risk of early rebleeding and worsens prognosis. Tranexamic acid (TXA), an antifibrinolytic agent, can prevent rebleeding; however, its effects on mortality and neurological outcomes remain controversial.
Methods:
This review evaluated the efficacy and safety of TXA with SAH. MEDLINE, CENTRAL, EMBASE, ICTRP, and ClinicalTrials.gov were systematically searched for randomized controlled trials (RCTs) and non-randomized studies of interventions (NRSIs) to assess TXA use in SAH. Studies comparing TXA with controls with SAH were included. The primary outcome was the mortality; secondary outcomes included neurological outcomes, rebleeding, thromboembolism, delayed cerebral ischemia (DCI), hydrocephalus, and adverse events. The certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach.
Results:
Fifteen RCTs (3,109 patients) and nine NRSIs (1,506 patients) were included. RCTs demonstrated that TXA likely does not reduce mortality (risk ratio [RR], 1.00; 95% confidence interval [CI], 0.82–1.22; moderate certainty) and neurological outcome, and may not increase thromboembolism and DCI. However, TXA probably reduces rebleeding but probably increases hydrocephalus. The NRSIs results were similar.
Discussion:
Although routine use is not supported, TXA may be considered for high-risk patients when early aneurysm treatment is unavailable.
Systematic review registration:
1 Introduction
Aneurysmal subarachnoid hemorrhage (SAH) accounts for 5% of all stroke incidents and has a poor prognosis (1). Rebleeding, which occurs most frequently within the first 24 h after the initial hemorrhage, markedly worsens prognosis (2). Although early aneurysm treatment is recommended to prevent rebleeding (2), immediate surgical or endovascular intervention is not always feasible for all patients (2). Tranexamic acid (TXA), an antifibrinolytic agent, has been considered a potential alternative to reduce the risk of rebleeding in such cases. Several studies have reported that TXA effectively decreases the incidence of rebleeding after SAH (3, 4).
Although TXA reduces re-bleeding, its long-term neurological effects remain unclear. Recent randomized controlled trials (RCTs) found no significant effect on neurological outcomes or mortality (5), leading to guideline recommendations against routine use (6). Nevertheless, TXA is still administered in certain settings due to uncertainties regarding the optimal duration (e.g., 24 vs. 72 h) and associated risks (7–9). Previous systematic reviews and meta-analyses (SR/MAs) often combined RCTs and observational studies without distinguishing study designs (9–11), contributing to clinical ambiguity.
This SR/MA evaluated the benefits and risks of TXA in SAH management by analyzing both RCTs and observational studies. Subgroup analyses based on treatment duration and application of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework assessed the impact of TXA on rebleeding, neurological outcomes, and complications such as thromboembolism and delayed cerebral ischemia (DCI).
2 Methods
2.1 Protocol and registration
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (Supplementary Table S1) (12). This study protocol has been made public under the Open Science Framework (accessible online: https://osf.io/yp78b/ [accessed on July 8, 2024]).
2.2 Eligibility criteria
RCTs and non-randomized studies of interventions (NRSI) on TXA for SAH were included regardless of publication status, language, country, observation period, or publication year. Studies on interventions other than TXA, comparisons of TXA with other clotting agents, and studies on intracranial hemorrhage other than spontaneous SAH were excluded. In addition, case reports and case series were excluded.
2.3 Participant types
Patients with symptoms of cerebral aneurysmal SAH confirmed by computed tomography (CT), magnetic resonance imaging (MRI), angiography, or cerebrospinal fluid (CSF) analysis and presenting within 72 h of symptom onset were included. Eligible patients were adults aged ≥ 18 years regardless of sex or racial background. Patients with intracranial hemorrhage due to trauma, arteriovenous malformations, or traumatic SAH were excluded.
2.3.1 Intervention type
TXA was administered orally or intravenously. We used a placebo, such as saline, or standard therapy alone as a control.
2.3.2 Outcome types
The following primary and secondary outcomes were evaluated.
2.3.2.1 Primary outcome
The primary outcome was all-cause mortality, including death from re-bleeding, cerebral ischemia, hydrocephalus, extracranial causes, and surgery- or anesthesia-related complications. Follow-up lasted for at least 3 months from the onset of cerebral aneurysmal SAH.
2.3.2.2 Secondary outcomes
The secondary outcomes included neurological outcomes, rebleeding, DCI, hydrocephalus, and adverse events.
Good neurological outcomes were defined as a favorable functional status based on the modified Rankin Scale (mRS) or Glasgow Outcome Scale (GOS), with mRS scores of 0–2 and GOS scores of 4–5 considered indicative of good outcomes (13). Follow-up lasted for at least 3 months from the onset of cerebral aneurysmal SAH.
Rebleeding was defined as bleeding confirmed by CT, autopsy, CSF analysis, sudden changes in vital signs, or neurological deterioration suggestive of rebleeding. The observation period included in-hospital events or those occurring within 24 h of SAH onset.
Thromboembolism includes any form of thrombosis, including clinically suspected or diagnosed deep vein thrombosis of the lower extremities and pulmonary embolism. Follow-up was conducted for a minimum of 3 months after the onset of SAH.
DCI was defined as cerebral ischemia or infarction identified through clinical assessment, CT or MRI, cerebral angiography, or relevant laboratory studies. The observation period included events that occurred during hospitalization.
Hydrocephalus was defined as a gradual onset of disorientation, CT-confirmed ventricular enlargement, and no alternative explanation for deterioration. Follow-up was conducted for a minimum of 3 months after the onset of SAH.
Adverse events were defined according to the criteria set by the original authors. The incidence proportion was calculated as the number of patients experiencing any adverse event, excluding events without specific definitions, divided by the total number of patients.
For all secondary outcomes, definitions provided by the original authors were accepted.
2.4 Search strategy and study selection
We searched MEDLINE (PubMed) from inception through May 30, 2024; the Cochrane Central Register of Controlled Trials (Cochrane Library); EMBASE (Dialog) from inception through May 15, 2024; and ongoing or unpublished trials, including the World Health Organization International Clinical Trials Platform Search Portal (WHO ICTRP) and ClinicalTrials.gov from inception through May 15, 2024 (Supplementary Table S2). The original authors were also asked for unpublished or additional data. Furthermore, the reference lists of eligible studies, relevant articles, and international guidelines (6) were reviewed.
Two reviewers (EI and HI) independently screened the titles and abstracts of all the identified studies. Articles selected during the abstract screening underwent a full-text review for eligibility. If needed, the original authors were contacted to resolve content-related discrepancies. Disagreements were resolved by consensus or by a third reviewer (HO).
2.5 Data extraction
Two independent reviewers (EI and HI) extracted the data from the included trials using a standardized data collection form. The collected data included the author, publication year, study design, setting, sample size, sex, age, eligibility criteria, country, TXA dosage, administration route, treatment duration, ischemia prevention methods, neurological status at admission, post-treatment course, and outcomes. Trials with missing data were requested from the study authors, and trials with unretrievable data were excluded.
2.6 Risk of bias assessment
Two reviewers (EI and HI) independently assessed the risk of bias using version 2 of the Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) (14). Their quality was evaluated using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (15). Discrepancies were resolved by consensus or by a third reviewer (HO). Risk-of-bias plots were generated using the robvis web application (16).
2.7 Measurement of treatment effects
For dichotomous variables, including mortality, neurological outcomes, rebleeding, thromboembolism, DCI, and hydrocephalus, random effects models were used to calculate the relative risk (RR) with a 95% confidence interval (CI). Adverse events, defined by the original authors, were summarized descriptively but were excluded from the meta-analysis.
2.8 Data synthesis and statistical analyses
All analyses were conducted using the Review Manager software (RevMan 5.4.2; Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark) to calculate pooled estimates and generate forest plots. Missing data not reported in the published manuscripts were requested by the original authors. For dichotomous data, the ITT analysis assumed that participants lost to follow-up before the event did not experience the event. Missing continuous data were not imputed, following the Cochrane Handbook (17). When only the median and interquartile range were reported, the median was converted to the mean and standard deviation using the Cochrane Handbook methods (17). Meta-analyses used data extracted from the original studies.
Statistical heterogeneity was assessed by forest plot inspection and I2 statistics. Heterogeneity was categorized as follows: 0–40% (likely unimportant), 30–60% (moderate heterogeneity), 50–90% (substantial heterogeneity), and 75–100% (considerable heterogeneity) (17). The Cochrane Chi2 test (Q-test) to assess the I2 statistic, with p-values < 0.10 considered statistically significant. Subgroup analyses of the primary outcomes in the older age group were conducted according to the protocol for substantial heterogeneity (I2 > 50%).
2.9 Reporting bias assessment
Clinical trial registries (ClinicalTrials.gov and WHO ICTRP) and literature were extensively searched for unpublished trials. Outcome reporting bias was assessed by comparing the trial protocols with published outcomes. Publication bias was evaluated using funnel plot inspection and Egger’s test, with p < 0.10 indicating statistical significance.
2.10 Subgroup analyses
Subgroup analyses examined the impact of TXA administration duration, categorized as ultra-early short-term use (≤24 h), short-term use (≤72 h), and long-term use (>72 h).
2.11 Sensitivity analyses
Sensitivity analyses assessed heterogeneity, evaluated the impact of bias on effect estimates, and excluded studies with a high risk of bias from the primary outcome analyses.
2.12 Certainty of evidence
The outcomes were summarized and their certainty of evidence was determined using the GRADEpro tool (McMaster University; Hamilton, ON, Canada), considering the risk of bias, imprecision, inconsistency, indirectness, and publication bias. Several NRSIs had a serious to critical risk of bias; therefore, the RCT and NRSI results were analyzed separately. Evidence from the included studies was listed, and outcome strength was evaluated according to the GRADE approach (18). GRADE recommendations were based solely on RCT data.
2.13 Difference between protocol and review
A subgroup analysis for DCI prevention was not conducted because only two studies explicitly reported the implementation of such measures (3, 5). Sensitivity analyses using imputed statistics for the primary outcome were not performed because no studies used imputed data. Analyses limited to participants with complete data were also not conducted, as none of the studies reported incomplete data.
3 Results
3.1 Search results
A total of 3,198 records were screened through May 2024, and 79 studies underwent full-text review (Figure 1). Ultimately, 15 RCTs (3,109 patients) and nine NRSIs (1,506 patients) from 37 reports were included in this review (Figure 1; Table 1). The reasons for excluding 42 reports from the second screening are listed in Supplementary Table S3. Fodstad 1980 (19) included two studies and was analyzed separately: one with 46 unique participants and the other overlapping with the Fodstad 1981–2 (20) publication (59 participants). Among the RCTs, one study (5) administered treatments within 24 h, nine studies (19–27) included treatments administered within 72 h, and four studies (3, 4, 28, 29) included treatments administered beyond 72 h. Among NRSI, one study (30) administered treatment within 24 h of onset, three studies (31–33) within 72 h, and two studies beyond 72 h (34, 35), whereas the timing of administration was unclear in four studies (Supplementary Table S4) (35–38).
Figure 1

PRISMA 2020 flow diagram. CENTRAL: Cochrane Central Register of Controlled Trials; ICTRP, International Clinical Trials Registry Platform; RCTs, Randomized Controlled Trials.
Table 1
| References | Country | Study types | Number of patients; total (intervention/control) | Intervention (drug dosage, route, duration) | Comparator | Time from symptom onset to treatment (h) | Ischemia prophylaxis |
|---|---|---|---|---|---|---|---|
| van Rossum et al. (28) | The Netherlands | Double-blind | 51 (26/25) | TXA 1 g/6 h, intravenously, 10 days or until surgery | Placebo (saline) | Within 0–14 days | Unclear |
| Chandra (4) | England | Double-blind | 39 (20/19) | TXA 1 g/4 h, intravenously, 14–21 days | Placebo (saline) | Within 7 days | Unclear |
| Maurice-Williams (29) | England | Non-blinded | 50 (25/25) | TXA 1.5 g/6 h, intravenously, 6 weeks or until operation, followed by 1.5 g/6 h, orally, 6 weeks or until operation | Standard therapy | Within 96 h | Unclear |
| Kaste and Ramsay (21) | Finland | Double-blind | 64 (32/32) | TXA 1 g/4 h, intravenously, until surgery or at least 21 days if no surgery | Placebo (saline) | Within 72 h | Unclear |
| Fodstad (19) | Sweden | Double-blind | 46 (23/23) | TXA 1 g/4 h, intravenously, 7 days, followed by 1 g/6 h, intravenously, day 8–35, then 1 g/8 h, orally, 6th week | Standard therapy | Within 72 h | Unclear |
| Fodstad et al. (20) | Sweden | Non-blinded | 41 (21/20) | TXA 1 g/4 h, intravenously, 7 days, followed by 1 g/6 h, intravenously, day 8–28 | Standard therapy | Unclear | Unclear |
| Fodstad et al. (20) | Sweden | Non-blinded | 59 (30/29) | TXA 1 g/4 h, intravenously, 7 days, then 1 g/6 h, intravenously, day 8–21, followed by 1.5 g/6 h, orally, 3–6th weeks | Standard therapy | Within 72 h | Unclear |
| Vermeulen et al. (22) | The Netherlands | Double-blind | 479 (241/238) | TXA 1 g/4 h, intravenously, 7 days, followed by 1 g/6 h, intravenously, day 8–28 | Placebo (saline) | Within 72 h | Unclear |
| Hijdra et al. (23) | The Netherlands | Non-blinded | 176 (88/88) | TXA 6 g/day, intravenously, 7 days, followed by 4 g/day, intravenously, day 8–28 | Standard therapy | Within 72 h | Unclear |
| Tsementzis et al. (25) | England | Double-blind | 100 (50/50) | TXA 1.5 g/4 h, intravenously, 7 days, followed by 1.5 g/4 h, orally, day 8–28 | Standard therapy | Within 72 h | Unclear |
| Menzies et al. (24) | England | Double-blind | 31 (17/14) | TXA 9 g/day, intravenously, 7 days, followed by 9 g/day, orally, day 8–21 | Standard therapy | Within 72 h | Unclear |
| Tsementzis et al. (26) | England | Double-blind | 19 (9/10) | TXA 1.5 g/4 h, intravenously, 7 days, followed by 1.5 g/4 h, orally, day 8–28 | Standard therapy | Within 72 h | Unclear |
| Roos and STAR Study Group (3) | The Netherlands | Double-blind | 462 (229/233) | TXA 1 g bolus + 1 g/4 h, intravenously, 7 days, followed by 1.5 g/6 h, other, day 8–21 | Standard therapy | Within 96 h | Administer nimodipine 60 mg/4 h, orally, 3 weeks |
| Hillman et al. (27) | Sweden | Open-label | 505 (254/251) | TXA 1 g bolus + 1 g/6 h, intravenously, 2 days | Standard therapy | within 48 h | Unclear |
| Post et al. (5) | The Netherlands | Open-label | 955 (480/475) | TXA 1 g bolus + 1 g/8 h, intravenously, 1 day or until aneurysm treatment | Standard therapy | Within 24 h | Nimodipine and normovolemia |
Characteristics of the included RCTs.
NI; no information, TXA; tranexamic acid.
3.2 Risk of bias of included studies
The Cochrane risk-of-bias tool indicated high concern for the included RCTs. The ROBINS-I tool identified a moderate to critical risk of bias in the included NRSI. The detailed assessment results are shown in Figure 2 and Supplementary Figures S1–S5.
Figure 2
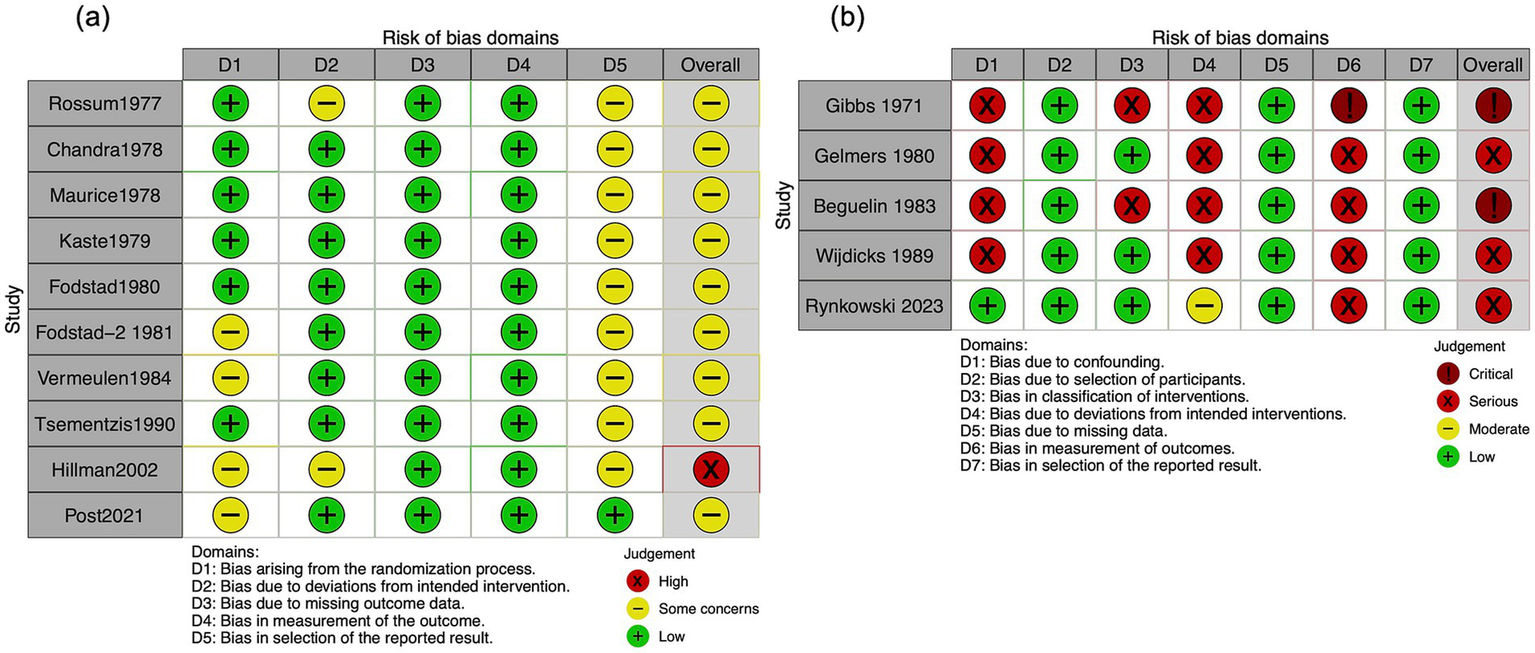
Risk of bias in the included studies evaluating the mortality. (a) Randomized controlled trials. (b) Non-randomized studies of interventions.
3.3 Primary outcomes
3.3.1 Mortality
A total of 10 RCTs (2,348 participants) were evaluated in our meta-analysis, showing that TXA likely has little to no difference in mortality (RR, 1.00; 95% CI, 0.82–1.22; I2 = 28%; moderate certainty; Table 2). Although included NRSIs exhibited high heterogeneity, they yielded findings consistent with RCTs (RR, 1.11; 95% CI, 0.42–2.89; I2 = 82%; serious to critical risk of bias; Figures 2, 3).
Table 2
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with control | Risk with TXA | |||||
| Mortality | 256 per 1,000 | 256 per 1,000 (210 to 313) | RR 1.00 (0.82 to 1.22) | 2,348 (10 RCTs) | ⨁⨁◯◯ Moderatea | TXA likely has little to no difference in mortality |
| Neurological outcomes | 588 per 1,000 | 547 per 1,000 (464 to 635) | RR 0.93 (0.79 to 1.08) | 1,736 (4 RCTs) | ⨁⨁⨁◯ Moderatea | TXA likely results have little to no difference in good neurological outcome |
| Rebleeding | 209 per 1,000 | 115 per 1,000 (88 to 150) | RR 0.55 (0.42 to 0.72) | 3,027 (13 RCTs) | ⨁⨁⨁◯ Moderatea | TXA likely reduces rebleeding |
| Thromboembolism | 47 per 1,000 | 55 per 1,000 (37 to 83) | RR 1.17 (0.78 to 1.75) | 1,746 (7 RCTs) | ⨁⨁◯◯ Lowa,b | TXA may have little to no difference in thromboembolism |
| DCI | 197 per 1,000 | 247 per 1,000 (195 to 312) | RR 1.25 (0.99 to 1.58) | 2,838 (9 RCTs) | ⨁⨁◯◯ Lowa,b | TXA may have little to no difference in DCI |
| Hydrocephalus | 370 per 1,000 | 414 per 1,000 (377 to 455) | RR 1.12 (1.02 to 1.23) | 2,184 (8 RCTs) | ⨁⨁⨁◯ Moderatea | TXA probably increases hydrocephalus slightly |
Summary of findings.
Summary of findings: Effect of TXA on outcomes in aneurysmal SAH from RCTs: Patient: Patients with aneurysmal SAH. Setting: Emergency departments, intensive care units, and hospital wards. Intervention: TXA. Comparison: Control (placebo or standard therapy). *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, confidence interval; DCI, delayed cerebral ischemia; RCT, randomized controlled trial; RR: risk ratio; SAH, subarachnoid hemorrhage; TAX, tranexamic acid. GRADE working group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect; however, there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Downgraded one point due to the risk of bias, which consists only of some concerns and high concerns.
Downgraded one point because the 95% confidence interval (CI) straddles the clinical threshold.
Figure 3
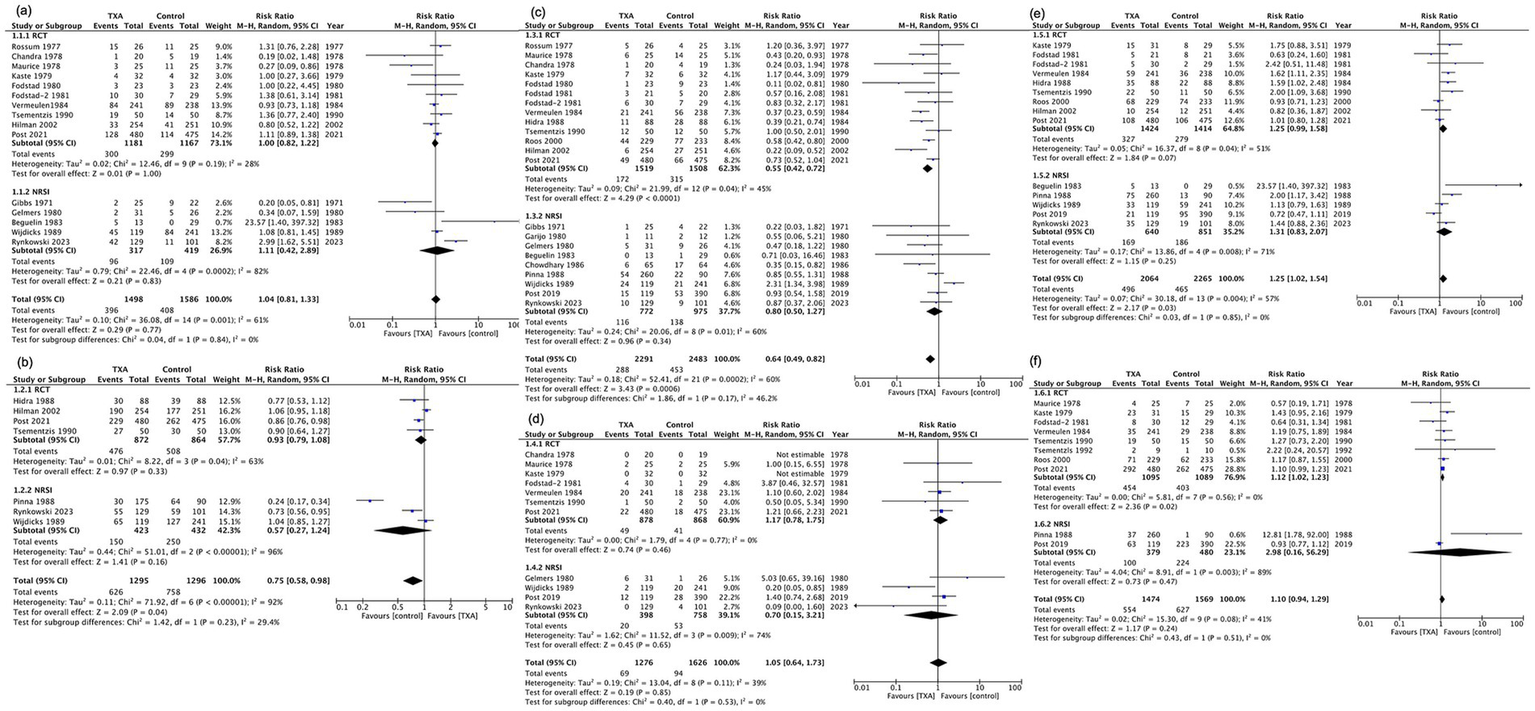
Forest plot of primary and secondary outcomes. (a) Mortality. (b) Good neurological outcomes. (c) Rebleeding. (d) Thromboembolism. (e) Delayed cerebral ischemia. (f) Hydrocephalus. CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel; SD, standard deviation; TXA, tranexamic acid.
3.4 Secondary outcomes
3.4.1 Neurological outcome
Four RCTs (1,736 participants) demonstrated that TXA likely has little to no difference in good neurological outcome (RR, 0.93; 95% CI, 0.79–1.08; I (2) = 63%; moderate certainty; Table 2; Figure 3; Supplementary Figure S1). Three NRSI (855 participants) yielded consistent results but exhibited high heterogeneity (RR, 0.75; 95% CI, 0.58–0.98; I2 = 96%; serious risk of bias; Figure 3; Supplementary Figure S1). Five of the seven studies used GOS-E (23, 25, 27, 32, 34), while two studies assessed outcomes using mRS (5, 33).
3.4.2 Rebleeding
Thirteen RCTs (3,027 participants) demonstrated that TXA likely reduces rebleeding (RR, 0.55; 95% CI, 0.42–0.72; I2 = 45%; moderate certainty; Table 2; Figure 3; Supplementary Figure S2). Nine NRSIs (1,747 participants) were pooled (RR, 0.80; 95% CI, 0.50–1.27; I2 = 60%; moderate to critical risk of bias). Both the RCTs and NRSIs displayed a similar direction of effect (Figure 3; Supplementary Figure S2).
3.4.3 Thromboembolism
Seven RCTs (1,746 participants) demonstrated that TXA may have little to no difference in thromboembolism (RR, 1.17; 95% CI, 0.78–1.75; I2 = 0%; low certainty; Table 2, Figure 3; Supplementary Figure S3). Four NRSIs (1,156 participants) were pooled (RR, 0.80; 95% CI, 0.70–3.21; I2 = 74%; serious to critical risk of bias). Although the RCTs and NRSIs displayed opposite effects, the NRSI results had a serious or critical bias and low reliability (Figure 3; Supplementary Figure S3).
3.4.4 DCI
Nine RCTs (2,838 participants) demonstrated that TXA may have little to no difference in DCI (RR, 1.25; 95% CI, 0.99–1.58; I2 = 51%; low certainty; Table 2; Figure 3; Supplementary Figure S4). Five NRSIs (1,491 participants) were pooled (RR, 1.31; 95% CI, 0.83–2.07; I2 = 71%; serious to critical risk of bias). Both the RCTs and NRSIs displayed a similar direction of effect (Figure 3; Supplementary Figure S4).
3.4.5 Hydrocephalus
Eight RCTs (2,184 participants) demonstrated that TXA probably increases hydrocephalus slightly (RR, 1.12; 95% CI, 1.02–1.23; I2 = 0%; moderate certainty; Table 2, Figure 3; Supplementary Figure S5). Two NRSIs (859 participants) were pooled (RR: 2.98; 95% CI, 0.16–56.29; I2 = 89%; moderate to serious risk of bias). Unlike the NRSIs, the pooled RR in the RCTs favored the control group (Figure 3; Supplementary Figure S5). Three studies reported a rebleeding RR < 1 and a hydrocephalus RR < 1; four studies reported a rebleeding RR < 1 and a hydrocephalus RR ≥ 1; and two studies reported both rebleeding and hydrocephalus RRs ≥ 1 (Supplementary Figure S6).
3.4.6 Adverse events
Only two studies reported adverse events other than primary and secondary outcomes. Two studies reported delirium, with incidence ranging from 2.0 to 13.5% in the TXA group and from 10.0 to 12.8% in the control group (5, 25). One study reported the following complication rates for TXA versus controls: severe hyponatremia (2.4% vs. 2.0%), pneumonia (12.6% vs. 14.6%), infectious meningitis (7.4% vs. 6.7%), urinary tract infection (9.6% vs. 9.1%), seizures (12.0% vs. 8.9%), and Terson syndrome (3.9% vs. 3.6%) (5).
3.5 Reporting bias assessment
Funnel plots (Supplementary Figures S7–S12) did not indicate publication bias. Egger’s test was performed for mortality and rebleeding, as both outcomes included more than 10 RCTs; however, no evidence of publication bias was observed (p = 0.32 and 0.58, respectively).
3.6 Subgroup analysis
In the RCTs, a subgroup analysis of the primary outcome was conducted based on the duration of TXA administration. One study (5) administered TXA within 24 h, six studies (19–22, 25, 27) within 72 h, and three studies (4, 28, 29) after 72 h. The pooled results remained consistent across all subgroups, showing no differences between the TXA and control groups (Table 1; Supplementary Figure S13).
3.7 Sensitivity analysis
One study with a high risk of bias was removed from the primary analysis. This exclusion did not affect the results, which showed no difference between the TXA and control groups (Supplementary Figure S14).
4 Discussion
This is the first meta-analysis to rigorously compare RCTs and NRSIs on TXA use for SAH. Fifteen RCTs (3,109 participants) were included to provide GRADE-based evidence on TXA’s efficacy and safety of TXA. Additionally, nine NRSIs (1,506 participants) were analyzed. Overall, TXA administration, regardless of the timing or method, did not affect mortality, favorable neurological outcomes, thromboembolism, or DCI. However, it likely reduced the risk of rebleeding while slightly increasing the incidence of hydrocephalus. As rebleeding occurs in less than 4% of patients (39), routine TXA use offers no apparent benefit. However, its use should be considered in patients with a high risk of rebleeding.
TXA may be beneficial when early surgical intervention is not feasible; however, its risks, including hydrocephalus, should be carefully considered. Therefore, routine administration is not supported. Although older studies used TXA for 3–4 weeks post-SAH (3, 26), recent trends favor its administration within 1–2 days or until surgery (5, 27). Subgroup analyses demonstrated no benefit from ultra-early (≤24 h) or short-term (≤72 h) administration, whereas prolonged use (>72 h) showed only a modest trend toward reduced rebleeding, with substantial heterogeneity. The widespread adoption of early, definitive interventions such as surgical clipping or endovascular coiling may have contributed to more consistent rebleeding prevention. Despite methodological variations, including the use of two different neurological outcome scales (mRS and GOS-E) across studies, recent studies have demonstrated diminishing differences in rebleeding and mortality over time between the TXA and control groups. Rebleeding-related mortality is approximately 80% (39) and because TXA reduced rebleeding incidence (RR ≈ 0.55), its use may be considered in high-risk patients, such as those with (1) increasing aneurysm size, (2) worsening neurological deficits, (3) angiography within 3 h of bleeding, (4) sentinel hemorrhage, and (5) loss of consciousness at initial bleed (39). Future studies should evaluate the impact of TXA in these high-risk populations to determine its role in reducing rebleeding, mortality, and neurological complications.
Unlike previous SR/MA, this study applied GRADE assessments to different TXA-related outcomes with certainty ranging from low to moderate. Recent SR/MA have faced limitations, including the inclusion of non-SAH hemorrhages (40), misclassification of observational studies as RCTs (9, 10), lack of GRADE assessments (41), inconsistencies between GRADE assessments and recommendations (42), and duplicate inclusion of the same study by Foodstad as two separate studies (43). These methodological issues have resulted in an unclear understanding of the role of TXA in SAH. By addressing these limitations, this study provides a more objective assessment. Specifically, we excluded non-SAH hemorrhages based solely on GRADE assessments of RCTs to minimize confounding and incorporated NRSIs to enhance the comprehensiveness of our analysis. The application of these methodological refinements established that the certainty of the GRADE assessments for different outcomes ranged from low to moderate. Consequently, this review included the largest number of RCTs and observational studies to date and provided the most detailed effect size estimates for TXA in SAH management.
This study had several limitations. First, several of the included studies were outdated, with longer intervals between SAH onset and study enrollment and prolonged TXA administration. However, our sensitivity analysis of recent RCTs demonstrated that studies with shorter enrollment and administration periods demonstrated similar trends in mortality, neurological outcomes, and rebleeding. Additionally, modern management strategies, such as venous thromboembolism prophylaxis and spinal drains for hydrocephalus prevention (30), may have influenced the comparability between older and recent studies. Second, seizure reporting was limited. Risk factors for TXA-associated seizures include renal impairment, female sex, epilepsy history, age > 70 years, and high-dose administration (>50 mg/kg) (44). Among the included studies, only one RCT (5) reported seizure, suggesting insufficient power to detect complications or difficulty in differentiating TXA-induced seizures from SAH-related seizures. Future studies should specifically investigate the seizure risk to elucidate the safety profile of TXA in SAH management. Third, a major limitation was the insufficient reporting of detailed patient admission status and TXA administration protocols across the included studies. Only four studies (16.7%) reported Fisher grades, nine (37.5%) reported Hunt-Hess grades, and two (8.3%) reported World Federation of Neurosurgical Societies (WFNS) grades, indicating substantial gaps in baseline clinical characterization. Furthermore, substantial heterogeneity in TXA administration parameters—including bolus use, infusion rate, total dosage, and timing relative to aneurysm treatment—was observed across studies, limiting comparability and synthesis of findings. This lack of granular data precluded a more refined analysis to establish objective criteria for patient selection and to elucidate the therapeutic balance between rebleeding prevention and hydrocephalus risk. Future research should prioritize standardized and detailed reporting of both admission characteristics and TXA protocols. In particular, dose–response meta-analyses incorporating these variables may help define optimal dosing strategies tailored to patient-specific risk profiles. Fourth, although CT was the primary modality for diagnosing rebleeding and hydrocephalus, other approaches (angiography, CSF analysis, autopsy, and clinical deterioration) were also used, which may have contributed to heterogeneity. We assessed outcome-measurement bias using RoB 2 and ROBINS-I, but future studies should standardize diagnostic criteria for TXA evaluation in SAH.
5 Conclusion
This meta-analysis reveals that TXA likely has little to no impact on mortality; however, it probably reduces rebleeding while slightly increasing hydrocephalus risk, with moderate-certainty evidence. In clinical practice, TXA should be reserved for selected patients with a high risk of rebleeding.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
EI: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. HI: Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. HmO: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AI: Supervision, Writing – review & editing. TT: Conceptualization, Project administration, Supervision, Writing – review & editing. HsO: Conceptualization, Project administration, Supervision, Writing – review & editing. TH: Project administration, Supervision, Writing – review & editing. YF: Conceptualization, Writing – review & editing. GF: Conceptualization, Writing – review & editing. YK: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by JSPS KAKENHI (Grant number 24K12153).
Acknowledgments
We extend our gratitude to René Post for providing additional information regarding the ULTRA trial, which contributed greatly to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fneur.2025.1754088.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1617817/full#supplementary-material
References
1.
Etminan N Chang HS Hackenberg K de Rooij NK Vergouwen MDI Rinkel GJE et al . Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and Meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
2.
Guo LM Zhou HY Xu JW Wang Y Qiu YM Jiang JY . Risk factors related to aneurysmal rebleeding. World Neurosurg. (2011) 76:292–8. doi: 10.1016/j.wneu.2011.03.025
3.
Roos Y . Antifibrinolytic treatment in subarachnoid hemorrhage: a randomized placebo-controlled trial. Neurology. (2000) 54:77–82. doi: 10.1212/wnl.54.1.77
4.
Chandra B . Treatment of subarachnoid haemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double-blind clinical trial. Ann Neurol. (1978) 3:502–4.
5.
Post R Germans MR Tjerkstra MA Vergouwen MDI Jellema K Koot RW et al . Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. (2021) 397:112–8. doi: 10.1016/S0140-6736(20)32518-6
6.
Treggiari MM Rabinstein AA Busl KM Caylor MM Citerio G Deem S et al . Guidelines for the Neurocritical Care Management of Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. (2023) 39:1–28. doi: 10.1007/s12028-023-01713-5
7.
Sorteberg A Romundstad L Sorteberg W . Timelines and rebleeds in patients admitted into neurosurgical care for aneurysmal subarachnoid haemorrhage. Acta Neurochir. (2021) 163:771–81. doi: 10.1007/s00701-020-04673-3
8.
Lauridsen SV Hvas CL Sandgaard E Gyldenholm T Tønnesen EK Hvas A-M . No hyperfibrinolysis following subarachnoid or intracerebral haemorrhage: a prospective cohort study. Blood Coagul Fibrinolysis. (2019) 30:341–9. doi: 10.1097/MBC.0000000000000845
9.
Fatima K Ur Rehman MEAE Asmar A Farooq H Ahmad NU Danial A et al . The efficacy of antifibrinolytic therapy in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Future Sci OA. (2023) 9:FSO866. doi: 10.2144/fsoa-2023-0014
10.
Ghaith HS Gabra MD Ebada MA Dada OE Al-Shami H Bahbah EI et al . Tranexamic acid for patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of 2991 patients. Int J Neurosci. (2022) 134:763–776. doi: 10.1080/00207454.2022.2148957
11.
Bouillon-Minois JB Croizier C Baker JS Pereira B Moustafa F Outrey J et al . Tranexamic acid in non-traumatic intracranial bleeding: a systematic review and meta-analysis. Sci Rep. (2021) 11:15275. doi: 10.1038/s41598-021-94727-y
12.
Page MJ McKenzie JE Bossuyt PM . Statement: an updated guideline for reporting systematic reviews. BMJ. (2020) 372:372. doi: 10.1136/bmj.n71
13.
Gaastra B Ren D Alexander S Awad IA Blackburn S Doré S et al . Evidence-based interconversion of the Glasgow outcome and modified Rankin scales: pitfalls and best practices. J Stroke Cerebrovasc Dis. (2022) 31:106845–6. doi: 10.1016/j.jstrokecerebrovasdis.2022.106845
14.
Sterne JAC Savović J Page MJ . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:1–8. doi: 10.1136/bmj.l4898
15.
Sterne JA Hernán MA Reeves BC Savović J Berkman ND Viswanathan M et al . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:4–10. doi: 10.1136/bmj.i4919
16.
McGuinness LA Higgins JPT . Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12:55–61. doi: 10.1002/jrsm.1411
17.
Higgins JPT Thomas J Chandler J . Cochrane handbook for systematic reviews of interventions second edition. Cochrane Database Systematic Review (2019).
18.
Guyatt G Oxman AD Akl EA Kunz R Vist G Brozek J et al . GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
19.
Fodstad H . Tranexamic acid (AMCA) in aneurysmal subarachnoid haemorrhage. J Clin Pathol. (1980) 33:68–73.
20.
Fodstad H Forssell A Liliequist B Schannong M . Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery. (1981) 8:158–65.
21.
Kaste M Ramsay M . Tranexamic acid in subarachnoid hemorrhage. A double-blind study. Stroke. (1979) 10:519–22.
22.
Vermeulen M Lindsay KW Murray GD Cheah F Hijdra A Muizelaar JP et al . Antifibrinolytic treatment in subarachnoid hemorrhage. N Engl J Med. (1984) 311:432–7.
23.
Hijdra A van Gijn J Nagelkerke NJ Vermeulen M van Crevel H . Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke. (1988) 19:1250–6.
24.
Menzies SA Hartley JA Hitchcock ER Rorke E Gill JS . The effect of tranexamic acid on bleeding time and haemostasis. Neurochirurgia (Stuttg). (1991) 34:141–3.
25.
Tsementzis SA Hitchcock ER Meyer CHA . Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms - a double-blind placebo-controlled study. Acta Neurochir. (1990) 102:1–10.
26.
Tsementzis SA Meyer CH Hitchcock ER . Cerebral blood flow in patients with a subarachnoid haemorrhage during treatment with tranexamic acid. Neurochirurgia (Stuttg). (1992) 35:74–8.
27.
Hillman J Fridriksson S Nilsson O Yu Z Säveland H Jakobsson KE . Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. (2002) 97:771–8. doi: 10.3171/jns.2002.97.4.0771
28.
van Rossum J Wintzen AR Endtz LJ Schoen JHR de Jonge H . Effect of tranexamic acid on rebleeding after subarachnoid hemorrhage: a double-blind controlled clinical trial. Ann Neurol. (1977) 2:242–5.
29.
Maurice-Williams RS . Prolonged antifibrinolysis: an effective non-surgical treatment for ruptured intracranial aneurysms?Br Med J. (1978) 1:945–7.
30.
Post R Germans MR Boogaarts HD Ferreira Dias Xavier B van den Berg R Coert BA et al . Short-term tranexamic acid treatment reduces in-hospital mortality in aneurysmal sub-arachnoid hemorrhage: a multicenter comparison study. PLoS One. (2019) 14:e0211868–9. doi: 10.1371/journal.pone.0211868
31.
Gelmers HJ . Prevention of recurrence of spontaneous subarachnoid haemorrhage by tranexamic acid. Acta Neurochir. (1980) 52:45–50.
32.
Wijdicks EFM Hasan D Lindsay KW Wijdicks EF Brouwers PJ Hatfield R et al . Short-term tranexamic acid treatment in aneurysmal subarachnoid hemorrhage. Stroke. (1989) 20:1674–9.
33.
Rynkowski CB Hegele V Soares PHR Tonello ML Petterson L Gomes FK et al . Effects of Tranexamic acid in patients with subarachnoid hemorrhage in Brazil: a prospective observational study with propensity score analysis. Neurocrit Care. (2023) 39:191–7. doi: 10.1007/s12028-023-01732-2
34.
Pinna G Pasqualin A Vivenza C Da Pian R . Rebleeding, ischaemia and hydrocephalus following anti-fibrinolytic treatment for ruptured cerebral aneurysms: a retrospective clinical study. Acta Neurochir. (1988) 93:77–87.
35.
Béguelin C Seiler R . Subarachnoid hemorrhage with normal cerebral panangiography. Neurosurgery. (1983) 13:409–11.
36.
Gibbs JR Corkill AG . Use of an anti-fibrinolytic agent (tranexamic acid) in the management of ruptured intracranial aneurysms. Postgrad Med J. (1971) 47:199–200.
37.
Alvarez Garijo JA Vilches JJ Aznar JA . Preoperative treatment of ruptured intracranial aneurysms with tranexamic acid and monitoring of fibrinolytic activity. J Neurosurg. (1980) 52:453–5.
38.
Chowdhary UM Sayed K . Prevention of early recurrence of aneurysmal subarchnoid haemorrhage by tranexamic acid: a controlled clinical trial. Vasc Surg. (1986) 20:8–13.
39.
Starke RM Connolly ES . Rebleeding after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2011) 15:241–6.
40.
Xiong Y Guo X Huang X Kang X Zhou J Chen C et al . Efficacy and safety of tranexamic acid in intracranial haemorrhage: a meta-analysis. PLoS One. (2023) 18:e0282726. doi: 10.1371/journal.pone.0282726
41.
Ren J Qian D Wu J Ni L Qian W Zhao G et al . Safety and efficacy of tranexamic acid in aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. Front Neurol. (2021) 12:710495. doi: 10.3389/fneur.2021.710495
42.
Shi M Yang C Chen Z-H Xiao L-F Zhao W-Y . Efficacy and safety of tranexamic acid in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of randomized controlled trials. Front Surg. (2021) 8:790149. doi: 10.3389/fsurg.2021.790149
43.
Al Zayer MO Al Johani FM Al Ghamdi SA . The effectiveness and safety of Tranexamic acid in treating aneurysmal subarachnoid hemorrhage: a systematic review and Meta-analysis. Healthc (Basel, Switzerland). (2024) 12:2452. doi: 10.3390/healthcare12232452
44.
Mergoum AM Mergoum AS Larson NJ Dries DJ Cook A Blondeau B et al . Tranexamic acid use in the surgical arena: a narrative review. J Surg Res. (2024) 302:208–21. doi: 10.1016/j.jss.2024.07.042
Summary
Keywords
meta-analysis, subarachnoid hemorrhage, systematic review, tranexamic acid, rebleeding
Citation
Imai E, Ito H, Okano H, Inoue A, Terayama T, Okamoto H, Hifumi T, Fujimoto Y, Fujiwara G and Kuroda Y (2025) Efficacy and safety of tranexamic acid administration for subarachnoid hemorrhage: a systematic review and meta-analysis. Front. Neurol. 16:1617817. doi: 10.3389/fneur.2025.1617817
Received
25 April 2025
Accepted
02 June 2025
Published
17 June 2025
Corrected
15 December 2025
Volume
16 - 2025
Edited by
Johannes Boltze, University of Warwick, United Kingdom
Reviewed by
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, Colombia
Luan Oliveira Ferreira, Federal University of Pará, Brazil
Updates
Copyright
© 2025 Imai, Ito, Okano, Inoue, Terayama, Okamoto, Hifumi, Fujimoto, Fujiwara and Kuroda.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiromu Okano, okanohiromu0121@gmail.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.