Abstract
Introduction:
Dementia prevalence increases with age, underscoring the importance of early intervention for mild cognitive impairment (MCI). However, standard treatment recommendations for MCI remain lacking. Herbal medicine and acupuncture have been proposed as potential alternatives. This study evaluated the feasibility and effectiveness of these interventions in patients with MCI.
Methods:
We conducted a retrospective cohort study using data from a public health promotion program for community-dwelling older adults in Korea who received acupuncture, with or without herbal medicine, between 2021 and 2023. Feasibility was assessed by completion of acupuncture sessions and follow-up. Exact propensity score matching was performed using age, sex, comorbidities, depression scores, and health-related behaviors to compare herbal medicine add-on versus acupuncture only groups. Clinical outcomes included cognitive function [the Cognitive Impairment Screening Test (CIST) and the Montreal Cognitive Assessment (MoCA)] and depression [Geriatric Depression Scale–Short Form (GDS-SF)].
Results:
Of 5,525 participants, 4,623 received acupuncture with or without herbal medicine. Feasibility was high, with 86.4% completing planned acupuncture sessions; among these, 93.8% also received herbal medicine. Loss-to-follow-up rate was lower in the herbal medicine add-on (4.1%) than in the acupuncture-only (12.1%). After matching, 2,242 participants were included (2,044 herbal medicine add-on and 198 acupuncture-only). Both groups showed significant improvements in CIST, MoCA, and GDS-SF scores, with the herbal medicine add-on group demonstrating significantly greater cognitive improvement in the CIST (coefficient: 0.58; 95% CI, 0.10–1.08).
Conclusion:
Herbal medicine combined with acupuncture appeared feasible and potentially effective for managing MCI, supporting its practicality in community settings. However, its therapeutic benefits need to be further validated through rigorously designed randomized controlled trials. Long-term studies are warranted to confirm these findings and clarify their role in dementia prevention.
1 Introduction
The prevalence and burden of dementia are rapidly increasing with the aging global population. By 2050, more than two billion people (22% of the global population) will be aged ≥ 60 years (1), and the number of individuals living with dementia is projected to reach 139 million (2). The associated economic costs, estimated at US $1.3 trillion in 2019, are expected to rise substantially, underscoring the urgent need for early diagnosis and preventive interventions (2).
Mild cognitive impairment (MCI), often considered a prodromal stage of dementia, is defined as measurable cognitive decline with largely preserved daily functioning (3). MCI affects about 22% of adults aged ≥ 65 years (4), and carries a clinically significant risk of progression to Alzheimer’s disease, with reported annual conversion rates ranging from 14 to 87%, depending on study design and diagnostic criteria (5).
Although early intervention for MCI is of major importance to public health, therapeutic options remain limited (6). The 2022 American Academy of Neurology guidelines concluded that evidence is insufficient for pharmacological therapies and recommended nonpharmacological strategies such as exercise and cognitive training (7). However, adherence to these strategies is often poor in older adults because of limited awareness of MCI, comorbid conditions, low social support, and restricted accessibility (8, 9).
Consequently, traditional medicine approaches have attracted considerable attention. Systematic reviews have suggested that herbal medicine (10) and acupuncture (11) may improve cognitive function in patients with MCI. However, most trials were small, heterogeneous, and of limited generalizability.
To address these gaps, we conducted a retrospective cohort study to evaluate the feasibility and effectiveness of an 8–10-week combined intervention of herbal medicine and acupuncture in a local government–initiated dementia prevention program for community-dwelling older adults with MCI, thereby generating real-world evidence for the potential integration of traditional medicine into public health strategies.
2 Materials and methods
2.1 Data sources
We used the Korean Medicine Senior Health Promotion Program (KSHPP) cohort, which includes participants aged 60 years and older who utilized Korean medicine public health services in Seoul, Republic of Korea. The KSHPP was conducted in 2017. In this study, we retrospectively analyzed KSHPP cohort data from 2021 to 2023. During the program, the KSHPP provides acupuncture and/or herbal treatments (decoction or granules) to elderly individuals at high risk of MCI. The study protocol was approved by the Institutional Review Board of Kyung Hee University, Korea (KHSIRB-23-418[EA]).
The overall KSHPP process and outcome measurements are shown in Supplementary Figure 1. Informed consent was obtained from all the participants at the start of the program.
2.2 Subjects
The inclusion criteria were as follows: (1) participants in the KSHPP between 2021 and 2023; (2) aged 60 years or older; (3) Montreal Cognitive Assessment (MoCA) score <23 at baseline; (4) those who received acupuncture only or combined treatment of herbal decoction and acupuncture; (5) received acupuncture treatment from 12 to 20 sessions; and (6) followed the standard treatment protocol. The subjects were retrospectively divided into two groups: the herbal medicine add-on group (acupuncture and herbal medicine) and the acupuncture-only group. For analysis, we excluded (1) those who did not have available pre- and post-treatment MoCA, Cognitive Impairment Screening Test (CIST), and Geriatric Depression Scale-Short Form (GDS-SF) scores and (2) those with no matching subjects between the two groups.
2.3 Acupuncture
The acupuncture protocol was standardized. Treatment acupoints were GV20, EX-HN1, LI4, LR3, ST36, HT7, and PC6. The duration of acupuncture was 25 min. The recommended number of acupuncture sessions was 12–20 (twice weekly).
2.4 Herbal medicine
Each participant received one of eight herbal medicines as a decoction for 15 days. These herbal medicines are multi-herbal formulas as follows: Guibi-tang (12 herbs), Modified Guibi-tang (14 herbs), Yukmijihwang-tang (6 herbs), Cheonwangbosim-dan (15 herbs), Jowiseungcheong-tang (14 herbs), Ondam-tang (8 herbs), Modified Ukgan-san (9 herbs), and Hwanglyeonhaedok-tang (4 herbs). The detailed compositions of these herbal formulas are summarized in Supplementary Table 1. The selection of a specific herbal formula for each participant was determined by a doctor of Korean medicine according to the principles of pattern identification.
2.5 Outcome measurements
Feasibility was primarily assessed by the proportion of participants who completed acupuncture treatment, which was the mandatory component of the program. In addition, we evaluated the proportion of participants who additionally received herbal medicine and follow-up completion rates, stratified by treatment group.
Clinical outcomes were assessed as secondary objectives. To observe the effectiveness of the KSHPP using herbal medicine and acupuncture, we assessed improvement rate and the pre-post changes in the CIST, MoCA, and GDS-SF scores.
The improvement rate was calculated as the proportion of participants whose change in CIST scores exceeded the minimal clinically important difference (MCID). The MCID for the CIST was defined as 0.5 standard deviation (SD) of the normative values based on the previous review (12).
CIST is a cognitive screening test developed by a public medical institution for use in the National Dementia Screening Program in Korea (13). The CIST is free to use, and the materials can be downloaded from the website of the National Institute of Dementia in Korea. The cutoff score was presented according to age and years of education (range: 10–27 scores). A higher CIST score indicated an improvement in cognition.
The MoCA is a screening tool for MCI, rather than dementia, in older adults. The cutoff score was 26 in the original version (14); however, currently, a cutoff score of 23 is recommended for better diagnostic accuracy (15, 16). The Korean version of the MoCA has been validated in a clinical setting, and a cutoff score of 23 has been adopted (17). A higher MoCA score indicated an improvement in cognition.
The GDS-SF is used to evaluate depressive symptoms in older adults. This scale distinguishes between symptoms caused by increasing age and those caused by depression and consists of simple and easily understandable questions for older adults. The previously developed 30-item binary scale (18) was simplified to include 15 items (19), and the short form has been validated with a cutoff score of five in Korea (20). A lower GDS-SF score indicated an improvement in geriatric depression.
2.6 Study covariates
Age, sex, comorbidities such as hypertension, diabetes, and hyperlipidemia based on concomitant medications collected through history taking at baseline, geriatric depression assessed by the GDS-SF, and health-related behaviors such as smoking, drinking, and exercise based on self-reporting at baseline were obtained as covariates in this study. Adjustments for medications affecting cognition, such as anti-inflammatory drugs (e.g., steroids or biologics) and cholinergic agents (e.g., cholinesterase inhibitors or anticholinergics), could not be performed because the medical records primarily documented indications rather than specific drug names.
2.7 Statistical analysis
Patient baseline characteristics were summarized using descriptive statistics. Continuous variables were compared between the two treatment groups using either an independent samples t-test for normally distributed data or the Mann–Whitney U test for non-normally distributed data. For categorical variables, group comparisons were performed using the Chi-square test or Fisher’s exact test when appropriate.
To assess the changes between the pre- and post-treatment values within each group, the Wilcoxon signed-rank test was applied, as the data exhibited non-normality and included outliers. This non-parametric test was used to compare pre- and post-intervention differences in cognition and depression outcomes within each group.
Given the non-normality and presence of outliers in the outcome data, attributable to the chart-based nature of the data, we employed a robust regression approach (M-estimation) to further analyze the treatment effects. Robust regression was performed using the rlm function in the MASS package of R. This method provides robust estimates of regression coefficients when the data contain outliers or violate the assumptions of normality.
Owing to the significant imbalance in sample sizes between the two groups—3,852 patients in the herbal medicine add-on group and 222 patients in the acupuncture-only group—sensitivity analysis was conducted to assess the impact of this discrepancy on the results. To address the potential bias from unequal sample sizes, two propensity score matching (PSM) methods were applied: 1:1 nearest-neighbor matching and 1:n exact matching. Propensity scores were estimated using logistic regression and the participants were matched based on these scores. The standardized mean difference (SMD) was calculated for all covariates to evaluate the balance achieved through matching; an SMD of less than 20% was considered indicative of successful balancing. After matching, robust regression was reapplied to assess the treatment effects in a more balanced sample, considering weight adjustment for the exact matching method.
To enhance the robustness of our findings and explore the consistency of treatment effects, we performed two sets of additional analyses on the propensity score-matched cohort. First, within the herbal add-on group, we used robust regression model (M-estimation) to compare changes in cognitive outcomes (ΔCIST) across major herbal prescriptions. The model was adjusted for the study covariates described in Section 2.6. This analysis allowed us to investigate whether specific herbal formulas demonstrated differential efficacy. Second, we conducted pre-specified subgroup analyses using robust regression stratified by sex (male vs. female) and baseline depressive symptom risk (GDS-SF ≥ 5 vs. <5). This allowed us to examine the consistency of treatment effects across these key patient characteristics. We also fitted interaction terms between treatment allocation and subgroup in the same robust regression framework to determine if the treatment effect significantly differed between them.
The significance of the results was evaluated using t-values and confidence intervals (CIs). Statistical significance was set at p < 0.05. All analyses were performed using R version 4.2.3, with the MASS and MatchIt packages used for robust regression and PSM, respectively.
3 Results
3.1 Characteristics of subjects
Of the 5,525 participants initially registered in the KSHPP cohort, 4, 870 were at risk for MCI and aged 60 years or older. Of these, 4,623 received acupuncture treatment, and 3,992 (86.4%) completed the treatment. After matching, we obtained a matched sample of 2,242 patients, consisting of 2,044 herbal medicine add-on users and 198 acupuncture-only users, for the final analysis (Figure 1). As adjusted SMD of each outcome variable was smaller than 0.2, baseline outcome balance after matching was confirmed. Table 1 presents the baseline characteristics of the two groups.
Figure 1

Flow chart of the study.
Table 1
| Characteristics | Herbal medicine add-on (n = 2,044) | Acupuncture- only (n = 198) |
|---|---|---|
| Demographic characteristics | ||
| Age [year, median (IQR)] | 74.0 [71.0, 78.0] | 75.0 [71.0, 80.0] |
| Women (n, %) | 1,884 (92.2) | 157 (79.3) |
| Education [year, median (IQR)] | 7.0 [6.0, 12.0] | 7.0 [6.0, 12.0] |
| Clinical characteristics | ||
| Comorbidities (n, %)a | 1,414 (69.2) | 158 (79.8) |
| Hypertension (n, %) | 1,043 (51.0) | 122 (61.6) |
| Diabetes mellitus (n, %) | 328 (16.0) | 63 (31.8) |
| Hyperlipidemia (n, %) | 582 (28.5) | 66 (33.3) |
| Geriatric depression (n, %)b | 1,433 (70.1) | 131 (66.2) |
| Health-related behaviorsc | ||
| Smoking (n, %) | 0 (0) | 0 (0) |
| Drinking (n, %) | 88 (4.3) | 21 (10.6) |
| Exercise (n, %) | 1,501 (73.4) | 135 (68.2) |
| Cognitive function and depression | ||
| CIST [median (IQR)] | 19.0 [16.0, 23.0] | 18.0 [15.0, 22.0] |
| MoCA [median (IQR)] | 19.0 [16.0, 21.0] | 17.0 [15.0, 20.0] |
| GDS-SF [median (IQR)] | 7.0 [4.0, 10.0] | 6.0 [3.0, 9.0] |
Baseline characteristics after exact propensity score matching.
CIST, Cognitive Impairment Screening Test; GDS-SF, Geriatric Depression Scale-Short Form; IQR, interquartile range; MoCA, Montreal Cognitive Assessment.
Comorbidities were identified based on concomitant medications collected through history taking at baseline.
Geriatric depression was assessed using the Geriatric Depression Scale-Short Form (GDS-SF) at baseline. A GDS-SF score of 5 or higher was considered indicative of risk for geriatric depression.
Health-related behaviors were presented based on self-reporting at baseline.
3.2 Feasibility
Because acupuncture was a mandatory component of the program, feasibility was primarily evaluated by completion of the acupuncture sessions. Among the 4,623 participants who initiated acupuncture, 86.4% (3,992/4,623) completed 12–20 sessions. Of these, 93.8% (3,744/3,992) also received herbal medicine. The loss-to-follow-up rate was 4.1% in the herbal medicine add-on group and 12.1% in the acupuncture-only group.
3.3 Cognitive function and depression before and after treatment
The improvement rates were 74.2% in the herbal medicine add-on group and 69.2% in the acupuncture-only group. Scores on the CIST, MoCA, and GDS-SF significantly improved from baseline to post-treatment in both groups (all p < 0.05). However, when comparing the change in each variable between groups, a significant difference was found only in the CIST (p < 0.05). These results are presented in Table 2.
Table 2
| Outcomes | Herbal medicine add-on (n = 2,044) | Acupuncture- only (n = 198) |
|---|---|---|
| Improvement (n, %) | 1,516 (74.2%) | 137 (69.2%) |
| CIST (mean, SD) | ||
| Pre | 19.1 ± 4.7 | 18.4 ± 4.9 |
| Post | 23.2 ± 4.4a | 21.9 ± 4.5a |
| Change | 4.0 ± 3.7 b | 3.4 ± 3.9 |
| MoCA (mean, SD) | ||
| Pre | 17.7 ± 3.7 | 17.0 ± 3.9 |
| Post | 21.4 ± 4.4a | 20.6 ± 4.7a |
| Change | 3.7 ± 3.4 | 3.6 ± 3.6 |
| GDS-SF (mean, SD) | ||
| Pre | 6.7 ± 3.9 | 6.3 ± 3.8 |
| Post | 5.3 ± 3.7a | 5.2 ± 3.9a |
| Change | −1.5 ± 3.0 | −1.1 ± 2.5 |
Changes in cognition and depression outcome after exact propensity score matching.
CIST, Cognitive Impairment Screening Test; GDS-SF, Geriatric Depression Scale-Short Form; MoCA, Montreal Cognitive Assessment; SD, standard deviation.
Within-group difference comparing pre- and post-values; Wilcoxon signed rank test, p < 0.05.
Between-group difference comparing change from baseline values in each group; Mann Whitney U test, p < 0.05.
3.4 Add-on effect of herbal medicine between groups
The use of herbal medicine was associated with cognition improvement measured by CIST (p < 0.05; coefficients of 0.58 [95% CI, 0.10 to 1.08]) (Table 3). Regarding different herbal medicine formulas, robust regression showed no statistically significant differences in changes in cognitive scores (ΔCIST) across major herbal prescriptions after adjusting for study covariates within the herbal add-on group. Compared with Guibi-tang as the reference group, other formulas such as Ondam-tang (coefficient 0.23 [95% CI, −0.50 to 0.95]) and Modified Ukgan-san (0.75 [95% CI, −0.19 to 1.69]) showed numerically higher scores, but these differences were not statistically significant (Supplementary Table 2).
Table 3
| Outcomes | Coefficients | Std. error | t value | 95% CI |
|---|---|---|---|---|
| CIST | 0.58 | 0.249 | 2.362 | (0.10, 1.08) |
| MoCA | 0.20 | 0.237 | 0.863 | (−0.26, 0.67) |
| GDS-SF | 0.10 | 0.201 | 0.469 | (−0.30, 0.49) |
Robust regression analysis between two groups after exact propensity score matching.
CIST, Cognitive Impairment Screening Test; GDS-SF, Geriatric Depression Scale-Short Form; MoCA, Montreal Cognitive Assessment.
The results of the robust regression analysis showed that the herbal medicine add-on group had an average increase of 0.20 in MoCA difference and an average increase of 0.10 in GDS-SF difference, neither of which was statistically significant (Table 3).
3.5 Subgroup analyses
Pre-specified subgroup analyses indicated that the association between herbal medicine add-on treatment and CIST improvement was generally consistent across subgroups (Figure 2). The coefficient for CIST improvement was 0.63 (95% CI, 0.04 to 1.22) for patients at high risk of depressive symptoms and 0.51 (95% CI, −0.35 to 1.37) for those at normal risk. Similarly, coefficients were 1.30 (95% CI, −0.47 to 3.07) for males and 0.53 (95% CI, 0.02 to 1.04) for females. Tests for interaction between treatment allocation and subgroup were not statistically significant (p for interaction = 0.829 for GDS-SF risk and 0.409 for sex), suggesting that the treatment effect was consistent across these strata.
Figure 2
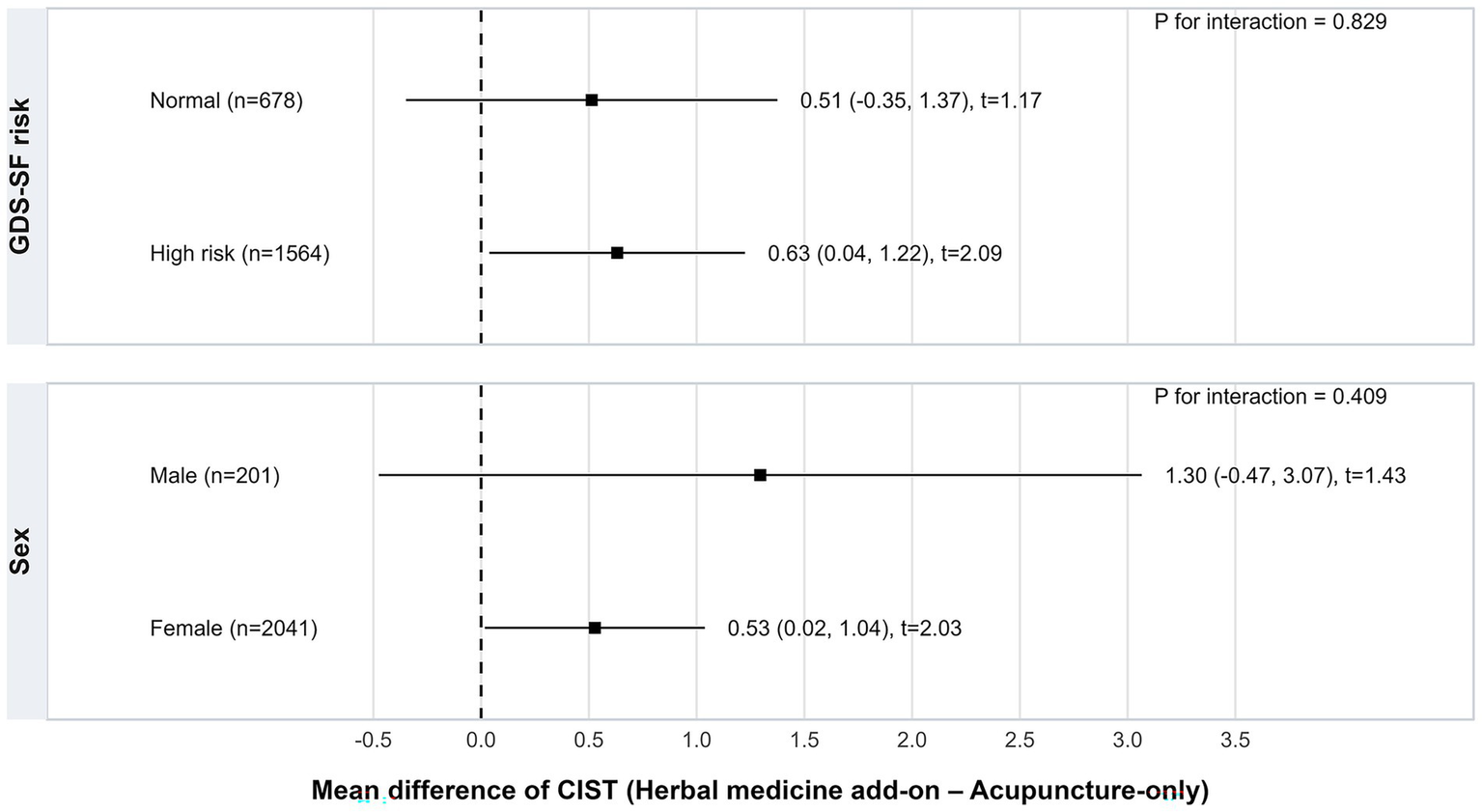
Subgroup analysis of adjusted treatment effects on CIST score change stratified by baseline GDS-SF risk and sex (exact-matched cohort). CIST, Cognitive Impairment Screening Test; GDS-SF, Geriatric Depression Scale-Short Form.
3.6 Sensitivity analyses
To assess the robustness of our findings and explore the consistency of treatment effects, we performed several additional analyses. We examined the results using 1:1 PSM with the same covariates from 422 participants (211 per group) and the association between herbal medicine use and cognitive improvement was consistent in the sensitivity tests (Supplementary Table 3). We also performed quantitative bias analyses, calculating E-values to determine the minimum strength of association an unmeasured confounder would need to have with both the treatment and the outcome to completely explain our findings. For the continuous outcome (ΔCIST), the point estimate had an E-value of 2.67, indicating that only a relatively strong unmeasured confounder could nullify the observed association.
4 Discussion
Although the clinical significance of managing MCI is well recognized, therapeutic options remain limited. Pharmacological interventions, such as cholinesterase inhibitors, have shown modest improvements in cognitive function but have not demonstrated efficacy in delaying or preventing progression to dementia (21, 22). Nonpharmacological strategies, including physical activity and cognitive training, are recommended (7, 23), but their effectiveness depends heavily on sustained patient engagement. This can be particularly challenging for older adults with MCI, who often experience cognitive limitations, low health literacy, or limited accessibility (8, 9).
Given this context, we examined herbal medicine and acupuncture, which are widely practiced and culturally accessible in East Asia, as alternative interventions. The herbal formulas (24–33) and acupuncture protocols (34, 35) used in this study have been previously reported to improve cognitive function. These approaches are supported by surveys of Korean medicine practitioners and are consistent with the Korean medicine clinical practice guidelines for dementia and MCI (36–38).
Systematic reviews have shown that most randomized controlled trials (RCTs) investigated either herbal medicine (39) or acupuncture (11) compared with conventional pharmacological agents such as donepezil or nimodipine. However, these two modalities are commonly combined in routine Korean medical practice. Therefore, evaluating the relative effectiveness of acupuncture alone versus combination therapy provides clinically relevant insights into real-world practice.
In this large-scale observational study, we demonstrated the feasibility and acceptability of these interventions among community-dwelling older adults with MCI. Of the 4,623 eligible participants, 86.4% (n = 3,992) completed acupuncture treatment, and among these, 93.8% also received herbal medicines. Importantly, the combination therapy group exhibited lower dropout rates compared with the acupuncture-only group (4.1% vs. 12.1%), indicating that integrated therapy may enhance adherence and feasibility in this population.
To address potential confounding, we conducted PSM using demographic, lifestyle, and comorbidity factors known to influence MCI progression. Previous studies have shown that advanced age and female sex increase the risk of conversion from MCI to Alzheimer’s disease, while chronic conditions such as hypertension, diabetes, and hyperlipidemia, as well as smoking and alcohol use, further accelerate decline (40, 41). Conversely, healthy behaviors—including mental activity, fruit consumption, and social engagement—promote recovery to cognitive normalcy, whereas depression predicts deterioration (42, 43). By adjusting for these covariates, we sought to provide more reliable estimates of treatment effects.
Because the CIST is a relatively new assessment tool developed in Korea in 2021, previous studies using the CIST are limited and have not established MCID. Thus, we defined “improvement” as an individual pre–post change exceeding 0.5 SD of the normative data, adjusted for age and education level, ranging from 0.57 to 2.41 points. Following matching, the herbal add-on group showed an average improvement of 4 points, whereas the acupuncture-only group showed an average improvement of 3.4 points. Based on our definition, 74.2% of participants in the herbal add-on group and 69.2% in the acupuncture-only group were classified as showing improvement.
Meanwhile, robust regression showed that the herbal medicine add-on group had significantly greater improvement in CIST scores (coefficient 0.58 [95% CI, 0.10–1.08]). Sensitivity analyses yielded an even larger effect size, reinforcing the robustness of the findings. The greater increase was observed in sensitivity analyses using 1:1 PSM (0.95 [95% CI, 0.30–1.60]). Our results align with prior evidence: an RCT reported that acupuncture combined with herbal medicine (Yishen granules) improved MoCA scores by 1.63 points compared with acupuncture plus placebo (44), and a systematic review showed that herbal medicine increased Mini Mental State Examination scores by 1.90 points compared with placebo (10). As noted above, it was not feasible to establish a single-point estimate for the MCID in our study. For other cognitive instruments such as the Mini Mental State Examination and MoCA, however, changes of approximately 1–2 points have been suggested as indicative of the MCID in populations with MCI (45, 46). The observed between-group difference of 0.58 points (95% CI, 0.10–1.08) falls below this suggested range. Although the upper bound of the confidence interval approached the lower limit of the MCID, the overlap indicates that the clinical significance remains uncertain. Taken together, the overall direction and magnitude of our findings are broadly consistent with prior herbal medicine trials, albeit with smaller effect sizes. The relatively short intervention period of 15 days, compared with the median 6-month duration in previous RCTs, may partly account for this difference.
Within the herbal add-on group, robust regression adjusting for study covariates revealed no statistically significant differences across major herbal prescriptions. Although Ondam-tang (coefficient 0.23 [95% CI, −0.50 to 0.95]) and Modified Ukgan-san (0.75 [95% CI, −0.19 to 1.69]) showed numerically higher improvements compared with Guibi-tang, these differences were not statistically significant.
We performed subgroup analyses based on sex and baseline depressive symptom risk because previous studies indicates that sex and geriatric depression may influence cognitive trajectories and responses to interventions in MCI. Epidemiological studies have shown sex differences in the prevalence, progression, and neuropathological features of MCI and dementia, with women often exhibiting higher risk of cognitive decline (47). Similarly, depressive symptoms are known to affect cognitive function, with evidence suggesting that individuals with higher depressive symptom burden may experience different cognitive outcomes compared with those at low risk (48, 49). Therefore, we explored these subgroups to assess whether the effects of the herbal add-on differed according to sex or baseline depression risk, while acknowledging that our findings are exploratory and interaction tests did not reach statistical significance.
The cognitive benefits observed in this study may be supported by several biological mechanisms of herbal medicine. MCI is thought to arise from multiple interacting mechanisms, including amyloid-β deposition and tau hyperphosphorylation leading to synaptic loss (50), oxidative stress, and mitochondrial dysfunction (51), deficits in neurotransmitter systems, particularly cholinergic dysfunction (52), chronic neuroinflammation (53), and reduced neurogenesis and neurotrophic support such as decreased brain-derived neurotrophic factor (54). Herbal medicines have been reported to inhibit amyloid aggregation (55), exert antioxidant effects via activation of Nrf2 (56), and regulate cholinergic neurotransmission, for example through acetylcholinesterase inhibition (57). In addition, herbal medicines can modulate neuroinflammation by regulating cytokine expression (58) and promote neurogenesis through upregulation of neurotrophic factors (59). Collectively, these multi-target actions provide plausible biological explanations for the cognitive improvements associated with herbal medicine add-on therapy observed in our study and support the clinical relevance of the findings.
These findings suggest that acupuncture and herbal medicine may contribute to delaying or attenuating cognitive decline in patients with MCI, supporting their potential role as a prevention program. South Korea is currently pursuing the “National Responsibility for Dementia” initiative through the 4th National Dementia Management Plan (2021–2025) (60), which establishes a nationwide framework for prevention, early diagnosis, and long-term care; however, preventive interventions remain limited. Our results provide preliminary evidence to consider incorporating public health promotion programs using acupuncture and herbal medicine into primary-care settings—including private clinics, public health centers, and dementia care centers—particularly for older adults (61). Integrating acupuncture and herbal medicine could help support a policy shift toward prevention-oriented dementia management.
Several limitations should be acknowledged. First, the retrospective observational design may not fully eliminate residual confounding, despite PSM adjustment for nine covariates. However, the E-value of 2.67 indicates that only an unmeasured confounder of moderate strength could explain away the observed association, suggesting that residual confounding is unlikely to substantially bias the results. Second, participants were grouped according to the treatment they received, resulting in unequal sample sizes between groups. Nevertheless, baseline balance was confirmed after exact PSM, with all outcome variables showing SMDs < 0.2. Additionally, 1:1 PSM was performed as a sensitivity analysis. Third, neither participants nor practitioners were not blinded, and cognitive outcomes were assessed by the same practitioners, which may have introduced performance and detection biases (62). Fourth, the short herbal treatment duration (15 days) and follow-up interval (8–10 weeks) contrast with prior RCTs that typically evaluated treatment over at least 2 months (10, 39). These design features may have led to underestimation of long-term benefits or, conversely, overestimation due to short-term learning effects. Future studies should adopt longer treatment durations and extended follow-up to better evaluate sustained outcomes. Lastly, in our study, although data were collected from multiple sites, site-level clustering was not explicitly modeled, and residual variation attributable to clinic-specific factors may exist. Future studies with larger sample sizes should consider multi-level modeling or generalized estimating equations to account for potential clinic-level effects. Moreover, the generalizability of our findings may be limited beyond the specific clinical settings included in this study. Differences in patient demographics, practitioner expertise, or treatment delivery across other sites could affect the observed outcomes. Therefore, caution is warranted when extrapolating these results to other populations or healthcare settings, and replication in broader and more diverse contexts is recommended.
5 Conclusion
In conclusion, our findings suggest that herbal medicine combined with acupuncture is a feasible and potentially effective intervention for managing MCI. High adherence and reduced dropout in the combination group highlight the practicality of this approach for community-based programs. Future well-designed, long-term studies are warranted to confirm these results and to determine whether integration of traditional medicine into clinical practice and public health strategies could contribute to dementia prevention.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the raw data supporting the conclusion of this article is available from the corresponding author, upon reasonable request. Requests to access these datasets should be directed to S-YC, lovepwr@khu.ac.kr.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Kyung Hee University, Korea. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
H-WS: Formal analysis, Data curation, Writing – original draft. JHL: Data curation, Conceptualization, Project administration, Writing – review & editing, Supervision. J-wS: Writing – review & editing. CP: Writing – review & editing. Y-SP: Writing – review & editing, Conceptualization, Project administration. S-YC: Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Seoul Korean Medicine Association and Seoul Metropolitan Government.
Acknowledgments
The authors would like to express their appreciation to all the doctors of Korean medicine who participated in the KSHPP as healthcare providers. We thank the Seoul Korean Medicine Association for supporting the collection of patient data from local clinics. We also thank Hyo-been Lee from the Healthcare Big Data Center, Research Institute of Clinical Medicine, Kyung Hee University Hospital at Gangdong, for assistance with the statistics used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1628794/full#supplementary-material
Abbreviations
CI, confidence interval; CIST, Cognitive Impairment Screening Test; GDS-SF, Geriatric Depression Scale-Short Form; IQR, interquartile range; KSHPP, Korean Medicine Senior Health Promotion Program; MCI, mild cognitive impairment; MCID, minimal clinically important difference; MoCA, Montreal Cognitive Assessment; PSM, propensity score matching; RCT, randomized controlled trial; SD, standard deviation; SMD, standardized mean difference.
References
1.
World Health Organization . Ageing and health [online]. (2022) Available online at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed November 9, 2024).
2.
World Health Organization . Global status report on the public health response to dementia. Geneva: World Health Organization (2021).
3.
Bradfield NI . Mild cognitive impairment: diagnosis and subtypes. Clin EEG Neurosci. (2023) 54:4–11. doi: 10.1177/15500594211042708
4.
Manly JJ Jones RN Langa KM Ryan LH Levine DA Mccammon R et al . Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. (2022) 79:1242–9. doi: 10.1001/jamaneurol.2022.3543
5.
Chen Y Qian X Zhang Y Su W Huang Y Wang X et al . Prediction models for conversion from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci. (2022) 14:840386. doi: 10.3389/fnagi.2022.840386
6.
Chen Y-X Liang N Li X-L Yang S-H Wang Y-P Shi N-N . Diagnosis and treatment for mild cognitive impairment: a systematic review of clinical practice guidelines and consensus statements. Front Neurol. (2021) 12:719849. doi: 10.3389/fneur.2021.719849
7.
Petersen R Lopez O Armstrong M Getchius T Ganguli M Gloss D et al . Practice guideline update summary: mild cognitive impairment. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
8.
Mohammad Hanipah J Mat Ludin AF Singh DKA Subramaniam P Shahar S . Motivation, barriers and preferences of lifestyle changes among older adults with frailty and mild cognitive impairments: a scoping review of qualitative analysis. PLoS One. (2025) 20:e0314100. doi: 10.1371/journal.pone.0314100
9.
Sun J Zhao Y Wang A . Barriers to self-management engagement among community-dwelling older adults with mild cognitive impairment: a qualitative study. Front Psychiatry. (2025) 15:1446344. doi: 10.3389/fpsyt.2024.1446344
10.
Liu L Zhang CS Zhang AL Cai Y Xue CC . The efficacy and safety of Chinese herbal medicine for mild cognitive impairment: a systematic review and meta-analysis of randomized placebo-controlled trials. Front Pharmacol. (2024) 15:1341074. doi: 10.3389/fphar.2024.1341074
11.
Lin G Yie SLJ Guo S Li X Xu L . Clinical evidence of acupuncture for amnestic mild cognitive impairment: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2024) 88:103114. doi: 10.1016/j.ctim.2024.103114
12.
Draak THP De Greef BTA Faber CG Merkies ISJ PeriNomS study group . The minimum clinically important difference: which direction to take. Eur J Neurol. (2019) 26:850–5. doi: 10.1111/ene.13941
13.
Ministry of Health and Welfare . Study to develop Korean dementia screening test. Sejong, Korea: The Ministry of Health and Welfare (2020).
14.
Nasreddine ZS Phillips NA Bédirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
15.
Carson N Leach L Murphy KJ . A re-examination of Montreal cognitive assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. (2018) 33:379–88. doi: 10.1002/gps.4756
16.
Luis CA Keegan AP Mullan M . Cross validation of the Montreal cognitive assessment in community dwelling older adults residing in the southeastern US. Int J Geriatr Psychiatry. (2009) 24:197–201. doi: 10.1002/gps.2101
17.
Lee J-Y Lee DW Cho S-J Na DL Jeon HJ Kim S-K et al . Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal cognitive assessment. J Geriatr Psychiatry Neurol. (2008) 21:104–10. doi: 10.1177/0891988708316855
18.
Yesavage JA Brink TL Rose TL Lum O Huang V Adey M et al . Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. (1982) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
19.
Yesavage JA Sheikh JI . 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol. (1986) 5:165–73.
20.
Kee B-S . A preliminary study for the standardization of geriatric depression scale short form-Korea version. J Korean Neuropsychiatr Assoc. (1996) 35:298–307.
21.
Kasper S Bancher C Eckert A Förstl H Frölich L Hort J et al . Management of mild cognitive impairment (MCI): the need for national and international guidelines. World J Biol Psychiatry. (2020) 21:579–94. doi: 10.1080/15622975.2019.1696473
22.
Pyun J-M Lee I Lee K Kim M-H Park C Yang H-J . Effect of choline Alfoscerate on the progression from mild cognitive impairment to dementia: distributed network analysis of a Multicenter Korean database using a common data model. Dement Neurocogn Disord. (2024) 23:202–11. doi: 10.12779/dnd.2024.23.4.202
23.
Ravona-Springer R. Korczyn A. Management of Mild Cognitive Impairment. Oxford, United Kingdom: Oxford University Press (2020).
24.
Arai H Suzuki T Sasaki H Hanawa T Toriizuka K Yamada H . A new interventional strategy for Alzheimer's disease by Japanese herbal medicine. Nihon Ronen Igakkai Zasshi. (2000) 37:212–5. doi: 10.3143/geriatrics.37.212
25.
Chen G Shan P Qiu X . The clinical study on Huanglianjiedu decoction on for patients with senile dementia, the type of hyperactivity of heart-fire and liver fire in TCM. Zhongguo Zhongyi Jizheng. (2007) 16:386–7. Available online at: https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=ZYJZ200704004&uniplatform=OVERSEA&v=8zKuUWGzNV-PYn6jnJeSETxXyPpW79oSgyGwQDJ6GBlBQfcTHY4Wjolv7DGUc6gK (Accessed October 16, 2025).
26.
Choi Y Kim A-R Lee J-Y Kim HS Yang C Kim JK et al . Herbal medicine for patients with cognitive impairment: an observational study. Neuropsychiatr Dis Treat. (2021) 17:3183–94. doi: 10.2147/NDT.S333569
27.
Katsumoto E Ishida T Kinoshita K Shimizu M Tsutsumi T Nagai Y et al . Yokukansankachimpihange is useful to treat behavioral/psychological symptoms of dementia. Front Nutr. (2021) 7:529390. doi: 10.3389/fnut.2020.529390
28.
Park K Lee WH Cho E Kong CH Min HS Kim MS et al . The effects of Cheonwangbosim-dan, a traditional herbal medicine prescription, on scopolamine-induced cognitive dysfunction in mice. J Ethnopharmacol. (2025) 343:119500. doi: 10.1016/j.jep.2025.119500
29.
Shin H-Y Kim H-R Jahng G-H Jin C Kwon S Cho S-Y et al . Efficacy and safety of kami-guibi-tang for mild cognitive impairment: a pilot, randomized, double-blind, placebo-controlled trial. BMC Complement Med Ther. (2021) 21:251. doi: 10.1186/s12906-021-03428-6
30.
Shin SJ Jeong Y-O Jeon SG Kim S Lee S-K Nam Y et al . Jowiseungchungtang inhibits amyloid-β aggregation and amyloid-β-mediated pathology in 5XFAD mice. Int J Mol Sci. (2018) 19:4026. doi: 10.3390/ijms19124026
31.
Watari H Shimada Y Matsui M Tohda C . Kihito, a traditional Japanese kampo medicine, improves cognitive function in Alzheimer’s disease patients. Evid Based Complement Alternat Med. (2019) 2019:4086749. doi: 10.1155/2019/4086749
32.
Yim T-B Jeon G-R Lee HJ Lee K-H Heo H-M Lee HG et al . The effectiveness of kami Guibi-tang for cognitive impairment patients: a retrospective chart review. Heliyon. (2023) 10:e23615. doi: 10.1016/j.heliyon.2023.e23615
33.
Zhang P Han Y Yao C Zou G Sui Y Liu L . Effect of Huanglian Wendan decoction on metabolic syndrome and cognitive function. Chinese J. Integr. Med. Cardio Cereb Dis. (2024) 22:2923–7. Available online at: https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2024&filename=ZYYY202416006&uniplatform=OVERSEA&v=0a3_7YUP5UTgQkWVMu7soDp9M6DVs5YAbrm_gdWv8oqKlXlV2rvWW3IH_0l4ipGv (Accessed October 16, 2025).
34.
Sun Yz ZP Zhang M Zhang Y . Clinical observation on Yuanluo Tongjing needling method for treatment of mild cognitive impairment. Chin Acupunct. Moxibust. (2007) 27:810–2. Available online at: https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=ZGZE200711008&uniplatform=OVERSEA&v=dvPiy8GilhQOyv-dwNHk6uIoSQefLZvLPszjjyTzZs2h9A4Go5FslOKyPFqvdXmR (Accessed October 16, 2025).
35.
Tan TT Wang D Huang JK Zhou XM Yuan X Liang JP et al . Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regen Res. (2017) 12:250–8. doi: 10.4103/1673-5374.200808
36.
Lee G-E Jeon W-K Heo E-J Yang HD Kang H-W . The study on the Korean traditional medical treatment and system of collaborative practice between Korean traditional medicine and western medicine for dementia: based on analysis of questionnaire survey in professional group. J Orient Neuropsychiatr. (2012) 23:49–68. doi: 10.7231/jon.2012.23.4.049
37.
Seo YK You DK Kim H Kim S Kim S-H Kang H-W et al . A survey of the recognition on the practice pattern, diagnosis, and treatment of Korean medicine of dementia and mild cognitive impairment-focusing on the differences between neuropsychiatrists of Korean medicine and general physicians. J Orient Neuropsychiatr. (2017) 28:263–74. doi: 10.7231/jon.2017.28.3.263
38.
The Society of Korean Medicine Neuropsychiatry . Korean medicine clinical practice guideline for dementia. Seoul: The Society of Korean Medicine Neuropsychiatry (2021).
39.
Liu L Zhang CS Zhang AL Cai Y Xue CC . Oral Chinese herbal medicine combined with donepezil for mild cognitive impairment: a systematic review and meta-analysis. J Am Geriatr Soc. (2024) 72:3890–902. doi: 10.1111/jgs.19125
40.
Jones A Ali MU Kenny M Mayhew A Mokashi V He H et al . Potentially modifiable risk factors for dementia and mild cognitive impairment: an umbrella review and meta-analysis. Dement Geriatr Cogn Disord. (2024) 53:91–106. doi: 10.1159/000536643
41.
Xue H Sun Q Liu L Zhou L-Y Liang R He R-L et al . Risk factors of transition from mild cognitive impairment to Alzheimer's disease and death: a cohort study. Compr Psychiatry. (2017) 78:91–7. doi: 10.1016/j.comppsych.2017.07.003
42.
Kaduszkiewicz H Eisele M Wiese B Prokein J Luppa M Luck T et al . Prognosis of mild cognitive impairment in general practice: results of the German AgeCoDe study. Ann Fam Med. (2014) 12:158–65. doi: 10.1370/afm.1596
43.
Sha F Zhao Z Wei C Yang Z Li B . Modifiable factors associated with reversion from mild cognitive impairment to cognitively normal status: a prospective cohort study. J Alzheimer's Dis. (2021) 86:1897–906. doi: 10.3233/JAD-215677
44.
Li N Li O Sha Z Wang Y Li Z Li Y et al . Efficacy and safety of acupuncture combined with Yishen granule in elderly adults with mild cognitive impairment: a multicenter, randomized controlled trial. Altern Ther Health Med. (2023) 29:340–9. Available online at: https://alternative-therapies.com/oa/index.html?fid=8151 (Accessed October 16, 2025).
45.
Cheng S Qin J Hou C Wu Y Du X Liu H et al . Linking cognitive screening tests in community-dwelling older adults: crosswalk between the Montreal cognitive assessment-basic and the Mini-mental state examination. J Am Med Dir Assoc. (2025) 26:105550. doi: 10.1016/j.jamda.2025.105550
46.
Muir RT Hill MD Black SE Smith EE . Minimal clinically important difference in Alzheimer's disease: rapid review. Alzheimers Dement. (2024) 20:3352–63. doi: 10.1002/alz.13770
47.
Levine DA Gross AL Briceño EM Tilton N Giordani BJ Sussman JB et al . Sex differences in cognitive decline among US adults. JAMA Netw Open. (2021) 4:e210169. doi: 10.1001/jamanetworkopen.2021.0169
48.
Livingston G Huntley J Liu KY Costafreda SG Selbæk G Alladi S et al . Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet. (2024) 404:572–628. doi: 10.1016/S0140-6736(24)01296-0
49.
Oh DJ Han JW Bae JB Kim TH Kwak KP Kim BJ et al . Chronic subsyndromal depression and risk of dementia in older adults. Aust N Z J Psychiatry. (2021) 55:809–16. doi: 10.1177/0004867420972763
50.
Hanseeuw BJ Betensky RA Jacobs HIL Schultz AP Sepulcre J Becker JA et al . Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. (2019) 76:915–24. doi: 10.1001/jamaneurol.2019.1424
51.
Song T Song X Zhu C Patrick R Skurla M Santangelo I et al . Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer's disease: a meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev. (2021) 72:101503. doi: 10.1016/j.arr.2021.101503
52.
Hampel H Mesulam MM Cuello AC Farlow MR Giacobini E Grossberg GT et al . The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain. (2018) 141:1917–33. doi: 10.1093/brain/awy132
53.
Nordengen K Kirsebom BE Henjum K Selnes P Gísladóttir B Wettergreen M et al . Glial activation and inflammation along the Alzheimer's disease continuum. J Neuroinflammation. (2019) 16:46. doi: 10.1186/s12974-019-1399-2
54.
Mori Y Tsuji M Oguchi T Kasuga K Kimura A Futamura A et al . Serum BDNF as a potential biomarker of Alzheimer's disease: verification through assessment of serum, cerebrospinal fluid, and medial temporal lobe atrophy. Front Neurol. (2021) 12:653267. doi: 10.3389/fneur.2021.653267
55.
Ganapathy AA Haripriya VM Acharya N Somappa SB Kumaran A . Ethnobotanical significance of medicinal plants: beta-amyloid and tau aggregation inhibitors against Alzheimer's disease. J Biochem Mol Toxicol. (2023) 37:e23339. doi: 10.1002/jbt.23339
56.
Gugliandolo A Bramanti P Mazzon E . Activation of Nrf2 by natural bioactive compounds: a promising approach for stroke?Int J Mol Sci. (2020) 21:4875. doi: 10.3390/ijms21144875
57.
Patel SS Raghuwanshi R Masood M Acharya A Jain SK . Medicinal plants with acetylcholinesterase inhibitory activity. Rev Neurosci. (2018) 29:491–529. doi: 10.1515/revneuro-2017-0054
58.
Jang J-H Jun HJ Lee C Sohn E Kwon O Kang D-H et al . Therapeutic potential of combined herbal medicine and electroacupuncture in mild cognitive impairment through cytokine modulation: an observational study. Neuropsychiatr Dis Treat. (2024) 21:1331–44. doi: 10.2147/NDT.S465650
59.
Yang W-T Zheng X-W Chen S Shan C-S Xu Q-Q Zhu J-Z et al . Chinese herbal medicine for Alzheimer’s disease: clinical evidence and possible mechanism of neurogenesis. Biochem Pharmacol. (2017) 141:143–55. doi: 10.1016/j.bcp.2017.07.002
60.
Ministry of Health and Welfare . The 4th National Dementia Management Plan (2021–2025). Sejong: Ministry of Health and Welfare (2020).
61.
Kim S Choi H Kim M Kang J . The current status and policy directions of dementia care in rural super-aged society. Naju, Republic of Korea: Korea Rural Economic Institute (KREI) (2025).
62.
Sterne JA Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
Summary
Keywords
cognitive dysfunction, mild cognitive impairment, herbal medicine, acupuncture therapy, propensity score
Citation
Suh H-W, Lim JH, Suh J-w, Park C, Park Y-S and Chung S-Y (2025) Herbal medicine and acupuncture for mild cognitive impairment: a retrospective study of 2,242 for older adults in Republic of Korea. Front. Neurol. 16:1628794. doi: 10.3389/fneur.2025.1628794
Received
20 May 2025
Accepted
06 October 2025
Published
29 October 2025
Volume
16 - 2025
Edited by
Jiu Chen, Nanjing University, China
Reviewed by
Ayon Nandi, Johns Hopkins University, United States
Mukaila Raji, University of Texas Medical Branch at Galveston, United States
Updates
Copyright
© 2025 Suh, Lim, Suh, Park, Park and Chung.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sun-Yong Chung, lovepwr@khu.ac.kr
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.