Abstract
Background:
Cenobamate (CNB) and lacosamide (LCM) are two common used third-generation anti-seizure medications (ASMs) for third-line treatment of the drug-resistant epilepsy. The real-world data on adverse events (AEs) related to them remains limited.
Methods:
All data obtained from the US Food and Drug Administration Adverse Event Reporting System (FAERS) database, covering the period from 2008 to 2024. The reporting odds ratio, proportional reporting ratio and bayesian confidence propagation neural network to assess and compare the safety signals of CNB and LCM for comparison.
Results:
A total of 50,323,324 AE reports were recorded, with 3,584 for CNB and 13,874 for LCM. The most significant signals were primarily in nervous system and psychiatric disorders, resembling those of traditional sodium channel blockers. Unreported AEs in the drug dispensatory were identified in LCM (multiple-drug resistance). Notable differences between LCM and CNB emerged: Certain numbers of AE signals associated with LCM were found in cardiac disorders, while no such relevant signals were detected for CNB; among the signals that detected in both drugs, most signals from CNB are stronger than those from LCM; The initial titration dose of CNB (12.5 mg, qd) reported a significantly higher number of AEs compared to the other dose groups.
Conclusion:
Choosing the right ASMs requires consideration of the type of epilepsy, the individual tolerance and potential severe toxicity of different medications. Although the disproportionality analysis is a hypothesis generating, we provide a reference for the clinical safety of CNB and LCM.
1 Introduction
Epilepsy is one of the most common and severe neurological disorders, affecting approximately 70 million people worldwide (1). Anti-seizure medications (ASMs) are the cornerstone of epilepsy treatment. Patients who fail to effectively control their seizures after trials with two properly dosed ASMs are classified as having drug-resistant epilepsy (DRE) (2). Focal seizures are the most common type of epileptic seizures and are more prone to drug resistance, with the drug resistance rate even exceeding 50% (3). Cenobamate (CNB) and lacosamide (LCM) are two commonly used third-generation ASMs for third-line treatment of focal DRE (4).
CNB, a novel oral ASM, was approved for monotherapy or adjunctive therapy in adults with focal seizures by the Food and Drug Administration (FDA) of United States in November 2019 and for adjunctive treatment of focal DRE by the European Medicines Agency (EMA) in March 2021 (5, 6). Studies also showed that CNB demonstrates remarkable superiority in the treatment of focal epilepsy (7, 8). CNB exerts its effect mainly through a dual mechanism. As a sodium channel blocker (SCB), it acts on voltage-gated sodium channels (VGSCs), suppressing persistent sodium currents instead of transient ones to diminish repetitive neuronal firing (9). Meanwhile, CNB serves as a positive allosteric modulator of γ-aminobutyric acid (GABA)-A receptors, augmenting neuronal inhibition (10). Adverse events (AEs) associated with CNB are generally mild to moderate, primarily involving nervous system symptoms such as somnolence, dizziness, diplopia, and gait or coordination issues (11). The incidence of treatment-emergent adverse events (TEAEs) may be related to faster titration rates and higher starting doses, with a higher incidence noted in patients using SCBs and benzodiazepines (e.g., clobazam) concurrently (12).
LCM, available in multiple formulations, received FDA and EMA approval in 2008 for the treatment of focal seizures, either as monotherapy or in combination (13). LCM is also the first third-generation ASM approved for sale in China. LCM is also a SCB, which selectively enhances the slow inactivation of VGSCs, thereby reducing pathological hyperexcitability without affecting the physiological activities of neurons (14). Additionally, it is reported that LCM interacts with collapsin response mediator protein-2 (CRMP-2), preventing abnormal neuronal connections linked to epilepsy (15). Common AEs associated with LCM include dizziness, headache, somnolence, diplopia, and arrhythmias, most of which are mild to moderate (16). Reducing the maintenance dose can alleviate or eliminate these adverse effects (17).
Patients with DRE typically require combination therapy with at least two ASMs, increasing the likelihood of drug interactions and adverse effects (18). However, real-world data on AEs related to newer-generation ASMs remains limited. The FDA Adverse Event Reporting System (FAERS), one of the largest pharmacovigilance databases globally, serves as a public, voluntary, spontaneous reporting system aimed at facilitating post-marketing safety monitoring (19). In this study, we extracted data from the FAERS database to identify safety signals, thoroughly assess, compare, and analyze real-world AEs related to CNB and LCM. We aim to enhance clinical awareness of the AEs associated with these two drugs and provide a reference for the clinical safety of novel ASMs.
2 Methods
2.1 Data source
All data for this study were obtained from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database, covering the period from Quarter 1 (Q1) of 2008 to Q3 of 2024. The raw data files were downloaded from the official FDA website.1 The FAERS database consists of seven primary data files: demographic and administrative information (DEMO), drug details (DRUG), reported adverse events (REAC), patient outcomes (OUTC), sources of reports (RPSR), drug therapy information (THER), and indications for drug use (INDI). The FAERS database follows international safety reporting guidelines from the International Conference on Harmonization, coding all AEs with preferred terms (PTs) from the Medical Dictionary of Regulatory Activities. PTs cover signs, symptoms, diagnoses, lab tests, and medical/family history. They can also be grouped into high-level group terms (HLGTs) and system organ classes (SOCs), or organized using Standardized MedDRA Queries (SMQs) for specific conditions.
2.2 Ethic approval
FARES database is a de-identified public database, thus this study not requiring any form of ethic approval.
2.3 Drug identification
Given the vast number of drug-related AE and drugs in the FAERS database, we focused our analysis on two specific drugs: the generic names “Cenobamate” and “Lacosamide,” along with their brand names “Xcopri,” “Motpoly XR” and “Vimpat.” This approach allowed us to effectively screen relevant reports for the targeted drugs.
2.4 Adverse event
In accordance with FDA guidelines for data deduplication, we selected the field labels PRIMARY_ID, CASE_ID, and FDA_DT from the DEMO table and sorted the data based on these labels. For reports that share the same CASE_ID, we retained only the report with the highest FDA_DT value. In instances where multiple reports have the same CASE_ID and FDA_DT, we kept only the report with the largest PRIMARY_ID value.
Two chief pharmacists categorized the AE reports based on standardized MedDRA queries (SMQs) and gathered clinical characteristics of patients, including gender, age, and AE outcomes. Importantly, this study places greater emphasis on AEs that are not documented in the descriptions of each drug or those that were previously undetected. With the assistance of these pharmacists and drug dispensatory, we were able to exclude drug indications from the AEs and identify AEs that were overlooked by healthcare professionals.
2.5 Statistical analysis
In this study, we employed the reporting odds ratio (ROR) and proportional reporting ratio (PRR) to assess the association between two groups of drugs. Higher values of ROR and PRR indicate a stronger relationship between the target drug and specific AEs. However, since relying on a single algorithm can introduce bias, we also utilized an alternative method known as the Bayesian Confidence Propagation Neural Network (BPNN) to further analyze the data and minimize false positive safety signals. A signal was identified if: (1) a ≥ 6, (2) ROR ≥ 2 with 95% CI > 1, (3) PRR > 2 with χ2 > 4, and (4) IC-2SD > 0. All disproportionality analyses in databases were followed READUS-PV guidelines (20). The ratio imbalance measurement algorithm was in Supplementary Table S1. Disproportionate measurement principles and signal detection standards were in Supplementary Table S2. Reports that did not meet these criteria were not considered signals and were excluded from this study.
After acquiring the enrollment data, we systematically compared the safety signals of PTs and SOCs for CNB and LCM. All analyses were conducted using Microsoft Excel 2019 or R (V4.1.2), while figures were created using python (v3.12).
3 Results
3.1 Baseline patient characteristics
The baseline characteristics of patients were presented in Table 1. From Q4 of 2008 to Q3 of 2024, a total of 50,323,324 AE reports were recorded from FAERS, in which 3,584 reports were associated with CNB, and 13,874 with LCM (Figure 1). This disparity is due to CNB’s later introduction, as it was added to the FAERS database in Q4 of 2020. The highest proportion of AE reports came from the United States, accounting for 58.2% of LCM-related reports and 89.6% of CNB-related reports. The number of AEs reported yearly after the marketing was shown in Figure 2.
Table 1
| Index | Lacosamide (%) | Cenobamate (%) |
|---|---|---|
| Number of events | 13,874 | 3,584 |
| Gender | ||
| Female | 6,724 (48.5) | 10 (0.3) |
| Male | 5,491 (39.6) | 13 (0.4) |
| Unknown | 1,659 (12.0) | 3,561 (99.4) |
| Age | ||
| <18 | 1,019 (7.3) | 1 (0.0) |
| 18–49 | 3,016 (21.8) | 14 (0.4) |
| 50–79 | 3,133 (22.6) | 4 (0.01) |
| ≥80 | 585 (4.2) | 0 (0) |
| Unknown | 6,121 (44.1) | 3,565 (99.5) |
| Serious outcomes | ||
| Death | 1,333 (9.6) | 49 (1.4) |
| Disability | 134 (1.0) | 7 (0.2) |
| Life-threatening | 367 (2.6) | 47 (1.3) |
| Hospitalization | 3,600 (25.9) | 436 (12.2) |
| Other | 4,866 (35.1) | 391 (10.8) |
| Unknown | 5 (0.0) | 2 (0.1) |
| Reporter country | ||
| United States | 8,068 (58.2) | 3,210 (89.6) |
| Japan | 1,179 (8.5) | 0 (0) |
| Germany | 1,114 (8.0) | 43 (1.2) |
| French/Britain | 610 (4.4) | 70 (2.0) |
| Other countries | 2,903 (20.9) | 261 (7.2) |
Characteristics of reports associated with lacosamide and cenobamate of the FAERS.
Figure 1

The flow chart of the study.
Figure 2
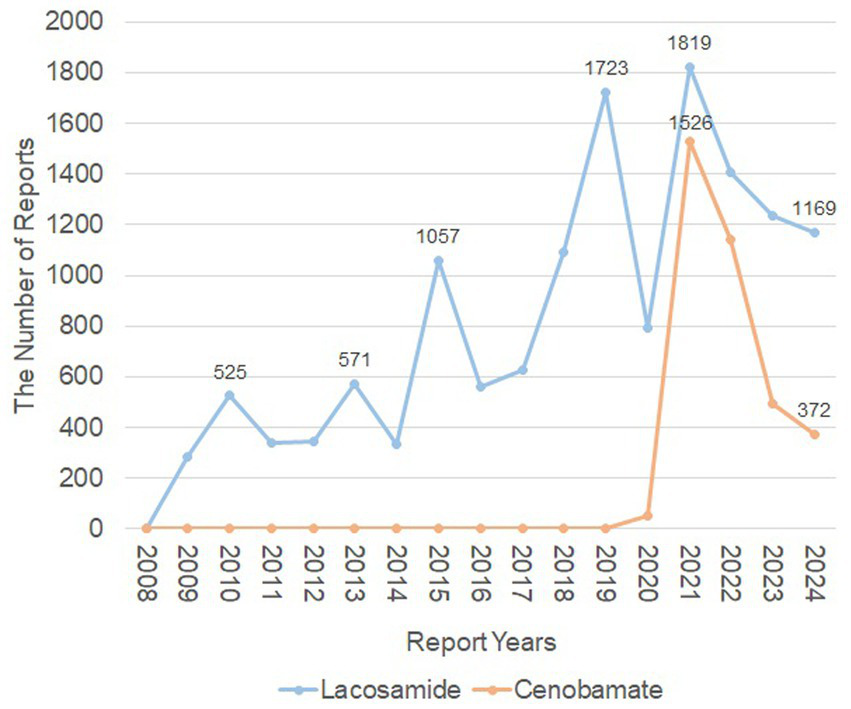
The number of adverse events reported yearly after the marketing of lacosamide and cenobamate. The orange line represented the reports of Cenobamate, while the blue line represented the reports of Lacosamide. X-axis shows the timeline when the drug was used, and Y-axis displays the number of reports per year.
CNB and LCM had the same top three indications, and the concomitant medications were also quite similar, including Levetiracetam, Lamotrigine and Topiramate (Table 2). Notably, CNB was frequently used in combination with LCM (3rd concomitant medication). The top 10 AEs were summarized in Table 3. The common AEs between CNB and LCM were in nervous system, such as somnolence (CNB n = 406, LCM n = 425), dizziness (CNB n = 344, LCM n = 694), balance disorder (CNB n = 140, LCM n = 213), memory impairment (CNB n = 120, LCM n = 289).
Table 2
| Index | Lacosamide (n) | Cenobamate (n) |
|---|---|---|
| Indications | Epilepsy (3823) | Epilepsy (986) |
| Seizure (3542) | Seizure (1069) | |
| Partial seizures (852) | Partial seizures (197) | |
| Concomitant medication | Levetiracetam (2404) | Levetiracetam (144) |
| Lamotrigine (894) | Lamotrigine (293) | |
| Carbamazepine (531) | Lacosamide (97) | |
| Topiramate (572) | Clobazam (245) | |
| Valproic acid (511) | Topiramate (113) |
Top 3 indications and top 5 concomitant medications and in AE reports of lacosamide and cenobamate.
Table 3
| Lacosamide | n | Cenobamate | n |
|---|---|---|---|
| Dizziness | 694 | Fatigue | 432 |
| Fall | 584 | Somnolence | 406 |
| Somnolence | 425 | Dizziness | 344 |
| Memory impairment | 289 | Fall | 213 |
| Bradycardia | 220 | Feeling abnormal | 172 |
| Balance disorder | 213 | Gait disturbance | 142 |
| Loss of consciousness | 208 | Balance disorder | 140 |
| Amnesia | 196 | Memory impairment | 120 |
| Multiple-drug resistance | 177 | Insomnia | 95 |
| Diplopia | 174 | Drug interaction | 91 |
Top 10 in the number of adverse event report of lacosamide and cenobamate.
The number of AE reports of CNB and LCM in patients with various daily dose were presented in Table 4. The lowest dose of CNB (12.5 mg) was reported the highest number of AEs, while the 200 ~ 400 mg dose of LCM was reported the most AEs.
Table 4
| Daily dose | Lacosamide (n) | Daily dose | Cenobamate (n) |
|---|---|---|---|
| ≤100 mg | 1209 | 12.5 mg | 1952 |
| 100 mg ~ 200 mg | 1540 | 25 mg | 363 |
| 200 mg ~ 400 mg | 1940 | 50 mg | 377 |
| >400 mg | 521 | 100 mg | 344 |
| 150 mg | 215 |
Number of adverse event reports of lacosamide and cenobamate in patients over 18 years old with various daily dose.
3.2 Disproportionality analyses
A total of 55 strong signals with an IC-2SD ≥ 1.0 were identified for CNB, while 98 were found for LCM, as shown in Table 5. First of all, nervous system disorders were the most prominent SOC in both medications, such as somnolence (CNB IC-2SD = 3.29, LCM IC-2SD = 1.55), balance disorder (CNB IC-2SD = 2.81, LCM IC-2SD = 1.65), dysarthria (CNB IC-2SD = 2.76, LCM IC-2SD = 1.01), eye movement disorder (CNB IC-2SD = 2.55, LCM IC-2SD = 1.12), sedation (CNB IC-2SD = 2.14, LCM IC-2SD = 1.24), ataxia (CNB IC-2SD = 1.94, LCM IC-2SD = 2.54), memory impairment (CNB IC-2SD = 1.88, LCM IC-2SD = 1.43) and aphasia (CNB IC-2SD = 1.85, LCM IC-2SD = 1.77). Other similar SOCs are psychiatric disorders (CNB 16 signals, IC-2SD range:1.02 ~ 2.99; LCM 21 signals, IC-2SD range: 1.00 ~ 2.89), general disorders and administration site conditions (CNB 9 signals, IC-2SD range:1.03 ~ 4.15; LCM 3 signals, IC-2SD range: 1.14 ~ 6.25), eye disorders (CNB 2 signals, IC-2SD range:1.71 ~ 4.12; LCM 1 signal: diplopia, IC-2SD = 3.8), injury, poisoning and procedural complications (CNB 1 signal: fall IC-2SD = 1.54, LCM 1 signal: fall IC-2SD = 1.27). CNB has 2 unique SOC classification of AE, such as social circumstances (2 signals, IC-2SD range: 1.20 ~ 1.46), respiratory, thoracic and mediastinal disorders (1 signal: hiccups, IC-2SD = 2.53). LCM has 6 unique SOC classification of AE, such as cardiac disorders (18 signals, IC-2SD range: 1.02 ~ 4.92), congenital, familial and genetic disorders (13 signals, IC-2SD range: 1.16 ~ 7.39), investigations (5 signals, IC-2SD range: 1.11 ~ 5.11), metabolism and nutrition disorders (3 signals, IC-2SD range: 1.04 ~ 1.84), pregnancy, puerperium and perinatal conditions (5 signals, IC-2SD range: 1.10 ~ 3.29), skin and subcutaneous tissue disorders (2 signals, IC-2SD range: 1.12 ~ 1.36), and vascular disorders (1 signal: systolic hypertension IC-2SD = 3.57). Additionally, signals with an IC-2SD ≥ 0 are also presented in Supplementary Table S3.
Table 5
| SOCs/PTs | Lacosamide | Cenobamate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | PRR | Chi_squared | ROR(CI025) | IC(IC-2SD) | N | PRR | Chi_squared | ROR(CI025) | IC(IC-2SD) | |
| Cardiac disorders | ||||||||||
| Atrial flutter | 37 | 7.07 | 191.94 | 7.08 (5.12 ~ 9.78) | 2.82 (2.35) | / | / | / | / | / |
| Atrioventricular block | 93 | 19.2 | 1580.72 | 19.25 (15.68 ~ 23.63) | 4.24 (3.94) | / | / | / | / | / |
| Atrioventricular block complete | 100 | 24.23 | 2185.12 | 24.29 (19.92 ~ 29.61) | 4.57 (4.28) | / | / | / | / | / |
| Atrioventricular block first degree | 36 | 13.14 | 399.56 | 13.15 (9.47 ~ 18.26) | 3.7 (3.22) | / | / | / | / | / |
| Atrioventricular block second degree | 52 | 27.73 | 1310.93 | 27.77 (21.09 ~ 36.56) | 4.76 (4.36) | / | / | / | / | / |
| Bradycardia | 220 | 6.39 | 996.87 | 6.42 (5.62 ~ 7.34) | 2.67 (2.48) | / | / | / | / | / |
| Bradycardia neonatal | 14 | 17.55 | 215.5 | 17.56 (10.36 ~ 29.76) | 4.11 (3.36) | / | / | / | / | / |
| Bundle branch block left | 24 | 9.18 | 173.69 | 9.19 (6.15 ~ 13.73) | 3.19 (2.61) | / | / | / | / | / |
| Bundle branch block right | 18 | 6.42 | 81.93 | 6.42 (4.04 ~ 10.21) | 2.68 (2.01) | / | / | / | / | / |
| Conduction disorder | 11 | 10.71 | 96.04 | 10.71 (5.92 ~ 19.4) | 3.41 (2.57) | / | / | / | / | / |
| Defect conduction intraventricular | 6 | 26.64 | 144.95 | 26.64 (11.87 ~ 59.81) | 4.71 (3.6) | / | / | / | / | / |
| Sinus arrest | 23 | 24.97 | 518.88 | 24.98 (16.53 ~ 37.76) | 4.61 (4.02) | / | / | / | / | / |
| Sinus bradycardia | 48 | 7.94 | 289.48 | 7.95 (5.99 ~ 10.56) | 2.98 (2.57) | / | / | / | / | / |
| Sinus node dysfunction | 34 | 27.7 | 856.15 | 27.73 (19.73 ~ 38.95) | 4.76 (4.27) | / | / | / | / | / |
| Supraventricular extrasystoles | 8 | 3.97 | 17.73 | 3.97 (1.98 ~ 7.95) | 1.99 (1.02) | / | / | / | / | / |
| Ventricular tachycardia | 41 | 4.07 | 94.68 | 4.07 (3 ~ 5.54) | 2.02 (1.58) | / | / | / | / | / |
| Electrocardiogram pr prolongation | 21 | 48.6 | 942.38 | 48.63 (31.44 ~ 75.21) | 5.55 (4.92) | / | / | / | / | / |
| Electrocardiogram qrs complex prolonged | 17 | 5.5 | 62.29 | 5.5 (3.42 ~ 8.86) | 2.45 (1.77) | / | / | / | / | / |
| Congenital, familial and genetic disorders | ||||||||||
| Atrial septal defect | 33 | 5.41 | 118.19 | 5.42 (3.85 ~ 7.62) | 2.43 (1.93) | / | / | / | / | / |
| Brugada syndrome | 9 | 13.81 | 105.77 | 13.81 (7.16 ~ 26.64) | 3.77 (2.85) | / | / | / | / | / |
| Cardiac septal defect | 10 | 20.28 | 180.4 | 20.29 (10.86 ~ 37.9) | 4.32 (3.44) | / | / | / | / | / |
| Coarctation of the aorta | 20 | 29.28 | 533.83 | 29.3 (18.8 ~ 45.65) | 4.84 (4.2) | / | / | / | / | / |
| Congenital hydronephrosis | 11 | 22.76 | 224.71 | 22.76 (12.54 ~ 41.33) | 4.48 (3.64) | / | / | / | / | / |
| Cryptorchism | 8 | 10.79 | 70.44 | 10.79 (5.38 ~ 21.64) | 3.42 (2.45) | / | / | / | / | / |
| Cytogenetic abnormality | 7 | 8.4 | 45.3 | 8.4 (3.99 ~ 17.66) | 3.06 (2.04) | / | / | / | / | / |
| Fetal malformation | 17 | 24.66 | 378.42 | 24.67 (15.26 ~ 39.87) | 4.6 (3.91) | / | / | / | / | / |
| Hepatic arteriovenous malformation | 7 | 545.77 | 2647.91 | 545.86 (224.55 ~ 1326.92) | 8.57 (7.39) | / | / | / | / | / |
| Multiple congenital abnormalities | 11 | 11.28 | 102.18 | 11.29 (6.23 ~ 20.44) | 3.48 (2.65) | / | / | / | / | / |
| Polydactyly | 7 | 8.84 | 48.32 | 8.84 (4.2 ~ 18.59) | 3.13 (2.11) | / | / | / | / | / |
| Spina bifida | 7 | 4.54 | 19.27 | 4.54 (2.16 ~ 9.54) | 2.18 (1.16) | / | / | / | / | / |
| Trisomy 18 | 6 | 24.78 | 134.28 | 24.79 (11.05 ~ 55.62) | 4.6 (3.5) | / | / | / | / | / |
| Eye Disorders | ||||||||||
| Diplopia | 174 | 10.46 | 1477.28 | 10.5 (9.04 ~ 12.2) | 3.38 (3.16) | 83 | 17.39 | 1277.97 | 17.51 (14.11 ~ 21.74) | 4.12 (3.8) |
| Vision blurred | / | / | / | / | / | 83 | 3.27 | 131.12 | 3.29 (2.65 ~ 4.08) | 1.71 (1.39) |
| General disorders and administration site conditions | ||||||||||
| Crying | / | / | / | / | / | 22 | 3.16 | 32.45 | 3.16 (2.08 ~ 4.81) | 1.66 (1.06) |
| Drug interaction | / | / | / | / | / | 91 | 3.08 | 127.83 | 3.09 (2.52 ~ 3.8) | 1.62 (1.32) |
| Drug intolerance | / | / | / | / | / | 51 | 2.71 | 54.88 | 2.71 (2.06 ~ 3.57) | 1.44 (1.03) |
| Fatigue | / | / | / | / | / | 432 | 2.87 | 531.85 | 2.94 (2.67 ~ 3.24) | 1.52 (1.38) |
| Feeling abnormal | / | / | / | / | / | 172 | 3.65 | 331.89 | 3.69 (3.17 ~ 4.29) | 1.87 (1.65) |
| Feeling drunk | 27 | 5.5 | 98.94 | 5.5 (3.77 ~ 8.03) | 2.45 (1.91) | 35 | 24.98 | 801.33 | 25.06 (17.96 ~ 34.95) | 4.63 (4.15) |
| Gait disturbance | / | / | / | / | / | 142 | 3.69 | 279.36 | 3.73 (3.16 ~ 4.4) | 1.88 (1.64) |
| Gait inability | / | / | / | / | / | 32 | 5.25 | 110.02 | 5.26 (3.72 ~ 7.45) | 2.39 (1.89) |
| Multiple-drug resistance | 177 | 95.75 | 15413.47 | 96.17 (82.51 ~ 112.09) | 6.48 (6.25) | / | / | / | / | / |
| Screaming | 15 | 3.64 | 28.58 | 3.64 (2.19 ~ 6.04) | 1.86 (1.14) | 7 | 5.93 | 28.66 | 5.93 (2.83 ~ 12.45) | 2.57 (1.55) |
| Injury, poisoning and procedural complications | ||||||||||
| Fall | 584 | 2.62 | 585.52 | 2.64 (2.43 ~ 2.87) | 1.39 (1.27) | 213 | 3.34 | 350.3 | 3.38 (2.95 ~ 3.87) | 1.74 (1.54) |
| Investigations | ||||||||||
| Anticoagulation drug level abnormal | 8 | 72.84 | 535.59 | 72.86 (35.71 ~ 148.64) | 6.11 (5.11) | / | / | / | / | / |
| Anticonvulsant drug level above therapeutic | 9 | 23.59 | 191.05 | 23.59 (12.2 ~ 45.62) | 4.53 (3.61) | / | / | / | / | / |
| Anticonvulsant drug level decreased | 14 | 16.86 | 206.06 | 16.86 (9.95 ~ 28.58) | 4.06 (3.31) | / | / | / | / | / |
| Anticonvulsant drug level increased | 15 | 15.08 | 194.82 | 15.08 (9.06 ~ 25.1) | 3.9 (3.17) | / | / | / | / | / |
| Blood sodium decreased | 35 | 3.02 | 47.29 | 3.02 (2.17 ~ 4.22) | 1.59 (1.11) | / | / | / | / | / |
| Metabolism and nutrition disorders | ||||||||||
| Cell death | 6 | 4.4 | 15.72 | 4.4 (1.98 ~ 9.82) | 2.13 (1.04) | / | / | / | / | / |
| Hyperammonaemia | 14 | 4.36 | 36.17 | 4.36 (2.58 ~ 7.38) | 2.12 (1.37) | / | / | / | / | / |
| Marasmus | 7 | 7.33 | 38.01 | 7.33 (3.49 ~ 15.4) | 2.87 (1.84) | / | / | / | / | / |
| Nervous system disorders | ||||||||||
| Eye movement disorder | 16 | 3.53 | 28.99 | 3.53 (2.16 ~ 5.77) | 1.82 (1.12) | 13 | 10.05 | 105.67 | 10.06 (5.83 ~ 17.34) | 3.33 (2.55) |
| Altered state of consciousness | 58 | 4 | 130.11 | 4 (3.09 ~ 5.18) | 2 (1.62) | / | / | / | / | / |
| Amnesia | 196 | 4.63 | 557.19 | 4.65 (4.04 ~ 5.35) | 2.21 (2) | 41 | 3.38 | 68.88 | 3.39 (2.5 ~ 4.61) | 1.76 (1.31) |
| Aphasia | 86 | 4.24 | 212.49 | 4.25 (3.44 ~ 5.25) | 2.08 (1.77) | 30 | 5.17 | 100.81 | 5.18 (3.62 ~ 7.41) | 2.37 (1.85) |
| Apraxia | 6 | 6.34 | 26.84 | 6.34 (2.84 ~ 14.14) | 2.66 (1.56) | / | / | / | / | / |
| Ataxia | 62 | 7.53 | 349.07 | 7.54 (5.87 ~ 9.68) | 2.91 (2.54) | 15 | 6.35 | 67.51 | 6.36 (3.83 ~ 10.55) | 2.66 (1.94) |
| Balance disorder | 213 | 3.61 | 401.1 | 3.62 (3.17 ~ 4.15) | 1.85 (1.65) | 140 | 8.3 | 897.83 | 8.39 (7.1 ~ 9.91) | 3.05 (2.81) |
| Brain fog | / | / | / | / | / | 9 | 6.14 | 38.68 | 6.14 (3.19 ~ 11.82) | 2.62 (1.7) |
| Cerebral disorder | 18 | 3.71 | 35.48 | 3.71 (2.33 ~ 5.89) | 1.89 (1.22) | / | / | / | / | / |
| Clumsiness | / | / | / | / | / | 6 | 11.28 | 56.07 | 11.29 (5.06 ~ 25.15) | 3.49 (2.4) |
| Cognitive disorder | 97 | 3.08 | 136.07 | 3.09 (2.53 ~ 3.77) | 1.62 (1.33) | / | / | / | / | / |
| Coordination abnormal | 30 | 3.49 | 53.17 | 3.49 (2.44 ~ 5) | 1.8 (1.28) | 21 | 8.55 | 139.68 | 8.56 (5.58 ~ 13.14) | 3.09 (2.48) |
| Dementia | 52 | 2.9 | 64.56 | 2.9 (2.21 ~ 3.81) | 1.53 (1.14) | |||||
| Disturbance in attention | 96 | 2.65 | 98.32 | 2.65 (2.17 ~ 3.24) | 1.4 (1.11) | 47 | 4.53 | 129.41 | 4.55 (3.41 ~ 6.06) | 2.18 (1.76) |
| Dizziness | / | / | / | / | / | 344 | 3.67 | 674.26 | 3.76 (3.37 ~ 4.18) | 1.88 (1.72) |
| Drop attacks | 14 | 35.5 | 456.37 | 35.51 (20.87 ~ 60.41) | 5.11 (4.35) | / | / | / | / | / |
| Drug withdrawal convulsions | 23 | 18.81 | 382.2 | 18.82 (12.47 ~ 28.42) | 4.21 (3.62) | / | / | / | / | / |
| Dysarthria | 63 | 2.59 | 61.34 | 2.59 (2.02 ~ 3.32) | 1.37 (1.01) | 61 | 8.77 | 419.62 | 8.82 (6.85 ~ 11.34) | 3.13 (2.76) |
| Dysgraphia | / | / | / | / | / | 8 | 6.12 | 34.24 | 6.13 (3.06 ~ 12.26) | 2.61 (1.65) |
| Dyslexia | 6 | 7.88 | 35.81 | 7.88 (3.53 ~ 17.58) | 2.97 (1.88) | / | / | / | / | / |
| Dysstasia | / | / | / | / | / | 29 | 5.08 | 94.87 | 5.09 (3.53 ~ 7.32) | 2.34 (1.81) |
| Febrile convulsion | 8 | 10.86 | 70.99 | 10.86 (5.41 ~ 21.79) | 3.43 (2.46) | / | / | / | / | / |
| Hypersomnia | / | / | / | / | / | 88 | 16.2 | 1250.72 | 16.31 (13.22 ~ 20.13) | 4.01 (3.71) |
| Lethargy | / | / | / | / | / | 58 | 5.44 | 210.21 | 5.46 (4.22 ~ 7.07) | 2.44 (2.07) |
| Loss of consciousness | 208 | 2.57 | 199.29 | 2.58 (2.25 ~ 2.95) | 1.36 (1.16) | / | / | / | / | / |
| Memory impairment | 289 | 3.04 | 395.77 | 3.06 (2.72 ~ 3.43) | 1.6 (1.43) | 120 | 4.41 | 317.26 | 4.45 (3.72 ~ 5.33) | 2.14 (1.88) |
| Motor dysfunction | / | / | / | / | / | 8 | 4.19 | 19.4 | 4.19 (2.09 ~ 8.38) | 2.07 (1.1) |
| Nystagmus | 27 | 8.05 | 165.6 | 8.05 (5.52 ~ 11.76) | 3 (2.45) | 7 | 7.27 | 37.79 | 7.27 (3.46 ~ 15.27) | 2.86 (1.84) |
| Sedation | 50 | 3.13 | 72.34 | 3.13 (2.37 ~ 4.14) | 1.64 (1.24) | 29 | 6.35 | 130.56 | 6.36 (4.42 ~ 9.16) | 2.66 (2.14) |
| Slow speech | / | / | / | / | / | 11 | 23.41 | 234.74 | 23.43 (12.95 ~ 42.39) | 4.54 (3.71) |
| Somnolence | 425 | 3.24 | 657.37 | 3.26 (2.96 ~ 3.59) | 1.69 (1.55) | 406 | 10.82 | 3622.68 | 11.18 (10.13 ~ 12.35) | 3.43 (3.29) |
| Speech disorder | 99 | 2.85 | 118.66 | 2.85 (2.34 ~ 3.48) | 1.51 (1.22) | 36 | 3.62 | 68.33 | 3.63 (2.62 ~ 5.04) | 1.86 (1.38) |
| Syncope | 157 | 2.38 | 125.98 | 2.39 (2.04 ~ 2.79) | 1.25 (1.02) | / | / | / | / | / |
| Tongue biting | 7 | 5.52 | 25.79 | 5.52 (2.63 ~ 11.6) | 2.46 (1.44) | / | / | / | / | / |
| Tremor | / | / | / | / | / | 79 | 2.53 | 73.12 | 2.54(2.03 ~ 3.17) | 1.34(1.01) |
| Bradyphrenia | / | / | / | / | / | 6 | 4.52 | 16.44 | 4.52(2.03 ~ 10.08) | 2.18(1.08) |
| Pregnancy, puerperium and perinatal conditions | ||||||||||
| Abortion spontaneous | 131 | 4.94 | 410.24 | 4.95 (4.17 ~ 5.88) | 2.3 (2.05) | / | / | / | / | / |
| Hydrops foetalis | 8 | 19.49 | 138.19 | 19.5 (9.7 ~ 39.2) | 4.26 (3.29) | / | / | / | / | / |
| Premature baby | 58 | 2.78 | 66.14 | 2.79 (2.15 ~ 3.6) | 1.47 (1.1) | / | / | / | / | / |
| Premature delivery | 49 | 4.22 | 120.14 | 4.23 (3.19 ~ 5.6) | 2.07 (1.67) | / | / | / | / | / |
| Stillbirth | 19 | 5.78 | 74.71 | 5.78 (3.68 ~ 9.07) | 2.52 (1.88) | / | / | / | / | / |
| Psychiatric disorders | ||||||||||
| Mental impairment | / | / | / | / | / | 18 | 3.86 | 38.2 | 3.87 (2.44 ~ 6.14) | 1.95 (1.29) |
| Abnormal behavior | 107 | 4.17 | 256.66 | 4.17 (3.45 ~ 5.05) | 2.05 (1.78) | 39 | 5.3 | 136.11 | 5.32 (3.88 ~ 7.28) | 2.41 (1.95) |
| Acute psychosis | 15 | 9.38 | 111.46 | 9.38 (5.65 ~ 15.59) | 3.22 (2.5) | / | / | / | / | / |
| Affective disorder | 23 | 4.42 | 60.77 | 4.43 (2.94 ~ 6.67) | 2.14 (1.55) | / | / | / | / | / |
| Aggression | 152 | 4.72 | 444.57 | 4.74 (4.04 ~ 5.56) | 2.24 (2) | 31 | 3.36 | 51.42 | 3.37 (2.37 ~ 4.79) | 1.75 (1.24) |
| Agitation | 127 | 2.68 | 133.85 | 2.69 (2.26 ~ 3.2) | 1.42 (1.17) | / | / | / | / | / |
| Anger | 89 | 3.91 | 192.66 | 3.92 (3.18 ~ 4.83) | 1.97 (1.66) | 34 | 5.23 | 116.1 | 5.24 (3.74 ~ 7.34) | 2.38 (1.9) |
| Apathy | / | / | / | / | / | 14 | 5.14 | 46.64 | 5.14 (3.05 ~ 8.69) | 2.36 (1.61) |
| Behavior disorder | 34 | 10.48 | 289 | 10.48 (7.48 ~ 14.69) | 3.38 (2.89) | 11 | 11.8 | 108.42 | 11.81 (6.53 ~ 21.34) | 3.56 (2.72) |
| Communication disorder | / | / | / | / | / | 6 | 8.57 | 40.07 | 8.58 (3.85 ~ 19.11) | 3.1 (2.01) |
| Confusional state | / | / | / | / | / | 77 | 2.55 | 72.95 | 2.56 (2.05 ~ 3.21) | 1.35 (1.02) |
| Delirium | 67 | 3.05 | 91.91 | 3.05 (2.4 ~ 3.88) | 1.6 (1.25) | / | / | / | / | / |
| Dysphemia | 11 | 3.77 | 22.37 | 3.78 (2.09 ~ 6.82) | 1.91 (1.08) | 11 | 13.22 | 123.84 | 13.23 (7.32 ~ 23.91) | 3.72 (2.89) |
| Emotional disorder | / | / | / | / | / | 20 | 3.36 | 33.22 | 3.37 (2.17 ~ 5.22) | 1.75 (1.12) |
| Homicidal ideation | 12 | 4.76 | 35.47 | 4.76 (2.7 ~ 8.39) | 2.25 (1.44) | / | / | / | / | / |
| Impulse-control disorder | 6 | 4.5 | 16.28 | 4.5 (2.02 ~ 10.03) | 2.17 (1.07) | / | / | / | / | / |
| Inappropriate affect | 6 | 5.58 | 22.46 | 5.58 (2.5 ~ 12.45) | 2.48 (1.38) | / | / | / | / | / |
| Irritability | 126 | 3.14 | 182.93 | 3.14 (2.64 ~ 3.74) | 1.65 (1.39) | 36 | 3.13 | 52.2 | 3.14 (2.26 ~ 4.35) | 1.65 (1.17) |
| Logorrhoea | 10 | 4.3 | 25.27 | 4.3 (2.31 ~ 8.01) | 2.1 (1.23) | / | / | / | / | / |
| Mood altered | / | / | / | / | / | 25 | 4.85 | 76.25 | 4.85 (3.28 ~ 7.19) | 2.28 (1.71) |
| Mood swings | / | / | / | / | / | 21 | 3.42 | 36 | 3.43 (2.23 ~ 5.26) | 1.77 (1.16) |
| Paranoia | 34 | 3.09 | 48.05 | 3.09 (2.21 ~ 4.33) | 1.63 (1.14) | / | / | / | / | / |
| Persecutory delusion | 10 | 6.45 | 45.85 | 6.45 (3.47 ~ 12.02) | 2.68 (1.81) | / | / | / | / | / |
| Personality change | 26 | 4.19 | 62.95 | 4.19 (2.85 ~ 6.16) | 2.06 (1.51) | 13 | 7.32 | 70.86 | 7.33 (4.25 ~ 12.63) | 2.87 (2.1) |
| Psychomotor retardation | 11 | 6.15 | 47.24 | 6.15 (3.4 ~ 11.13) | 2.62 (1.78) | / | / | / | / | / |
| Psychotic disorder | 71 | 3.81 | 146.65 | 3.81 (3.02 ~ 4.81) | 1.93 (1.59) | / | / | / | / | / |
| Staring | 14 | 9.27 | 102.53 | 9.27 (5.48 ~ 15.69) | 3.2 (2.45) | 7 | 16.17 | 99.23 | 16.18 (7.7 ~ 33.98) | 4.01 (2.99) |
| Suicidal ideation | 141 | 2.36 | 110.72 | 2.37 (2.01 ~ 2.79) | 1.24 (1) | 54 | 3.16 | 79.96 | 3.17 (2.43 ~ 4.15) | 1.66 (1.27) |
| Respiratory, thoracic and mediastinal disorders | ||||||||||
| Hiccups | / | / | / | / | / | 14 | 9.71 | 109.12 | 9.72 (5.75 ~ 16.42) | 3.28 (2.53) |
| Skin and subcutaneous tissue disorders | ||||||||||
| Drug eruption | 34 | 3.04 | 46.58 | 3.05 (2.18 ~ 4.27) | 1.6 (1.12) | / | / | / | / | / |
| Lichenoid keratosis | 7 | 5.22 | 23.8 | 5.22 (2.49 ~ 10.97) | 2.38 (1.36) | / | / | / | / | / |
| Social circumstances | ||||||||||
| Impaired quality of life | / | / | / | / | / | 11 | 4.09 | 25.67 | 4.09 (2.27 ~ 7.4) | 2.03 (1.2) |
| Impaired work ability | / | / | / | / | / | 18 | 4.35 | 46.42 | 4.36(2.74 ~ 6.92) | 2.12(1.46) |
| Vascular disorders | ||||||||||
| Systolic hypertension | 7 | 24.67 | 155.87 | 24.67(11.68 ~ 52.13) | 4.6(3.57) | / | / | / | / | / |
Comparison of adverse event signals between lacosamide and cenobamate in various system organ classes.
PTs, Preferred Terms; SOCs, System Organ Classes; N is the number of reported adverse events; IC (IC-2SD), information component (lower end of the 95% confidence interval); /, IC-2SD value of the adverse event is less than 1.0.
3.3 Comparison of safety signals between LCM and CNB
Sankey diagram was used to compare safety signals between CNB and LCM (Figure 3). The length of each bar reflects the frequency of the respective signals. Different signals converge in the central bar, representing the corresponding SOCs. Compared with LCM, CNB’s AE had fewer types of SOCs and more concentrated on nervous system, psychiatric disorders, general disorders and administration site condition, and eye disorders.
Figure 3
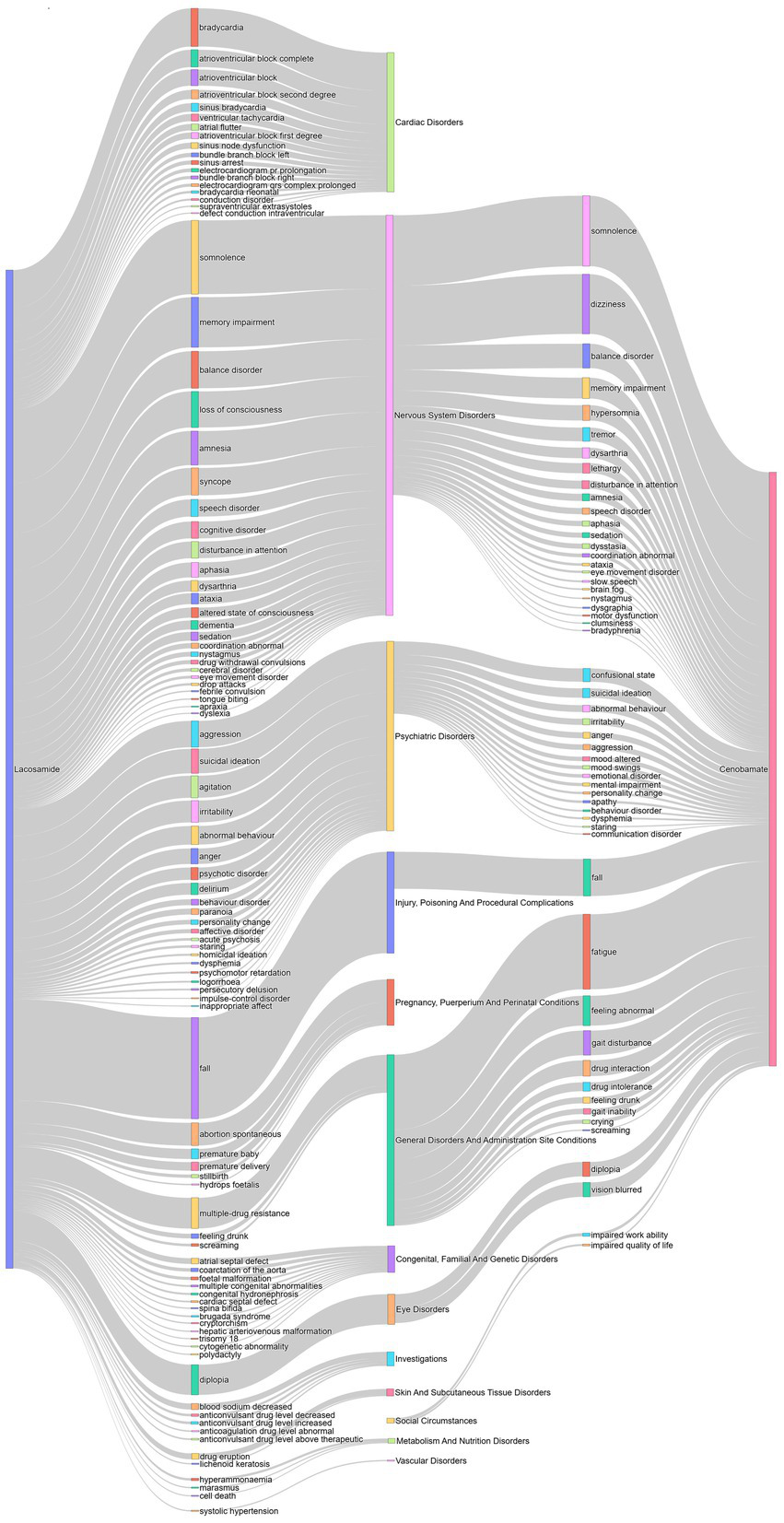
Comparison of adverse event signals between lacosamide and cenobamate. The lines on both ends correspond to the drug and its adverse events signals or the signals and its systems organ classes. The length of each bar indicates the number of the corresponding signals. The signals for Lacosamide emerge from the left, while those for Cenobamate originate from the right. The different signals converge in the middle bar, which represents the corresponding system organ classes.
The forest plot of the ROR for LCM and CNB compared the signal strength of the two drugs, highlighting significant differences in the risk of specific AEs. Despite its shorter time on the market and fewer total AE reports, CNB exhibited stronger signal strength for most AEs (Figure 4).
Figure 4
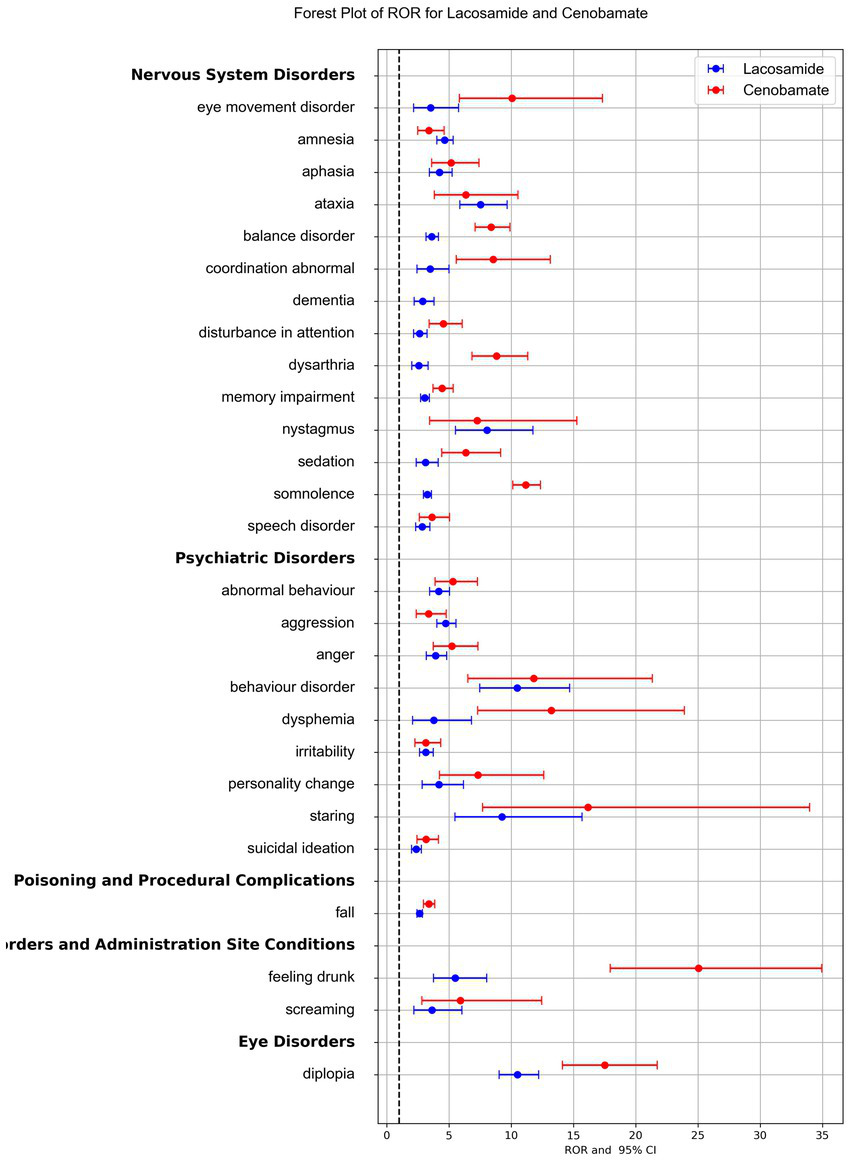
Reporting Odds Ratios (RORs) comparison for adverse events associated with lacosamide and cenobamate. The blue line represented the reports of Lacosamide, and the red line represented the reports of Cenobamate. Only adverse events signal appearing in both drugs were presented here for RORs comparison.
4 Discussion
Based on the FAERS database from 2008 to 2024, we comprehensively compared the AE risks of CNB and LCM. The same AE of these two drugs were similar to traditional SCB reactions, such as dizziness, ataxia, feeling drunk, balance disorder, and diplopia (21). Unreported AEs in the drug dispensatory were identified, such as high signals for multiple-drug resistance in LCM. Notably, AEs between the two drugs showed differences: (1) Certain numbers of AE signals associated with LCM were found in cardiac disorders, reproductive toxicity, and genetic disorders, et al., while no such relevant signals were detected for CNB; (2) Among the signals that detected in both drugs, most signals from CNB are stronger than those from LCM, such as eye movement disorder, coordination abnormal, dysarthria, somnolence, feeling drunk, dysphemia, diplopia, et al.; (3) The initial titration dose of CNB (12.5 mg, qd) reported a significantly higher number of AEs compared to the other dose groups.
4.1 Nervous system disorders and psychiatric disorders
Nervous system disorders and psychiatric disorders are the most common AEs of ASMs, sometimes reaching up to 20%, and are linked to poor patient compliance and treatment discontinuation (22). Some AEs associated with CNB were the same as the traditional SCBs, such as dizziness, diplopia, and gait disturbance, and the incidence is even higher when it is used in combination with SCBs (23). It is worth noting that the half-maximal inhibitory concentration of the persistent sodium current is approximately 53 mM, while the half-maximal effective concentration of the GABA-A receptor is between 42 and 194 mM. Due to the dual mechanisms of CNB, as the dose of CNB increases, more GABA-A receptors will be involved, which will not only produce therapeutic effects but also cause potential AEs in the nervous system (11). Central nervous system-related AEs are frequently reported for CNB, including somnolence, dizziness, headache, and fatigue, with an incidence of 5.4%, primarily mild to moderate, and more frequent in patients on multiple ASMs (12, 24). These AEs typically appear during titration (usually in the first days/week) and can resolve spontaneously, but dosage reduction of concomitant may be required (12, 24). Our analysis also found that initial titration dose of CNB were reported more AEs than other dose. Dizziness and somnolence are significant concerns for patients and major reasons for discontinuation with an incidence of about 22%, which might due to its effect on GABA-A receptors. In our study, the signal for somnolence and dizziness with CNB was also notably higher than with LCM. Clinical data indicate that slower titration can reduce the incidence of somnolence, dizziness, and fatigue in patients taking CNB (25). Experts recommend evening dosing or reducing the dosage of co-administered ASMs to mitigate AEs, especially when combined with benzodiazepines or SCBs, to lower the risk of synergistic adverse effects (26).
LCM also causes AEs similar to traditional SCBs, such as dizziness, headache, fatigue, diplopia, ataxia, and balance disorder. The most frequent TEAEs leading to discontinuation are dizziness and ataxia. Dizziness is a common AE associated with LCM, with an incidence of 8.3–55%, mostly mild (16). The incidence of dizziness and discontinuation increases with dosage, with severe dizziness reported in higher-dose groups (600 mg/day) (17). Dizziness is most likely to occur in the first 3 months of LCM use, with a rate during titration 3 ~ 4 times higher than during maintenance (27). Somnolence occurs less frequently than dizziness and headache, with an incidence of about 1.6 ~ 15% (28). Recent studies on children with epilepsy under 4 years old suggest that somnolence is a common AE (29). Most somnolence AEs occur during titration, with no clear correlation to dosage (30).
Patients with refractory epilepsy are more likely to experience psychiatric disorder (31). In our study, there was a notable signal for LCM associated acute psychosis. It was reported that the incidence of psychiatric AEs in patients treated with LCM ranges from 0.3 to 3.1%, often leading to discontinuation or serious consequences (16, 31). Study showed no significant relationship between LCM dosage and psychiatric disorder (32). Psychiatric disorder typically occur within hours to 2 weeks after administration, with a higher incidence in patients with pre-existing mental disorders (33). Large genomic studies indicate that various voltage-gated sodium channels are involved in the pathogenesis of psychosis, and over activity of CRMP-2 is also linked to psychiatric symptoms (34). LCM may enhance the slow inactivation of voltage-gated sodium channels and modulate CRMP-2, potentially leading to negative psychiatric AEs in patients.
4.2 Cardiac and vascular disorders
Increasing evidence indicates that certain ASMs, particularly SCBs, are linked to a higher risk of cardiac disorder (35). ASMs prolongs the QT interval, either by closing ion channels or delaying their opening to affect heart rhythm, which is a pathophysiological basis for ASM induced arrhythmias. Specifically, SCBs act on VGSCs, which is crucial ion channels responsible for generating action potentials, playing a significant role in neuronal excitability and epilepsy. Besides nervous system, VGSCs are also expressed in cardiac tissue and crucial for maintaining heart rhythm (36). Disruption of these channels can lead to arrhythmias and conduction blocks.
LCM’s inhibition of sodium channels may delay cardiac conduction below the atrioventricular bundle level, triggering arrhythmias. Although cardiac sodium channels are less sensitive to LCM blockade than neuronal ones, in vitro experiments confirm that LCM affect both neuronal and cardiac activities (37). Research confirms LCM affects cardiac sodium channels (hNav1.5) and is associated with electrocardiograph (ECG) changes, atrial fibrillation, and AV block (38). It can inhibit conduction in the His-Purkinje system, resulting in QRS prolongation and potentially causing recurrent arrhythmias and ventricular tachycardia (39). Consequently, the FDA contraindicates LCM for patients with existing second-degree or complete AV block and warns of increased atrial arrhythmias in those with diabetes or cardiovascular disease (40). Reports of sinus bradycardia and AV block have surfaced since LCM’s introduction, with severe cases leading to atrial fibrillation, QRS prolongation and cardiac arrest (41). This is largely consistent with our research findings, where strong signal were found in LCM associated cardiac AEs, such as various degrees of atrioventricular block, electrocardiogram PR prolongation, electrocardiogram QRS complex prolongation, sinus arrest, and bradycardia.
The correlation between cardiac AEs and LCM dose remains controversial. Some studies suggest that cardiac AEs are dose dependent, especially under loading doses (42–45). However, other research indicates that low dose LCM also led to cardiac AEs (42, 46). Moreover, most cardiac AEs related to LCM occur during the titration period, and their incidence shows a downward trend over time (47). Our study found no clear relationship between the reported number of AEs and the LCM dose. The reported number of AEs showed a slight upward trend with the increase in LCM dose, but dropped sharply in the group with a dosage > 400 mg. This could be attributed to the inherent limitations of the database, given that the proportion of unreported dosages is substantial.
Several large-scale studies have found that the overall incidence of cardiac adverse events (AEs) related to oral Lacosamide (LCM) ranges from 0.7 to 1.2%, with mild symptoms. Most of them do not require intervention or can be relieved after drug discontinuation (48, 49). There are certain differences between the AEs of intravenous and oral administrations. A study in South Korea showed that the incidence of cardiac AEs was relatively high (32.9%) when LCM was rapidly administered intravenously (400 mg within 10 to 20 min), especially the first-degree atrioventricular block (22.4%), which led to a prolonged average PR interval (50). However, some studies suggest that although intravenous LCM may have certain impacts on electrocardiogram parameters and blood pressure, the changes are mild and clinically insignificant, and there’s no need to stop LCM or implant a pacemaker. Considering the risk of seizures, slow intravenous administration of LCM seems to be a relatively safe option. When the infusion time of LCM is slowed down to 30 min, the cardiac side effects are low and it does not affect the effectiveness of seizure control (51). But for emergency situations like status epilepticus, a faster infusion rate may be required, and the safety of LCM in such cases needs further research. In our study, the AE signals of bradycardia and ventricular tachycardia were relatively high. Studies have shown that ventricular tachycardia is common among the arrhythmias caused by LCM (45). ST segment elevation has been reported after using LCM (52). Thus, clinically, prior to LCM administration, it’s crucial to ascertain patients’ heart disease history. For those with arrhythmia predisposing factors like cardiac conduction disease history, concurrent use of cardiac conduction affecting drugs, or diabetic neuropathy, LCM should be used cautiously. After LCM is administered, attention should be paid to monitoring patients’ cardiac function and electrocardiogram changes, especially in the first month after starting treatment. If necessary, the dosage should be reduced or the drug should be discontinued. In addition, when other SCBs like carbamazepine and phenytoin are used simultaneously with LCM, they may increase cardiac AEs, so caution should be exercised when combining them.
4.3 Pregnancy, puerperium and perinatal conditions
The incidence of congenital malformations in pregnant women with epilepsy not taking ASMs is similar to that of the general population, around 2 ~ 4% (53). During pregnancy, especially in the first trimester, the incidence of congenital malformations associated with ASM use is 2 ~ 3 times higher, approximately 4 ~ 8% (53, 54). An observational study of pregnant women exposed to ASMs indicated that LCM is the most commonly used third-generation ASM (13). Most patients were exposed to LCM early in pregnancy, which is associated with an increased risk of malformations, as this period is critical for embryonic organ development. The overall incidence of congenital malformations with LCM combination therapy is higher than with monotherapy, consistent with reports on other ASMs (53, 54). A case report showed that three pregnant women exposed to LCM, showing good efficacy and safety during pregnancy and breastfeeding, with no teratogenic or potential toxic effects (53). However, high concentrations of LCM can pharmacologically affect the placenta, impacting fetal folate supply, so plasma levels must be closely monitored during pregnancy to adjust the dosage dynamically (55). Our study identified reproduction toxicity high-signal related to LCM, including fetal malformation, hydrops foetalis, bradycardia neonatal, premature baby, and abortion spontaneous, consistent with existing literature. This finding was drawn without restricting the analysis to either LCM monotherapy or its combination with other drugs. There is currently no clear data on whether CNB is teratogenic. For pregnant patients, it is recommended to use ASMs with relatively high safety, such as lamotrigine, levetiracetam, or oxcarbazepine (56). Additionally, pregnant women should avoid combining multiple ASMs, especially high-risk teratogenic medications like valproic acid. A careful balance of risks and benefits should be considered in the context of effective seizure control and pregnancy toxicity when determining the treatment plan.
4.4 Drug interaction with other ASMs
Although monotherapy is the first choice for epilepsy treatment, some patients may require two or more ASMs. For patients with focal epilepsy, combination therapy is more effective after the first anti-epileptic treatment fails. As LCM and CNB are often used in DRE patients, who typically use multiple ASMs, increasing the number of combined ASMs heighten the risk of AEs due to pharmacokinetic and pharmacodynamic interactions. In such cases, a common approach is to adjust the doses of existing ASMs rather than discontinue the newly added ASM. This can improve patient tolerance and safety while titrating the new ASM to an effective dose. Using ASMs with similar mechanisms lead to excessive pharmacodynamic effects and AEs, particularly during later titration phases when high doses of similar mechanism ASMs are employed, such as SCBs and benzodiazepines (e.g., clobazam) (57). These interactions occur at the ASM target sites, altering pharmacological effects without changing plasma concentrations. LCM and CNB both carry a higher risk of AEs when used with SCBs (1). Our AE signal analysis found that the low-dose CNB group (12.5 mg, qd) reported more AEs, likely due to a higher incidence of AEs during early titration (1st week) (12, 24). In contrast, the 200 mg ~ 400 mg dose range of LCM reported the most numbers of AEs, possibly because this range is the most commonly used maintenance dose with the largest population of patients (40).
While LCM and CNB do not have significant clinical pharmacokinetic interactions, the incidence of dose-related nervous system AEs (such as dizziness, somnolence, and ataxia) increases with combination therapy, likely due to pharmacodynamic interactions, as both act on voltage-gated sodium channels, albeit differently (9). If the combined LCM dose is high (≥500 mg/day), these interactions may occur relatively early in CNB titration. Therefore, it is recommended to actively reduce the LCM dose early in CNB titration (e.g., decrease by 25% every 2 weeks as needed) to mitigate potential adverse effects from pharmacodynamic interactions (57). If patients are already on two or more SCBs, it is advisable to proactively lower the SCB dose or discontinue them when adding CNB (18).
It is worth noting that clobazam is a long-acting benzodiazepine. It can bind to GABA-A receptors to enhance GABAergic neurotransmission. When combining used with CNB, it has an additive effect on the action of GABA, increasing the inhibitory effect on neurons and resulting in significant somnolence. In addition, CNB significantly increase the concentration of clobazam and/or its active metabolite (N-desmethylclobazam) by 2–6 times via inhibiting CYP2C19. Due to the pharmacokinetic and pharmacodynamic interactions between the two drugs, their combination may lead to dual interactions and thus cause serious adverse events (SAEs) (58, 59). Our results also indicate that clobazam is a commonly used drug in combination with CNB (ranking fourth), so caution should be exercised to avoid or carefully manage the use of benzodiazepines and their derivatives (40). Studies have found that after the initiation of CNB treatment, among the different classes of concomitant ASMs, clobazam shows the greatest reduction in drug load (60, 61). When CNB is used in combination with clobazam, reducing the dose of clobazam as early as possible helps CNB achieve the optimal titration dose (57, 62). When CNB is combined with a high dose of clobazam (≥40 mg/d), reducing the latter to a low dose of 5-10 mg is beneficial for reducing AEs and controlling epileptic seizures (18, 58, 63).
4.5 Other AEs signals
Our study also identified a strong signal for multiple drug-resistant AEs related to LCM, which is not mentioned in its FDA labeling. The mechanisms of ASM resistance are unclear, with past studies suggesting hypotheses like target alteration, transport proteins, and pharmacokinetic changes (64). LCM’s anticonvulsant effect primarily involves binding to and inactivating sodium channel subunits. If the expression or structure of these subunits is altered, LCM may lose its efficacy, leading to drug-resistant epilepsy (65). Recent studies on pediatric epilepsy patients have shown an increasing rate of LCM resistance (65). Patients with a long standing illness or those unresponsive to initial ASM treatment, irrespective of prior drugs, are at an elevated risk of developing drug resistance. Moreover, a protracted disease course correlates with an increased ASM resistance risk (66). Furthermore, choosing an inappropriate ASM monotherapy early in the disease course may severely affect the sensitivity to later drugs. Resistance is highly specific to the type of ASM (67). Animal studies have shown that repeated early administration of sodium - channel - blocking ASMs promote drug resistant chronic seizures (68). Nevertheless, we need to note that since LCM is a third-line treatment for focal DRE, it is not used as a first- or second-line option. This finding of multiple drug-resistant AEs may not be exclusive to LCM; it could also reflect the practice related to associated ASMs that patients used previously. It is also commonly observed that ASMs reduce seizures but may increase AEs (69).
Our results indicated high signals for diplopia with both LCM and CNB, which is often associated with SCBs (70). If diplopia persists for ≥ 3 days, it’s recommended to reduce the dosage of SCBs. In this study, CNB was associated with AEs related to eye disorders, such as vision blurred (n = 83, IC-2SD = 1.39) and diplopia (n = 83, IC-2SD = 3.8), while LCM primarily showed diplopia with slightly lower signal strength (n = 174, IC-2SD = 3.16). Studies have demonstrated that diplopia is a common ophthalmic AEs (16), which is consistent with our findings.
5 Limitation
Several limitations of our study should be noted. First, the FAERS database, being a spontaneous reporting system, may contain duplicate reports and inconsistent symptom descriptions, leading to inaccuracies in AE incidence calculations. Missing information on complications, dosage, and medical history in AE reports limits the ability to assess safety comprehensively. Second, AE reports primarily come from the U. S. and Europe, which may not represent all populations due to ethnic differences. Third, methods like ROR, PRR and BPNN indicate statistical associations, not causality, necessitating further clinical studies for validation. Fourth, epilepsy patients often use multiple medications, increasing the risk of interactions. This study focused on monotherapy and did not account for combinations or specific dosages, suggesting future research should incorporate combined drug signal detection. Fifth, varying market entry times for LCM and CNB may lead to reporting bias, complicating direct safety comparisons. Larger future trials may uncover more adverse signals for CNB. Lastly, since this study does not specifically use a registry for pregnant women, assumptions about adverse effects related to these drugs in pregnancy is limited.
6 Conclusion
Early AEs affect compliance, diminish quality of life, and delay the achievement of optimal therapeutic doses. Research indicates that AEs associated with ASMs remain a leading cause of treatment failure and reduced quality of life in epilepsy patients (71). Achieving successful epilepsy management hinges on balancing effective seizure control with minimizing AEs. Choosing the right ASM requires consideration of the type of epilepsy, as well as the individual tolerance and potential severe toxicity of different medications in patients. By thoroughly analyzing and comparing the AEs of LCM and CNB, we provide valuable insights for assessing the clinical safety of the two medications. Our study emphasizes the importance of vigilant monitoring of patients undergoing treatment and contributes to optimizing the therapeutic use of ASMs in clinical practice.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: www.FDA.gov.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SS: Writing – review & editing, Writing – original draft, Funding acquisition. DC: Visualization, Software, Formal analysis, Methodology, Validation, Writing – review & editing. ZS: Writing – review & editing. CZ: Writing – review & editing. QC: Writing – review & editing, Formal analysis, Data curation, Methodology. LX: Writing – original draft. MY: Validation, Writing – review & editing. YZ: Writing – review & editing. WL: Writing – review & editing. FZ: Conceptualization, Writing – review & editing, Writing – original draft. AY: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by National Natural Science Foundation of China (82304576); China Postdoctoral Science Foundation (2022M713859); Nature Science Foundation of Hubei Province (2022CFB879); Knowledge Innovation Project of Wuhan city (2022020801020521); Open foundation of Hubei Key Laboratory of Central Nervous System Tumor and Intervention (ZZYKF202304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1625612/full#supplementary-material
Footnotes
1.^ www.FDA.gov
References
1.
Beghi E . The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
2.
Kwan P Arzimanoglou A Berg AT Brodie MJ Allen Hauser W Mathern G et al . Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x
3.
Gilioli I Vignoli A Visani E Casazza M Canafoglia L Chiesa V et al . Focal epilepsies in adult patients attending two epilepsy centers: classification of drug-resistance, assessment of risk factors, and usefulness of “new” antiepileptic drugs. Epilepsia. (2012) 53:733–40. doi: 10.1111/j.1528-1167.2012.03416.x
4.
Mao J Takahashi K Cheng M Xu C Boca A Song Y et al . Real-world anti-seizure treatment and adverse events among individuals living with drug-resistant focal epilepsy in the United States. Epilepsia Open. (2024) 9:1311–20. doi: 10.1002/epi4.12967
5.
US Food and Drug Administration . FDA approves new treatment for adults with partial-onset seizures. Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-partial-onset-seizures.
6.
European Medicines Agency . Ontozry Cenobamate. Available online at: https://www.ema.europa.eu/en/documents/scientific-conclusion/ontozry-h-c-psusa-00010921-202303-epar-scientific-conclusions-and-grounds-variation-terms-marketing-authorisation_en.pdf (Accessed 30 July 2024).
7.
Beltrán-Corbellini Á Romeral-Jiménez M Mayo P Sánchez-Miranda Román I Iruzubieta P Chico-García JL et al . Cenobamate in patients with highly refractory focal epilepsy: a retrospective real-world study. Seizure. (2023) 111:71–7. doi: 10.1016/j.seizure.2023.07.026
8.
Makridis KL Kaindl AM . Real-world experience with cenobamate: a systematic review and meta-analysis. Seizure. (2023) 112:1–10. doi: 10.1016/j.seizure.2023.09.006
9.
Nakamura M Cho JH Shin H Jang IS . Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. (2019) 855:175–82. doi: 10.1016/j.ejphar.2019.05.007
10.
Sharma R Nakamura M Neupane C Jeon BH Shin H Melnick SM et al . Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. (2020) 879:173117. doi: 10.1016/j.ejphar.2020.173117
11.
Roberti R De Caro C Iannone LF Zaccara G Lattanzi S Russo E . Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs. (2021) 35:609–18. doi: 10.1007/s40263-021-00819-8
12.
Krauss GL Klein P Brandt C Lee SK Milanov I Milovanovic M et al . Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. (2020) 19:38–48. doi: 10.1016/S1474-4422(19)30399-0
13.
Hoeltzenbein M Slimi S Fietz AK Stegherr R Onken M Beyersmann J et al . Increasing use of newer antiseizure medication during pregnancy: an observational study with special focus on lacosamide. Seizure. (2023) 107:107–13. doi: 10.1016/j.seizure.2023.02.015
14.
Rogawski MA Tofighy A White HS Matagne A Wolff C . Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. (2015) 110:189–205. doi: 10.1016/j.eplepsyres.2014.11.021
15.
Wilson SM Khanna R . Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct impairment of its canonical function: implications for the therapeutic potential of lacosamide. Mol Neurobiol. (2015) 51:599–609. doi: 10.1007/s12035-014-8775-9
16.
Li J Sun M Wang X . The adverse-effect profile of lacosamide. Expert Opin Drug Saf. (2020) 19:131–8. doi: 10.1080/14740338.2020.1713089
17.
Biton V Gil-Nagel A Isojarvi J Doty P Hebert D Fountain NB . Safety and tolerability of lacosamide as adjunctive therapy for adults with partial-onset seizures: analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy Behav. (2015) 52:119–27. doi: 10.1016/j.yebeh.2015.09.006
18.
Steinhoff BJ Ben-Menachem E Klein P Peltola J Schmitz B Thomas RH et al . Therapeutic strategies during cenobamate treatment initiation: Delphi panel recommendations. Ther Adv Neurol Disord. (2024) 17:733. doi: 10.1177/17562864241256733
19.
Huang L Guo T Zalkikar JN Tiwari RC . A review of statistical methods for safety surveillance. Ther Innov Regul Sci. (2014) 48:98–108. doi: 10.1177/2168479013514236
20.
Fusaroli M Salvo F Begaud B AlShammari TM Bate A Battini V et al . The reporting of a disproportionality analysis for drug safety signal detection using individual case safety reports in PharmacoVigilance (READUS-PV): development and statement. Drug Saf. (2024) 47:575–84. doi: 10.1007/s40264-024-01421-9
21.
Steinhoff BJ Georgiou D Intravooth T . The cenobamate KORK study-A prospective monocenter observational study investigating cenobamate as an adjunctive therapy in refractory epilepsy, with comparisons to historical cohorts treated with add-on lacosamide, perampanel, and brivaracetam. Epilepsia Open. (2024) 9:1502–14. doi: 10.1002/epi4.12992
22.
Krauss GL Chung SS Ferrari L Stern S Rosenfeld WE . Cognitive and psychiatric adverse events during adjunctive cenobamate treatment in phase 2 and phase 3 clinical studies. Epilepsy Behav. (2024) 151:109605. doi: 10.1016/j.yebeh.2023.109605
23.
Lauxmann S Heuer D Heckelmann J Fischer FP Schreiber M Schriewer E et al . Cenobamate: real-world data from a retrospective multicenter study. J Neurol. (2024) 271:6596–604. doi: 10.1007/s00415-024-12510-1
24.
Sperling MR Klein P Aboumatar S Gelfand M Halford JJ Krauss GL et al . Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. (2020) 61:1099–108. doi: 10.1111/epi.16525
25.
Sperling MR Abou-Khalil B Aboumatar S Bhatia P Biton V Klein P et al . Efficacy of cenobamate for uncontrolled focal seizures: post hoc analysis of a phase 3, multicenter, open-label study. Epilepsia. (2021) 62:3005–15. doi: 10.1111/epi.17091
26.
Rosenfeld WE Abou-Khalil B Aboumatar S Bhatia P Biton V Krauss GL et al . Dose adjustments to concomitant antiseizure medications: post-hoc analysis of a phase 3, open-label study of cenobamate for treatment of unco2021ntrolled focal seizure. Neurology. (2021) 62:3016–28. doi: 10.1111/epi.17092
27.
Liu H Xu X . Influence of adjunctive lacosamide in patients with seizures: a systematic review and meta-analysis. Int J Neurosci. (2018) 128:670–6. doi: 10.1080/00207454.2017.1408619
28.
Villanueva V Giráldez BG Toledo M De Haan GJ Cumbo E Gambardella A et al . Lacosamide monotherapy in clinical practice: a retrospective chart review. Acta Neurol Scand. (2018) 138:186–94. doi: 10.1111/ane.12920
29.
Makedonska I Ng YT Beller C Bozorg A Csikós J McClung C et al . Efficacy and tolerability of adjunctive lacosamide in patients aged <4 years with focal seizures. Ann Clin Transl Neurol. (2024) 11:768–79. doi: 10.1002/acn3.52004
30.
Zadeh WW Escartin A Byrnes W Tennigkeit F Borghs S Li T et al . Efficacy and safety of lacosamide as first add-on or later adjunctive treatment for uncontrolled partial-onset seizures: a multicentre open-label trial. Seizure. (2015) 31:72–9. doi: 10.1016/j.seizure.2015.07.001
31.
Chen B Choi H Hirsch LJ Katz A Legge A Buchsbaum R et al . Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. (2017) 76:24–31. doi: 10.1016/j.yebeh.2017.08.039
32.
Hong Z Inoue Y Liao W Meng H Wang X Wang W et al . Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: A randomized, double-blind, placebo-controlled study. Epilepsy Res. (2016) 127:267–75. doi: 10.1016/j.eplepsyres.2016.08.032
33.
Li KY Huang LC Chang YP Yang YH . The effects of lacosamide on cognitive function and psychiatric profiles in patients with epilepsy. Epilepsy Behav. (2020) 113:107580. doi: 10.1016/j.yebeh.2020.107580
34.
Nomoto M Konopaske GT Yamashita N Aoki R Jitsuki-Takahashi A Nakamura H et al . Clinical evidence that a dysregulated master neural network modulator may aid in diagnosing schizophrenia. Proc Natl Acad Sci U S A. (2021) 118:32118. doi: 10.1073/pnas.2100032118
35.
Wang J Huang P Yu Q Lu J Liu P Yang Y et al . Epilepsy and long-term risk of arrhythmias. Eur Heart J. (2023) 44:3374–82. doi: 10.1093/eurheartj/ehad523
36.
Curia G Biagini G Perucca E Avoli M . Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. (2009) 23:555–68. doi: 10.2165/00023210-200923070-00002
37.
Rudd GD Haverkamp W Mason JW Wenger T Jay G Hebert D et al . Lacosamide cardiac safety: clinical trials in patients with partial-onset seizures. Acta Neurol Scand. (2015) 132:355–63. doi: 10.1111/ane.12414
38.
Delaunois A Colomar A Depelchin BO Cornet M . Cardiac safety of lacosamide: the non-clinical perspective. Acta Neurol Scand. (2015) 132:337–45. doi: 10.1111/ane.12413
39.
Tisdale JE Chung MK Campbell KB Hammadah M Joglar JA Leclerc J et al . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. (2020) 142:e214–33. doi: 10.1161/CIR.0000000000000905
40.
UCB Inc. VIMPAT® (lacosamide) US Prescribing Information. Available online at: https://www.ucb-usa.com/vimpat-prescribing-information.pdf (Accessed Jun 5, 2023).
41.
Wechsler RTS . Lacosamide In: WyllieE, editor. Treatment of epilepsy. Philadelphia: Wolters Kluwer (2021). 641–5.
42.
Lachuer C Corny J Bézie Y Ferchichi S Durand-Gasselin B . Complete atrioventricular block in an elderly patient treated with low-dose lacosamide. Cardiovasc Toxicol. (2018) 18:579–82. doi: 10.1007/s12012-018-9467-x
43.
Krause LU Brodowski KO Kellinghaus C . Atrioventricular block following lacosamide intoxication. Epilepsy Behav. (2011) 20:725–7. doi: 10.1016/j.yebeh.2011.02.006
44.
Berei TJ Lillyblad MP Almquist AK . Lacosamide-induced recurrent ventricular tachycardia in the acute care setting. J Pharm Pract. (2018) 31:222–6. doi: 10.1177/0897190017700557
45.
Yadav R Schrem E Yadav V Jayarangaiah A das S Theetha Kariyanna P . Lacosamide-related arrhythmias: a systematic analysis and review of the literature. Cureus. (2021) 13:e20736. doi: 10.7759/cureus.20736
46.
Shibata M Hoshino R Shimizu C Sato M Furuta N Ikeda Y . Lacosamide-induced sinus node dysfunction followed by severe agranulocytosis. BMC Neurol. (2021) 21:217. doi: 10.1186/s12883-021-02253-1
47.
Yang C Zhao W Chen H Yao Y Zhang J . Cardiac adverse events associated with lacosamide: a disproportionality analysis of the FAERS database. Sci Rep. (2024) 14:16202. doi: 10.1038/s41598-024-67209-0
48.
Ben-Menachem E Biton V Jatuzis D Abou-Khalil B Doty P Rudd GD . Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. (2007) 48:1308–17. doi: 10.1111/j.1528-1167.2007.01188.x
49.
Vossler DG Wechsler RT Williams P Byrnes W Therriault S the ALEX‐MT study group . Long-term exposure and safety of lacosamide monotherapy for the treatment of partial-onset (focal) seizures: results from a multicenter, open-label trial. Epilepsia. (2016) 57:1625–33. doi: 10.1111/epi.13502
50.
Kim HK Lee H Bae EK Kim DW . Cardiac effects of rapid intravenous loading of lacosamide in patients with epilepsy. Epilepsy Res. (2021) 176:106710. doi: 10.1016/j.eplepsyres.2021.106710
51.
Lu YT Lin CH Ho CJ Hsu CW Tsai MH . Evaluation of cardiovascular concerns of intravenous lacosamide therapy in epilepsy patients. Front Neurol. (2022) 13:891368. doi: 10.3389/fneur.2022.891368
52.
Goodnough R Badea A Geier C Lynch KL LeSaint KT . Lacosamide induced brugada I morphology in the setting of septicemia: a case report. Medicine. (2021) 100:e25577. doi: 10.1097/MD.0000000000025577
53.
Lattanzi S Cagnetti C Foschi N Provinciali L Silvestrini M . Lacosamide during pregnancy and breastfeeding. Neurol Neurochir Pol. (2017) 51:266–9. doi: 10.1016/j.pjnns.2017.03.003
54.
Kinney MO Smith PEM Craig JJ . Preventing teratogenicity in women with epilepsy. Semin Neurol. (2022) 42:679–92. doi: 10.1055/s-0042-1759579
55.
Berman E Kohn E Berkovitch M Kovo M Eyal S . Lacosamide effects on placental carriers of essential compounds in comparison with valproate: studies in perfused human placentas. Epilepsia. (2022) 63:2949–57. doi: 10.1111/epi.17395
56.
Pack AM Oskoui M Williams Roberson S Donley DK French J Gerard EE et al . Teratogenesis, perinatal, and neurodevelopmental outcomes after in utero exposure to Antiseizure medication: practice guideline from the AAN, AES, and SMFM. Neurology. (2024) 102:e209279. doi: 10.1212/WNL.0000000000209279
57.
Smith MC Klein P Krauss GL Rashid S Seiden LG Stern JM et al . Dose adjustment of concomitant antiseizure medications during cenobamate treatment: expert opinion consensus recommendations. Neurol Ther. (2022) 11:1705–20. doi: 10.1007/s40120-022-00400-5
58.
Osborn M Abou-Khalil B . The cenobamate-clobazam interaction- evidence of synergy in addition to pharmacokinetic interaction. Epilepsy Behav. (2023) 142:109156. doi: 10.1016/j.yebeh.2023.109156
59.
Elakkary S Hagemann A Klimpel D Bien CG Brandt C . A retrospective non-interventional study evaluating the pharmacokinetic interactions between cenobamate and clobazam. Epilepsia. (2023) 64:e36–42. doi: 10.1111/epi.17515
60.
Aboumatar S Ferrari L Stern S Wade CT Weingarten M Connor GS et al . Reductions in concomitant antiseizure medication drug load during adjunctive cenobamate therapy: post-hoc analysis of a subset of patients from a phase 3, multicenter, open-label study. Epilepsy Res. (2024) 200:107306. doi: 10.1016/j.eplepsyres.2024.107306
61.
Becker DA Demko SA . Dose reduction and discontinuation of concomitant antiseizure medications after initiating cenobamate: A retrospective review. Epilepsy Res. (2023) 197:107242. doi: 10.1016/j.eplepsyres.2023.107242
62.
Carreño M Gil-Nagel A Serratosa JM Toledo M Rodriguez-Uranga JJ Villanueva V . Spanish consensus on the management of concomitant antiseizure medications when using cenobamate in adults with drug-resistant focal seizures. Epilepsia Open. (2024) 9:1051–8. doi: 10.1002/epi4.12936
63.
Serrano-Castro PJ Rodríguez-Uranga JJ Cabezudo-García P García-Martín G Romero-Godoy J Estivill-Torrús G et al . Cenobamate and clobazam combination as personalized medicine in autoimmune-associated epilepsy with anti-GAD65 antibodies. Neurol Neuroimmunol Neuroinflamm. (2023) 10:e200151. doi: 10.1212/NXI.0000000000200151
64.
Hung CC Chen CC Lin CJ Liou HH . Functional evaluation of polymorphisms in the human ABCB1 gene and the impact on clinical responses of antiepileptic drugs. Pharmacogenet Genomics. (2008) 18:390–402. doi: 10.1097/FPC.0b013e3282f85e36
65.
Zhao T Li HJ Feng J Zhang HL Ting-ting W Ma L et al . Impact of ABCB1 polymorphisms on Lacosamide serum concentrations in Uygur pediatric patients with epilepsy in China. Ther Drug Monit. (2022) 44:455–64. doi: 10.1097/FTD.0000000000000927
66.
Bjørke AB Nome CG Falk RS Gjerstad L Taubøll E Heuser K . Evaluation of long-term antiepileptic drug use in patients with temporal lobe epilepsy: assessment of risk factors for drug resistance and polypharmacy. Seizure. (2018) 61:63–70. doi: 10.1016/j.seizure.2018.07.011
67.
Zierath D Mizuno S Barker-Haliski M . Frontline Sodium Channel-blocking Antiseizure medicine use promotes future onset of drug-resistant chronic seizures. Int J Mol Sci. (2023) 24:4848. doi: 10.3390/ijms24054848
68.
Pawluski JL Kuchenbuch M Hadjadj S Dieuset G Costet N Vercueil L et al . Long-term negative impact of an inappropriate first antiepileptic medication on the efficacy of a second antiepileptic medication in mice. Epilepsia. (2018) 59:e109–13. doi: 10.1111/epi.14454
69.
Luoni C Bisulli F Canevini MP De Sarro G Fattore C Galimberti CA et al . Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. (2011) 52:2181–91. doi: 10.1111/j.1528-1167.2011.03325.x
70.
Androsova G Krause R Borghei M Wassenaar M Auce P Avbersek A et al . Comparative effectiveness of antiepileptic drugs in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. (2017) 58:1734–41. doi: 10.1111/epi.13871
71.
Perucca P Gilliam FG . Adverse effects of antiepileptic drugs. Lancet Neurol. (2012) 11:792–802. doi: 10.1016/S1474-4422(12)70153-9
Summary
Keywords
epilepsy, cenobamate, lacosamide, adverse events, pharmacovigilance
Citation
Shang S, Chen D, Song Z, Zhou C, Chang Q, Xiang L, Yu M, Zhao Y, Li W, Zhou F and Yu A (2025) Real-world safety comparison between cenobamate and lacosamide: a pharmacovigilance study based on the FDA Adverse Event Reporting System. Front. Neurol. 16:1625612. doi: 10.3389/fneur.2025.1625612
Received
09 May 2025
Accepted
14 August 2025
Published
02 September 2025
Volume
16 - 2025
Edited by
Lécio Figueira Pinto, University of São Paulo, Brazil
Reviewed by
Rudá Alessi, Faculdade de Medicina do ABC, Brazil
Nitish Chourasia, University of Tennessee Health Science Center (UTHSC), United States
Updates
Copyright
© 2025 Shang, Chen, Song, Zhou, Chang, Xiang, Yu, Zhao, Li, Zhou and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Airong Yu, yarfwy@163.com; Fan Zhou, 49886971@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.